Abstract
Ion channels from bovine neurohypophysial secretory granules (NSG) were incorporated into artificial lipid bilayers. Specific antibodies against identified synaptic vesicle proteins were tested on such incorporated channel activity and on peptide release from rat permeabilized neurohypophysial terminals. Both the NSG cation channel and Ca2+-dependent release were inhibited by only SY-38, a monoclonal antibody directed against the C-terminus of synaptophysin. SY-38 and Ca2+ altered both the gating and conductance of the NSG cation channel, but in opposite ways. The close correlation between SY-38 effects on Ca2+-dependent channel activity and release leads us to conclude that this synaptophysin-like NSG channel is directly involved in peptide secretion from these central nervous system terminals.
Neuropeptides and neurotransmitters are secreted via fusion of the neurosecretory granule (NSG) or synaptic vesicle membrane with the plasma membrane of nerve terminals following the invasion of action potentials (Douglas & Poisner, 1964). In recent years, many molecules, including both membrane and soluble proteins, have been implicated in exocytosis (Rothman, 1994; Sudhof, 1995; Rahamimoff & Fernandez, 1997; Brunger, 2000). Synaptophysin, for example, is the most abundant integral membrane protein in synaptic vesicles (Navone et al. 1986; De Camilli & Jahn, 1990) and is also located in large dense core granules (Lowe et al. 1988; Obendorf et al. 1988; Lah & Burry, 1993). It has been reported to form voltage-dependent channels (Thomas et al. 1988), whose apparent conductance and gating could be modified by a monoclonal antibody, SY-38, against the C-terminus of synaptophysin (Knaus & Betz, 1990).
Ion channels have been reported in vesicle membranes (Lemos & Nordmann, 1986; DeRiemer et al. 1987; Rahamimoff et al. 1988; Stanley et al. 1988; Lemos et al. 1989; Lee et al. 1992; Sato et al. 1992; Kelly & Woodbury, 1996), but their functional role in release is still unclear (Rahamimoff et al. 1989; Woodbury, 1995). In mast cells, studies have shown that the first step in exocytosis is the formation of a fusion pore with conductance properties similar to those of gap junctions (Chandler & Heuser, 1980; Breckenridge & Almers, 1987). Furthermore, ion channels can greatly accelerate the fusion of vesicles with the lipid bilayer (Woodbury & Hall, 1988). Thus it has been suggested that ion channels located on the vesicle membrane could act as either a hemichannel involved in forming a fusion pore that leads to transmitter release (Thomas et al. 1988; Lemos et al. 1989; Almers, 1990) or a channel that might function to increase the osmolarity inside the vesicles to promote exocytotic fusion (Stanley & Ehrenstein, 1985; Finkelstein et al. 1986). On the other hand, ion channels have been suggested to increase the number of counter ions inside synaptic vesicles and thus displace the charged secretory products that were initially present on the vesicular matrix, forcing them to be released through the fusion pore (Rahamimoff & Fernandez, 1997).
Previously, we (Lemos & Nordmann, 1986; Lee et al. 1992; Yin et al. 1995) have shown that there are at least two types of channels in neurosecretory granule (NSG) membranes, one of ∼30 pS conductance and the other of multiple conductances (62–232 pS). The latter is a calcium-dependent cation channel. Here we have examined more thoroughly the calcium regulation of this NSG channel. Furthermore, in order to determine if the larger NSG channel could be one of the well-characterized vesicular proteins, a number of specific antibodies directed against identified proteins associated with vesicles, such as synaptophysin, were tested on channel activity and on peptide release. This combination of approaches allowed us to determine if the NSG channel is functionally involved in calcium-dependent secretion from these central nervous system (CNS) terminals.
METHODS
Isolation of NSG
The NSG of bovine posterior pituitary glands (collected from Arena Bros. slaughterhouse in Hopkington, MA, USA) were obtained by differential centrifugation as previously reported (Nordmann et al. 1979; Lemos & Nordmann, 1986). Briefly, bovine posterior pituitary glands were dissected and stored in homogenizing solution (HS), which contained 0.3 m sucrose and 10 mm Tris-Hepes (N-2-hydroxyethyl piperazine-N′-2-ethane-sulphonic acid), at pH 7.0. They were cleansed with Listerine and HS several times, finely minced, and then homogenized. The homogenate was sequentially centrifuged for 5 min at 300 g, and for 10 min at 3400 g. The final supernatant, with added HS, was then centrifuged for 20 min at 27 000 g. The pellet was resuspended in the isosmotic Percoll (Pharmacia) solution (30 % Percoll, 0.3 m sucrose and 10 mm Hepes), and centrifuged for 45 min at 27 000 g. The two broad bands from the middle of the resulting gradient were collected, resuspended in HS, and centrifuged for 40 min at 27 000 g. The pellet was resuspended in 10 mm Tris-Hepes (pH 7.0), and ultracentrifuged for 30 min at 125 000 g. The final pellet was resuspended in 200 mm KCl and 20 mm Tris-Hepes, at pH 7.0. In order to enhance channel protein concentration per vesicle, the samples were frozen and thawed three times before using. Similar electrophysiological results were obtained with rat posterior pituitaries (Lemos & Nordmann, 1986).
Immunoblots
Sodium dodecyl sulfate-containing polyacrylamide gels (SDS-PAGE) and blots were performed according to published procedures (Laemmli, 1970; Towbin et al. 1979). Subcellular fractions of bovine neurohypophyses were prepared as described previously (Nordmann et al. 1979) and above. Monoclonal antibody SY-38 to synaptophysin (Boehringer Mannheim, Germany) was used for detecting the proteins with an enhanced chemiluminescence (ECL) system (Boehringer Mannheim) in accordance with the manufacturer's instructions.
Preparation of lipid mixture and liposome
Brain phosphatidylethanolamine and phosphatidylserine (Avanti Polar Lipid, Inc., Pelham, AL, USA) were mixed in a 3:1 ratio (weight: weight), and dried with nitrogen. This mixture was either resuspended in an equal volume of decane by vortexing and used for the formation of lipid bilayers, or resuspended in an equal volume of 20 mm Tris-Hepes (pH 7.0) and sonicated until the solution became transparent. An NSG membrane preparation was then added to a final protein concentration of 1 mg ml−1 and mixed by vortexing before use.
Electrophysiology
Planar lipid bilayers are formed by brushing the lipid mixture onto an orifice (diameter about 200 μm) on the side wall of a polystyrene tube (Sarstedt, Princeton, NC, USA), which was situated in one side of a two-compartment Teflon chamber filled with experimental solutions. The cis well solution contained 150 mm KCl, 10 mm Hepes and variable free Ca2+ concentrations, and the trans well solution contained 50 mm KCl, 10 mm Hepes and 2 mm EGTA. The NSG membrane preparation was added to the cis compartment and a stir bar was continuously moved by a small magnetic bar under the cis chamber until a channel was incorporated into the lipid membrane.
The single-channel activity was recorded with conventional patch clamp techniques (Hamill et al. 1981). Data were digitized using a Pulse Code Modulator (Sony PCM-501ES) and analysed with pCLAMP6 software (Axon Instruments, Foster City, CA, USA). In all figures currents flowing into the trans well are shown as downward (or negative) deflections and vice versa. Frequency/ amplitude graphs were obtained by digitizing at 10 kHz current records of at least 20 s duration, which were filtered with an 8-pole Bessel filter at 200–1000 Hz. The sampled current amplitude was then used to form an all-points amplitude histogram (see right-hand sides of Figs 2, 4 and 6). For simplicity, the total open probability (NPo), which is a combination of all the open level probabilities, was normally utilized. The data were usually expressed as mean values ± standard error of the mean. The statistical significance of the difference between means was assessed by Student's t test, for paired or unpaired data as indicated. For trend analysis (Fig. 5), a two-way analysis of variance was utilized. Differences were considered statistically significant when P was lower than 0.05.
Figure 2. Effects of SY-38 mAb on NSG cation channel activity.
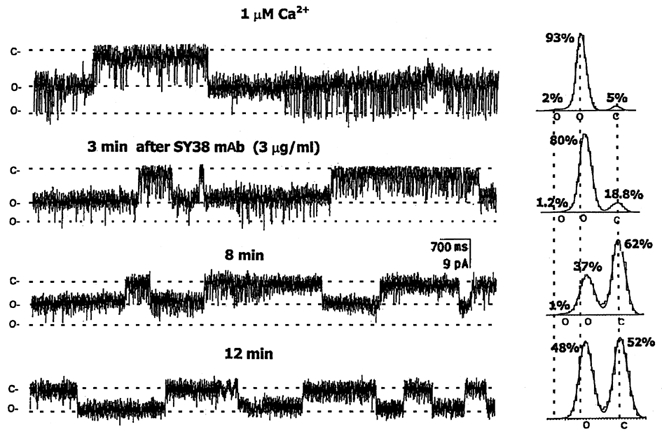
The cation channel shown here was obtained from fusion of purified NSG membranes with an artificial bilayer (see Methods). Holding potential was −5 mV for all of these traces. Two open (o) and one closed (c) states are indicated on the left of each trace and under the appropriate peaks of the all-points amplitude histograms summarizing each condition, shown on the right. The cis solution contained 150 mm KCl, 1.887 mm CaCl2, 2 mm EGTA and 10 mm Hepes, while the trans solution contained 50 mm KCl, 10 mm Hepes and 2 mm EGTA, both adjusted to pH 7.4. Channel currents were filtered at 600 Hz. Before addition of SY-38 mAb, channel openings were predominantly to the smaller conductance state, but with frequent openings to the largest conductance. After addition of 1 μg (3 μg ml−1) SY-38 mAb, almost all of the channel openings were to only the lower amplitude substate and the total open probability was gradually reduced over time. The different open (o) and closed (c) state levels are indicated by dotted lines both in the recordings and in the histograms, where they are aligned with the peaks of the control all-points amplitude histogram (at top). The corresponding mean current amplitudes were 0 pA for c, and −5.85 and −10.23 pA for the two o states. Note that the conductance (peak-to-peak on the histogram) of the channel is also reduced (by 20.3 %).
Figure 4. Ca2+ modulation of the NSG cation channel.
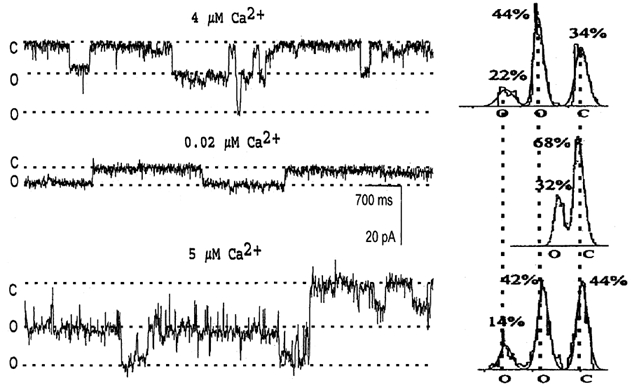
After the large conductance NSG cation channel was incorporated into a lipid bilayer, the Ca2+ concentration in cis solution was adjusted with EGTA (Lee et al. 1992). The holding potential was −10 mV for all traces. The cis solution contained 150 mm KCl and 10 mm Hepes, with indicated concentrations of free Ca2+, while the trans solution contained 50 mm KCl, 10 mm Hepes and 2 mm EGTA, both adjusted to pH 7.4. Channel currents were filtered at 200 Hz. Two open (o) and one closed (c) states are indicated on the left of each trace and under the appropriate peaks of the all-points amplitude histograms summarizing each condition, shown on the right. The top recording, in 4 μm Ca2+, shows that the NSG channel can open to either conductance level. After chelating the free Ca2+ to 0.02 μm, the larger conductance open state was eliminated, with channel openings only to the lower amplitude state, and the total open probability was reduced (middle trace). The conductance of the NSG channel was also decreased (by 41.2 %). After restoring the free Ca2+ to 5 μm, the open probability was increased and the larger conductance open state was recovered (bottom trace). The different open and closed state levels are indicated by dotted lines both in the recordings and in the histograms, as in Fig. 2. Corresponding currents: 0 pA for c, −9.42 pA for the first o state, and −16.76 pA for the second o state.
Figure 6. Ca2+vs. SY-38 effects on NSG channel activity.
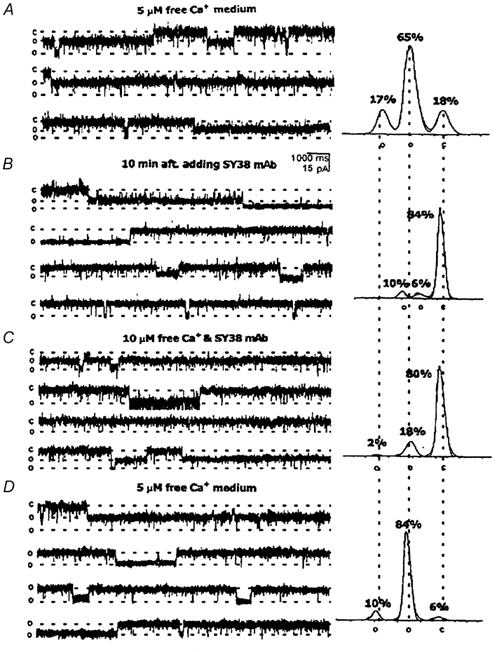
After incorporation of an NSG cation channel into an artificial bilayer, the holding potential was −10 mV for all of these traces. Two open (o) and one closed (c) states are indicated on the left of each trace and under the appropriate peaks of the all-points amplitude histograms summarizing each condition, shown on the right, as in Fig. 2. Corresponding currents: 0 pA for c, −9.23 pA for the first o state, and −16.92 pA for the second o state. A, the cis solution contained 150 mm KCl, 1.981 mm CaCl2, 2 mm EGTA and 10 mm Hepes, while the trans solution contained 50 mm KCl, 10 mm Hepes and 2 mm EGTA, both adjusted to pH 7.4. B, 10 min after addition of 1 μg SY-38 mAb, both the channel's conductance and open probability were reduced. There was no change, however, in its selectivity (data not shown). C, increasing the free [Ca2+] in the cis solution to 10 μm partially recovered the channel's conductance and open probability, even in the continued presence of the SY-38 mAb. D, washing the cis side with 5 μm free Ca2+ solution almost completely recovered the relative distribution of channel amplitudes and open probability. Currents were filtered at 400 Hz.
Figure 5. Ca2+ -regulated SY-38 effects on AVP release from permeabilized nerve terminals.
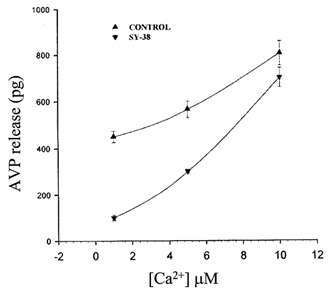
Plot of the relationship between the amount (pg) ± s.e.m. of arginine vasopressin (AVP) released from digitonin-permeabilized neurohypophysial nerve terminals in response to different concentrations of internal Ca2+ in the absence (▴) or presence (▾) of 3 μg ml−1 SY-38. There appears to be an interaction or ‘interference’ between the antibody and increasing concentrations of this second messenger. The maximal inhibition of AVP release by SY-38 mAb was observed at 1 μm free [Ca2+], but by increasing the Ca2+ concentration, less inhibition of AVP release by the SY-38 mAb was obtained.
Permeabilized nerve terminals and release
Rat neurohypophysial terminal endings were isolated and perfused as described previously (Cazalis et al. 1987a). The animals were anaesthetized with CO2 and then decapitated (as approved by UMMS animal protocol A-1031). Rats were used because of our extensive experiments with release from terminals of this species. Furthermore, peptide release mechanisms of bovine and rat neurohypophysial terminals appear identical (Cazalis et al. 1987b). The purified nerve endings were loaded onto 0.45 μm filters (Acrodisc LC 13, Gelman Sciences, Ann Arbor, MI, USA) and permeabilized as previously described (Cazalis et al. 1987b). Arginine vasopressin (AVP) secretion was triggered with different Ca2+ concentrations (see Results) buffered by EGTA. All the agents tested were added to the perfusion medium 10–20 min before the Ca2+ challenge. Monoclonal antibodies tested were against synapsin-1, SV-2, agrin, or against synaptophysin: p38 and SY-38 (Boehringer Mannheim). All antibodies were brought to the same concentration using Centriprep concentrators (Millipore, MA, USA). AVP release was measured using a radioimmunoassay as previously described (Cazalis et al. 1987a, b; Lee et al. 1992).
RESULTS
Neurosecretory granules (NSG) were purified from bovine neurohypophyses (see Methods). The purity (90–95 %) of the NSG preparation has been confirmed in previous biochemical and morphological studies (Nordmann et al. 1979; Nordmann & Cazalis, 1986; Lemos et al. 1989). The identity of the proteins included in the NSG preparation was further verified by immunoblotting (Fig. 1). SY-38, a monoclonal antibody (mAb) raised specifically against the C-terminal domain of synaptophysin, could detect strong signals from bands of the proper molecular weight from NSG, microvesicle (MV) or plasma membrane (PM) preparations. It is known that synaptophysin, an integral membrane protein on both the microvesicle and neurosecretory granule membrane, is incorporated into the plasma membrane after the vesicle membrane is fused with it during exocytosis (Navone et al. 1986). Control blots with only secondary antibody, however, could not detect any significant signal except for background, high molecular weight bands that appeared common to all preparations. Thus Fig. 1 demonstrates that the obtained NSG membrane preparation is derived from secretory granules and confirms that they contain synaptophysin (see also Lowe et al. 1988). The purified NSG membranes were then examined for the presence of ion channels using planar lipid bilayers (see Methods).
Figure 1. Immunoblot of neurohypophysial terminal membrane proteins.
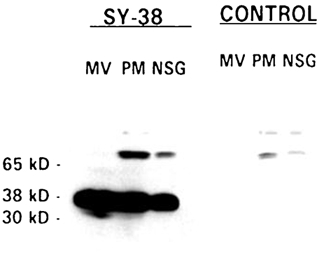
Proteins were isolated as described in Methods. Equal amounts (40 μg) of purified neurohypophysial secretory granule (NSG), microvesicle (MV) and plasma membrane (PM) proteins were loaded on a 10 % SDS-PAGE, transferred and probed with a monoclonal antibody raised against synaptophysin (SY-38). The relative molecular weights are indicated on the left. NSG, PM and MV contain synaptophysin (at 38 kDa). Three other lanes loaded in the same manner but without primary antibody (CONTROL) were treated only with the enhanced chemiluminescence (ECL) system.
Effects of SY-38 mAb on NSG cation channels
The non-specific cation channel found in purified NSG from the neurohypophysis has a large conductance (62–236 pS) and exhibits multiple open states (e.g. Fig. 2). In order to determine if the cation channel could be a known NSG integral membrane protein, a battery of antibodies against identified vesicular proteins were tested. First, we utilized SY-38, a monoclonal antibody (mAb) raised specifically against the C-terminal domain of synaptophysin (Wiedenman & Franke, 1985). For these initial studies (Fig. 2), the Ca2+ concentration in the cis (NSG side) well was kept at a constant level (1 μm) during the entire experiment. After the NSG multiple-conductance cation channel was incorporated into a lipid bilayer, SY-38 mAb was added to the cis solution at 3 μg ml−1. After 8 min of continuous agitation, the larger conductance openings of the NSG cation channel were gradually reduced and subsequently, after ∼12 min, completely eliminated. The all-points amplitude histogram (see right-hand side of Fig. 2) reveals that the time spent in the closed state is increased and that the two open states (i.e. the total open probability) are reduced. Finally, only two states are left, the closed and the smaller open conductance. On average (n = 11), the total open probability (NPo) is reduced by 60 ± 6.8 % after exposure to 1 μm SY-38 mAb. Significant (P <0.001), but smaller (38.2 ± 7.3 %) inhibition was seen in 5 μm Ca2+ (see Table 1). Moreover, the effects of SY-38 are specific to the larger cation channel because in patches that contained both types of NSG channels it has no significant effect on the NPo of the smaller anion channel (Table 1). Preliminary experiments (n = 2) revealed no effects by SY-38 when added to the trans side of the bilayer.
Table 1.
Effect of antibodies against synaptic proteins on NSG channel activity
| NSG anion channel open probabilities | ||
|---|---|---|
| Condition | Mean ± s.e.m. | n |
| Before SY-38 | 0.075 ± 0.005 | 3 |
| After SY-38 | 0.084 ± 0.006 | |
| NSG cation channel open probabilities | ||
|---|---|---|
| Condition | Mean ± s.e.m. | n |
| Before SY-38 | 0.71 ±0.05 | 15 |
| After SY-38 | 0.44 ±0.033* | |
| Before p38 | 0.558 ±0.11 | 4 |
| After p38 | 0.563 ± 0.11 | |
| Before SV-2 | 0.439 ±0.06 | 4 |
| After SV-2 | 0.433 ±0.057 | |
| Before IgG | 0.45 ± 0.05 | 3 |
| After IgG | 0.48 ± 0.06 | |
The open probabilities of neurohypophysial NSG anion (top) and cation (bottom) channels, recorded in the presence of 5μm Ca2+, before and after addition of monoclonal antibodies (at 3 μg ml−1) against synaptic membrane protein 2 (SV-2), or against agrin (IgG), or against two different epitopes (SY-38 and p38) of synaptophysin (see Methods) are shown. Analysis using Student's pairedt tests indicates that there is no statistically significant (P > 0.5) change in open probability (NPo) after addition of the SV-2, IgG or p38 antibodies.
However, there is a highly significant (P < 0.001) inhibition of NPo after addition of SY-38, but only on the NSG cation channel.
In contrast, another antibody (p38), also directed against synaptophysin but with a different targeting domain from that of SY-38, does not significantly (P > 0.5) inhibit the NSG large conductance cation channel (Table 1). Other antibodies, such as IgG (against agrin) and SV-2 (against synaptic vesicle protein 2), also have no significant (P > 0.5 for both) effects on the channel open probability (Table 1). These results demonstrate that the large cation channel appears to be a vesicular protein, with possible homology to synaptophysin.
Antibody effects on arginine vasopressin (AVP) release
In order to test for any role of the NSG cation channel in neuropeptide release, the effects of different monoclonal antibodies (mAb) on release were studied in permeabilized nerve terminals purified from rat neurohypophyses (see Methods). After permeabilization with 1 μm digitonin, the nerve terminals were then challenged with a 1 μm Ca2+ buffer with or without pre-incubation of antibodies at 3 μg ml−1 for 10 min. The collected elute was analysed by radioimmunoassay (see Methods). Figure 3 shows that of several monoclonal antibodies specific to different synaptic vesicle membrane or associated proteins, only SY-38 shows a significant (P < 0.001) inhibition (83 ± 8.1 %) of AVP secretion. Furthermore, freezing (FRZ) this antibody, which is known to reduce its effective titre (Wiedenmann & Franke, 1985), significantly reduces (to 51 ± 6.5 %) its ability to inhibit release. Other monoclonal antibodies, such as SV-2, and SYN-1 (against synapsin-1), as well as a control IgG antibody against a non-vesicular protein (agrin) all fail to show significant (P > 0.05) effects on AVP secretion (Fig. 3).
Figure 3. The effect of SY-38 mAb on AVP release from permeabilized nerve terminals.
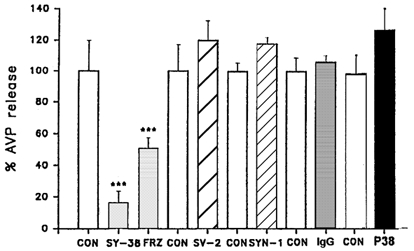
Nerve terminals isolated from rat neurohypophyses were permeabilized with digitonin, and incubated with either of two antibodies against synaptophysin (SY-38 and p38) or with antibodies against other synaptic proteins (SV-2, synaptic vesicle membrane protein 2; SYN-1, synapsin 1; IgG, agrin antibody). All were monoclonal antibodies pre-incubated for 10 min at a concentration of 3 μg ml−1. Arginine vasopressin (AVP) release (normalized to per cent of matched controls ± s.e.m.) was evoked with 1 μm free Ca2+. Only SY-38 showed a significant inhibition of evoked secretion. Furthermore, freezing (FRZ) this antibody, which is known to reduce its effective titre, significantly reduced its inhibitory effect. Controls (CON; with no added antibodies) are shown. Significant differences (***), compared with the respective controls, are at the P < 0.001 level (Student's t test).
Most importantly, a different synaptophysin antibody, p38, does not show any significant (P > 0.05) effect on AVP secretion (Fig. 3). Furthermore, SY-38 only acts intracellularly, since it has no effects on high (100 mm) K+-stimulated release (control = 1.03 ± 0.04 vs. SY-38 = 0.96 ± 0.06) from unpermeabilized neurohypophysial terminals. The data suggest a specific inhibitory, intracellular effect of SY-38 mAb on AVP release that is comparable to its effects on the NSG cation channel (see above).
Ca2+ regulation of NSG channel activity
To further characterize the SY-38-sensitive NSG channel, the Ca2+ concentration in the cis well was adjusted with EGTA. As previously reported (Lee et al. 1992) for the large NSG cation channel, multiple open states are observed in the presence of high (4 μm) Ca2+ (Fig. 4). At this Ca2+ concentration, the channel is predominantly in the subconductance open state, but frequently opens (NPo = 0.68 ± 0.03; n = 7) to its maximum conductance. The all-points amplitude histogram thus exhibits three peaks (right-hand side of Fig. 4). The percentages given at the top of each peak represent the time that the channel spent in each state. After reducing the Ca2+ concentration to 0.02 μm in the cis well with appropriate amounts of EGTA (Lee et al. 1992), the conductance and the open probability of the channel are reduced, and the largest open-state conductance of the channel is totally eliminated. The all-points amplitude histogram thus shows only two peaks (Fig. 4). The selectivity of the channel does not change (n = 3), however (see also Lee et al. 1992). After decreasing the Ca2+ concentration the open probability is reduced, on average (n = 4), to 39 ± 7.7 % of the control. Meanwhile, the channel conductance also becomes smaller (see Fig. 4). Upon returning the Ca2+ concentration to 5 μm in the cis well, the conductance and open probability of the channel are both increased. These results not only confirm our previous observation (Lee et al. 1992) that the NSG cation channel is regulated by Ca2+, but also further demonstrate that Ca2+ affects both the conductance and the open probability of the channel.
Ca2+-regulated effects of SY-38 mAb on AVP release
From the above experiments, it is clear that Ca2+ and SY-38 mAb have opposite effects on the activity of the NSG synaptophysin-like channel. In order to further assess any such possible interference between Ca2+ and SY-38 mAb, AVP secretion was measured from digitonin-permeabilized neurohypophysial nerve terminals in response to different concentrations of internal Ca2+ in the presence/absence of 3 μg ml−1 SY-38 mAb (Fig. 5). It is evident that the effect of SY-38 mAb on AVP secretion is countered by increasing cytosolic Ca2+. At the highest Ca2+ (10 μm) concentration tested here, which corresponds to maximal secretion (Lee et al. 1992), the block by SY-38 mAb of AVP release is almost absent in contrast to its potent inhibitory effect (77 %) in the presence of 1 μm Ca2+ (Fig. 5). These data further indicate a possible interference between Ca2+ and SY-38 mAb on the NSG cation channel and on subsequent release.
Ca2+-regulated effects of SY-38 mAb on NSG cation channel activity
In order to test the above hypothesis, the effect of SY-38 mAb on the NSG cation channel activity was tested with different Ca2+ concentrations. After incorporation of the NSG channel into the lipid bilayer, initially in the presence of 5 μm Ca2+, SY-38 mAb was added to the cis solution at 3 μg ml−1 and the solution was stirred for 5 min before acquisition of data. Figure 6 is a specific example of the general results (n = 5) obtained. Figure 6A shows that the NSG cation channel again has three states, one closed and two open, but mainly remains in the subconductance state. Ten minutes after adding SY-38 mAb, the open probability and conductance of the NSG cation channel are both reduced. Total open probability (NPo) is reduced by 80.2 % (Fig. 6B). However, upon increasing the Ca2+ concentration in the cis well from 5 to 10 μm, the inhibitory effects of SY-38 mAb on the NSG cation channel activity are reversed and the largest conductance state as well as the total open probability are partially recovered (Fig. 6C). Washing off SY-38 mAb with a 5 μm Ca2+ solution almost fully recovers both the conductance and open probability (Fig. 6D) of the NSG channel to control levels. The results indicate that Ca2+ and SY-38 mAb seem to interfere with each other's effects on the NSG cation channel activity.
Furthermore, in the presence of 10 μm free Ca2+, it was observed that the activity (NPo = 0.27 ± 0.05) of the cation channel does not show any significant change (P > 0.05; n = 3) upon addition of SY-38 mAb (NPo = 0.23 ± 0.03). This result indicates that the elevated Ca2+ can apparently diminish the antibody's inhibitory effects, possibly through interference at the C-terminal domain of synaptophysin. Again, these results are consistent with the release results (Fig. 5) in that SY-38 mAb could not significantly inhibit AVP secretion triggered by 10 μm Ca2+ from permeabilized nerve terminals.
DISCUSSION
Previous studies from our own and other laboratories have provided indirect evidence that channels located on the secretory granules/synaptic vesicles could function in release (Lee et al. 1992; Rahamimoff et al. 1988; Woodbury & Hall, 1988). Other studies have suggested that synaptophysin could form ‘fusion-pore’-like channels (Thomas et al. 1988; Lemos et al. 1989; Almers, 1990). The present results extend these findings by directly demonstrating that both the activity of individual Ca2+-regulated NSG channels and Ca2+-dependent AVP secretion from nerve endings can be modulated by a mAb specific to synaptophysin. Thus a synaptophysin-like NSG channel appears to be involved in Ca2+-dependent peptide release.
The non-specific cation NSG channel described here shows similarities to activity reconstituted using the purified vesicular integral membrane protein synaptophysin (Thomas et al. 1988), although we were unable to confirm those results using recombinant synaptophysin (from R. Leube, University of Mainz, Germany). SY-38 mAb changed the properties of channels reconstituted into bilayer membranes by incorporation of purified synaptophysin from rat brain. The elementary conductance of the synaptophysin channel appeared to decrease, as did the number of channel openings (Thomas et al. 1988). Those results are consistent with the findings of the present study, and we have further shown that SY-38 mAb reduces both the subconductances and open probability of the neurohypophysial NSG channel (Fig. 2) and that the inhibition by SY-38 could be counteracted by increasing the free Ca2+ concentration (Fig. 6).
Most importantly, SY-38 mAb significantly reduces AVP release from digitonin-permeabilized neurohypophysial nerve terminals (Fig. 3). It could be that, by directly binding to the C-terminus of synaptophysin, SY-38 mAb reduces ion permeation through the channel formed by synaptophysin or a similar NSG protein, thus interfering with its functional role in secretion. One possible artifact in this kind of release study is that the antibody could be sterically hindering the vesicle docking and fusing with the plasma membrane during exocytosis rather than through a direct interference with the function of synaptophysin. However, this possibility is not likely, since another synaptophysin antibody, p38, but one not directed against its C-terminus, does not inhibit AVP secretion (see Fig. 3). Furthermore, antibodies directed against other synaptic vesicle proteins, SV-2 and synapsin, also did not show any significant inhibition of AVP release. If steric hindrance was a problem, these should also affect AVP release, but we have only seen significant inhibition of AVP release by the SY-38 antibody, thus strongly suggesting that this synaptophysin-like NSG channel is involved in Ca2+-activated AVP secretion from neurohypophysial terminals.
Interestingly, the effects on AVP release by SY-38 are modulated by Ca2+. Increasing Ca2+ concentrations, in the range of 1 to 10 μm, correspondingly reduce the inhibitory effects of SY-38 on AVP secretion (Fig. 5). Synaptophysin has been reported to be a Ca2+ binding protein, and Ca2+ binding was reported to be on its C-terminal domain (Wiedenmann & Franke, 1985; Rehm et al. 1986). This possible binding site is located near the SY-38 mAb epitope (Leube et al. 1987; Knaus & Betz, 1990). SY-38 mAb was raised against the C-terminal domain, amino acids 269–289, of synaptophysin (Leube et al. 1987; Knaus & Betz, 1990). More recently, however, no Ca2+ binding was observed for synaptophysin (Brose et al. 1992), casting doubts on the capability of Ca2+ to bind directly to synaptophysin.
From our experimental results, Ca2+ increases both the conductance and the open probability of the NSG cation channel. Possible explanations for Ca2+ regulation of the activity of the synaptophysin-like channel are either a direct role of Ca2+ or indirect effects through other molecules in the neurosecretory granule associated with the cation channel. For example, we cannot exclude the possibility that other molecules, such as synaptotagmin, a known Ca2+ sensor (Brose et al. 1992) implicated in regulated transmitter release (Geppert et al. 1994), interact with synaptophysin-like or associated proteins (Edelmann et al. 1995) and thus provide Ca2+ sensitivity to the NSG channel.
Ca2+ can increase both the conductance levels and open probability of the synaptophysin-like NSG channel (Fig. 4), while SY-38 has just the opposite effects (Fig. 2). Concurrently, AVP release from the neurohypophysial nerve endings can be strongly inhibited by SY-38 mAb (Fig. 3), and this effect is also highly sensitive to Ca2+ (Fig. 5). Taken together, the data presented here show interference between the Ca2+ and SY-38 mAb effects on both the NSG cation channel (Fig. 6 and Table 1) and AVP secretion (Fig. 5).
SY-38 mAb has also been found to block reconstituted Ca2+-mediated glutamate secretion from oocytes (Alder et al. 1992a) and to decrease synaptic transmission at neuromuscular junctions after injection into presynaptic neurons (Alder et al. 1992b). On the other hand, SY-38 did not appear to inhibit exocytosis from PC-12 cells (Elferink et al. 1993). However, the antibody used in the latter study had a much lower (∼1 %) effective titre (L. Elferink, personal communication) than the one used here. Furthermore, the p38 mAb, which was ineffective on peptide release from neurohypophysial terminals (Fig. 3), is also ineffective on dopamine release from PC-12 cells (G. Dayanithi, unpublished observations). On the other hand, in mice with genetically disrupted synaptophysin, there are no observable morphological changes in brain structures (Eshkind & Leube, 1995) or in synaptic transmission (McMahon et al. 1996). One plausible explanation is a complementary role by other unknown molecules, such as synaptoporin, synaptogyrin or granulophysin, for the loss of synaptophysin during development (Yin et al. 1997).
In conclusion, the results presented here show that the large, multiple-conductance, Ca2+-regulated cation channel in neurohypophysial NSG has similarities to the vesicular membrane protein synaptophysin. Most importantly, this NSG channel appears to be directly involved in the mechanism of Ca2+-dependent peptide release from these CNS terminals.
Acknowledgments
Preliminary experiments were conducted by Dr Cheol J. Lee, who is now Head of the Department of Cardiothoracic Surgery, Inha General Hospital, Inha University College of Medicine, 3309–327 Taepyungdong, Seongnam City, Korea and Dr Orit Braha, now at the Department of Biochemistry, University of Texas A&M, College Station, TX, USA. We would like to thank the following for generous gifts of specific antibodies used in this study: Dr Kathy Buckley, Harvard Medical School (SV-2 and SYN-1), Dr R. Jahn, Yale University (p38), Dr J. Fallon, Brown University (antibody to agrin), and Dr John Bicknell, Babraham Institute (antisera to AVP). This work was supported by grants from the Muscular Dystrophy Foundation and NIH (NS24970) to J. R. L.
REFERENCES
- Alder J, Lu B, Valtorta F, Greengard P, Poo MM. Calcium-dependent transmitter secretion reconstituted in Xenopus oocytes: requirement for synaptophysin. Science. 1992a;257:657–661. doi: 10.1126/science.1353905. [DOI] [PubMed] [Google Scholar]
- Alder J, Xie ZP, Valtorta F, Greengard P, Poo MM. Antibodies to synaptophysin interfere with transmitter secretion at neuromuscular synapses. Neuron. 1992b;9:759–768. doi: 10.1016/0896-6273(92)90038-f. [DOI] [PubMed] [Google Scholar]
- Almers W. Exocytosis. Annual Review of Physiology. 1990;52:607–624. doi: 10.1146/annurev.ph.52.030190.003135. [DOI] [PubMed] [Google Scholar]
- Breckenridge LJ, Almers W. Currents through the fusion pore that forms during exocytosis of a secretory vesicle. Nature. 1987;328:814–817. doi: 10.1038/328814a0. [DOI] [PubMed] [Google Scholar]
- Brose N, Petrenko AG, Sudhof TC, Jahn R. Synaptotagmin: a Ca2+ sensor on the synaptic vesicle surface. Science. 1992;256:1021–1025. doi: 10.1126/science.1589771. [DOI] [PubMed] [Google Scholar]
- Brunger AT. Structural insights into the molecular mechanism of Ca2+-dependent exocytosis. Current Opinion in Neurobiology. 2000;10:293–302. doi: 10.1016/s0959-4388(00)00098-2. [DOI] [PubMed] [Google Scholar]
- Cazalis M, Dayanithi G, Nordmann JJ. Requirements for hormone release from permeabilized nerve endings isolated from the rat neurohypophysis. Journal of Physiology. 1987a;390:71–91. doi: 10.1113/jphysiol.1987.sp016687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cazalis M, Dayanithi G, Nordmann JJ. Hormone release from isolated nerve endings of the rat neurohypophysis. Journal of Physiology. 1987b;390:55–70. doi: 10.1113/jphysiol.1987.sp016686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandler DE, Heuser JE. Arrest of membrane fusion in mast cells by quick-freezing. Journal of Cell Biology. 1980;86:666–674. doi: 10.1083/jcb.86.2.666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Camilli P, Jahn R. Pathways to regulated exocytosis in neurons. Annual Review of Physiology. 1990;52:625–645. doi: 10.1146/annurev.ph.52.030190.003205. [DOI] [PubMed] [Google Scholar]
- DeRiemer SA, Martin R, Rahamimoff R, Sakmann B, Stadler H. Ion Channels in Synaptic Vesicles. New York: Plenum Press; 1987. pp. 407–414. [Google Scholar]
- Douglas WW, Poisner AM. Stimulus-secretion coupling in a neurosecretory organ: the role of calcium in the release of vasopressin from neurohypophysis. Journal of Physiology. 1964;172:1–18. doi: 10.1113/jphysiol.1964.sp007399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edelmann L, Hanson PI, Chapman ER, Jahn R. Synaptobrevin binding to synaptophysin: a potential mechanism for controlling the exocytotic fusion machine. EMBO Journal. 1995;14:224–231. doi: 10.1002/j.1460-2075.1995.tb06995.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elferink LA, Peterson MR, Scheller RH. A role for synaptotagmin (p65) in regulated exocytosis. Cell. 1993;72:153–159. doi: 10.1016/0092-8674(93)90059-y. [DOI] [PubMed] [Google Scholar]
- Eshkind LG, Leube RE. Mice lacking synaptophysin reproduce and form typical synaptic vesicles. Cell and Tissue Research. 1995;282:423–433. doi: 10.1007/BF00318874. [DOI] [PubMed] [Google Scholar]
- Finkelstein A, Zimmerberg J, Cohen F. Osmotic swelling of vesicles: its role in the fusion of vesicles with planar phospholipid bilayer membrane and its possible role in exocytosis. Annual Review of Physiology. 1986;48:163–174. doi: 10.1146/annurev.ph.48.030186.001115. [DOI] [PubMed] [Google Scholar]
- Geppert M, Goda Y, Hammer RE, Li C, Rosahl TW, Stevens CF, Sudhof TC. Synaptotagmin I: A major Ca2+ sensor for transmitter release at a central synapse. Cell. 1994;79:717–727. doi: 10.1016/0092-8674(94)90556-8. [DOI] [PubMed] [Google Scholar]
- Hamill OP, Marty A, Neher E, Sakmann B, Sigworth FJ. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflügers Archiv. 1981;391:85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Kelly ML, Woodbury DJ. Ion channels from cholinergic synaptic vesicle fragments reconstituted into lipid bilayers. Biophysical Journal. 1996;70:2593–2599. doi: 10.1016/S0006-3495(96)79830-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knaus P, Betz H. Mapping of a dominant immunogenic region of synaptophysin, a major membrane protein of synaptic vesicles. FEBS Letters. 1990;261:358–360. doi: 10.1016/0014-5793(90)80591-6. [DOI] [PubMed] [Google Scholar]
- Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lah JJ, Burry RW. Neuronotypic differentiation results in reduced levels and altered distribution of synaptophysin in PC12 cells. Journal of Neurochemistry. 1993;60:503–512. doi: 10.1111/j.1471-4159.1993.tb03178.x. [DOI] [PubMed] [Google Scholar]
- Lee CL, Dayanithi G, Nordmann JJ, Lemos JR. Possible role during exocytosis of a Ca2+-activated channel in neurohypophysial granules. Neuron. 1992;8:335–342. doi: 10.1016/0896-6273(92)90299-s. [DOI] [PubMed] [Google Scholar]
- Lemos JR, Nordmann JJ. Ionic channels and hormone release from peptidergic nerve terminals. Journal of Experimental Biology. 1986;124:53–72. doi: 10.1242/jeb.124.1.53. [DOI] [PubMed] [Google Scholar]
- Lemos JR, Ocorr KA, Nordmann JJ. Possible role for ionic channels in neurosecretory granules of the rat neurohypophysis. In: Armstrong C, Oxford G, editors. Secretion and its Control. New York, NY, USA: The Rockefeller University Press; 1989. pp. 334–347. [Google Scholar]
- Leube RE, Kaiser P, Seiter A, Zimbelmann R, Franke WW. Synaptophysin: molecular organization and mRNA expression as determined from cloned cDNA. EMBO Journal. 1987;6:3261–3268. doi: 10.1002/j.1460-2075.1987.tb02644.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowe AW, Madeddu L, Kelly RB. Endocrine secretory granules and neuronal synaptic vesicles have three integral membrane proteins in common. Journal of Cell Biology. 1988;106:51–59. doi: 10.1083/jcb.106.1.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMahon HT, Bolshakov VY, Janz R, Hammer RE, Siegelbaum SA, Sudhof TC. Synaptophysin, a major synaptic vesicle protein, is not essential for neurotransmitter release. Proceedings of the National Academy of Sciences of the USA. 1996;93:4760–4764. doi: 10.1073/pnas.93.10.4760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navone F, Jahn R, Digioia G, Stukenbrok H, Greengard P, Decamilli P. Protein p38: an integral membrane protein specific for small vesicles of neurons and neuroendocrine cells. Journal of Cell Biology. 1986;103:2511–2527. doi: 10.1083/jcb.103.6.2511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nordmann JJ, Cazalis M. Characterization of newly formed and aged granules in the neurohypophysis. Journal of Neurochemistry. 1986;47:1534–1543. doi: 10.1111/j.1471-4159.1986.tb00791.x. [DOI] [PubMed] [Google Scholar]
- Nordmann JJ, Louis F, Morris SJ. Purification of two structurally and morphologically distinct populations of rat neurohypophysial secretory granules. Neuroscience. 1979;4:1367–1375. doi: 10.1016/0306-4522(79)90164-7. [DOI] [PubMed] [Google Scholar]
- Obendorf D, Schwarzebrunner U, Fischer-Colbrie R, Laslop A, Winkler H. In adrenal medulla synaptophysin (protein p38) is present in chromaffin granules and in a special vesicle population. Journal of Neurochemistry. 1988;51:1573–1580. doi: 10.1111/j.1471-4159.1988.tb01127.x. [DOI] [PubMed] [Google Scholar]
- Rahamimoff R, Deriemer SA, Ginsburg S, Kaiserman I, Sakmann B, Shapira R, Stadler H, Yakir N. Ionic channels in synaptic vesicles: are they involved in transmitter release? Quarterly Journal of Experimental Physiology. 1989;74:1019–1031. doi: 10.1113/expphysiol.1989.sp003330. [DOI] [PubMed] [Google Scholar]
- Rahamimoff R, Fernandez JM. Pre- and postfusion regulation of transmitter release. Neuron. 1997;18:17–27. doi: 10.1016/s0896-6273(01)80043-x. [DOI] [PubMed] [Google Scholar]
- Rehm H, Wiedenmann B, Betz H. Molecular characterization of synaptophysin, a major calcium-binding protein of the synaptic vesicle membrane. EMBO Journal. 1986;6:535–541. doi: 10.1002/j.1460-2075.1986.tb04243.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothman JE. Mechanisms of intracellular protein transport. Nature. 1994;372:55–63. doi: 10.1038/372055a0. [DOI] [PubMed] [Google Scholar]
- Sato M, Inoue K, Kasai M. Ion channel on synaptic vesicle membranes studied by planar lipid bilayer method. Biophysical Journal. 1992;63:1500–1505. doi: 10.1016/S0006-3495(92)81731-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanley EF, Ehrenstein G. A model for exocytosis based on the opening of calcium-activated potassium channels in vesicles. Life Sciences. 1985;37:1985–1995. doi: 10.1016/0024-3205(85)90029-3. [DOI] [PubMed] [Google Scholar]
- Stanley EF, Ehrenstein G, Russell GT. Evidence for anion channels in secretory vesicles. Neuroscience. 1988;25:1035–1039. doi: 10.1016/0306-4522(88)90056-5. [DOI] [PubMed] [Google Scholar]
- Sudhof TC. The synaptic vesicle cycle: a cascade of protein-protein interactions. Nature. 1995;375:645–653. doi: 10.1038/375645a0. [DOI] [PubMed] [Google Scholar]
- Thomas L, Hartung K, Langosch E, Rehm H, Bamverg E, Betz H. Identification of synaptophysin as a hexameic channel protein of the synaptic vesicle membrane. Science. 1988;242:1050–1052. doi: 10.1126/science.2461586. [DOI] [PubMed] [Google Scholar]
- Towbin H, Staehelin T, Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proceedings of the National Academy of Sciences of the USA. 1979;76:4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiedenmann B, Franke WW. Identification and localization of synaptophysin, an integral membrane glycoprotein of M 38,000 characteristic of presynaptic vesicles. Cell. 1985;4:1017–1028. doi: 10.1016/s0092-8674(85)80082-9. [DOI] [PubMed] [Google Scholar]
- Woodbury DJ. Evaluation of the evidence for ion channels in synaptic vesicles (review) Journal of Membrane Biology. 1995;12:165–171. doi: 10.3109/09687689509027504. [DOI] [PubMed] [Google Scholar]
- Woodbury DJ, Hall JE. The role of channels in the fusion of vesicles with a planar bilayer. Biophysical Journal. 1988;54:1053–1063. doi: 10.1016/S0006-3495(88)83042-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin Y, Harrison R, Mullikin-Kilpatrick D, Lemos JR, Melloni R. Knock-out of synaptophysin in PC-12 cells. Society for Neuroscience Abstracts. 1997;23:1169. [Google Scholar]
- Yin Y, Lee CJ, Dayanithi G, Nordmann JJ, Lemos JR. Synaptophysin antibody specifically affects both a neurosecretory granule channel and peptide release. Biophysical Journal. 1995;68:A395. [Google Scholar]


