Abstract
The expression of mRNA for acid sensing ion channels (ASIC) subunits ASIC1a, ASIC2a and ASIC2b has been reported in hippocampal neurons, but the presence of functional hippocampal ASIC channels was never assessed. We report here the first characterization of ASIC-like currents in rat hippocampal neurons in primary culture. An extracellular pH drop induces a transient Na+ current followed by a sustained non-selective cation current. This current is highly sensitive to pH with an activation threshold around pH 6.9 and a pH0.5 of 6.2. About half of the total peak current is inhibited by the spider toxin PcTX1, which is specific for homomeric ASIC1a channels. The remaining PcTX1-resistant ASIC-like current is increased by 300 μm Zn2+ and, whereas not fully activated at pH 5, it shows a pH0.5 of 6.0 between pH 7.4 and 5. We have previously shown that Zn2+ is a co-activator of ASIC2a-containing channels. Thus, the hippocampal transient ASIC-like current appears to be generated by a mixture of homomeric ASIC1a channels and ASIC2a-containing channels, probably heteromeric ASIC1a+2a channels. The sustained non-selective current suggests the involvement of ASIC2b-containing heteromeric channels. Activation of the hippocampal ASIC-like current by a pH drop to 6.9 or 6.6 induces a transient depolarization which itself triggers an initial action potential (AP) followed by a sustained depolarization and trains of APs. Zn2+ increases the acid sensitivity of ASIC channels, and consequently neuronal excitability. It is probably an important co-activator of ASIC channels in the central nervous system.
H+-gated cation channels are present in sensory neurons and in neurons of the central nervous system (CNS). Several H+-gated cation channel subunits (ASIC, acid sensing ionic channels) have been cloned and functionally expressed so far: ASIC1a (Waldmann et al. 1997b), ASIC1b (Chen et al. 1998), ASIC2a (Price et al. 1996; Waldmann et al. 1996), ASIC2b (Lingueglia et al. 1997), ASIC3 (Waldmann et al. 1997a; de Weille et al. 1998; Babinski et al. 1999). Very recently, another putative ASIC subunit (ASIC4) was identified which seems not to be activated by acidic pH (Akopian et al. 2000; Grunder et al. 2000).
Functional ASIC channels are thought to be tetrameric assemblies of ASIC subunits (Coscoy et al. 1998; Waldmann et al. 1999). Both homomeric and heteromeric ASIC channels can be formed (Bassilana et al. 1997; Waldmann & Lazdunski, 1998; Waldmann et al. 1999; Babinski et al. 2000), thus contributing to the functional diversity of neuronal ASIC-like channels (Grantyn & Lux, 1988; Ueno et al. 1992; Varming, 1999; Escoubas et al. 2000; Sutherland et al. 2000a).
Some ASIC subunits are specifically expressed in sensory neurons, like ASIC1b (Chen et al. 1998) and ASIC3 (Waldmann et al. 1997a; Voilley et al. 2001), whereas ASIC1a (Bassilana et al. 1997; Olson et al. 1998; Voilley et al. 2001), ASIC2a (Price et al. 1996, 2000; Bassilana et al. 1997; Lingueglia et al. 1997; Garcia-Anoveros et al. 2001), ASIC2b (Lingueglia et al. 1997) and ASIC4 (Akopian et al. 2000; Grunder et al. 2000) are also found in central neurons.
In sensory neurons, ASIC currents are thought to play an important role in nociception during a tissue acidosis and inflammation (Olson et al. 1998; Waldmann & Lazdunski, 1998; Benson et al. 1999; Kress & Zeilhofer, 1999; Waldmann et al. 1999; Sutherland et al. 2000a, b; Voilley et al. 2001), and ASIC2a has been proposed to participate in touch sensation (Price et al. 2000; Garcia-Anoveros et al. 2001).
The role of ASIC1a, ASIC2a, ASIC2b and ASIC4 in central neurons (Price et al. 1996; Waldmann et al. 1996; Bassilana et al. 1997; Garcia-Anoveros et al. 1997) remains to be established. An acidosis accompanies brain ischaemia or epilepsy, and ASIC currents might contribute to the associated neuronal death. However, pH fluctuations also occur in normal brain function. Several studies with brain slices indicate that neuronal activity gives rise to significant and rapid changes in extracellular pH (Krishtal et al. 1987; Chesler & Kaila, 1992). Because of the limited spatial and temporal resolution of pH microelectrodes used in those studies, the global pH variations that have been reported may underestimate the actual pH changes occurring within or in the vicinity of the synaptic cleft (Chesler & Kaila, 1992). The content of synaptic vesicles is acidic (Ozkan & Ueda, 1998) and synaptic release during neuronal activity is expected to create an extracellular acidification in the vicinity of the synaptic cleft. For hippocampal neurons, an intravesicular pH of 5.7 has been measured with a pH-sensitive fluorescent probe (Miesenbock et al. 1998). The same study showed a transient decrease in the extracellular pH to about 6.4 after secretion of synaptic vesicles.
Synaptic vesicles of hippocampal glutamatergic neurons also contain a high amount of Zn2+, particularly in the dentate granule cells and their projections, the mossy fibres (Frederickson, 1989). Vesicular Zn2+ is co-released with the neurotransmitter (i.e. glutamate), resulting in a transient increase of the local synaptic Zn2+ concentration up to 100–300 μm from resting levels below 500 nm (Assaf & Chung, 1984; Howell et al. 1984; Smart et al. 1994; Budde et al. 1997; Weiss et al. 2000). Zn2+ is known to exert a variety of effects on ion channels, among which are AMPA and NMDA receptors, and was thus proposed to be a neuromodulator of the excitatory glutamatergic synapse (Harrison & Gibbons, 1994; Smart et al. 1994; Henze et al. 2000; Vogt et al. 2000).
In order to understand the function of the brain ASIC subunits, particularly in hippocampus which is involved in memory processes and in the physiopathology of ischaemia (Schmidt-Kastner & Freund, 1991; Henze et al. 2000), we recorded and characterized the molecular constitution of the ASIC-like current in hippocampal neurons. We previously showed that Zn2+ potentiates the activation of ASIC2a-containing ASIC channels (Baron et al. 2001). We demonstrate here that the hippocampal ASIC-like current is coactivated by Zn2+ resulting in an increase in neuronal excitability.
METHODS
Primary cultured hippocampal neurons
Primary cell cultures derived from rat embryonic hippocampi, containing mainly neurons over astrocytes, were established as described previously (Goslin & Banker, 1991; Lauritzen et al. 1997). In brief, 18- to 19-day-pregnant Wistar rats were stunned and killed by decapitation, according to national and institutional guidelines. Embryos were removed and hippocampi were dissected, incubated in 0.125% trypsin for 35 min at 37°C, and dissociated mechanically. Cells were plated in modified Eagle's medium (MEM) containing 10 % dialysed inactivated horse serum (Sigma), 6 g l−1 glucose, 50 U ml−1 penicillin, and 50 μg ml−1 streptomycin. Cells were plated (day 0) at a density of ∼750 000 cells per 35-mm poly-l-lysine-coated tissue culture plates (Falcon). After 48 h, the culture medium was replaced with serum-free MEM with 2 % B27 supplement (Gibco) and 6 g l−1 glucose, and kept in 95 % air-5 % CO2 at 37 °C. Cells were used for electrophysiological recordings 7–20 days after plating. Neurons with triangular-shaped cell bodies, a typical feature of pyramidal neurons, were selected for recording.
Patch-clamp recordings of hippocampal ASIC-like currents
Ion currents were recorded using the whole-cell patch-clamp technique (Hamill et al. 1981). Data were sampled at 500 Hz and low-pass filtered at 3 kHz using pCLAMP 8 software (Axon Instruments, USA). The statistical significance of differences between sets of data was estimated by the single-sided Student test. The pipette solution contained (mm): KCl 140, NaCl 5, MgCl2 2, EGTA 5, K2ATP 2, Hepes 10 (pH 7.35), and the bath solution contained (mm): NaCl 150, KCl 5, MgCl2 2, CaCl2 2, glucose 10 mm, Hepes 10 (pH 7.45). CNQX 20 μm, kynurenic acid 10 μm, MgCl2 7 mm and bicuculline 10 μm were added in order to inhibit glutamate- and GABA-induced currents. MES or acetate were used instead of Hepes to buffer bath solution pH ranging from 6 to 5, and from 4.5 to 3, respectively. ZnCl2 was added to the bath solution at 300 μm (Baron et al. 2001). Changes in extracellular pH were induced by shifting one out of six outlets of a microperfusion system in front of the cell. Experiments were carried out at room temperature (20–24 °C). Bovine serum albumin (0.1 %) was added in extracellular solutions containing the spider toxin PcTX1 (Escoubas et al. 2000) to prevent its adsorption to tubing and containers. Amiloride, CNQX, bicuculline, capsaicin, capsazepine and kynurenic acid were all from Sigma.
RESULTS
ASIC-like current of primary cultured hippocampal neurons
The expression of ASIC1a and ASIC2a was previously studied in hippocampal neurons by in situ hybridization. A high level of overlapping expression of the two subunits was particularly found in the pyramidal neurons of CA1 and CA3 subfields of the hippocampus (Bassilana et al. 1997; Waldmann et al. 1997a, b). However, the presence of functional hippocampal ASIC channels was never assessed. When the neurons were held at −50 mV, acidification of the extracellular medium triggered a transient inward current that was frequently followed by a plateau phase (Fig. 1A and C). At −50 mV, a drop to pH 5 induced a mean peak current of 17.15 ± 1.86 pA pF−1 and a mean plateau current of 1.41 ± 0.33 pA pF−1 (cell capacitance: 39.8 ± 2.7 pF, n = 52). The I-V curve shows that the peak transient current (Fig. 1A and B, ○) is highly selective for Na+ (Erev (reversal potential) = +80 mV) whereas the plateau current was non-selective (Erev = +15 mV; Fig. 1A and B, •).
Figure 1. ASIC-like current in hippocampal neurons.
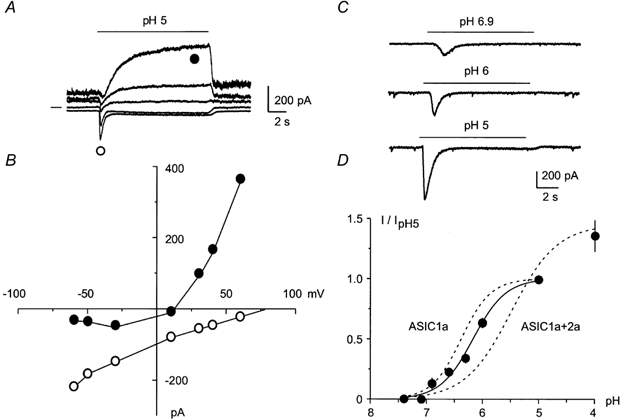
A, hippocampal ASIC-like currents activated at pH 5 and recorded at −60, −30, +10, +30 and +60 mV. The 0 pA current level is indicated on the left. B, current-potential relationship of hippocampal ASIC-like current obtained from traces shown in A. ○, peak current; •, plateau current. C, hippocampal ASIC-like currents induced by pH 6.9, 6 and 5. Currents were recorded at −50 mV. D, pH-dependent activation of the hippocampal ASIC-like current. Current amplitude was expressed as a fraction of the current induced by pH 5 (I/IpH 5), and plotted as mean ± s.e.m., n ranging from 8 to 18. Between pH 7.4 and 5, data could be fitted by a sigmoidal curve, showing a pH0.5 of 6.2 and a Hill coefficient of 1.48. The dashed curves represent the pH-dependent activation of the homomeric ASIC1a and of the heteromeric ASIC1a+2a (1:1) currents expressed in Xenopus oocytes (Baron et al. 2001).
The hippocampal ASIC-like peak current showed a high sensitivity to pH, with an activation threshold around pH 6.9 (Fig. 1C and D). Between pH 7.4 and 5, the activation of the peak current could be fitted by a sigmoidal curve, with a half-maximal activation at pH 6.2 and a Hill slope factor (nH) of 1.48 (Fig. 1D). These activation properties are intermediate between those of heterologously expressed homomeric ASIC1a and heteromeric ASIC1a+2a currents (pH0.5 = 6.4 and nH = 1.65 for ASIC1a, pH0.5 = 5.5 and nH = 1.10 for ASIC1a+2a (Baron et al. 2001; Fig. 1D). This suggests that the hippocampal ASIC-like current flows through a mixture of different channels. This view is supported by the fact that the current is not fully activated at pH 5 and can be further increased at pH 4.
Pharmacological properties of hippocampal ASIC-like current
The peak current was fully inhibited by 500 μm amiloride, a known blocker of ASIC channels (Lingueglia et al. 1993; Waldmann et al. 1997b, 1999), whereas the plateau current was not fully suppressed (Fig. 2A). The hippocampal ASIC-like current was not inhibited by 10 μm capsazepine, and 100 μm capsaicin evoked only a small inward current in hippocampal neurons (0.7 ± 0.3 pA pF−1, n = 5, not shown), showing that a contamination of the ASIC-like current by the pH-activated VR1 current (Tominaga et al. 1998; Szallasi & Di Marzo, 2000) can be ruled out. In the majority (80 %) of the recorded neurons, the hippocampal ASIC-like peak current induced at pH 6 was inhibited by 46 ± 8 % (n = 12) by the tarantula toxin PcTX1, a specific blocker of homomeric ASIC1a channels (Escoubas et al. 2000) (Fig. 2B), whereas the inhibition was complete in the remaining 20 % of cells. This result shows that the hippocampal ASIC-like current is due to a mixture of homomeric ASIC1a channels and at least one other PcTx1-resistant ASIC channel.
Figure 2. Pharmacological properties of the hippocampal ASIC-like current.
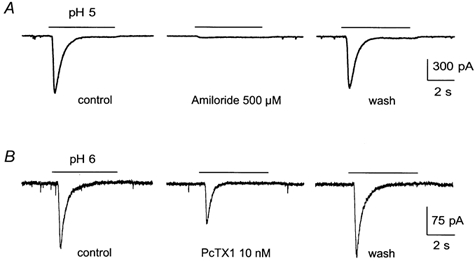
A, reversible inhibition of hippocampal ASIC-like current by 500 μm amiloride. B, reversible inhibition of hippocampal ASIC-like current by 10 nm of the toxin PcTX1. Currents were recorded at −50 mV. Amiloride and PcTX1 were given before and during the pH drop.
Effect of Zn2+ on hippocampal ASIC-like current
Previous work with recombinant channels has shown that Zn2+ co-applied with acidic pH increases the amplitude of ASIC2a-containing currents (Baron et al. 2001). We tested the effect of Zn2+ on the hippocampal ASIC-like current with two purposes in mind. The first was to use Zn2+ as a pharmacological tool to reveal the involvement of ASIC2a subunits in hippocampal ASIC assemblies. The second was to establish a possible physiological role for Zn2+ in the activation of these channels. In 80 % of the recorded cells, 300 μm Zn2+ increased the peak hippocampal ASIC-like current, with no effect on the sustained current (Fig. 3A). The PcTX1-resistant peak current was more sensitive to Zn2+ than the whole ASIC-like current (Fig. 3B), consistent with the fact that the homomeric ASIC1a current is insensitive to Zn2+ (Baron et al. 2001). In the presence of PcTX1, 300 μm Zn2+ increased the amplitude of the peak ASIC-like current by a factor of 4.74 ± 0.73 (n = 4) at pH 6.9, by a factor of 1.86 ± 0.19 (n = 6) at pH 6, and by a factor of 1.33 ± 0.11 (n = 4) at pH 5. These values are similar to those obtained for the ASIC1a+2a current expressed in Xenopus oocytes or in COS cells (Baron et al. 2001). The few Zn2+-insensitive currents were highly (90–100 %) inhibited by PcTX1, and would thus correspond to homomeric ASIC1a currents.
Figure 3. Effect of Zn2+ on the hippocampal ASIC-like current.
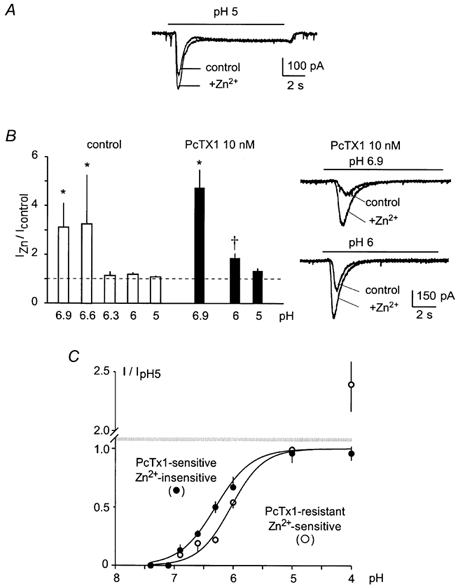
A, 300 μm Zn2+ co-applied with acidic pH increases the amplitude of the peak hippocampal ASIC-like current without affecting the plateau phase. Currents were recorded at −50 mV. B, effect of 300 μm Zn2+ on hippocampal ASIC-like currents induced by pH ranging from 6.9 to 5. Current amplitude ratio (IZn/Icontrol) was measured for each pH value, in the absence (□) and in the presence of 10 nm PcTX1 (▪), and plotted as mean ± s.e.m., n ranging from 4 to 22; * significantly different from corresponding pH 5 ratio (P < 0.05); † Significantly different from corresponding control (no PcTX1) pH ratio (P < 0.005). Currents were recorded at −50 mV. On the right side on the histogram, original current traces are shown to illustrate the effect of Zn2+ on hippocampal ASIC-like current induced by pH 6.9 (top) and pH 6 (bottom) in the presence of PcTX1 (holding potential: −50 mV). C, pH-dependent activation of the PcTX1-sensitive Zn2+-insensitive ASIC-like current (•) and of the PcTX1-resistant Zn2+-sensitive ASIC-like current (○). Data for PcTX1-resistant ASIC-like current were obtained from the same experiments as in B. Data for PcTX1-sensitive Zn2+-insensitive ASIC-like current were obtained from neurons (20 % of total recorded neurons) showing an ASIC-like current highly sensitive to PcTX1 (90–100 % inhibited) and not potentiated by Zn2+. Current amplitude was expressed as a fraction of the current induced by pH 5 (I/IpH5), and plotted as mean ± s.e.m., n ranging from 4 to 18. Between pH 7.4 and 5, data could be fitted by sigmoidal curves, showing a pH0.5 of 6.3 for the PcTX1-sensitive Zn2+-insensitive ASIC-like current and a pH0.5 of 6.0 for the PcTX1-resistant Zn2+-sensitive ASIC-like current.
To investigate the molecular association involved in hippocampal Zn2+-sensitive ASIC-like current, we measured the pH sensitivity of the PcTX1-resistant Zn2+-sensitive ASIC-like current (Fig. 3C, ○) and compared it with the pH sensitivity of the PcTx1-sensitive Zn2+-insensitive ASIC-like current (Fig. 3C, •). The PcTx1-sensitive Zn2+-insensitive current showed a pH0.5 of 6.3 with a maximal activation at pH 5, which would be expected from homomeric ASIC1a current. In contrast, the PcTX1-resistant Zn2+-sensitive current was not maximal at pH 5 and greatly increased at pH 4. Between pH 7.4 and 5, a pH0.5 of 6.0 was obtained, a value significatively lower than that for both the PcTX1-sensitive Zn2+-insensitive current (ASIC1a-like) or the whole ASIC-like current. These results support the suggestion that ASIC2a-containing heteromers are involved between pH 7.4 and 5, whereas the further increase in current at pH 4 could be mainly due to homomeric ASIC2a channels.
The Zn2+ sensitivity of the peak hippocampal ASIC-like current thus appears to be conferred by a PcTX1-resistant current, probably flowing through ASIC1a+2a channels.
Effect of Zn2+ and ASIC-like current on the membrane potential of hippocampal neurons
Membrane potential variations have been recorded in the current-clamp mode. Hippocampal neurons had a resting potential of −49.8 ± 2.0 mV (n = 29) which usually prevented the triggering of spontaneous action potentials (APs) (Fig. 4A and D). Acidification of the extracellular medium induced a biphasic depolarization with a transient phase and a plateau (Fig. 4Aa–c), compatible with kinetics of the hippocampal ASIC-like current (Fig. 4Ad). The transient depolarizations induced by pH shifts from 7.4 to 6.6 or 5 triggered an initial AP (Fig. 4Ab and c, enlargements shown), whereas the threshold of the AP was not reached by the transient depolarization induced by a pH drop to 6.9 (Fig. 4Aa). However, spontaneous AP trains could often be recorded during the plateau of the depolarization induced by pH drops to 6.9 or to 6.6 (Fig. 4Aa and b), whereas lower pH values (pH 5 in Fig. 4Ac) induced a sustained depolarization to around −20 mV but did not produce any AP. This can be easily explained by an inactivation of the voltage-sensitive Na+ channels by the sustained depolarization, and the prevention of any AP triggering.
Figure 4. Effect of ASIC current activation on membrane potential of hippocampal neurons.
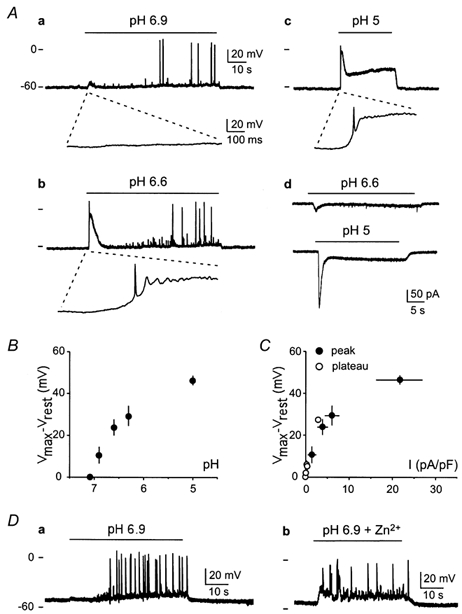
A, membrane depolarization induced by pH 6.9 (a), pH 6.6 (b) and pH 5 (c) in a single neuron. The initial acid-induced depolarization and AP is shown on a higher scale (100-fold) under each recording. Membrane potential was recorded in current-clamp mode with 0 pA current. Resting potential was −63 mV. Ticks on the left side of recordings represent the 0 mV level (upper tick) and the −60 mV level (lower tick). On the same neuron, ASIC-like currents activated by pH 6.6 and 5 were subsequently recorded in voltage-clamp mode at −60 mV (d). B, mean maximal membrane depolarization induced by ASIC-like current activation (Vmax −Vrest) as a function of extracellular pH. Recordings with a resting membrane potential (Vrest) between −45 and −55 mV were selected, and action potentials were excluded of measurements. Mean ± s.e.m. values are shown, n ranging from 3 to 12. C, mean maximal membrane depolarization induced by ASIC-like current activation (Vmax −Vrest) as a function of current density. For membrane potential measurements, recordings with a resting membrane potential between −45 and −55 mV (Vrest) were selected, and action potentials were excluded of measurements. ASIC-like current density (pA pF−1) was subsequently measured on the same neurons, at holding potential between −45 and −55 mV. Membrane potential and ASIC-like current amplitude were measured during the transient phase (•) and during the sustained plateau phase (○) for the same pH value. Mean ± s.e.m. values are shown, n ranging from 3 to 12. D, effect of 300 μm Zn2+ on membrane depolarization induced by ASIC-like current activation. Membrane depolarization induced by pH 6.9 (a) and pH 6.9 + 300 μm Zn2+ (b) on a single neuron. Membrane potential was recorded in current-clamp mode with 0 pA current. Resting membrane potential was −53 mV. Ticks on the left side of recordings represent the 0 mV level (upper tick) and the −60 mV level (lower tick).
The transient peak of the acid-induced depolarization at pH 5 (46.4 ± 2.3 mV; n = 12) corresponds to a membrane potential close to 0 mV, whereas the maximal depolarization induced by a pH drop from 7.4 to 6.9 (10.6 ± 4.0 mV; n = 9) would bring the membrane potential to around −40 mV (Fig. 4B). Figure 4C, showing the relationship between the ASIC current density and the membrane depolarization induced by the same pH drop on the same neuron, illustrates the fact that 25 % of the maximal ASIC current could induce 50 % of the maximal depolarization. Thus, a slight drop of the extracellular pH and a submaximal activation of ASIC channels may cause important changes in the excitability of hippocampal neurons.
Zn2+ potentiates the hippocampal ASIC-like current. It also increases the submaximal acid-induced transient depolarizations. Figure 4D shows the effect of 300 μm Zn2+ co-applied with pH 6.9. In the absence of Zn2+, APs were only recorded during the plateau phase of the depolarization induced by pH 6.9 (Fig. 4Da), whereas, in the presence of 300 μm Zn2+, APs were also triggered during the increased transient depolarization (Fig. 4Db). The depolarization induced by a pH drop to 5 was not significantly modified by Zn2+ (not shown). This was expected considering that a pH change of this magnitude already induced a quasi-maximal depolarization (Fig. 4C).
DISCUSSION
ASIC-like currents in CNS neurons
Several ASIC-like currents have been recorded in different types of central neurons. However, the available data show an important diversity of ASIC-like currents depending on the neuronal type. In mouse cerebellar granule cells, the half-maximal activation of ASIC-like current was obtained at pH0.5 = 6.4, and the current was nearly completely inhibited by PcTX1 (IC50 = 0.7 nm). These two properties suggested that the ASIC-like current mainly flows through homomeric ASIC1a channels in cerebellar granule cells (Escoubas et al. 2000). With a half-maximal activation at pH 6.8 (Grantyn & Lux, 1988), ASIC-like currents in rat tectal neurons also seem to flow through homomeric ASIC1a channels, although a pharmacological analysis using PcTX1 would be needed to confirm this. In rat ventromedial hypothalamic neurons (Ueno et al. 1992), the properties of ASIC-like currents are quite different with a threshold at pH 6.5, a maximal activation at pH 4 and pH0.5 = 4.9. These properties seem closer to those of the heteromeric ASIC1a+2a current (Baron et al. 2001). In mouse cortical neurons, low pH0.5 values also suggest the involvement of heteromeric ASIC1a+2a channels (Varming, 1999). However, here again, because no pharmacological tools or antibodies were available, it was impossible to elucidate the exact molecular nature of the ASIC channels.
We report here the first characterization of ASIC-like current in hippocampal neurons. Extracellular acidification induces a biphasic current, i.e. a transient Na+ current followed by a sustained non-selective cation current. The partial block by the ASIC1a-specific toxin PcTX1 (Escoubas et al. 2000), the pH dependence and the Zn2+ co-activation of the hippocampal current suggest that the transient current flows through a mixture of PcTX1-sensitive Zn2+-insensitive homomeric ASIC1a channels and of PcTX1-resistant Zn2+-sensitive ASIC2a-containing channels. The biphasic pattern of the PcTX1-resistant current activation curve (Fig. 3C, ○) suggests that the PcTX1-resistant current involves as least two different channel types: putative heteromeric ASIC1a+2a with a pH0.5 of 6.0, and putative homomeric ASIC2a channels mainly responsable for the increase in current amplitude at pH 4. The pH sensitivity of heteromeric ASIC1a+2a channels is highly dependent on the stoichiometry of the two subunits. Whereas homomeric ASIC1a current shows a pH0.5 of 6.4 and the homomeric ASIC2a shows a pH0.5 of 4.4, the heteromeric ASIC1a+2a (1:1) current shows a pH0.5 of 5.5 and the heteromeric ASIC1a+2a (1:2) current shows a pH0.5 of 5.1 (Baron et al. 2001; A. Baron, unpublished data). Thus, the pH0.5 of 6.0 obtained for the PcTX1-resistant Zn2+-sensitive hippocampal current activated between pH 7.4 and 5 suggests that the heteromeric ASIC1a+2a channels would contain a higher proportion of ASIC1a subunits than of ASIC2a subunits. This hypothesis is supported by semi-quantitative RT-PCR experiments performed on the same hippocampal neurons as those used in patch-clamp experiments, which showed a 10-fold lower level of ASIC2a mRNA compared to ASIC1a mRNA (N. Voilley, unpublished data). This suggests a higher probability of a heteromeric association between ASIC1a and ASIC2a subunits rather than a homomeric ASIC2a association, and that heterotetrameric ASIC1a+2a channels could involve more ASIC1a than ASIC2a subunits.
ASIC2b is known to induce a sustained outward-rectifying non-selective cationic current when associated with other ASIC subunits (Lingueglia et al. 1997). Since ASIC1a, ASIC2a and ASIC2b are expressed in hippocampal neurons (Price et al. 1996; Waldmann et al. 1996; Bassilana et al. 1997; Garcia-Anoveros et al. 1997), it is likely that heteromeric ASIC2b-containing channels are responsible for the sustained non-selective cation current recorded in hippocampal neurons. However, it is difficult to postulate the stoichiometry or the other subunits involved in these heteromeric channels (ASIC1a+2b, ASIC2a+2b or even ASIC1a+2a+2b). The participation of ASIC2b in the plateau phase is thus highly probable but the involvement of another channel type cannot be ruled out. Further biochemical evidence will be needed to confirm the presence and the molecular composition of functional heteromeric ASIC channels in hippocampal neurons.
Effects of extracellular pH variations on neuronal activity
There are situations in which acidic pH may lead to a decrease in neuronal excitability (Hsu et al. 2000). Protons inhibit NMDA currents and voltage-dependent Ca2+ currents, whereas GABAA currents are stimulated (Chesler & Kaila, 1992). In contrast, a rapid external acidification has been shown to excite a variety of neurons, due to the activation of ASIC-like currents (Gruol et al. 1980; Krishtal et al. 1987; Jarolimek et al. 1989; Walz, 1989; Chesler & Kaila, 1992; Varming, 1999). Neurons of rat ventral medulla oblongata have been shown to respond to small extracellular pH variations by a transient increase in AP frequency (Jarolimek et al. 1990). A rapid acid-triggered depolarization and the generation of spikes was also reported in mouse cortical spinal neurons (Gruol et al. 1980; Varming, 1999).
We show that ASIC-like current activation triggers a membrane depolarization and trains of APs in hippocampal neurons. Small pH changes, compatible with local transient acidifications reported in the CNS (Krishtal et al. 1987; Jarolimek et al. 1989; Chesler & Kaila, 1992; Miesenbock et al. 1998), can increase neuronal excitability by raising the membrane potential to a level near the voltage-dependent Na+ channel threshold.
Under certain pathological conditions, such as brain ischaemia, the local extracellular pH becomes quite acidic, reaching values around 6.5 (Ohno et al. 1989; Nedergaard et al. 1991). Activation of sustained ASIC currents is then expected to produce an intense firing of neurons which itself might contribute to neuronal death during an ischemic insult. Very recently, variations of expression of ASIC subunits has been related to physiopathological processes. Ischaemia induces the increase of ASIC2a expression in neurons of hippocampus and cortex (Johnson et al. 2001), whereas epilepsy induces a marked decrease in ASIC2b mRNA levels in all hippocampus areas and in ASIC1a mRNA levels in the CA1–2 fields (Biagini et al. 2001). Taken altogether, these results suggest an important role for ASIC subunits in both normal and pathological activity of hippocampus.
Effect of Zn2+ on ionic currents and neuronal excitability
Ever since the discovery that Zn2+ is present in large amounts in hippocampus, there has been intense speculation concerning a possible synaptic signalling role for this metal in brain function. In general, exogenous Zn2+ tends to lead to excitatory bursting (Harrison & Gibbons, 1994; Reece et al. 1994; Henze et al. 2000). Some of this excitatory effect is likely to be due to an inhibition of voltage-dependent K+ channels as well as an inhibition of GABA channels and a potentiation of AMPA receptors. In contrast to its excitatory effects, Zn2+ blocks NMDA receptors (Harrison & Gibbons, 1994; Smart et al. 1994; Henze et al. 2000). Zn2+ was also reported to play a role in neurodegeneration associated with pathologies such as ischaemia and epilepsia (Harrison & Gibbons, 1994; Smart et al. 1994; Choi & Koh, 1998; Weiss et al. 2000).
We show that hippocampal ASIC-like current activation and the potentiation by Zn2+ modulates neuronal excitability by increasing the membrane depolarization induced by small pH changes. This effect was observed at Zn2+ concentrations compatible with the physiological range of synaptically released Zn2+ (100–300 μm) (Assaf & Chung, 1984; Howell et al. 1984; Smart et al. 1994; Budde et al. 1997; Weiss et al. 2000). ASIC channels might thus be important physiological targets of Zn2+ in the hippocampus.
Acknowledgments
We thank V. Lopez for excellent technical assistance, M. Jodar for performing primary cultures, I. Lauritzen for helpful advice about hippocampal neurons, and N. Voilley and J. Mamet for helpful comments. This work was supported by the Centre National de la Recherche Scientifique (CNRS), the Institut National de la Santé et de la Recherche Médicale (INSERM), the Association Française contre les Myopathies (AFM), the Conseil Regional (PACA), The Ministère de la Recherche (ACI: Molécules et Cibles Thérapeutiques) and the Association pour la Recherche sur le Cancer (ARC).
REFERENCES
- Akopian AN, Chen CC, Ding Y, Cesare P, Wood JN. A new member of the acid-sensing ion channel family. NeuroReport. 2000;11:2217–2222. doi: 10.1097/00001756-200007140-00031. [DOI] [PubMed] [Google Scholar]
- Assaf SY, Chung SH. Release of endogenous Zn2+ from brain tissue during activity. Nature. 1984;308:734–736. doi: 10.1038/308734a0. [DOI] [PubMed] [Google Scholar]
- Babinski K, Catarsi S, Biagini G, Seguela P. Mammalian ASIC2a and ASIC3 subunits co-assemble into heteromeric proton-gated channels sensitive to Gd3+ Journal of Biological Chemistry. 2000;275:28519–28525. doi: 10.1074/jbc.M004114200. [DOI] [PubMed] [Google Scholar]
- Babinski K, Le KT, Seguela P. Molecular cloning and regional distribution of a human proton receptor subunit with biphasic functional properties. Journal of Neurochemistry. 1999;72:51–57. doi: 10.1046/j.1471-4159.1999.0720051.x. [DOI] [PubMed] [Google Scholar]
- Baron A, Schaefer L, Lingueglia E, Champigny G, Lazdunski M. Zn2+ and H+ are coactivators of acid sensing ion channels. Journal of Biological Chemistry. 2001;276:35361–35367. doi: 10.1074/jbc.M105208200. [DOI] [PubMed] [Google Scholar]
- Bassilana F, Champigny G, Waldmann R, de Weille JR, Heurteaux C, Lazdunski M. The acid-sensitive ionic channel subunit ASIC and the mammalian degenerin MDEG form a heteromultimeric H+-gated Na+ channel with novel properties. Journal of Biological Chemistry. 1997;272:28819–28822. doi: 10.1074/jbc.272.46.28819. [DOI] [PubMed] [Google Scholar]
- Benson CJ, Eckert SP, McCleskey EW. Acid-evoked currents in cardiac sensory neurons: A possible mediator of myocardial ischemic sensation. Circulation Research. 1999;84:921–928. doi: 10.1161/01.res.84.8.921. [DOI] [PubMed] [Google Scholar]
- Biagini G, Babinski K, Avoli M, Marcinkiewicz M, Seguela P. Regional and subunit-specific downregulation of acid-sensing ion channels in the pilocarpine model of epilepsy. Neurobiology Disease. 2001;8:45–58. doi: 10.1006/nbdi.2000.0331. [DOI] [PubMed] [Google Scholar]
- Budde T, Minta A, White JA, Kay AR. Imaging free zinc in synaptic terminals in live hippocampal slices. Neuroscience. 1997;79:347–358. doi: 10.1016/s0306-4522(96)00695-1. [DOI] [PubMed] [Google Scholar]
- Chen CC, England S, Akopian AN, Wood JN. A sensory neuron-specific, proton-gated ion channel. Proceeding of the National Academy of Science of the USA. 1998;95:10240–10245. doi: 10.1073/pnas.95.17.10240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chesler M, Kaila K. Modulation of pH by neuronal activity. Trends in Neurosciences. 1992;15:396–402. doi: 10.1016/0166-2236(92)90191-a. [DOI] [PubMed] [Google Scholar]
- Choi DW, Koh JY. Zinc and brain injury. Annual Review of Neuroscience. 1998;21:347–375. doi: 10.1146/annurev.neuro.21.1.347. [DOI] [PubMed] [Google Scholar]
- Coscoy S, Lingueglia E, Lazdunski M, Barbry P. The Phe-Met-Arg-Phe-amide-activated sodium channel is a tetramer. Journal of Biological Chemistry. 1998;273:8317–8322. doi: 10.1074/jbc.273.14.8317. [DOI] [PubMed] [Google Scholar]
- de Weille JR, Bassilana F, Lazdunski M, Waldmann R. Identification, functional expression and chromosomal localisation of a sustained human proton-gated cation channel. FEBS Letters. 1998;433:257–260. doi: 10.1016/s0014-5793(98)00916-8. [DOI] [PubMed] [Google Scholar]
- Escoubas P, De Weille JR, Lecoq A, Diochot S, Waldmann R, Champigny G, Moinier D, Menez A, Lazdunski M. Isolation of a tarantula toxin specific for a class of proton-gated Na+ channels. Journal of Biological Chemistry. 2000;275:25116–25121. doi: 10.1074/jbc.M003643200. [DOI] [PubMed] [Google Scholar]
- Frederickson CJ. Neurobiology of zinc and zinc-containing neurons. International Review of Neurobiology. 1989;31:145–238. doi: 10.1016/s0074-7742(08)60279-2. [DOI] [PubMed] [Google Scholar]
- Garcia-Anoveros J, Derfler B, Neville-Golden J, Hyman BT, Corey DP. BNaC1 and BNaC2 constitute a new family of human neuronal sodium channels related to degenerins and epithelial sodium channels. Proceedings of the National Academy of Sciences of the USA. 1997;94:1459–1464. doi: 10.1073/pnas.94.4.1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Anoveros J, Samad TA, Woolf CJ, Corey DP. Transport and localization of the DEG/ENaC ion channel BNaC1alpha to peripheral mechanosensory terminals of dorsal root ganglia neurons. Journal of Neuroscience. 2001;21:2678–2686. doi: 10.1523/JNEUROSCI.21-08-02678.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goslin K, Banker G. Rat hippocampal neurons in low-density culture. In: Banker G, Goslin K, editors. Culturing Nerve Cells. Cambridge, MA, USA: Massachussets Institute of Technology; 1991. pp. 251–283. [Google Scholar]
- Grantyn R, Lux HD. Similarity and mutual exclusion of NMDA- and proton-activated transient Na+-currents in rat tectal neurons. Neuroscience Letters. 1988;89:198–203. doi: 10.1016/0304-3940(88)90381-3. [DOI] [PubMed] [Google Scholar]
- Grunder S, Geissler HS, Bassler EL, Ruppersberg JP. A new member of acid-sensing ion channels from pituitary gland. Neuroreport. 2000;11:1607–1611. doi: 10.1097/00001756-200006050-00003. [DOI] [PubMed] [Google Scholar]
- Gruol DL, Barker JL, Huang LY, Macdonald JF, Smith TG., Jr Hydrogen ions have multiple effects on the excitability of cultured mammalian neurons. Brain Research. 1980;183:247–252. doi: 10.1016/0006-8993(80)90138-9. [DOI] [PubMed] [Google Scholar]
- Hamill OP, Marty A, Neher E, Sakmann B, Sigworth FJ. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflügers Archiv. 1981;391:85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Harrison NL, Gibbons SJ. Zn2+: an endogenous modulator of ligand- and voltage-gated ion channels. Neuropharmacology. 1994;33:935–952. doi: 10.1016/0028-3908(94)90152-x. [DOI] [PubMed] [Google Scholar]
- Henze DA, Urban NN, Barrionuevo G. The multifarious hippocampal mossy fiber pathway: a review. Neuroscience. 2000;98:407–427. doi: 10.1016/s0306-4522(00)00146-9. [DOI] [PubMed] [Google Scholar]
- Howell GA, Welch MG, Frederickson CJ. Stimulation-induced uptake and release of zinc in hippocampal slices. Nature. 1984;308:736–738. doi: 10.1038/308736a0. [DOI] [PubMed] [Google Scholar]
- Hsu KS, Liang YC, Huang CC. Influence of an extracellular acidosis on excitatory synaptic transmission and long-term potentiation in the CA1 region of rat hippocampal slices. Journal of Neuroscience Research. 2000;62:403–415. doi: 10.1002/1097-4547(20001101)62:3<403::AID-JNR11>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- Jarolimek W, Misgeld U, Lux HD. Activity dependent alkaline and acid transients in guinea pig hippocampal slices. Brain Research. 1989;505:225–232. doi: 10.1016/0006-8993(89)91447-9. [DOI] [PubMed] [Google Scholar]
- Jarolimek W, Misgeld U, Lux HD. Neurons sensitive to pH in slices of the rat ventral medulla oblongata. Pflügers Archiv. 1990;416:247–253. doi: 10.1007/BF00392060. [DOI] [PubMed] [Google Scholar]
- Johnson MB, Jin K, Minami M, Chen D, Simon RP. Global ischemia induces expression of acid-sensing ion channel 2a in rat brain. Journal of Cerebral Blood Flow and Metabolism. 2001;21:734–740. doi: 10.1097/00004647-200106000-00011. [DOI] [PubMed] [Google Scholar]
- Kress M, Zeilhofer HU. Capsaicin, protons and heat: new excitement about nociceptors. Trends in Pharmacological Sciences. 1999;20:112–118. doi: 10.1016/s0165-6147(99)01294-8. [DOI] [PubMed] [Google Scholar]
- Krishtal OA, Osipchuk YV, Shelest TN, Smirnoff SV. Rapid extracellular pH transients related to synaptic transmission in rat hippocampal slices. Brain Research. 1987;436:352–356. doi: 10.1016/0006-8993(87)91678-7. [DOI] [PubMed] [Google Scholar]
- Lauritzen I, De Weille JR, Lazdunski M. The potassium channel opener (-)-cromakalim prevents glutamate-induced cell death in hippocampal neurons. Journal of Neurochemistry. 1997;69:1570–1579. doi: 10.1046/j.1471-4159.1997.69041570.x. [DOI] [PubMed] [Google Scholar]
- Lingueglia E, de Weille JR, Bassilana F, Heurteaux C, Sakai H, Waldmann R, Lazdunski M. A modulatory subunit of acid sensing ion channels in brain and dorsal root ganglion cells. Journal of Biological Chemistry. 1997;272:29778–29783. doi: 10.1074/jbc.272.47.29778. [DOI] [PubMed] [Google Scholar]
- Lingueglia E, Voilley N, Waldmann R, Lazdunski M, Barbry P. Expression cloning of an epithelial amiloride-sensitive Na+ channel. A new channel type with homologies to Caenorhabditis elegans degenerins. FEBS Letters. 1993;318:95–99. doi: 10.1016/0014-5793(93)81336-x. [DOI] [PubMed] [Google Scholar]
- Miesenbock G, De Angelis DA, Rothman JE. Visualizing secretion and synaptic transmission with pH-sensitive green fluorescent proteins. Nature. 1998;394:192–195. doi: 10.1038/28190. [DOI] [PubMed] [Google Scholar]
- Nedergaard M, Kraig RP, Tanabe J, Pulsinelli WA. Dynamics of interstitial and intracellular pH in evolving brain infarct. American Journal of Physiology. 1991;260:R581–588. doi: 10.1152/ajpregu.1991.260.3.R581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohno M, Obrenovitch TP, Hartell N, Barratt S, Bachelard HS, Symon L. Simultaneous recording of tissue PCO2, interstitial pH and potassium activity in the rat cerebral cortex during anoxia and the subsequent recovery period. Neurology Research. 1989;11:153–159. doi: 10.1080/01616412.1989.11739882. [DOI] [PubMed] [Google Scholar]
- Olson TH, Riedl MS, Vulchanova L, Ortiz-Gonzalez XR, Elde R. An acid sensing ion channel (ASIC). localizes to small primary afferent neurons in rats. NeuroReport. 1998;9:1109–1113. doi: 10.1097/00001756-199804200-00028. [DOI] [PubMed] [Google Scholar]
- Ozkan ED, Ueda T. Glutamate transport and storage in synaptic vesicles. Japanese Journal of Pharmacology. 1998;77:1–10. doi: 10.1254/jjp.77.1. [DOI] [PubMed] [Google Scholar]
- Price MP, Lewin GR, McIlwrath SL, Cheng C, Xie J, Heppenstall PA, Stucky CL, Mannsfeldt AG, Brennan TJ, Drummond HA, Qiao J, Benson CJ, Tarr DE, Hrstka RF, Yang B, Williamson RA, Welsh MJ. The mammalian sodium channel BNC1 is required for normal touch sensation. Nature. 2000;407:1007–1011. doi: 10.1038/35039512. [DOI] [PubMed] [Google Scholar]
- Price MP, Snyder PM, Welsh MJ. Cloning and expression of a novel human brain Na+ channel. Journal of Biological Chemistry. 1996;271:7879–7882. doi: 10.1074/jbc.271.14.7879. [DOI] [PubMed] [Google Scholar]
- Reece LJ, Dhanjal SS, Chung SH. Zinc induces hyperexcitability in the hippocampus. NeuroReport. 1994;5:2669–2672. doi: 10.1097/00001756-199412000-00066. [DOI] [PubMed] [Google Scholar]
- Schmidt-Kastner R, Freund TF. Selective vulnerability of the hippocampus in brain ischemia. Neuroscience. 1991;40:599–636. doi: 10.1016/0306-4522(91)90001-5. [DOI] [PubMed] [Google Scholar]
- Smart TG, Xie X, Krishek BJ. Modulation of inhibitory and excitatory amino acid receptor ion channels by zinc. Progress in Neurobiology. 1994;42:393–341. doi: 10.1016/0301-0082(94)90082-5. [DOI] [PubMed] [Google Scholar]
- Sutherland SP, Benson CJ, Adelman JP, McCleskey EW. Acid-sensing ion channel 3 matches the acid-gated current in cardiac ischemia-sensing neurons. Proceedings of the National Academy of Sciences of the USA. 2000a;98:711–716. doi: 10.1073/pnas.011404498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutherland SP, Cook SP, McCleskey EW. Chemical mediators of pain due to tissue damage and ischemia. Progress in Brain Research. 2000b;129:21–38. doi: 10.1016/S0079-6123(00)29003-1. [DOI] [PubMed] [Google Scholar]
- Szallasi A, Di Marzo V. New perspectives on enigmatic vanilloid receptors. Trends in Neurosciences. 2000;23:491–497. doi: 10.1016/s0166-2236(00)01630-1. [DOI] [PubMed] [Google Scholar]
- Tominaga M, Caterina MJ, Malmberg AB, Rosen TA, Gilbert H, Skinner K, Raumann BE, Basbaum AI, Julius D. The cloned capsaicin receptor integrates multiple pain-producing stimuli. Neuron. 1998;21:531–543. doi: 10.1016/s0896-6273(00)80564-4. [DOI] [PubMed] [Google Scholar]
- Ueno S, Nakaye T, Akaike N. Proton-induced sodium current in freshly dissociated hypothalamic neurones of the rat. Journal of Physiology. 1992;447:309–327. doi: 10.1113/jphysiol.1992.sp019004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varming T. Proton-gated ion channels in cultured mouse cortical neurons. Neuropharmacology. 1999;38:1875–1881. doi: 10.1016/s0028-3908(99)00079-9. [DOI] [PubMed] [Google Scholar]
- Vogt K, Mellor J, Tong G, Nicoll R. The actions of synaptically released zinc at hippocampal mossy fiber synapses. Neuron. 2000;26:187–196. doi: 10.1016/s0896-6273(00)81149-6. [DOI] [PubMed] [Google Scholar]
- Voilley N, de Weille J, Mamet J, Lazdunski M. Nonsteroid anti-inflammatory drugs inhibit both the activity and the inflammation-induced expression of acid-sensing ion channels in nociceptors. Journal of Neuroscience. 2001;21:8026–8033. doi: 10.1523/JNEUROSCI.21-20-08026.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waldmann R, Bassilana F, Weille J, Champigny G, Heurteaux C, Lazdunski M. Molecular cloning of a non-inactivating proton-gated Na+ channel specific for sensory neurons. Journal of Biological Chemistry. 1997a;272:20975–20978. doi: 10.1074/jbc.272.34.20975. [DOI] [PubMed] [Google Scholar]
- Waldmann R, Champigny G, Bassilana F, Heurteaux C, Lazdunski M. A proton-gated cation channel involved in acid-sensing. Nature. 1997b;386:173–177. doi: 10.1038/386173a0. [DOI] [PubMed] [Google Scholar]
- Waldmann R, Champigny G, Lingueglia E, De Weille JR, Heurteaux C, Lazdunski M. H+-gated cation channels. Annals of the New York Academy of Sciences. 1999;868:67–76. doi: 10.1111/j.1749-6632.1999.tb11274.x. [DOI] [PubMed] [Google Scholar]
- Waldmann R, Champigny G, Voilley N, Lauritzen I, Lazdunski M. The mammalian degenerin MDEG, an amiloride-sensitive cation channel activated by mutations causing neurodegeneration in Caenorhabditis elegans. Journal of Biological Chemistry. 1996;271:10433–10436. doi: 10.1074/jbc.271.18.10433. [DOI] [PubMed] [Google Scholar]
- Waldmann R, Lazdunski M. H+-gated cation channels: neuronal acid sensors in the NaC/DEG family of ion channels. Current Opinion in Neurobiology. 1998;8:418–424. doi: 10.1016/s0959-4388(98)80070-6. [DOI] [PubMed] [Google Scholar]
- Walz W. pH shifts evoked by neuronal stimulation in slices of rat hippocampus. Canadian Journal of Physiology and Pharmacology. 1989;67:577–581. doi: 10.1139/y89-092. [DOI] [PubMed] [Google Scholar]
- Weiss JH, Sensi SL, Koh JY. Zn2+: a novel ionic mediator of neural injury in brain disease. Trends in Pharmacological Sciences. 2000;21:395–401. doi: 10.1016/s0165-6147(00)01541-8. [DOI] [PubMed] [Google Scholar]


