Abstract
P2X2 receptor currents are potentiated by acidic pH and zinc. To identify residues necessary for proton and zinc modulation, alanines were singly substituted for each of the nine histidines in the extracellular domain of the rat P2X2 receptor. Wild-type and mutant receptors were expressed in Xenopus oocytes and analysed with two-electrode voltage clamp. All mutations caused less than a 2-fold change in the EC50 of the ATP concentration-response relation. Decreasing the extracellular pH from 7.5 to 6.5 potentiated the responses to 10 μm ATP of wild-type P2X2 and eight mutant receptors more than 4-fold, but the response of the mutant receptor H319A was potentiated only 1.4-fold. The H319A mutation greatly attenuated the maximal potentiation that could be produced by a drop in pH, shifted the pKa (-log of dissociation constant) of the potentiation to a more basic pH as compared with P2X2 and revealed a substantial pH-dependent decrease in the maximum response with a pKa near 6.0. Substituting a lysine for H319 reduced the EC50 for ATP 40-fold. Zinc (20 μm) potentiated the responses to 10 μm ATP of wild-type P2X2 and seven histidine mutants by ∼8-fold but had virtually no effect on the responses of two mutants, H120A and H213A. Neither H120A nor H213A removed the voltage-independent inhibition caused by high concentrations of zinc. The observation that different mutations selectively eliminated pH or zinc potentiation implies that there are two independent sites of action, even though the mechanisms of pH and zinc potentiation appear similar.
P2X receptors are cation channels gated by extracellular ATP. The widespread expression of these receptors in both neuronal and non-neuronal tissue suggests that P2X receptors are likely to play a role in a variety of processes such as pain sensation, auditory transduction and cardiovascular regulation (Brake & Julius, 1996; Ralevic & Burnstock, 1998; Housley, 2000; Burnstock, 2001; Khakh, 2001). Two endogenous modulators that differentially affect the seven P2X receptor subtypes that have been cloned from rat are extracellular pH and zinc (Brake & Julius, 1996; Ralevic & Burnstock, 1998: Khakh, 2001). Proton and zinc modulation have both been suggested to occur by allosteric regulation of the P2X receptors rather than by an effect on ATP, because changes in the species of ATP present do not correlate with changes in receptor properties (King et al. 1996; Li et al. 1996; Stoop et al. 1997).
Acidic extracellular pH (e.g. pH 6.5) potentiates P2X2 and P2X2/3 receptor currents and decreases the EC50 of the ATP concentration-response relation (King et al. 1996, 1997; Nakazawa et al. 1997; Stoop et al. 1997; Wildman et al. 1997, 1998); however, the same pH change inhibits the responses of P2X1, P2X3 and P2X4 receptors (Stoop et al. 1997; Wildman et al. 1999a, b). The pH sensitivity of P2X receptors is within the range of pH values seen under physiological conditions (e.g. inflammation, seizures, hypoxia), which suggests that pH modulation of P2X receptors has a significant role in vivo (Chesler, 1990; Reeh & Steen, 1996). For example, pH modulation of P2X receptors may allow respiratory neurons to detect changes in blood CO2 levels via changes in blood pH (Thomas & Spyer, 2000).
Extracellular zinc (< 100 μm) potentiates the responses of homomeric P2X2, P2X3 and P2X4 receptors as well as those of heteromeric P2X2/6 and P2X4/6 receptors, but inhibits the responses of P2X1 and P2X7 receptors (Nakazawa et al. 1997; Nakazawa & Ohno, 1997; Le et al. 1998; Wildman et al. 1998, 1999a, b; King et al. 2000). The physiological significance of zinc modulation of P2X receptors is unclear, but the co-localization of P2X receptors and zinc in the nervous system (e.g. in the hippocampus and cerebral cortex) suggests that this modulator may play an important role in vivo (Smart et al. 1994; Kanjhan et al. 1999).
The pH-response relation for P2X2 has a pKa reported to be between 7.0 and 7.9 (King et al. 1997; Stoop et al. 1997; Ding & Sachs, 1999). The amino acid with a pKa closest to this range is histidine. The pKa of free histidine is 6.0 (Tanokura, 1983), but in proteins the pKa of histidines can range from 5.0 to 8.0 (Kao et al. 2000), making histidines obvious candidates for the proton-binding site involved in pH potentiation. However, diethylpyrocarbonate (DEPC), a compound that covalently modifies histidines and makes them unable to be protonated, has no effect on pH modulation of P2X2 (Stoop et al. 1997; Wildman et al. 1998) or P2X4 (Clarke et al. 2000) receptors, suggesting that histidines are not involved in pH potentiation. An alternative explanation for these results is that DEPC did not gain access to some essential histidine in P2X2, and this explanation gained support when it was reported that a histidine in P2X4 receptors is necessary for proton inhibition of P2X4 receptor currents (Clarke et al. 2000). Histidine residues may also be involved in zinc binding to P2X2 receptors. Histidines are prominent in the zinc-binding domains of many enzymes (Christianson, 1991), and histidines are essential for the zinc sensitivity of GABA (Wang et al. 1995; Wooltorton et al. 1997), NMDA (Choi & Lipton, 1999; Paoletti, 2000) and glycine (Harvey et al. 1999) receptors.
To test whether histidines are involved in modulation of P2X2 receptors by extracellular protons and zinc, we singly substituted alanines for each of the nine histidines in the extracellular domain of the rat P2X2 receptor. Our results demonstrate that mutating one histidine (H319) greatly reduced pH potentiation with no effect on zinc modulation. In contrast, mutating either one of two histidines (H120 and H213) nearly eliminated zinc potentiation without altering pH modulation.
METHODS
Mutagenesis
Rat P2X2 cDNA in pcDNA1 was obtained from Dr D. Julius, University of California, San Francisco, CA, USA (Brake et al. 1994). Mutagenic oligonucleotides were obtained from Operon Technologies, Inc. (Alameda, CA, USA), and contained the base changes necessary for a single amino acid substitution and for the introduction or deletion of a restriction enzyme recognition site. Most mutant P2X2 receptors were made using the QuickChange Site-Directed Mutagenesis kit (Stratagene, LaJolla, CA, USA). For some mutations, we used overlap extension by polymerase chain reaction (Ho et al. 1989; Vallejo et al. 1995). Putative mutants were first screened by restriction enzyme digestion and then confirmed by DNA sequencing (University of Michigan DNA Sequencing Core, Ann Arbor, MI, USA). Each mutant is referred to by the original amino acid (one letter code) followed by the residue number and the substituted amino acid (one letter code). The oligonucleotide sequences and the diagnostic restriction sites introduced or deleted were:
H120A: 5′-GAGCATGAGGGTTGCTAGCTCTACCTGCCA-3′ (adds Nhe I);
H125A: 5′-AGCTCTACCTGCGCTTCAGACG-3′ (adds Hha I);
H146A: 5′-AAGGCAATGGGATCCGGACAGGGGCCTGTGTACCC TAT-3′ (adds BamH I);
H152A: 5′-TGTACCCTATTACGCTGGGGACTCCAAG-3′ (removes Nco I);
H174A: 5′-GAACTTCTGACAACGCATTTCTGGGTAAAATG-3′ (removes Pflm I);
H192A: 5′-AAGAACAGCATCGCCTACCCCAAGTTCAAATTCTCAAAG-3′ (adds Apo I);
H213A: 5′-CCTCAAGGCTTGTACATTTGATCAGG-3′ (adds Bsr GI);
H245A: 5′-TTCACAGAACTGGCAGCCAAGGGCGGTGTCAT-3′ (adds Sty I);
H319A: 5′-GTTATCGTGGCGGGACAGGCAG-3′ (adds Bsm FI);
H319K: 5′-TGTTATCGTGAAGGGACAGGCAGGGAAATT-3′ (adds BsmFI).
Expression of P2X2 receptors
P2X2 receptors were expressed in defolliculated stage V-VI Xenopus laevis oocytes. Oocytes were harvested by procedures approved by the University of Michigan Committee on the Use and Care of Vertebrate Animals, and have been described in detail elsewhere (Zhou & Hume, 1998). Frogs were anaesthetized by immersion in well water containing 3-aminobenzoic acid ethyl ester (1 g l−1, Sigma) and were humanely killed after the final collection of oocytes.
P2X2 and mutant receptor RNA were synthesized using the T7 mRNA Message Machine kit from Ambion (Austin, TX, USA) and quantified both spectroscopically and on ethidium bromide gels. Fifty nanolitres of RNA (typically 2–5 ng μl−1) were injected into each oocyte. Two-electrode voltage clamp experiments were carried out 1–5 days after RNA injection. To compare the maximum responses of the different constructs, we injected 50 nl of a high concentration of RNA (100 ng μl−1) to saturate translation, thereby eliminating variation due to differences in the quality of the RNA. In the data reported in Table 1, all oocytes came from a single frog, and were studied after 2 days.
Table 1.
Parameters of the ATP concentration–response relations for wild-type and mutant P2X2 receptors
| EC50 (μm) | Hill coefficient | Maximum current (μA) | |
|---|---|---|---|
| P2X2 | 30.1 ± 2.5 | 1.96 ± 0.23 | 25.2 ± 2.7 |
| H120A | 39.2 ± 0.8 | 2.00 ± 0.05 | 18.3 ± 0.8 |
| H125A | 26.9 ± 5.9 | 2.80 ± 1.25 | 21.1 ± 1.1 |
| H146A | 40.1 ± 11.8 | 1.33 ± 0.31 | 22.3 ± 2.1 |
| H152A | 22.2 ± 1.1 | 2.31 ± 0.22 | 21.0 ± 1.0 |
| H174A | 26.2 ± 2.3 | 1.73 ± 0.19 | 22.1 ± 1.4 |
| H192A | 23.2 ± 3.6 | 2.35 ± 0.70 | 17.7 ± 1.8 |
| H213A | 35.3 ± 0.1 | 2.65 ± 0.02 | 18.9 ± 1.6 |
| H245A | 22.0 ± 4.1 | 2.74 ± 1.22 | 25.9 ± 1.7 |
| H319A | 16.1 ± 2.5 | 2.02 ± 0.54 | 4.1 ± 0.3 |
The half-maximal effective concentrations (EC50) and Hill coefficients of wild-type and mutant receptors in response to ATP (5–500 μm) were determined as described in Methods using 5–7 oocytes per concentration of ATP for each construct (30–50 total oocytes per construct). To compare the maximum response of these receptors to ATP, oocytes from a single batch were injected with a large amount of RNA (5 ng oocyte−1). After incubation for 2 days, the current amplitudes in response to 1 mm ATP of 7–10 oocytes per construct were measured. All oocytes were held at −50 mV.
Solutions
The base oocyte recording solution (ORS) contained (mm): 90 NaCl, 1 KCl, 1.32 MgCl2, 10 Hepes, pH 7.5. We compensated for the chelation of Mg2+ by ATP by adding MgCl2 to the solutions used such that all solutions contained 1 mm free Mg2+, as determined by the program Bound and Determined (Brooks & Storey, 1992). The ATP stock solution consisted of disodium adenosine 5′-triphosphate (ATP; Sigma-Aldrich, St Louis, MO, USA) dissolved at 100 mm in double-distilled H2O; aliquots were stored at −20 °C for 1–3 months. The ATP concentrations of all solutions were verified by taking spectroscopic measurements at 259 nm. A 1 m stock of ZnCl2 was dissolved in acidic double-distilled H2O in order to prevent precipitation.
For recording, ATP stock solutions were diluted in ORS with the required zinc and magnesium, followed by adjustment of the pH to the required level. All solutions were used within 48 h.
Electrophysiological recordings and data analysis
P2X2 and mutant receptor currents were investigated using two-electrode voltage clamp (in most experiments an npi TurboTec 3 apparatus was used; in the remainder an Axon Instruments Geneclamp 500B apparatus was used). All experiments were done at room temperature (22 oC), and unless otherwise stated the holding potential was −50 mV. The rate of P2X2 receptor current desensitization is slow, concentration dependent and varies between batches of oocytes (Zhou et al. 1998). More importantly, once fully desensitized, P2X2 receptors recover very slowly (Zhou et al. 1998). Thus, obtaining a concentration-response relation for P2X2 receptors by sequentially applying higher concentrations of ATP to the same oocytes is likely to cause significant desensitization. Therefore, the final concentration-response relation will be that for a mixture of desensitized and non-desensitized P2X2 receptors, which will result in an underestimation of the EC50, because desensitized receptors have a higher affinity for ATP. To obtain the most accurate estimate of the concentration-response relations (reported in Table 1), our strategy was to expose each oocyte to only two concentrations of ATP: a small non-desensitizing concentration of ATP (10 μm) immediately followed by a test concentration of ATP (5–200 μm). The ratio between these current amplitudes was then averaged among five to seven oocytes for each test concentration of ATP (30–50 total oocytes per construct). The plot of these averaged ratios was fitted using the Hill equation, and re-normalized to a maximum of 100 %. The EC50 obtained for wild-type P2X2 receptors using this method (30 μm; Table 1) was 136 % of that obtained by estimating the EC50 with sequential 20 s applications of ATP to single oocytes (22 μm; Figs 3C, 5D and 8A). Since average concentration-response relations of individual oocytes were not obtained using this protocol, we report the standard error of the regression from Sigma Plot 5.0 as a measure of the precision of the fit. In other experiments, we used an ascending series of ATP concentrations on single oocytes to measure changes in the maximum response of a cell with pH or zinc.
Figure 3. The ATP concentration-response relations for wild-type and H319A change with pH.
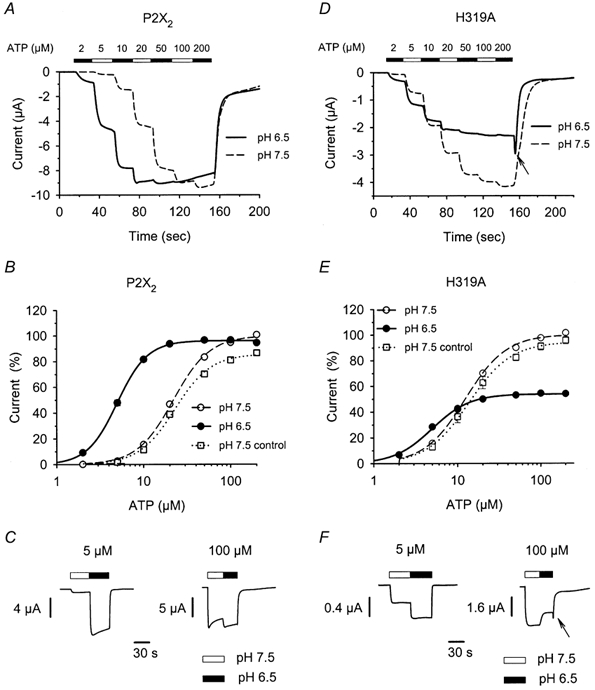
A and D, representative current responses of single oocytes expressing P2X2 (A) or H319A (D) to increasing concentrations of ATP at pH 7.5 and then at pH 6.5 after allowing a 5 min recovery between traces. The numbers and the filled and open bars above the graphs indicate the concentration and duration of ATP application. B and E, the ATP concentration-response relations for P2X2 (B) and H319A (E) were derived from experiments like those shown in A and D. All currents were normalized to the maximum current amplitude of the initial concentration response at pH 7.5. The concentration-response relations at pH 7.5 following a 5 min incubation (pH 7.5 control) were similar to the initial concentration-response relations at pH 7.5. C and F, sample traces of the responses of oocytes expressing wild-type (C) or H319A (F) receptors to either 5 or 100 μm ATP at pH 7.5 (open bars) and pH 6.5 (filled bars). The time scales in C and F apply to both traces. The arrows in D and F indicate a transient increase in the current response of H319A after switching from a high concentration of ATP at pH 6.5 back to the recording solution without ATP at pH 7.5. For some points the error bars (s.e.m.) were smaller than the symbols.
Figure 5. Additional characterization of mutations at H319.
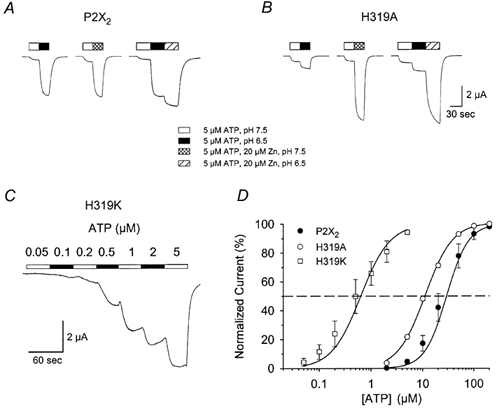
A and B, comparison of the effect of zinc and pH on single oocytes expressing P2X2 (A) or H319A (B). For P2X2 the potentiation elicited by switching from 5 μm ATP at pH 7.5 to either 5 μm ATP plus 20 μm zinc at pH 7.5 or to 5 μm ATP alone at pH 6.5 was very similar, but for H319A the potentiation elicited by switching to acidic pH was much smaller than the potentiation that could be elicited by zinc. In addition, potentiation by 20 μm zinc was mostly occluded by pH 6.5 potentiation in P2X2 but not in H319A. C, currents elicited from an oocyte expressing H319K in response to step concentration increases in ATP at pH 7.5. D, concentration-response relations of a series of oocytes from a single frog expressing P2X2, H319A or H319K. Each graph is the average for 5–6 oocytes studied as in C. For some points the error bars (s.e.m.) were smaller than the symbols.
Figure 8. ATP concentration-response relations for P2X2 (A), H120A (B) and H213A (C) with and without 20 μm zinc.
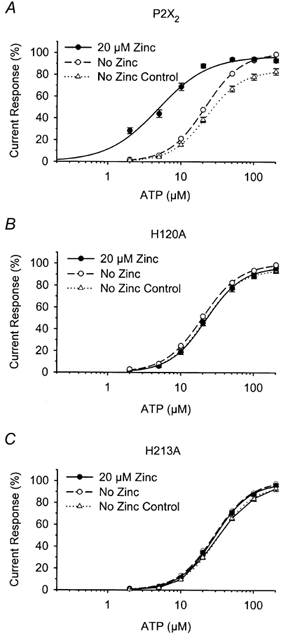
After obtaining a concentration-response relation in the absence of zinc (○, No Zinc), 5 min was allowed for recovery and then a second concentration-response relation was obtained in either the absence (▵, No Zinc Control) or presence of 20 μm zinc (•). All currents were normalized to the maximum current amplitude of the initial concentration-response relation in the absence of zinc.
Data are expressed as means ± s.e.m. unless otherwise specified. Box plots of potentiation ratios (Fig. 2 and Fig. 7) display the median values and the 10 %, 25 %, 75 % and 90 % percentiles because the data were not normally distributed.
Figure 2. Potentiation of currents by protons was greatly diminished for H319A.
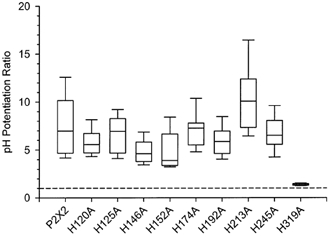
The pH potentiation ratio is the ratio of the current amplitude in the presence of 10 μm ATP at pH 6.5 to the response at pH 7.5. Lines within the boxes indicate the median potentiation ratio (n = 7–27 oocytes for each mutant construct, n = 52 oocytes for wild-type). The upper and lower boundaries of each box plot demarcate the 75th and 25th percentiles, respectively. The horizontal lines above and below each box indicate the 90th and 10th percentiles. The dashed line indicates a ratio of 1 (no potentiation).
Figure 7. Zinc potentiation was greatly reduced in two histidine mutants, H120A and H213A.
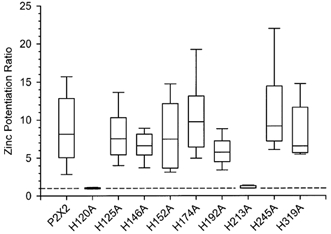
The zinc potentiation ratio is the ratio of the current amplitude in the presence of 10 μm ATP and 20 μm zinc to the current amplitude in response to 10 μm ATP alone. Lines within the boxes indicate the median potentiation ratio (n = 15–25 oocytes per mutant construct, 55 oocytes for wild-type). The upper and lower boundaries of each box demarcate the 75th and 25th percentiles, respectively. The horizontal lines above and below each box indicate the 90th and 10th percentiles. The dashed line indicates a ratio of 1 (no potentiation).
RESULTS
Effect of histidine mutations on the ATP concentration-response relation
To test whether any of the nine histidine mutations altered ATP responsiveness, we measured the ATP concentrationresponse relations of the mutants expressed in Xenopus oocytes, as described in Methods. The histidine mutations only slightly altered the EC50 of the receptor to ATP (Table 1). The most left-shifted mutant was H319A and the most right-shifted mutant was H146A, but these changes in the EC50 were less than 2-fold different from wild-type P2X2. To test whether the maximum response of these mutants was altered we injected oocytes with a high concentration of RNA (5 ng oocyte−1) to saturate translation and assayed oocytes after similar incubation times. The maximum responses of eight histidine mutants were not different from wild-type; however, the maximum response of H319A was reduced 6-fold compared with wild-type (Table 1).
H319A altered the pH sensitivity of P2X2 receptor currents
As an initial screen to identify mutants that altered the sensitivity of P2X2 receptors to pH, we tested whether the responses of mutant receptors to 10 μm ATP were potentiated when the extracellular pH was dropped from 7.5 to 6.5 (Fig. 1 and Fig. 2). The potentiation ratios for individual oocytes expressing wild-type P2X2 receptors varied from 4- to 16-fold. The source of this variation is not yet clear, but the potentiation ratios for eight of the nine histidine mutants also fell within this range (median potentiation ratios varying from 4- to 10-fold). In contrast, the responses to 10 μm ATP of individual oocytes expressing H319A were never potentiated by more than 1.9-fold (median potentiation ratio = 1.37, n = 14).
Figure 1. Potentiation of wild-type and mutant receptor currents by pH.
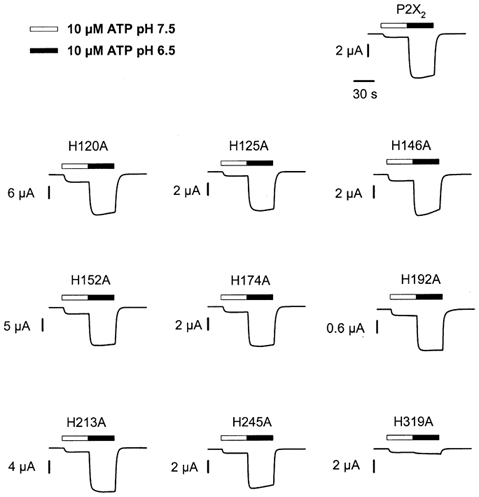
Each trace is the response of a single oocyte expressing wild-type or mutant receptors to 10 μm ATP at pH 7.5 and pH 6.5. The bars above each trace indicate the duration of the applications. The time scale applies to all traces.
The failure of H319A to potentiate in response to acidic pH might result from several different mechanisms. The simplest case would be if the H319A mutant was insensitive to protons. However, Fig. 3 demonstrates that the actual situation was more complex. The potentiation of wild-type P2X2 by pH is characterized by a substantial decrease in the EC50, with little change in the maximum current (King et al. 1996, 1997; Nakazawa et al. 1997; Stoop et al. 1997). This is most readily demonstrated by applying an ascending series of concentrations of ATP (Fig. 3A and B) to the same oocyte at two different pH values. Although this procedure produced a slight underestimation of the actual EC50 (see Methods), it had the advantage that the amplitudes of the maximal responses could be more easily compared under the two conditions. For each oocyte, we determined the ATP concentration-response relation at pH 7.5, waited 5 min to allow for recovery from desensitization, and then determined the ATP concentration-response relation at either pH 6.5 or 7.5 (control). The similarity of the initial and control concentration-response relations at pH 7.5 (Fig. 3B) indicated that relatively little long-term desensitization occurred under the conditions used. Reducing the pH from 7.5 to 6.5 decreased the EC50 of wild-type P2X2 by about 4-fold (from 22.5 ± 0.7 to 5.1 ± 0.2 μm, n = 7; Fig. 3B) and the pH potentiation ratios predicted from the curves in Fig. 3B matched very well with the actual pH potentiation ratios obtained. The curves shown in Fig. 3B predict pH potentiation ratios of 14.1 and 1.18 at 5 and 100 μm ATP, respectively. When individual oocytes were ‘jumped’ directly from pH 7.5 to 6.5 at these ATP concentrations, the median ratios were 16.8 (n = 8) and 1.23 (n = 8), respectively (Fig. 3C).
Like wild-type P2X2, the concentration-response relation of H319A showed a leftward shift in the EC50 from 12.4 ± 0.6 to 4.9 ± 0.1 μm (n = 7; Fig. 3D and E). However, this did not produce very much potentiation, because the maximal response to ATP was decreased about 2-fold at pH 6.5. As a consequence, decreasing the pH from 7.5 to 6.5 potentiated the response of H319A to 5 μm ATP by only 2.37 ± 0.20-fold (n = 14) and inhibited the response to 100 μm ATP (potentiation ratio of 0.71 ± 0.02, n = 8; Fig. 3F). Interestingly, changing from solutions with a high concentration of ATP (100 or 200 μm ATP) at pH 6.5 to the bath recording solution at pH 7.5 produced a transient increase in the current (after-spike) before the current returned to baseline (arrows in Fig. 3D and F).
We investigated the sensitivity of the H319A mutant to pH by examining relative current amplitudes across a range of pH values. Since pH has different effects at different concentrations of ATP, we studied the pH-response relations of H319A and P2X2 at a low and a high concentration of ATP: 5 and 100 μm (Fig. 4A and B). At 5 μm ATP, the pH-response relation of H319A (pKa = 7.71 ± 0.11, n = 6) was shifted compared with that of wild-type P2X2 (pKa = 6.83 ± 0.003, n = 5). At 100 μm ATP, H319A receptor currents decreased with decreasing pH (pKa = 5.98 ± 0.12, n = 5) with no indication of potentiation at any pH tested (Fig. 4B). In contrast, for 100 μm ATP the relation between P2X2 receptor currents and pH was bell-shaped with a maximum response at pH 7.0. This bell-shaped relation results from the combined potentiating and inhibiting effects of pH. We used the inhibitory pH-response relation of H319A to scale P2X2 currents ratios at 100 μm ATP and calculated the pKa of the potentiating effect to be 7.60 ± 0.21 (n = 5).
Figure 4. The relation between pH and current responses of P2X2 and H319A receptors at 5 μm ATP (A) and 100 μm ATP (B).
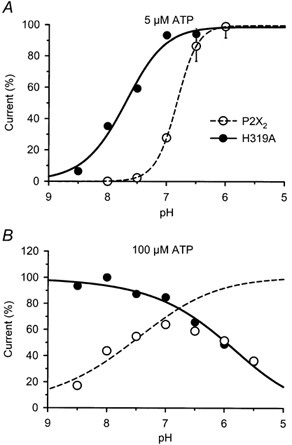
The symbol legend in A also applies to B. All currents were normalized so that the maximum ratio of the fit was 100 %. The curves shown for responses to 5 μm ATP and for H319A at 100 μm ATP were fitted to the data using the Hill equation. To fit the data for potentiation of wild-type P2X2 at 100 μm ATP, we scaled the data using the inhibitory pH-response relation of H319A at 100 μm. The fit of this adjusted pH response of P2X2 receptor currents is shown as a dashed line in B.
To explore further the mechanism of action of the H319A mutation, we compared the effect of zinc and pH potentiation on oocytes expressing P2X2 or H319A (Fig. 5A). For wild-type P2X2, the maximal potentiation that could be achieved by optimal levels of zinc or protons was similar. With 5 μm ATP at pH 7.5, the average potentiation to 20 μm zinc was 14.9 ± 4.5-fold, and the average potentiation in pH 6.5 solution was 12.6 ± 3.6-fold (n = 5). For a series of five cells, the average ratio of zinc potentiation to pH potentiation was 1.14 ± 0.04. A second important feature of the responses of wild-type P2X2 was that the amplitude of the maximally potentiated current was similar to the current that was elicited by a high concentration of ATP (200 μm) at pH 7.5 with no zinc (see Fig. 3C and Fig. 8A). Finally, the two types of potentiation were not independent. At pH 6.5, a level that caused nearly maximal pH potentiation of the response of P2X2 to 5 μm ATP but relatively little inhibition (see Fig. 4A), very little additional potentiation was produced by adding 20 μm zinc (1.22 ± 0.04-fold, n = 5). Thus the potentiation caused by these two modulators occlude each other. When zinc and pH potentiation was compared in H319A-expressing oocytes the results were quite different (Fig. 5B). For 5 μm ATP, the potentiation of H319A by 20 μm zinc at pH 7.5 was 15.1 ± 1.6-fold, nearly identical to the zinc potentiation of P2X2. However, as noted above, the maximal potentiation that could be achieved at pH 6.5 was much smaller (2.34-fold) and for a series of five cells, the average ratio of zinc potentiation to pH potentiation was 5.06 ± 0.38. Furthermore, pH potentiation did not effectively occlude the response of H319A to zinc. At pH 6.5, addition of 20 μm zinc caused the response to 5 μm ATP to increase 4.22 ± 0.22-fold (n = 5). Thus the mechanism that produces the residual pH potentiation in H319A is much less efficacious than the mechanisms that produce zinc potentiation in H319A and pH potentiation in P2X2.
One additional property that was altered in the H319A mutation was the desensitization evident with high agonist concentration (see for example Figs 1, 3 and 6). The rate and magnitude of desensitization of P2X2 varies widely between oocytes studied under identical conditions (Zhou et al. 1998), but a substantial number of the P2X2-expressing oocytes we studied showed detectable desensitization (time constant of 7–60 s) and in these oocytes the current at steady state declined to less than 80 % of the initial current. In contrast, the observed desensitization of H319A to high agonist concentration was uniformly slow (time constant of > 60 s) and the steady state currents were typically 90–100 % of peak currents.
Figure 6. Potentiation of wild-type and mutant receptors by zinc.
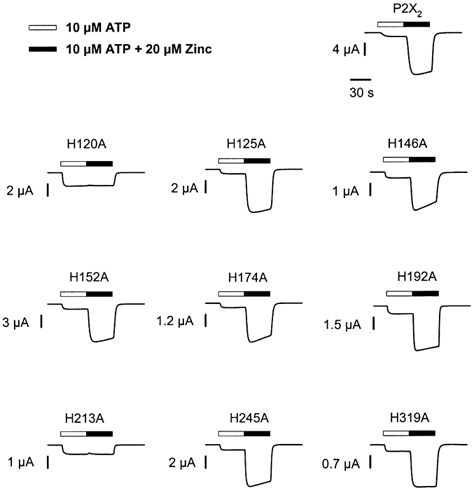
Each trace is the response of a single oocyte expressing wild-type or mutant receptor to 10 μm ATP followed by 10 μm ATP with 20 μm zinc. The bars above each trace indicate the duration of the applications. The time scale applies to all traces.
A final set of experiments concerning the mechanism of pH potentiation examined the properties of the H319K mutation. The rationale for these experiments was that if H319 is the proton sensor responsible for normal pH potentiation, then a positive charge at this position should mimic protonation of this histidine, and produce a leftward shift of the concentration-response curve at pH 7.5. Indeed, there was a leftward shift of over 40-fold. The mean EC50 obtained from applying gradually increasing concentrations of ATP (Fig. 5C) was 0.63 ± 0.23 μm, compared with 29.1 ± 5.9 μm for oocytes from the same frog injected with RNA encoding P2X2 (Fig. 5D). As was the case for the H319A mutation, when the ATP concentration was low relative to the EC50, there was a residual pH-dependent potentiation of the H319K currents. When 0.2 μm ATP was used (a concentration that produced nearly the same fractional response as 5 μm ATP did on H319A), the pKa for the potentiation was 7.6 ± 0.3 (n = 5), and the potentiation was nearly maximal at pH 7.0. Like the pH potentiation in H319A, the residual potentiation was not very efficacious. The current achieved with 0.2 μm ATP at pH 7.0 (maximal pH potentiation) was much smaller (28 %) than the current that could be elicited by increasing ATP to 5 μm at pH 7.5. Finally, as was the case with P2X2 and H319A, an inhibitory action of protons became apparent at quite acidic pH. For H319K studied with 0.2 μm ATP, the inhibition was sufficiently powerful that by pH 6.5 it dominated over the potentiation so that the ratio of current at pH 6.5 to the current at pH 7.5 was 0.59 ± 0.2 (n = 3).
In summary, these data clearly demonstrate that H319 plays an important role in pH potentiation but not zinc potentiation. Whether the properties of the H319A and H319K mutations are consistent with H319 serving as the primary sensor for the pH modulation observed in P2X2 is explored in the Dicussion.
Two histidine mutations reduced zinc potentiation of P2X2 receptor currents
We next tested whether any of the nine histidine mutations reduced the ability of zinc to modulate P2X2 receptor currents (Fig. 6 and Fig. 7). The response of P2X2 receptors to 10 μm ATP was potentiated ∼8-fold in the presence of 20 μm zinc. Strikingly, zinc had little or no effect on the responses of two histidine mutant receptors (H120A and H213A). The remaining seven histidine mutant receptor currents exhibited zinc potentiation similar to wild-type.
Zinc potentiation of P2X2 receptor currents results from a decrease in the EC50 of the ATP concentration-response relation with little change in the maximum response (Wildman et al. 1998; Xiong et al. 1999), similar to the effect of low pH described above. We therefore tested whether zinc alters the ATP concentration-response relations of either H120A or H213A (Fig. 8). These experiments were performed on single oocytes in order to also compare the maximum response of these receptors with and without zinc. We first applied ascending concentrations of ATP in the absence of zinc. Then, after a 5 min recovery period, we applied another ascending series of ATP concentrations either with 20 μm zinc or without (control). As was shown above, the initial and control ATP concentration-response relations were not significantly different from each other. For P2X2, the EC50 was decreased 4-fold from 21.34 ± 0.54 μm (n = 11) in the absence of zinc to 5.11 ± 0.78 μm (n = 6) in the presence of 20 μm zinc. In contrast, the ATP concentration- response relations of H120A and H213A were not significantly altered by 20 μm zinc.
To further characterize the zinc sensitivity of H120A and H213A, we determined the concentration-response relations for zinc (Fig. 9). Both H120A and H213A currents were inhibited by zinc with IC50 values of 112 ± 13 and 110 ± 24 μm and Hill coefficients of 1.50 ± 0.25 and 1.26 ± 0.33, respectively. The zinc concentration- response relation for P2X2 was bell-shaped, reflecting the combined effects of zinc potentiation and inhibition. We used the inhibitory zinc-response relations of H120A and H213A to scale P2X2 currents ratios and calculated that the zinc concentration-response relation for potentiation at 10 μm ATP had an EC50 of 16.5 ± 4.5 μm and a Hill coefficient of 0.92 ± 0.16.
Figure 9. Zinc concentration-response relations for H120A, H213A and wild-type P2X2 in the presence of 10 μm ATP.
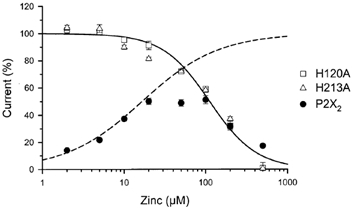
All currents were normalized to the currents induced by ATP in the absence of zinc, and each point represents the mean ± s.e.m. of 5–13 oocytes. The continuous line represents a fit to all data from H120A and H213A (IC50 = 112 μm, Hill coefficient = 1.38). The dashed line represents the predicted relation between zinc concentration and potentiation of wild-type P2X2 (EC50 = 16.5 μm, Hill coefficient = 0.92) based on the assumption that an inhibitory process identical to that seen in H120A and H213A also occurs in wild-type, but is partially masked by the potentiating effect of ATP.
The reduced potentiation of H120A and H213A receptor currents by zinc allowed us to investigate the nature of the inhibitory zinc binding site. Inhibition of P2X2 receptor currents by high concentrations of zinc may be due to either an allosteric modification or to a block of the channel pore, similar to the effect of magnesium on NMDA receptors. If zinc inhibits current responses by blocking the channel pore and the zinc binding site lies within the membrane field, this inhibition should be voltage dependent. However, Fig. 10 shows that this inhibitory effect was not voltage dependent. In summary, H120 and H213 are required for zinc potentiation but are not necessary for voltage-independent, low-affinity zinc inhibition.
Figure 10. The inhibition of H120A and H213A mutant receptor currents by high concentrations of zinc (200 μm) is voltage independent.
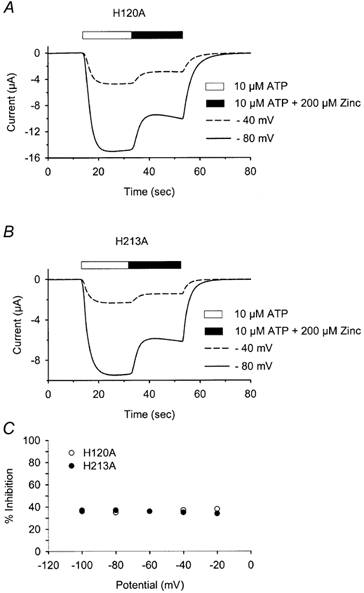
A and B, the response of H120A (A) and H213A (B) to 10 μm ATP followed by 10 μm ATP with 200 μm zinc at two different potentials. The lengths of the bars above the graphs indicate the duration of application. C, percentage inhibition of mutant receptor currents by 200 μm zinc at different potentials.
DISCUSSION
In this study, we used site-directed mutagenesis to test whether any of the nine histidines in the extracellular domain of P2X2 receptors are necessary for zinc or proton potentiation. One histidine mutation (H319A) dramatically reduced pH potentiation of receptor currents with no effect on zinc potentiation; two mutations (H120A, H213A) eliminated zinc potentiation with no effect on pH potentiation. The ability to eliminate one type of potentiation with no effect on the other suggests that zinc and protons act to potentiate the receptor at independent sites.
Inhibition by high concentrations of zinc and protons
Zinc potentiation of P2X2 responses to 10 μm ATP attenuates at zinc concentrations greater than 100 μm. Similarly, pH potentiation of P2X2 responses to 100 μm ATP peaks at pH 7 and then falls with lower pH. Thus, zinc and pH potentiation in wild-type P2X2 receptors mask inhibitory effects of zinc and protons. By eliminating zinc potentiation, H120A and H213A mutants revealed this inhibitory effect of zinc even at low concentrations of zinc. Likewise, the basic shift in the pKa of the pH potentiation caused by mutating H319 revealed an inhibitory effect of protons at pH 6.5, a pH value that greatly potentiates wild-type P2X2 receptor currents. Since zinc inhibition was maintained in H120A and H213A, and proton inhibition in H319A, these results suggest that the lower affinity inhibitory sites for protons and zinc are independent of the higher affinity potentiating sites for these modulators. At present we do not know what residues might be required for this inhibition. However, a promising approach to identifying such residues is to make double mutants (H319A plus another residue or H120A plus another residue) to probe pH or zinc inhibition, respectively.
The inhibitory effect of protons on the maximum response of H319A receptors can explain why H319A currents induced by high concentrations of ATP at pH 6.5 briefly increased (after-spike) before returning to baseline when the solution was changed to zero ATP at pH 7.5. This is expected to occur if the rate of deprotonation of the inhibitory site is faster than the decline in the ATP concentration. In Fig. 3B, a change in pH from 6.5 to 7.5 corresponds to a change in proton concentration of 0.285 μm; the change in ATP concentration was 200 μm. Given the low affinity of the inhibitory pH action (pKa = 5.98, corresponding to a proton concentration of almost exactly 1 μm) and the slow perfusion rate (time constant of exchange of ∼4 s), it is quite likely that the receptor deprotonated before ATP could unbind.
The H319A mutation must have also caused an additional, pH-independent decrease in the maximum response, since the 6-fold decrease in the maximum response of H319A compared with wild-type P2X2 at pH 7.5 (Table 1) is more than expected from the inhibitory pH response alone. Distinguishing whether this pH-independent reduction of the maximal response was due to a reduction in the number of functional channels, the single-channel conductance or the probability of the channel being open will require single-channel recordings.
The proton binding site?
A simple model for pH sensing would be that protonation of a single histidine is necessary and sufficient to account for pH potentiation. One prediction of this model is that replacing this histidine with a positively charged residue should lock the receptor in the potentiated state, which would be characterized by a greatly left-shifted ATP concentration-response relation at pH 7.5. This is exactly what was observed with the H319K mutant. However, this simple model also predicts that replacing this histidine with an uncharged residue should lock the receptor in the unpotentiated state, which would be expected to produce a rightward shift of the concentration-response relation at pH 7.5. However, the H319A mutation produced a slight leftward shift. An even more serious apparent problem with this model is that both H319A and H319K still display potentiation as the pH becomes more acidic. Thus H319 cannot be the only pH sensor associated with potentiation in P2X2.
The properties of the residual potentiation observed in H319A and H319K were different in three important ways from the potentiation shown in wild-type P2X2. First, the pKa was shifted by nearly a full pH unit, from 6.8 in P2X2 to 7.6 in the H319 mutants. Second, the maximum potentiation that was achieved in these mutants was far less than could be achieved in the wild-type. Third, this potentiation did not effectively occlude zinc potentiation. Interestingly, treating wild-type P2X2 receptors with the histidyl-modifying agent DEPC also created receptors that retained the ability to be potentiated by pH, but showed a much smaller maximum response (Stoop et al. 1997; Wildman et al. 1998), suggesting that this inhibitory action of DEPC may occur by modification of H319. It seems possible that the residual potentiation observed when H319 is replaced may be the result of a separate process that is usually hidden beneath a more efficacious process that requires H319. If this idea is correct, then there would actually be three separate pH-dependent processes in P2X2: a low efficacy potentiation process with a pKa near 7.6, a high efficacy potentiation process with a pKa near 6.8 that requires H319 and an inhibitory process with a pKa near 6.0. Another possibility is that mutation of H319 indirectly altered pH potentiation. For example, protonation of H319 might stabilize a closed state of P2X2 through an interaction dependent upon the chemical structure of the imidazole side-chain. Since an alanine is a poor substitute for an imidazole group and a lysine is an even worse substitute, both H319A and H319K (as well as the reaction of H319 with DEPC) would favour the open state of P2X2. This would be expected to decrease the EC50 of the ATP concentration-response relation and increase the pKa of pH potentiation as observed. However, this mechanism does not readily explain why the maximal response obtainable with pH potentiation was decreased in the H319A and H319K mutants.
Regardless of whether the H319 mutations have completely eliminated one of two types of pH potentiation or only modified the way a single potentiation process is expressed, the residual potentiation and the inhibition apparent in these mutants indicates that there must be additional amino acids involved in pH sensing. Several possibilities remain open. First, the other extracellular histidines may not be sufficient to have a discernable effect when mutated by themselves, but their role might become more apparent when H319 is absent. Second, other amino acids may be involved. Although the pKa of potentiation of P2X2 receptor currents by pH is outside the range of pKa values for the side groups of other free amino acids (aspartate, 3.85; glutamate, 4.25; cysteine, 8.5; tyrosine, 10.05; lysine, 10.53; arginine, 12.48) (Chang, 1981), it is possible that the local electrostatic environment may bring the pKa of one of these amino acids into the range seen for pH modulation of P2X2 receptors. Third, one must consider the effects of pH on ATP itself, since the tertiary phosphate group of ATP titrates with a pKa of 6.95 (Alberty, 1968). Calculations by three groups (Li et al. 1996; King et al. 1997; Stoop et al. 1997) have shown that the pH-dependent changes in the species of ATP do not correlate with changes in receptor properties; however, it is possible that ATP bound to the receptor may bind protons with a different affinity than free ATP.
The zinc binding site?
Our finding that H120 and H213 are necessary for zinc modulation of P2X2 receptors is in keeping with the role of histidines in zinc modulation of other ion channels (Wang et al. 1995; Wooltorton et al. 1997; Choi & Lipton, 1999; Harvey et al. 1999; Paoletti et al. 2000). Interestingly, H120 and H213 are not conserved in rat P2X4, which also potentiates to zinc: H120 is replaced with a lysine and H213 is replaced by serine. Although both serine and lysine residues are found in some zinc-binding domains (Christianson, 1991; Paoletti et al. 2000), we do not know if mutations at these residues alter zinc potentiation of P2X4 receptor currents. The lack of identity between histidines in P2X2 and P2X4 receptors may explain the smaller zinc potentiation of P2X4 receptor currents compared with those of P2X2 as well as the differential regulation of these subtypes by copper (Xiong et al. 1999; Acuna-Castillo et al. 2000). A human P2X2 receptor has recently been cloned and characterized (Lynch et al. 1999). In human P2X2, H120 is conserved but H213 is replaced by an arginine; whether human P2X2 receptor currents are potentiated by zinc is not known.
As has been eloquently argued, whether a residue participates in ligand binding or a later step in receptor gating cannot be dissociated from an analysis of macroscopic currents (Colquhoun, 1998). One argument against a role for these histidines in binding zinc is that DEPC, which renders histidines incapable of binding zinc, does not affect zinc potentiation (Wildman et al. 1998). The lack of an effect of DEPC could be explained if H120 and H213 alter responsiveness to zinc remotely from the binding site, since DEPC modification might not alter the key features of such a remote site. However, the lack of an effect of DEPC on zinc potentiation could also be explained if H120 and H213 are part of the zinc binding site, but the access pathway to this site is too narrow for DEPC to enter. A direct role for H120 and H213 in zinc binding is consistent with the well-known role of histidines in the zinc binding sites of many transcription factors, enzymes and structural proteins (Christianson, 1991). In addition, mutations at H120 and H213 do not dramatically alter the response to ATP or protons, suggesting that these mutations did not dramatically disrupt receptor gating. In order to participate in the same zinc binding site, H120 and H213 must be close together in the three-dimensional protein structure. In most zinc-binding proteins, zinc complexes with either three or four residues (Christianson, 1991). Thus, if we assume that H120 and H213 represent bona fide zinc-binding residues, zinc should bind to one or two additional sites. Besides additional sites on the receptor, the terminal phosphates of ATP can also complex with zinc, similar to the binding to magnesium (Orioli et al. 1980). Thus, zinc might be bound between H120 and H213 on the receptor and the two terminal phosphates of ATP.
Regardless of the molecular mechanism by which the mutations at H120, H213 and H319 alter zinc or pH potentiation, the identification of mutant receptors in which either zinc or pH potentiation is eliminated with relatively little change in other receptor properties provides exciting new opportunities. For instance, it focuses attention on these sites as potential targets of drugs that selectively block these modulations, but do not alter normal ATP-activated signalling. The availability of such agents would allow one to more directly assess the physiological importance of these modulatory sites than has so far been possible.
Acknowledgments
We would like to thank members of the Hume laboratory for helpful comments on this work. We also would like to thank Jamila Ware for helpful technical assistance. This research was supported by PHS grant NS 39196.
REFERENCES
- Acuna-Castillo C, Morales B, Huidobro-Toro JP. Zinc and copper modulate differentially the P2X4 receptor. Journal of Neurochemistry. 2000;74:1529–1537. doi: 10.1046/j.1471-4159.2000.0741529.x. [DOI] [PubMed] [Google Scholar]
- Alberty RA. Effect of pH and metal ion concentration on the equilibrium hydrolysis of adenosine triphosphate to adenosine diphosphate. Journal of Biological Chemistry. 1968;243:1337–1343. [PubMed] [Google Scholar]
- Brake AJ, Julius D. Signaling by extracellular nucleotides. Annual Review of Cell and Developmental Biology. 1996;12:519–541. doi: 10.1146/annurev.cellbio.12.1.519. [DOI] [PubMed] [Google Scholar]
- Brake AJ, Wagenbach MJ, Julius D. New structural motif for ligand-gated ion channels defined by an ionotropic ATP receptor. Nature. 1994;371:519–523. doi: 10.1038/371519a0. [DOI] [PubMed] [Google Scholar]
- Brooks SP, Storey KB. Bound and determined: a computer program for making buffers of defined ion concentrations. Analytical Biochemistry. 1992;201:119–126. doi: 10.1016/0003-2697(92)90183-8. [DOI] [PubMed] [Google Scholar]
- Burnstock G. Purine-mediated signalling in pain and visceral perception. Trends in Pharmacological Sciences. 2001;22:182–188. doi: 10.1016/s0165-6147(00)01643-6. [DOI] [PubMed] [Google Scholar]
- Chang R. Physical Chemistry with Application to Biological Systems. New York: Macmillan Publishing Co. Inc.; 1981. [Google Scholar]
- Chesler M. The regulation and modulation of pH in the nervous system. Progress in Neurobiology. 1990;34:401–427. doi: 10.1016/0301-0082(90)90034-e. [DOI] [PubMed] [Google Scholar]
- Choi Y-B, Lipton SA. Identification and mechanism of action of two histidine residues underlying high-affinity Zn2+ inhibition of the NMDA receptor. Neuron. 1999;23:171–180. doi: 10.1016/s0896-6273(00)80763-1. [DOI] [PubMed] [Google Scholar]
- Christianson DW. Structural biology of zinc. Advances in Protein Chemistry. 1991;42:281–355. doi: 10.1016/s0065-3233(08)60538-0. [DOI] [PubMed] [Google Scholar]
- Clarke CE, Benham CD, Bridges A, George AR, Meadows HJ. Mutation of histidine 286 of the human P2X4 purinoceptor removes extracellular pH sensitivity. Journal of Physiology. 2000;523:697–703. doi: 10.1111/j.1469-7793.2000.00697.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colquhoun D. Binding, gating, affinity and efficacy: the interpretation of structure-activity relationships for agonists and of the effects of mutating receptors. British Journal of Pharmacology. 1998;125:924–947. doi: 10.1038/sj.bjp.0702164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding S, Sachs F. Single channel properties of P2X2 purinoceptors. Journal of General Physiology. 1999;113:695–720. doi: 10.1085/jgp.113.5.695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey RJ, Thomas P, James CH, Wilderspin A, Smart TG. Identification of an inhibitory Zn2+ binding site on the human glycine receptor α1 subunit. Journal of Physiology. 1999;520:53–64. doi: 10.1111/j.1469-7793.1999.00053.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho SN, Hunt HD, Horton RM, Pullen JK, Pease LR. Site-directed mutagenesis by overlap extension using the polymerase chain reaction. Gene. 1989;77:51–59. doi: 10.1016/0378-1119(89)90358-2. [DOI] [PubMed] [Google Scholar]
- Housley GD. Physiological effects of extracellular nucleotides in the inner ear. Clinical and Experimental Pharmacology and Physiology. 2000;27:575–580. doi: 10.1046/j.1440-1681.2000.03314.x. [DOI] [PubMed] [Google Scholar]
- Kanjhan R, Housley GD, Burton LD, Christie DL, Kippenberger A, Thorne PR, Luo L, Ryan AF. Distribution of the P2X2 receptor subunit of the ATP-gated ion channels in the rat central nervous system. Journal of Comparative Neurology. 1999;407:11–32. [PubMed] [Google Scholar]
- Kao YH, Fitch CA, Bhattacharya S, Sarkisian CJ, Lecomte JT, Garcia-Moreno EB. Salt effects on ionization equilibria of histidines in myoglobin. Biophysical Journal. 2000;79:1637–1654. doi: 10.1016/S0006-3495(00)76414-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khakh BS. Molecular physiology of P2X receptors and ATP signaling at synapses. Nature Neuroscience Reviews. 2001;2:165–174. doi: 10.1038/35058521. [DOI] [PubMed] [Google Scholar]
- King BF, Townsend-Nicholson A, Wildman SS, Thomas T, Spyer KM, Burnstock G. Coexpression of rat P2X2 and P2X6 subunits in Xenopus oocytes. Journal of Neuroscience. 2000;20:4871–4877. doi: 10.1523/JNEUROSCI.20-13-04871.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King BF, Wildman SS, Ziganshina LE, Pintor J, Burnstock G. Effects of extracellular pH on agonism and antagonism at a recombinant P2X2 receptor. British Journal of Pharmacology. 1997;121:1445–1453. doi: 10.1038/sj.bjp.0701286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King BF, Ziganshina LE, Pintor J, Burnstock G. Full sensitivity of P2X2 purinoceptor to ATP revealed by changing extracellular pH. British Journal of Pharmacology. 1996;117:1371–1373. doi: 10.1111/j.1476-5381.1996.tb15293.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le K-T, Babinski K, Seguela P. Central P2X4 and P2X6 channel subunits coassemble into a novel heteromeric ATP receptor. Journal of Neuroscience. 1998;18:7152–7159. doi: 10.1523/JNEUROSCI.18-18-07152.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C, Peoples RW, Weight FF. Proton potentiation of ATP-gated ion channel responses to ATP and Zn2+ in rat nodose ganglion neurons. Journal of Neurophysiology. 1996;76:3048–3058. doi: 10.1152/jn.1996.76.5.3048. [DOI] [PubMed] [Google Scholar]
- Lynch KJ, Touma E, Niforatos W, Kage KL, Burgard EC, Van Biesen T, Kowaluk EA, Jarvis MF. Molecular and functional characterization of human P2X(2) receptors. Molecular Pharmacology. 1999;56:1171–1181. doi: 10.1124/mol.56.6.1171. [DOI] [PubMed] [Google Scholar]
- Nakazawa K, Liu M, Inoue K, Ohno Y. pH dependence of facilitation by neurotransmitters and divalent cations of P2X2 purinoceptor/channels. European Journal of Pharmacology. 1997;337:309–314. doi: 10.1016/s0014-2999(97)01293-4. [DOI] [PubMed] [Google Scholar]
- Nakazawa K, Ohno Y. Effects of neuroamines and divalent cations on cloned and mutated ATP-gated channels. European Journal of Pharmacology. 1997;325:101–108. doi: 10.1016/s0014-2999(97)00107-6. [DOI] [PubMed] [Google Scholar]
- Orioli P, Cini R, Donati D, Mangani S. X-ray structure of the ternary complex Zn(II)-ATP-2,2′-bipyridyl and possible model for ATP transport. Nature. 1980;283:691–693. doi: 10.1038/283691a0. [DOI] [PubMed] [Google Scholar]
- Paoletti P, Florent P-D, Fayyazuddin A, Le Goff A, Callebaut I, Neyton J. Molecular organization of a zinc binding N-terminal modulatory domain in a NMDA receptor subunit. Neuron. 2000;28:911–925. doi: 10.1016/s0896-6273(00)00163-x. [DOI] [PubMed] [Google Scholar]
- Ralevic V, Burnstock G. Receptors for purines and pyrimidines. Pharmacological Reviews. 1998;50:413–492. [PubMed] [Google Scholar]
- Reeh PW, Steen KH. Tissue acidosis in nociception and pain. Progress in Brain Research. 1996;113:143–151. doi: 10.1016/s0079-6123(08)61085-7. [DOI] [PubMed] [Google Scholar]
- Smart TG, Xie X, Krishek BJ. Modulation of inhibitory and excitatory amino acid receptor ion channels by zinc. Progress in Neurobiology. 1994;42:393–441. doi: 10.1016/0301-0082(94)90082-5. [DOI] [PubMed] [Google Scholar]
- Stoop R, Surprenant A, North RA. Different sensitivities to pH of ATP-induced currents at four cloned P2X receptors. Journal of Neurophysiology. 1997;78:1837–1840. doi: 10.1152/jn.1997.78.4.1837. [DOI] [PubMed] [Google Scholar]
- Tanokura M. 1H-NMR study on the tautomerism of the imidazole ring of histidine residues. I. Microscopic pK values and molar ratios of tautomers in histidine-containing peptides. Biochimica et Biophysica Acta. 1983;742:576–585. doi: 10.1016/0167-4838(83)90276-5. [DOI] [PubMed] [Google Scholar]
- Thomas T, Spyer KM. ATP as a mediator of mammalian central CO2 chemoreception. Journal of Physiology. 2000;523:441–447. doi: 10.1111/j.1469-7793.2000.00441.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vallejo AN, Pogulis RJ, Pease LR. Mutagenesis and synthesis of novel recombinant genes using PCR. In: Dieffenbach CW, Dveksler GS, editors. PCR Primer: A Laboratory Manual. Plainview, New York: Cold Spring Harbor Laboratory Press; 1995. pp. 603–612. [Google Scholar]
- Wang T-L, Hackam A, Guggino WB, Cutting GR. A single histidine residue is essential for zinc inhibition of GABA rho 1 receptors. Journal of Neuroscience. 1995;15:7684–7691. doi: 10.1523/JNEUROSCI.15-11-07684.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wildman SS, King BF, Burnstock G. Potentiation of ATP-responses at a recombinant P2x2 receptor by neurotransmitters and related substances. British Journal of Pharmacology. 1997;120:221–224. doi: 10.1038/sj.bjp.0700903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wildman SS, King BF, Burnstock G. Zn2+ modulation of ATP-responses at recombinant P2X2 receptors and its dependence on extracellular pH. British Journal of Pharmacology. 1998;123:1214–1220. doi: 10.1038/sj.bjp.0701717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wildman SS, King BF, Burnstock G. Modulation of ATP-responses at recombinant rP2X4 receptors by extracellular pH and zinc. British Journal of Pharmacology. 1999a;126:762–768. doi: 10.1038/sj.bjp.0702325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wildman SS, King BF, Burnstock G. Modulatory activity of extracellular H+ and Zn2+ on ATP-responses at rP2X1 and rP2X3 receptors. British Journal of Pharmacology. 1999b;128:486–492. doi: 10.1038/sj.bjp.0702802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wooltorton JR, McDonald BJ, Moss SJ, Smart TG. Identification of a Zn2+ binding site on the murine GABAA receptor complex: dependence on the second transmembrane domain of β subunits. Journal of Physiology. 1997;505:633–640. doi: 10.1111/j.1469-7793.1997.633ba.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong K, Peoples RW, Montgomery JP, Chiang Y, Stewart RR, Weight FF, Li C. Differential modulation by copper and zinc of P2X2 and P2X4 receptor function. Journal of Neurophysiology. 1999;81:2088–2094. doi: 10.1152/jn.1999.81.5.2088. [DOI] [PubMed] [Google Scholar]
- Zhou Z, Hume RI. Two mechanisms for inward rectification of current flow through the purinoceptor P2X2 class of ATP-gated channels. Journal of Physiology. 1998;507:353–364. doi: 10.1111/j.1469-7793.1998.353bt.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Z, Monsma LR, Hume RI. Identification of a site that modifies desensitization of P2X2 receptors. Biochemical and Biophysical Research Communications. 1998;252:541–545. doi: 10.1006/bbrc.1998.9689. [DOI] [PubMed] [Google Scholar]


