Abstract
We have employed in vitro physiological methods to investigate dopaminergic modulation of excitatory synaptic transmission in monkey prefrontal cortex (PFC) circuits. We show that combined activation of D1-like and D2-like dopamine receptors results in the reduction of extracellular stimulation-evoked isolated EPSCs in layer 3 pyramidal neurons. Using paired recordings from synaptically connected pyramidal neurons we have determined the basic properties of unitary synaptic connections between layer 3 pyramids in the primate PFC and, interestingly, we found that dopamine does not reduce synaptic transmission between nearby pairs of synaptically coupled PFC pyramidal neurons. This input specificity may be a critical aspect of the dopaminergic regulation of recurrent excitatory circuits in the PFC.
Dopaminergic modulation of neuronal activity in the dorsolateral prefrontal cortex (PFC) of primates is critical for the proper functioning of the PFC. Depletion of dopamine (DA) in the monkey PFC disrupts working memory (Brozoski et al. 1979), and activity of PFC neurons during the delay of delayed-response tasks is influenced by changes in the level of DA D1 receptor activation (Sawaguchi & Goldman-Rakic, 1994; Williams & Goldman-Rakic, 1995). However, the cellular mechanisms by which DA modulates PFC function are largely unknown.
The analysis of the functional role of DA in the PFC is complicated by species-specific features of PFC architecture. The dorsolateral PFCs of humans and macaque monkeys are thought to be homologous; however, there are significant anatomical differences between primate dorsolateral PFC and rodent medial PFC (Preuss, 1995). Dopaminergic projections to rat and primate PFC differ in their laminar specificity (Berger et al. 1991; Lewis & Sesack, 1997). Also, the lattice-like patterns of intrinsic horizontal connections found in the primate PFC (Levitt et al. 1993; Kritzer & Goldman-Rakic, 1995; Pucak et al. 1996) have not been observed in rodents. These species-specific differences in DA innervation and intrinsic circuitry make the analysis of DA modulation of PFC function in primates necessary for understanding the role of the PFC in cognitive function.
Previously we have shown that DA increases the excitability of layer 3 pyramidal cells in the primate PFC (Henze et al. 2000), an effect that may be important for sustaining the activity of PFC neurons during the delay of delayed-response tasks. Here we continue our analysis of the regulation of PFC circuits by DA by looking at the DA modulation of excitatory inputs to PFC neurons. Layer 3 pyramidal neurons in the primate PFC receive a variety of excitatory synaptic connections, including local and long range projections from pyramidal cells of various layers located in the same cortical region, associational and callosal projections from other cortical regions, and projections from the mediodorsal thalamus (Melchitzky et al. 1998, 1999, 2001). Any or all of these excitatory connections are potential targets for dopaminergic modulation, a phenomenon that has been observed previously in several preparations (Law-Tho et al. 1994; Koga & Momiyama, 2000; Seamans et al. 2001; Gao et al. 2001).
METHODS
PFC slices were obtained from 16 young adult (3.5–6 kg, 4–5 years old) male cynomolgus monkeys (Macaca fascicularis) according to procedures described previously (Henze et al. 2000; Gonzalez-Burgos et al. 2000). Animals were treated according to the guidelines in the National Institutes of Health Guide to the Care and Use of Animals, and with the approval of the University of Pittsburgh's Institutional Animal Care and Use Committee. Animals were treated with ketamine hydrochloride (25 mg kg−1, intramuscular), dexamethasone phosphate (0.5 mg kg−1, intramuscular) and atropine sulfate (0.05 mg kg−1, subcutaneous), intubated and then placed in a stereotaxic frame. Anaesthesia was maintained with 1 % halothane in 28 % O2-air. A craniectomy was performed over the dorsal PFC, and a small block of tissue was excised containing both medial and lateral banks of the caudal principal sulcus (Brodman's area 46). The tissue block was placed in an ice-cold modified artificial cerebrospinal fluid (ACSF; composed of (mm): 230 sucrose, 1.9 KCl, 1.2 Na2HPO4, 33 NaHCO3, 6 MgCl2, 0.5 CaCl2, 10 glucose, and 2 kynurenic acid; pH 7.3–7.4 when bubbled with 95 % O2-5 % CO2 gas mixture). Animals recovered quickly after the discontinuation of anaesthesia, without impairments in eating, drinking or other behaviours, and were treated with an analgesic (hydromorphone 0.02 mg kg−1, intramuscular) and a systematic antibiotic (chloramphenicol 15 mg kg−1, intramuscular) three times a day for 3 days. In most cases, the same procedure was performed 2–4 weeks later to obtain tissue from the opposite hemisphere. During the second procedure, after the craniectomy, the animal was given an overdose of pentobarbital (30 mg kg−1) and was perfused through the heart with ice-cold modified ACSF. A tissue block was excised that contained rostral area 46 and the adjacent area 9 in the contralateral PFC to the first biopsy. Tissue from these same animals was used in other studies (Gonzalez-Burgos et al. 2000; Henze et al. 2000; Melchitzky et al. 2001).
The PFC tissue blocks were cut into 400-μm-thick sections. Slices were maintained for at least 2 h prior to use in a standard ACSF containing (mm): 126 NaCl, 2 KCl, 1.2 Na2HPO4, 10 glucose, 2.5 NaHCO3, 6.0 MgCl2 and 1.0 CaCl2. During recordings, slices were superfused with oxygenated ACSF at 33 °C with 1.5 mm MgCl2 and 2.5 mm CaCl2. In addition, all recordings were done in the continuous presence of 75 μm sodium metabisulfite to reduce oxidation of DA in solution.
Pyramidal neurons in layer 3 were identified with IR-DIC optics (Gonzalez-Burgos et al. 2000). For voltage clamp recordings, patch pipettes (3–7 MΩ) were filled with an internal solution containing (mm): 120 caesium gluconate, 10 KCl, 10 Hepes, 0.1 EGTA, 4 Mg-ATP, 0.3 GTP, 10 sodium phosphocreatine, and in some cases the chloride channel blocker DIDS (5 mm). For current clamp recordings, the internal solution contained (mm): 120 potassium gluconate, 10 KCl, 10 Hepes, 0.1 EGTA, 4 Mg-ATP, 0.3 GTP and 10 sodium phosphocreatine. Recordings were obtained with Axoclamp-2A or Axopatch-1D amplifiers and membrane potential was not corrected for junction potential. Data acquisition and analysis were performed using LabView (National Instruments, Austin, TX, USA). All group data are presented as means ± s.e.m. Calculations of the coefficient of variation (CV = standard deviation/mean) included failures, although these were few in number (< 5 % of responses). P values of < 0.05 were considered to be significant. Extracellular stimulation was performed using bipolar stimulating electrodes made of nichrome wire, 26 or 62 μm in diameter, at a frequency of 0.1 Hz.
DA, SCH23390, SKF38393, SKF81297, quinpirole and sulpiride were obtained from RBI (Natick, MA, USA). All other drugs were from Sigma (St Louis, MO, USA).
RESULTS
Visually identified pyramidal neurons were recorded from layer 3 of primate PFC slices. After a recording was obtained, stimulation locations (∼500 μm from the recording electrode) and intensities were tested until an isolated EPSC was observed in the cell being recorded. Pharmacological isolation of EPSCs was not possible because GABAA receptor blockade resulted in epileptiform activity in the slices. To determine whether GABAergic IPSCs contributed to the postsynaptic current, we stimulated synaptic inputs while holding the cell at voltages positive to the chloride reversal potential (∼ −65 mV with our internal solution). When an outward (chloride) current was observed, the stimulation intensity was lowered or the stimulating electrode was moved, until stimulation no longer resulted in inhibition (see Fig. 1A). Only then experiments examining the effects of DA and DA agonists were begun. The effect of DA was tested on EPSCs recorded at −75 mV, a membrane potential at which glutamatergic currents are mediated primarily by AMPA receptors.
Figure 1. DA depresses extracellular stimulation-evoked EPSCs in layer 3 pyramidal cells of the monkey PFC.
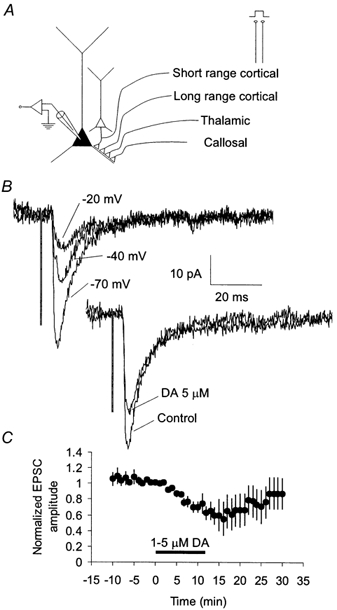
A, axons from several sources travel in the superficial layers of the PFC and form excitatory synapses onto layer 3 pyramidal neurons. Extracellular stimulation in superficial layers of the PFC may activate axons that originate in the contralateral PFC (callosal), the dorsomedial thalamus (thalamic), or from the cortex (long range cortical, either the PFC or other cortical areas which project to the PFC). Extracellular stimulation also may activate synapses made by pyramidal cells within 100–200 μm of the recorded cell via an anti/ortho-dromic route (short range cortical). B, extracellular stimulation-evoked postsynaptic currents (PSCs) were screened to obtain isolated EPSCs. Only PSCs showing no evidence of outward current at holding potentials positive to the chloride reversal potential were selected. Dopamine reduced the amplitude of isolated EPSCs. C, time course showing the average effect of dopamine (1–5 μm) on the peak amplitude of isolated extracellular stimulation-evoked EPSCs.
Addition of DA to the external bathing solution reduced the isolated EPSC for concentrations as low as 200–250 nm (60 ± 10 % of control for 200–250 nm, n = 4, Fig. 2) as well as by higher concentrations of DA (71 ± 5 % of control for 5 μm, n = 8, Fig. 2). Similar reductions were observed in experiments in which EPSPs were recorded. Significant EPSC reduction also was observed with application of the DA agonist apomorphine (78 ± 5 % of control, n = 7, 200 nm, Fig. 2), which activates both D1- and D2-type DA receptors. The reduction of EPSC amplitude by DA was blocked by the addition of either the D2-specific antagonist sulpiride (98 ± 7 % of control, n = 5, 2.5 μm) or the D1-specific antagonist SCH23390 (103 ± 9 % of control, n = 8, 5 μm), neither of which had any effect on baseline synaptic transmission (Fig. 2). These results suggest that the reduction of EPSCs required activation of both D1 and D2 family DA receptors.
Figure 2. Pharmacology of the reduction of EPSCs in layer 3 pyramidal cells.
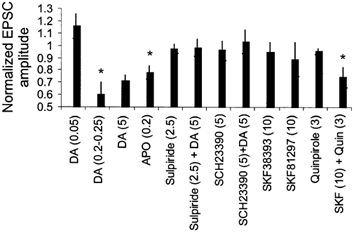
Bar graph showing the effects of various DA agonists and antagonists (concentrations in micromolar) on the amplitude of extracellular stimulation-evoked EPSCs recorded in layer 3 pyramidal neurons. *P > 0.05 when compared to control.
To confirm that the DA-induced reduction of EPSC amplitude requires activation of both D1-like and D2-like DA receptors we specifically activated subtypes of DA receptors. Neither the D1-specific agonists SKF38393 (95 ± 8 % of control, n = 9, 10 μm) or SKF81297 (88 ± 14 % of control, 10 μm, n = 6) nor the D2-specific agonist quinpirole (95 ± 2 % of control, n = 12, 3 μm) had any effect on EPSC amplitude (Fig. 2). However, the combined application of quinpirole and SKF38393 at these concentrations reduced EPSCs recorded in layer 3 pyramidal cells (74 ± 8 % of control, n = 5, Fig. 2). These data confirm that the reduction of EPSCs recorded in layer 3 pyramidal cells by DA requires activation of both D1-like and D2-like DA receptors.
The extracellular stimulation used in the above experiments may result in activation of several populations of axons forming synapses on layer 3 pyramidal cells (see Discussion) which may be modulated by DA in an input-specific manner. Activation of selective populations of axons in a slice preparation is difficult, because the somata of the cells that give rise to many of these axons are not preserved in the slice. However, layer 3 pyramidal cells send axon collaterals horizontally in layer 3 that contact neighbouring layer 3 pyramids (Melchitzky et al. 2001). Thus, to determine whether the reduction of EPSCs by DA was specific for excitatory synapses originating from a particular class of cells, we tested the effects of DA on synaptic transmission between pairs of synaptically connected nearby pyramidal cells in layer 3.
Pairs of layer 3 cells with somata less than 50 μm apart were recorded simultaneously in current clamp mode and tested for connectivity. Simultaneous recordings were obtained from approximately 40 pairs of nearby pyramidal cells and of these six pairs were connected (none reciprocally). These six connections had an average EPSP amplitude of 0.66 ± 0.29 mV and an average CV of 0.16 ± 0.03. Of these pairs, in four cases recordings from both of the cells remained stable long enough for the effect of DA to be tested (6–12 min of recording in the presence of DA). During DA application cells tended to depolarize requiring that resting membrane potential be maintained by injecting small constant current. In these four pairs, DA had no significant effect on the amplitude of EPSPs (EPSP amplitude: 115 ± 14 % of control; P > 0.05, n = 4, Fig. 3). Combined with the above data from experiments in which EPSCs were evoked by extracellular stimulation, these data suggest that DA reduces synaptic transmission at excitatory synapses in a synapse-specific manner.
Figure 3. Excitatory connections between nearby layer 3 pyramidal neurons are unaffected by addition of DA.

A, left, schematic showing recording configuration. Two pyramidal neurons with somata less than 50 μm apart were recorded simultaneously. Right, activation of an action potential in one cell evoked a unitary EPSP in the other. Traces show average pre- and postsynaptic responses from 10 consecutive sweeps recorded under control conditions (black line - a) or in the presence of dopamine (5 μm, grey line - b). B, time course of the DA effect. The amplitude of each unitary EPSP is plotted as a function of time from whole cell break-in. DA had no observable effect on EPSP amplitude at this unitary connection. Markings (a) and (b) show the time at which example traces in A were recorded. C, histogram showing amplitudes of the unitary EPSPs recorded in control conditions and in the presence of DA. D, group data showing effect of DA application on unitary EPSPs.
DISCUSSION
We have shown that DA causes a synapse-specific reduction of the strength of excitatory synaptic transmission at synapses onto pyramidal cells in the superficial layers of monkey PFC. Extracellular stimulation-evoked isolated EPSCs in layer 3 pyramidal neurons were reduced by the bath application of DA. This effect requires the combined activation of D1-like and D2-like DA receptors. The reduction of excitatory transmission is specific to one or more classes of excitatory inputs onto PFC pyramidal neurons, as indicated by the fact that unitary synaptic connections between nearby pyramidal neurons were unaffected by DA.
Together our data suggest that DA reduces inputs received by a pyramidal neuron in superficial layers of the dorsolateral PFC in an input-specific manner. We cannot, however, eliminate absolutely the possibility that the lack of DA effect on unitary EPSPs in the present study could be due to other factors. For example, despite our efforts to isolate EPSCs when using extracellular stimulation, these responses may have included a small inhibitory component. In this case, an increase by DA of an outward current could then be responsible for the apparent reduction in the inward current we observed. Although effects of DA on IPSCs have been observed previously (Seamans et al. 2001), D1 and D2 agonists have opposite short-term effects on ISPCs. Alternatively, whole cell dialysis of the presynaptic cell in the paired cell experiments could prevent a presynaptic effect of DA on the EPSP. However, presynaptic effects of DA have been observed previously in paired recordings (Gao et al. 2001) making this explanation unlikely.
There are several possible mechanisms for an input-specific reduction of EPSCs by DA. First, glutamate release at different classes of axon terminals may be modified differentially by DA, perhaps because DA receptors are present on certain axon terminals but not on others (Bergson et al. 1995). A second possibility is that the specificity of DA may be due to a spine-specific modulation of the postsynaptic response to glutamate. In the primate PFC, some spines receive both excitatory synaptic input and convergent synaptic input from dopaminergic terminals, forming ‘synaptic triads’ (Goldman-Rakic et al. 1989). The number of dopaminergic synapses made onto both spines and shafts is quite small (less than 90 per cell; Krimer et al. 1997), suggesting that triads may play a highly specific, but limited, role in mediating DA's effects on PFC pyramidal neurons. Therefore, the DA effect on excitatory inputs mediated via triads is likely to be restricted to a particular class of excitatory inputs. DA actions on glutamatergic inputs might also be selective if DA receptors are expressed in spines that receive a specific type of excitatory input but that lack DA synaptic input.
The present data along with our previous results (Henze et al. 2000) provide insight into how DA may facilitate recurrent excitation in the primate dorsolateral PFC. Periods of elevated DA in the PFC, such as those observed during delayed response tasks (Watanabe et al. 1997), will cause increased pyramidal neuron excitability. This increased excitability will promote sustained action potential discharge during the delay period of the delay task (Durstewitz et al. 2000). However, increased excitability will make pyramidal neuron responses less specific because equal levels of excitatory synaptic input would drive pyramidal neurons more strongly. As a result, there will be a decrease in the signal-to-noise ratio, making working memory storage unreliable (Camperi & Wang, 1998). The increased cell excitability together with selective depression of excitatory synaptic input by DA could increase the relative weight of specific synaptic inputs. For example, after the initial stimulus-driven activity pattern is established in the PFC network, DA may decrease the strength of those inputs that are most reliably coupled with the initial stimulus. This may prevent interference with the maintenance of specific activity patterns being held in working memory. This decrease in input would normally cause an overall decrease in firing rate; however, sustained firing in the PFC may be maintained by recurrent excitation combined with the DA-mediated increase in pyramidal cell excitability. Further testing of this model requires that the source of the synapses downregulated by DA application, which may include long-range intrinsic connections, ipsi- or contralateral cortical projections, or afferents from the thalamus, be identified.
Acknowledgments
This work was supported by NIMH Predoctoral Fellowship MH10474 (to D.A.H.), a HHMI Predoctoral Fellowship (to N.N.U.), and the NIMH Center for the Neuroscience of Mental Disorders (MH45156).
REFERENCES
- Berger B, Gaspar P, Verney C. Dopaminergic innervation of the cerebral cortex: unexpected differences between rodents and primates. Trends in Neurosciences. 1991;14:21–27. doi: 10.1016/0166-2236(91)90179-x. [DOI] [PubMed] [Google Scholar]
- Bergson C, Mrzljak L, Smiley JF, Pappy M, Levenson R, Goldman-Rakic PS. Regional, cellular, and subcellular variations in the distribution of D1 and D5 dopamine receptors in primate brain. Journal of Neuroscience. 1995;15:7821–7836. doi: 10.1523/JNEUROSCI.15-12-07821.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brozoski TJ, Brown RM, Rosvold HE, Goldman PS. Cognitive deficit caused by regional depletion of dopamine in prefrontal cortex of rhesus monkey. Science. 1979;205:929–932. doi: 10.1126/science.112679. [DOI] [PubMed] [Google Scholar]
- Camperi M, Wang XJ. A model of visuospatial working memory in prefrontal cortex: recurrent network and cellular bistability. Journal of Computational Neuroscience. 1998;5:383–405. doi: 10.1023/a:1008837311948. [DOI] [PubMed] [Google Scholar]
- Durstewitz D, Seamans JK, Sejnowski TJ. Neurocomputational models of working memory. Nature Neuroscience. 2000;3:1184–1191. doi: 10.1038/81460. [DOI] [PubMed] [Google Scholar]
- Gao WJ, Krimer LS, Goldman-Rakic PS. Presynaptic regulation of recurrent excitation by D1 receptors in prefrontal circuits. Proceedings of the National Academy of Sciences of the USA. 2001;98:295–300. doi: 10.1073/pnas.011524298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman-Rakic PS, Leranth C, Williams SM, Mons N, Geffard M. Dopamine synaptic complex with pyramidal neurons in primate cerebral cortex. Proceedings of the National Academy of Sciences of the USA. 1989;86:9015–9019. doi: 10.1073/pnas.86.22.9015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Burgos G, Barrionuevo G, Lewis DA. Horizontal synaptic connections in monkey prefrontal cortex: an in vitro electrophysiological study. Cerebral Cortex. 2000;10:82–92. doi: 10.1093/cercor/10.1.82. [DOI] [PubMed] [Google Scholar]
- Henze DA, Gonzalez-Burgos GR, Urban NN, Lewis DA, Barrionuevo G. Dopamine increases excitability of pyramidal neurons in primate prefrontal cortex. Journal of Neurophysiology. 2000;84:2799–2809. doi: 10.1152/jn.2000.84.6.2799. [DOI] [PubMed] [Google Scholar]
- Koga E, Momiyama T. Presynaptic dopamine D2-like receptors inhibit excitatory transmission onto rat ventral tegmental dopaminergic neurones. Journal of Physiology. 2000;523:163–173. doi: 10.1111/j.1469-7793.2000.t01-2-00163.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krimer LS, Jakab RL, Goldman-Rakic PS. Quantitative three-dimensional analysis of the catecholaminergic innervation of identified neurons in the macaque prefrontal cortex. Journal of Neuroscience. 1997;17:7450–7461. doi: 10.1523/JNEUROSCI.17-19-07450.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kritzer MF, Goldman-Rakic PS. Intrinsic circuit organization of the major layers and sublayers of the dorsolateral prefrontal cortex in the rhesus monkey. Journal of Comparative Neurology. 1995;359:131–143. doi: 10.1002/cne.903590109. [DOI] [PubMed] [Google Scholar]
- Law-Tho D, Hirsch JC, Crepel F. Dopamine modulation of synaptic transmission in rat prefrontal cortex: an in vitro electrophysiological study. Neuroscience Research. 1994;21:151–160. doi: 10.1016/0168-0102(94)90157-0. [DOI] [PubMed] [Google Scholar]
- Levitt JB, Lewis DA, Yoshioka T, Lund JS. Topography of pyramidal neuron intrinsic connections in macaque monkey prefrontal cortex (areas 9 and 46) Journal of Comparative Neurology. 1993;338:360–376. doi: 10.1002/cne.903380304. [DOI] [PubMed] [Google Scholar]
- Lewis DA, Sesack SR. Dopamine systems in the primate brain. In: Bloom FE, Bjorklund A, editors. The Primate Nervous System. New York: Elsevier; 1997. pp. 263–275. [Google Scholar]
- Melchitzky DS, Gonzalez-Burgos G, Barrionuevo G, Lewis DA. Synaptic targets of the intrinsic axon collaterals of supragranular pyramidal neurons in monkey prefrontal cortex. Journal of Comparative Neurology. 2001;430:209–221. doi: 10.1002/1096-9861(20010205)430:2<209::aid-cne1026>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- Melchitzky DS, Sesack SR, Lewis DA. Parvalbumin-immunoreactive axon terminals in macaque monkey and human prefrontal cortex: laminar, regional, and target specificity of type I and type II synapses. Journal of Comparative Neurology. 1999;408:11–22. [PubMed] [Google Scholar]
- Melchitzky DS, Sesack SR, Pucak ML, Lewis DA. Synaptic targets of pyramidal neurons providing intrinsic horizontal connections in monkey prefrontal cortex. Journal of Comparative Neurology. 1998;390:211–224. doi: 10.1002/(sici)1096-9861(19980112)390:2<211::aid-cne4>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- Preuss TM. Do rats have a prefrontal cortex? The Rose-Woosley-Akert program reconsidered. Journal of Cognitive Neuroscience. 1995;7:1–24. doi: 10.1162/jocn.1995.7.1.1. [DOI] [PubMed] [Google Scholar]
- Pucak ML, Levitt JB, Lund JS, Lewis DA. Patterns of intrinsic and associational circuitry in monkey prefrontal cortex. Journal of Comparative Neurology. 1996;376:614–630. doi: 10.1002/(SICI)1096-9861(19961223)376:4<614::AID-CNE9>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- Sawaguchi T, Goldman-Rakic PS. The role of D1-dopamine receptor in working memory: local injections of dopamine antagonists into the prefrontal cortex of rhesus monkeys performing an oculomotor delayed-response task. Journal of Neurophysiology. 1994;71:515–528. doi: 10.1152/jn.1994.71.2.515. [DOI] [PubMed] [Google Scholar]
- Seamans JK, Durstewitz D, Christie BR, Stevens CF, Sejnowski TJ. Dopamine D1/D5 receptor modulation of excitatory synaptic inputs to layer V prefrontal cortex neurons. Proceedings of the National Academy of Sciences of the USA. 2001;98:301–306. doi: 10.1073/pnas.011518798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe M, Kodama T, Hikosaka K. Increase of extracellular dopamine in primate prefrontal cortex during a working memory task. Journal of Neurophysiology. 1997;78:2795–2798. doi: 10.1152/jn.1997.78.5.2795. [DOI] [PubMed] [Google Scholar]
- Williams GV, Goldman-Rakic PS. Modulation of memory fields by dopamine D1 receptors in prefrontal cortex. Nature. 1995;376:572–575. doi: 10.1038/376572a0. [DOI] [PubMed] [Google Scholar]


