Abstract
Experiments were designed to examine if ion-flow through α-amino-3-hydroxy-5-methyl-isoxazolepropionic acid (AMPA) or kainate receptors interferes with protein structures associated with the gating machinery. Gating was studied using ultra-fast drug perfusion of outside-out patches containing rat GluR-A or GluR6 subunits excised from transfected human embryonic kidney cells. Deactivation rates of GluR6 kainate receptors observed following brief l-glutamate (10 mm Glu, 1 ms) applications differed by two to threefold in high (405 mm symmetrical Na+, τdecay = 2.7 ms at −100 mV) and low ionic strength (55 mm, τdecay = 1.1 ms) solutions. In comparison, GluR-A AMPA receptors were much less sensitive. Ion effects on GluR6 receptors did not reflect surface potential screening or ion-agonist competition at the agonist-binding site since deactivation rates were slower in high ionic strength solutions. Moreover, the apparent agonist affinity did not decrease with increasing ionic strength (e.g. 55 mm, EC50 = 110 μmvs. 405 mm, EC50 = 61 μm). GluR6 responses were strongly dependent on ions present on the external, but not the internal, side of the plasma membrane. Decay kinetics was regulated by the type of ion present suggesting that the chemical nature of the solution, not its ionic strength, governed channel behaviour. Both external anions and cations modulated the amplitude and decay kinetics of GluR6 responses in a concomitant manner. AMPA receptor responses recorded in identical ionic conditions did not exhibit this behaviour. These results identify a novel mechanism that distinguishes AMPA and kainate receptors. External ions regulate the gating machinery of kainate receptors through an allosteric mechanism that involves both anions and cations.
Compared to voltage-gated ion channels (Yellen, 1998; Yi & Jan, 2000), our understanding of the molecular events that govern desensitization of AMPA- and kainate-type glutamate receptors is still emerging (Dingledine et al. 1999). Recent analysis of the crystal structure of the ligand-binding core of AMPA (GluR2 or GluR-B) receptors has provided a novel perspective on the conformational events that lead into desensitization. Full and partial agonists have been shown to close the agonist-binding domain to different degrees (Armstrong et al. 1998; Armstrong & Gouaux, 2000). The extent of domain closure has been proposed to account for the different activation and desensitization behaviours of full and partial agonists at neuronal AMPA receptors (Patneau & Mayer, 1990, 1991; Patneau et al. 1992). However, it remains to be established how conformational changes in the agonist-binding domain are translocated to the other protein structures that form the gating machinery. It is possible that the extent of domain closure is not the only factor directing openings and closures of the pore region. For example ion-flow through the pore region of glutamate receptors may also regulate channel behaviour through electrostatic or physical interactions with gating machinery structures.
It has been known for sometime that ion-channels fail to gate normally under different ionic conditions (Yellen, 1997). Ascher and colleagues (Ascher et al. 1978) were the first to observe this effect, showing that the stability of the acetylcholine receptor in the open state is dependent on the permeant ion species. This observation was later explained by assuming that permeant ions in the pore prevent channel closure (Marchais & Marty, 1979). Swenson and Armstrong (1981) reported a similar observation for voltage-gated K+-channels, suggesting that the time an ion resides in the pore during permeation determines channel open time. In effect, permeant ions restrict channel closure, indicating that gating and permeation can be coupled. A number of channel blockers have also been shown to hinder channel closure in much the same way as permeant ions (Armstrong, 1971; Yeh & Armstrong, 1978; Cahalan & Almers, 1999). Yeh and Armstrong (1978) coined the expression ‘foot in the door’ for the action of pancuronium on K+-channels that has now become a general term to describe this phenomenon on other channels.
Channel block of kainate receptors has been shown to regulate channel gating (Bähring & Mayer, 1998; Bowie et al. 1998; Bowie et al. 1999) suggesting that events in the pore region may interact with structures associated with the channel gate. For example the cytoplasmic channel blocker, spermine, destabilizes the open state of the kainate receptor, GluR6 (Bowie et al. 1998). Spermine affects GluR6 gating properties only at membrane potentials where channel block occurs, suggesting that polyamines may hasten channel closure whilst occupying the pore (Bowie et al. 1998). Although the precise nature of this mechanism is still not understood, one possibility is that polyamines entering the pore region deplete it of permeant cations and, as a result, promote channel closure. An important implication of this hypothesis is that ions in the permeation pathway stabilize GluR6 receptors in the open state much like the ‘foot in the door’ effect described for K+ channels (Yeh & Armstrong, 1978). Despite this observation, the effect of permeant ions on glutamate receptor gating properties has not been examined extensively. A careful study of neuronal N-methyl-d-aspartate (NMDA) receptors has not identified ion-dependent gating (Antonov et al. 1998), though a single point mutation in the pore region does confer strong coupling between permeation and gating processes (Schneggenburger & Ascher, 1997). I show that external anions and cations regulate, in a concomitant manner, the behaviour of kainate but not AMPA receptors. Experiments described here suggest that permeant and non-permeant ions regulate kainate receptors through an allosteric mechanism rather than by ‘foot in the door’ effect.
METHODS
Cell culture and expression of recombinant receptors
HEK 293 cells (CRL 1573; American Type Culture Collection, Manassas, VA, USA) and tsA201 cells (provided by Dr R. Horn, Jefferson Medical College, PA, USA) were maintained at a confluency of 70–80 % in minimal essential medium with Earle's salts, 2 mm glutamine and 10 % fetal bovine serum. Cells plated at low density were transfected using the calcium phosphate technique as described previously (Bowie et al. 1998) with cDNAs encoding GluR-A and GluR6 receptor subunits (supplied by Drs M. L. Mayer and K. M. Partin at the National Institutes of Health, MD, USA and Colorado State University, CO, USA, respectively) The cDNA for green fluorescent protein (GFP S65T mutant) was routinely co-transfected to help identify transfected cells.
Electrophysiology and recording solutions
All outside-out patch recordings were made with an Axopatch 200B amplifier (Axon Instruments Inc., CA, USA) using thin-walled borosilicate glass pipettes (2–5 MΩ) coated with dental wax to reduce electrical noise. Control and L-Glu (1 or 50 ms duration) solutions were rapidly applied to outside-out patches excised from HEK293 or tsA201 cells expressing GluR-A or unedited GluR6 subunits as described previously (Bowie et al. 1998). Solution exchange (10–90 % rise-time = 25–50 μs) was determined routinely by measuring a liquid junction current at the end of the experiment (Bowie et al. 1998). External solutions used in initial experiments (Figs 1–4) contained 55, 150 or 405 mmNaCl, 5 mm Hepes, 0.1 mm each of CaCl2 and MgCl2, pH 7.3. Internal solutions of 55, 150 and 405 mm Na+, contained 10, 115 or 360 mmNaCl respectively, with 10 mm NaF, 5 mm Hepes, 5 mm Na4BAPTA, 0.5 mm CaCl2, 1 mm MgCl2, 10 mm Na2ATP, pH 7.3. Osmotic pressure was adjusted to 295 mOsm for 55 and 150 mmNaCl solutions with sucrose and to 750 mOsm for 405 mmNaCl. The osmotic pressure did not influence channel gating. In experiments where different external anions were compared, 150 mm solutions containing the sodium salt of various halides (F−, Cl−, Br−, I−) and monovalent anions (propionate, nitrate, acetate) were prepared and the pH adjusted with NaOH. Similarly, for different alkali metal ions, 150 mm solutions of the chloride salt of different cations (Li+, Na+, K+, Rb+ and Cs+) were prepared and the pH adjusted with the corresponding hydroxide solution (e.g. LiOH for LiCl solutions). In all experiments, the reference electrode was connected to the bath via an agar bridge of 3 m KCl. Current records were filtered at 5 kHz, digitized at 25–50 kHz and series resistances (3–10 MΩ) compensated by 95 %. Data acquisition and analysis was performed using pClamp7 software (Axon Instruments Inc., CA, USA) and data illustrated using Origin 6.1 (OriginLab Corporation, MA, USA). All experiments were performed at room temperature.
Figure 1. Ions affect the gating properties of kainate receptors.
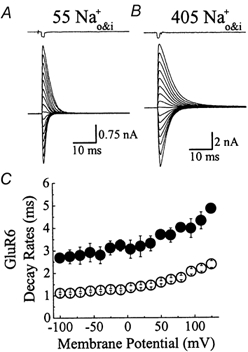
Membrane currents activated by brief (1 ms) applications of 10 mm l-Glu at various membrane potentials (−100 to + 125 mV, 15 mV increments) in 55 mm (A) and 405 mm (B) symmetrical NaCl. In each case, the top trace shows the junction current (1 ms) recorded with an open patch electrode at the end of the experiment. C, summary plot comparing the deactivation rates measured in low (55 mm Na+, ○, n = 11) and high (405 mm Na+, •, n = 3) ionic strength.
Figure 4. External ions do not affect AMPA receptor kinetics.
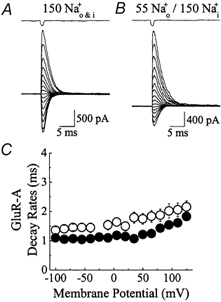
A and B, Glu responses (10 mm Glu, 1 ms duration) evoked at a range of membrane potentials (−100 to + 125 mV, 15 mV increments) in external NaCl solutions of 150 mm (A) and 55 mm (B). Responses in each ionic condition were recorded from the same patch. Unlike kainate receptors, lowering NaCl concentration did not accelerate deactivation rates but produced a modest slowing of kinetics. The junction current (1 ms) recorded at the end of the experiment with an open patch electrode is shown above each trace. C, summary of the effect of external ions on GluR-A deactivation rates in 150 mm (n = 6, •) and 55 mm (n = 5, ○) external NaCl.
RESULTS
Ion effects on kainate receptors
To investigate if permeant ions stabilize the kainate receptor in the open state, the rate of channel closure in solutions of different ionic strength was determined. It has been tentatively assumed that ion concentration in the channel lumen can be altered by changes in the bulk ion concentration. Figure 1A and B show typical membrane currents elicited by 1 ms pulses of 10 mm Glu at a range of membrane potentials (−100 to +125 mV, 15 mV increments) in solutions of symmetrical 55 mm and 405 mmNaCl respectively. Since internal and external concentrations of the main permeant ion, sodium, were identical, the reversal potentials in each case were close to 0 mV. Due to the brief duration of the agonist pulse, the rate of current decay observed following removal of Glu reflected, primarily, the rate of channel closure (i.e. deactivation). Consistent with the hypothesis that permeant ions stabilize the open state, deactivation rates were faster in solutions of low ionic strength. For example at −100 mV, currents activated by 10 mm Glu in 55 and 405 mmNaCl decayed with time constants of 1.11 ± 0.04 ms (n = 11) and 2.69 ± 0.04 ms (n = 3), respectively (Fig. 1C). Similar observations were made at all membrane potentials tested suggesting that GluR6 receptor gating is modulated in a voltage-independent manner (Fig. 1C).
Although these results are consistent with sodium ions acting like a ‘foot in the door’ to prevent channel closure (Yeh & Armstrong, 1978), a number of other mechanisms involving sodium and/or chloride ions may also account for these observations. Moreover, it is possible that the ionic species involved are not important per se but rather their total ionic charge or strength. It is unlikely that ion effects on GluR6 gating result from screening of surface potential (Hille, 1992) or ion-agonist competition (Akk & Auerbach, 1996) at the agonist-binding site. First, deactivation rates are slower not faster at higher ionic strengths. Second, the apparent agonist affinity does not decrease with increasing ionic strength, as would be expected for either mechanism. Figure 2 summarizes the ion effects on peak Glu dose-response relationships in symmetrical solutions of 55, 150 and 405 mmNaCl. EC50 (Hill slope, nH) values were estimated to be 110 ± 13 μm (nH = 1.0, n = 8), 694 ± 75 μm (nH = 1.1, n = 4) and 61 ± 4 μm (nH = 1.4, n = 5) for symmetrical 55, 150 and 405 mmNaCl solutions, respectively.
Figure 2. Ion effects on kainate receptor dose-response relationships.
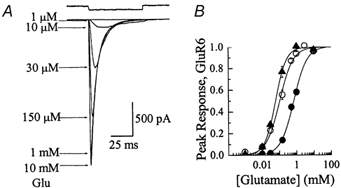
A, typical responses evoked by Glu in symmetrical 405 mmNaCl on a patch that exhibited an EC50 value and Hill slope of 61.3 μm and 1.15 respectively. The top trace shows the junction current (50 ms) recorded with an open patch electrode at the end of the experiment. B, dose-response relationships for 55 (○, n = 8), 150 (•, n = 4) and 405 mmNaCl (▴, n = 5). In each ionic condition, response rundown was not appreciable; however, to minimize this effect, all dose-response relationships were constructed by alternating between high and low agonist concentrations. Data are expressed as the mean ± s.e.m.
External not internal ions affect kainate receptors
Before investigating whether the ion effects were due to the ionic strength of the solution or the chemical nature of its ions, the sensitivity of GluR6 receptors to ions on both or only one side of the plasma membrane was tested. Figure 3 summarizes experiments examining the effect of reducing external or internal NaCl on response amplitude and deactivation rates. Reducing external NaCl from 150 mm to 55 mm had three effects on GluR6 responses. First, as expected from the Nernst relationship, the reversal potential shifted from 0 mV (Fig. 3A) in symmetrical 150 mm Na+ to around −25 mV (Fig. 3B) in 55 mm external Na+/150 mm internal Na+ (Fig. 3D). Reversal potentials estimated from current-voltage relationships constructed in 150 mm (filled circle) and 55 mm (open circle) external Na+ in the same patch (n = 3) are shown in Fig. 3D. Second, peak Glu responses were reduced at both positive and negative membrane potentials in 55 mm external NaCl (Fig. 3A and B). Due to the hyperpolarizing shift in the reversal potential, inward currents would be expected to be smaller in amplitude; however, the reduction of outward currents suggests that external ions modulate GluR6 response amplitude in a voltage-independent manner (Fig. 3D). Third, as shown previously (Fig. 1C), deactivation rates were accelerated at low ionic strength (55 mm, Fig. 3B) compared to the control (150 mm, Fig. 3A). For example at −100 mV, currents activated by 10 mm Glu in 55 and 150 mmNaCl decayed with time constants of 1.00 ± 0.08 ms (n = 3) and 2.55 ± 0.18 ms (n = 9) respectively, representing a 2.55-fold acceleration in the decay of the control (Fig. 3E). In contrast, lowering internal NaCl had no effect on deactivation rates (Fig. 3C) suggesting that ions affect GluR6 receptors only from the external side of the plasma membrane. Figure 3E summarizes the effect of reducing external (open triangle) or internal NaCl (open circle) on the kinetic properties of GluR6. It is worth noting that despite the reversal potential difference, the deactivation rates for GluR6 in symmetrical 55 mm Na+ (Fig. 1C) and 55 mm external Na+/ 150 mm internal Na+ (Fig. 3E) were identical.
Figure 3. Ions affect kainate receptor kinetics from the external but not internal membrane surface.
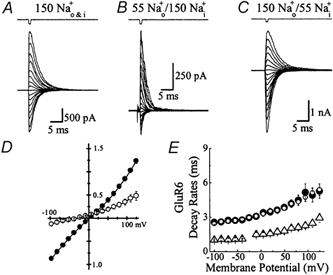
A and B, Glu responses (10 mm, 1 ms duration) evoked at different membrane potentials (−100 to +125 mV, 15 mV increments) in external NaCl solutions of 150 mm (A) and 55 mm (B). Data were obtained from the same outside-out patch recording. Lowering external NaCl concentration produced an expected shift in the reversal potential (−25 mV) but also a voltage-independent acceleration of decay kinetics and a reduction of peak responses. C, in another patch, lowering internal NaCl (55 mm) shifted the reversal potential to more positive values (+25 mV) but did not affect decay kinetics. D, Glu current-voltage relationships comparing symmetrical 150 mmNaCl (•, n = 3) with 55 mm external NaCl (○, n = 3) in the same patch shows the hyperpolarizing reversal potential shift and voltage-independent reduction in current amplitude. E, plot summarizing the effects of external and internal NaCl on GluR6 kinetics. Compared to 150 mmNaCl control (•, n = 9), lowering external NaCl (55 mm, ▵, n = 3) accelerated GluR6 deactivation kinetics whereas lowering internal NaCl (55 mm, ○, n = 6) had no effect. Data are expressed as mean ± s.e.m.
External ion effects on AMPA receptors
Although kainate- and AMPA-type Glu receptors exhibit distinct pharmacological profiles, they are closely related modular proteins, sharing many basic properties such as membrane topology, ionic selectivity and channel block behaviour as well as gating similarly in response to Glu (Dingledine et al. 1999). In view of this, whether AMPA receptors are also modulated by external ions was tested. As described for kainate receptors (Fig. 3), the effect of lowering external NaCl on the AMPA receptor composed of GluR-A subunits was examined (Fig. 4). Replacement of 150 mm external NaCl with 55 mmNaCl in the same patch produced a shift in the reversal potential from 0 mV to around −25 mV membrane potential but did not accelerate deactivation rates as described for kainate receptors (Fig. 4A and B). Instead, deactivation rates in 55 mm external NaCl were slowed modestly in comparison with control responses in 150 mm external NaCl. For example at −100 mV, currents activated by 10 mm Glu in 55 and 150 mmNaCl decayed with time constants of 1.35 ± 0.12 ms (n = 5) and 1.10 ± 0.04 ms (n = 6) respectively representing a 1.2-fold slowing in the control decay rate (Fig. 4C). Figure 4C summarizes the effect of external NaCl on deactivation rates of GluR-A channels and reveals that the modest ion effect on the AMPA receptor was observed at all membrane potentials tested.
Ionic strength does not regulate kainate receptors
To understand if the effects of external ions on kainate receptors were imparted by the chemical nature of individual ions or from changes in ionic strength, GluR6 responses were examined in solutions where the ionic strength was kept constant but the ion composition was altered (Fig. 5). For comparison, ion-substitution experiments were repeated with GluR-A receptors (Fig. 6). The external monovalent cation, Na+, was replaced by alkali metal ions (Li+, K+, Rb+, Cs+) that have similar permeability through non-NMDA receptors (Burnashev et al. 1996). External Cl− ions were replaced with a number of monovalent anions (F−, Br−, I−, propionate, nitrate, acetate). Unlike cations, unedited Q-forms of GluR-A and GluR6 are impermeable to anions (Burnashev et al. 1996). Experiments outlined below describe the effect of external cations and anions on desensitization kinetics observed using long (50 ms duration) agonist applications. Interestingly, external ions similarly affected deactivation rates (data not shown), suggesting that protein conformations associated with channel closure and desensitization are probably coupled, as proposed previously (Trussell & Otis, 1996; Partin et al. 1996).
Figure 5. External cations and anions modulate amplitude and kinetics of kainate receptors.
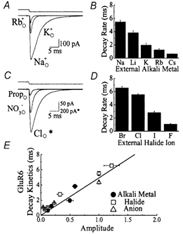
A, typical Glu responses (10 mm Glu, 50 ms duration, Vh=−20 mV) evoked in the same patch in 150 mm external Na, K or RbCl solutions. Replacement of external Na+ with K+ or Rb+ ions elicited a reversible, voltage-independent reduction in response amplitude and acceleration of desensitization kinetics. Upper trace shows the open tip current recorded at the end of the experiment. B, plot summarizing the effect of external alkali metal ions on the entry rate of GluR6 channels into desensitization. Statistical information is provided in Table 1. C, Glu responses (10 mm Glu, 50 ms duration, Vh=−20 mV) evoked in the same patch in the presence of 150 mm external chloride, nitrate or propionate sodium solutions. Similar to alkali metal ions, replacement of external chloride with nitrate or propionate elicited a reversible, voltage-independent reduction in response amplitude and acceleration of desensitization kinetics. Note that the agonist response observed in external Cl− (marked by *) is significantly larger in amplitude and has been plotted at a different scale to permit comparison. Upper trace shows the open tip current. D, plot summarizing the effect of external halide ions on the entry rate of GluR6 channels into desensitization. Statistical information is provided in Table 2. E, plot comparing GluR6 response amplitude and desensitization kinetics under different external ion conditions. Data have been fit by linear regression analysis through the origin (continuous line, y = 4.94x, r = 0.83).
Figure 6. The effect of external cations and anions on AMPA receptor amplitude and kinetics.
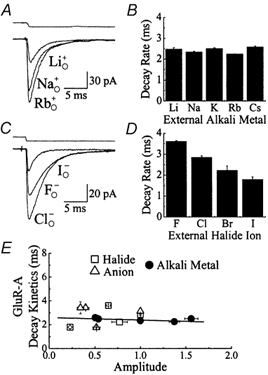
A, 10 mm Glu responses (50 ms duration, Vh=−20 mV) evoked in the same patch in the presence of 150 mm external Na, Rb or LiCl solutions. Replacement of external Na+ with Rb+ or Li+ ions elicited a reversible, voltage-independent increase or reduction in response amplitude respectively but did not affect desensitization kinetics. Upper trace shows the open tip current recorded at the end of the experiment. B, plot showing that external alkali metal ions do not influence the onset of GluR-A desensitization. Statistical information is provided in Table 1. C, typical Glu responses (10 mm Glu, 50 ms duration, Vh=−20 mV) evoked in the same patch in the presence of 150 mm external Cl−, F− or I− Na solutions. Replacement of external Cl− with F− or I− elicited a reversible, voltage-independent effect on desensitization kinetics and a reduction in response amplitude. Upper trace shows the open tip current. D, plot summarizing the effect of external halide ions on GluR-A desensitization rates. Statistical information is provided in Table 2. E, GluR-A response amplitude and desensitization kinetics in different external ion conditions are plotted for comparison. Data have been fit by linear regression analysis (continuous line, y =−0.22x + 2.6, r =−0.19).
Figure 5A shows the effect of substituting 150 mm external Na+ with equimolar K+ or Rb+ ions on GluR6 responses on the same patch (10 mm Glu, 50 ms duration, Vh−20 mV). Compared to control, external K+ or Rb+ elicited an apparently concomitant and reversible reduction in the peak response amplitude and the rate of desensitization (Fig. 5A, Table 1). Similar observations were made with Li+ and Cs+ (Fig. 5B, Table 1). These observations suggest that kainate receptors are sensitive to the external cation composition and not the solution's ionic strength. Unlike kainate receptors, the onset of GluR-A receptor desensitization was not dependent on the external alkali metal (Fig. 6A and B, Table 1). Figure 6A shows typical GluR-A responses evoked by 10 mm Glu in 150 mm external Rb+, Na+ and Li+. The decay kinetics were similar in each case; however, the peak response amplitude was cation-dependent (Table 1). The effect of external alkali metals on AMPA and kainate receptors was voltage-insensitive with no apparent shift in the reversal potential (data not shown). Kainate receptors, however, are not modulated exclusively by external cations. Experiments described below reveal that external anions also influence the kinetic behaviour of GluR6.
Table 1.
Summary of desensitization time constants and the relative amplitude of AMPA and kainate receptors in different alkali metal ions
| Li+ | Na+ | K+ | Rb+ | Cs+ | |
|---|---|---|---|---|---|
| GluR6 | |||||
| 3.84 ± 0.29 ms | 5.47 ± 0.23 ms | 1.97 ± 0.28 ms | 1.22 ± 0.19 ms | 0.65 ± 0.02 ms | |
| 0.59 ± 0.04 | 1 | 0.50 ± 0.06 | 0.18 ± 0.01 | 0.12 ± 0.01 | |
| (n = 10) | (n = 29) | (n = 10) | (n = 7) | (n = 3) | |
| GluR-A | |||||
| 2.48 ± 0.07 ms | 2.34 ± 0.07 ms | 2.50 ± 0.07 ms | 2.24 ± 0.02 ms | 2.58 ± 0.06 ms | |
| 0.54 ± 0.02 | 1 | 1.56 ± 0.07 | 1.37 ± 0.06 | 0.50 ± 0.03 | |
| (n = 16) | (n = 34) | (n = 9) | (n = 4) | (n = 5) | |
Data are expressed as means ± s.e.m.n, number of observations.
Similar to external Na+ effects, replacement of external Cl− with other monovalent anions modulated GluR6 receptor response amplitude and desensitization rates (Fig. 5C and D, Table 2). A typical Glu response evoked in 150 mm external NaCl is compared in Fig. 5C with responses observed in external nitrate and propionate on the same patch. Similar observations were obtained with external halide ions Br−, I− and F− (Fig. 5D). As described previously for cations, external anions also reversibly modulated GluR6 response amplitude and decay kinetics in a voltage-independent manner with no apparent shift in the reversal potential (data not shown).
Table 2.
Summary of desensitization time constants and the relative amplitude of AMPA and kainate receptors in different monovalent anions
| F− | Cl− | Br− | I− | Propionate | Nitrate | Acetate | |
|---|---|---|---|---|---|---|---|
| GluR6 | |||||||
| 1.05 ± 0.10 ms | 5.54 ± 0.12 ms | 6.56 ± 0.24 ms | 2.79 ± 0.24 ms | 1.06 ± 0.06 ms | 1.63 ± 0.04 ms | 0.76 ± 0.06 ms | |
| 0.12 ± 0.02 | 1 | 1.22 ± 0.13 | 0.33 ± 0.05 | 0.04 ± 0.00 | 0.23 ± 0.03 | 0.07 ± 0.01 | |
| (n = 9) | (n = 21) | (n = 8) | (n = 7) | (n = 5) | (n = 8) | (n = 5) | |
| GluR-A | |||||||
| 3.60 ± 0.05 ms | 2.84 ± 0.07 ms | 2.22 ± 0.21 ms | 1.79 ± 0.02 ms | 3.43 ± 0.20 ms | 1.76 ± 0.04 ms | 3.41 ± 0.51 ms | |
| 0.65 ± 0.02 | 1 | 0.77 ± 0.09 | 0.23 ± 0.02 | 0.40 ± 0.02 | 0.52 ± 0.04 | 0.34 ± 0.03 | |
| (n = 8) | (n = 30) | (n = 14) | (n = 8) | (n = 10) | (n = 10) | (n = 8) | |
Data are expressed as means ± s.e.m.n, number of observations.
It is interesting that both external cations and anions modulate the response amplitude and decay kinetics of GluR6 to a similar degree. To examine this relationship for GluR6 receptors more closely, response amplitude and decay kinetics in each ionic condition were plotted for comparison (Fig. 5E). Linear regression analysis through the origin fit the data well (continuous line, y = 4.94x, r = 0.83, Fig. 5E), suggesting that external ions probably influence GluR6 response amplitude and decay kinetics similarly. Linear regression fits were improved when the line was not forced through the origin (y = 3.89x + 0.39, r = 0.92, data not shown). Although external anions modulate GluR-A decay kinetics (Fig. 6C and D, Table 2), the relationship between the response amplitude and decay kinetics in different ionic conditions is different from kainate receptors (Fig. 6E). Linear regression analysis of GluR-A experiments fit the data poorly (continuous line, y =−0.22x + 2.6, r = −0.19, Fig. 6E), suggesting that the effects of external ions on GluR-A response amplitude and decay kinetics are unrelated.
DISCUSSION
Like NMDA receptors, both AMPA- and kainate-type glutamate receptors are subject to allosteric regulation by a number of ions found in the extracellular milieu that bathes neurons (Dingledine et al. 1999). However, unlike NMDA receptors, there has been much less success in demonstrating physiological or pathological roles for these ions. AMPA and kainate receptors are regulated by a number of exogenous cations including H+ (Mott & Dingledine, 1999; Ihle & Patneau, 2000), Zn2+ (Rassendren et al. 1990; Mott & Dingledine, 1999), Ca2+ (Perouansky & Grantyn, 1989; Bowie & Mayer, 1996), Mg2+ (Bowie & Mayer, 1996) as well as Hg2+ (Kiskin et al. 1986; Umbach & Gundersen, 1989) ions. Perhaps more surprising for cation-selective ion channels, the anion thiocyanate also regulates AMPA (Bowie & Smart, 1993; Arai et al. 1995; Partin et al. 1996) and kainate (D. Bowie, unpublished observation) receptor gating behaviour. In this study, external anions and cations are shown to influence the gating properties and response amplitude of both recombinant AMPA and kainate receptors. Interestingly, external ions apparently regulate both deactivation and desensitization similarly, supporting previous findings that channel closure and desensitization are coupled (Trussell & Otis, 1996; Partin et al. 1996). Individual anions or cations determine the amplitude and decay kinetics of kainate receptors in a concomitant manner whereas this behaviour is not retained by AMPA receptors. Whether this difference supports the existence of disparate gating mechanisms for AMPA and kainate receptors remains to be established.
How do external anions and cations modulate kainate receptors?
Experiments described in this study suggest that neither ion-agonist competition at the agonist-binding domain nor the ionic strength of the solution influences the gating behaviour of kainate receptors. Moreover, ion effects are unlikely to reflect channel block since kainate receptors are regulated in a voltage-independent manner by both permeant and non-permeant ions. Instead, a comparison of a series of anions and cations suggested that the chemical nature of the solution probably governed the gating behaviour of kainate receptors.
Classical work on voltage-dependent Na+ (Hille et al. 1975; Dani et al. 1983) and K+ (Kao & Stanfield, 1968) channels has shown that both cations and anions can selectively regulate gating behaviour (Hille, 1992). Each of these effects was attributed to the screening of surface charge located on or in the vicinity of the voltage sensor (Kao & Stanfield, 1968; Hille et al. 1975; Dani et al. 1983; Hille, 1992). Interestingly, it is possible to predict the cation selectivity observed in this study from the electrostatic behaviour of different cations at a single anion-binding site on the kainate receptor (Eisenman, 1962; Hille, 1992). Assuming that cation binding stabilizes the open state of the channel, the rank order of potency is Na+ > Li+ > K+ > Rb+ > Cs+, which corresponds to sequence X of the Eisenman series, favouring the binding of smaller rather than larger cations (Eisenman, 1962; Hille, 1992).
Despite this interesting correlation amongst external cations, it is nonetheless difficult to account for the behaviour of anions. As expected for a charge-screening mechanism, the effects of cations and anions on Na+ and K+ channels have been shown to be distinct, suggesting that several non-identical, local surface charges are located on each protein structure (Dani et al. 1983; Hille, 1992). However in this study, anions and cations elicited apparently identical effects and, contrary to the charge-screening mechanism, these results favour a common site of action for ions of different charge. One possibility is that external anions modulate kainate receptors from anion-specific binding sites but, like cations, regulate gating behaviour through a common pathway. An alternative possibility is suggested from experiments designed to understand the weak cation permeability of anion channels (Franciolini & Nonner, 1987; Hille, 1992). To account for their observation, Franciolini and Nonner (1987) proposed that anion channels possess a site of net negative charge (in the pore region) that can attract and bind small cations. The pairing of the cation with this negative charge establishes a dipole that can, in turn, attract anions that are present in the solution (Franciolini & Nonner, 1987). Since the cation will interact differently with various anions, this mechanism for cation permeation of anion channels could also account for the observations described in this study.
Identifying the regulatory site(s) on kainate receptors
At present, it is difficult to speculate on whether anions and cations regulate kainate receptors in the vicinity of the pore region or at other sites on the protein structure. There are two apparently conflicting pieces of evidence. Channel block by cytoplasmic spermine accelerates gating kinetics, suggesting that the depletion of permeant ions in the pore might occur (Bowie et al. 1998). In contrast, the ion effects described in this study are voltage-insensitive and occur with both permeant and non-permeant ions, favouring a location outside the membrane electric field. One possibility is that both spermine and external ions act at discrete sites that are functionally linked in much the same way that agonist binding is coupled to channel activation (Colquhoun, 1998). Alternatively, allosteric ion effects and channel block may regulate kainate receptors by independent mechanisms. GluR6 receptor activation promotes channel openings of at least two or three subconductance levels (Swanson et al. 1996; Howe, 1996); however, their sensitivity to polyamine block or kinetic behaviour in different ionic conditions has not been investigated (Dingledine et al. 1999). Assuming subconductance states are kinetically distinct, allosteric modulation by external ions or channel block by spermine may affect kainate receptors by shifting the relative proportions of sublevels. Consistent with this, recent non-stationary variance analysis has revealed that the weighted unitary GluR6 conductance is dependent on the external ion composition (D. Bowie, unpublished observation). However, whether this observation reflects an ion-dependent shift in the relative contributions of several sublevels, as speculated, awaits future analysis of unitary events.
Acknowledgments
I thank Dr P. Seeburg for permission to use GluR-A and GluR6 cDNAs, Drs K. Partin and M. L. Mayer for providing them, Dr R. Horn for the tsA201 cell line and Dr G. D. Lange for enlivening discussions. I am indebted to Dr S. F. Traynelis for support and Dr R. Dingledine for the piezo-electric stack. Preliminary experiments identifying GluR6 ion effects were performed in Dr M. L. Mayer's laboratory. This work was supported by the National Institute of Health (RO1 MH62144, D. Bowie and RO1 NS36654, S. F. Traynelis).
REFERENCES
- Akk G, Auerbach A. Inorganic, monovalent cations compete with agonists for the transmitter binding site of nicotinic acetylcholine receptors. Biophysical Journal. 1996;70:2652–2658. doi: 10.1016/S0006-3495(96)79834-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antonov SM, Gmiro VE, Johnson JW. Binding sites for permeant ions in the channel of NMDA receptors and their effects on channel block. Nature Neuroscience. 1998;1:451–456. doi: 10.1038/2167. [DOI] [PubMed] [Google Scholar]
- Arai A, Silberg J, Kessler M, Lynch G. Effect of thiocyanate on AMPA receptor mediated responses in excised patches and hippocampal slices. Neuroscience. 1995;66:815–827. doi: 10.1016/0306-4522(94)00616-d. [DOI] [PubMed] [Google Scholar]
- Armstrong CM. Interaction of tetraethylammonium ion derivatives with the potassium channels of giant axons. Journal of General Physiology. 1971;58:413–437. doi: 10.1085/jgp.58.4.413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong N, Gouaux E. Mechanisms for activation and antagonism of an AMPA-sensitive glutamate receptor: crystal structures of the GluR2 ligand binding core. Neuron. 2000;28:165–181. doi: 10.1016/s0896-6273(00)00094-5. [DOI] [PubMed] [Google Scholar]
- Armstrong N, Sun Y, Chen G-Q, Gouaux E. Structure of a glutamate-receptor ligand-binding core in complex with kainate. Nature. 1998;395:913–917. doi: 10.1038/27692. [DOI] [PubMed] [Google Scholar]
- Ascher P, Marty A, Neild TO. Life time and elementary conductance of the channels mediating the excitatory effects of acetylcholine in Aplysia neurones. Journal of Physiology. 1978;278:177–206. doi: 10.1113/jphysiol.1978.sp012299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bähring R, Mayer ML. An analysis of philanthotoxin block for recombinant rat GluR6(Q) glutamate receptor channels. Journal of Physiology. 1998;509:635–650. doi: 10.1111/j.1469-7793.1998.635bm.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowie D, Bähring R, Mayer ML. Block of kainate and AMPA receptors by polyamines and arthropod toxins. In: Jonas P, Monyer H, editors. Handbook of Experimental Pharmacology; Ionotropic Glutamate Receptors in the CNS. Berlin: Springer-Verlag; 1999. pp. 251–373. [Google Scholar]
- Bowie D, Lange GD, Mayer ML. Activity-dependent modulation of glutamate receptors by polyamines. Journal of Neuroscience. 1998;18:8175–8185. doi: 10.1523/JNEUROSCI.18-20-08175.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowie D, Mayer ML. Dual blockade of glutamate receptors by external divalent cations and internal polyamines. Society for Neuroscience Abstracts. 1996;22:236.14–236.10. [Google Scholar]
- Bowie D, Smart TG. Thiocyanate ions selectively antagonize AMPA-evoked responses in Xenopus laevis oocytes microinjected with rat brain mRNA. British Journal of Pharmacology. 1993;109:779–787. doi: 10.1111/j.1476-5381.1993.tb13642.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnashev N, Villarroel A, Sakmann B. Dimensions and ion selectivity of recombinant AMPA and kainate receptor channels and their dependence on Q/R site residues. Journal of Physiology. 1996;496:165–173. doi: 10.1113/jphysiol.1996.sp021674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cahalan MD, Almers W. Block of sodium conductance and gating current in squid giant axons poisoned with quaternary strychinine. Biophysical Journal. 1999;27:57–74. doi: 10.1016/S0006-3495(79)85202-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colquhoun D. Binding, gating, affinity and efficacy: The interpretation of structure-activity relationships for agonists and of the effects of mutating receptors. British Journal of Pharmacology. 1998;125:923–948. doi: 10.1038/sj.bjp.0702164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dani JA, Sanchez JA, Hille B. Lyotropic anions: Na channel gating and Ca electrode response. Journal of General Physiology. 1983;81:255–281. doi: 10.1085/jgp.81.2.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dingledine R, Borges K, Bowie D, Traynelis SF. The glutamate receptor ion channels. Pharmacology Reviews. 1999;51:7–61. [PubMed] [Google Scholar]
- Eisenman G. Cation selective glass electrodes and their mode of operation. Biophysical Journal. 1962;2(Suppl. 2):259–323. doi: 10.1016/s0006-3495(62)86959-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franciolini F, Nonner W. Anion and cation permeability of a chloride channel in rat hippocampal neurons. Journal of General Physiology. 1987;90:453–478. doi: 10.1085/jgp.90.4.453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hille B. Ionic Channels of Excitable Membranes. Sunderland: Sinauer Associates; 1992. [Google Scholar]
- Hille B, Woodhull AM, Shapiro BI. Negative surface near sodium channels of nerve: divalent ions, monovalent ions, and pH. Philosophical Transactions of the Royal Society. 1975;270:301–318. doi: 10.1098/rstb.1975.0011. [DOI] [PubMed] [Google Scholar]
- Howe JR. Homomeric and heteromeric ion channels formed from kainate-type subunits GluR6 and KA2 have very small, but different, unitary conductances. Journal of Neurophysiology. 1996;76:510–519. doi: 10.1152/jn.1996.76.1.510. [DOI] [PubMed] [Google Scholar]
- Ihle EC, Patneau DK. Modulation of alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor desensitization by extracellular protons. Molecular Pharmacology. 2000;58:1204–1212. doi: 10.1124/mol.58.6.1204. [DOI] [PubMed] [Google Scholar]
- Kao CY, Stanfield PR. Actions of some anions on lectrical properties and mechanical threshold of frog twitch muscle. Journal of Physiology. 1968;198:291–309. doi: 10.1113/jphysiol.1968.sp008607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiskin NI, Krishtal OA, Tsyndrenko AY, Akaike N. Are sulfhydryl groups essential for function of the glutamate-operated receptor-ionophore complex. Neuroscience Letters. 1986;66:305–310. doi: 10.1016/0304-3940(86)90036-4. [DOI] [PubMed] [Google Scholar]
- Marchais D, Marty A. Interaction of permeant ions with channels activated by acetylcholine in Aplysia. Journal of Physiology. 1979;297:9–45. doi: 10.1113/jphysiol.1979.sp013025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mott DD, Dingledine R. Similarity in modulation of kainate and NMDA receptors. Society for Neuroscience Abstracts. 1999:594.3. [Google Scholar]
- Partin KM, Fleck MW, Mayer ML. AMPA receptor flip/flop mutants affecting deactivation,desensitization, and modulation by cyclothiazide,aniracetam,and thiocyanate. Journal of Neuroscience. 1996;16:6634–6647. doi: 10.1523/JNEUROSCI.16-21-06634.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patneau DK, Mayer ML. Structure-activity relationships for amino acid transmitter candidates acting at N-methyl-D-aspartate and quisqualate receptors. Journal of Neuroscience. 1990;10:2385–2399. doi: 10.1523/JNEUROSCI.10-07-02385.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patneau DK, Mayer ML. Kinetic analysis of interactions between kainate and AMPA: evidence for activation of a single receptor in mouse hippocampal neurons. Neuron. 1991;6:785–798. doi: 10.1016/0896-6273(91)90175-y. [DOI] [PubMed] [Google Scholar]
- Patneau DK, Mayer ML, Jane DE, Watkins JC. Activation and desensitization of AMPA/kainate receptors by novel derivatives of willardiine. Journal of Neuroscience. 1992;12:595–606. doi: 10.1523/JNEUROSCI.12-02-00595.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perouansky M, Grantyn R. Separation of quisqualate- and kainate-selective glutamate receptors in cultured neurons from rat superior colliculus. Journal of Neuroscience. 1989;9:70–80. doi: 10.1523/JNEUROSCI.09-01-00070.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rassendren FA, Lory P, Pin J-P, Nargeot J. Zinc has opposite effects on NMDA and non-NMDA receptors in Xenopus oocytes. Neuron. 1990;4:733–740. doi: 10.1016/0896-6273(90)90199-p. [DOI] [PubMed] [Google Scholar]
- Schneggenburger R, Ascher P. Coupling of permeation and gating in an NMDA-channel pore mutant. Neuron. 1997;18:167–177. doi: 10.1016/s0896-6273(01)80055-6. [DOI] [PubMed] [Google Scholar]
- Swanson GT, Feldmeyer D, Kaneda M, Cull-Candy SG. Effect of RNA editing and subunit co-assembly on single-channel properties of recombinant kainate receptors. Journal of Physiology. 1996;492:129–142. doi: 10.1113/jphysiol.1996.sp021295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swenson RP, Armstrong CM. K+ channels close more slowly in the presence of external K + and Rb+ Nature. 1981;291:427–429. doi: 10.1038/291427a0. [DOI] [PubMed] [Google Scholar]
- Trussell LO, Otis TS. Physiology of AMPA receptors: Biophysical characterisitics that subserve integrative roles of synapses. In: Conti F, Hicks TP, editors. Excitatory Amino Acids and the Cerebral Cortex. Cambridge, MA, USA: MIT Press; 1996. pp. 63–72. [Google Scholar]
- Umbach JA, Gundersen CB. Mercuric ions are potent noncompetitive antagonists of human brain kainate receptors expressed in Xenopus oocytes. Molecular Pharmacology. 1989;36:582–588. [PubMed] [Google Scholar]
- Yeh JZ, Armstrong CM. Immobilisation of gating charge by a substance that simulates inactivation. Nature. 1978;273:387–389. doi: 10.1038/273387a0. [DOI] [PubMed] [Google Scholar]
- Yellen G. Single channel seeks permeant ion for brief but intimate relationship. Journal of General Physiology. 1997;110:83–85. doi: 10.1085/jgp.110.2.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yellen G. The moving parts of voltage-gated ion channels. Quarterly Review Biophysics. 1998;31:239–295. doi: 10.1017/s0033583598003448. [DOI] [PubMed] [Google Scholar]
- Yi BA, Jan LY. Taking apart the gating of voltage-gated K+ channels. Neuron. 2000;27:423–425. doi: 10.1016/s0896-6273(00)00052-0. [DOI] [PubMed] [Google Scholar]


