Abstract
Membrane fusion plays a central role in the synaptic vesicle cycle. While many of the pre- and postfusion events have been investigated at room temperature, few researchers have investigated these processes at more physiologically relevant temperatures. We have used autaptic cultures of hippocampal neurons to investigate changes in the size and refilling rate of the readily releasable pool (RRP) of synaptic vesicles brought about by an increase in temperature from 25 to 35 °C. We have also examined temperature-dependent changes in spontaneous and action potential (AP)-evoked release as well as the fraction of the RRP that is released during an AP. Although we found a threefold increase in the refilling rate of the RRP at the higher temperature, there was no apparent change in the size of the RRP with increased temperature. Moreover, we observed a slight but significant decrease in the quanta released during an AP. This increased refilling rate and decreased release probability resulted in a reduction of both the degree and time course of synaptic depression during high frequency stimulation at the higher temperature. This reduction in synaptic depression was accompanied by an increased maintenance of the synchronous component of release during high frequency stimulation. These findings indicate that the dynamics of vesicular supply and release in hippocampal neurons at room temperature are significantly different at near physiological temperatures and could affect our present understanding of the way in which individual neurons and networks of neurons process information.
The efficiency of synaptic transmission depends on a variety of pre- and postsynaptic processes involving vesicular release and recruitment as well as the postsynaptic response to the neurotransmitter. The temperature dependence of vesicular release at the vertebrate synapse, including the rate of spontaneous transmitter release (Fatt & Katz, 1952), the duration of the action potential (Katz & Miledi, 1965), the time course of transmitter release after stimulation (Katz & Miledi, 1965) and the rate of decay of facilitation (Eccles et al. 1941), has been well known for almost 50 years. More recent work in a variety of mammalian secretory cells indicates that vesicular recruitment is enhanced at higher temperatures (Thomas et al. 1993; Renström et al. 1996; Dinkelacker et al. 2000). Although a handful of recent studies have investigated directly the temperature dependence of synaptic transmission in neuronal preparations (Allen & Stevens, 1994; Sabatini & Regehr, 1996; Hardingham & Larkman, 1998; Taschenberger & von Gersdorff, 2000), most have been performed at room temperature (Liu & Tsien, 1995; Stevens & Tsujimoto, 1995; Rosenmund & Stevens, 1996; Murthy et al. 1997; Ryan et al. 1997; von Gersdorff & Matthews, 1997). Considering that protein conformation, enzyme kinetics, cytoskeletal dynamics and neuronal excitability are known to be profoundly sensitive to changes in temperature, it would be surprising if such a drastic difference between the experimental temperature (typically 22 °C) and the physiological temperature of mammalian preparations (37 °C) did not affect the efficiency of synaptic transmission.
Of the few studies that have directly investigated the temperature dependence of synaptic transmission, most have reported conflicting findings. Specifically, vesicular and synaptic release probabilities in hippocampal cultures or slices at room temperature are surprisingly low (Hessler et al. 1993; Rosenmund et al. 1993; Allen & Stevens, 1994; Rosenmund & Stevens, 1996). In contrast to these findings, most studies using paired recordings in cortical slices at near physiological temperatures have observed very reliable transmission, suggesting higher than expected release probabilities (Thomson et al. 1993a, b; Hardingham & Larkman, 1998). Specifically, Hardingham & Larkman (1998) observed an increase in the reliability of transmission (reduction of failures) upon an increase in temperature in slice preparations of rat visual cortex. They concluded that this increase in reliability results from an increase in the synaptic release probability that occurs with increasing temperature. However, earlier work indicated that synaptic reliability in slices of CA1 hippocampal pyramidal neurons is unaffected by changes from room to physiological temperature (Allen & Stevens, 1994). Although the differences in the experimental preparations and the complexity of polysynaptic slice preparations may be responsible for the differences in findings, it may also be that release probability does not alone mediate the temperature-dependent efficiency of synaptic transmission. Additional work examining the calyx of Held synapse found that increasing the temperature from 21–23 to 35 °C reduced the duration of the presynaptic action potential (AP) and decreased the depression of EPSCs in response to high frequency afferent stimulation, allowing some synapses to follow stimuli reliably at frequencies of 800 Hz (Taschenberger & von Gersdorff, 2000). Unfortunately, the mechanisms mediating this response are unclear and, in light of previous work in other preparations, vesicular maturation and recruitment as well as changes in release probability could all profoundly affect the efficiency of synaptic transmission in response to either a single stimulus or multiple stimuli.
In this study we took advantage of the autaptic preparation of hippocampal neurons to investigate electrophysiologically the temperature dependence of isolated pre- and postsynaptic vesicular dynamics and integrate findings from different preparations and experiments. Our findings suggest that the probability of neurotransmitter release at physiological temperature is slightly but significantly reduced, but more stable and with higher temporal precision over a variety of action potential frequencies. The increased stability of transmission over a variety of frequencies is likely to be mediated by the threefold increase in vesicular recruitment to the readily releasable pool that we observed at the higher temperature. No doubt these findings affect our present understanding of the way in which neuronal information transfer occurs in vivo.
METHODS
Cell culture
Microislands of astrocyte feeder cells were prepared 4–5 days before plating hippocampal neurons. Briefly, islands of substrate (a 1 : 4 mixture of collagen and poly-d-lysine) were applied to 18 mm glass coverslips using a stamp containing regularly spaced squares (200 × 200 μm). To obtain astrocytes and hippocampal neurons, newborn rats and mice were decapitated according to the rules of the state and animal welfare committee. Brains were removed and cleaned of meninges and vascular tissue. To obtain hippocampal neurons, the hippocampi were removed in physiological salt solution and enzymatically dissociated in papain (2 units ml−1) in DMEM for 60 min at 37 °C. To obtain astrocytes, the cortexes of separate animals were removed in physiological salt solution, similarly dissociated and preplated at a density of 5000 cm−2 in DMEM containing 10 % fetal calf serum. Once astrocytes reached confluency, 5-fluoro-2′deoxyuridine (10 μm) was added to inhibit further proliferation. Before plating the dissociated hippocampal neurons at a density of 500 cm−2, the medium of the astrocyte feeder cells was replaced with serum-free medium (Neurobasal medium A supplemented with B27). Neurons were allowed to mature for 14–21 days before they were used for experiments, and only islands containing single neurons were examined. Cell culture solutions were purchased from Gibco (Grand Island, NY, USA).
Solutions
Patch-pipette solutions contained (mm): 135 potassium gluconate, 10 Hepes, 1 EGTA, 4.6 MgCl2, 4 NaATP, 15 creatine phosphate and 50 U ml−1 phosphocreatine kinase (300 mosmol l−1, pH 7.3). The extracellular saline solution contained (mm): 140 NaCl, 2.4 KCl, 10 Hepes, 10 glucose, 4 CaCl2 and 4 MgCl2 (300 mosmol l−1, pH 7.3). External divalent concentrations of 4 mm Ca2+ and 4 mm Mg2+ were used to increase the stability of the patch clamp recordings, which was critical to obtain reliable recordings over multiple temperature changes. The elevated Mg2+ concentration also prevented excessive NMDA receptor activation during repetitive synaptic stimuli. Additionally, the concentrations of Ca2+ and Mg2+ were chosen so that the release probability seen in our preparation was identical to that seen in traditional Krebs buffer (2.5 mm Ca2+ and 1.3 mm Mg2+). Although future experiments should be done with solutions that better reflect the in vivo divalent concentrations, since such changes have been shown to affect findings in in vitro recordings (Sanchez-Vives & McCormick, 2000), the results obtained in this study are more comparable to the findings of others who have used traditional extracellular recording solutions because they reflect merely the physiological effects of changes in temperature. All chemicals, except for TTX (Sankyo Co., Ltd, Tokyo, Japan), cyclothiazide, and kynurenic acid (Tocris Cookson, Bristol, UK) were purchased from Sigma.
Experimental set up
All solutions were applied using a fast-flow system that provides reliable and precise solution exchanges with time constants of approximately 20–30 ms as described previously (Rosenmund et al. 1995). The temperature of the hippocampal neuron was controlled by heating the superfused solution as it exited the flow pipe via a resistive wire wrapped around the array of flow pipes and connected to a feedback temperature controller. Current was passed through the wire in order to maintain the desired temperature as measured by a temperature sensor placed near the exit of the flow pipes. The flow pipe was positioned 100–200 μm away from the neuron. In this way, we were able to increase the solution temperature from 25 to 35 °C in approximately 8–15 s and cool solutions from 35 to 25 °C in approximately 20 s. In order to ensure that the solution temperature was indeed the temperature measured by the sensor on the flow pipe, we occasionally placed a second temperature sensor in the solution flow experienced by the cells. In this manner, we verified that the recorded temperature was within ±0.5 °C of the actual temperature.
Electrophysiology
Cells were whole-cell voltage clamped at −85 mV with a PC-505 amplifier (Warner Instruments, Hamden, CT, USA) under control of the Clampex 8.0 program (Axon Instruments). Currents were low-pass filtered at 1 or 5 kHz and stored at either 10 or 20 kHz. The series resistance was compensated 60–70 %. Only cells with series resistances below 15 MΩ were analysed.
The size of the readily releasable pool (RRP) of synaptic vesicles was determined by a 3 or 4 s application of the external saline solution made hypertonic by the addition of 500 mm sucrose. The RRP was defined as those quanta released during the transient burst of exocytotic activity that followed application of the hypertonic solution. The Q10 for peak vesicular release was determined by dividing the peak vesicular release rate calculated at 35 °C by the value calculated at 25 °C. The refilling rate of the RRP was determined by applying paired pulses of hypertonic solutions with varying interpulse intervals and comparing the degree to which the charge of the transient component recovered. The Q10 for recovery was determined by dividing the time constants of recovery at 25 °C by those at 35 °C. Refilling data were first collected from autaptic cultures of rat hippocampal neurons and subsequently collected from similar cultures of mouse hippocampal neurons. Data were individually analysed, not found to be significantly different (P = 0.30–0.96 for each interpulse interval time) and, therefore, pooled for the final analysis. All other experiments were performed entirely using autaptic cultures of mouse hippocampal neurons and were performed on the same cell at both temperatures.
Recordings of mEPSCs were performed in the presence of 200 nm TTX. Spontaneous events were detected using the event detection feature available as part of Axograph 4.1 (Axon Instruments). Briefly, the events were detected by sliding a predefined template (consisting of a linear summation of two exponents) along the recorded trace. The template was automatically offset to account for fluctuations in baseline, and the amplitude scaled to fit the analysis window. Only events with amplitudes 3.5 times greater than the standard deviation of the baseline noise were detected. After computer detection, events were individually examined to verify that they satisfied the detection criteria. Events were then averaged to obtain a mean amplitude, charge and decay time per cell at each temperature. The Q10 for mEPSC frequency was determined by dividing the mEPSC frequency at 35 °C by that at 25 °C.
EPSCs were evoked by depolarizing the cell from −85 to −15 mV for 1 or 2 ms every 5 s (0.2 Hz). Temperature-dependent changes in the amplitude, charge and decay time were compared for each cell at 25 and 35 °C. Additionally, the change in amplitude, area and decay time normalized to the value at 25 °C were plotted as a function of increasing temperature. The effect of high frequency stimulation on the amplitude of the EPSC was measured by applying depolarizations at frequencies of 10, 20 and 40 Hz for a period of 5 s. The EPSC amplitude normalized to the first response was plotted as a function of time for each temperature and stimulation frequency.
The synchronous and asynchronous components of release during high frequency stimulation were also calculated and plotted as a function of time. Specifically, we integrated each stimulus episode (with durations of 100, 50 and 25 ms for stimulation frequencies of 10, 20 and 40 Hz, respectively) to calculate the charge or total release as a function of time and stimulus number. Such traces show an initial linear increase in charge due to stimulus-uncoupled or asynchronous release. This asynchronous component increases with stimulus number as evidenced by the increasing steepness of the initial linear segment with successive stimuli. After application of the depolarizing pulse, the charge increases exponentially. Thus, the cumulative charge at the end of the stimulus episode represents a sum of the asynchronous (stimulus-uncoupled) and synchronous (stimulus-coupled) release. The total contribution of asynchronous release was calculated for each stimulus episode by fitting the linear part of the trace with a best-fit line. To calculate the synchronous component of release, the extrapolated value of asynchronous release was subtracted from the total release calculated for that stimulus episode. The determined values for synchronous and asynchronous release were plotted as a function of time (or stimulus episode).
Statistical analyses
All experiments (except those performed to analyse the RRP refilling rate) were performed on the same cell at both temperatures. Therefore, statistical analyses were performed using the Wilcoxon rank sum test for paired, non-parametric data. Temperature-dependent changes were considered to be significant only when P < 0.05. In other cases, statistical analyses were performed using the Wilcoxon rank sum test for unpaired, non-parametric data. All values are presented as the means ± s.e.m. For data fitted with exponential curves, the goodness of fit for each curve fitting was determined by comparing both the χ2 and sum of squares errors.
RESULTS
Using a fast-flow system that allows rapid application and exchange of external solutions in conjunction with a recently designed temperature regulation system, we originally examined various parameters of synaptic transmission over a variety of temperatures (ranging from 22 to 40 °C). However, the stability of recordings decreased tremendously with multiple temperature changes and temperatures above 35 °C. Therefore, to obtain recordings from the same cell at different temperatures and to allow direct calculation of Q10 values, we decided to perform the final experiments at 25 °C (near room temperature) and 35 °C (near physiological temperature). Furthermore, in about half of the experiments the temperature was increased (from 25 to 35 °C) and in the other half it was decreased (from 35 to 25 °C). In a few cases, data were obtained with multiple changes in temperature. In such cases, the averaged response at each temperature was used for the final analysis. Additionally, in autaptic cultures, excitatory glutamatergic neurons present the majority of cells, and for this reason we limited our analysis to glutamatergic neurons only. However, inhibitory GABAergic neurons were occasionally examined and found to show similar trends as those presented below.
Refilling of the RRP
To explore changes in the RRP, we applied pulses of hypertonic solution. Although the exact mechanism of hypertonically mediated release is unknown, it appears to be at least partly mediated by mechanical stress on integrins that link ligands in the extracellular matrix with active zone structures in the terminals (Kashani et al. 2001). More importantly, hypertonically mediated release functions independently of Ca2+ (Rosenmund & Stevens, 1996; Kashani et al. 2001). Therefore, measurement of the refilling of the RRP using hypertonically mediated release does not cause an evoked increase in intracellular Ca2+ that could, in turn, affect the refilling rate (Stevens & Wesseling, 1998). We measured refilling of the RRP by applying paired pulses of hypertonic solution (each for 3 s) with varying interpulse time intervals. To allow sufficient time for the RRP to recover, pairs of pulses were separated by a 30–60 s interval. Typical raw traces at each temperature are shown in Fig. 1A. Pairs of pulses with varying interpulse time intervals are superimposed to show the recovery of the response as a function of time. As expected, the response of the second pulse steadily increases with increasing interpulse time intervals; however, this degree of recovery appears remarkably faster at the higher temperature (Fig. 1A). Additionally, the steady-state current evoked by application of the hypertonic solution is larger at the higher temperature. In these recordings, the response (charge) of the first pulse was remarkably stable for both temperatures, despite successive applications of hypertonic solution over the recording period. In line with these findings, Stevens & Wesseling (1998) have documented the decline of the hypertonically mediated response size to be less than 1.2 % min−1. However, to ensure that rundown or other unknown factors did not confound our analysis, we performed experiments with both increasing and decreasing interpulse intervals. Furthermore, the second response was always compared to the first response of that pair.
Figure 1. Refilling of the RRP is faster at the higher temperature.
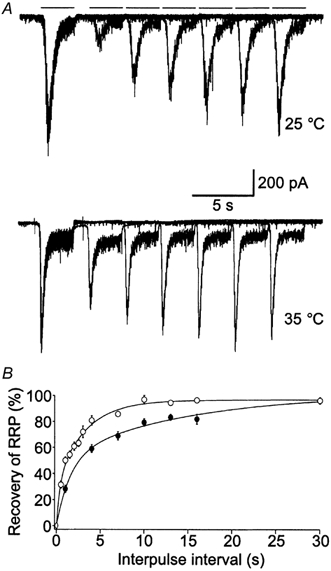
A, example trace of paired hypertonically mediated responses at 25 and 35 °C. Bars represent 3 s applications of 500 mm sucrose. Interpulse intervals are 1, 4, 7, 10, 13 and 16 s. Additionally, the total time of one episode (paired pulse) was between 30 and 60 s in order to allow adequate time for refilling of the RRP. Since refilling was significantly faster at the higher temperature, additional interpulse interval times (0.5, 1.5, 2, 2.5, 3 and 3.5 s) were also examined at 35 °C in order to obtain better resolution of the fast component of refilling (raw traces not shown). B, the degree to which the charge of the second response (A2) had recovered relative to the charge of the first response (A1) was plotted [1 - (A1 - A2)/A1] as a function of the interpulse interval time for both 35 (○) and 25 °C (•). The data were best fitted by biexponential curves, indicating that refilling of the RRP involves both a fast and a slow component (n = 9–24 for each temperature point).
To obtain plots that could be fitted by exponential curves, we calculated the percentage to which the response of the second pulse (A2) had recovered relative to the first pulse (A1) and plotted this value [1 - (A1 - A2)/A1] as a function of the interpulse interval (Fig. 1B). For both temperatures, we assumed that the second response was entirely eliminated by the first (0 % recovery) when the interpulse time was 0 s because we know that higher concentrations of sucrose (above 500 mosmol l−1) fail to produce larger responses (Rosenmund & Stevens, 1996). The recovery data were best fitted by biexponential curves, indicating that recovery of the RRP involves both a fast (τ25 °C = 1.51 s, 49.6 %; τ35 °C = 0.49 s, 40.2 %; Q10 = 3.1) and slow component (τ25 °C = 13.0 s; τ35 °C = 4.28 s; Q10 = 3.0) (n = 9–24 for each temperature point). Both time constants were approximately three times faster at 35 than at 25 °C and, therefore, indicate that refilling is most probably dependent on enzymatic processes that become enhanced at the higher temperature (Precht et al. 1973). Furthermore, analysis of our findings indicates that at 35 °C the RRP is more than 90 % recovered within 10 s. These findings are in agreement with those of Stevens & Tsujimoto (1995), who found that the RRP recovered within 10 s at 36 °C, and with those of Liu & Tsien (1995), who found that the RRP required more than 30 s to recover at 23 °C. Moreover, Dinkelacker et al. (2000) also reported a greater than twofold increase in the rate of recovery following depletion of the RRP in adrenal chromaffin cells upon an increase in temperature from 22 to 37 °C.
Release of the RRP
Spontaneous release was measured in the presence of 200 nm TTX as described in the Methods. A total of 903 events at 25 °C and 1177 events at 35 °C from 13 cells were analysed. Typical raw traces are shown for the same cell at each temperature (Fig. 2A). The averaged mEPSC events from the same cell as shown in Fig. 2A at each temperature are shown superimposed (Fig. 2B). As Fig. 2B exemplifies, at 35 °C the amplitude and charge of the mEPSCs were significantly larger than their respective values at 25 °C (Table 1 and Fig. 2B and C). The mean monoexponential decay time was also significantly reduced at 35 compared to 25 °C (Table 1 and Fig. 2C). The Q10 (determined from the increase in mEPSC frequency) was 1.5, indicating that physical processes such as enhanced diffusion are most likely to mediate the temperature-dependent increase in spontaneous release (Precht et al. 1973). For subsequent experiments, the mean charge of the mEPSC at each temperature (−135.2 fC at 25 °C and −166.5 fC at 35 °C) was assumed to represent the total charge of a single quantum and used to quantify the size of the RRP and the quantal release rates in terms of vesicles.
Figure 2. Changes in the shape and frequency of mEPSCs occur with increasing temperature.
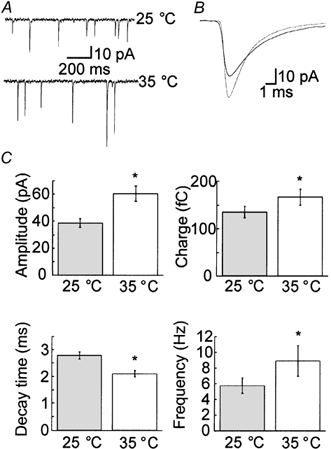
A, example traces of mEPSCs recorded in the presence of 200 nm TTX from the same cell at both 25 and 35 °C. B, superimposed traces of the average mEPSC from the same cell as shown in A at 25 (continuous line) and 35 °C (dotted line). C, statistical analysis indicated that the amplitude, charge and frequency of release increased significantly at the higher temperature (n = 13; Table 1). Furthermore, the decay time decreased significantly at the higher temperature (n = 13; Table 1). Asterisks indicate changes that were statistically significant (P < 0.05).
Table 1.
Temperature-dependent changes of the mEPSC and EPSC (–85 mV holding potential)
| 25 °C | 35 °C | Trend | |
|---|---|---|---|
| Characteristics of the mEPSC | |||
| Amplitude (pA) | 38.9 ± 3.3 | 60.6 ± 5.9 | + |
| Charge (fC) | 138.2 ± 11.9 | 166.5 ± 17.0 | + |
| Decay time (ms) | 2.80 ± 0.13 | 2.11 ± 0.18 | − |
| Frequency (Hz) | 5.8 ± 1.0 | 8.9 ± 1.9 | + |
| Characteristics of the EPSC | |||
| Amplitude (nA) | 5.16 ± 0.60 | 6.62 ± 0.77 | + |
| Charge (pC) | 45.6 ± 6.0 | 35.8 ± 4.7 | − |
| Decay time (ms) | 6.30 ± 0.69 | 3.75 ± 0.41 | − |
| Vesicular release | 330 ± 44 | 215 ± 28 | − |
In addition to detecting spontaneous release, we also monitored release mediated by the application of hypertonic solutions. Application of hypertonic solutions evokes an initially fast rate of release of those vesicles that constitute the RRP (Fig. 3A). This release then decays monoexponentially to a steady-state level of release that represents release of vesicles newly refilled to the RRP. Moreover, while application of hypertonic solutions increases the frequency of spontaneous release, it does not affect the measured quantal size (Stevens & Tsujimoto, 1995; Stevens & Wesseling, 1998). Therefore, we can assess temperature-dependent changes in hypertonically mediated release in terms of total charge transfer as well as number of quanta. In our experiments, the peak amplitude and, thus, peak vesicular release rate was an average of 58.0 ± 6.6 % larger at the higher temperature (from −2.88 ± 0.49 nA at 25 °C to −4.34 ± 0.66 nA at 35 °C; Fig. 3A). By dividing by the average quantal charge at each temperature, we calculated an increase from 21 ± 4 vesicles ms−1 released during peak release at 25 °C to 26 ± 4 vesicles ms−1 at 35 °C. Also, the time to peak release was reduced by an average of 35.7 ± 2.3 % at the higher temperature (from 1.2 ± 0.1 s at 25 °C to 0.8 ± 0.1 s at 35 °C; Fig. 3A). The Q10 for the peak vesicular release was determined to be 1.2 and, as found for spontaneous release, indicates that changes in hypertonically mediated release are dependent on physical, and not enzymatic, processes (Precht et al. 1973). The temperature dependence of these physical processes is most likely to arise from changes in the rate of osmolarity-induced shrinkage of the nerve terminal (via water fluxing from the terminal) that supplies the mechanical force to drive release of fusion-competent vesicles (Kashani et al. 2001).
Figure 3. The size of the RRP remains constant at both temperatures.
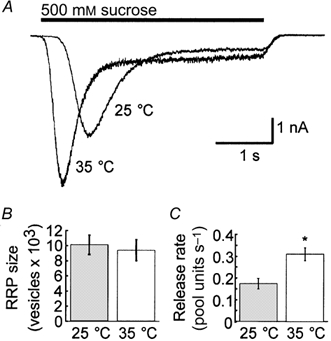
A, example traces of the hypertonically mediated release of quanta upon application of 500 mm sucrose from the same cell at both 25 and 35 °C. The response rises monoexponentially to a peak rate of release and then decays monoexponentially to a steady-state level of release that represents release of vesicles newly refilled to the RRP. B, by dividing the charge of the transient response by the mean quantal charge at the appropriate temperature, we obtained the size of the RRP of vesicles (n = 25). We found no significant difference in the size of the RRP between the two temperatures. C, by examining the steady-state component of the response, we were able to determine the fraction of the pool refilled per second (n = 8). As expected from Fig. 1C, this value increased significantly (* P < 0.05) at the higher temperature.
By dividing the charge of the transient response by the mean quantal charge at the appropriate temperature, we obtained the size of the RRP in vesicles. We found no significant difference in the size of the RRP between the two temperatures and calculated the average pool size to be 10 054 ± 1227 vesicles at 25 °C and 9414 ± 1393 vesicles at 35 °C (n = 25; Fig. 3B). To verify that postsynaptic receptor desensitization or saturation did not affect our calculation of the size of the RRP, we also performed a separate set of experiments in which we calculated the charge of the RRP and mEPSC at both 25 and 35 °C in the absence and presence of both 100 μm cyclothiazide (CTZ), a potent blocker of AMPA receptor desensitization, and 1 mm kynurenic acid (KYN), an AMPA receptor antagonist. We found no significant difference in the number of vesicles in the RRP at either temperature or condition (25 °C: 14 100 ± 3200 vesicles in the absence of CTZ/KYN; 11 322 ± 3342 vesicles in the presence of CTZ/KYN; 35 °C: 16 105 ± 3220 vesicles in the absence of CTZ/KYN; 12 100 ± 3200 vesicles in the presence of CTZ/KYN; n = 5).
For cells in which the steady-state component of the hypertonically mediated response was especially well resolved, we were also able to determine the fraction of the pool (pool units) that was released during steady-state conditions. To calculate this value, we integrated the steady-state current over a 1 s interval to determine the vesicular charge that was released per second. We then divided this value by the charge of the RRP (the charge of the transient response) in order to determine the fraction of the pool that was released per second during sustained release. Since the steady-state component of release is thought to reflect release of vesicles newly refilled to the RRP, such values provide another measure of the refilling rate of the RRP. As expected from the results of paired pulses of hypertonic solution (Fig. 1B), this value increased significantly from 0.20 ± 0.03 pool units s−1 at 25 °C to 0.35 ± 0.03 pool units s−1 at 35 °C (Fig. 3C; n = 8).
We also examined the AP-evoked response during low frequency (0.2 Hz) stimulation (n = 10; Fig. 4). Changes in the EPSC response were compared at both temperatures (Table 1 and Fig. 4A) as well as by measuring instantaneous changes during temperature increases or decreases during recording (Fig. 4B). Like the mEPSCs, the magnitude of the EPSC amplitude increased significantly (by approximately 30 %) at the higher temperature, possibly reflecting more synchronous release of neurotransmitter. Taschenberger & von Gersdorff (2000) observed a similar increase in the EPSC amplitude after increasing the temperature from 21–23 to 35 °C. Also, the monoexponential decay time decreased significantly upon increasing the temperature. However, the charge of the EPSC decreased significantly upon increasing temperature and presumably reflects a decrease in the release probability. These findings are summarized in Table 1 and Fig. 4B. We noticed the same temperature-dependent increase in the EPSC amplitude and decrease in decay time in all cells and the decrease in EPSC charge in all but one cell (n = 10). Hardingham & Larkman (1998) also observed an approximate doubling of the EPSC amplitude upon a temperature increase from 23 to 36 °C in slices of rat visual cortex. Unfortunately, these authors did not analyse temperature-dependent changes in the EPSC charge or decay time. We also observed a decrease in the delay of the peak response following the onset of somatic depolarization from 6.1 ± 0.3 ms at 25 °C to 4.1 ± 0.3 ms at 35 °C. Such findings have been observed previously for cerebellar synapses (Sabatini & Regehr, 1996).
Figure 4. The release probability decreases at the higher temperature.
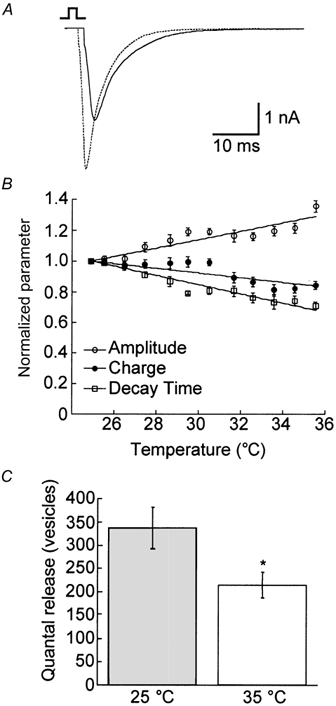
A, example traces of the EPSC from the same cell at both 25 (continuous line) and 35 °C (dotted line). B, plot of the instantaneous change in the EPSC amplitude (○), charge (•) and decay time (□) (n = 10). The amplitude increased significantly with increasing temperature, while the charge and decay time decreased significantly with increasing temperature (Table 1). C, by dividing the charge of the EPSC by the mean quantal charge at each temperature in the absence (n = 10) and presence (n = 11) of CTZ, we quantified the quanta released per EPSC. For both conditions, we observed a significant (* P < 0.05) decrease in the release probability at the higher temperature. We also observed a significant increase in the total number of quanta released at both temperatures in the presence of CTZ.
To calculate the number of vesicles released per single AP, we divided the charge of the evoked response by the mean quantal charge at the appropriate temperature for each cell at each temperature and then compared the change in quantal release for each cell at 25 and 35 °C. We observed a statistically significant decrease in the quantal release at 35 °C (mean reduction of 36 %) regardless of whether the temperature was raised to 35 from 25 °C or lowered from 35 to 25 °C (Table 1 and Fig. 4C).
The decreased charge of the EPSC at the higher temperature is at first glance indicative of a reduced release probability. However, increased postsynaptic receptor desensitization and/or reduced saturation at the higher temperature could also be responsible for the observed reduction in charge. To investigate the role of postsynaptic receptor desensitization, we compared the AP-evoked and hypertonically mediated release from the same cells at both temperatures in the absence and presence of 100 μm CTZ. We observed a mean reduction in the fraction of the RRP released per AP at the higher temperature in both the absence and presence of CTZ (n = 10). To quantify the quantal release per AP at both temperatures in the presence of CTZ, we measured the charge of the mEPSCs at both temperatures in the presence of TTX and 100 μm CTZ (n = 5). In the presence of CTZ, we again observed an approximately 1.5-fold increase in the charge of the mEPSC at 35 °C (439.34 fC) compared to 25 °C (289.14 fC). While we observed a comparable decrease in the quanta released per AP in the presence of CTZ (36 %) as in its absence, we also observed an approximate doubling of the total quanta released per AP at both temperatures in the presence of CTZ (845 ± 120 at 25 °C, n = 12; 540 ± 111 at 35 °C, n = 11). Thus, although these data suggest that postsynaptic receptor desensitization is not responsible for the observed reduction in release probability, they support previous findings that CTZ may also exert presynaptic effects (Barnes-Davies & Forsythe, 1995; Diamond & Jahr, 1995; Dittman & Regehr, 1998; Bellingham & Walmsley, 1999; Ishikawa & Takahashi, 2001). For this reason, these data should be interpreted with caution, especially since they are being used to assay the effects of desensitization on measurements of release probabilities.
We also investigated the effects of postsynaptic receptor saturation using a similar protocol, in which we compared the fraction of the RRP released during an AP from the same cell at both temperatures in the presence and absence of a rapidly dissociating AMPA receptor antagonist, either 1.5 mm γ-d-glutamylglycine (γ-DGG; n = 5; Liu et al. 1999) or 300 μm KYN (n = 5; Diamond & Jahr, 1997). For both antagonists, we observed a mean reduction in the fraction of the RRP released per AP at the higher temperature (data not shown). These findings are congruent with studies suggesting that glutamate within the synaptic cleft is well below saturating concentrations following release (Diamond & Jahr, 1997; Liu et al. 1999; McAllister & Stevens, 2000).
Maintenance of synaptic transmission during high frequency stimulation
Because of the increased refilling rate, increased amplitude of the evoked EPSC and reduced release probability observed at 35 °C, we predicted that cells would be better able to maintain synaptic transmission during high frequency stimulation at the higher temperature. Such increased maintenance of release could, in turn, affect many forms of short-term synaptic plasticity that are seen in preparations of hippocampal neurons. Therefore, we monitored changes in the EPSC amplitude and charge during high frequency (10, 20 and 40 Hz) stimulation (Fig. 5). At all stimulation frequencies and at both temperatures the response of the EPSC normalized to the initial value was dominated by depression, similar to previous work (Mennerick & Zorumski, 1995; Brody & Yue, 2000). However, the degree of EPSC depression after 5 s of stimulation was markedly reduced at the higher temperature. Specifically, during 10 Hz stimulation the amplitude decreased to approximately 50 % of its starting value at 25 °C in contrast to only 75 % at 35 °C (Fig. 5A; n = 17). During 20 Hz stimulation, the amplitude decreased to approximately 20 % of its initial value at 25 °C and to 40 % of its initial value at 35 °C (Fig. 5B; n = 10). During 40 Hz stimulation, it appeared that rapid depletion of the RRP occurred at both temperatures. Interestingly, the steady-state level of release was approximately 9 % of its original value at 25 °C, in contrast to 15 % at 35 °C (Fig. 5C; n = 10). This increase in the steady-state level of release at the higher temperature no doubt results from the increased refilling rate (Fig. 1) that serves to maintain release during trains of APs. Biexponential curves with an added constant could be fitted to graphs plotting the normalized EPSC as a function of time for each temperature and stimulation frequencies of 20 and 40 Hz (Table 2). A biexponential curve without an added constant was used to fit the data obtained during 10 Hz stimulation, presumably because depression had not reached a level that allowed fitting of an added constant (Table 2). These findings are similar to those of Taschenberger & von Gersdorff (2000), who found a decrease in the depression of the EPSC in response to trains of APs at frequencies of up to 800 Hz as the temperature was raised from 21–23 to 35 °C.
Figure 5. Depression of the synaptic response during high frequency stimulation is reduced at the higher temperature.
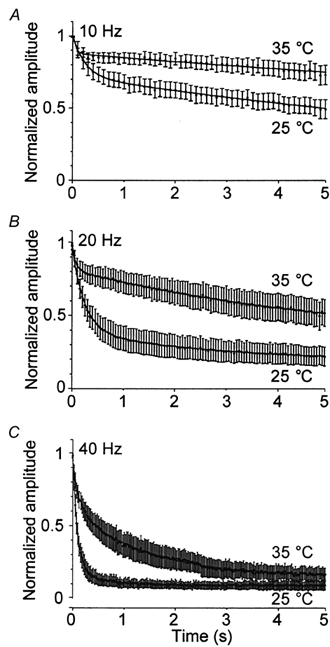
The amplitude of the EPSC normalized to the amplitude of the first response is plotted as a function of time (n = 10–17). Stimulations at a frequency of 10 (A), 20 (B) and 40 Hz (C) were performed for 5 s at both 25 and 35 °C. Biexponential curves with an added time constant could be fitted to graphs plotting the normalized EPSC as a function of time for each temperature and stimulation frequencies of 20 and 40 Hz (Table 2). A biexponential curve without an added constant was used to fit the data obtained during 10 Hz stimulation, presumably because depression had not reached a level that allowed fitting of an added constant (Table 2).
Table 2.
Biexponential fittings of the amplitude depression during high frequency stimulation at 25 and 35 °C
| τFast (s) | Amplitude (%) | τSlow (s) | Amplitude (%) | Constant (%) * | |
|---|---|---|---|---|---|
| 10 Hz, 25 °C | 0.241 | 27.6 | 13.12 | 72.3 | — |
| 10 Hz, 35 °C | 0.0566 | 11.8 | 31.0 | 87.7 | — |
| 20 Hz, 25 °C | 0.240 | 57.0 | 2.65 | 21.0 | 18.8 |
| 20 Hz, 35 °C | 0.0685 | 16.8 | 5.45 | 50.2 | 30.9 |
| 40 Hz, 25 °C | 0.0894 | 80.0 | 0.569 | 10.4 | 8.95 |
| 40 Hz, 35 °C | 0.116 | 33.2 | 1.42 | 46.7 | 14.7 |
Steady-state response of the EPSC normalized to the first response.
We also noted an increase in the decay time constant of the EPSC and lowering of the baseline amplitude during the course of high frequency stimulation. Figure 6A compares the response of the same cell at the start and end (after 5 s) of 20 Hz stimulation at both temperatures. Interestingly, the increase in decay time of the EPSC and lowering of the baseline were not as pronounced at the higher temperature. To ensure that the reduction in baseline was not due to residual current from previous release, we fitted the first EPSC with a single exponential curve. During both 10 and 20 Hz stimulation, we could safely conclude that the response had decayed sufficiently before the next stimulation began. Furthermore, control experiments performed in the presence of d-2-amino-5-phosphonovalerate (d-APV), a specific antagonist of NMDA receptors, indicated that the change in the EPSC shape was not altered by contribution of charge from NMDA receptors (data not shown). Moreover, all experiments were performed in the presence of 4 mm Mg2+, which should completely block NMDA receptors. Therefore, we suspected the increase in decay time and decrease in the baseline amplitude to be indicative of asynchronous vesicular release. We quantified the fraction of synchronous and asynchronous release during high frequency stimulation as described in the Methods and plotted these values as a function of time for the results obtained during 20 Hz stimulation (Fig. 6B and C, respectively). After 2 s of stimulation at 20 Hz, almost half of the total release was asynchronous at 25 °C. In contrast, only approximately 20 % of the total release was asynchronous at 35 °C.
Figure 6. Maintenance of synchronous release during high frequency stimulation is improved at the higher temperature.
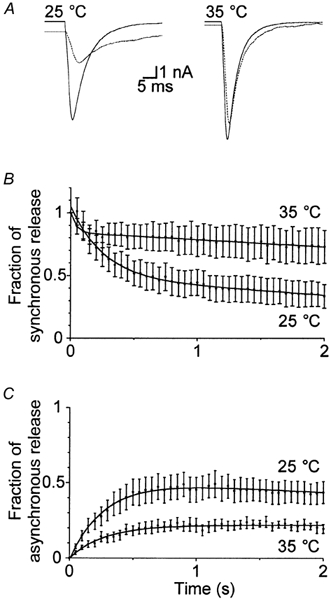
A, comparison of the response of the same cell at the start (continuous line) and end (after 5 s; dotted line) of 20 Hz stimulation at both temperatures. Interestingly, the increase in decay time of the EPSC and lowering of the baseline were not as pronounced at the higher temperature. The components of synchronous (B) and asynchronous (C) release were quantified as described in the Methods and plotted as a function of time for the first 2 s of stimulation at 20 Hz at both 25 and 35 °C (n = 10).
DISCUSSION
In these experiments, we have electrophysiologically isolated key events of the synaptic vesicle cycle, examining the processes of vesicular supply and release in preparations of isolated hippocampal neurons grown on microisland beds of glial cells (Bekkers & Stevens, 1991). When grown in isolation, the cell forms synapses only with itself (hence, autapses). By using such monosynaptic preparations, we ensure that we are monitoring release from the same set of synapses regardless of the stimulation method (spontaneous, hypertonically mediated or action potential evoked) or temperature under investigation and in the absence of complex polysynaptic circuitry. Moreover, such cultures are already known to display the many types of short- and long-term plasticity seen in slice preparations (Mennerick & Zorumski, 1995; Tong et al. 1996). Thus, autaptic preparations provide a reliable means of identifying the temperature-dependent dynamics involved in transmitter release so that the implications of performing experiments well below physiologically relevant temperatures can be understood.
Investigation of spontaneous release provides a means of monitoring both the postsynaptic and presynaptic behaviour of neurons. Specifically, the shape of the mEPSC is most likely to be due to the changes in postsynaptic channel behaviour. An increased open probability or conductance of the postsynaptic receptor would account for the increase in mEPSC amplitude and charge that we observed at the higher temperature. In agreement with these conclusions, Stiles et al. (1999) also observed an increase in the miniature endplate current (mEPC) amplitude and a decrease in the decay time with increased temperature in preparations of reptilian neuromuscular junction. By using optimized electrophysiological recordings and Monte Carlo simulations, these researchers identified channel gating as the most significant factor affecting the temperature dependence of mEPCs. However, single channel recordings would be necessary to illustrate similar findings in preparations of autaptic hippocampal neurons.
Indicative of additional temperature-dependent changes in the presynaptic neural behaviour, we observed a significant increase in the mEPSC frequency at the higher temperature. Indeed, Hardingham & Larkman (1998) observed an even more substantial (fourfold) increase in the mEPSC frequency in their examination of rat brain slices. The increase in spontaneous mEPCs at the frog neuromuscular junction upon increase in temperature was first described by Fatt & Katz (1952) and has subsequently been well documented. This increase in spontaneous release most probably represents a decrease in the energy barrier of spontaneous fusion as a result of the increased temperature. Interestingly, we also noticed an increase in the maximal release rate and a decrease in the onset of maximal release evoked by application of hypertonic solutions at the higher temperature. Such findings are also indicative of a decreased energy requirement for Ca2+-independent fusion of the synaptic vesicle, possibly due to an increase in the fluidity of the membrane lipid bilayer.
In addition to investigating the temperature-dependent changes underlying spontaneous release of the RRP, we also investigated refilling of this pool. While our results do not allow the elucidation of the underlying molecular machinery of vesicular refilling, they do suggest that refilling of the RRP is not only dramatically faster at physiological temperatures but also involves both a fast and a slow component. Surprisingly, previous studies indicate that refilling of the RRP occurs monoexponentially. Differences in the experimental protocol used to determine the extent of refilling, specifically the fewer number of interpulse intervals analysed in these earlier studies (5 in Liu & Tsien, 1995; 4 in Stevens & Wesseling, 1998; 7–12 in this study) may be responsible for this discrepancy. Importantly, the multiple time constants observed in this study are not related to some form of Ca2+-dependent adaptation process since hypertonically mediated release is known to occur in a Ca2+-independent manner (Rosenmund & Stevens, 1996). Therefore, the multiple time components are most likely to represent the varying rates of refilling of the RRP from various reserve pools. Indeed, the existence of multiple vesicular pools has been reported previously for a variety of preparations (von Gersdorff & Matthews, 1997; Voets et al. 1999). In hippocampal neurons, hypertonically mediated release may distinguish between either physically distinct pools, such as those closer to or further from the active zone, or functionally distinct pools, such as those vesicles that are rapidly refilled but reluctantly released as opposed to more readily releasable vesicles (Wu & Borst, 1999). Furthermore, recent work also suggests that depleted vesicles of the RRP may be replenished directly from recently released vesicles rather than from a reserve pool (Pyle et al. 2000). Thus, the faster time constant could also reflect release of rapidly recycled, previously released vesicles. Interestingly, application of hypertonic solutions also indicated that the size of the RRP does not change at the higher temperature. Such findings suggest that, despite faster supply, the number of occupied release sites does not change upon an increase or decrease in temperature and is, thus, most probably saturated at both temperatures.
We did, however, notice a marked decrease in quantal content of the EPSC at the higher temperature, indicating a reduced release probability. These findings could also result from increased postsynaptic receptor desensitization and/or decreased saturation at the higher temperature. Unfortunately, research has not conclusively shown whether the concentration of glutamate released into the synaptic cleft is high enough to saturate postsynaptic AMPA receptors (Frerking & Wilson, 1996; Diamond & Jahr, 1997; Liu et al. 1999; McAllister & Stevens, 2000) and even less is known about the temperature dependence of postsynaptic AMPA receptor desensitization. However, experiments performed in the presence of either CTZ, a potent inhibitor of postsynaptic receptor desensitization, or the low-affinity AMPA receptor antagonists γ-DGG and KYN suggest that the observed reduction in the charge of the EPSC at the higher temperature is not due to either of these postsynaptic phenomena. Unfortunately, possible presynaptic effects of CTZ (Barnes-Davies & Forsythe, 1995; Diamond & Jahr, 1995; Dittman & Regehr, 1998; Bellingham & Walmsley, 1999; Ishikawa & Takahashi, 2001), potentially mediated by either antagonism of a presynaptic inhibitory mechanism that normally underlies paired pulse depression (Bellingham & Walmsley, 1999) or suppression of presynaptic potassium channels (Ishikawa & Takahashi, 2001), make conclusive interpretation of these data difficult. Therefore, further experiments, in which desensitization is blocked without changing the presynaptic release probability, should be performed to confirm the reduction in release probability we observed at the higher temperature. In addition to possible postsynaptic effects, the observation of a reduced release probability could also result from an increased likelihood of AP conduction failures at the higher temperature. However, we consider this possibility unlikely since we consistently observed larger Na+ currents with accelerated kinetics together with a shortened delay between the somatic action potential and detection of the postsynaptic currents. Such observations are consistent with more reliable AP conduction in the axon. Furthermore, Allen & Stevens (1994) and Hardingham & Larkman (1998) observed, respectively, no change or a decrease in the failure rate of synaptic transmission in slice preparations, again suggesting that the likelihood of conduction failures does not increase at higher temperatures.
In light of these findings, we suspect that the amount of Ca2+ available for triggered fusion is reduced due to reduced Ca2+ influx into the presynaptic terminal, more efficient buffering or faster extrusion, leading to smaller, shorter and/or steeper concentration profiles within nerve terminals. In line with this hypothesis, Borst & Sakmann (1998) reported Ca2+ currents during APs in giant brainstem terminals that had twice the peak amplitude and half the width at 36 than at 24 °C, resulting in a slight but significant reduction of the total Ca2+ influx. Additional evidence from fluorometric measurement of presynaptic Ca2+ also indicates a slight but significant reduction in the total Ca2+ influx during an AP at physiological temperature compared to room temperature (Borst & Helmchen, 1998). Given the steep Ca2+ dependency of release, this reduced Ca2+ influx is consistent with the reduction of quantal content released during an AP that we observed in our preparation. The finding that release probability is actually lower at physiological temperature is not necessarily in contrast to a recent study in cortical slices (Hardingham & Larkman, 1998), in which the authors hypothesized that an increased release probability was responsible for the observed increase in the reliability of synaptic transmission between pairs of neurons at 36 °C. Reliability of evoked EPSPs may depend more on timed, synchronous release rather than the absolute number of quanta released.
Understanding the prefusion process of vesicular refilling is also critical to our understanding of the many forms of synaptic plasticity apparent in hippocampal and other preparations. For example, short-term depression is often attributed to the depletion of the RRP of vesicles (Zucker, 1999). As our work shows, the degree of refilling is substantially increased (threefold) at 35 °C. In line with our current understanding of short-term synaptic depression, we noticed a substantial decrease in the degree and time course of depression of the EPSC amplitude during high frequency stimulation at the higher temperature. We also observed an improved release capacity upon an increase in temperature. While the total release (synchronous and asynchronous) seen during 5 s of 10 Hz stimulation was lower at 35 than at 25 °C, we observed an increased total release during 5 s of 40 Hz stimulation at 35 than at 25 °C (data not shown). Thus, the maintenance of synaptic transmission at the higher temperature during trains of stimuli represents the combined effect of the increased refilling rate of the RRP and the decreased release probability. Moreover, we noticed that the synchronous component of high frequency release is better maintained at the higher temperature. Given that the progressive increase of asynchronous release in response to high frequency stimulation is primarily attributed to the build up of Ca2+ within the presynaptic terminal, the notion of reduced Ca2+ influx during an AP or better buffering of intracellular Ca2+ at the higher temperature is again consistent with our observation of more synchronous release at 35 °C.
Ultimately, at the higher temperature release is more stable over a variety of frequencies and durations due to an increase in the refilling rate of the RRP and a reduction in the release probability. Our findings from autaptically cultured neurons no doubt affect our understanding of how neurons interact as part of polysynaptic networks. Importantly, our results are consistent with the observations of Taschenberger & von Gersdorff (2000), who found that mature (P14) calyx synapses are able to follow input rates as high as 800 Hz only at near physiological temperatures (35 °C). The authors explain that these findings are most likely to be due to the observed shortening of the presynaptic AP that occurs irreversibly during development as well as reversibly during an increase in the recording temperature and may ultimately serve to reduce the release probability and decrease the synaptic delay. Their findings explain how this important auditory synapse can maintain the fast, precise and sustained synaptic transmission that is required for sound localization. Furthermore, neurons that are able to maintain synchronous release over a variety of frequencies would be better able to encode temporal cues (Singer, 1999), potentially explaining the rapid temporal precision also observed in cortical networks (DeCharms et al. 1998; Kilgard & Merzenich, 1998) at frequencies of 50 and even 100 Hz (Rager & Singer, 1998). Furthermore, the increased maintenance of synchronous release and the lowered release probability observed at the physiological temperature would allow improved synaptic integration, since many neurons would be required to act cooperatively in order to determine the response of the target neuron (Allen & Stevens, 1994; Stevens, 1994).
It is not difficult to imagine that rapid temporal precision in combination with improved synaptic integration are crucial for higher level cognitive processing and yet, surprisingly, researchers still observe strong synaptic responses and transmission that is, at the very least, not entirely unreliable in mammalian central neurons held at room temperature. However, severe symptoms of mammalian hypothermia, including semiconsciousness or unconsciousness, begin when the core body temperature falls below 32 °C. It is exciting to speculate that subtle changes in neuronal processing, such as those described above, are responsible for distinguishing the conscious from the unconscious state.
Acknowledgments
We thank Ina Herfort for her assistance in the preparation of cell cultures and E. Neher, T. Sakaba and V. Dinkelacker for reviewing the manuscript. This work was supported by a grant from the Deutsche Forschungsgemeinschaft Ro 1296/6–1 and the Max-Planck-Gesellschaft. S. J. P. was a Fulbright Scholar and C. R. is a Heisenberg Fellow.
REFERENCES
- Allen CA, Stevens CF. An evaluation of causes for unreliability of synaptic transmission. Proceedings of the National Academy of Sciences of the USA. 1994;91:10380–10383. doi: 10.1073/pnas.91.22.10380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes-Davies M, Forsythe ID. Pre- and postsynaptic glutamate receptors at a giant excitatory synapse in rat auditory brainstem slices. Journal of Physiology. 1995;488:387–406. doi: 10.1113/jphysiol.1995.sp020974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bekkers JM, Stevens CF. Excitatory and inhibitory autaptic currents in isolated hippocampal neurons maintained in cell culture. Proceedings of the National Academy of Sciences of the USA. 1991;88:7834–7838. doi: 10.1073/pnas.88.17.7834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellingham MC, Walmsley B. A novel presynaptic inhibitory mechanism underlies paired pulse depression at a fast central synapse. Neuron. 1999;23:159–170. doi: 10.1016/s0896-6273(00)80762-x. [DOI] [PubMed] [Google Scholar]
- Borst JG, Helmchen F. Calcium influx during an action potential. Methods in Enzymology. 1998;293:352–371. doi: 10.1016/s0076-6879(98)93023-3. [DOI] [PubMed] [Google Scholar]
- Borst JG, Sakmann B. Calcium current during a single action potential in a large presynaptic terminal of the rat brainstem. Journal of Physiology. 1998;506:143–157. doi: 10.1111/j.1469-7793.1998.143bx.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brody DL, Yue DT. Release-independent short-term synaptic depression in cultured hippocampal neurons. Journal of Neuroscience. 2000;20:2480–2494. doi: 10.1523/JNEUROSCI.20-07-02480.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeCharms RC, Blake DT, Merzenich MM. Optimizing sound features for corticol neurons. Science. 1998;280:1439–1443. doi: 10.1126/science.280.5368.1439. [DOI] [PubMed] [Google Scholar]
- Diamond JS, Jahr CE. Asynchronous release of synaptic vesicles determines the time course of the AMPA receptor-mediated EPSC. Neuron. 1995;15:1097–1107. doi: 10.1016/0896-6273(95)90098-5. [DOI] [PubMed] [Google Scholar]
- Diamond JS, Jahr CE. Transporters buffer synaptically released glutamate on a submillisecond time scale. Journal of Neuroscience. 1997;17:4672–4687. doi: 10.1523/JNEUROSCI.17-12-04672.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinkelacker V, Voets T, Neher E, Moser T. The readily releasable pool of vesicles in chromaffin cells is replenished in a temperature-dependent manner and transiently overfills at 37 °C. Journal of Neuroscience. 2000;20:8377–8383. doi: 10.1523/JNEUROSCI.20-22-08377.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dittman JS, Regehr WG. Calcium dependence and recovery kinetics of presynaptic depression at the climbing fiber to Purkinje cell synapse. Journal of Neuroscience. 1998;18:6147–6162. doi: 10.1523/JNEUROSCI.18-16-06147.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eccles JC, Katz B, Kuffler SW. Nature of the end-plate potential in curarized muscle. Journal of Neurophysiology. 1941;4:362–387. [Google Scholar]
- Fatt P, Katz B. Spontaneous subthreshold activity at motor nerve endings. Journal of Physiology. 1952;117:109–128. [PMC free article] [PubMed] [Google Scholar]
- Frerking M, Wilson M. Saturation of postsynaptic receptors at central synapses? Current Opinions in Neurobiology. 1996;6:395–403. doi: 10.1016/s0959-4388(96)80125-5. [DOI] [PubMed] [Google Scholar]
- Hardingham NR, Larkman AU. The reliability of excitatory synaptic transmission in slices of rat visual cortex in vitro is temperature dependent. Journal of Physiology. 1998;507:249–256. doi: 10.1111/j.1469-7793.1998.249bu.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hessler NA, Shirke AM, Malinow R. The probability of transmitter release at a mammalian central synapse. Nature. 1993;366:569–572. doi: 10.1038/366569a0. [DOI] [PubMed] [Google Scholar]
- Ishikawa T, Takahashi T. Mechanisms underlying presynaptic facilitatory effect of cyclothiazide at the calyx of Held of juvenile rats. Journal of Physiology. 2001;533:423–431. doi: 10.1111/j.1469-7793.2001.0423a.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kashani AH, Chen B-M, Grinnell AD. Hypertonic enhancement of transmitter release from frog motor nerve terminals: Ca2+ independence and the role of integrins. Journal of Physiology. 2001;530:243–252. doi: 10.1111/j.1469-7793.2001.0243l.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz B, Miledi R. The measurement of synaptic delay at the neuromuscular junction. Journal of Physiology. 1965;181:656–670. doi: 10.1113/jphysiol.1965.sp007790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilgard MP, Merzenich MM. Plasticity of temporal information processing in the primary auditory cortex. Nature Neuroscience. 1998;8:727–731. doi: 10.1038/3729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu G, Choi S, Tsien RW. Variability of neurotransmitter concentration and nonsaturation of postsynaptic AMPA receptors at synapses in hippocampal cultures and slices. Neuron. 1999;22:395–409. doi: 10.1016/s0896-6273(00)81099-5. [DOI] [PubMed] [Google Scholar]
- Liu G, Tsien RW. Properties of synaptic transmission at single hippocampal synaptic boutons. Nature. 1995;375:404–408. doi: 10.1038/375404a0. [DOI] [PubMed] [Google Scholar]
- McAllister AK, Stevens CF. Nonsaturation of AMPA and NMDA receptors at hippocampal synapses. Proceedings of the National Academy of Sciences of the USA. 2000;97:6173–6178. doi: 10.1073/pnas.100126497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mennerick S, Zorumski CF. Presynaptic influence on the time course of fast excitatory currents in cultured hippocampal cells. Journal of Neuroscience. 1995;15:3178–3192. doi: 10.1523/JNEUROSCI.15-04-03178.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murthy VN, Sejnowski TJ, Stevens CF. Heterogeneous release properties of visualized individual hippocampal synapses. Neuron. 1997;18:599–612. doi: 10.1016/s0896-6273(00)80301-3. [DOI] [PubMed] [Google Scholar]
- Precht H, Laudien H, Havesteen B. The normal temperature range. In: Precht H, Christophersen J, Hensel H, Larcher W, editors. Temperature and Life. New York, NY, USA: Springer; 1973. pp. 302–325. [Google Scholar]
- Pyle JL, Kavalali ET, Piedras-Renteria ES, Tsien RW. Rapid reuse of readily releasable pool vesicles at hippocampal synapses. Neuron. 2000;25:221–231. doi: 10.1016/s0896-6273(00)00098-2. [DOI] [PubMed] [Google Scholar]
- Rager G, Singer W. The response of the cat visual cortex to flicker stimuli of variable frequency. European Journal of Neuroscience. 1998;10:1856–1877. doi: 10.1046/j.1460-9568.1998.00197.x. [DOI] [PubMed] [Google Scholar]
- Renstrenstrenstrenström E, Eliasson L, Bokvist K, Rorsman P. Cooling inhibits exocytosis in single mouse pancreatic B-cells by suppression of granule mobilization. Journal of Physiology. 1996;494:41–52. doi: 10.1113/jphysiol.1996.sp021474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenmund C, Clements JD, Westbrook GL. Nonuniform probability of glutamate release at a hippocampal synapse. Science. 1993;262:754–757. doi: 10.1126/science.7901909. [DOI] [PubMed] [Google Scholar]
- Rosenmund C, Feltz A, Westbrook GL. Synaptic NMDA receptor channels have a low open probability. Journal of Neuroscience. 1995;15:2788–2795. doi: 10.1523/JNEUROSCI.15-04-02788.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenmund C, Stevens CF. Definition of a readily releasable pool of vesicles at hippocampal synapses. Neuron. 1996;16:1197–1207. doi: 10.1016/s0896-6273(00)80146-4. [DOI] [PubMed] [Google Scholar]
- Ryan TA, Reuter H, Smith SJ. Optical detection of a quantal presynaptic membrane turnover. Nature. 1997;388:478–482. doi: 10.1038/41335. [DOI] [PubMed] [Google Scholar]
- Sabatini BL, Regehr WG. Timing of neurotransmission at fast synapses in the mammalian brain. Nature. 1996;384:170–172. doi: 10.1038/384170a0. [DOI] [PubMed] [Google Scholar]
- Sanchez-Vives MV, McCormick DA. Cellular and network mechanisms of rhythmic recurrent activity in neocortex. Nature Neuroscience. 2000;3:1027–1034. doi: 10.1038/79848. [DOI] [PubMed] [Google Scholar]
- Singer W. Neuronal synchrony: a versatile code for the definition of relations? Neuron. 1999;24:49–65. doi: 10.1016/s0896-6273(00)80821-1. [DOI] [PubMed] [Google Scholar]
- Stevens CF. Neuronal communication: cooperativity of unreliable neurons. Current Biology. 1994;4:268–269. doi: 10.1016/s0960-9822(00)00062-2. [DOI] [PubMed] [Google Scholar]
- Stevens CF, Tsujimoto T. Estimates for the pool size of releasable quanta at a single central synapse and for the time required to refill the pool. Proceedings of the National Academy of Sciences of the USA. 1995;92:846–849. doi: 10.1073/pnas.92.3.846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens CF, Wesseling JF. Activity-dependent modulation of the rate at which synaptic vesicles become available to undergo exocytosis. Neuron. 1998;21:415–424. doi: 10.1016/s0896-6273(00)80550-4. [DOI] [PubMed] [Google Scholar]
- Stiles JR, Kovyazina IV, Salpeter EE, Salpeter MM. The temperature sensitivity of miniature endplate currents is mostly governed by channel gating: evidence from optimized recordings and Monte Carlo simulations. Biophysics Journal. 1999;77:1117–1187. doi: 10.1016/S0006-3495(99)76969-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taschenberger H, von Gersdorff H. Fine-tuning an auditory synapse for speed and fidelity: developmental changes in presynaptic waveform, EPSC kinetics, and synaptic plasticity. Journal of Neuroscience. 2000;20:9162–9173. doi: 10.1523/JNEUROSCI.20-24-09162.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas P, Wong JG, Lee AK, Almers W. A low affinity Ca2+ receptor controls the final steps in peptide secretion from pituitary melanotrophs. Neuron. 1993;11:93–104. doi: 10.1016/0896-6273(93)90274-u. [DOI] [PubMed] [Google Scholar]
- Thomson AM, Deuchars J, West DC. Large, deep layer pyramid-pyramid single axon EPSPs in slices of rat motor cortex display paired pulse and frequency-dependent depression, mediated presynaptically and self-facilitation, mediated postsynaptically. Journal of Neurophysiology. 1993a;70:2354–2369. doi: 10.1152/jn.1993.70.6.2354. [DOI] [PubMed] [Google Scholar]
- Thomson AM, Deuchars J, West DC. Single axon excitatory postsynaptic potentials in neocortical interneurons exhibit pronounced paired pulse facilitation. Neuroscience. 1993b;54:347–360. doi: 10.1016/0306-4522(93)90257-g. [DOI] [PubMed] [Google Scholar]
- Tong G, Malenka RC, Nicoll RA. Long-term potentiation in cultures of single hippocampal cells: a presynaptic form of plasticity. Neuron. 1996;16:1147–1157. doi: 10.1016/s0896-6273(00)80141-5. [DOI] [PubMed] [Google Scholar]
- Voets T, Neher E, Moser T. Mechanisms underlying phasic and sustained secretion in chromaffin cells from mouse adrenal slices. Neuron. 1999;23:607–615. doi: 10.1016/s0896-6273(00)80812-0. [DOI] [PubMed] [Google Scholar]
- von Gersdorff H, Matthews G. Depletion and replenishment of vesicle pools at a ribbon-type synaptic terminal. Journal of Neuroscience. 1997;17:1919–1927. doi: 10.1523/JNEUROSCI.17-06-01919.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu LG, Borst JG. The reduced release probability of releasable vesicles during recovery from short-term synaptic depression. Neuron. 1999;23:821–832. doi: 10.1016/s0896-6273(01)80039-8. [DOI] [PubMed] [Google Scholar]
- Zucker RS. Calcium- and activity-dependent synaptic plasticity. Current Opinion in Neurobiology. 1999;9:305–313. doi: 10.1016/s0959-4388(99)80045-2. [DOI] [PubMed] [Google Scholar]


