Abstract
The effects of phosphocreatine (PCr) on sarcoplasmic reticulum (SR) Ca2+ regulation were investigated in saponin-permeabilized rat ventricular myocytes. Cells were perfused continuously with weakly Ca2+-buffered solutions approximating to the intracellular milieu. Ca2+ release from the SR was detected using Fura-2 or Fluo-3. Withdrawal of PCr reduced the frequency of spontaneous Ca2+ release by 12.8 ± 3.4 % (n = 9) and the amplitude of the spontaneous Ca2+ transient by 17.4 ± 3.1 % (n = 9). Stepwise reductions in [PCr] progressively increased the time for the spontaneous Ca2+ transient to rise from 25 to 100 % of the maximum value (TP75) and to fall by 75 % of the peak level (DT75). Following complete PCr withdrawal, the TP75 and the DT75 were 147.1 ± 13.2 and 174.8 ± 23.2 % of the control values, respectively. Experiments involving confocal microscopy showed that PCr withdrawal decreased the propagation velocity of spontaneous Ca2+ waves. PCr withdrawal also reduced the frequency and amplitude, but increased the duration of spontaneous Ca2+ sparks. Rapid application of 20 mm caffeine was used to assess the SR Ca2+ content at the point of spontaneous Ca2+ release. In the absence of PCr, the amplitude of the caffeine-induced Ca2+ transient was 18.4 ± 2.7 % (n = 9) lower than in the presence of 10 mm PCr. This suggests that PCr withdrawal reduces the maximum SR Ca2+ content that can be sustained before spontaneous Ca2+ release occurs. These results suggest that local ADP buffering by PCr is essential for normal Ca2+ regulation by the SR. Prolongation of the descending phase of the spontaneous Ca2+ transient is consistent with a reduction in the efficiency of the SR Ca2+ pump due to ADP accumulation. The fact that spontaneous Ca2+ release occurs at a lower SR Ca2+ content in the absence of PCr suggests that the Ca2+ release mechanism may also be affected. These effects may be of relevance in circumstances where PCr depletion and Ca2+ overload occur, such as myocardial ischaemia or anoxia.
Previous studies have established that the creatine kinase (CK) reaction serves to maintain cytosolic ATP levels following the onset of anoxia or ischaemia in cardiac muscle (reviewed by Allen & Orchard, 1987). However, ATP synthesis via the CK reaction may also have an important role in supporting Ca2+ regulation during normal excitation-contraction coupling (for review see Wallimann et al. 1992). It has been shown that a subfraction of CK isoenzymes is bound within muscle cells at locations of high energy utilization, including the sarcoplasmic reticulum (SR) membrane (Rossi et al. 1990). Experiments on isolated SR vesicles and permeabilized muscle fibres suggest that bound CK is functionally coupled to the SR Ca2+-ATPase. For example, in the presence of millimolar levels of cytosolic ATP, introduction of PCr markedly increased the Ca2+ uptake rate and the maximum SR Ca2+ content (Korge et al. 1993; Minajeva et al. 1996). Furthermore, in these experiments, an exogenous ATP regenerating system (phosphoenol pyruvate and pyruvate kinase) was less effective at supporting SR Ca2+ uptake than PCr acting in conjunction with bound CK.
It has also been suggested that ATP synthesized locally by CK may have preferential access to the SR Ca2+-ATPase (Arrio-Dupont et al. 1992). Similarly, much of the ADP produced by cellular ATPases appears to be rephosphorylated locally by CK and is not released into the bulk solution. This apparent compartmentalization of ATP and ADP might reflect the formation of a complex between CK and the SR ATPase, or an indirect effect involving the local flux through an unstirred layer. Whatever the underlying mechanism, these studies suggest that: (i) bound CK may supply much of the ATP utilized by the SR Ca2+-ATPase under normal conditions; and (ii) depletion of PCr might be expected to impair SR Ca2+ uptake, despite the presence of millimolar levels of cytosolic ATP.
There is limited evidence that the CK reaction may also influence the SR Ca2+ release process in striated muscle. In myotubes from CK-deficient mice, impaired SR Ca2+ uptake was accompanied by an apparent decrease in sensitivity of the SR Ca2+ release mechanism (Steeghs et al. 1997). There are a number of possible mechanisms that could explain this effect. It has been shown that CK is also bound to the junctional regions of the SR, which contain the ryanodine receptor (RyR), but lack ATPase activity (Rossi et al. 1990). Therefore, CK may contribute to ADP buffering in the junctional space, in close proximity to the RyR. A decrease in [ATP] and the resultant increase in [Mg2+] would both be expected to inhibit activation of the RyR (Fabiato & Fabiato, 1975; Rousseau et al. 1986; Yang & Steele, 2000). Furthermore, in the absence of local ADP buffering, the reduced efficiency of the Ca2+-ATPase might result in a decrease in the SR Ca2+ content (Minajeva et al. 1996). The open probability of the RyR and the gain of the Ca2+-induced Ca2+ release (CICR) mechanism are both strongly influenced by the luminal [Ca2+] (Sitsapesan & Williams, 1994; Bassani et al. 1995). Therefore, a decrease in luminal [Ca2+] could have an indirect inhibitory influence on SR Ca2+ release.
The aim of the present study was to investigate the influence of PCr withdrawal on the SR uptake and release mechanisms. Experiments were carried out on saponin-permeabilized cardiac myocytes and SR Ca2+ release was detected using Fluo-3 or Fura-2. Withdrawal of PCr reduced the frequency and amplitude of spontaneous Ca2+ transients. This was accompanied by a marked prolongation of the Ca2+ transient. Most of these effects may be explained by impaired SR Ca2+ uptake in the absence of PCr. However, PCr also decreased the maximum SR Ca2+ content that could be sustained before spontaneous Ca2+ release occurred. This effect suggests that the sensitivity of the RyR may be altered following PCr withdrawal. The underlying mechanisms and the possible relevance of these effects to Ca2+ regulation in anoxia or ischaemia are discussed.
METHODS
Ventricular myocyte isolation and permeabilization
Rats were killed by intraperitoneal overdose of sodium pentobarbitone and hearts rapidly excised into oxygenated physiological saline solution. Hearts were mounted on a Langendorff apparatus and perfused at 5 ml min−1 at 37 °C with a series of solutions based on an ‘isolation solution’ of the following composition (mm): NaCl, 130; KCl, 5.4; MgCl2, 1.4; NaH2PO4, 0.4; Hepes, 5; glucose, 10; taurine, 20; and creatine, 10; pH adjusted to 7.3 by addition of 1 m KOH. The first solution, containing 750 μm CaCl2, was perfused for 4 min. The heart was then perfused for 4 min with Ca2+-free isolation solution containing 100 μm Na2EGTA. Finally, perfusion was switched to the isolation solution containing 200 μm CaCl2 and collagenase (Worthington type 2; 0.1 mg ml−1) for 9–12 min. The left ventricle was dissected and finely chopped in a collagenase-containing solution with 1 % bovine serum albumin (BSA), and gently agitated in a water bath at 37 °C. Aliquots of the cell suspension were examined every 5 min until a > 80 % yield of rod-shaped cells with a clear striation pattern was obtained. Myocytes were collected by filtration through nylon gauze and gentle centrifugation. The myocytes were then exposed to saponin (10 μg ml−1) in a mock intracellular solution (as below, but containing 0.2 mm EGTA) for 6 min, before centrifugation and resuspension. All experiments were done at room temperature (20–22 °C).
Composition of mock intracellular solutions
Unless otherwise stated, chemicals were obtained from Sigma. H+ and Ca2+ were buffered with Hepes and EGTA respectively. Solutions used during and after saponin treatment contained ATP to support the activity of the SR and myofilaments. In all experiments, the ionic composition of the solution was adjusted to maintain [Ca2+], [Mg2+], [Na+], [K+] and pH constant. In brief, for most experiments, a basic solution was prepared containing KCl (100 mm), Hepes (25 mm), EGTA (0.05 mm), phosphocreatine (0–10 mm), ATP (5 mm) and Fura-2 (4 μm) or Fluo-3 (5 μm). MgCl2 was added (from 1 m stock solution) to produce a free concentration of 1.0 mm. The free [Ca2+] was adjusted to the desired level by addition of CaCl2 (1 m stock solution).
In experiments where [PCr] was reduced from 10 to 0 mm, 20 mmNaCl was added to maintain the [Na+] at 30 mm. As a consequence, the [Cl−] increased by 20 mm, from 111.6 to 131.2 mm. The [MgCl2] was reduced by approximately 0.2 mm in PCr-free solution to maintain the free Mg2+ at a constant level. However, we found that the effect of PCr withdrawal was similar, whether or not 20 mmNaCl was added to the solution lacking PCr. This suggests that: (i) compensation for the change in [Na+] that accompanied PCr withdrawal had little effect; and (ii) a 20 mm increase in [Cl−] had no apparent influence on the phenomena. Furthermore, increasing the [Cl−] from 112 to 132 mm at a constant [PCr] also had no significant effect on the caffeine-induced Ca2+ transients. In control experiments, we studied the effects PCr withdrawal when potassium propionate was used in place of KCl. However, the effects of PCr withdrawal were the same whether propionate or Cl− was used as the principal anion. In order to avoid possible inaccuracies in calculating the free [Mg2+] using binding constant data obtained under different ionic conditions, the concentration was measured directly using Mg2+-selective Fura-2 (Furaptra). The total concentrations of Na+ and K+ were 30 and 130 mm respectively and the pH was adjusted to 7.0 by addition of 1 m KOH.
Where necessary, the equilibrium concentrations of metal ions in the calibration solutions were calculated using the affinity constants for H+, Ca2+ and Mg2+ for EGTA as previously reported (Fabiato & Fabiato, 1979; Smith & Miller, 1985) using the Windows-based REACT program (Duncan et al. 1999). Corrections for ionic strength, details of pH measurement, allowance for EGTA purity and the principles of the calculations are as described by Miller & Smith (1984). In some experiments, 5 mm azide (BDH) was included in the solutions to inhibit possible mitochondrial activity. However, azide had no apparent influence on the effects of ATP reported in this study.
[Ca2+] measurement using Fura-2
The apparatus used for measurement of [Ca2+] in ventricular myocytes has been described previously (Yang & Steele, 2000). Briefly, the cells were placed in a cylindrical bath (5 mm diameter) in a Perspex block. The bottom of the bath was formed by attaching a coverslip to the underside of the block using epoxy resin. A drop of solution containing cells was placed at the bottom of the bath and a tightly fitting Perspex column inserted into the well until the lower surface was close to myocytes that had come to rest on the coverslip. During this procedure, some displaced solution rose up the side of the column, but most cells remained at the bottom of the bath. Perfusion was achieved by pumping solution (at 0.3 ml min−1) down a narrow bore running longitudinally through the centre of the column. After passing through the bath, the solution flowed continuously up the side of the column, where it was collected and taken to waste. In some experiments, solution with caffeine (20 mm) was rapidly applied via a narrow injection duct, which joined the column above the upper surface of the Perspex block.
The bath was placed on the stage of a Nikon Diaphot Eclipse inverted microscope and the cells were viewed using a ×40 oil immersion lens (Nikon Plan Flour ×40 DLL). In most experiments, the preparation was alternately illuminated with light of wavelengths 340 and 380 nm at 40 Hz frequency using a spinning wheel spectrophotometer (Cairn Research, Faversham, Kent, UK). The average [Ca2+] within the visual field containing the preparation was indicated by the ratio of light intensities emitted at > 500 nm. Light emitted from areas of the field to each side of the cell was eliminated using a variable rectangular window on the side-port of the microscope and long-pass filtered at > 480 nm before entering the photomultiplier. In these experiments, Fura-2 was used in preference to Fluo-3, because ratiometric Ca2+ measurement is less prone to possible movement artefacts. However, in experiments involving confocal microscopy, Fluo-3 was used.
Ca2+ measurement using confocal microscopy
The bath was placed on the stage of a Nikon Diaphot Eclipse inverted microscope and the cells were viewed using a ×40 oil immersion lens (Nikon Plan Fluor DLL, NA 1.3) or a ×60 water immersion lens (Nikon, Plan Apo, NA 1.2). A confocal laser-scanning unit (Microradiance 2000, Bio-Rad, Hemel Hempsted, Herts, UK) was attached to the side-port of the microscope. The X-Y resolution of the system was 0.45 μm, as measured from the point-spread function of fluorescent microspheres (diameter, 0.175 μm; Molecular Probes). The aperture size was set to the size of the Airy disc (1.8 mm for ×60 objective, 1.07 mm for ×40 objective) to optimize the Z-axis resolution (approx. 2 × X-Y resolution). Fluo-3 fluorescence was excited with the 488 nm line of an argon ion laser and emitted fluorescence was measured at > 515 nm. Images were acquired in line scan mode (at intervals of 6 or 2 ms) along the longitudinal axis of the cell. To reduce possible laser damage, the position of the line was changed after two or three scans. Sparks were identified by applying an automatic spark detection program to the line scan images as previously described (Cheng et al. 1999). As in previous studies on skinned cells, events were detected with the detection threshold set at 2.6 × s.d. (Lukyanenko & Gyorke, 1999). Image processing and analysis were done using IDL (Research Systems Inc., Boulder, CO, USA) and Laserpix (Bio-Rad, Hemel Hempsted, Herts, UK) software. Gaussian curves were fitted using Origin (Microcal, Northampton, MA, USA).
Data recording and analysis
The ratio signal and the individual wavelength intensities were digitized at 40–100 Hz as appropriate, using a Data Translation 2801A computer card in a 120 MHz Pentium PC, using in-house software. Data are presented as mean values ± s.e.m. Where necessary, statistical significance was determined using Student's t test (Microsoft Excel).
RESULTS
Effects of PCr withdrawal on spontaneous Ca2+ release
Saponin-permeabilized rat ventricular myocytes were perfused with solutions weakly buffered with 0.05 mm EGTA, in the presence of 10 mm PCr. The solutions also contained 4 μm Fura-2 to allow SR Ca2+ release to be detected. In the presence of 260 nm Ca2+, cells exhibited repetitive spontaneous Ca2+ release due to the cyclic uptake and release of Ca2+ from the SR. Figure 1A shows steady-state fluorescence transients under control conditions (upper trace), following complete withdrawal of PCr from the bathing medium (middle trace) and after reintroduction of 10 mm PCr (lower trace). Superimposed and normalized Ca2+ transients, obtained in the presence and absence of PCr, are shown on an expanded time scale in Fig. 1B. Withdrawal of PCr markedly prolonged the descending phase of the spontaneous Ca2+ transients. Most of this prolongation occurred early in the descending phase, resulting in a characteristic flattening of the peak of the Ca2+ transient. Since the Ca2+ transients were smaller in the absence of PCr, it is unlikely that the flattening of the transient reflects saturation of the dye. The duration of the rising phase also increased slightly in the absence of PCr. These effects on the time course of the transient were fully reversible on reintroduction of PCr.
Figure 1. Effect of cytosolic PCr on spontaneous Ca2+ release.
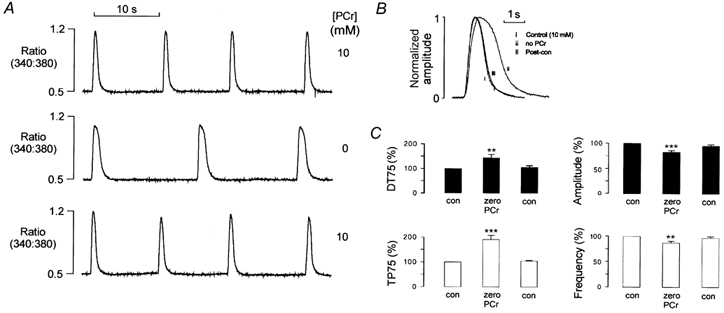
A, records of the Fura-2 fluorescence ratio from a saponin-permeabilized ventricular myocyte. In the presence of 10 mm PCr, transient increases in the fluorescence ratio occurred due to spontaneous SR Ca2+ release (top trace). Withdrawal of PCr resulted in a decrease in the amplitude and frequency of the spontaneous transients (middle trace). This effect was reversible on reintroduction of 10 mm PCr (bottom trace). B, spontaneous fluorescence transients in the presence and absence of PCr are shown superimposed and normalized on an expanded time scale. C, accumulated data from 9 myocytes showing the effects of PCr withdrawal on the mean time for the spontaneous transient to rise from 25 to 100 % of the maximum value (TP75) or to fall by 75 % of the peak level (DT75). Also shown is the effect of PCr withdrawal on the amplitude and frequency of spontaneous Ca2+ release. Each point represents the mean + s.e.m.
The accumulated data (Fig. 1C) show the mean time for the transient to rise from 25 to 100 % of the maximum value attained (TP75) or fall by 75 % of the peak level (DT75) in the presence and absence of PCr. Following withdrawal of PCr, the DT75 increased by 90.8 ± 16.7 % (mean ± s.e.m., n = 9, P < 0.001) and the TP75 increased by 44.8 ± 12.6 % (mean ± s.e.m., n = 9, P < 0.01), while the amplitude was reduced by 17.4 ± 3.1 % (mean ± s.e.m., n = 9, P < 0.001) and the frequency was reduced by 12.8 ± 3.4 % (mean ± s.e.m., n = 9, P < 0.01). These parameters returned to control levels on reintroduction of PCr.
Concentration-dependent effects of PCr on spontaneous CICR
In order to establish the concentration dependence of these effects, [PCr] of the bathing solution was decreased in a stepwise manner from 10 to 0 mm. Representative steady-state fluorescence records at each [PCr] are shown normalized and superimposed in Fig. 2A. The prolongation of the descending phase became apparent at [PCr] less than 2.5 mm and was near maximal at 0.1 mm PCr.
Figure 2. Concentration dependence of PCr effects.
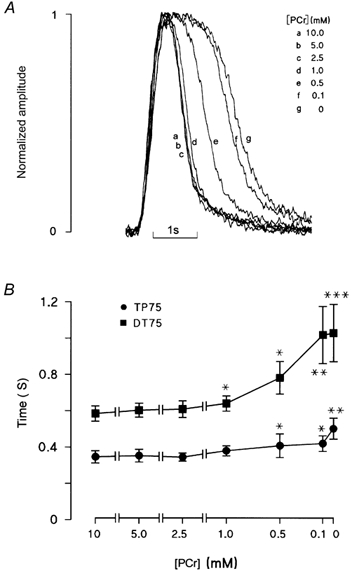
A, spontaneous Ca2+ transients at various [PCr] are shown superimposed and normalized on an expanded time scale. All transients were obtained from the same ventricular myocyte following equilibration at each [PCr]. B, accumulated data from 7 myocytes showing the mean time for the Ca2+ transient to rise from 25 to 100 % of the maximum value (TP75) or to fall by 75 % of the peak level (DT75) over a range of [PCr]. Each point represents the mean ± s.e.m. (n = 7). Reducing [PCr] to 1.0 mm caused a small but significant prolongation of the descending phase of the Ca2+ transient, without affecting the rising phase. At 0.5 mm PCr and below, the descending and ascending phases were both markedly prolonged. *P < 0.05, **P < 0.02 and ***P < 0.001.
The accumulated data (Fig. 2B) show that, as PCr decreased from 10 to 1.0 mm, DT75 increased significantly from 584 ± 42 to 639 ± 42 ms (P < 0.05). A further reduction in [PCr] to 0.5 and then 0.1 mm resulted in corresponding increases in DT75, reaching a maximum at 1028 ± 158 ms (P < 0.001) in the complete absence of PCr. The time to peak of the transient was not significantly affected until [PCr] was reduced to 0.5 mm. On introduction of 0.5 mm PCr, the TP75 increased from 346 ± 34 to 407 ± 65 ms (P < 0.05). Following complete withdrawal of PCr, the TP75 increased to 501 ± 58 ms (P < 0.01).
Confocal imaging of spontaneous Ca2+ waves before and after PCr withdrawal
Depending on the conditions, myocytes can exhibit spontaneous activity ranging from localized abortive Ca2+ waves to large waves, which propagate across the entire cell (Cheng et al. 1996). Therefore, changes in the amplitude or time course of the spatially averaged Ca2+ transients could potentially result from more complete wave propagation, or changes in localized Ca2+ release events.
The properties of spontaneous SR Ca2+ release and the effects of PCr were investigated in more detail using confocal microscopy. This involved repeated longitudinal scans along a single line at 1.3 ms intervals. Successive lines were placed one below the other to produce a ‘waterfall’ plot. In Fig. 3A, a permeabilized cell was perfused with a solution containing 260 nm Ca2+ and 10 mm PCr. A localized Ca2+ release occurred (left of centre), which then propagated longitudinally in both directions to the edges of the myocyte. Progression of the wave front produced sharply defined diagonal lines of increased fluorescence, joining the initiation site to both ends of the cell. Therefore, the gradient of the diagonal line defined by the wave front is an index of the propagation velocity. In the same myocyte, Ca2+ release responses at lower [PCr] (1.0, 0.5 and 0 mm) were characterized by prolonged time courses.
Figure 3. Effects of PCr on spontaneous Ca2+ waves in permeabilized myocytes.
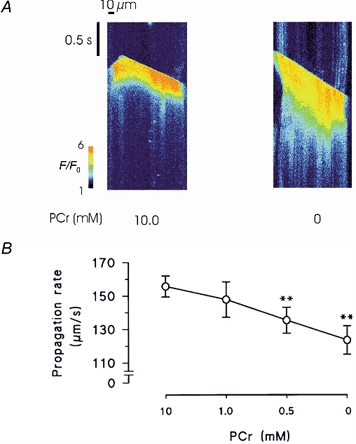
A, line scan images of Fluo-3 florescence during spontaneous Ca2+ waves in the presence and absence of PCr. Following withdrawal of PCr, spontaneous waves were markedly prolonged and the propagation rate was reduced. Both responses were from the same permeabilized myocyte. B, accumulated data showing changes in the propagation velocity of spontaneous Ca2+ waves following equilibration with solutions containing a range of [PCr]. Error bars represent the means ± s.e.m., n = 8. **P < 0.02.
The conduction velocity was calculated by fitting a linear function to the diagonal line defined by the wave front. In some cells, where contraction resulted in large changes in myocyte length, the linear function was fitted to the early phase of the wave, which preceded significant shortening. Accumulated data of the changes in conduction velocity are shown in Fig. 3B. On average, decreasing [PCr] to 0.5 and 0 mm resulted in a significant reduction in the Ca2+ wave propagation velocity from 155.7 ± 6.3 (control) to 135.4 ± 7.8 and 123.5 ± 8.4 μm s−1 respectively (n = 8, both P < 0.02).
Relationship between the [PCr] and SR Ca2+ content in permeabilized myocytes
The protocol shown in Fig. 4 was designed to investigate the relationship between the [PCr] and the SR Ca2+ content. Cells were initially equilibrated with solutions containing 260 nm Ca2+ and 10 mm PCr until regular spontaneous transients were observed. The frequency of the release was then measured and a high concentration of caffeine (20 mm) rapidly applied when a spontaneous Ca2+ release would otherwise have occurred. The amplitude of the caffeine-induced Ca2+ release was used as an index of the SR Ca2+ content at the point of spontaneous release. Figure 4A (left trace) shows the last spontaneous Ca2+ transient followed by a caffeine-induced Ca2+ transient. This protocol was then repeated following complete withdrawal of PCr (middle trace) and then after reintroduction of 10 mm PCr (right trace). In the absence of PCr, the amplitude of the caffeine-induced Ca2+ transient at the point of spontaneous Ca2+ release was consistently smaller. This suggests that spontaneous Ca2+ release occurs at a lower SR Ca2+ content in the absence of PCr.
Figure 4. SR Ca2+ content at the point of spontaneous Ca2+ release.
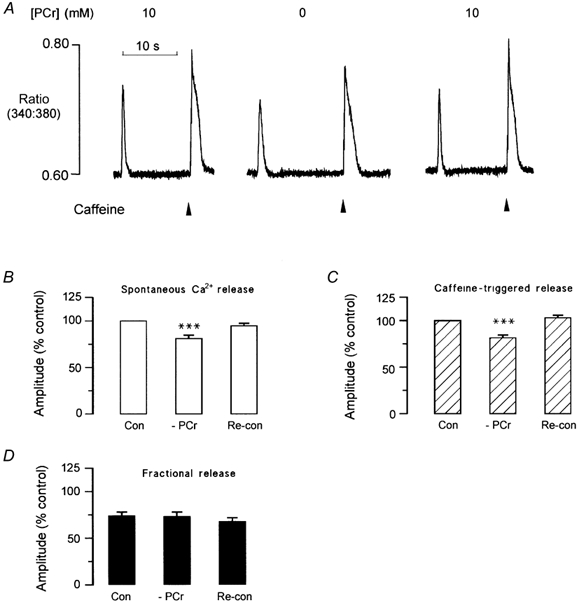
A, a spontaneous Ca2+ release is shown, followed by a release induced by rapid application of caffeine when spontaneous release would otherwise have occurred (left trace). The amplitude of the caffeine-induced Ca2+ transient was used as an index of the SR Ca2+ content at the point of spontaneous release. Withdrawal of PCr resulted in a decrease in the amplitude of the spontaneous Ca2+ transient and the caffeine-induced transient (middle trace). Following reintroduction of PCr, the responses returned to control levels (right trace). B, accumulated data showing the effects of PCr withdrawal and reintroduction on the amplitude of the spontaneous Ca2+ transient. C, the amplitude of the caffeine-induced Ca2+ transient. D, accumulated data showing the effects of PCr withdrawal and reintroduction on the fractional release (i.e. amplitude of caffeine-induced Ca2+ transient/amplitude of spontaneous Ca2+ transient). ***P < 0.002, n = 6–15.
The accumulated data (Fig. 4B) show that following withdrawal of PCr, the amplitudes of spontaneous Ca2+ release and caffeine-induced Ca 2+ transients decreased by 19.0 ± 3.5 and 18.4 ± 2.7 % respectively (both P < 0.01; n = 9) while the fractional Ca2+ release (spontaneous Ca2+ transient amplitude/caffeine-induced transient amplitude) remained constant. On reapplication of 10 mm PCr, the amplitude of both the spontaneous Ca2+ transient and the caffeine-triggered release returned to control levels.
Effect of ADP on the spontaneous Ca2+ release in the absence of PCr
Under normal conditions, PCr buffers ADP within the cell by rephosphorylating ADP to ATP, catalysed by CK. Therefore, the effects of PCr on SR Ca2+ regulation may indirectly result from local accumulation of ADP within the cell. This was investigated by the addition of exogenous ADP in the absence of PCr. Figure 5A shows that addition of 0.2 mm ADP to a solution lacking PCr resulted in a further prolongation of the descending phase of the Ca2+ transient. As with PCr withdrawal, most of this prolongation occurred early in the descending phase, resulting in a characteristic flattening of the peak of the Ca2+ transient. This result supports the possibility that local accumulation of ADP may underlie the changes in the time course of the spontaneous Ca2+ transient following PCr withdrawal. This effect was fully reversible on the removal of exogenous ADP.
Figure 5. Effects of ADP on spontaneous Ca2+ release in the absence of PCr.
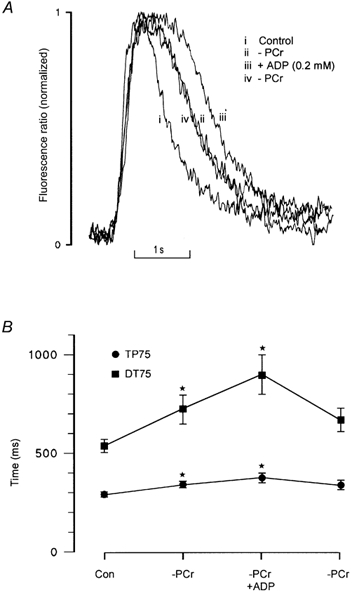
A, spontaneous Ca2+ transients are shown superimposed and normalized on an expanded time scale: (i) in the presence of 10 mm PCr; (ii) in the absence of PCr; (iii) in the absence of PCr, but with 0.2 mm ADP added; and (iv) following reintroduction of a solution lacking PCr (zero ADP). B, the accumulated data show that after withdrawal of PCr, addition of 0.2 mm ADP further increased TP75 by 10.4 ± 4.6 % (mean ± s.e.m., n = 6) and DT75 by 24.2 ± 5.2 % (mean ± s.e.m., n = 6; *P < 0.01) respectively.
The accumulated data (Fig. 5B) show again that withdrawal of PCr results in a marked increase in DT75 and TP75, from 536.7 ± 32.8 and 289.5 ± 6.5 to 726.3 ± 73.8 and 339.6 ± 13.5 ms respectively (both P < 0.01). Addition of 0.2 mm ADP in PCr-free perfusing solution resulted in a further increase in DT75 and TP75 to 900.4 ± 100.5 (P < 0.05, n = 6) and 666.3 ± 59.2 ms (P < 0.05, n = 6).
Effects of [PCr] on spontaneous Ca2+ sparks in permeabilized myocytes
Figure 6 shows line scan images of spontaneous Ca2+ sparks in the presence and absence of PCr. In these experiments, [EGTA] was increased to 0.36 mm, while free [Ca2+] was maintained at ∼260 nm. This level of EGTA has been shown to block propagation of Ca2+ sparks without significantly altering the amplitude, time course or spatial characteristics. In the presence of 10 mm PCr, spontaneous Ca2+ sparks are clearly visible in the line scan image. Following equilibration with a solution lacking PCr, sparks were noticeably prolonged and the frequency decreased significantly. Selected plots of F/F0 (resting fluorescence/peak fluorescence) were obtained by averaging over three pixels, centered on a Ca2+ release event (Fig. 6A, right).
Figure 6. Effects of PCr on spontaneous Ca2+ sparks in permeabilized myocytes.
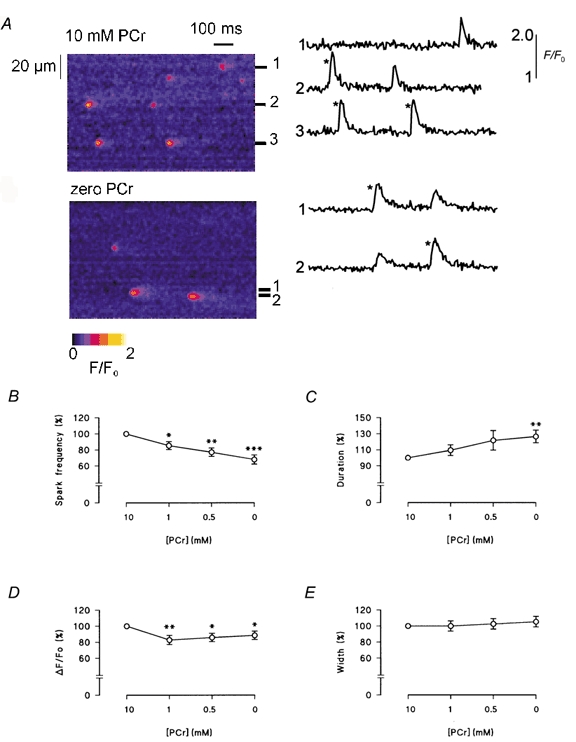
A, representative longitudinal line scan images of Fluo-3 fluorescence from the same myocyte in the presence of PCr (top) and in its absence (bottom). Selected plots of F/F0 (to the right of each image) were obtained by averaging over 3 pixels, centered on a Ca2+ release event. Comparison of the largest Ca2+ sparks obtained under both conditions (*) shows that sparks were typically smaller and more prolonged in the absence of PCr. B-E, accumulated data illustrating the effects of PCr on the frequency, duration, amplitude and width of spontaneous Ca2+ sparks. Error bars represent the means ± s.e.m., n = 8. *P < 0.05, **P < 0.02 and ***P < 0.005.
Both line scan images and the resulting F/F0 plots contain large (bright) sparks and smaller, apparently briefer events. As suggested in previous studies, out of focus sparks arising outside the focal plane contribute to the apparent variability in the characteristics of the Ca2+ sparks. However, comparison of the largest events in the F/F0 plots shows that spark amplitude was reduced slightly in the absence of PCr and the descending phase was clearly prolonged.
Accumulated data relating to the effects of PCr on spark characteristics are shown in Fig. 6B-E. On average, reducing [PCr] to 1.0 mm or less significantly reduced spark frequency and amplitude. The frequency decreased gradually to 68.0 ± 5.7 % (n = 8, P < 0.005) of the control value when [PCr] was reduced to 0 mm, while the amplitude remained significantly lower than the control value (n = 8, P < 0.05). Correspondingly, spark duration increased progressively from 31.2 ± 1.9 ms at 10 mm [PCr] to 38.6 ± 1.8 ms at 0 mm [PCr] (n = 8, P < 0.02). This is consistent with a slowing of the Ca2+ uptake process by SR due to the inhibition of the Ca2+ pump (Gomez et al. 1996). The mean spark width did not change significantly.
DISCUSSION
Under normal physiological conditions, spontaneous Ca2+ sparks do not propagate to neighbouring regions of the SR. However, when the SR becomes Ca2+ overloaded, localized Ca2+ sparks can induce a propagated Ca2+ wave (Cheng et al. 1996). Spontaneous release occurs cyclically and appears to be initiated when the SR Ca2+ content reaches a critical threshold. The mechanism by which a rise in luminal [Ca2+] triggers spontaneous Ca2+ release remains uncertain. Experiments on isolated SR Ca2+ channels have shown that the open probability of isolated RyRs increases in response to a rise in luminal [Ca2+]. This may occur due to Ca2+ binding to a regulatory site on the luminal face of the RyR (Sitsapesan & Williams, 1994; Lukyanenko et al. 1996). Alternatively, Ca2+ may pass through the open channel and bind to cytosolic Ca2+ activation and inactivation sites, thereby influencing the opening probability of the RyR (Xu & Meissner, 1999).
Previous work has shown that interventions modifying the activity of the SR Ca2+-ATPase and RyR have different effects on spontaneous SR Ca2+ release. Inhibition of the Ca2+-ATPase with cyclopiazonic acid reduces the frequency and prolongs the descending phase of spontaneous Ca2+ waves, but does not affect the amplitude or rising phase (Kawai et al. 1998; Yang & Steele, 2000). The reduction in frequency, without influence on the amplitude, supports the suggestion that propagated waves occur when the SR Ca2+ content reaches a critical threshold. In this situation, pump inhibition simply increases the time required to reach the threshold SR Ca2+ content. Prolongation of the descending phase of the transient shows that active SR Ca2+ accumulation contributes to the decline in [Ca2+] following spontaneous release.
Inhibition of RyR with tetracaine, or by a decrease in [ATP], prolongs the rising phase, increases the amplitude and decreases the frequency of spontaneous Ca2+ release (Gyorke et al. 1997; Overend et al. 1997; Yang & Steele, 2000). These studies also showed that desensitization of the RyR allows a higher SR Ca2+ content to be sustained before spontaneous Ca2+ release occurs. Therefore, the reduction in the frequency of spontaneous Ca2+ release following desensitization of the RyR probably reflects the additional time required for the SR Ca2+ content to reach a higher threshold level. Confocal imaging of spontaneous Ca2+ waves, in the presence of low [ATP], has shown that the reduced rate of rise of the spatially averaged Ca2+ transient results from a decrease in propagation velocity (Steele & Yang, 2001). Sensitization of the Ca2+-induced Ca2+ release mechanism with caffeine produces essentially the opposite effects, and is typically associated with a decrease in amplitude and an increase in the frequency of spontaneous Ca2+ release (Kort et al. 1985; Miura et al. 1999).
Evidence of impaired SR Ca2+ uptake following PCr withdrawal
The differing characteristics of interventions that influence the SR Ca2+-ATPase and the RyR provide a basis for interpreting the effects of PCr reported in the present study. As shown in Fig. 1, withdrawal of PCr was followed by a decrease in the frequency of spontaneous release and prolongation of the descending phase of the Ca2+ transient. It seems likely that a reduction in the rate of Ca2+ uptake by the SR Ca2+-ATPase underlies both of these effects. This is consistent with previous work showing that the efficiency of the Ca2+ pump is reduced in the absence of local ATP regeneration via the CK reaction.
In theory, the effect of PCr withdrawal on net SR Ca2+ uptake could occur as a consequence of a local decrease in [ATP] or an increase in [ADP]. However, we have shown previously (Yang & Steele, 2000) that in the presence of PCr, the SR Ca2+ uptake rate is essentially unaffected by large decreases in [ATP] from 5 to ∼0.2 mm ATP. This suggests that a local decrease in [ATP] is unlikely to explain the effects of PCr withdrawal. However, as shown in Fig. 5, addition of micromolar levels of ADP to the bathing medium further prolonged the descending phase of the spontaneous Ca2+ transient. This suggests that inhibition of the SR Ca2+-ATPase may reflect local ADP accumulation, which could result in ‘slippage’, inhibition or reversal of the Ca2+-ATPase (Makinose & Hasselbach, 1971; Inesi & de Meis, 1989; Smith & Steele, 1992).
Does PCr withdrawal influence the SR Ca2+ channel?
In addition to prolongation of the spontaneous Ca2+ transient and a decrease in release frequency, PCr withdrawal also reduced the maximum Ca2+ content of the SR that could be sustained before spontaneous Ca2+ release occurred (Fig. 4). Such an effect is not consistent with inhibition of the SR Ca2+-ATPase, which reduces the frequency of release but does not affect the transient amplitude (Yang & Steele, 2000). The decrease in the maximum Ca2+ content suggests that the luminal [Ca2+] threshold for initiation of spontaneous Ca2+ release has been reduced. By analogy with the effects of caffeine (see above), such an effect appears most consistent with an increase in the sensitivity of the SR Ca2+ channel.
There are a number of possible mechanisms by which PCr depletion might affect the sensitivity of the SR Ca2+ channel. As suggested in previous studies, PCr withdrawal could indirectly affect the gating of the RyR by altering ATP : ADP.Pi in the junctional space (Steeghs et al. 1997). Any decrease in [ATP] would also result in an increase in [Mg2+], due to reduced buffering. However, these factors would be expected to reduce the sensitivity of the RyR (Meissner & Henderson, 1987). An alternative possibility is that a local increase in [ADP] might influence the sensitivity of the RyR to activation by Ca2+. Experiments on isolated channels suggest that ADP acts as a partial agonist at the adenine nucleotide-binding site on the RyR, which could not explain the present findings (Kermode et al. 1998). However, in permeabilized cardiac trabeculae, Ca2+ release triggered by flash photolysis of caged Ca2+ was increased in the presence of ADP (Xiang & Kentish, 1995). This occurred despite a decrease in SR Ca2+ content (due to ADP-induced inhibition of net Ca2+ uptake) and was apparent in the presence of millimolar [ATP]. Furthermore, the apparent ability of ADP to sensitize the CICR mechanism was greater in the presence of Pi. While the mechanism underlying this effect of ADP remains uncertain, it might explain the apparent reduction in the threshold for spontaneous Ca2+ release reported in the present study.
One final possibility is that the apparent change in the threshold for spontaneous Ca2+ release may reflect an increase in local [Ca2+]. This might be expected to influence the response of neighbouring Ca2+ channels to activation by Ca2+ and the characteristics of wave propagation. Further experiments involving measurement of localized [Ca2+] using confocal microscopy are required to investigate this possibility.
Effects of PCr depletion on spontaneous Ca2+ sparks and waves
In the present study, PCr withdrawal reduced the amplitude and frequency of spontaneous Ca2+ sparks. Previous work has shown that the frequency of spontaneous Ca2+ sparks is dependent upon the SR Ca2+ content (Lukyanenko et al. 1996). Furthermore, spontaneous Ca2+ sparks are particularly sensitive to reductions in SR Ca2+ content to 10–20 % below the maximum SR Ca2+ content achievable in the presence of 5 mm ATP (Z. Yang & D. S. Steele, unpublished observations). Therefore, it seems likely that the reduction in spark frequency is secondary to the decrease in SR Ca2+ content that occurs following PCr withdrawal. Similarly, the decrease in spark amplitude may also reflect the decrease in SR Ca2+ content.
Further experiments using confocal microscopy showed that PCr withdrawal resulted in increased temporal spread of Ca2+ waves in line scan images and slightly reduced the propagation velocity. The increased temporal spread is consistent with a reduction of the rate of SR Ca2+ uptake (see above). However, the decrease in propagation velocity is less easy to understand. Inhibition of the SR Ca2+ uptake mechanism would be expected to increase the propagation velocity. Similarly, if PCr results in a slight increase in the sensitivity of the RyR to rising levels of luminal Ca2+, this might also be expected to increase the propagation velocity. However, the propagation velocity is also sensitive to the SR Ca2+ load and the amount of released Ca2+ (Miura et al. 1999). The decrease in SR Ca2+ content (Fig. 4) and localized Ca2+ release (Fig. 6) following PCr withdrawal would be expected to reduce the propagation velocity. Therefore, it is possible that the small decrease in propagation velocity occuring after PCr withdrawal reflects the net influence of these opposing factors.
Possible physiological relevance
Human congestive heart failure is associated with slowed rates of contraction and relaxation, which appear to reflect changes in cellular Ca2+ handling (for review see Lehnart et al. 1998). A number of factors may contribute to impaired Ca2+ uptake and release, including altered expression of the SR Ca2+-ATPase or the sarcolemmal Na+-Ca2+ exchanger (e.g. Dipla et al. 1998). However, expression of CK isoenzymes is markedly reduced in failing hearts. In animal models of heart failure, changes in CK isoform expression have been linked to abnormal energy metabolism, including a decrease in the PCr : ATP ratio, reduced CK forward flux rate and a rise in intracellular [ADP] (Ye et al. 2001). Furthermore, saponin-permeabilized fibres from animals in heart failure exhibited a reduced capacity to store Ca2+ and the ability of PCr to enhance Ca2+ uptake was halved (De Sousa et al. 1999). The present data support the proposal that reduced local ATP synthesis via the CK reaction impairs SR Ca2+ uptake. Additionally, the results suggest that local ADP accumulation may alter the sensitivity of the SR Ca2+ release mechanism, resulting in a decrease in the luminal threshold for spontaneous Ca2+ release. This may be important in heart failure, because spontaneous Ca2+ release has been shown to activate a transient inward current (Iti), leading to after-depolarizations and ‘triggered’ arrhythmias (Wit & Rosen, 1992).
Acknowledgments
This work was supported by the British Heart Foundation.
REFERENCES
- Allen DG, Orchard CH. Myocardial contractile function during ischemia and hypoxia. Circulation Research. 1987;60:153–168. doi: 10.1161/01.res.60.2.153. [DOI] [PubMed] [Google Scholar]
- Arrio-Dupont M, Bechet JJ, d’Albis A. A model system of coupled activity of co-immobilized creatine kinase and myosin. European Journal of Biochemistry. 1992;207:951–955. doi: 10.1111/j.1432-1033.1992.tb17129.x. [DOI] [PubMed] [Google Scholar]
- Bassani JWM, Yuan WL, Bers DM. Fractional SR Ca2+ release is regulated by trigger Ca2+ and SR Ca2+ content in cardiac myocytes. American Journal of Physiology. 1995;37:C1313–C1319. doi: 10.1152/ajpcell.1995.268.5.C1313. [DOI] [PubMed] [Google Scholar]
- Cheng H, Lederer MR, Lederer WJ, Cannell MB. Ca2+ sparks and [Ca2+]i waves in cardiac myocytes. American Journal of Physiology. 1996;39:C148–C159. doi: 10.1152/ajpcell.1996.270.1.C148. [DOI] [PubMed] [Google Scholar]
- Cheng H, Song LS, Shirokova N, Gonzalez A, Lakatta EG, Rios E, Stern MD. Amplitude distribution of calcium sparks in confocal images: theory and studies with an automatic detection method. Biophysical Journal. 1999;76:606–617. doi: 10.1016/S0006-3495(99)77229-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Sousa E, Veksler V, Minajeva A, Kaasik A, Mateo P, Mayoux E, Hoerter J, Bigard X, Serrurier B, Ventura-Clapier R. Subcellular creatine kinase alterations. Implications in heart failure. Circulation Research. 1999;85:68–76. doi: 10.1161/01.res.85.1.68. [DOI] [PubMed] [Google Scholar]
- Dipla K, Mattiello JA, Jeevanandam V, Houser SR, Margulies KB. Myocyte recovery after mechanical circulatory support in humans with end-stage heart failure. Circulation. 1998;97:2316–2322. doi: 10.1161/01.cir.97.23.2316. [DOI] [PubMed] [Google Scholar]
- Duncan L, Burton FL, Smith GL. REACT: Calculation of free metal and ligand concentrations using a Windows-based computer program. Journal of Physiology. 1999;517.P:2P. [Google Scholar]
- Fabiato A, Fabiato F. Effects of magnesium on contractile activation of skinned cardiac cells. Journal of Physiology. 1975;249:497–517. doi: 10.1113/jphysiol.1975.sp011027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabiato A, Fabiato F. Calculator programs for computing the composition of the solutions containing multiple metals and ligands used for experiments in skinned muscle cells. Journal de Physiologie. 1979;75:463–505. [PubMed] [Google Scholar]
- Gomez AM, Cheng H, Lederer WJ, Bers DM. Ca2+ diffusion and sarcoplasmic reticulum transport both contribute to [Ca2+]i decline during Ca2+ sparks in rat ventricular myocytes. Journal of Physiology. 1996;496:575–581. doi: 10.1113/jphysiol.1996.sp021708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gyorke S, Lukyanenko V, Gyorke I. Dual effects of tetracaine on spontaneous calcium release in rat ventricular myocytes. Journal of Physiology. 1997;500:297–309. doi: 10.1113/jphysiol.1997.sp022021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inesi G, De Meis L. Regulation of steady state filling in sarcoplasmic reticulum. Roles of back-inhibition, leakage, and slippage of the calcium pump. Journal of Biological Chemistry. 1989;264:5929–5936. [PubMed] [Google Scholar]
- Kawai M, Hussain M, Orchard CH. Cs+ inhibits sponteneous Ca2+ release from the sarcoplasmic reticulum of skinned cardiac myocytes. American Journal of Physiology. 1998;275:H422–H430. doi: 10.1152/ajpheart.1998.275.2.H422. [DOI] [PubMed] [Google Scholar]
- Kermode H, Williams AJ, Sitsapesan R. The interactions of ATP, ADP and inorganic phosphate with the sheep cardiac ryanodine receptor. Biophysical Journal. 1998;74:1296–1304. doi: 10.1016/S0006-3495(98)77843-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korge P, Byrd SK, Campbell KB. Functional coupling between sarcoplasmic-reticulum-bound creatine kinase and Ca2+-ATPase. European Journal of Biochemistry. 1993;213:973–980. doi: 10.1111/j.1432-1033.1993.tb17842.x. [DOI] [PubMed] [Google Scholar]
- Kort AA, Capogrossi MC, Lakatta EG. Frequency, amplitude, and propagation velocity of spontaneous Ca2+-dependent contractile waves in intact adult rat cardiac muscle and isolated myocytes. Circulation Research. 1985;57:844–855. doi: 10.1161/01.res.57.6.844. [DOI] [PubMed] [Google Scholar]
- Lehnart SE, Schillinger W, Pieske B, Prestle J, Just H, Hasenfuss G. Sarcoplasmic reticulum proteins in heart failure. Annals of the New York Academy of Sciences. 1998;853:220–230. doi: 10.1111/j.1749-6632.1998.tb08270.x. [DOI] [PubMed] [Google Scholar]
- Lukyanenko V, Gyorke I, Gyorke S. Regulation of Ca2+ release by calcium inside the sarcoplasmic reticulum in ventricular myocytes. Pflügers Archiv. 1996;432:1047–1054. doi: 10.1007/s004240050233. [DOI] [PubMed] [Google Scholar]
- Lukyanenko V, Gyorke S. Ca2+ sparks and Ca2+ waves in saponin-permeabilized rat ventricular myocytes. Journal of Physiology. 1999;521:575–585. doi: 10.1111/j.1469-7793.1999.00575.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makinose M, Hasselbach W. ATP synthesis by the reversal of the sarcoplasmic calcium pump. FEBS Letters. 1971;12:271–272. doi: 10.1016/0014-5793(71)80196-5. [DOI] [PubMed] [Google Scholar]
- Meissner G, Henderson JS. Rapid calcium release from cardiac sarcoplasmic reticulum vesicles is dependent on Ca2+ and is modulated by Mg2+, adenine nucleotide, and calmodulin. Journal of Biological Chemistry. 1987;262:3065–3073. [PubMed] [Google Scholar]
- Miller DJ, Smith GL. EGTA purity and the buffering of calcium ions in physiological solutions. American Journal of Physiology. 1984;246:C160–C166. doi: 10.1152/ajpcell.1984.246.1.C160. [DOI] [PubMed] [Google Scholar]
- Minajeva A, Ventura-Clapier R, Veksler V. Ca2+ uptake by cardiac sarcoplasmic reticulum ATPase in situ strongly depends on bound creatine kinase. Pflügers Archiv. 1996;432:904–912. doi: 10.1007/s004240050214. [DOI] [PubMed] [Google Scholar]
- Miura M, Boyden PA, Ter Keurs HE. Ca2+ waves during triggered propagated contractions in intact trabeculae. Determinants of the velocity of propagation. Circulation Research. 1999;84:1459–1468. doi: 10.1161/01.res.84.12.1459. [DOI] [PubMed] [Google Scholar]
- Overend CL, Eisner DA, O'Neill SC. The effect of tetracaine on spontaneous Ca2+ release and sarcoplasmic reticulum calcium content in rat ventricular myocytes. Journal of Physiology. 1997;502:471–479. doi: 10.1111/j.1469-7793.1997.471bj.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi AM, Eppenberger HM, Volpe P, Cotrufo R, Wallimann T. Muscle-type MM creatine kinase is specifically bound to sarcoplasmic reticulum and can support Ca2+ uptake and regulate local ATP/ADP ratios. Journal of Biological Chemistry. 1990;265:5258–5266. [PubMed] [Google Scholar]
- Rousseau E, Smith JS, Henderson JS, Meissner G. Single channel and Ca2+-45 flux measurements of the cardiac sarcoplasmic-reticulum calcium-channel. Biophysical Journal. 1986;50:1009–1014. doi: 10.1016/S0006-3495(86)83543-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sitsapesan R, Williams AJ. Regulation of the gating of the sheep cardiac SR Ca2+ release channel by luminal Ca2+ Journal of Membrane Biology. 1994;137:215–226. doi: 10.1007/BF00232590. [DOI] [PubMed] [Google Scholar]
- Smith GL, Miller DJ. Potentiometric measurements of stoichiometric and apparent affinity constants of EGTA for protons and divalent ions including calcium. Biochimica et Biophysica Acta. 1985;839:287–299. doi: 10.1016/0304-4165(85)90011-x. [DOI] [PubMed] [Google Scholar]
- Smith GL, Steele DS. Inorganic phosphate decreases the Ca2+ content of the sarcoplasmic reticulum in saponin-treated rat cardiac trabeculae. Journal of Physiology. 1992;458:457–473. doi: 10.1113/jphysiol.1992.sp019427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steeghs K, Benders A, Oerlemans F, De Haan A, Heerschap A, Ruitenbeek W, Jost C, Van Deursen J, Perryman B, Pette D, Bruckwilder M, Koudijs J, Jap P, Veerkamp J, Wieringa B. Altered Ca2+ responses in muscles with combined mitochondrial and cytosolic creatine kinase deficiencies. Cell. 1997;89:93–103. doi: 10.1016/s0092-8674(00)80186-5. [DOI] [PubMed] [Google Scholar]
- Steele DS, Yang Z. Effects of cytosolic ATP on Ca2+ waves and Ca2+ sparks in permeabilized cardiac myocytes. Biophysical Journal. 2001;80:2709. doi: 10.1161/hh1801.096264. [DOI] [PubMed] [Google Scholar]
- Wallimann T, Wyss M, Brdiczka D, Nicolay K, Eppenberger HM. Intracellular compartmentation, structure and function of creatine kinase isoenzymes in tissues with high and fluctuating energy demands: the ‘phosphocreatine circuit’ for cellular energy homeostasis. Biochemical Journal. 1992;281:21–40. doi: 10.1042/bj2810021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wit AL, Rosen MR. Afterdepolarisations and triggered activity: Distinction from automaticity as an arrythmogenic mechanism. In: Fozzard HA, editor. The Heart and the Cardiovascular System. New York: Raven Press Ltd; 1992. [Google Scholar]
- Xiang JZ, Kentish JK. Effects of inorganic phosphate and ADP on Ca2+ handling by the sarcoplasmic reticulum in rat skinned cardiac muscles. Cardiovascular Research. 1995;29:391–400. [PubMed] [Google Scholar]
- Xu L, Meissner G. Regulation of cardiac muscle Ca2+ release channel by sarcoplasmic reticulum lumenal Ca2+ Biophysical Journal. 1999;75:2302–2312. doi: 10.1016/S0006-3495(98)77674-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Z, Steele DS. Effects of cytosolic ATP on spontaneous and triggered Ca2+-induced Ca2+ release in permeabilised rat ventricular myocytes. Journal of Physiology. 2000;523:29–44. doi: 10.1111/j.1469-7793.2000.00029.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye Y, Gong G, Ochiai K, Liu J, Zhang J. High-energy phosphate metabolism and creatine kinase in failing hearts: a new porcine model. Circulation. 2001;103:1570–1576. doi: 10.1161/01.cir.103.11.1570. [DOI] [PubMed] [Google Scholar]


