Abstract
Electrophysiological and pharmacological properties of glycine receptors were characterized in hippocampal organotypic slice cultures. In the presence of ionotropic glutamate and GABAB receptor antagonists, pressure-application of glycine onto CA3 pyramidal cells induced a current associated with increased chloride conductance, which was inhibited by strychnine. Similar chloride currents could also be induced with β-alanine or taurine. Whole-cell glycine responses were significantly greater in CA3 pyramidal cells than in CA1 pyramidal cells and dentate granule cells, while responses to GABA were similar among these three cell types. Although these results demonstrate the presence of functional glycine receptors in the hippocampus, no evidence for their activation during synaptic stimulation was found. Gabazine, a selective GABAA receptor antagonist, totally blocked evoked IPSCs in CA3 pyramidal cells. Glycine receptor activation is not dependent on transporter-controlled levels of extracellular glycine, as no chloride current was observed in response to sarcosine, an inhibitor of glycine transporters. In contrast, application of guanidinoethanesulfonic acid, an uptake inhibitor of β-alanine and taurine, induced strychnine-sensitive chloride current in the presence of gabazine. These data indicate that modulation of transporters for the endogenous amino acids, β-alanine and taurine, can regulate tonic activation of glycine receptors, which may function in maintenance of inhibitory tone in the hippocampus.
Glycine is a major inhibitory neurotransmitter in the nervous system (Langosch et al. 1990). Inhibition occurs by activation of glycine receptors that gate integral chloride channels consisting of pentamers of α or combinations of α and β subunits (Kuhse et al. 1995). In the brainstem, glycinergic inhibitory signalling is dominant (Kandler & Friauf, 1995; Golding & Oertel, 1996; Stevens et al. 1996; Singer et al. 1998; Donato & Nistri, 2000; Smith et al. 2000), whereas in cortical and subcortical brain areas GABA is the major inhibitory neurotransmitter (Sivilotti & Nistri, 1991). Strychnine-sensitive glycine receptors are, however, widely expressed throughout the brain (Betz, 1991). In the hippocampus, where glycinergic inhibitory synaptic signalling has not been observed, in situ hybridization has revealed the presence of α2, α3, and β-glycine receptor subunits (Malosio et al. 1991). Immunohistochemical studies have provided conflicting results as to whether glycine or glycine receptors are, in fact, expressed in the hippocampus (Wenthold et al. 1987; van den Pol & Gorcs, 1988; Araki et al. 1988; Pourcho et al. 1992; Fujiwara et al. 1998). Nevertheless, electrophysiological responses to exogenously applied glycine have consistently been observed in the hippocampus both in young rats (up to postnatal day (P)14) (Ito & Cherubini, 1991; Shirasaki et al. 1991; Trombley & Shepherd, 1994; Schönrock & Bormann, 1995) and in adult rats (from P21) (Ye et al. 1999; Chattipakorn & McMahon, 2000). In addition to glycine, at least two other endogenous amino acids activate glycine receptors, β-alanine and taurine. Both agonists can bind to either glycine or GABAA receptors to increase membrane chloride conductance (Krishtal et al. 1988; Horikoshi et al. 1988; Tokutomi et al. 1989; Langosch et al. 1990; Kuhse et al. 1995; Boehm et al. 1997; Shen et al. 2000). Moreover, the extracellular concentrations of these amino acids in the hippocampus are relatively high, in the micromolar range (Shibanoki et al. 1993), and may thus tonically activate glycine receptors. Further indication for a role of β-alanine and taurine in modulating neuronal responses is suggested by the expression of glial and neuronal transporters, which control their concentrations in the brain (Smith et al. 1992b; Liu et al. 1993a). Thus, although functional glycine receptors are clearly present in the hippocampus, the endogenous agonists and the possible role of these receptors remain unknown. A difficult problem in answering these questions has been the lack of specific pharmacological tools, in particular antagonists which unequivocally differentiate between GABAA and glycine receptors.
In this electrophysiological study we have characterized the properties of strychnine-sensitive glycine receptors in the hippocampus. Experiments were designed to evaluate the possible role of glycine receptors both as mediators of synaptic transmission and as sensors of ambient levels of endogenous agonists that might tonically modulate neuronal activity.
METHODS
Preparation of slice cultures
Slice cultures were prepared from 6- to 7-day-old Wistar rat pups killed by decapitation following a protocol approved by the Veterinary Department of the Canton of Zurich, and maintained as previously described (Gähwiler et al. 1998). In brief, 400 μm thick hippocampal slices were attached to glass coverslips using clotted chicken plasma, placed in sealed test tubes with serum-containing medium, and maintained in a roller-drum incubator at 36 °C for 14–28 days. After this time, the cultured hippocampus has differentiated, displaying a cytoarchitecture closely resembling that in situ (Gähwiler et al. 1997).
Electrophysiological recordings
Cultures were then transferred to a recording chamber mounted under an upright microscope (Axioskop FS1; Zeiss, Jena, Germany) and superfused with an external solution (pH 7.4) containing 148.8 mm Na+, 2.7 mm K+, 149.2 mmCl−, 2.8 mm Ca2+, 2.0 mm Mg2+, 11.6 mm HCO3−, 0.4 mm H2PO4−, 5.6 mm d-glucose and 10 mg l−1 Phenol Red (pH 7.4). In all experiments, neurones were voltage clamped at 0 mV at a temperature of 28 °C. Glycine, GABA, β-alanine or taurine (all at 0.3 mm pipette concentration) was pressure-applied locally via a glass pipette positioned about 50 μm from the soma of the recorded cell to obtain a maximal peak response (49.0 kPa for 0.5–1 s; Neuro Phore; Medical Systems, Greenvale, NY, USA). Synaptic currents were evoked with monopolar metal electrodes using single pulses (100 μs, 0.5–30 μA). Recordings were obtained from CA1 and CA3 pyramidal cells, and dentate granule cells (Axopatch 200B amplifier; Axon Instruments, Foster City, CA, USA) with patch pipettes (2–5 MΩ) filled with a solution containing: 130 mm caesium methanesulphonate; 10 mm Hepes; 10 mm ethylene glycol-bis (2-aminoethyl)-N,N,N″,N″tetraacetic acid (EGTA); 5 mm QX-314-Cl; 2 mm Mg-ATP, pH 7.25. The actual membrane potentials were corrected for the liquid junction potential of −8.3 mV for the internal solution. Series resistance (typically between 5 and 15 MΩ) was regularly monitored, and if a change of more than 10 % occurred, cells were excluded.
Drugs and chemicals
6-Cyano-7-nitroquinoxaline−2,3(1H,4H)-dione (CNQX, 40 μm), 3-((R)-2-carboxypiperazin−4yl)-propyl-1-phosphonic acid (CPP, 40 μm) and CGP 62349 (5 μm) were always present in the bathing fluid. Tetrodotoxin (TTX, 0.5 μm) was also present in the bath, except for the experiments in which IPSCs were recorded (Fig. 6). CNQX was purchased from Tocris Cookson (Bristol, UK); ATP, EGTA, GABA, glycine, β-alanine, taurine, picrotoxin, sarcosine and strychnine from Sigma/Fluka (Buchs, Switzerland); TTX from Latoxan (Rossans, France); gabazine (SR 95531) from Sanofi-Synthelabo (Paris, France); QX-314 from Alomone Labs (Jerusalem, Israel), and guanidinoethanesulfonic acid (GES) from Toronto Research Chemicals (Toronto, Canada). CPP and CGP 62349 were kindly provided by Novartis AG (Basel, Switzerland).
Figure 6. Glycine receptors do not mediate fast IPSCs in CA3 pyramidal cells.

A, gabazine (10 μm) completely blocks electrically evoked IPSCs. Pressure applications of GABA and synaptic stimulation were alternated every 60 s in a CA3 pyramidal cell held at 0 mV. The horizontal bar indicates when gabazine was applied. Responses (a-d) are expanded below (a, c: GABA responses; b, d: evoked IPSCs). Transient suppression of spontaneous IPSCs after extracellular electrical stimulation is probably due to depolarization-induced suppression of inhibition (Alger & Pitler, 1995). B, effect of strychnine on the IPSC evoked in a CA3 pyramidal cell at a holding potential of 0 mV. The trace labelled ‘strychnine’ was recorded after a 5 min perfusion with strychnine (3 μm). Decay time constants for each trace are indicated. Bar graphs for decay time constants (middle) and peak amplitudes (right) show means from four cells. The asterisk indicates statistical significance (Student's t test).
Data acquisition and analysis
Signals were filtered at 2 kHz, digitally recorded on a computer using Clampex 7 software (Axon Instruments) and stored on tape for later analysis. Interpolated Erev of the currents was calculated by fitting data points of the current-voltage relationship with third-order polynominals. Numerical data in the text are expressed as means ± s.e.m. Student's t test or ANOVA with Fisher's least-significant difference test was used to compare values when appropriate. P < 0.05 was considered significant.
RESULTS
Glycine receptor-mediated chloride currents in the hippocampus
Pressure-application of glycine induced a transient outward current in CA3 pyramidal cells voltage clamped at 0 mV in the presence of CNQX (40 μm), CPP (40 μm), CGP 62349 (5 μm) and TTX (0.5 μm) in the bath solution to block, respectively, AMPA/kainate, NMDA and GABAB receptors and voltage-dependent Na+ channels (Fig. 1). The amplitude of the current increased with duration of glycine application (Fig. 1A). Plots of the I-V relationship of the glycine-induced current revealed a reversal potential of −88.8 ± 2.4 mV (Fig. 1B) (n = 5), close to the theoretical equilibrium potential calculated for chloride of −88.3 mV (assuming a permeability ratio of HCO3− relative to Cl− of 0.11; Bormann et al. 1987), indicating an underlying increase in chloride conductance (5.35 ± 1.10 nS, n = 5). To determine the specificity of the glycine receptor antagonist strychnine and the GABAA receptor antagonist gabazine, we examined their effects on currents induced by glycine and GABA applied alternately at 1 min intervals to the same CA3 pyramidal cell. Significantly larger currents were induced by GABA as compared to glycine (GABA, 4158.5 ± 868.7 pA, n = 6; glycine, 698.3 ± 98.1 pA, n = 6; Fig. 2 and Fig. 5). Bath-application of strychnine preferentially inhibited glycine-induced currents with an IC50 of 0.30 μm (n = 4, Fig. 2A). The Hill coefficient of less than 1 (0.81) might be due to the heterogeneity of glycine receptors with different affinities for strychnine. At a concentration greater than 1.0 μm, strychnine also inhibited GABA-induced currents (at 10 μm, by 8.5 ± 2.1 %, P < 0.01, n = 4) as reported previously (Shirasaki et al. 1991; Trombley & Shepherd, 1994; Jonas et al. 1998). Gabazine preferentially inhibited GABA-induced currents with an IC50 of 0.34 μm (n = 8, Fig. 2B). Gabazine did not significantly inhibit glycine-induced currents up to a concentration of 10 μm (at 10 μm, by 5.4 ± 10.6 %, P = 0.64, n = 4; Fig. 2B). These results demonstrate that exogenous application of glycine selectively activates postsynaptic glycine receptors.
Figure 1. Glycine-induced currents in CA3 pyramidal cells.

A, currents recorded from a typical cell held at 0 mV in response to three different durations of glycine pressure-application (glycine pipette concentration, 0.3 mm, applied at 1 min intervals at time indicated by arrow). CNQX (40 μm), CPP (40 μm), CGP 62349 (5 μm) and TTX (0.5 μm) were present in the bathing solution. B, an example of the glycine-induced currents at different voltages (range, from −100 to +20 mV in 20 mV increments) (top) and a plot of the current-voltage relationship for this cell (bottom). Each trace and data point represents an average of three sweeps.
Figure 2. The effects of strychnine and gabazine on glycine- and GABA-induced currents in CA3 pyramidal cells.

Glycine and GABA (pipette concentration, 0.3 mm) were pressure-applied alternately at 1 min intervals from different pipettes approximately equidistant to the same cell. A, typical responses of glycine- and GABA-induced currents in a cell at a holding potential of 0 mV before, 5 min after strychnine (1 μm) perfusion, and after 30 min of wash (top). Graph shows reduction of glycine- (n = 4) and GABA-induced current (n = 4) as a function of strychnine concentration. The concentration-dependent effect of strychnine on glycine responses was fitted with a curve calculated by the Hill equation (glycine: IC50 = 0.30 μm, Hill coefficient, nH = 0.81). B, typical responses to glycine and GABA recorded before, 5 min after gabazine (1 μm) perfusion, and after 30 min of wash (top). Graph shows reduction of glycine- (n = 4) and GABA-induced currents (n = 8) as a function of gabazine concentration. The concentration-dependent effect of gabazine on GABA responses was fitted with a curve calculated by the Hill equation (IC50 = 0.34 μm, nH = 0.80).
Figure 5. Glycine-induced currents in different hippocampal cell types.

Application parameters were set to obtain maximal amplitude glycine and GABA responses. A, typical responses of glycine-induced and GABA-induced currents recorded at a holding potential of 0 mV in a CA3 pyramidal cell (CA3), a dentate granule cell (DG) and a CA1 pyramidal cell (CA1). B, summary bar graphs. Each column shows the mean response in six cells. Cell capacitance was 228.4 ± 18.0 pF in CA3 pyramidal cells, 109.9 ± 9.2 pF in dentate granule cells, and 217.9 ± 34.3 pF in CA1 pyramidal cells. s.e.m. is indicated and asterisks denote statistically significant differences for the current amplitude in CA3 cells (ANOVA with Fisher's least-significant difference test).
An indication of the subunit composition of the glycine receptors was obtained by testing the effects of picrotoxin, which is much more effective in blocking homomeric α subunit-containing glycine receptors than heteromeric αβ glycine receptors (Pribilla et al. 1992; Yoon et al. 1998). Picrotoxin (100 μm) incompletely inhibited the glycine-induced current (by 81.4 ± 1.9 %, P < 0.01, n = 4) consistent with the expression of not only homomeric α subunit-containing glycine receptors but also heteromeric αβ glycine receptors in CA3 pyramidal cells (Fig. 3).
Figure 3. Effect of picrotoxin on glycine-induced currents.

Glycine (0.3 mm) was pressure-applied at 1 min intervals. A, traces recorded from a cell at a holding potential of 0 mV before and 5 min after perfusion of picrotoxin (100 μm) are superimposed. B, summary bar graph showing mean for four cells. Asterisk indicates statistical significance (Student's t test).
In addition to glycine, the amino acids β-alanine and taurine are reported to be endogenous partial agonists at strychnine-sensitive glycine receptors (Langosch et al. 1990; Boehm et al. 1997). We therefore determined whether this is also the case in hippocampus. Because β-alanine and taurine also activate GABAA receptors (Horikoshi et al. 1988; Puopolo et al. 1998; Shen et al. 2000), the responses of these agonists were examined in the presence of gabazine (10 μm). In CA3 pyramidal cells held at 0 mV, pressure-application of β-alanine or taurine induced transient outward chloride currents, which were, however, smaller than the glycine-induced currents (β-alanine, 215.7 ± 135.8 pA, n = 4; taurine, 39.4 ± 7.5 pA, n = 5; glycine, 593.5 ± 75.2 pA, n = 4) (Fig. 4). Both β-alanine and taurine responses were inhibited by strychnine (1 μm) (data not shown).
Figure 4. Responses to glycine receptor agonists in CA3 pyramidal cells.
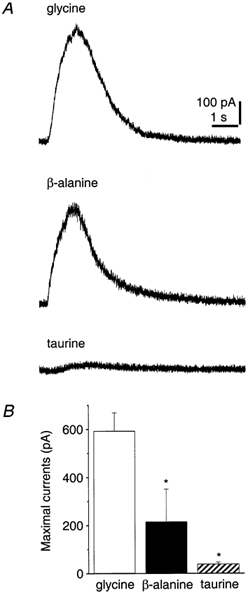
A, currents induced by the glycine receptor agonists, glycine, β-alanine and taurine (concentrations in application pipette: 0.3 mm), at a holding potential of 0 mV. Gabazine (10 μm) was present in the bathing solution. B, bar graph showing mean effects in four to five cells. s.e.m. is indicated and the asterisks denote statistically significant differences for the current amplitude induced by glycine (ANOVA with Fisher's least-significant difference test).
Functional evidence for glycine receptor expression was also obtained for CA1 pyramidal cells and dentate granule cells. A comparison of whole-cell glycine-induced responses among the three principal excitatory hippocampal cells showed significantly larger currents in CA3 pyramidal cells (698.3 ± 98.1 pA; current density, 3.0 ± 0.4 A F−1; n = 6) versus CA1 pyramidal (274.1 ± 75.1 pA; current density, 1.2 ± 0.2 A F−1; n = 6) or dentate granule cells (375.4 ± 69.4 pA; current density 3.5 ± 0.6 A F−1; n = 6) (Fig. 5). As a reference, the much larger GABA-induced currents, which were not significantly different among the three cell types (P = 0.34), are shown superimposed on the glycine responses.
Glycine receptors do not mediate synaptic currents in CA3 pyramidal cells
To determine whether glycine receptors, which are expressed most strongly on CA3 pyramidal cells, are involved in synaptic transmission, we characterized the pharmacological profile of the receptors mediating inhibitory postsynaptic currents (IPSCs) evoked by brief extracellular stimulation (100 μs, single current pulse up to 30 μA) in the stratum radiatum of the CA3 region in the presence of CNQX (40 μm), CPP (40 μm) and CGP 62349 (5 μm). First, the sensitivity of the IPSC to GABAA receptor blockade by gabazine was tested. Evoked IPSCs and responses to GABA puffs were alternately induced. The degree of GABAA receptor blockade was monitored by examining the effect of gabazine on responses induced by GABA puffs, whose peak amplitudes were adjusted to match those of the alternately evoked IPSCs. As shown in Fig. 6A, bath application of gabazine (10 μm) completely blocked the IPSC and inhibited the GABA-induced currents by 98.7 ± 0.3 % (n = 7). Application of gabazine also abolished spontaneous IPSCs. The incomplete blockade of responses to exogenous GABA by the competitive antagonist gabazine probably reflects the prolonged presence of GABA resulting from the puff applications, which may saturate GABA transporters, since even with addition of gabazine to the GABA application pipette, GABA-induced currents could not be blocked completely. The hippocampus contains a large variety of interneurones, all of which are considered to mediate inhibition by release of GABA (Freund & Buzsáki, 1996). Consistent with these findings, evoked IPSCs always appeared to be mediated entirely by GABA, even when the stimulating electrode was moved from stratum radiatum to stratum oriens (n = 3), stratum pyramidale (n = 2) or dentate hilus (n = 4).
Even though these experiments suggest that the IPSC is mediated entirely by GABAA receptors, we checked whether a glycine-dependent component could be detected by application of strychnine. Bath-perfusion of strychnine (3 μm, a relatively high concentration) partially inhibited the IPSC (control, 907.3 ± 168.9 pA; after perfusion of strychnine, 631.5 ± 129.2 pA, n = 4) (Fig. 6B). However, the decay time constants of the IPSCs before and after application of strychnine were the same (36.3 ± 6.5 and 37.0 ± 5.9 ms, respectively, P = 0.35; n = 4). Significant differences have been reported for the time constants of decay for glycine receptor-mediated IPSCs versus GABAA receptor-mediated IPSCs. In spinal cord or brainstem, glycine receptor-mediated IPSCs exhibit a significantly shorter decay time constant than GABAA receptor-mediated IPSCs (Jonas et al. 1998; Donato & Nistri, 2000), while the opposite is the case in retinal ganglion cells (Protti et al. 1997). The reduction in IPSC amplitude is likely to be due to an action of strychnine at GABAA receptors, similar to the effect on GABA-induced responses (Fig. 2A). Taken together, these results indicate that activation of glycine receptors does not contribute to IPSCs in CA3 pyramidal cells.
Are glycine receptors activated by endogenous agonists?
If glycine receptors are not involved in inhibitory synaptic transmission, the question arises whether they are modulated by ambient levels of extracellular glycine. Bath-perfusion of strychnine (1 μm), however, had no effect on the holding current of CA3 pyramidal cells clamped at 0 mV (n = 4; data not shown). Thus, glycine receptors appear not to be tonically activated under these experimental conditions, in which the basal concentrations of endogenous agonists for glycine receptors are not supplemented to in vivo levels (micromolar range; Shibanoki et al. 1993). In further experiments, we checked for a possible role of glycine transporters in the modulation of glycinergic responses. Bath-application of sarcosine (300 μm), a specific inhibitor of glycine transporter function, did not, however, change the holding current or the kinetics of the current induced by a pressure puff of glycine (n = 4; Fig. 7A). Raising the concentration of sarcosine to 1 mm still had no effect (n = 2; data not shown), indicating that glycine transporters do not modulate extracellular glycine concentrations in a range relevant for gating of chloride currents in vitro.
Figure 7. Tonic activation of glycine receptors by inhibiting the β-alanine/taurine transporter.

A, currents induced by pressure-application of glycine were recorded in a CA3 pyramidal cell held at 0 mV. Horizontal bar indicates when sarcosine (300 μm) was applied. Traces labelled with letters are shown at an expanded time scale below and the three traces superimposed are shown on the right. B, application of GES (300 μm) induces an outward current in a CA3 pyramidal cell held at 0 mV. Horizontal bars indicate the time of perfusion of GES (300 μm), gabazine (10 μm) and strychnine (1 μm). Strychnine was applied after perfusion of gabazine when currents had maintained a plateau level for 2 min. A portion of the trace after gabazine perfusion is shown enlarged below. C, outward current induced by perfusion of GES (300 μm) in the presence of gabazine (10 μm) (left). The holding potential was alternated between 0 and +10 mV every 5 s. Horizontal bars indicate the time of perfusion of gabazine (10 μm), GES (300 μm) and strychnine (1 μm). D, summary bar graph. Each column represents mean responses in nine cells 1 min before application of strychnine (1 μm) and 2 min after currents attained a steady level, in the presence of gabazine (10 μm) and GES (300 μm). The asterisk indicates statistical significance (Student's t test).
On the other hand, bath-application of GES (300 μm), an amidino analogue of taurine that inhibits a transporter for β-alanine, taurine and GABA (Li & Lombardini, 1990; Smith et al. 1992b) without exhibiting agonist activity at glycine receptors (Flint et al. 1998), induced a persistent outward current (725.3 ± 94.1 pA, n = 4). Most of this current was rapidly blocked by gabazine (10 μm), indicating that a major part of the current is mediated by GABAA receptors activated by extracellular accumulation of GABA (Fig. 7B). In these experiments, however, a residual current persisted (14.5 ± 4.0 pA, n = 4), which was inhibited by strychnine (1 μm). GES applied in the presence of gabazine (10 μm) also induced a strychnine-sensitive outward current (29.0 ± 12.9 pA, n = 5; Fig. 7C). This sensitivity of the GES-induced gabazine-insensitive current to strychnine (before strychnine, 22.6 ± 7.4 pA; after strychnine 5.1 ± 3.5 pA; P < 0.01; n = 9, Fig. 7D) is consistent with glycine receptor activation resulting from extracellular accumulation of β-alanine and/or taurine.
DISCUSSION
The principal findings of this study are that functional strychnine-sensitive glycine receptors expressed in the hippocampus, predominantly on CA3 pyramidal cells, do not appear to be involved in inhibitory synaptic transmission and that glycine does not attain sufficient ambient concentrations to induce chloride currents. Rather, our experiments suggest that β-alanine and taurine are the primary agonists at hippocampal strychnine-sensitive glycine receptors, and that membrane transporters are critical in regulating their extracellular concentration.
The properties of glycine receptors in the hippocampus
A major difficulty in the functional investigation of GABAergic and glycinergic inhibitory responses is the lack of absolutely specific antagonists. It was therefore necessary in our initial experiments to determine the specificity of the best available antagonists, strychnine and gabazine, in the hippocampus. Although brief applications of GABA and glycine were used in our experiments, outside-out patches in combination with a fast-application system would be necessary to avoid all desensitization. Glycine-induced current was preferentially inhibited by strychnine with an IC50 of 0.30 μm (Fig. 2A), which is relatively high compared to the IC50 typically reported for strychnine-sensitive glycine receptors (less than 0.1 μm) (Krishtal et al. 1988; Tokutomi et al. 1989; Shirasaki et al. 1991; Boehm et al. 1997; Jonas et al. 1998). In light of the finding that neonatal forms of the glycine receptor are strychnine resistant (Becker et al. 1988; Langosch et al. 1990), our data suggest that the glycine receptors expressed in 3-week-old hippocampal slice cultures may include the neonatal phenotype. Moreover, the incomplete blockade of responses by picrotoxin (Fig. 3) indicates that these glycine receptors are composed both of homomeric α subunits and heteromeric αβ subunits (Pribilla et al. 1992; Yoon et al. 1998).
Glycine receptors do not contribute to synaptic inhibitory responses
The complete blockade of evoked IPSCs by the specific GABAA receptor antagonist gabazine (Fig. 6A) strongly suggests that this response is mediated entirely by GABA receptors. The glycine receptor antagonist strychnine at a relatively high concentration of 3 μm also reduced the amplitude of evoked IPSCs (Fig. 6B). Strychnine, however, is not an absolutely specific antagonist. As shown in Fig. 2A and as previously reported (Shirasaki et al. 1991; Trombley & Shepherd, 1994; Jonas et al. 1998), strychnine also inhibits GABAA receptors. In addition, strychnine did not change the kinetics of the IPSC component (Fig. 6B). If the glycine response were inhibited by strychnine, then the residual strychnine-insensitive component should exhibit altered decay kinetics (Protti et al. 1997; Jonas et al. 1998; Donato & Nistri, 2000). These results indicate that glycine receptors are not involved in synaptic transmission in the hippocampus. A similar finding has been reported in rat cerebellar granule cells, where GABA- and glycine-induced responses are observed, but spontaneous IPSCs are mediated exclusively by GABA (Kaneda et al. 1995).
Amino acid transporters and the activation of hippocampal glycine receptors
Extracellular concentrations of endogenous neuroactive agents must be tightly regulated by membrane transporters or catabolic enzymes that terminate signalling (Nelson, 1998). A number of membrane transporters have been identified that control the levels of the amino acids acting as agonists at hippocampal glycine receptors. GLYT1 and GLYT2 are two transporters specific for glycine, with only GLYT1 expressed in the hippocampus by the glial cells (Smith et al. 1992a; Zafra et al. 1995). In our study, however, sarcosine, an inhibitor of GLYT1, did not induce a current reflecting the activation of strychnine-sensitive glycine receptors nor was there a change in the kinetics of the current induced by pressure-application of glycine (Fig. 7A), indicating that GLYT1 does not play a significant role in modulating glycine-dependent chloride currents in the hippocampus.
β-Alanine and taurine, the other endogenous agonists activating strychnine-sensitive glycine receptors, are not substrates for GLYT1 and GLYT2 (Smith et al. 1992a; Liu et al. 1993b). These amino acids are transported by a specific membrane transporter (Smith et al. 1992b; Liu et al. 1992) and by the GABA transporters, GAT3 and GAT4 (Liu et al. 1993a), which do not, however, transport glycine. Our observation of a tonic current after inhibition of β-alanine and taurine transport (Fig. 7B, C and D) suggests that ambient levels of these amino acids could act at glycine receptors to modulate neuronal processing. Tissue concentrations of taurine are in the millimolar range within most of the brain and both the synthesizing enzyme and transporter for taurine are present in glia (Almarghini et al. 1991; Liu et al. 1992). Thus, glial transport may control extracellular concentrations of taurine as reported in rat neonatal neocortex (Flint et al. 1998), although a hypotonic stimulus (Deleuze et al. 1998) or ischaemia (Saransaari & Oja, 1999) can also lead to taurine release.
Functional role of glycine receptors in the hippocampus
Compared to GABAA receptor-mediated currents, glycine responses are much smaller (Fig. 2 and Fig. 5), and we found no evidence for intrahippocampal glycinergic synaptic transmission (Fig. 6). Our experiments do not, however, rule out an input from an as yet unidentified extrahippocampal glycinergic pathway. An alternative function for hippocampal glycine receptors may lie in the modulation of excitability in neuronal circuits. Although activation of non-synaptic glycine receptors may not change resting membrane potential, a tonic background chloride current could act to shunt synaptic signals, changing the amplitude and kinetics of fast synaptic potentials. The fact that whole-cell glycinergic responses were largest in CA3 pyramidal cells, which represent a central relay synapse in the hippocampal synaptic network, suggests that non-synaptic glycine receptors may play an important role in regulating hippocampal information processing.
Acknowledgments
We thank L. Heeb, L. Rietschin, S. Giger, H. Kasper, R. Dürr and R. Schöb for excellent technical assistance and P. Benquet, K. Fischer, C. Gee and M. Scanziani for valuable discussions and critical reading of the manuscript. This work was supported by the Swiss National Science Foundation (U.G.), and the Ministry of Education, Science, and Culture of Japan (M.M.).
REFERENCES
- Alger BE, Pitler TA. Retrograde signaling at GABAA-receptor synapses in the mammalian CNS. Trends in Neurosciences. 1995;18:333–340. doi: 10.1016/0166-2236(95)93923-l. [DOI] [PubMed] [Google Scholar]
- Almarghini K, Remy A, Tappaz M. Immunocytochemistry of the taurine biosynthesis enzyme, cysteine sulfinate decarboxylase, in the cerebellum: evidence for a glial localization. Neuroscience. 1991;43:111–119. doi: 10.1016/0306-4522(91)90421-j. [DOI] [PubMed] [Google Scholar]
- Araki T, Yamano M, Murakami T, Wanaka A, Betz H, Tohyama M. Localization of glycine receptors in the rat central nervous system: an immunocytochemical analysis using monoclonal antibody. Neuroscience. 1988;25:613–624. doi: 10.1016/0306-4522(88)90263-1. [DOI] [PubMed] [Google Scholar]
- Becker CM, Hoch W, Betz H. Glycine receptor heterogeneity in rat spinal cord during postnatal development. EMBO Journal. 1988;7:3717–3726. doi: 10.1002/j.1460-2075.1988.tb03255.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Betz H. Glycine receptors: heterogeneous and widespread in the mammalian brain. Trends in Neurosciences. 1991;14:458–461. doi: 10.1016/0166-2236(91)90045-v. [DOI] [PubMed] [Google Scholar]
- Boehm S, Harvey RJ, Von Holst A, Rohrer H, Betz H. Glycine receptors in cultured chick sympathetic neurons are excitatory and trigger neurotransmitter release. Journal of Physiology. 1997;504:683–694. doi: 10.1111/j.1469-7793.1997.683bd.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bormann J, Hamill OP, Sakmann B. Mechanism of anion permeation through channels gated by glycine and gamma-aminobutyric acid in mouse cultured spinal neurones. Journal of Physiology. 1987;385:243–286. doi: 10.1113/jphysiol.1987.sp016493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chattipakorn SC, McMahon LL. Activation of glycine-gated chloride channels depresses excitatory and inhibitory transmission recorded in rat hippocampus. Society for Neuroscience Abstracts. 2000;26:93. [Google Scholar]
- Deleuze C, Duvoid A, Hussy N. Properties and glial origin of osmotic-dependent release of taurine from the rat supraoptic nucleus. Journal of Physiology. 1998;507:463–471. doi: 10.1111/j.1469-7793.1998.463bt.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donato R, Nistri A. Relative contribution by GABA or glycine to Cl−-mediated synaptic transmission on rat hypoglossal motoneurons in vitro. Journal of Neurophysiology. 2000;84:2715–2724. doi: 10.1152/jn.2000.84.6.2715. [DOI] [PubMed] [Google Scholar]
- Flint AC, Liu X, Kriegstein AR. Nonsynaptic glycine receptor activation during early neocortical development. Neuron. 1998;20:43–53. doi: 10.1016/s0896-6273(00)80433-x. [DOI] [PubMed] [Google Scholar]
- Freund TF, Buzsáki G. Interneurons of the hippocampus. Hippocampus. 1996;6:347–470. doi: 10.1002/(SICI)1098-1063(1996)6:4<347::AID-HIPO1>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- Fujiwara K, Kaminishi Y, Inoue Y, Yabuuchi M. Monoclonal antibody monospecific to glycine for brain immunocytochemistry. Brain Research. 1998;806:210–218. doi: 10.1016/s0006-8993(98)00744-6. [DOI] [PubMed] [Google Scholar]
- Gähwiler BH, Capogna M, Debanne D, McKinney RA, Thompson SM. Organotypic slice cultures: a technique has come of age. Trends in Neurosciences. 1997;20:471–477. doi: 10.1016/s0166-2236(97)01122-3. [DOI] [PubMed] [Google Scholar]
- Gähwiler BH, Thompson SM, McKinney RA, Debanne D, Robertson RT. Culturing nerve cells. In: Banker G, Goslin K, editors. Organotypic Slice Cultures of Neural Tissue. Cambridge, MA, USA: MIT Press; 1998. pp. 461–498. [Google Scholar]
- Golding NL, Oertel D. Context-dependent synaptic action of glycinergic and GABAergic inputs in the dorsal cochlear nucleus. Journal of Neuroscience. 1996;16:2208–2219. doi: 10.1523/JNEUROSCI.16-07-02208.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horikoshi T, Asanuma A, Yanagisawa K, Anzai K, Goto S. Taurine and β-alanine act on both GABA and glycine receptors in Xenopus oocyte injected with mouse brain messenger RNA. Brain Research. 1988;464:97–105. doi: 10.1016/0169-328x(88)90002-2. [DOI] [PubMed] [Google Scholar]
- Ito S, Cherubini E. Strychnine-sensitive glycine responses of neonatal rat hippocampal neurones. Journal of Physiology. 1991;440:67–83. doi: 10.1113/jphysiol.1991.sp018696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonas P, Bischofberger J, Sandkühler J. Corelease of two fast neurotransmitters at a central synapse. Science. 1998;281:419–424. doi: 10.1126/science.281.5375.419. [DOI] [PubMed] [Google Scholar]
- Kandler K, Friauf E. Development of glycinergic and glutamatergic synaptic transmission in the auditory brainstem of perinatal rats. Journal of Neuroscience. 1995;15:6890–6904. doi: 10.1523/JNEUROSCI.15-10-06890.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaneda M, Farrant M, Cull-Candy SG. Whole-cell and single-channel currents activated by GABA and glycine in granule cells of the rat cerebellum. Journal of Physiology. 1995;485:419–435. doi: 10.1113/jphysiol.1995.sp020739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishtal OA, Osipchuk YuV, Vrublevsky SV. Properties of glycine-activated conductances in rat brain neurones. Neuroscience Letters. 1988;84:271–276. doi: 10.1016/0304-3940(88)90519-8. [DOI] [PubMed] [Google Scholar]
- Kuhse J, Betz H, Kirsch J. The inhibitory glycine receptor: architecture, synaptic localization and molecular pathology of a postsynaptic ion-channel complex. Current Opinion in Neurobiology. 1995;5:318–323. doi: 10.1016/0959-4388(95)80044-1. [DOI] [PubMed] [Google Scholar]
- Langosch D, Becker CM, Betz H. The inhibitory glycine receptor: a ligand-gated chloride channel of the central nervous system. European Journal of Biochemistry. 1990;194:1–8. doi: 10.1111/j.1432-1033.1990.tb19419.x. [DOI] [PubMed] [Google Scholar]
- Li YP, Lombardini JB. Guanidinoethanesulfonic acid - inhibitor of GABA uptake in rat cortical synaptosomes. Brain Research. 1990;510:147–149. doi: 10.1016/0006-8993(90)90742-t. [DOI] [PubMed] [Google Scholar]
- Liu QR, López-Corcuera B, Mandiyan S, Nelson H, Nelson N. Molecular characterization of four pharmacologically distinct gamma-aminobutyric acid transporters in mouse brain. Journal of Biological Chemistry. 1993a;268:2106–2112. [PubMed] [Google Scholar]
- Liu QR, López-Corcuera B, Mandiyan S, Nelson H, Nelson N. Cloning and expression of a spinal cord- and brain-specific glycine transporter with novel structural features. Journal of Biological Chemistry. 1993b;268:22802–22808. [PubMed] [Google Scholar]
- Liu QR, López-Corcuera B, Nelson H, Mandiyan S, Nelson N. Cloning and expression of a cDNA encoding the transporter of taurine and β-alanine in mouse brain. Proceedings of the National Academy of Sciences of the USA. 1992;89:12145–12149. doi: 10.1073/pnas.89.24.12145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malosio ML, Marqueze-Pouey B, Kuhse J, Betz H. Widespread expression of glycine receptor subunit mRNAs in the adult and developing rat brain. EMBO Journal. 1991;10:2401–2409. doi: 10.1002/j.1460-2075.1991.tb07779.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson N. The family of Na+/Cl− neurotransmitter transporters. Journal of Neurochemistry. 1998;71:1785–1803. doi: 10.1046/j.1471-4159.1998.71051785.x. [DOI] [PubMed] [Google Scholar]
- Pourcho RG, Goebel DJ, Jojich L, Hazlett JC. Immunocytochemical evidence for the involvement of glycine in sensory centers of the rat brain. Neuroscience. 1992;46:643–656. doi: 10.1016/0306-4522(92)90151-q. [DOI] [PubMed] [Google Scholar]
- Pribilla I, Takagi T, Langosch D, Bormann J, Betz H. The atypical M2 segment of the beta subunit confers picrotoxinin resistance to inhibitory glycine receptor channels. EMBO Journal. 1992;11:4305–4311. doi: 10.1002/j.1460-2075.1992.tb05529.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Protti DA, Gerschenfeld HM, Liano I. GABAergic and glycinergic IPSCs in ganglion cells of rat. Journal of Neuroscience. 1997;17:6075–6085. doi: 10.1523/JNEUROSCI.17-16-06075.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puopolo M, Kratskin I, Belluzzi O. Direct inhibitory effect of taurine on relay neurones of the rat olfactory bulb in vitro. Neuroreport. 1998;9:2319–2323. doi: 10.1097/00001756-199807130-00031. [DOI] [PubMed] [Google Scholar]
- Saransaari P, Oja SS. Characteristics of ischemia-induced taurine release in the developing mouse hippocampus. Neuroscience. 1999;94:949–954. doi: 10.1016/s0306-4522(99)00384-x. [DOI] [PubMed] [Google Scholar]
- Schönrock B, Bormann J. Modulation of hippocampal glycine receptor channels by protein kinase C. Neuroreport. 1995;6:301–304. doi: 10.1097/00001756-199501000-00019. [DOI] [PubMed] [Google Scholar]
- Shen W, Mennerick S, Covey DF, Zorumski CF. Pregnenolone sulfate modulates inhibitory synaptic transmission by enhancing GABAA receptor desensitization. Journal of Neuroscience. 2000;20:3571–3579. doi: 10.1523/JNEUROSCI.20-10-03571.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibanoki S, Kogure M, Sugahara M, Ishikawa K. Effect of systemic administration of N-methyl-D-aspartic acid on extracellular taurine level measured by microdialysis in the hippocampal CA1 field and striatum of rats. Journal of Neurochemistry. 1993;61:1698–1704. doi: 10.1111/j.1471-4159.1993.tb09806.x. [DOI] [PubMed] [Google Scholar]
- Shirasaki T, Klee MR, Nakaye T, Akaike N. Differential blockade of bicuculline and strychnine on GABA- and glycine-induced responses in dissociated rat hippocampal pyramidal cells. Brain Research. 1991;561:77–83. doi: 10.1016/0006-8993(91)90751-g. [DOI] [PubMed] [Google Scholar]
- Singer JH, Talley EM, Bayliss DA, Berger AJ. Development of glycinergic synaptic transmission to rat brain stem motoneurons. Journal of Neurophysiology. 1998;80:2608–2620. doi: 10.1152/jn.1998.80.5.2608. [DOI] [PubMed] [Google Scholar]
- Sivilotti L, Nistri A. GABA receptor mechanisms in the central nervous system. Progress in Neurobiology. 1991;36:35–92. doi: 10.1016/0301-0082(91)90036-z. [DOI] [PubMed] [Google Scholar]
- Smith AJ, Owens S, Forsythe ID. Characterisation of inhibitory and excitatory postsynaptic currents of the rat medial superior olive. Journal of Physiology. 2000;529:681–698. doi: 10.1111/j.1469-7793.2000.00681.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith KE, Borden LA, Hartig PR, Branchek T, Weinshank RL. Cloning and expression of a glycine transporter reveal colocalization with NMDA receptors. Neuron. 1992a;8:927–935. doi: 10.1016/0896-6273(92)90207-t. [DOI] [PubMed] [Google Scholar]
- Smith KE, Borden LA, Wang CH, Hartig PR, Branchek TA, Weinshank RL. Cloning and expression of a high affinity taurine transporter from rat brain. Molecular Pharmacology. 1992b;42:563–569. [PubMed] [Google Scholar]
- Stevens DR, Gerber U, McCarley RW, Greene RW. Glycine-mediated inhibitory postsynaptic potentials in the medial pontine reticular formation of the rat in vitro. Neuroscience. 1996;73:791–796. doi: 10.1016/0306-4522(96)00046-2. [DOI] [PubMed] [Google Scholar]
- Tokutomi N, Kaneda M, Akaike N. What confers specificity on glycine for its receptor site? British Journal of Pharmacology. 1989;97:353–360. doi: 10.1111/j.1476-5381.1989.tb11961.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trombley PQ, Shepherd GM. Differential modulation by zinc and copper of amino acid receptors from rat olfactory bulb neurons. Journal of Neurophysiology. 1994;76:2536–2546. doi: 10.1152/jn.1996.76.4.2536. [DOI] [PubMed] [Google Scholar]
- Van Den Pol AN, Gorcs T. Glycine and glycine receptor immunoreactivity in brain and spinal cord. Journal of Neuroscience. 1988;8:472–492. doi: 10.1523/JNEUROSCI.08-02-00472.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wenthold RJ, Huie D, Altschuler RA, Reeks KA. Glycine immunoreactivity localized in the cochlear nucleus and superior olivary complex. Neuroscience. 1987;22:897–912. doi: 10.1016/0306-4522(87)92968-x. [DOI] [PubMed] [Google Scholar]
- Ye JH, Schaefer R, Wu WH, Liu PL, Zbuzek VK, McArdle JJ. Inhibitory effect of ondansetron on glycine response of dissociated rat hippocampal neurons. Journal of Pharmacology and Experimental Therapeutics. 1999;290:104–111. [PubMed] [Google Scholar]
- Yoon KW, Wotring VE, Fuse T. Multiple picrotoxinin effect on glycine channels in rat hippocampal neurons. Neuroscience. 1998;87:807–815. doi: 10.1016/s0306-4522(98)00158-4. [DOI] [PubMed] [Google Scholar]
- Zafra F, Gomeza J, Olivares L, Aragón C, Giménez C. Regional distribution and developmental variation of the glycine transporters GLYT1 and GLYT2 in the rat CNS. European Journal of Neuroscience. 1995;7:1342–1352. doi: 10.1111/j.1460-9568.1995.tb01125.x. [DOI] [PubMed] [Google Scholar]


