Abstract
Sensory inputs from the whiskers reach the primary somatosensory thalamus through the medial lemniscus tract. The main role of the thalamus is to relay these sensory inputs to the neocortex according to the regulations dictated by behavioural state. Intracellular recordings in urethane-anaesthetized rats show that whisker stimulation evokes EPSP-IPSP sequences in thalamic neurons. Both EPSPs and IPSPs depress with repetitive whisker stimulation at frequencies above 2 Hz. Single-unit recordings reveal that during quiescent states thalamic responses to repetitive whisker stimulation are suppressed at frequencies above 2 Hz, so that only low-frequency sensory stimulation is relayed to the neocortex. In contrast, during activated states, induced by stimulation of the brainstem reticular formation or application of acetylcholine in the thalamus, high-frequency whisker stimulation at up to 40 Hz is relayed to the neocortex. Sensory suppression is caused by the depression of lemniscal EPSPs in relatively hyperpolarized thalamocortical neurons. Sensory suppression is abolished during activated states because thalamocortical neurons depolarize and the depressed lemniscal EPSPs are able to reach firing threshold. Strong IPSPs may also contribute to sensory suppression by hyperpolarizing thalamocortical neurons, but during activated states IPSPs are strongly reduced altogether. The results indicate that the synaptic depression of lemniscal EPSPs and the level of depolarization of thalamocortical neurons work together in thalamic primary sensory pathways to suppress high-frequency sensory inputs during non-activated (quiescent) states while permitting the faithful relay of high-frequency sensory information during activated (processing) states.
All sensory information, excluding olfaction, must pass through the thalamus before reaching the neocortex. The main function of the thalamus is to control the flow of sensory information to the neocortex during different behavioural states (Singer, 1977; Sherman & Koch, 1986; Steriade & McCarley, 1990; Steriade, 1993; McCormick & Bal, 1994; Sherman & Guillery, 1996; Steriade et al. 1997). One important aspect of sensory information flow that is particularly sensitive to behavioural state is the relay of high-frequency inputs. Poggio & Mountcastle (1963) demonstrated that the capacity for frequency-following of tactile stimuli is dramatically different for thalamic cells in the waking as compared with the anaesthetized monkey. In anaesthetized rats some studies have shown that thalamocortical neurons can follow whisker stimulation at up to 12 Hz (Simons, 1985; Simons & Carvell, 1989; Hartings & Simons, 1998), while other studies using similar methods report strong frequency-dependent depression at frequencies above 5 Hz (Diamond et al. 1992; Lee et al. 1994b) or 2 Hz (Ahissar et al. 2000). Taken together, these results suggest that during quiescent states the thalamic relay of high-frequency sensory inputs is impeded, but allowed during activated states. This may be due to the effects of neuromodulators released in the thalamus during behavioural activation. Several neuromodulatory systems from the brainstem project to the ventrobasal thalamus. In particular, cholinergic fibres innervate the rodent ventrobasal thalamus to different degrees depending on the species and nucleus (Hallanger et al. 1990; Bennett-Clarke et al. 1999). Cholinergic cell populations discharge vigorously during activated states (Steriade et al. 1990) and thus the levels of acetylcholine increase in the thalamus during activated states (Williams et al. 1994a).
The present study used the rodent whisker system to test the hypothesis that the relay of high-frequency sensory inputs through the thalamus is allowed during activated states by the actions of neuromodulators. The rodent whisker thalamus receives its primary sensory input from the principal trigeminal nucleus through the medial lemniscus tract. These lemniscal terminals form glutamatergic synaptic contacts with the soma and proximal dendrites of neurons in the ventroposterior medial (VPM) thalamus (Spacek & Lieberman, 1974; Feldman & Kruger, 1980; Chiaia et al. 1991a; Liu et al. 1995; Williams et al. 1994b; Diamond, 1995; Veinante & Deschenes, 1999). Electrophysiological and pharmacological studies in vivo have shown that stimulation of the lemniscal pathway produces a glutamatergic short latency EPSP followed by a longer latency GABergic IPSP (Andersen et al. 1966; Iwamura & Inubushi, 1974; Tsumoto & Nakamura, 1974; Baldissera & Margnelli, 1979; Salt, 1987; Salt & Eaton, 1990; Chiaia et al. 1991b; Mishima, 1992). In rodent VPM the long latency IPSP is generated by feedback inhibition from the nucleus reticularis thalami (nRt), because there are no inhibitory interneurons within the ventrobasal thalamus of rodents (Spacek & Lieberman, 1974; Barbaresi et al. 1986; Harris & Hendrickson, 1987; Ohara & Lieberman, 1993). Many studies in rodents have extensively characterized receptive field properties of thalamic neurons using whisker stimulation and extracellular recordings (Waite, 1973; Shosaku, 1985; Rhoades et al. 1987; Ito, 1988; Simons & Carvell, 1989; Armstrong-James & Callahan, 1991; Chiaia et al. 1991b; Diamond et al. 1992; Nicolelis et al. 1993; Lee et al. 1994a; Hartings & Simons, 1998, 2000; Friedberg et al. 1999). Recent work in vitro using slices of adult rodent ventrobasal thalamus has investigated the properties of lemniscal EPSPs in the ventrobasal thalamus (Castro-Alamancos, 2002). Stimulation of lemniscal fibres produces large amplitude, highly secure unitary EPSPs, which depress when stimulated at frequencies above 2 Hz. Also, acetylcholine, which is an important thalamic modulator, was found not to have any effect on the efficacy of lemniscal EPSPs, but depolarized thalamocortical neurons and strongly depressed feedback IPSPs and corticothalamic EPSPs. This work in vitro suggests that during activated states, when neuromodulators are released in the thalamus, the relay of high-frequency sensory inputs is facilitated mainly by the depolarization of thalamocortical neurons and the depression of feedback inhibition. The present study tested this hypothesis in vivo by recording the responses to whisker stimulation of single neurons in the VPM of anaesthetized rodents. To induce thalamic activation, typical of the waking state, stimulation was delivered to the brainstem reticular formation or acetylcholine was infused into the thalamus.
METHODS
Adult Sprague-Dawley rats (300 g) were anaesthetized with urethane (1.5 g kg−1 intraperitoneally) and placed in a stereotaxic frame. All skin incisions and frame contacts with the skin were injected with lidocaine (2 %). A unilateral craniotomy extended over a large area of the parietal cortex. Small incisions were made in the dura as necessary and the cortical surface was covered with artificial cerebrospinal fluid (ACSF) containing (mm): NaCl (126), KCl (3), NaH2PO4 (1.25), NaHCO3 (26), MgSO4 7H2O (1.3), dextrose (10), CaCl2 2H2O (2.5). Body temperature was automatically maintained constant with a heating pad. The level of anaesthesia was monitored with field recordings and limb-withdrawal reflexes and kept constant at about stage III/3 using supplemental doses of urethane (Friedberg et al. 1999). Extracellular recordings were made using electrodes (5-10 MΩ) filled with ACSF; single units and field potentials were recorded simultaneously via this same electrode. Intracellular recordings were performed using sharp electrodes (60-100 MΩ) filled with 3 m potassium acetate. Intracellular and extracellular recordings were obtained from the VPM at the following co-ordinates (Paxinos & Watson, 1982) from bregma: posterior = 3.5, lateral = 3, depth = 5-6. Co-ordinates for the brainstem reticular formation (i.e. laterodorsal tegmentum) bipolar stimulating electrode (250 μm diameter, concentric; FHC, ME) were posterior = 9, lateral = 0.7, depth = 5-6. The sensory stimulation consisted of deflecting several (2-4) caudal whiskers simultaneously. The selected whiskers were inserted into a glass micropipette (1 mm diameter) that was glued to the membrane of a miniature speaker (3.5 cm diameter; Electrosonic, ON, Canada). Application of a 1 ms square current pulse to the speaker deflected the micropipette and the whiskers inside (≈300 μm). To apply drugs into the VPM thalamus during recordings a microdialysis probe (250 μm diameter, 2 mm long) was placed at the following co-ordinates: posterior = 3, lateral = 2, depth = 4-6 as previously described (Castro-Alamancos, 2000). ACSF was continuously infused through the probe at 2-4 μl min−1. Drugs were dissolved in the ACSF at doses about 10 times higher than used in vitro. Acetylcholine was applied at 50-100 mm, bicuculline methobromide (BMI) at 400 μm, CGP35348 at 10 mm and scopolamine at 1 mm. The spike sorting and analysis were accomplished using Experimenters Workbench and Origin software. At the end of the experiments the animals were given an overdose of sodium pentobarbital. The Animal Care Committee of McGill University approved protocols for all experiments.
RESULTS
Thalamic sensory responses
Intracellular recordings were made from VPM neurons (n = 32) in vivo. The resting membrane potential of the cells was −64 ± 5 mV (mean ± s.d.). The input resistance measured at resting potential was 22.4 ± 5 MΩ (50 ms current pulse of −0.3 nA) and the action potential amplitude was 68 ± 4 mV. In the cases illustrated in this section the cells were hyperpolarized to produce only subthreshold responses. Whisker deflections produced two types of subthreshold responses in VPM neurons: short latency (3-6 ms), large amplitude and fast rising EPSPs followed by an IPSP (Fig. 1B) or a long latency IPSP alone (Fig. 1C). The short latency, large amplitude EPSPs depressed with repetitive stimulation (Fig. 1A). Sensory-evoked IPSPs also depressed with repetitive stimulation (Fig. 1B and C). IPSP depression also occurs with electrical stimulation in the cat thalamus (Steriade & Timofeev, 1997). Since VPM is devoid of inhibitory interneurons, the source of these IPSPs is feedback inhibition from the nRt. The observation that some cells responded only with IPSPs indicates that feedback inhibition does not only affect the thalamocortical neurons that discharge to the sensory stimulus but also adjacent neurons whose excitatory receptive field is not being stimulated. Interestingly, there is anatomical evidence for such a mechanism of lateral inhibition in rats (Pinault & Deschenes, 1998). Depression of sensory-evoked EPSPs was observed for frequencies above 2 Hz (Fig. 2A). These results in vivo are similar to those obtained previously in vitro; isolated lemniscal EPSPs in vitro consist of fast rising, large amplitude unitary events that also depress in response to repetitive stimulation above 2 Hz (Castro-Alamancos, 2002). In some cases, the large amplitude EPSPs evoked by low-frequency whisker stimulation could be composed of two large amplitude unitary events and thus unitary events summed to form the sensory EPSP. This is illustrated in Fig. 2B, where spontaneously occurring unitary events and the sensory-evoked EPSP are overlaid. The large amplitude spontaneous events presumably correspond to the spontaneous firing of a single lemniscal fibre. In this case the sensory-evoked response seems to be composed of the sum of two unitary events (Fig. 2B). This type of response was found in several neurons and it may reflect the onset and offset of the 1 ms whisker stimulus or the activation of two lemniscal fibres with slightly different conduction velocities.
Figure 1. Intracellular correlates of whisker-evoked responses in VPM neurons.
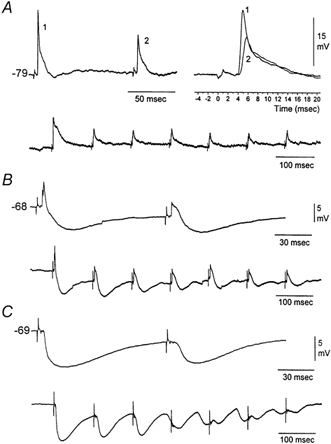
A, example of a typical EPSP induced by whisker stimulation and its depression by a second stimulus delivered 100 ms later. Notice the fast rise and large amplitude of the evoked response and the strong synaptic depression of the second response (right panel). Below is the effect of seven sensory stimuli delivered at 10 Hz. Notice that the degree of synaptic depression is constant after the third stimulus. The neuron was hyperpolarized to about −80 mV using −0.5 nA current injection. B, sensory-evoked EPSPs are followed by IPSPs, which themselves depress with repetition. Notice that despite the strong depression of IPSPs the EPSP also remains depressed, indicating that EPSP depression was not due to strong inhibition. The neuron was at resting potential. C, sensory-evoked responses in some VPM neurons consist of IPSPs alone. Notice the robust IPSPs and their frequency-dependent depression. Intracellular recordings were performed using K+-filled pipettes. The AC-coupled square pulses on the traces mark the whisker stimuli.
Figure 2. Frequency-dependent depression of whisker-evoked EPSPs in VPM neurons.
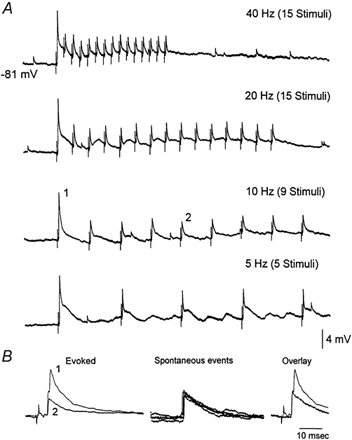
A, application of whisker stimulation at 40, 20, 10 and 5 Hz depresses thalamic EPSPs. Traces are the average of 3-5 trials. The cell was hyperpolarized to −81 mV with current injection (-0.5 nA). B, overlaid are the first (1) and fifth (2) evoked responses for the 10 Hz stimulus train shown in A (left panel). From the same recording four spontaneously occurring large amplitude events are shown, which presumably correspond to spontaneous activity in lemniscal afferents (middle panel). An overlay between the sensory-evoked response to the first stimulus and a spontaneous event reveals a close fit, which suggests that the evoked response is composed of two spontaneous events.
The present study deals with the relay of high-frequency sensory inputs through the lemniscal pathway. Considering that EPSPs evoked in VPM neurons by repetitive whisker stimulation above 2 Hz depress, it was expected that thalamic firing to repetitive whisker stimulation at these frequencies would also depress. As shown below, single-unit extracellular recordings revealed a strong sensory suppression of VPM responses to whisker stimulation above 2 Hz. The following experiments explored the possibility that the level of activation could affect sensory suppression.
Effect of brainstem reticular formation stimulation
Extracellular recordings were made from single VPM neurons. In the present study, single-unit (n = 54 units) responses evoked by whisker stimulation consisted of a single spike evoked reliably (> 80 % probability of firing) at short latency (between 3 and 6 ms) by low-frequency whisker stimulation (0.1 Hz). Repetitive whisker stimulation at frequencies above 2 Hz caused depression of the thalamic sensory response. Thus, the spike only occurred in response to the first stimulus at frequencies above 2 Hz. Figure 3 shows the response of a typical single unit in the VPM to whisker deflections delivered at 10 Hz and at 2 Hz (sum of 14 trials). The neuron responds with short latency and high reliability to the low-frequency sensory stimuli, but very poorly to the whisker deflections delivered at 10 Hz (Fig. 3B). Note that when the spike fails to occur in the second through fourth whisker stimuli delivered at 10 Hz, a small event can be observed which corresponds to the depressed large amplitude and fast rising EPSPs described in the previous section using intracellular recordings; these extracellular events have been termed ‘prepotentials’ (Gottschaldt et al. 1983). Thus, thalamic sensory responses are suppressed for stimuli delivered at frequencies above 2 Hz. The relay of sensory inputs through the thalamus was compared between quiescent states and activated states. To test the effect of thalamic activation on the relay of sensory inputs a stimulating electrode was implanted in the brainstem reticular formation (RF). The RF stimulation (100 Hz, 1 s, 100-200 μA) caused thalamic activation, abolishing the slow oscillatory activity observed in the thalamic field potential and increasing the firing rate of the thalamic units (Fig. 3A). Interestingly, thalamic activation caused by RF stimulation eliminated the thalamic suppression of high-frequency sensory stimuli (Fig. 3C). A spectrum analysis revealed the effects of RF stimulation on low-frequency and high-frequency thalamic sensory responses (Fig. 4). The probability of firing during the short latency window of 3-6 ms after a whisker deflection was calculated for 10-20 stimulus trials at each frequency (n = 5) before and after RF stimulation. After RF stimulation the baseline unit activity increased due to the spontaneous firing caused by the depolarization. Consequently, there was a slight increase (8.7 %) in the firing probability at 3-6 ms latency evoked by low-frequency sensory stimuli. In addition, the firing probability for high-frequency sensory stimuli was strongly enhanced so that sensory suppression was no longer apparent. The short latency firing probability after a whisker deflection at 10 Hz was 11.5 % under control conditions, while after RF stimulation the probability was 100 %. This means that sensory suppression at 10 Hz was completely eliminated by RF stimulation. The spectrum analysis in Fig. 4B shows that sensory suppression was completely abolished for frequencies of 10 Hz and lower (P < 0.0001). The amount of sensory suppression at 20 Hz was also significantly reduced, so that the probability of firing after a whisker deflection changed from 9.3 % during control conditions to 84 % during thalamic activation. This was also the case for stimuli delivered at 40 Hz, so that the probability of firing after a whisker deflection changed from 0 % during control conditions to 31 % during thalamic activation (P < 0.0001). It is important to note that by stimulus 4 the probability of firing had reached a steady state because there was no significant difference between stimulus 4 and stimulus 15 for the frequencies tested (not shown). During control conditions the relay of sensory inputs through the thalamus is effective for stimuli delivered up to 2 Hz, while during thalamic activation the relay of sensory stimuli is effective for stimuli delivered up to at least 20 Hz and significantly enhanced for stimuli at 40 Hz. Thus, during non-activated states sensory suppression works like a low-pass filter set at 2 Hz, and during activated states this filter is opened by one order of magnitude.
Figure 3. Effect of thalamic activation induced by RF stimulation on whisker-evoked responses in VPM neurons.
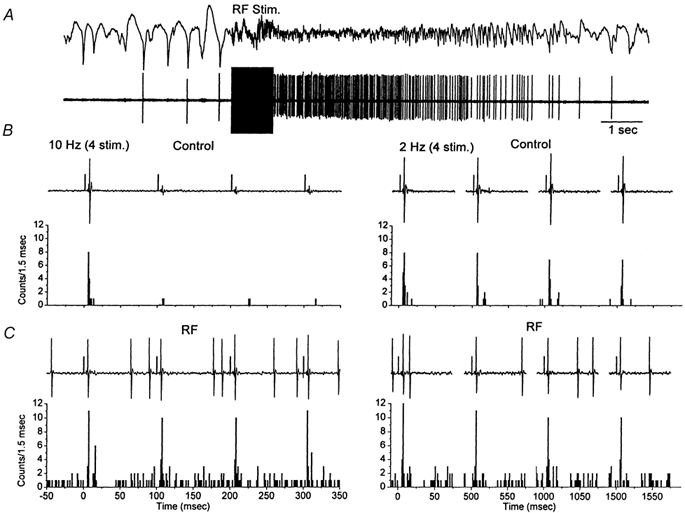
A, RF stimulation (100 Hz, 1 s) induces thalamic activation which was monitored by field potential (above) and single-unit recordings (below) using the same electrode placed in the VPM thalamus. B, the effect of four whisker stimuli (1 ms pulses) delivered at 20 Hz and at 2 Hz is shown as a raw trace for one trial (above) and as the binned sum of 14 trials (below). C, the response to the same whisker stimulation as in B is displayed during thalamic activation induced by RF stimulation. The 1 ms positive square pulses on the raw traces mark the time of the sensory stimulus. Notice that the x-axis for the 2 Hz stimulation is not continuous.
Figure 4. Spectrum analysis of whisker-evoked responses during quiescent and activated states in VPM neurons.
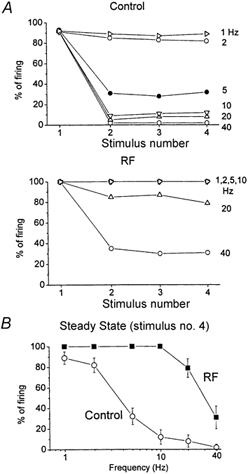
A, the probability of single-unit firing for 10-20 stimulus trials during the 3-6 ms time window after the whisker stimulation is displayed. The average response to four stimuli delivered at 1, 2, 5, 10, 20 and 40 Hz is shown for five single units during quiescent conditions (control) and during thalamic activation caused by RF stimulation. B, a spectrum analysis was performed during control conditions and during thalamic activation using the fourth response of the stimulus train. The average firing probability to the fourth stimulus for each frequency is displayed. The data correspond to the mean ± s.d. of five experiments.
Effect of acetylcholine
Reticular formation stimulation is a non-specific way to produce thalamic activation because very likely several neuromodulators are simultaneously released in the thalamus. One of the main neurotransmitters released in the thalamus by fibres from the brainstem reticular formation during behavioural activation is acetylcholine (Williams et al. 1994a). Thus, the following experiments tested if application of acetylcholine into the VPM would mimic the effect of RF stimulation with regards to the relay of high-frequency whisker stimulation. Acetylcholine was infused into the VPM using microdialysis. Single units (n = 7) were recorded in VPM and whisker stimulation consisted of 15 stimuli delivered at different frequencies (1, 2, 5, 10, 20 and 40 Hz; inter-trial interval was 10 s) each for 30 trials before, during and after application of acetylcholine. In every neuron tested, application of acetylcholine eliminated sensory suppression, so that VPM neurons could follow high-frequency whisker stimulation at up to 40 Hz. Figure 5 shows the responses of a single neuron to whisker stimulation delivered at different frequencies before, during and after the application of acetylcholine. Note that under control conditions the neuron responds only to the first whisker stimulus at 40 and 20 Hz, and depresses strongly at 10 Hz. In contrast, during application of acetylcholine the neuron responds to almost every one of the 15 whisker stimuli delivered at 40 and 20 Hz, and the depression at 10 Hz is significantly reduced. Moreover, these effects of acetylcholine are reversible. Figure 6A shows population data corresponding to the average of seven neurons before and during the application of acetylcholine. Figure 6B shows a spectrum analysis of the probability of firing at short latency (between 3 and 7 ms) after the 15th whisker stimulus. Acetylcholine enhanced the response to high-frequency whisker stimulation much more than the response to low frequencies. For instance, the probability of firing at 1 Hz was 85 % before and 93 % after acetylcholine; the efficacy of relay increased by 8 %. In contrast, the probability of firing at 40 Hz was 4 % before and 70 % after acetylcholine, the efficacy of relay increased by 66 %. This resulted in a strong enhancement in the responsiveness to stimuli arriving at high frequencies. In conclusion, thalamic activation induced by acetylcholine or by reticular formation stimulation eliminates sensory suppression in the thalamus and allows the relay of high-frequency sensory inputs.
Figure 5. Effect of acetylcholine on whisker-evoked responses in a VPM neuron.
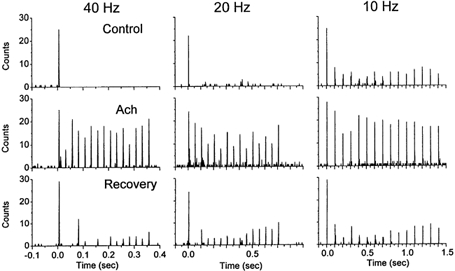
Single-unit response to whisker stimulation displayed as the binned (2 ms bins) sum of 30 trials each consisting of 15 whisker stimuli delivered at 40 Hz (left panels), 20 Hz (middle) and 10 Hz (right), before (upper panels), during (middle) and after (lower) application of acetylcholine to VPM. Notice that the single unit responds to whisker stimulation at up to 40 Hz during acetylcholine application, but not before or after. The first stimulus is delivered at time zero.
Figure 6. Population data on the effect of acetylcholine on whisker-evoked responses in VPM neurons.
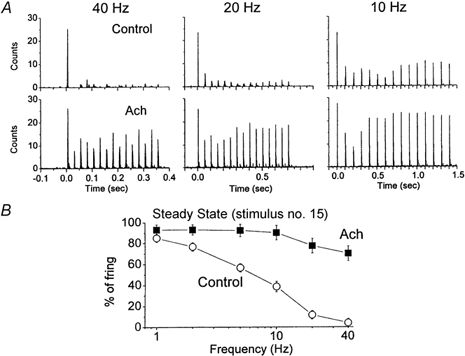
A, mean counts of seven single units displayed as the binned (2 ms bins) sum of 30 trials each consisting of 15 whisker stimuli delivered at 40 Hz (left panels), 20 Hz (middle) and 10 Hz (right), before (upper panels) and during (lower) application of acetylcholine to VPM. B, spectrum analysis of whisker-evoked responses in VPM neurons before and during the application of acetylcholine. The average firing probability to 30 trials during the 3-7 ms time window after the 15th stimulus is displayed for each frequency. The data correspond to the mean ± s.d. of seven experiments.
Effect of postsynaptic depolarization and disinhibition
Both RF stimulation or acetylcholine application can produce complex effects in the thalamus. Studies in vivo and in vitro have shown that acetylcholine depolarizes thalamocortical neurons by an increase in apparent input resistance due to a reduction in a leak potassium current (McCormick & Prince, 1987; Curro et al. 1991; Deschenes & Hu, 1990; McCormick, 1992), and that acetylcholine suppresses inhibition by hyperpolarizing nRt neurons (Ben Ari et al. 1976; McCormick & Prince, 1986; Curro et al. 1992). Also, a recent study in vitro showed that acetylcholine depresses IPSPs directly in the VPM (Castro-Alamancos, 2002). The next experiments explored the relative importance (i.e. necessity) of both of these effects of acetylcholine (i.e. depolarization and disinhibition) on the relay of high-frequency sensory inputs. To test if depolarization alone was sufficient to eliminate sensory suppression in the thalamus, intracellular recordings were performed and the responses of thalamocortical neurons to whisker stimulation were compared at different membrane potentials (Fig. 7). At resting membrane potentials thalamocortical neurons displayed sensory suppression; they would only produce an action potential in response to the first whisker stimulus of a high-frequency train. However, postsynaptic depolarization by ≈10 mV from rest using current injection (Fig. 7) was sufficient to eliminate the suppression of thalamic firing to high-frequency sensory inputs (n = 4 neurons). This was the case if IPSPs evoked by the whisker stimulation were relatively small. However, if the IPSPs were robust they could strongly hyperpolarize thalamocortical neurons and impede the relay of sensory inputs despite of postsynaptic depolarization (Fig. 8). In these cases the relay of sensory inputs was blocked during the initial stimuli before IPSPs depressed (Fig. 8B). As IPSPs were depressed by activity the relay of sensory stimuli was regained if the neuron was depolarized. These results demonstrate two effects. On the one hand, they show that under circumstances in which feedback inhibition is very strong, postsynaptic depolarization alone is not sufficient to overcome thalamic sensory suppression. On the other hand, they also point to the importance of depolarization because, as shown in Fig. 8B, if the cell is not depolarized (0 DC trace) thalamic relay is effective only for the first sensory stimulus. This is despite the fact that for stimuli 10-15, IPSPs are completely abolished and the membrane potential is at the same level as for the first stimulus. The lack of relay is a consequence of the low amplitude of the depressed lemniscal EPSPs, which are unable to reach threshold to produce an action potential; note that stimuli 10-15 are able to relay the sensory stimulus when slightly depolarized (+0.4 DC).
Figure 7. Postsynaptic depolarization can eliminate thalamic sensory suppression.
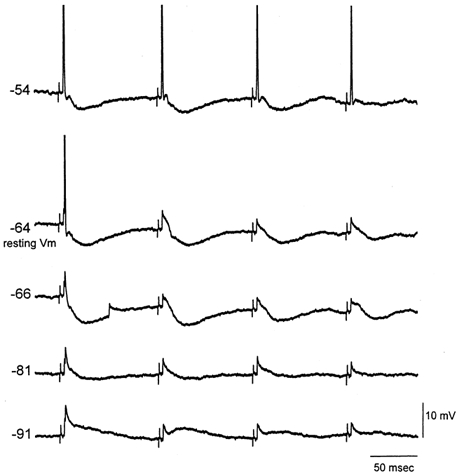
Intracellular recording from a VPM neuron responding to whisker stimulation. When the neuron is at resting membrane potential (around−65 mV) and four stimuli are applied at 10 Hz, the neuron only responds with an action potential to the first stimulus. However, when the neuron is depolarized by 10 mV the whisker stimulation is effective at driving the neuron at 10 Hz. Shown are also the sensory responses at membrane potentials hyperpolarized using current injection. Applied DC curents were: 0.4, 0, −0.2, −0.7 and −1.4 nA (top to bottom traces). Action potentials are truncated.
Figure 8. Robust whisker-evoked IPSPs can restrict the ability of postsynaptic depolarization to eliminate thalamic sensory suppression.
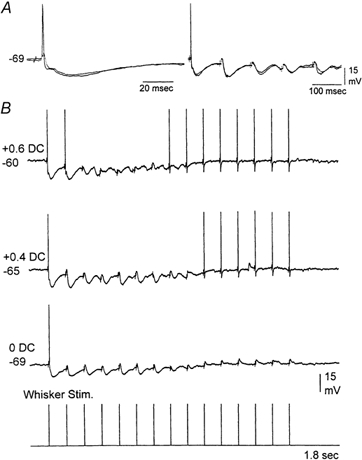
A, intracellular recording from a VPM neuron that responds to whisker stimulation with an EPSP followed by a robust IPSP. Both the EPSPs and IPSPs depress with repetition at 10 Hz (right panel). B, when sensory-evoked IPSPs are very robust, postsynaptic depolarization is not effective in eliminating thalamic suppression during the initial stimulus trials when inhibition has not yet depressed. Notice that at resting potential (0 DC) the cell only produces an action potential to the first stimulus, and despite strong depression of IPSPs there is no relay of sensory inputs during the latter stimuli. As the neuron is depolarized from resting the latter stimuli are able to relay the sensory input, but the initial stimuli are not capable because of the strong sensory-evoked IPSPs. Whisker stimulation consisted of 15 stimuli at 10 Hz. Action potentials are truncated.
As described above, during activated states neuromodulators are released in the thalamus and produce effects that are more complex than the simple depolarization of VPM neurons. Thus, during activated states high-frequency sensory relay might be aided by the suppression of sensory-evoked IPSPs. Indeed, previous work has shown that suppressing inhibition in the ventrobasal thalamus enhances the relay of sensory inputs (Gottschaldt et al. 1983; Lee et al. 1994b; Hartings & Simons, 2000). We recorded intracellularly from neurons (n = 3) that responded to whisker stimulation only with IPSPs and applied RF stimulation. As described above, whisker stimulation in these neurons produced large IPSPs that depressed with repetition (Fig. 9). RF stimulation resulted in the activation of these neurons which depolarized them by 9 ± 2 mV. In addition, RF stimulation produced the complete abolition of the IPSPs evoked by whisker stimulation (Fig. 9). Thus, during thalamic activation the relay of high-frequency sensory inputs is not only facilitated by depolarizing thalamocortical neurons, but also by suppressing feedback inhibition.
Figure 9. Whisker-evoked IPSPs are suppressed by thalamic activation.
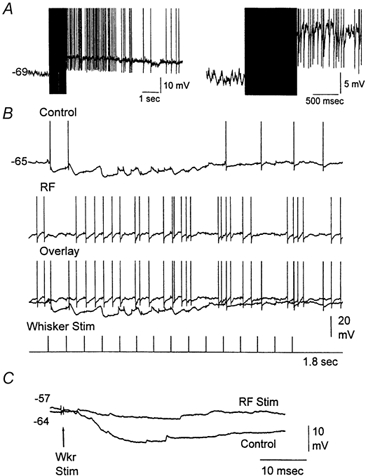
A, example of an intracellular recording from a VPM neuron showing the effect of stimulating the brainstem reticular formation (100 Hz, 1 s). The right trace is a close-up. B, the neuron responded to whisker stimulation (15 stimuli, 10 Hz) with robust IPSPs that hyperpolarized the cell during the initial sensory stimuli (control). Thalamic activation produced by stimulating the brainstem reticular formation blocked the sensory-evoked IPSPs in the VPM neuron (RF). Action potentials are truncated. C, IPSP evoked by a single whisker stimulus delivered before (Control) and after RF stimulation. Both traces are overlaid for comparison. The membrane potential values for the beginning of each trace (before the whisker stimulus) are displayed.
Finally, in order to determine if the suppression of feedback inhibition that occurs during activated states in the VPM is sufficient on its own to enhance the relay of high-frequency sensory inputs through the thalamus, single units (n = 3) were recorded before and during application of GABAA and GABAB receptor antagonists (BMI + CGP35348). As shown in Fig. 10, disinhibition was indeed effective in facilitating the relay of high-frequency sensory stimuli, much like acetylcholine. However, disinhibition in the thalamus changes thalamic excitability (Castro-Alamancos, 1999). In order to test if disinhibition was causing an enhanced relay of high-frequency stimuli by revealing or unmasking the effect of neuromodulators such as acetylcholine that would depolarize thalamic neurons, a muscarinic antagonist (scopolamine) was infused into the thalamus in the presence of disinhibition. Interestingly, this restored sensory suppression at frequencies above 2 Hz (Fig. 10) in the presence of disinhibition. Thus, disinhibition can enhance the relay of high-frequency sensory inputs and eliminate sensory suppression. The action of scopolamine suggests that disinhibition may uncover a tonic cholinergic action, which mediates the enhanced sensory transmission. However an alternative possibility is that scopolamine could re-establish sensory suppression through a non-specific action that reduces neuronal excitability. A specific effect of scopolamine on muscarinic receptors is more likely since application of 1 mm scopolamine with microdialysis results in the diffusion of a small concentration of the drug (5-10 %) to the brain surrounding the microdialysis probe due to concentration gradients. The dose of scopolamine that diffuses (50-100 μm) is commonly used to block specifically muscarinic receptors. Finally, it is worth noting that regardless of the specificity of scopolamine the factor that controls the transmission of high-frequency sensory inputs is the level of depolarization of thalamocortical neurons, which is imposed by several neuromodulators in addition to acetylcholine. Thus, these results indicate that thalamocortical neurons become excited by disinhibition because the balance is tilted towards the depolarizing effects of acetylcholine or other modulators and this mediates the enhanced relay of high-frequency stimuli produced by disinhibition.
Figure 10. Effect of disinhibition and subsequent muscarinic receptor block on whisker-evoked responses in VPM neurons.
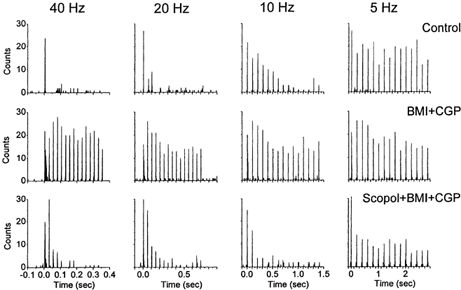
Single-unit response in VPM to whisker stimulation displayed as the binned (2 ms bins) sum of 30 trials each consisting of 15 whisker stimuli delivered at 40 Hz (left panels), 20 Hz (middle) and 10 Hz (right), before (upper panels) and during the application of BMI and CGP35348 (middle) and during the application of BMI, CGP35348 and scopolamine (lower) to VPM. Notice that the single unit responds to whisker stimulation at up to 40 Hz during disinhibition produced by BMI and CGP35348, but that this effect is eliminated by the muscarinic antagonist scopolamine.
DISCUSSION
The present experiments reveal that the relay of sensory activity through the thalamus at frequencies above 2 Hz requires sufficient postsynaptic depolarization to overcome synaptic depression. During quiescent states in vivo sensory inputs are low-pass filtered at 2 Hz. However, during thalamic activation as a consequence of postsynaptic depolarization and reduction of feedback inhibition this low-pass filter is opened to at least 40 Hz. In conclusion, lemniscal synaptic depression and postsynaptic depolarization combine to gate the flow of sensory inputs to the cortex as behavioural contingencies demand.
Rodent lemniscal synapses have been described at the electron microscope level as large terminals with numerous closely spaced synaptic contacts (Spacek & Lieberman, 1974; Williams et al. 1994b). Lemniscal synapses studied in vitro produce large amplitude unitary EPSPs that depress at frequencies above 2 Hz (Castro-Alamancos, 2002). Intracellular recordings revealed that whisker-evoked EPSPs depress in VPM in vivo at frequencies above 2 Hz. This depression results in the suppression of single-unit responses in VPM evoked by whisker stimulation at frequencies above 2 Hz. However, as shown here application of acetylcholine to VPM or RF stimulation eliminates sensory suppression, so that whisker stimulation can be followed by VPM neurons at up to 40 Hz. This effect cannot be attributed to a direct action of acetylcholine on the efficacy of lemniscal synapses, because lemniscal EPSPs are insensitive to acetylcholine; previous work has shown that application of acetylcholine does not affect the efficacy of lemniscal synapses in vitro (Castro-Alamancos, 2002). In contrast, acetylcholine and RF stimulation produce two other effects that serve to explain why the relay of high-frequency sensory inputs is possible: both depolarize VPM neurons and depress IPSPs.
In an attempt to dissociate these two factors (depolarization and disinhibition) we performed several experiments. First, direct depolarization of VPM neurons by ≈10 mV from rest using intracellular current injection was sufficient to eliminate sensory suppression.
Second, when feedback IPSPs are strong sensory, suppression may still be present despite postsynaptic depolarization of VPM neurons. This is illustrated in Fig. 8; in this case when the cell is depolarized (middle trace, +0.4 DC) it is able to relay the sensory stimulus but only when inhibition is abolished by frequency (i.e. stimulus 10 and higher). Thus, feedback inhibition can regulate sensory suppression and interfere with the effects of depolarization.
Third, the results also indicate that disinhibition alone is not sufficient to allow sensory relay at high frequencies. The evidence for this assertion is derived from two observations. First, IPSPs depress with frequency and thus the relay of sensory inputs could be facilitated at resting membrane potentials when IPSPs are depressed by frequency. However, as clearly shown in Fig. 8 (lower trace, 0 DC), this is not the case if the neuron is not sufficiently depolarized. Despite the absence of feedback inhibition after whisker stimulus 10, and at the same membrane potential as the first stimulus, the whisker stimulation is unable to drive the neuron to produce action potentials. This failure of sensory relay at resting membrane potential and in the absence of IPSPs is clearly attributable to the synaptic depression of the lemniscal EPSP. Second, further evidence that disinhibition is not enough on its own to eliminate sensory suppression was provided by blocking GABAA and GABAB receptors. This manipulation initially resulted in the elimination of sensory suppression, but a muscarinic antagonist blocked this effect. Therefore, the elimination of sensory suppression by disinhibition had been a consequence of the unmasking of an excitatory cholinergic effect and not of disinhibition per se.
Frequency-dependent depression at lemniscal synapses serves as a low-pass filter to block the flow of sensory information during quiescent states. As behavioural demands engage a sensory modality, brainstem afferents release modulators, such as acetylcholine, in the thalamus which depolarize thalamocortical neurons and depress feedback inhibition. Consequently thalamic sensory suppression is eliminated due to postsynaptic depolarization, which brings the depressed lemniscal EPSPs close to firing threshold, and also due to the block of feedback inhibition, which eliminates the hyperpolarization caused by IPSPs.
These findings serve to explain previous work by Poggio and Mountcastle in monkeys (Poggio & Mountcastle, 1963) and recent work in rodents (Fanselow & Nicolelis, 1999) showing that the capacity for frequency-following of tactile stimuli is dramatically different for thalamic cells during waking as compared with quiescent states. In anaesthetized rats, previous studies agree that the first-order neurons in the trigeminal nucleus do not display suppression to high-frequency sensory inputs (Simons, 1985; Ahissar et al. 2000). In contrast, studies disagree about the degree of sensory suppression observed in the thalamus. For instance, some studies have shown that thalamocortical neurons can follow whisker stimulation up to 12 Hz (Simons & Carvell, 1989; Hartings & Simons, 1998), while other studies report strong frequency-dependent depression at frequencies above 5 Hz (Diamond et al. 1992; Lee et al. 1994b) or 2 Hz (Ahissar et al. 2000). The present study shows that these discrepancies can be explained by the membrane potential of thalamocortical neurons and the degree of feedback inhibition, which are probably different between studies because of the use of different methods (e.g. types of anaesthesia).
Thalamic activation is known to enhance sensory transmission (Steriade & Demetrescu, 1960; Singer, 1977; Eysel et al. 1986; Francesconi et al. 1988; Pare et al. 1990; Steriade & McCarley, 1990; Humphrey & Saul, 1992; Uhlrich et al. 1995; Warren & Jones, 1994). The present study further demonstrates that an important effect of thalamic activation is to facilitate the relay of high-frequency sensory inputs to the neocortex. This occurs by means of the interplay between synaptic depression of sensory afferents and postsynaptic depolarization, which serves as a gating mechanism of sensory information flow.
Acknowledgments
This work was supported by the Medical Research Council of Canada, Natural Sciences and Engineering Council of Canada, Fonds de la Recherche en Sante du Quebec, Canadian Foundation for Innovation and Savoy Foundation. M. Maalouf is thanked for advice on the whisker stimulator device.
REFERENCES
- Ahissar E, Sosnik R, Haidarliu S. Transformation from temporal to rate coding in a somatosensory thalamocortical pathway. Nature. 2000;406:302–306. doi: 10.1038/35018568. [DOI] [PubMed] [Google Scholar]
- Andersen P, Andersson SA, Landgren S. Some properties of the thalamic relay cells in the spino-cervico-lemniscal path. Acta Physiologica Scandinavica. 1966;68:72–83. [Google Scholar]
- Armstrong-James M, Callahan CA. Thalamo-cortical processing of vibrissal information in the rat. II. spatiotemporal convergence in the thalamic ventroposterior medial nucleus (VPm) and its relevance to generation of receptive fields of S1 cortical ‘barrel’ neurones. Journal of Comparative Neurology. 1991;303:211–224. doi: 10.1002/cne.903030204. [DOI] [PubMed] [Google Scholar]
- Baldissera F, Margnelli M. Disynaptic IPSPs of lemniscal origin in relay-cells of thalamic ventrobasal nuclear complex of the cat. Brain Research. 1979;163:171–175. doi: 10.1016/0006-8993(79)90162-8. [DOI] [PubMed] [Google Scholar]
- Barbaresi P, Spreafico R, Frassoni C, Rustioni A. GABAergic neurons are present in the dorsal column nuclei but not in the ventroposterior complex of rats. Brain Research. 1986;382:305–326. doi: 10.1016/0006-8993(86)91340-5. [DOI] [PubMed] [Google Scholar]
- Ben Ari Y, Dingledine R, Kanazawa I, Kelly JS. Inhibitory effects of acetylcholine on neurones in the feline nucleus reticularis thalami. Journal of Physiology. 1976;261:647–671. doi: 10.1113/jphysiol.1976.sp011579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett-Clarke CA, Chiaia NL, Rhoades RW. Differential expression of acetylcholinesterase in the brainstem, ventrobasal thalamus and primary somatosensory cortex of perinatal rats, mice, and hamsters. Somatosensory and Motor Research. 1999;16:269–279. doi: 10.1080/08990229970339. [DOI] [PubMed] [Google Scholar]
- Castro-Alamancos MA. Neocortical synchronized oscillations induced by thalamic disinhibition in vivo. Journal Neuroscience. 1999;19, RC27:1–7. doi: 10.1523/JNEUROSCI.19-18-j0005.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castro-Alamancos MA. Origin of synchronized oscillations induced by neocortical disinhibition in vivo. Journal of Neuroscience. 2000;20:9195–9206. doi: 10.1523/JNEUROSCI.20-24-09195.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castro-Alamancos MA. Properties of primary sensory (lemniscal) synapses in the ventrobasal thalamus and the relay of high-frequency sensory inputs. Journal of Neurophysiology. 2002;87:946–953. doi: 10.1152/jn.00426.2001. [DOI] [PubMed] [Google Scholar]
- Chiaia NL, Rhoades RW, Bennett-Clarke CA, Fish SE, Killackey HP. Thalamic processing of vibrissal information in the rat. I. Afferent input to the medial ventral posterior and posterior nuclei. Journal of Comparative Neurology. 1991a;314:201–216. doi: 10.1002/cne.903140202. [DOI] [PubMed] [Google Scholar]
- Chiaia NL, Rhoades RW, Fish SE, Killackey HP. Thalamic processing of vibrissal information in the rat: II. Morphological and functional properties of medial ventral posterior nucleus and posterior nucleus neurons. Journal of Comparative Neurology. 1991b;314:217–236. doi: 10.1002/cne.903140203. [DOI] [PubMed] [Google Scholar]
- Curro DR, Pare D, Steriade M. Short-lasting nicotinic and long-lasting muscarinic depolarizing responses of thalamocortical neurons to stimulation of mesopontine cholinergic nuclei. Journal of Neurophysiology. 1991;65:393–406. doi: 10.1152/jn.1991.65.3.393. [DOI] [PubMed] [Google Scholar]
- Curro DR, Pare D, Steriade M. Various types of inhibitory postsynaptic potentials in anterior thalamic cells are differentially altered by stimulation of laterodorsal tegmental cholinergic nucleus. Neuroscience. 1992;47:279–289. doi: 10.1016/0306-4522(92)90244-v. [DOI] [PubMed] [Google Scholar]
- Deschenes M, Hu B. Membrane resistance increase induced in thalamic neurons by stimulation of brainstem cholinergic afferents. Brain Research. 1990;513:339–342. doi: 10.1016/0006-8993(90)90478-t. [DOI] [PubMed] [Google Scholar]
- Diamond ME. Somatosensory thalamus of the rat. In: Jones EG, Diamond IT, editors. Cerebral Cortex, The Barrel Cortex of Rodents. Vol. 11. New York: Plenum; 1995. pp. 189–220. [Google Scholar]
- Diamond ME, Armstrong-James M, Ebner FF. Somatic sensory responses in the rostral sector of the posterior group (POm) and in the ventral posterior medial nucleus (VPM) of the rat thalamus. Journal of Comparative Neurology. 1992;318:462–476. doi: 10.1002/cne.903180410. [DOI] [PubMed] [Google Scholar]
- Eysel UT, Pape HC, Van Schayck R. Excitatory and differential disinhibitory actions of acetylcholine in the lateral geniculate nucleus of the cat. Journal of Physiology. 1986;370:233–254. doi: 10.1113/jphysiol.1986.sp015932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fanselow EE, Nicolelis MA. Behavioral modulation of tactile responses in the rat somatosensory system. Journal of Neuroscience. 1999;19:7603–7616. doi: 10.1523/JNEUROSCI.19-17-07603.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldman SG, Kruger L. An axonal transport study of the ascending projection of medial lemniscal neurons in the rat. Journal of Comparative Neurology. 1980;192:427–454. doi: 10.1002/cne.901920305. [DOI] [PubMed] [Google Scholar]
- Francesconi W, Muller CM, Singer W. Cholinergic mechanisms in the reticular control of transmission in the cat lateral geniculate nucleus. Journal of Neurophysiology. 1988;59:1690–1718. doi: 10.1152/jn.1988.59.6.1690. [DOI] [PubMed] [Google Scholar]
- Friedberg MH, Lee SM, Ebner FF. Modulation of receptive field properties of thalamic somatosensory neurons by the depth of anesthesia. Journal of Neurophysiology. 1999;81:2243–2252. doi: 10.1152/jn.1999.81.5.2243. [DOI] [PubMed] [Google Scholar]
- Gottschaldt K-M, Vahle-Hinz C, Hicks TP. Electrophysiological and micropharmacological studies on mechanisms of input-output transformation in single neurons of the somatosensory thalamus. In: Macchi G, Rustioni A, Spreafico R, editors. Somatosensory Integration in the Thalamus. A Re-evaluation Based on New Methodological Approaches. Amsterdam: Elsevier; 1983. pp. 199–216. [Google Scholar]
- Hallanger AE, Price SD, Lee HJ, Steininger TL, Wainer BH. Ultrastructure of cholinergic synaptic terminals in the thalamic anteroventral, ventroposterior, and dorsal lateral geniculate nuclei of the rat. Journal of Comparative Neurology. 1990;299:482–492. doi: 10.1002/cne.902990408. [DOI] [PubMed] [Google Scholar]
- Harris RM, Hendrickson AE. Local circuit neurons in the rat ventrobasal thalamus – a GABA immunocytochemical study. Neuroscience. 1987;21:229–236. doi: 10.1016/0306-4522(87)90335-6. [DOI] [PubMed] [Google Scholar]
- Hartings JA, Simons DJ. Thalamic relay of afferent responses to 1- to 12-Hz whisker stimulation in the rat. Journal of Neurophysiology. 1998;80:1016–1019. doi: 10.1152/jn.1998.80.2.1016. [DOI] [PubMed] [Google Scholar]
- Hartings JA, Simons DJ. Inhibition suppresses transmission of tonic vibrissa-evoked activity in the rat ventrobasal thalamus. Journal of Neuroscience. 2000;20:RC100. doi: 10.1523/JNEUROSCI.20-19-j0001.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humphrey AL, Saul AB. Action of brain stem reticular afferents on lagged and nonlagged cells in the cat lateral geniculate nucleus. Journal of Neurophysiology. 1992;68:673–691. doi: 10.1152/jn.1992.68.3.673. [DOI] [PubMed] [Google Scholar]
- Ito M. Response properties and topography of vibrissa-sensitive VPM neurons in the rat. Journal of Neurophysiology. 1988;60:1181–1197. doi: 10.1152/jn.1988.60.4.1181. [DOI] [PubMed] [Google Scholar]
- Iwamura Y, Inubushi S. Functional organization of receptive fields in thalamic ventrobasal neurons examined by neuronal response to iterative electrical stimulation of skin. Journal of Neurophysiology. 1974;37:920–926. doi: 10.1152/jn.1974.37.5.920. [DOI] [PubMed] [Google Scholar]
- Lee SM, Friedberg MH, Ebner FF. The role of GABA-mediated inhibition in the rat ventral posterior medial thalamus. I. Assessment of receptive field changes following thalamic reticular nucleus lesions. Journal of Neurophysiology. 1994a;71:1702–1715. doi: 10.1152/jn.1994.71.5.1702. [DOI] [PubMed] [Google Scholar]
- Lee SM, Friedberg MH, Ebner FF. The role of GABA-mediated inhibition in the rat ventral posterior medial thalamus. II. Differential effects of GABAA and GABAB receptor antagonists on responses of VPM neurons. Journal of Neurophysiology. 1994b;71:1716–1726. doi: 10.1152/jn.1994.71.5.1716. [DOI] [PubMed] [Google Scholar]
- Liu XB, Honda CN, Jones EG. Distribution of four types of synapse on physiologically identified relay neurons in the ventral posterior thalamic nucleus of the cat. Journal of Comparative Neurology. 1995;352:69–91. doi: 10.1002/cne.903520106. [DOI] [PubMed] [Google Scholar]
- McCormick DA. Cellular mechanisms underlying cholinergic and noradrenergic modulation of neuronal firing mode in the cat and guinea pig dorsal lateral geniculate nucleus. Journal of Neuroscience. 1992;12:278–289. doi: 10.1523/JNEUROSCI.12-01-00278.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormick DA, Bal T. Sensory gating mechanisms of the thalamus. Current Opinion in Neurobiology. 1994;4:550–556. doi: 10.1016/0959-4388(94)90056-6. [DOI] [PubMed] [Google Scholar]
- McCormick DA, Prince DA. Acetylcholine induces burst firing in thalamic reticular neurones by activating a potassium conductance. Nature. 1986;319:402–405. doi: 10.1038/319402a0. [DOI] [PubMed] [Google Scholar]
- McCormick DA, Prince DA. Actions of acetylcholine in the guinea-pig and cat medial and lateral geniculate nuclei, in vitro. Journal of Physiology. 1987;392:147–165. doi: 10.1113/jphysiol.1987.sp016774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishima K. Facilitatory and inhibitory processes in the thalamic ventrobasal nucleus of the rat. Japanese Journal of Physiology. 1992;42:193–210. doi: 10.2170/jjphysiol.42.193. [DOI] [PubMed] [Google Scholar]
- Nicolelis MA, Lin RC, Woodward DJ, Chapin JK. Dynamic and distributed properties of many-neuron ensembles in the ventral posterior medial thalamus of awake rats. Proceedings of the National Academy of Sciences of the USA. 1993;90:2212–2216. doi: 10.1073/pnas.90.6.2212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohara PT, Lieberman AR. Some aspects of the synaptic circuitry underlying inhibition in the ventrobasal thalamus. Journal of Neurocytology. 1993;22:815–825. doi: 10.1007/BF01181326. [DOI] [PubMed] [Google Scholar]
- Pare D, Steriade M, Deschenes M, Bouhassira D. Prolonged enhancement of anterior thalamic synaptic responsiveness by stimulation of a brain-stem cholinergic group. Journal of Neuroscience. 1990;10:20–33. doi: 10.1523/JNEUROSCI.10-01-00020.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. New York: Academic Press; 1982. [Google Scholar]
- Pinault D, Deschenes M. Anatomical evidence for a mechanism of lateral inhibition in the rat thalamus. European Journal of Neuroscience. 1998;10:3462–3469. doi: 10.1046/j.1460-9568.1998.00362.x. [DOI] [PubMed] [Google Scholar]
- Poggio GF, Mountcastle VB. The functional properties of ventrobasal thalamic neurons studied in unanesthetized monkeys. Journal of Neurophysiology. 1963;26:775–806. doi: 10.1152/jn.1963.26.5.775. [DOI] [PubMed] [Google Scholar]
- Rhoades RW, Belford GR, Killackey HP. Receptive-field properties of rat ventral posterior medial neurons before and after selective kainic acid lesions of the trigeminal brain stem complex. Journal of Neurophysiology. 1987;57:1577–1600. doi: 10.1152/jn.1987.57.5.1577. [DOI] [PubMed] [Google Scholar]
- Salt TE. Excitatory amino acid receptors and synaptic transmission in the rat ventrobasal thalamus. Journal of Physiology. 1987;391:499–510. doi: 10.1113/jphysiol.1987.sp016752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salt TE, Eaton SA. Postsynaptic potentials evoked in ventrobasal thalamus neurones by natural sensory stimuli. Neuroscience Letters. 1990;114:295–299. doi: 10.1016/0304-3940(90)90579-x. [DOI] [PubMed] [Google Scholar]
- Sherman SM, Guillery RW. Functional organization of thalamocortical relays. Journal of Neurophysiology. 1996;76:1367–1395. doi: 10.1152/jn.1996.76.3.1367. [DOI] [PubMed] [Google Scholar]
- Sherman SM, Koch C. The control of retinogeniculate transmission in the mammalian lateral geniculate nucleus. Experimental Brain Research. 1986;63:1–20. doi: 10.1007/BF00235642. [DOI] [PubMed] [Google Scholar]
- Shosaku A. A comparison of receptive field properties of vibrissa neurons between the rat thalamic reticular and ventro-basal nuclei. Brain Research. 1985;347:36–40. doi: 10.1016/0006-8993(85)90886-8. [DOI] [PubMed] [Google Scholar]
- Simons DJ. Temporal and spatial integration in the rat SI vibrissa cortex. Journal of Neurophysiology. 1985;54:615–635. doi: 10.1152/jn.1985.54.3.615. [DOI] [PubMed] [Google Scholar]
- Simons DJ, Carvell GE. Thalamocortical response transformation in the rat vibrissa/barrel system. Journal of Neurophysiology. 1989;61:311–330. doi: 10.1152/jn.1989.61.2.311. [DOI] [PubMed] [Google Scholar]
- Singer W. Control of thalamic transmission by corticofugal and ascending reticular pathways in the visual system. Physiological Reviews. 1977;57:386–420. doi: 10.1152/physrev.1977.57.3.386. [DOI] [PubMed] [Google Scholar]
- Spacek J, Lieberman AR. Ultrastructure and three-dimensional organization of synaptic glomeruli in rat somatosensory thalamus. Journal of Anatomy. 1974;117:487–516. [PMC free article] [PubMed] [Google Scholar]
- Steriade M. Central core modulation of spontaneous oscillations and sensory transmission in thalamocortical systems. Current Opinion in Neurobiology. 1993;3:619–625. doi: 10.1016/0959-4388(93)90064-6. [DOI] [PubMed] [Google Scholar]
- Steriade M, Datta S, Pare D, Oakson G, Curro Dossi RC. Neuronal activities in brain-stem cholinergic nuclei related to tonic activation processes in thalamocortical systems. Journal of Neuroscience. 1990;10:2541–2559. doi: 10.1523/JNEUROSCI.10-08-02541.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steriade M, Demetrescu M. Unspecific systems of inhibition and facilitation of potentials evoked by intermittent light. Journal of Neurophysiology. 1960;23:602–617. [Google Scholar]
- Steriade M, Jones EG, McCormick DA. Thalamus. New York: Elsevier; 1997. [Google Scholar]
- Steriade M, McCarley RW. Brainstem Control of Wakefulness and Sleep. New York: Plenum; 1990. [Google Scholar]
- Steriade M, Timofeev I. Short-term plasticity during intrathalamic augmenting responses in decorticated cats. Journal of Neuroscience. 1997;17:3778–3795. doi: 10.1523/JNEUROSCI.17-10-03778.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsumoto T, Nakamura S. Inhibitory organization of the thalamic ventrobasal neurons with different peripheral representations. Experimental Brain Research. 1974;21:195–210. doi: 10.1007/BF00234389. [DOI] [PubMed] [Google Scholar]
- Uhlrich DJ, Tamamaki N, Murphy PC, Sherman SM. Effects of brain stem parabrachial activation on receptive field properties of cells in the cat's lateral geniculate nucleus. Journal of Neurophysiology. 1995;73:2428–2447. doi: 10.1152/jn.1995.73.6.2428. [DOI] [PubMed] [Google Scholar]
- Veinante P, Deschenes M. Single- and multi-whisker channels in the ascending projections from the principal trigeminal nucleus in the rat. Journal of Neuroscience. 1999;19:5085–5095. doi: 10.1523/JNEUROSCI.19-12-05085.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waite PM. The responses of cells in the rat thalamus to mechanical movements of the whiskers. Journal of Physiology. 1973;228:541–561. doi: 10.1113/jphysiol.1973.sp010099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren RA, Jones EG. Glutamate activation of cat thalamic reticular nucleus: effects on response properties of ventroposterior neurons. Experimental Brain Research. 1994;100:215–226. doi: 10.1007/BF00227192. [DOI] [PubMed] [Google Scholar]
- Williams JA, Comisarow J, Day J, Fibiger HC, Reiner PB. State-dependent release of acetylcholine in rat thalamus measured by in vivo microdialysis. Journal of Neuroscience. 1994a;14:5236–5242. doi: 10.1523/JNEUROSCI.14-09-05236.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams MN, Zahm DS, Jacquin MF. Differential foci and synaptic organization of the principal and spinal trigeminal projections to the thalamus in the rat. European Journal of Neuroscience. 1994b;6:429–453. doi: 10.1111/j.1460-9568.1994.tb00286.x. [DOI] [PubMed] [Google Scholar]


