Abstract
Intracellular recordings were made from narrow-field, bistratified AII amacrine cells in the isolated, superfused retina-eyecup of the rabbit. Pharmacological agents were applied to neurons to dissect the synaptic pathways subserving AII cells so as to determine the circuitry generating their off-surround responses. Application of the GABA antagonists, picrotoxin, bicuculline and 1,2,5,6-tetrahydropyridine-4-yl methylphosphinic acid (TPMPA) all increased the on-centre responses of AII amacrine cells, but attenuated the off-surround activity. At equal concentrations, picrotoxin was approximately twice as effective as bicuculline or TPMPA in modifying the response activity of AII amacrine cells. These results indicate that the mechanism underlying surround inhibition of AII amacrine cells includes activation of both GABAA and GABAC receptors in an approximately equal ratio. Application of the GABA antagonists also increased the size of on-centre receptive fields of AII amacrine cells. Again, picrotoxin was most effective, producing, on average, a 54 % increase in the size of the receptive field, whereas bicuculline and TPMPA produced comparable 34 and 33 % increases, respectfully. Application of the voltage-gated sodium channel blocker TTX produced effects on AII amacrine cells qualitatively similar to those of the GABA blockers. Intracellular application of the chloride channel blocker 4,4′-dinitro-stilbene-2,2′-disulphonic acid (DNDS) abolished the direct effects of GABA on AII amacrine cells. Moreover, DNDS increased the amplitude of both the on-centre and off-surround responses. The failure of DNDS to block the off-surround activity indicates that it is not mediated by direct GABAergic inhibition. Taken together, our results suggest that surround receptive fields of AII amacrine cells are generated indirectly by the GABAergic, reciprocal feedback synapses from S1/S2 amacrine cells to the axon terminals of rod bipolar cells.
In the mammalian retina, rod and cone photoreceptors synapse with different bipolar cell types, thereby segregating their signals within separate and parallel vertical streams (Boycott & Dowling, 1969; Boycott & Kolb, 1973). Whereas 8–11 different morphological types of cone bipolar cells have been described, thus offering photopic signals a variety of paths to third-order amacrine and ganglion cells, only a single type of rod bipolar cell exists (Boycott & Wässle, 1991; Euler & Wässle, 1995). The axon terminals of the rod bipolar cell rarely make contact directly with ganglion cells, but instead innervate three types of amacrine cells (Strettoi et al. 1990). The first two are the wide-field S1 and S2 amacrine cells (corresponding to the A17 cells in cat retina), that maintain synapses with rod bipolar cell axons at dendritic boutons lying at the most vitreal margin of the inner plexiform layer (IPL) (Sandell et al. 1989; Strettoi et al. 1990). The output of the S1/S2 amacrine cells, however, takes the form of reciprocal synapses at these same boutons, providing feedback inhibition of the rod bipolar cell axons that innervate them. The third target of rod bipolar cells is the narrow-field, bistratified AII cell (Famiglietti & Kolb, 1975; Dacheux & Raviola, 1986; Strettoi et al. 1990). In turn, AII cells form sign-inverting, glycinergic chemical synapses with off-centre cone bipolar cells in the scleral region of the IPL, and sign-conserving electrical synapses, in the form of gap junctions, with on-centre cone bipolar cells in sublamina b. In this way, opposite polarity rod-mediated signals utilize cone circuitry in the IPL before reaching either on- or off-centre ganglion cells. The AII amacrine cell is thus a vital element in the rod pathway in that most, if not all, rod signals must pass through them first before leaving the retina for higher brain centres.
As the most abundant subtype of amacrine cell in mammalian retina, the AII cells have been the subject of numerous physiological studies. Under scotopic light conditions, AII cells show robust on-centre/off-surround receptive fields (Nelson, 1982; Dacheux & Raviola, 1986; Bloomfield, 1992, 1997; Xin & Bloomfield, 1999). In a recent study, we examined the synaptic circuitry responsible for the centre- and surround-mediated responses of AII cells (Bloomfield & Xin, 2000). Whereas our data indicated that the centre-mediated activity was derived from direct, excitatory inputs from rod bipolar cells, several lines of evidence suggested that surround responses were generated by circuitry in the inner retina. First, we found no evidence for antagonistic surround responses from rod bipolar cells using the same stimuli that evoked robust surround responses in AII cells. Second, although the selective glutamate receptor agonist l-(+)-2-amino-4-phosphonobutyric acid (l-AP4) blocked both centre- and surround-mediated activity, application of the selective glutamate receptor antagonists 6-cyano-7-nitroquinoxaline-2,3-dione (CNQX) or 6,7-dinitro-quinoxaline-2,3-dione (DNQX) enhanced the on-centre responses of AII cells, but attenuated the off-surrounds. These opposite effects on the centre- and surround-mediated activity indicated that they could not both reflect signals crossing rod bipolar-to-AII cell synapses. Third, application of GABA receptor antagonists attenuated or completely blocked AII surround responses, but had no effect on horizontal cells thereby implicating GABAergic circuitry in the IPL and not feedback circuitry to photoreceptors thought responsible for surround receptive fields of cone bipolar cells (cf. Baylor et al. 1971; Burkhardt, 1977; Toyoda & Tonosaki, 1978). Finally, application of TTX attenuated the surround activity of AII cells indicating that spiking neurons are part of the generating circuitry.
Taken together, our data suggested that an on-centre, GABAergic, spiking amacrine cell(s) plays a vital role in the generation of AII cell surround activity under dark-adapted conditions. Although the circuitry subserving AII amacrine cells has been well established (reviewed by Bloomfield & Dacheux, 2001), the identity of the putative GABAergic amacrine cell remains uncertain. The dendrites of AII amacrine cells in sublamina b receive a massive chemical input from a homogeneous population of amacrine cell dendritic profiles (Strettoi et al. 1992). These inhibitory processes, from as yet unidentified amacrine cells, may provide a lateral, feedforward inhibition responsible for AII cell surround receptive fields. However, as aforementioned, rod bipolar cell axon terminals receive GABAergic feedback inhibition from S1/S2 cells that could serve to modulate the release of glutamate from rod bipolar cells and thereby create the AII cell surround responses.
The purpose of the present study was to determine which of these two alternative circuits is responsible for the surround responses of AII amacrine cells. To answer this question, we extended our study of the effects of selective GABAA and GABAC receptor blockers to determine the role of these receptors, which are differentially distributed in the IPL, in the generation of AII surrounds. Further, to differentiate between feedforward vs. feedback inhibition, we examined the effects of the novel chloride channel blocker 4,4′-dinitro-stilbene-2,2′-disulphonic acid (DNDS) that exerts its effect from the intracellular side and can thereby be used to distinguish direct from indirect inhibition (cf. Dudek & Friedlander, 1996; Shao & Burkhalter, 1996). Taken together, our results indicate that feedback inhibition from S1/S2 amacrine cells to rod bipolar cell axon terminals underlies the antagonistic surround receptive fields of AII amacrine cells. This feedback pathway underlying rod-mediated surround responses is thus analogous to the horizontal cell-to-cone photoreceptor feedback circuit thought responsible for surround receptive fields of cone bipolar cells.
METHODS
The general methods used in this study have been described previously (Bloomfield & Miller, 1982; Bloomfield et al. 1997; Bloomfield & Xin, 2000). Procedures were in accordance with the guidelines of the National Institutes of Health and the Institutional Animal Care Committee at New York University School of Medicine. Adult, Dutch-belted rabbits (1.5–3.0 kg) were anaesthetized with an intraperitoneal injection of ethyl carbamate (2.0 g kg−1) and a local injection of 2 % lidocaine hydrochloride into the tissue surrounding the eyelids. The eye was removed under dim red illumination and hemisected. The vitreous humour was removed with an ophthalmic sponge and the resultant retina-eyecup was mounted in a superfusion chamber. The chamber was then placed in a light-tight Faraday cage and superfused at a flow rate of 30 ml min−1 with a mammalian Ringer solution (Bloomfield & Miller, 1982). The superfusate was maintained at 35 °C with oxygenation and pH 7.4 was maintained by bubbling with a gaseous mixture of 95 % O2-5 % CO2. Following enucleations, animals were killed by an intracardial, bolus injection of ethyl carbamate.
Light stimulation
Two 100 W quartz-iodide lamps provided light for a dual beam optical bench. Light intensity could be reduced up to 7 log units with calibrated neutral density filters placed in the light path of both beams. The maximum irradiance of both beams was equalized at 2.37 mW cm−2. The beams were combined with a collecting prism and focused onto the vitreal surface of the retina- eyecup by means of a final focusing lens.
The bottom beam provided small concentric spot stimuli (50 μm to 6.0 mm diameter) as well as a 50 μm wide ×6.0 mm long rectangular slit of light that was moved along its minor axis (parallel to the visual streak) in steps as small as 3 μm. Alignment of the electrode tip with stimuli was accomplished visually with the aid of a dissecting microscope mounted in the Faraday cage. However, after impaling a cell, the spot stimulus that evoked the largest amplitude centre-mediated response was considered centred over the cell and adjustment of stimuli position was made accordingly. All retinas were left in complete darkness for at least 45 min prior to recording. In the search for cells, light stimuli of log −6.0 or −5.5 intensity (approximately 1 log unit above rod threshold) were presented only once every 10 s to limit any light adaptation.
Electrical recordings
Intracellular recordings were obtained with microelectrodes fashioned from standard borosilicate glass tubing (Sutter Instruments, Novato, CA, USA). Electrodes were filled first with 4 % N-(2-amino-ethyl)-biotinamide hydrochloride, Neurobiotin (Vector Laboratories, Burlingame, CA, USA), in 0.1 m Tris buffer (pH 7.6) and then backfilled with 4 m potassium chloride to form a reversible junction with a Ag-AgCl contact. The final DC resistances of electrodes ranged from 250 to 450 MΩ. In some experiments, the chloride channel blocker 4,4′-dinitro-stilbene-2,2′-disulphonic acid (DNDS) (Pfaltz and Bauer, Inc., Waterbury, CT, USA) was added to the microelectrode via the Neurobiotin- Tris solution and allowed to leak into impaled neurons. Following physiological characterization of a cell, Neurobiotin was iontophoresed into the neuron using a combination of sinusoidal (3 Hz, 0.8 nA peak to peak) and DC current (0.4 nA) applied simultaneously; this method allowed passage of tracer through the electrode without polarization. Recordings were displayed on an oscilloscope, recorded on magnetic tape, and digitized off-line at a sampling rate of 2 kHz for computer analyses. Recordings were low-pass filtered at 500 Hz (-3 dB) at the amplifier to prevent aliasing during digitilization. For pharmacological studies, drugs were applied by switching from the control solution described previously to one containing a known concentration of drug. Drugs and their sources were: 1,2,5,6-tetrahydropyridine-4-yl methylphosphinic acid (TPMPA; Research Biochemicals International, Natick, MA, USA); GABA, picrotoxin, (-)bicuculline methochloride, tetrodotoxin (TTX) (Sigma, St Louis, MO, USA).
Receptive field measurements
To measure the centre-receptive field of a cell, the 50 μm wide ×6.0 mm long rectangular slit of light was moved along its minor axis (parallel to the visual streak) in discrete steps in both directions from the central position (Bloomfield, 1992). The position of the slit at which it evoked the largest amplitude response was considered being centred over the cell. Peak response amplitudes of slow potentials were plotted against stimulus position and the extent of a neuron's centre-receptive field was taken to be the diameter of the Gaussian function fitted to the data (Origin, Microcal, Northampton, MA, USA). The Gaussian diameter was defined as 0.849 times the width (w) of the Gaussian at half-height (w ≈ 2σ).
Histology
One hour after labelling the last cell in an experiment, the retina was fixed in a cold (4 °C) solution of 4 % paraformaldehyde-0.1 % glutaraldehyde in 0.1 m phosphate buffer (pH 7.3) for 12 min. The retina was then detached, trimmed, and fixed onto a gelatinized glass coverslip and left in fixative overnight at 4 °C. Retinas were then washed in phosphate buffer and reacted with the Elite ABC kit (Vector Laboratories) and 1 % Triton X-100 in 10 mm sodium phosphate-buffered saline (9 % saline, pH 7.6). Retinas were subsequently processed for peroxidase histochemistry using 3,3′-diaminobenzidine (DAB, Sigma, St Louis, MO,USA) with cobalt intensification. Retinas were then dehydrated, cleared and flatmounted for light microscopy.
RESULTS
The data in this report reflect intracellular recordings from 112 AII amacrine cells. All impalements were made in the vicinity of the visual streak region; soma eccentricities ranged from 0.8 to 2.8 mm from the optic disc. All cells were injected with Neurobiotin and identification was based on established morphological criteria, including: (1) a relatively large somata in the proximal inner nuclear layer (INL); (2) a distinctive bistratified dendritic arbor with lobular appendages in sublamina a and finer dendrites stratifying primarily in the vitreal portion of sublamina b; and (3) homologous tracer coupling to other AII somata as well as smaller cone bipolar cells in the more distal INL (Famiglietti & Kolb, 1975; Vaney et al. 1991; Hampson et al. 1992; Strettoi et al. 1992; Bloomfield et al. 1997; Xin & Bloomfield, 1997, 1999).
Effects of GABA blockers on AII cell responses
The effects of the non-selective GABA receptor blocker picrotoxin, the selective GABAA receptor blocker bicuculline and the selective GABAC receptor blocker TPMPA were examined on the light-evoked responses of AII amacrine cells. All three antagonists showed the same basic effects on AII cell activity, but to varying extents. This included a 5–8 mV depolarization of the dark membrane potential, an increase in the amplitude of the on-centre response, and a diminution or complete abolition of the off-surround activity (Fig. 1). We did observe changes in the response waveforms following drug applications, such as enhanced transient or sustained phases, but these changes were highly variable and no clear trends emerged.
Figure 1. Effects of GABA blockers on AII amacrine cell light responses.
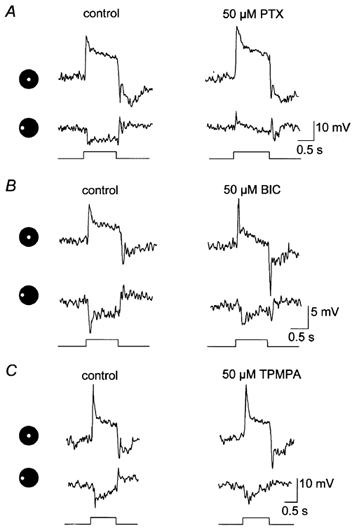
Each panel shows the on-centre and off-surround responses of an AII cell prior to (control) and after a 5 min application of a drug. The icons to the left of each panel and below each response indicate presentation of a 75 μm diameter spot of light either centred over the cell or translated 100 μm peripherally. Stimulus intensity = log −5.5; maximum intensity (log 0.0 = 2.37 mW cm−2). A, effects of 50 μm picrotoxin (PTX) on the light-evoked responses of an AII cell. Picrotoxin enhanced the on-centre response whereas the surround response was abolished revealing a complex depolarizing response. This depolarization is probably a centre-mediated response due to light scatter into the centre-receptive field region. B, application of 50 μm bicuculline (BIC) increased predominantly the transient component of the on-centre response of this cell, but attenuated the off-surround response. C, application of 50 μm TPMPA produced a modest increase in the amplitude of the on-centre response and a reduction in off-surround activity.
At equal concentrations of 50 μm, picrotoxin clearly had the most potent effects on AII cell activity. On average, the on-centre responses were increased in amplitude by 65 %, whereas the off-surrounds decreased by 52 % (Fig. 2A). On rare occasions (2/27 cells), application of 50 μm picrotoxin appeared to completely eliminate the off-surround response, leaving no light-evoked response or revealing a rather complex depolarization to light (Fig. 1A). This depolarization was presumably an on-centre response evoked by light scatter into the centre-receptive field that emerged following substantial attenuation of the antagonistic surround. Application of picrotoxin at higher concentrations of 250–500 μm produced a consistent, complete blockade of the surround responses of AII amacrine cells.
Figure 2. Summary of the effects of the GABA blockers on AII cells.

A, summary of the effects of the GABA blockers on the amplitude of the on-centre and off-surround responses of AII cells. At equal concentrations of 50 μm, picrotoxin (PTX) was most effective, producing an average 65 % increase in the on-centre response and a 52 % diminution of the off-surround. Bicuculline (BIC) and TPMPA showed similar effects, including an average 27 and 23 % increase in the centre-mediated responses and a 23 and 15 % reduction in the amplitude of the off-surround responses. B, at equal concentrations of 50 μm, PTX produced a 54 % increase in the size of the centre-receptive field of AII cells, whereas BIC and TPMPA only enhanced the receptive field by an average 34 and 33 %, respectively. The standard deviations are represented as a line above each data set. Individual drug treatments (n) = 24 for PTX, 22 for BIC and 23 for TPMPA.
Application of 50 μm bicuculline or TPMPA produced AII cell response amplitude changes that were similar in magnitude, but considerably less than those for picrotoxin. Bicuculline produced a 27 % increase in the amplitude of the on-centre responses, whereas TPMPA produced a 23 % increase (Fig. 2A). In contrast, bicuculline and TPMPA reduced AII cell off-surround responses by an average of 23 and 15 %, respectively. Once again, we found no consistent changes in response waveform, other than those in amplitude, following application of these blockers. We applied bicuculline in concentrations up to 250 μm (the highest concentration under which the rabbit retina remained viable) and were able to attenuate the off-surround responses up to 65 %, but never completely. Application of TPMPA up to 100 μm attenuated the off-surround responses up to about 30 %; unfortunately, use of higher concentration of TPMPA in our set-up was cost prohibitive. All drug effects were completely reversed after 4–6 min back in the control superfusate. Further, although these drugs had opposite effects on the centre- and surround-mediated responses, the timing of these actions and their reversals on individual cells were similar.
Effects of GABA blockers on AII cell centre-receptive field size
To determine the size of the centre-receptive field of a cell, we measured on-responses to a narrow 50 μm wide × 6.0 mm long slit of light that was displaced in discrete steps from the central position. By comparing the relative peak response amplitude to stimulus displacement, the centre-receptive field was calculated as the diameter (d) of the Gaussian functions fitted to the data (Fig. 3). Figure 3A shows the effect of picrotoxin on the receptive field of an AII cell. The Gaussian diameter of this cell prior to drug application was calculated to be 219 μm, whereas after a 5 min application of picrotoxin the diameter showed nearly a 68 % increase to 367 μm. On average, 50 μm picrotoxin increased the centre-receptive fields of dark-adapted AII cells by 54 % (Fig. 2B). Application of bicuculline and TPMPA had almost identical effects on the centre-receptive fields of AII cells: an averaged increase of 34 and 33 %, respectively. Thus, like their actions on the centre- and surround-mediated response amplitudes, picrotoxin was nearly twice as effective as bicuculline and TPMPA in increasing the centre-receptive fields of AII amacrine cells.
Figure 3. Effects of GABA blockers on the on-centre receptive fields of AII cells.

Scatterplots compare the normalized on-centre responses of an AII cell to a 50 μm wide × 6.0 mm long rectangular slit of light presented at different distances from the centre of the cell (0 μm). Values next to each curve indicate diameter (d) of Gaussian functions fitted to the data. A, effect of a 5 min application of 50 μm picrotoxin (PTX) on the centre-receptive field. Prior to application of the drug (control), the cell displayed a centre-receptive field of 219 μm. PTX increased the receptive field by 148 μm or 68 %. B, application of 50 μm bicuculline (BIC) produced a 41 % increase in the centre-receptive field of this AII cell. C, application of 50 μm TPMPA produced a 33 μm increase in the centre-receptive field of this AII cell. Number of drug treatments (n) = 18 for PTX, 19 for BIC and 18 for TPMPA.
Effects of TTX
In a previous study, we reported that application of TTX had effects qualitatively similar to those of GABA blockers on the responses of AII cells (Bloomfield & Xin, 2000). These included an increase in the amplitude of the on-centre responses coupled with attenuation of off-surround activity (Fig. 4A). In this study, we quantified the effects of TTX to compare them directly with those of the GABA antagonists. Application of 0.5 μm TTX produced a 3–5 mV depolarization of the dark membrane potential of AII cells coupled with an averaged 41 % increase in the amplitude of the on-centre response and an attenuation of the off-surrounds by 35 % (Fig. 4C). Similar to the actions of the GABA blockers, application of TTX also enlarged the centre-receptive fields of AII cells as indicated by an increase in the diameter of the Gaussian function fitted to response amplitude vs. stimulus position data. Figure 4B shows the data for an AII cell for which spike blockade in the retina by TTX produced an 83 μm or 37 % increase in the Gaussian diameter. On average, TTX produced a 43 % increase in the centre-receptive fields of AII amacrine cells (Fig. 4C). Overall, then, the magnitude of the effects of TTX on the amplitudes of centre- and surround-mediated responses and receptive field size fell between those of picrotoxin and bicuculline/TPMPA. Yet, despite being different in absolute values, the effects of TTX on these parameters were proportionately similar to those of the GABA blockers. This relationship suggests that the actions of the GABA blockers and TTX reflected effects on the same neuronal circuitry (see Discussion).
Figure 4. Effects of TTX on AII cell responses.

A, application of 0.5 μm TTX increased the amplitude of the on-centre response of this AII cell, whereas the off-surround response was completely abolished. Conventions are the same as Fig. 1. B, application of 50 μm TTX increased the size of the centre-receptive field of this AII cell by 83 μm. Conventions are the same as in Fig. 3. C, summary of the effects of TTX on AII cells (n = 16). TTX increased the on-centre responses of AII cells by an average of 41 %, reduced the off-surround responses by 35 % and enlarged the centre-receptive fields by an average of 43 %. Standard deviations are indicated by lines on top of each data set.
Effects of DNDS
The present results are consistent with previous findings from our laboratory (Bloomfield & Xin, 2000) that a spiking, GABAergic neuron plays a fundamental role in generating the surround inhibition of AII amacrine cells. Based on the numerous descriptions of the synaptic pathways subserved by AII cells, we can narrow the possible surround-generating circuits to two (Fig. 5). The first possible circuit includes a homogeneous population of amacrine cells that synapse directly upon the dendrites of AII cells in sublamina b of the IPL (Strettoi et al. 1992). Although the identity of these amacrine cells is presently unknown, they could provide a feedforward GABAergic inhibition to AII cells that generates their antagonistic surround receptive fields. The second possible circuit comprises the S1/S2 amacrine cells that provide GABAergic feedback inhibition to the same axon terminals of rod bipolar cells that innervate them (Dacheux & Raviola 1986; Strettoi et al. 1990). This feedback inhibition could, through modulation of glutamate release from rod bipolar cell axon terminals, produce the antagonistic surround-mediated responses of AII cells (see Discussion).
Figure 5. Schematic diagram of feedforward and feedback inhibitory circuits subserving AII cells in the IPL.

The on-centre receptive field of AII amacrine cells is produced via the rods → rod bipolar cell (RB) → AII amacrine cell pathway. Illumination of the periphery of the AII cell receptive field results in a characteristic off-surround response. There are two possible circuits underlying the surround response. In the first, peripheral signals are carried to the inner retina by the rods →rod bipolar cell → S1-S2 amacrine cell route. In turn, these peripherally generated signals are carried centrally, providing direct GABAergic feedback to rod bipolar cell axon terminals (large circle). Alternatively, an unidentified amacrine cell (?) may provide surround inhibition via direct feedforward inhibitory inputs onto AII amacrine cell dendrites (small circle). Open arrowheads, sign-conserving excitatory synapses; filled arrowheads, converting sign-inverting inhibitory synapses; AII, AII amacrine cell; S1-S2, S1-S2 amacrine cells; OPL, outer plexiform layer; INL, inner nuclear layer; IPL, inner plexiform layer.
To differentiate between these two possibilities, we examined the effects of the chloride channel blocker DNDS on AII cell response activity. Delivered by the recording electrode, DNDS has been shown to eliminate the effects of GABA receptor activation by blocking the chloride channel complex in cortical neurons from the intracellular side (Dudek & Friedlander, 1996; Shao & Burkhalter, 1996). Our strategy in using DNDS was to differentiate direct (feedforward) from indirect (feedback) inhibition. If the surround activity of AII amacrine cells is derived by feedforward inhibition, then application of DNDS should abolish, or at least attenuate, the activity by blocking the GABA-activated chloride conductance. Conversely, if feedback inhibition from S1/S2 amacrine cells to rod bipolar cells generates AII cell surrounds, then application of DNDS to a single AII cell through the recording electrode should have no effect on the surround activity as it would reflect a modulation of a glutamate-mediated cation conductance from rod bipolar cells rather than direct GABAergic inhibition. That is, with feedback inhibition, the GABA-mediated chloride conductance occurs on the presynaptic rod bipolar axon terminal and not the dendrites of AII amacrine cells.
In addition to ligand-gated chloride conductances, DNDS has also been shown to block a number of intrinsic chloride channels in both neural and non-neural tissues (Mayer, 1985; Madison et al. 1986; Schoppa et al. 1989; Singh et al. 1991; Hansen & Skydsgaard, 1992). It was thus imperative to first determine whether DNDS blocked GABA-activated responses in AII amacrine cells. Figure 6 illustrates an experiment in which an AII cell was impaled with an electrode loaded with 500 μm DNDS. The cell was bathed continuously with a high magnesium and low calcium solution to block synaptic transmission and thereby ensure that GABA effects were direct. Application of GABA within 1 min following impalement of the cell produced a 10–15 mV hyperpolarization that was readily reversed following return to the control superfusate. However, when this same procedure was followed 20 min later, after DNDS had leaked into the cell, GABA failed to modulate the dark membrane potential. The ability of DNDS to block GABA effects was a consistent finding (5/5 cells), indicating that it blocked GABA-gated chloride channels in AII cells.
Figure 6. Effect of DNDS on GABA-mediated responses of AII cells.

A, application of a high Mg2+/low Ca2+ Ringer solution blocked synaptic transmission resulting in a hyperpolarization of the dark membrane potential of the AII cell and a complete blockage of light-evoked on-centre activity. B, during continuous synaptic blockade with the high Mg2+/low Ca2+ solution, application of 250 μm GABA within 1 min of cell impalement produced a 10–15 mV hyperpolarization that was readily reversed following return to the control superfusate. C, a repeated application of GABA 20 min later, after DNDS was allowed to leak into the cell, failed to modulate the dark membrane potential of the cell.
DNDS also had robust and consistent effects on the on-centre and off-surround responses of AII amacrine cells. After allowing 500 μm DNDS to leak into the impaled AII cell for 20–30 min, we observed a 4–7 mV depolarization of the dark membrane potential and a sizeable increase in amplitude in both the centre- and surround-mediated responses as well as the background noise. As illustrated in Fig. 7, DNDS also occasionally brought out spontaneous spike-like impulses. As DNDS was delivered via the recording electrode, it was not possible to determine if these drug effects were reversible. However, the amplitude increase in the light-evoked activity and background noise was a very robust effect that occurs in each of the 35 AII amacrine cells that we studied. Despite the robust effects on response amplitude, we found that DNDS did not significantly change (3.6 ± 1.2 % (mean ± s.d.); P > 0.1, Mann-Whitney paired U test) the centre-receptive field size of AII amacrine cells.
Figure 7. Effects of DNDS on AII cell response activity.

Immediately after impalement with a microelectrode loaded with 500 μm DNDS, this AII cell showed a clear on-centre and off-surround responses to a 75 μm diameter spot of light either centred (right) or translated laterally by 100 μm (left). A slight enhancement of the light-evoked responses was detected following 10 min of impalement during which DNDS leaked into the cell. After 20 min, an additional increase in the amplitudes of both the centre- and surround-mediated responses was observed. Application of DNDS also resulted in increased dark background noise and brought out spike activity.
Following the experimental strategy described above, the failure of DNDS to attenuate the surround-mediated responses of AII cells is consistent with an indirect feedback inhibition being the generating mechanism rather than a direct feedforward inhibition. However, one concern with this interpretation involves the fact that AII cells are electrically coupled (Famiglietti & Kolb, 1975; Vaney et al. 1991; Bloomfield et al. 1997). This coupling raises the possibility that DNDS may have, in fact, blocked the surround activity in the impaled cell with the surviving surround responses actually being those generated in neighbouring AII cells that propagated to the impaled AII cell through the interconnecting gap junctions. To eliminate this possibility, it was therefore necessary to repeat the experiments with DNDS on uncoupled AII cells. We assessed the effects of several uncoupling agents by first applying them to the retina for 30 min and then injecting an AII cell with Neurobiotin and comparing the extent of tracer coupling to that under control conditions (Fig. 8). Application of the dopamine D1 receptor agonist SKF38393 (cf. Hampson et al. 1992; Mills & Massey, 1995) and retinoic acid (Weiler et al. 2001) were most effective, but their uncoupling actions were incomplete as evidenced by a few remaining tracer-coupled cells (Fig. 8B and C). However, we found that co-application of SKF38393 and retinoic acid for at least 30 min produced complete uncoupling of AII amacrine cells (Fig. 8D). Following the uncoupling of AII cells with this mixture, we found that application of DNDS produced a similar enhancement in the amplitude of the on-centre and off-surround responses and dark noise as seen in control retinas (Fig. 9). These results thus indicate that the failure of DNDS to attenuate the surround activity did not reflect surround signals from neighbouring AII cells reaching impaled AII cells via gap junctions.
Figure 8. Effects of SKF38393 and retinoic acid on the coupling pattern of AII cells.
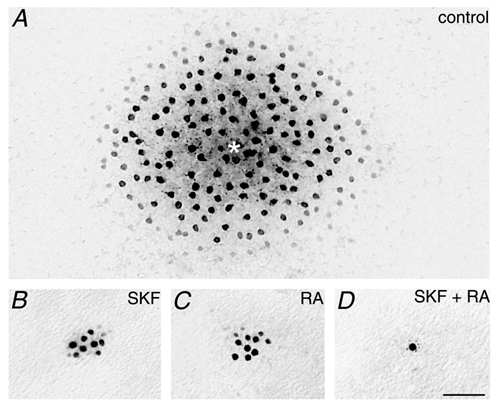
A, typical tracer-coupled array of AII amacrine cells following injection of Neurobiotin into a single cell (*). Retina was adapted with a dim background light of log −6.0. B, application of the dopamine D1 receptor agonist SKF38393 (400 μm) for 45 min prior to Neurobiotin injection resulted in a large reduction in tracer coupling of AII cells, although some coupling remained. Same adapting conditions as in A. C, application of 150 μm retinoic acid (RA) also reduced the tracer coupling of AII cells from control conditions. D, co-application of both SKF38393 and RA resulted in a complete uncoupling of AII cells so that only the cell injected with Neurobiotin is labelled. Adapting conditions were the same as in A. Calibration bar for all panels = 50 μm.
Figure 9. Effects of DNDS on the light-evoked responses of uncoupled AII cells.

Intracellular recordings from the uncoupled AII cell illustrated in Fig. 8D. Immediately after impalement with a microelectrode loaded with 500 μm DNDS, this AII cell showed a clear on-centre and off-surround response to a 75 μm diameter spot of light either centred (right panel) or translated laterally by 100 μm (left panel). Beginning at 10 min after impalement and reaching a maximum effect at 20 min, leakage of DNDS into the cell produced a sizeable increase in both the centre- and surround-mediated responses as well as the dark membrane noise. Therefore, DNDS had the same qualitative effects on both coupled and uncoupled AII amacrine cells.
DISCUSSION
Under dark-adapted conditions, the AII amacrine cells show robust on-centre/off-surround receptive fields. In a previous study, we provided strong evidence that whereas the centre-mediated responses reflect excitatory drive by presynaptic rod bipolar cells, the surround responses are generated in the inner retina (Bloomfield & Xin, 2000). The present results extend those findings to show clearly that a spiking, GABAergic amacrine cell plays a vital role in generating the AII cell surround responses and that both GABAA and GABAC receptors are activated in this process. Furthermore, our results indicate that feedback inhibition in the IPL is responsible for the generation of AII cell surround activity, thus offering a clear function for the S1/S2 to rod bipolar cell axon terminal circuitry in the proximal retina.
Drug effects on centre/surround responses of AII cells
Application of DNDS produced a robust enhancement of both the centre and surround responses in every AII cell we examined. These effects increased gradually and took 20–40 min to reach their maximum, thus paralleling the temporal effects of DNDS applied intracellularly to cortical neurons (Dudek & Friedlander, 1996; Shao & Burkhalter, 1996). The increases in the response amplitudes concurred with an increase in dark noise. Taken together, the most parsimonious explanation for these actions is that DNDS reduced a tonic chloride conductance across AII cell membranes and increased the cells' input resistance; the increased input resistance can also explain why application of DNDS also occasionally brought out spike activity in AII cells. One source of this tonic chloride conductance might be a basal release of GABA via the feedforward inhibitory pathway to AII cells described in Fig. 5. Our finding that DNDS blocked GABA-mediated voltage changes in AII cells is consistent with this idea. However, as aforementioned, in addition to GABA-mediated chloride currents, DNDS blocks a variety of different chloride channels, and so the increased input resistance may reflect closure of a combination of ligand-gated and intrinsic chloride conductances.
Similar to the effects of DNDS, application of both GABA blockers and TTX also increased the centre responses of AII cells. However, following application of these drugs, the surround activity was attenuated and there was usually no change in background noise levels. Thus, the application of GABA blockers and TTX, which presumably deactivated the feedback circuitry generating AII surround activity (see later), resulted in effects that diverge from those of DNDS directly on AII cells. We also found that application of GABA blockers or TTX produced an increase in the size of the cells' receptive fields, whereas DNDS had no effect. The GABA blockers increased the centre-receptive fields of AII cells an average of 33–54 %, with the order of potency: picrotoxin > bicuculline ≈ TPMPA. Further, the effects of these antagonists and TTX on receptive field size was proportional to their ability to reduce AII cell surround activity. These data suggest that tonic, indirect feedback inhibition of AII cells acts to circumscribe their centre-receptive fields. Thus, changes in the strength of the surround of AII amacrine cells could modulate the spatial acuity of individual cells, analogous to the manner in which light-induced changes in electrical coupling modulate spatial acuity of coupled AII cell groups (Bloomfield et al. 1997).
Generation of AII cell surround activity
Taken together, the present results provide circumstantial but compelling evidence that feedback inhibition from S1/S2 amacrine cells to rod bipolar axon terminals generates AII cell surround-receptive fields. First, our results show clearly that a GABAergic neuron plays a vital role in generating the AII cells surrounds and that both GABAA and GABAC receptors are activated about equally. Second, although application of DNDS was shown to block the hyperpolarizing effects of GABA on the AII cell membrane potential, it failed to attenuate the surround activity, thereby eliminating direct feedforward inhibition as the generating mechanism. Third, we found that application of TTX had effects on light-evoked activity and receptive field size that paralleled those of the GABA blockers, thus indicating that a spiking neuron is involved in the surround-generating circuitry. These data clearly implicate the feedback circuit from S1/S2 amacrine cells to rod bipolar cell axon terminals as being directly responsible for generating the AII cell surround receptive fields. Both S1 and S2 cells are GABAergic and capable of impulse behaviour (Massey et al. 1992; Bloomfield, 1996). Further, both GABAA and GABAC receptors have been localized to rod bipolar cell axon terminals (Euler & Wässle, 1998; Fletcher et al. 1998). There is also clear evidence that GABAC receptors are localized specifically to the S1-S2 cell reciprocal synapses to rod bipolar cells. Localization of GABAA receptors to these synapses is less certain (Fletcher & Wässle, 1999).
Combining our present physiological results with the established synaptic circuitry of AII cells, the events underlying their surround-mediated responses can be described. In this scheme, stimulation of the surround receptive field of an AII amacrine cell gives rise to depolarizing on-responses in peripheral rod bipolar cells that, likewise, produce a depolarizing on-response in the wide-field S1/S2 cells. This light-evoked signal is then propagated both actively and passively within the S1/S2 cell dendrites to activate the GABAergic feedback synapses to the axon terminals of centrally placed rod bipolar cells. This inhibition then shunts the membrane of the rod bipolar cell, thereby reducing the release of glutamate to postsynaptic AII cells and thus hyperpolarizing them. One caveat to this model is that there must be a tonic release of glutamate from rod bipolar cell axon terminals in the dark to both AII and S1/S2 cells that, in turn, would provide a basal release of GABA from S1/S2 cells back onto the rod bipolar cells. This baseline release of glutamate could then be reduced by activation of S1/S2-to-rod bipolar cell feedback synapses following peripheral stimulation. The temporal characteristics of transmitter release from rod bipolar cells are presently unclear, but evidence for continuous release at other bipolar cell terminals has been reported (Lagnado et al. 1996; Rouze & Schwartz, 1998).
Functional significance
The present results confirm prior reports that AII cells display a robust surround-receptive field under dark-adapted conditions (Nelson, 1982; Dacheux & Raviola, 1986; Bloomfield et al. 1997). Interestingly, we showed recently that the surround-mediated responses of AII cells vanish following light adaptation of the retina (Xin & Bloomfield, 1999). Thus, AII cell surround responses appear to reflect rod-mediated, but not cone-mediated, signals. This scheme appears opposite to that found for brisk ganglion cells whose surrounds are present under light-adapted conditions, but are greatly reduced or abolished by dark adaptation (Barlow et al. 1957; Rodieck & Stone, 1965; Muller & Dacheux, 1997). The surround receptive fields displayed by AII cells thus may be unique in that they appear exclusively under scotopic light conditions. It is interesting to note that it is widely maintained that the synaptic feedback circuitry from horizontal cells to photoreceptors, thought responsible for generating the surround responses of retinal neurons, occurs onto cones but not rods (Baylor et al. 1971; Burkhardt, 1977; but see Linberg & Fisher, 1988). This would necessitate different synaptic circuits to generate the rod-driven surround responses of AII cells since the appropriate feedback circuits do not exist in the outer retina. Whereas the amacrine-to-rod bipolar cell feedback synapses may be dedicated to the rod pathway, recent evidence indicates that with lateral inhibition derived from spiking, GABAergic amacrine cells play an important role in generating the surround responses of certain ganglion cells, even under light-adapted conditions (Cook & McReynolds, 1998; Taylor, 1999). These results suggest that inhibitory circuits in the IPL play a major role creating both rod- and cone-mediated antagonistic surround receptive fields.
Finally, although we have lumped S1 and S2 amacrine cells together since they show the same reciprocal feedback circuitry with rod bipolar cells, the question remains open whether both types of cells are responsible for the AII surrounds. In this regard it is interesting to note that the S2 cells have a significantly greater number of reciprocal contacts with rod bipolar cells and show ultrastructural features that differ from those of S1 cells (Sandell et al. 1989; Strettoi et al. 1992). Interestingly, Fletcher & Wässle (1999) reported that whereas both S1 and S2 dendrites are presynaptic to GABAC receptors on rod bipolar cells, not every reciprocal synapse was associated with a GABAC cluster. It will thus be important to determine how GABAA receptors fit into the arrangement of feedback synaptic circuitry and any differential patterning related to S1 vs. S2 amacrine cell inputs.
Acknowledgments
This study was supported by NIH Grant EY07360 awarded to S. A. Bloomfield, a Postdoctoral Fellowship from the Fight for Sight division of Prevent Blindness America, Inc. awarded to Béla Völgyi, a NIH Postdoctoral Fellowship EY06689 awarded to D. Xin, and Research to Prevent Blindness, Inc.
REFERENCES
- Barlow HB, Fitzhugh R, Kuffler SW. Change of organization of receptive fields of the cat's retina during dark adaptation. Journal of Physiology. 1957;137:338–354. doi: 10.1113/jphysiol.1957.sp005817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baylor DA, Fuortes MGF, O'Bryan PM. Receptive field of cones in the retina of turtle. Journal of Physiology. 1971;214:265–294. doi: 10.1113/jphysiol.1971.sp009432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloomfield SA. Relationship between receptive and dendritic field size of amacrine cells in the rabbit retina. Journal of Neurophysiology. 1992;68:711–725. doi: 10.1152/jn.1992.68.3.711. [DOI] [PubMed] [Google Scholar]
- Bloomfield SA. Effect of spike blockade on the receptive-field size of amacrine and ganglion cells in the rabbit retina. Journal of Neurophysiology. 1996;75:1878–1893. doi: 10.1152/jn.1996.75.5.1878. [DOI] [PubMed] [Google Scholar]
- Bloomfield SA, Dacheux RF. Rod vision: pathways and processing in the mammalian retina. Progress in Retina and Eye Research. 2001;20:351–384. doi: 10.1016/s1350-9462(00)00031-8. [DOI] [PubMed] [Google Scholar]
- Bloomfield SA, Miller RF. A physiological and morphological study of the horizontal cell types in the rabbit retina. Journal of Comparative Neurology. 1982;208:288–303. doi: 10.1002/cne.902080306. [DOI] [PubMed] [Google Scholar]
- Bloomfield SA, Xin D. Surround inhibition of mammalian AII amacrine cells is generated in the proximal retina. Journal of Physiology. 2000;523:771–783. doi: 10.1111/j.1469-7793.2000.t01-1-00771.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloomfield SA, Xin D, Osborne T. Light-induced modulation of coupling between AII amacrine cells in the rabbit retina. Visual Neuroscience. 1997;14:565–576. doi: 10.1017/s0952523800012220. [DOI] [PubMed] [Google Scholar]
- Boycott BB, Dowling JE. Organization of the primate retina: light microscopy. Philosophical Transactions of the Royal Society B. 1969;255:109–184. [Google Scholar]
- Boycott BB, Kolb H. The connections between bipolar cells and photoreceptors in the retina of the domestic cat. Journal of Comparative Neurology. 1973;148:91–114. doi: 10.1002/cne.901480106. [DOI] [PubMed] [Google Scholar]
- Boycott BB, Wässle H. Morphological classification of bipolar cells in macaque monkey retina. European Journal of Neuroscience. 1991;3:1069–1088. doi: 10.1111/j.1460-9568.1991.tb00043.x. [DOI] [PubMed] [Google Scholar]
- Burkhardt DA. Responses and receptive field organization of cones in perch retina. Journal of Neurophysiology. 1977;40:53–62. doi: 10.1152/jn.1977.40.1.53. [DOI] [PubMed] [Google Scholar]
- Cook PB, McReynolds JS. Lateral inhibition in the inner retina is important for spatial tuning of ganglion cells. Nature Neuroscience. 1998;1:714–719. doi: 10.1038/3714. [DOI] [PubMed] [Google Scholar]
- Dacheux RF, Raviola E. The rod pathway in the rabbit retina: a depolarizing bipolar and amacrine cell. Journal of Neuroscience. 1986;6:331–345. doi: 10.1523/JNEUROSCI.06-02-00331.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudek SM, Friedlander MJ. Intracellular blockade of inhibitory synaptic responses in visual cortical layer IV neurons. Journal of Neurophysiology. 1996;75:2167–2173. doi: 10.1152/jn.1996.75.5.2167. [DOI] [PubMed] [Google Scholar]
- Euler T, Wässle H. Immunocytochemical identification of cone bipolar cells in the rat retina. Journal of Comparative Neurology. 1995;361:461–478. doi: 10.1002/cne.903610310. [DOI] [PubMed] [Google Scholar]
- Euler T, Wässle H. Different contributions of GABAA and GABAC receptors to rod and cone bipolar cells in a rat retinal slice preparation. Journal of Neurophysiology. 1998;79:1384–1395. doi: 10.1152/jn.1998.79.3.1384. [DOI] [PubMed] [Google Scholar]
- Famiglietti EV, Kolb H. A bistratified amacrine cell and synaptic circuitry in the inner plexiform layer of the retina. Brain Research. 1975;84:293–300. doi: 10.1016/0006-8993(75)90983-x. [DOI] [PubMed] [Google Scholar]
- Fletcher EL, Koulen P, Wässle H. GABAA and GABAC receptors on mammalian rod bipolar cells. Journal of Comparative Neurology. 1998;396:351–365. doi: 10.1002/(sici)1096-9861(19980706)396:3<351::aid-cne6>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- Fletcher EL, Wässle H. Indoleamine-accumulating amacrine cells are presynaptic to rod bipolar cells through GABA(C) receptors. Journal of Comparative Neurology. 1999;413:155–167. doi: 10.1002/(sici)1096-9861(19991011)413:1<155::aid-cne11>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- Hampson EC, Vaney DI, Weiler R. Dopaminergic modulation of gap junction permeability between amacrine cells in mammalian retina. Journal of Neuroscience. 1992;12:4911–4922. doi: 10.1523/JNEUROSCI.12-12-04911.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen M, Skydsgaard JM. Stilbene disulphonates inhibit apparently separate chloride transporters in skeletal muscle of Rana temporaria. Journal of Physiology. 1992;448:383–395. doi: 10.1113/jphysiol.1992.sp019047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lagnado L, Gomis A, Job C. Continuous vesicle cycling in the synaptic terminal of retinal bipolar cells. Neuron. 1996;17:957–967. doi: 10.1016/s0896-6273(00)80226-3. [DOI] [PubMed] [Google Scholar]
- Linberg KA, Fisher SK. Ultrastructural evidence that horizontal cell axon terminals are presynaptic in the human retina. Journal of Comparative Neurology. 1988;268:281–297. doi: 10.1002/cne.902680211. [DOI] [PubMed] [Google Scholar]
- Madison DV, Malenka DC, Nicoll RA. Phorbol esters block voltage-sensitive chloride current in hippocampal pyramidal cells. Nature. 1986;321:695–697. doi: 10.1038/321695a0. [DOI] [PubMed] [Google Scholar]
- Massey SC, Mills SL, Marc RE. All indoleamine-accumulating cells in the rabbit retina contain GABA. Journal of Comparative Neurology. 1992;322:275–291. doi: 10.1002/cne.903220213. [DOI] [PubMed] [Google Scholar]
- Mayer ML. A calcium-activated chloride current generates the after-depolarization in rat sensory neurones in culture. Journal of Physiology. 1985;364:217–239. doi: 10.1113/jphysiol.1985.sp015740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mills SL, Massey SC. Differential properties of two gap junctional pathways made by AII amacrine cells. Nature. 1995;377:734–737. doi: 10.1038/377734a0. [DOI] [PubMed] [Google Scholar]
- Muller JF, Dacheux RF. Alpha ganglion cells of the rabbit retina lose antagonistic surround responses under dark adaptation. Visual Neuroscience. 1997;14:395–401. doi: 10.1017/s0952523800011512. [DOI] [PubMed] [Google Scholar]
- Nelson R. AII amacrine cells quicken time course of rod signals in cat retina. Journal of Neurophysiology. 1982;47:928–947. doi: 10.1152/jn.1982.47.5.928. [DOI] [PubMed] [Google Scholar]
- Rodieck RW, Stone J. Analysis of receptive fields of cat retinal ganglion cells. Journal of Neurophysiology. 1965;28:833–849. doi: 10.1152/jn.1965.28.5.833. [DOI] [PubMed] [Google Scholar]
- Rouze NC, Schwartz EA. Continuous and transient vesicle cycling at a ribbon synapse. Journal of Neuroscience. 1998;18:8614–8624. doi: 10.1523/JNEUROSCI.18-21-08614.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandell JH, Masland RH, Raviola E, Dacheux RF. Connections of indoleamine-accumulating cells in the rabbit retina. Journal of Comparative Neurology. 1989;283:303–313. doi: 10.1002/cne.902830210. [DOI] [PubMed] [Google Scholar]
- Schoppa N, Shorofsky SR, Jow F, Nelson DJ. Voltage-gated chloride currents in cultured canine tracheal epithelial cells. Journal of Membrane Biology. 1989;108:73–90. doi: 10.1007/BF01870427. [DOI] [PubMed] [Google Scholar]
- Shao Z, Burkhalter A. Different balance of excitation and inhibition in forward and feedback circuits of rat visual cortex. Journal of Neuroscience. 1996;16:7353–7365. doi: 10.1523/JNEUROSCI.16-22-07353.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh AK, Afink GB, Venglarik CJ, Wang RP, Bridges RJ. Colonic Cl channel blockade by three classes of compounds. American Journal of Physiology. 1991;261:51–63. doi: 10.1152/ajpcell.1991.261.1.C51. [DOI] [PubMed] [Google Scholar]
- Strettoi E, Dacheux RF, Raviola E. Synaptic connections of rod bipolar cells in the inner plexiform layer of the rabbit. Journal of Comparative Neurology. 1990;295:449–466. doi: 10.1002/cne.902950309. [DOI] [PubMed] [Google Scholar]
- Strettoi E, Dacheux RF, Raviola E. Synaptic connections of the narrow-field, bistratified rod amacrine cell (AII) in the rabbit retina. Journal of Comparative Neurology. 1992;5:449–466. doi: 10.1002/cne.903250203. [DOI] [PubMed] [Google Scholar]
- Taylor WR. TTX attenuates surround inhibition in rabbit retinal ganglion cells. Visual Neuroscience. 1999;16:285–290. doi: 10.1017/s0952523899162096. [DOI] [PubMed] [Google Scholar]
- Toyoda J-I, Tonosaki K. Effect of polarization of horizontal cells on the on-centre bipolar cell of carp retina. Nature. 1978;276:399–400. doi: 10.1038/276399a0. [DOI] [PubMed] [Google Scholar]
- Vaney DI, Young HM, Gynther IC. The rod circuit in the rabbit retina. Visual Neuroscience. 1991;7:141–154. doi: 10.1017/s0952523800011019. [DOI] [PubMed] [Google Scholar]
- Weiler R, Pottek M, Schultz K, Janssen-Bienhold U. Retinoic acid, a neuromodulator in the retina. Progress in Brain Research. 2001;131:309–318. doi: 10.1016/s0079-6123(01)31025-7. [DOI] [PubMed] [Google Scholar]
- Xin D, Bloomfield SA. Tracer coupling pattern of amacrine and ganglion cells in the rabbit retina. Journal of Comparative Neurology. 1997;383:512–528. doi: 10.1002/(sici)1096-9861(19970714)383:4<512::aid-cne8>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- Xin D, Bloomfield SA. Comparison of the responses of AII amacrine cells in the dark- and light-adapted rabbit retina. Visual Neuroscience. 1999;16:653–665. doi: 10.1017/s0952523899164058. [DOI] [PubMed] [Google Scholar]


