Abstract
The functional properties of the three most widely distributed α subunit isoforms of the Na,K-ATPase are not well known, particularly concerning the voltage dependence of their activity and cation binding kinetics. We measured the electrogenic activity generated by Na,K-ATPases resulting from co-expression of the rat α1, α2* or α3* subunits with the rat β1 subunit in Xenopus oocytes; α2* and α3* are ouabain-resistant mutants of the α2 and α3 isoform, which allowed selective inhibition of the endogenous Na+,K+-pump of the oocyte. In oocytes expressing the three isoforms of the α subunit, K+ induced robust outward currents that were largely ouabain-sensitive. In addition, ouabain-sensitive inward currents were recorded for all three isoforms in sodium-free and potassium-free acid solutions. The very similar voltage dependence of the Na+,K+-pump activity observed in the absence of extracellular Na+ indicated a similar stoichiometry of the transported cations by the three isoforms. The affinity for extracellular K+ was slightly lower for the α2* and α3* than for the α1 isoform. The α2* isoform was, however, more sensitive to voltage-dependent inhibition by extracellular Na+, indicating a higher affinity of the extracellular Na+ site in this isoform. We measured and controlled [Na+]i using a co-expressed amiloride-sensitive Na+ channel. The intracellular affinity for Na+ was slightly higher in the α2* than in the α1 or α3* isoforms. These results suggest that the α2 isoform could have an activity that is strongly dependent upon [Na+]o and [K+]o. These concentrations could selectively modulate its activity when large variations are present, for instance in the narrow intercellular spaces of brain or muscle tissues.
The Na,K-ATPase is the active transport system responsible for maintenance of the Na+ and K+ gradient across the cell membrane. In vertebrates, four different genes coding for Na,K-ATPase α subunits have been identified. Each of them has been shown to support sodium- and potassium-stimulated ATPase activity and is expected to carry out the exchange of intracellular Na+ for extracellular K+ using the energy provided by the hydrolysis of ATP. Although their complex tissue specific distribution, in particular in the nervous system (Sweadner, 1992; Zahler et al. 1992; Cameron et al. 1994; Blanco & Mercer, 1998; Wetzel & Sweadner, 2001) suggests different roles, the specific functional properties of each isoform, such as cation transport stoichiometry, cation apparent affinity and voltage dependence, are not yet completely characterized. Most of the available data concern the apparent affinity for Na+, K+ and ATP.
Apparent Na+ and K+ affinities were first studied in preparations from native tissues known to express different isoform contents. In these studies, it has been reported consistently that tissues expressing only the α1 isoform have a lower apparent Na+ affinity than tissues expressing α2 and α3 in addition to α1 (Sweadner, 1989; Shyjian et al. 1990). In contrast, in studies in which the various isoforms were expressed artificially in mammalian cells (HeLa cells) or in insect cells (SF9), the α3 isoform showed a lower apparent affinity for Na+ than did the α1 and α2 isoforms (Jewell & Lingrel, 1991; Munzer et al. 1994; Therien et al. 1996; Zahler et al. 1997; Blanco & Mercer, 1998). Attempts to resolve this apparent contradiction by comparing naturally and artificially expressed isoforms in well controlled experimental conditions failed to provide a simple explanation (Munzer et al. 1994; Therien et al. 1996). The observed differences could not be explained solely by the isoforms of the α subunit present in the various preparations.
The results were also somewhat divergent concerning the activation by K+. While experiments with HeLa cells showed a higher apparent K+ affinity with the α3 than with the α1 or α2 isoforms (Jewell & Lingrel, 1991; Munzer et al. 1994; Therien et al. 1996), α2 and α3 isoforms expressed in SF9 cells had a lower apparent affinity for K+ than the α1 isoform (Blanco & Mercer, 1998).
Recently, Crambert et al. (2000) reported the physiological properties of nine isozymes of the human Na,K-ATPase. This study showed the importance of the interaction between α and β subunits, especially concerning the affinity for external K+. The most striking differences were observed between the α2 β2 isozyme, which had the lowest apparent affinity for external K+, and all the other isozymes, and between the α3 β1 isozyme and the α1 β1 and α2 β1 isozymes, the former having an approximately three-fold decrease in the apparent intracellular Na+ affinity when compared with the latter two. The studies also disclosed a difference in the current-voltage (I-V) relationship of the Na+/K+-pump activity between the α1β1, α2 β1 and α3 β1 isozymes.
Because the Na+/K+-pump is electrogenic, its overall activity is expected to be voltage dependent. Furthermore, several steps in the transport cycle are thought to be voltage dependent. The best defined cases are the release of extracellular Na+, which has the highest estimated voltage dependence (with a voltage coefficient (γ) of about 0.7 for the first Na+ ion and 0.2 for the two other Na+ ions) and the binding of extracellular K+ (γ of about 0.2: Rakowski et al. 1997; Apell & Karlish, 2001).
The apparent affinity of one of the substrates of a multisubstrate enzyme such as the Na,K-ATPase depends in a complex manner not only upon the intrinsic affinity of the binding site itself, but also upon the equilibrium between its different conformations (Jaisser et al. 1994; Wang et al. 1996). To attempt to gain a better understanding of the differences in the transport cycle that may be responsible for the differences in apparent affinity for Na+ and K+ observed for the three major α isoforms of the Na,K-ATPase, we have studied the voltage dependence of the rat Na+/K+-pump, and its activation by external K+ and internal Na+ in a system that allows the control of membrane potential, ion concentrations and minimal contamination by endogenous components.
METHODS
Expression of the rat Na,K-ATPase isoforms in Xenopus oocytes
Stage V/VI Xenopus laevis oocytes were obtained from the ovarian tissue of frogs under MS222 (2 g l−1; Sandoz, Basel, Switzerland) anaesthesia; frogs were humanely killed after the final collection. These procedures were approved by the ‘Service Vétérinaire Cantonal’ of the State of Vaud (authorization no. 904.2).
The three isoforms of the Na,K-ATPase were expressed as described earlier (Jaisser et al. 1994) by co-injection in the oocytes of 7 ng cRNA of the various α subunit isoforms of the rat Na,K-ATPase with 1 ng cRNA of the rat β1 subunit. In order to eliminate the endogenous Xenopus Na,K-ATPase activity we used the ouabain-resistant forms of the rat α isoforms. The α1 isoform was the wild-type rat α1 isoform, which is naturally ouabain-resistant. The α2 and α3 were the ouabain-resistant mutants of these isoforms, designated as α2* and α3*, which contain a mutation in the first extracellular loop that makes them resistant to ouabain (Jewell & Lingrel, 1991). All three clones were a generous gift from J. B. Lingrel. In all experiments, the oocytes were incubated for 2–3 days to allow for a high level of exogenous Na+/K+-pump expression, and were then incubated overnight before the measurements were made in a potassium-free solution containing 200 nm ouabain.
To evaluate the electrogenic signals attributable to transport systems other than the expressed rat Na,K-ATPase, potassium-induced currents were also measured in non-injected oocytes, or in oocytes injected with the β subunit of the rat Na,K-ATPase alone. Part of these oocytes were exposed to the same sodium-loading solution as the oocytes expressing the rat α and β subunits with 200 nm ouabain. Another group was sodium-loaded in the absence of ouabain to estimate the amplitude of the endogenous Na+/K+-pump current.
Measurements of the electrogenic activity of the Na+/K+-pump
In order to measure the Na+/K+-pump activity under conditions of Vmax with regard to [Na+]i, the oocytes were loaded with Na+ by overnight incubation in a potassium-free solution (Jaisser et al. 1994). The composition of this solution was (mm) Na+ 87.4, K+ 0.0, Ca2+ 0.4, Mg2+ 0.8, N-methyl-d-glucamine (NMDG) 5, Cl− 86.0, HCO3− 2.4, Hepes 10.0. Whole-oocyte currents were measured by the classical two-electrode voltage-clamp method at a holding potential of −50 mV. The I-V relationship was studied by recording currents during a series of nine 250 ms voltage steps ranging from −130 to +30 mV. The composition of the standard experimental solution was (mm) Na+ 100, K+ 0, Cl− 22.4, gluconate 100, NMDG 10, Hepes 10, Mg2+ 1.0, Ca2+ 0.4, Ba2+ 5.0, TEA 10.0, pH 7.4 (adjusted with NMDG base or Hepes acid). The sodium-free solution was similar except that 100 mm sodium gluconate was replaced by 100 mm NMDG gluconate. For [Na+]i, measurements were made in a 10 mm Na+ solution (90 mm NMDG gluconate and 10 mm sodium gluconate). Various K+ concentrations were obtained by addition of appropriate amounts of potassium gluconate. Barium and TEA were used to block K+ channels present in the oocyte membrane and thus to minimize the K+ currents due to these channels when [K+]o was changed. A low-chloride solution (gluconate replacement) was used to reduce the baseline oocyte membrane conductance.
Measurements of the apparent affinity for extracellular K+
The apparent affinity for extracellular K+ was calculated from the measurements of the currents induced by four different concentrations of K+ (0.02, 0.1, 0.5, and 5 mm in the absence of extracellular Na+, or 0.3, 1.0, 3.0 and 10.0 mm in the presence of 100 mm Na+). A different set of K+ concentrations was chosen in the sodium-containing and sodium-free solutions because the apparent affinity for K+ is known to be about 3–5 times higher in the absence of extracellular Na+. As described earlier (Jaisser et al. 1994), the potassium half-activation constant (K1/2) was determined by fitting the Hill equation parameters to the [K+]o-I curve using a Hill coefficient of 1.6 for the measurements performed in the presence of extracellular Na+ and 1.0 for those performed in the absence of extracellular Na+.
Effect of extracellular Na+ on the potassium-induced Na+/K+-pump current
To estimate the affinity of the extracellular Na+ binding site, we measured the outward current induced by a non-saturating concentration of K+ (1 mm), in a nominally sodium-free solution, and then in solutions containing 30 and then 100 mm Na+. The different Na+ concentrations were obtained by replacement of NMDG gluconate by sodium gluconate.
Ouabain-blocked currents in sodium- and potassium-free solutions at pH 6.0
To measure the amplitude of the current carried by protons in the nominal absence of extracellular Na+ and K+ (Wang & Horisberger, 1995), we measured first the current activated by 5 mm K+ in a sodium-free solution and then exposed the oocyte to the same sodium-free and potassium-free solution titrated to pH 6.0, and measured the current inhibited by 2 mm ouabain.
[Na+]i and affinity measurements
To evaluate the apparent affinity of the intracellular Na+ site, we used the co-expression of the amiloride-sensitive epithelial Na+ channel (ENaC), as described recently (Hasler et al. 1998). The principle of this approach is to use the co-expressed ENaC first to measure the intracellular Na+ activity from the reversal of the amiloride-sensitive current, and second to allow the progressive and controlled sodium-loading of the oocyte by the Na+ inflow through ENaC. In addition to the Na,K-ATPase α and β subunit cRNA, oocytes were also injected with 0.3 ng of cRNA of each of the three subunits of the rat epithelial Na+ channel (Canessa et al. 1993, 1994). The oocytes were then kept for 2 days in a modified incubation solution containing 10 mm Na+ (NMDG replacement). On the day before the measurement, to reduce [Na+]i to low values, the oocytes were incubated in a sodium-free solution containing 40 mm K+.
The electrophysiological measurements were commenced in a solution containing 10 mm Na+, no K+ and 10 μm amiloride and the potential was held at −50 mV. After a stable current was recorded, two I-V curves were obtained, one during a brief removal of amiloride and the second one immediately after re-addition of amiloride. The reversal potential of the amiloride-sensitive current was determined from the intersection of these two I-V curves. Within 20 s after the recording of the second I-V curve (in the presence of amiloride), 10 mm K+ was added to record the Na+/K+-pump potassium-activated current. We have shown earlier that the current activated by 10 mm K+ at −50 mV is a reliable measure of the Na+/K+-pump activity (Jaunin et al. 1992; Jaisser et al. 1994). This sequence of measurements of amiloride current and K+-activated current was repeated 5–8 times after various levels of Na+ loading. Na+ loading was allowed to occur by removing amiloride for 1–3 min periods, first in the 10 mm Na+ solution, then in a 100 mm Na+ solution, and finally, by clamping the membrane potential to −100 or −120 mV to obtain large entering Na+ currents, before the last measurements (this procedure is illustrated by the example current trace shown in Fig. 7). Kinetic parameters (K1/2, and maximal current, Imax) were calculated using the non-linear fit routine of the Kaleidagraph program (Synergy Software, Reading, PA, USA) and the Hill equation, with a Hill coefficient of 2.5 for intracellular Na+. Knowing [Na+]o (10 mm), [Na+]i was calculated from the reversal potential of the amiloride-sensitive current (Vrev,amil) as
Figure 7. Apparent affinity for intracellular Na+.
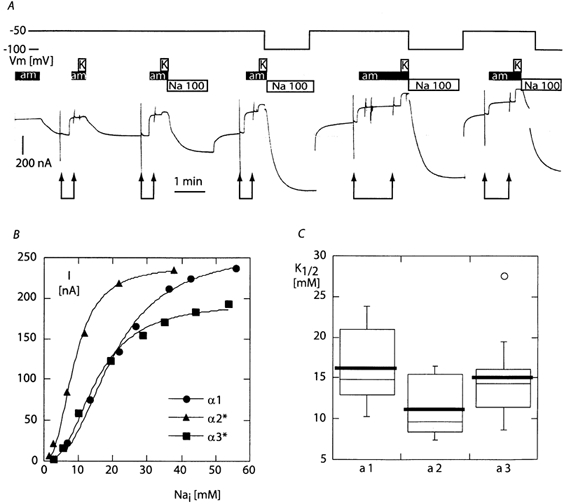
A, original current trace obtained with an sodium-deprived oocyte expressing the α3*β1 isoform of the rat Na,K-ATPase. The trace shows the successive recordings of I-V curves without and with 10 mm amiloride (pairs of arrows), and of the current induced by 10 mm K+ immediately thereafter, in a 10 mm Na+ experimental solution. Between these recordings, the oocyte was then exposed to a 100 mm Na+ solution in the absence of amiloride to allow for an increase in [Na+]i. As indicated by the top line, the voltage was maintained at −50 mV, except for the recording of the I-V curves and during the three last sodium-loading periods, during which it was set to −100 mV to increase the rate of Na+ entry. Because of the large amplitude of the current flowing through the epithelial Na+ channel in the absence of amiloride and the relatively small size of the potassium-induced current, it was not possible to read accurately the potassium-induced current values from the paper chart. The amiloride I-V curves were recorded and analysed using the PClamp data acquisition system to define the amiloride-sensitive current reversal potential, and the potassium-induced current was recording by hand directly from the digital reading of the voltage-clamp apparatus. In this example, the potassium-induced currents were 10, 42, 112, 206, 248 and 295 nA (the last value is not visible on the trace). B, example of the potassium-activated current versus [Na+]i relationship for each isoform, and the corresponding best-fitting model based on the Hill equation with a Hill coefficient of 2.5. C, mean K1/2 parameter in the ‘box and whiskers’ representation (thick line: mean, thin line: median, bottom and top of the box: 25th and 75th percentile, vertical bars: range, circle: outlier). The maximal current obtained from the fit was 255 ± 36 nA (n = 12), 238 ± 68 nA (n = 7), and 186 ± 17 nA (n = 16) for the α1, α2* and α3* groups, respectively. The difference in the mean K1/2 parameter between the α1 and α2* was statistically significant (t (degrees of freedom = 17): 2.1098, P≤ 0.05). The other differences were not significant.
where F, R and T have their usual meaning. We considered only oocytes for which at least five pairs of [Na+]i and Na+/K+-pump current measurements had been obtained and for which [Na+]i had risen to at least 25 mm.
Presentation of the results
The results are presented as mean ± s.e.m. (n = number of observations). Differences between means were considered statistically significant when a P value of ≤ 0.05 was obtained with the Student's t test for unpaired values (or for paired values if appropriate, as indicated in the text). Since the largest source of variability in the results was the level of expression of the protein, all current results are normalized for each oocyte to the amplitude of the current induced in this oocyte by the maximal concentration of K+ used (10 mm in the presence of extracellular Na+ and 5 mm in the absence of Na+), at 0 mV; the mean of the normalization values for each group is indicated in the figure legends.
RESULTS
The effectiveness of inhibition of the endogenous Xenopus Na+/K+-pump was tested in non-injected oocytes or in oocytes injected with the cRNA of the β1 subunit alone of the rat Na,K-ATPase. All oocytes were Na+ loaded by overnight exposure to a potassium-free solution. As no difference could be detected between non-injected oocytes and oocytes injected with the β subunit alone, the results of these two groups are pooled. Figure 1 shows example current recordings and the mean I-V curve of the current activated by 10 mm K+ in oocytes that had not been exposed to ouabain and in oocytes that had been exposed to 200 nm ouabain overnight. No ouabain was present in the solutions used for the electrophysiological measurements. A potassium-activated outward current with a mean amplitude of about 60 nA at low membrane potential was recorded in oocytes that had not been exposed to ouabain. This potassium-activated current is due to the endogenous Na,K-ATPase of the oocyte (Horisberger et al. 1991; Canessa et al. 1992). Overnight exposure to 0.2 mm ouabain resulted in a nearly complete inhibition of the potassium-activated current. Measurements of the potassium-activated current were repeated at 5 min intervals to demonstrate the slow recovery from ouabain inhibition. Even though the potassium-activated outward current tended to increase with time, it reached only a few nanoamps after a 10–12 min interval in continuously flowing solutions containing no ouabain. These values are to be compared with the 150–250 nA current generated by the exogenously expressed ouabain-resistant Na,K-ATPase, as described in the following paragraphs. Thus, because of the high affinity of ouabain for the Xenopus Na,K-ATPase and the slow dissociation rate constant (koff) of ouabain from its binding site (Canessa et al. 1992) pre-exposure to a low ouabain concentration in the absence of extracellular K+ was sufficient to obtain a long-duration inhibition of the endogenous Na+/K+-pump. These results also show that after Na+/K+-pump inhibition, the addition of extracellular K+ does not induce other currents such as the inward current that might be due to K+ conductances, demonstrating the effective inhibition of the oocyte K+ channels under our experimental conditions.
Figure 1. Potassium-induced current due to the endogenous Na,K-ATPase and after its inhibition by pre-exposure to 200 nm ouabain in the incubation solution.
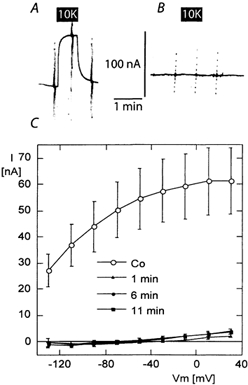
Original current recordings in oocytes injected with the βsubunit of the rat Na,K-ATPase alone after overnight exposure to a potassium-free solution without ouabain (A) or from a different oocyte incubated in a solution containing 200 nm ouabain (B). I-V curves recorded before and after the addition of 10 mm potassium gluconate (10K) to a previously potassium-free solution. No ouabain was present at the time of measurement. C, mean I-V curves of the potassium-induced current (I-V in the presence of 10 mm K+; the mean of the I-V curve in the potassium-free solution before and after) in 10 oocytes without ouabain pre-exposure (Co, open symbols) showing the electrogenic activity of the endogenous Na+/K+-pump, and in 17 (8 non-injected and 9 injected with the β subunit alone) oocytes pre-exposed to 200 nm ouabain (black symbols). Measurements were performed the first time 1–2 min after the oocytes had been removed from the 0.2 mm ouabain-containing incubation solution and placed in the control experimental solution, which contained no ouabain: (1 min) and repeated 5–6 min (6 min) and 10–12 min (11 min) later to demonstrate the very slow dissociation rate constant (koff) of ouabain from the Xenopus oocyte endogenous Na,K-ATPase.
Experiments aimed at testing the K+ activation and ouabain inhibition of the electrogenic activity of the Na+/K+-pump were performed as described in the examples shown in Fig. 2 (current traces; data recorded in sodium-free solutions). Starting from a potassium-free solution, [K+]o was increased in a stepwise manner and then a high concentration of ouabain (2 mm) was added to the highest [K+]o. I-V curves were recorded under each condition. The current traces shown in Fig. 2 show that 2 mm ouabain inhibited practically all of the potassium-induced current for the α1 isoform (Fig. 2A), while the inhibition was only partial for the α2* isoform (Fig. 2B). Figure 2D shows examples of the current-[K+]o relationship and the best fitting curves which in this examples yielded K1/2 values of 0.078, 0.086, 0.110, 0.143 and 0.208 mm for the −130, −90, −50, −10 and +30 mV membrane potentials, respectively. Mean values of the potassium-induced and ouabain-sensitive currents are shown in Fig. 3.
Figure 2. Potassium-activated and ouabain-sensitive currents.
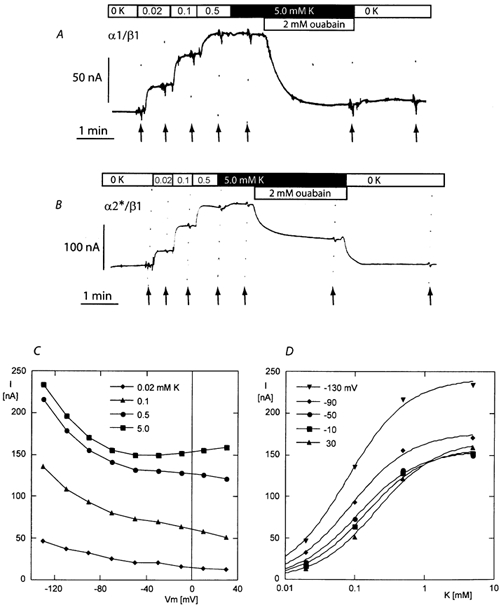
A and B, original current recordings obtained during exposure to increasing concentrations of K+ (0, 0.02, 0.1 0.5 and 5.0 mm) and to 2 mm ouabain with 5.0 mm K+ in a sodium-free experimental solution (0K). The traces in A and B were obtained with oocytes expressing the α1 β1 and the α2*β1 isoforms of the rat Na,K-ATPase, respectively. The holding potential was −50 mV and I-V curves were recorded under each condition at the time indicated by the arrows. C, I-V relationship for the current induced by four different concentrations of K+ recorded in the example given in B (α2*β1 isoform). D, relationship between the potassium-induced current to the K+ concentration for five membrane potentials in the same set of data (trace in B).
Figure 3. Voltage dependence of the ouabain-sensitive currents (A and C) and potassium-activated currents (B and D) measured in sodium-rich solutions (A and B) and in sodium-free solutions (C and D) in oocytes expressing the α1, α2* and α3* isoform together with the β1 subunit of the rat Na,K-ATPase.
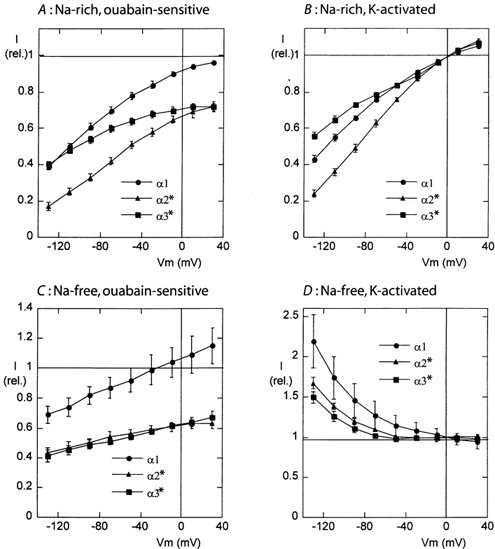
In all cases the current values are normalized to the amplitude of the current induced by the highest K+ concentration at a membrane potential of 0 mV. For the experiments shown in the sodium-rich solution (A and B), the mean currents induced by 10 mm K+ were 201 ± 41 nA (α1, n = 11), 282 ± 41 nA (α2*, n = 10) and 234 ± 32 nA (α3*, n = 10). For the experiments in the sodium-free solution (C and D), the mean currents induced by 5 mm K+ were 299 ± 4 nA (α1, n = 17), 261 ± 41 nA (α2*, n = 15) and 174 ± 20 nA (α3*, n = 15).
With oocytes expressing the α and β subunits of the Na,K-ATPase, the mean values of the current induced by 10 mm K+ at −50 mV, in the presence of extracellular Na+ (raw data not shown), were 254 ± 36 nA (n = 17), 198 ± 32 nA (n = 15), and 148 ± 18 nA (n = 15), for the α1, α2* and α3* isoforms, respectively. In the same groups of oocytes, the mean ouabain-sensitive currents obtained in the presence of 10 mm K+ were 238 ± 34, 136 ± 25 and 114 ± 14 nA, respectively (raw data not shown). The smaller amplitude of the ouabain-sensitive current when compared to the potassium-induced current can be understood as resulting from the rather low sensitivity to ouabain of the used forms of the enzyme, indicating that 2 mm ouabain inhibited approximately 92 %, 69 % and 73 % of the potassium-induced current in the α1, α2* and α3* isoforms, respectively.
Voltage dependence of the Na+/K+-pump currents
The voltage dependence of the Na+/K+-pump activity was studied either as the ouabain-sensitive current or as the current activated by addition of external K+. Examples traces are shown in Fig. 2, and the results are summarized in Fig. 3.
In the presence of a physiological [Na+]o (Fig. 3A and B), the Na+/K+-pump current showed a voltage dependence that was qualitatively similar to that described earlier for several other Na,K-ATPase isoforms or preparations (Rakowski & Paxson, 1988; Schweigert et al. 1988; Gadsby & Nakao, 1989; Jaisser et al. 1994; Sagar & Rakowski, 1994; Argüello et al. 1996; Wang et al. 1998): a shallow voltage dependence at depolarized membrane potentials that becomes steeper at high negative membrane potentials. More precise examination of the I-V curves indicates that the voltage-dependence of the Na+/K+-pump activity was quantitatively different between the α1, α2*, and α3* isoforms. For the ouabain-sensitive current (Fig. 3A) as well as for the potassium-activated current (Fig. 3B), the voltage dependence was less pronounced for the α3* subunit and more pronounced for the α2* subunit, when compared to the α1 subunit. The reasons for this difference in voltage sensitivity will be examined further below.
In contrast, when studied in the absence of extracellular Na+, the ouabain-sensitive current (Fig. 3C) presented a very similar voltage dependence along the whole potential range for the three isoforms. The amplitude of the current decreased by 20–25 % for a 100 mV change. The amplitude of the ouabain-sensitive current (in comparison with the potassium-induced current at 0 mV) was lower by about 40 % in the α2* and α3* isoforms, when compared to the α1 isoform, indicating, as mentioned above, a lower sensibility to ouabain of these mutant isoforms. In contrast to the observation made with extracellular Na+, in the absence of extracellular Na+ the potassium-activated currents (Fig. 3D) were clearly different from the ouabain-sensitive currents (Fig. 3C). The potassium-induced I-V curve had a negative slope over the whole negative potential range, and at high negative membrane potentials, the current induced by K+ was much larger than that inhibited by ouabain. This effect was present for the three isoforms, even though the potassium-induced current was slightly larger for the α1 isoform than for the α2* and α3* isoforms.
We have already shown that in the absence of extracellular Na+ and K+, the Na+/K+-pump carries a ouabain-sensitive inward current that is dependent upon the extracellular proton concentration and most probably reflects an inward proton current (Wang & Horisberger, 1995). We therefore also measured the ouabain-sensitive current at pH 6.0, in sodium-free and potassium-free solution. As shown in Fig. 4, all three isoforms presented large ouabain-sensitive inward currents with a qualitatively similar I-V curve.
Figure 4. Ouabain-sensitive inward current in a sodium-free, low-pH solution.
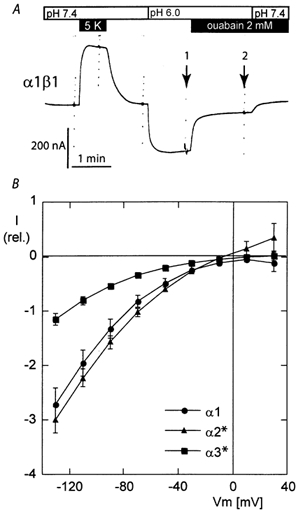
A, the current trace recorded at −50 mV in sodium-free solutions with an oocyte expressing the α1 β1 isoform of the rat Na,K-ATPase. The activation of the Na+/K+-pump current by 5 mm K+ was first recorded in a solution at pH 7.4. Change to a pH 6.0, potassium-free solution induced an inward current that could be largely inhibited by 2 mm ouabain. B, the mean values of the ouabain-sensitive current (i.e. the difference between the I-V curves recorded without and with ouabain (arrows 1 and 2 in A, respectively). All of the values are expressed relative to the current induced by 5 mm K+ at pH 7.4. The mean normalization values were 201 ± 41 nA (n = 11), 282 ± 41 nA (n = 10) and 234 ± 32 nA (n = 10) for the α1, α2* and α3* groups, respectively.
Apparent affinity for external K+
The K1/2 for external K+ was measured in the presence and in the absence of extracellular Na+ by recording currents induced by the application of increasing concentrations of K+, as shown in the examples in Fig. 2. In the absence of external Na+ (Fig. 5A), the K1/2 showed the usual voltage dependence that has already been described (Rakowski et al. 1991; Jaisser et al. 1994). The apparent affinity for external K+ was high at negative membrane potentials, with a K1/2 around 60 μm for the α1 and α3* isoforms and slightly lower for the α2* isoform (K1/2 around 80 μm). These K1/2 values are significantly lower than those measured in amphibian Na+/K+-pumps under the same experimental conditions (Jaisser et al. 1994), but are close to that reported for mammalian Na+/K+-pumps under similar conditions (Peluffo & Berlin, 1997; Peluffo et al. 2000).
Figure 5. Voltage dependence of the activation of the current by external K+.
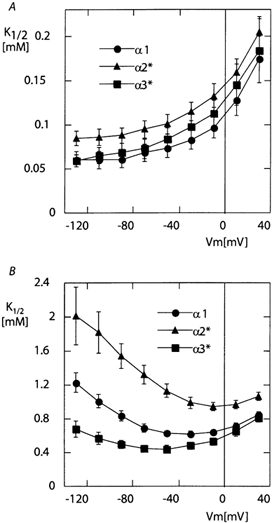
A, sodium-free solution. B, 100 mm Na+ solution. K1/2 values were obtained by fitting the Hill equation to the set of five current amplitudes measured at five values of [K+]o for each oocyte and at each membrane potential. The mean K1/2 values as a function of membrane potential are shown in A (sodium-free solution) and B (100 mm Na+ solution). One example of the relationship between [K+]o and the induced current at five different membrane potentials is given in D of Fig. 2.
In the presence of 100 mm extracellular Na+ (Fig. 5B), a biphasic voltage dependence, with higher K1/2 at positive and high negative membrane potentials, was observed with the three isoforms, similar to what has been described in Na+/K+-pumps from other species (Jaisser et al. 1994; Sagar & Rakowski, 1994; Peluffo et al. 2000). There was, however, a significant difference between the threse isoforms. The minimal values of the K1/2 were 0.62 ± 0.03 mm (n = 12) recorded at −30 mV for the α1 isoform and significantly smaller 0.44 ± 0.04 mm (n = 10) at −50 mV for the α3* isoform (P ≤ 0.01). The affinity for the α2* isoform was much lower over the whole potential range, and the minimal K1/2 value, 0.95 ± 0.05 mm (n = 11), recorded close to 0 mV, was significantly larger than those of the α1 and α3* isoforms (P ≤ 0.001 in both cases).
Effect of extracellular Na+ on the Na+/K+-pump activity
Because the Na+/K+-pump activity was rather similar between the three isoforms in the absence of extracellular Na+, but showed significant differences in voltage dependence or apparent affinity for K+ when extracellular Na+ was present, we suspected that the major difference might be due to the kinetics of extracellular Na+ binding or release. According to the standard model of the Na+/K+-pump transport cycle, Na+ must be released into the extracellular medium by the E2-P conformation of the enzyme to allow K+ ions to bind and promote dephosphorylation and completion of the transport cycle. As the release of Na+ is a reversible step, extracellular Na+ competes with K+ for binding to the E2-P conformation of the enzyme (whether or not the binding sites are the same; Sagar & Rakowski, 1994). We can thus use the competition between extracellular Na+ and K+ to evaluate the affinity of Na+ for its extracellular binding site (Vasilets et al. 1993).
The 1 mm potassium-induced currents measured in the presence of 0, 30, and 100 mm [Na+]o for the three isoforms are shown in Fig. 6. While the three isoforms had rather similar potassium-induced I-V curves in the absence of Na+ (small black symbols with solid line), a significant difference appeared between the α2* isoform and the α1 and α3* isoforms at 30 mm (open symbols), which was even more obvious with 100 mm extracellular Na+ (large black symbols with dashed line). In the presence of 100 mm Na+, the potassium-induced current was voltage-dependent for all three isoforms, but the mid-point potential (i.e. the potential at which the potassium-induced current was reduced by half compared to its maximal value at a positive membrane potential) was strongly shifted to the right by about 50–60 mV for the α2* isoform. Thus, the voltage-dependent inhibition of the potassium-induced current occurred at a lower potential, or at a lower [Na+]o, for the α2* isoform. In fact, the I-V curve at 30 mm Na+ for the α2* isoform was similar to that obtained at 100 mm Na+ for the α1 and α3* isoforms. These results could be explained by an approximately three-fold increase in affinity for extracellular Na+ by the α2* isoform. A smaller difference separates the α1 from the α2* isoform: extracellular Na+ inhibition curves with a shift of about 15 mV to the right for the α3* I-V curve. Since only three concentrations of Na+ were used, and because these concentrations are not sufficient to obtain a close to maximal effect, it was not possible to determine a precise value for the apparent Na+ affinity.
Figure 6. Effect of [Na+]o on the potassium-activated current.
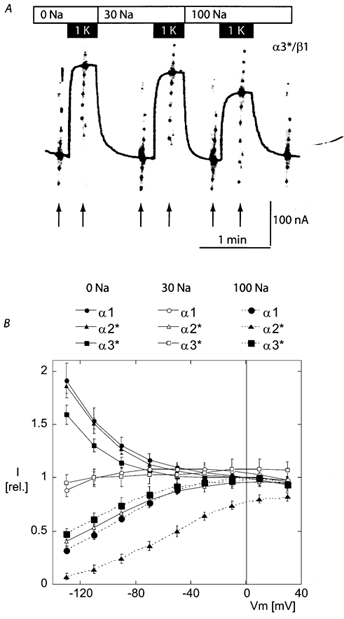
A, original current recordings showing the current induced by a [K+]o of 1 mm in the presence of extracellular solutions containing 0, 30 and 100 mm[Na+]o (effected by N-methyl-d-glucamine replacement). This recording was obtained with an oocyte expressing the α3*β1 isoform of the rat Na,K-ATPase. B, mean potassium-induced current expressed as relative to the current induced by 1 mm K+ measured at 0 mV in the sodium-free solution. The mean normalization value was 160 ± 25 nA (n = 12), 165 ± 25 nA (n = 12) and 154 ± 24 nA (n = 12) for the α1, α2*, and α3* groups, respectively. While the α1 and α3* isoforms present a rather similar voltage-dependent inhibition by external Na+, this inhibition was clearly more pronounced for the α2* isoform.
Apparent affinity for intracellular Na+
The Na+/K+-pump currents induced by a saturating [K+]o (10 mm) in a 10 mm Na+ solution were recorded during progressive Na+ loading of the oocytes at a holding membrane potential of −50 mV, as illustrated in the example of Fig. 7A. Figure 7B shows a typical example of individual concentration-response curves for each of the three isoforms, and Fig. 7C shows the mean values of K1/2 obtained from the measurements with α1 (n = 12), α2* (n = 7), and α3* (n = 16) isoforms. The apparent affinity for intracellular Na+ was slightly higher for the α2* isoform when compared to the α1 isoform (P ≤ 0.05); the other comparisons, α1 vs. α3* and α2* vs. α3*, did not show statistically significant differences.
DISCUSSION
The three isoforms of the rat α subunit of the Na,K-ATPase were well expressed in Xenopus oocytes when associated with the rat β1 subunit, with activity levels much larger than that of the remaining endogenous Na+/K+-pump, thus allowing us to study their physiological properties in this model. In all cases, large outward ouabain-sensitive currents could be stimulated by extracellular K+. This observation indicates that the three isoforms are indeed electrogenic Na+/K+ exchange pumps. The Na+/K+-pump is known to exchange three Na+ for two K+ for each hydrolyzed ATP (De Weer, 1985) under a large range of experimental conditions, however most of the measurements of this stoichiometry have been obtained with preparations from tissue naturally expressing either mostly the α1 isoform or an ill-defined mixture of isoforms. Even though our results are not direct stoichiometry measurements, the largely similar voltage dependence of the Na+/K+-pump activity observed under most circumstances (except for the measurements in the presence of Na+ and at a low K+ concentration, see discussion of this point below) makes it very unlikely that one isoform has a cation stoichiometry different from the others. Obviously, a 2:2 K+:Na+ ratio or a 3:3 K+:Na+ ratio can be excluded by the presence of the electrogenic signal. A 3:1 Na+:K+ ratio, with two charges translocated during each cycle, would be expected to modify considerably the voltage dependency of the Na+/K+-pump activity. Thus, the most parsimonious explanation for our results is that the three isoforms of the Na,K-ATPase function with a similar cation stoichiometry. However, the stoichiometry of the Na+/K+-pump and of the H+/K+-pump has been shown to be altered under specific experimental conditions such as low [Na+]i or [H+]i (Blostein, 1985; Polvani & Blostein, 1988, 1989; Blostein & Harvey, 1989), and our results do not allow us to predict the behaviour of each isoform under such conditions.
The ouabain-sensitive current presented a similar shallow voltage dependence along the whole membrane potential range, in the absence of extracellular Na+. This observation differs from results obtained under similar conditions either with myocardial cells or with sheep Na,K-ATPase expressed in HeLa cells, for which an essentially flat ouabain-sensitive I-V curve was observed (Nakao & Gadsby, 1989; Peluffo et al. 2000). The reason for this discrepancy is not clear, but a number of experimental differences (temperature, use of extra- or intracellular TEA, species, expression system) could explain it. On the other hand, this small voltage dependence is in good agreement with observations supporting the existence of a weakly voltage dependent conformational transition in the Na+/K+-pump cycle (Wuddel & Apell, 1995; Rakowski et al. 1997; Apell & Karlish, 2001). Our data suggest that this property is similar between the three rat Na,K-ATPase isoforms.
In the absence of extracellular Na+, potassium-induced currents were significantly larger than ouabain-sensitive currents at membrane potentials more negative than −50 mV, and the potassium-activated current I-V curve had a negative slope in the high negative potential range. A similar negative slope has been observed with the Xenopus, Bufo or Torpedo Na+/K+-pumps (Rakowski et al. 1991; Jaisser et al. 1994; Vasilets et al. 1991) expressed in Xenopus oocytes, however the relative amplitude of the current at negative membrane potential was much larger for the three rat isoforms used in this study.
These results are in contrast with observations made in other preparations, in which there was practically no difference between the potassium-induced currents and the ouabain-sensitive currents (Peluffo & Berlin, 1997; Peluffo et al. 2000). The reasons for this difference are not clear. As the negative slope has been observed only in Na+/K+-pumps expressed in Xenopus oocytes, it appears possible that this phenomenon is specific to that expression system. However, the experiments with heart cells or HeLa cells have not explored the large negative potential range in which the differences between the ouabain-sensitive current and potassium-induced current are most apparent.
Extracellular cation binding
When studied in the absence of extracellular Na+, a condition that eliminates the competitive effect of extracellular Na+ ions, and may therefore reflect more directly the intrinsic affinity of the external K+ binding site, the activation by extracellular K+ is roughly similar for the three isoforms, with a slightly lower apparent affinity for the α2* isoform. The known voltage dependence of the apparent K+ affinity (Rakowski et al. 1991; Jaisser et al. 1994; Peluffo et al. 2000) is observed with the three isoforms, and our data do not show any significant difference between isoforms for this parameter.
In the presence of extracellular Na+, the voltage dependence of the apparent affinity for K+ was qualitatively similar, with a maximal affinity at low membrane potentials (−10 to −50 mV) and lower affinity both at high negative and high positive membrane potentials. This shape of the voltage-apparent affinity curve is attributed to the double voltage dependence of the competing Na+ and K+ ions (Sagar & Rakowski, 1994; Rakowski et al. 1997). The apparent affinity for K+ was higher for the α3* subunit and lower for the α2* subunit when compared to the α1 subunit. The measurements of the influence of [Na+]o on the current induced by 1 mm [K+]o indicated that the three isoforms differed mainly in their sensitivity to the voltage-dependent effect of extracellular Na+. Measurements of currents activated by a low concentration of K+ confirm that the lower apparent affinity for K+ of the α2* isoform results mostly from extracellular Na+ competing with a higher affinity for this isoform. In fact, the lower apparent affinity for extracellular K+ in the α2* isoform can be explained mostly by a stronger voltage-dependent inhibition by extracellular Na+, with a small additional contribution from a slightly lower intrinsic affinity for K+. Similarly, the higher apparent affinity of the α3* isoform seems to result from a lower sensitivity to inhibition by extracellular Na+.
Inward pump-mediated currents in the absence of Na+ and K+
Large inward ouabain-sensitive currents can be recorded in the absence of extracellular Na+ and K+ at low pH (Efthymiadis et al. 1993) and these currents have been attributed to an inward proton current (Wang & Horisberger, 1995). These currents were present in the three isoforms. The amplitude of these currents was significantly smaller for the α3* isoform than for the α1 and α2* isoforms. It is not possible, however, to draw safe conclusions about these amplitudes because the effect of ouabain was only partial on the α2* and α3* isoforms and the affinity of ouabain under these conditions may be different from that observed in the presence of extracellular Na+. As described for the Xenopus Na+/K+-pump, the shape of the I-V curve indicated inward rectification and there was no significant difference between the isoforms in this regard.
Activation by intracellular Na+
Our measurements of the apparent affinity for activation by intracellular Na+ only show a modest difference between the α2* isoform and the α1 and α3* isoforms. This result is in opposition to several earlier reports in which different methods were used: ATPase activity in membrane preparations (Jewell & Lingrel, 1991; Therien et al. 1996; Blanco & Mercer, 1998), rubidium uptake in whole cells (Munzer et al. 1994), rate of intracellular Na+ decrease (Zahler et al. 1997) and expression systems such as HeLa cells (Jewell & Lingrel, 1991; Munzer et al. 1994; Therien et al. 1996; Zahler et al. 1997; Segall et al. 2001) or SF9 cells (Blanco & Mercer, 1998). In all these reports a lower apparent affinity for intracellular Na+ was observed with the α3 isoform, when compared to the α1 or α2 isoform. Differences in the same direction were also observed using the same technique as the present work applied to expression of the human α1, α2 and α3 isoforms (Crambert et al. 2000). The reasons for this discrepancy are not clear. Except for the case of the human isoforms, all other experiments have been performed with the same clones (wild-type rat α1 and ouabain-resistant mutant of the α2* and α3* rat isoforms) originating from the same laboratory. Some important experimental differences are evident, however: (1) ATPase activity measurements in membrane preparations convey a different type of information as both sides of the membrane are exposed to the same solution and the membrane potential is not controlled and (2) rubidium uptake measurements do not allow the membrane potential to be controlled. Considering the important effect of membrane potential and [Na+]o, different experimental conditions could explain some of the differences observed. Precise measurements of intracellular ion concentrations are not easy, and ion-sensitive electrodes or ion-sensitive fluorescent dye techniques yield a bulk solution concentration. The existence of unstirred layers leading to significant local concentration differences related to high transport rates of osmolytes (Duquette et al. 2001) or Na+ (Abriel & Horisberger, 1999) have been well demonstrated. Our technique has the advantage of estimating precisely (due to the extremely high Na+ selectivity of the epithelial Na+ channel) the sub-membrane Na+ concentration. Thus, although we cannot exclude that we have missed a slightly larger affinity value in the α3* isoform, our results indicate that there is no major difference in intracellular Na+ activation kinetics due exclusively to sequence differences between the three α subunits of the rat Na,K-ATPase under our experimental conditions (i.e. polarized membrane at −50 mV and activation by a high (10 mm) [K+]o). The discrepancy between measurements obtained with the human isoforms using the same technique (Crambert et al. 2000) could be explained by species differences or by the fact that the endogenous Xenopus component could not be inhibited (because the human isoforms are highly ouabain-sensitive) and the function of the expressed human Na+/K+-pumps was obtained by subtracting the endogenous component evaluated by measurement in native oocytes, a process that may have introduced some errors.
The most striking difference that we observed was the sensitivity to extracellular Na+, which was about three-fold higher for the α2* isoform, and made this form of the enzyme more sensitive to the membrane potential than α1 and α3* over the physiological range of membrane potential. A recent report by Segall et al. (2001) indicates that the strong shift towards the E1 form of the α2* isoform is the main distinguishing property of the α2* isoform in comparison to α1. This shift is due, at least in part, to a faster E2(K) to E1 transition (Munzer et al. 1994). The higher voltage-dependent affinity for extracellular Na+ that we have demonstrated in the present work must also contribute to a shift from E2-P to E1 through the sodium-transporting limb of the pump cycle.
This property could have physiological relevance in cells that express the α2 isoform and may undergo physiological depolarization such as the heart or skeletal muscle cells, neurones or glial cells, which may be depolarized by the electrogenic neurotransmitter re-uptake transport systems. As can be seen in Fig. 6B, the activity of the α2 isoform would increase by about three-fold between a resting membrane potential of about −90 mV and a depolarized potential, while the change would be much smaller for the α1 or the α3 isoform.
Acknowledgments
This work was supported by the Swiss ‘Fonds National de la Recherche Scientifique’, Grant no. 31–45867.95. We are grateful to K. Geering, P. Béguin and G. Crambert for helpful suggestions and careful reading of the manuscript.
REFERENCES
- Abriel H, Horisberger J-D. Feedback inhibition of amiloride-sensitive epithelial sodium channel in Xenopus laevis oocytes. Journal of Physiology. 1999;516:31–43. doi: 10.1111/j.1469-7793.1999.031aa.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apell H-J, Karlish SJ. Functional properties of Na,K-ATPase, and their structural implications, as detected with biophysical techniques. Journal of Membrane Biology. 2001;180:1–9. doi: 10.1007/s002320010053. [DOI] [PubMed] [Google Scholar]
- Argüello JM, Peluffo RD, Feng JN, Lingrel JB, Berlin JR. Substitution of glutamic 779 with alanine in the Na,K-ATPase alpha subunit removes voltage dependence of ion transport. Journal of Biological Chemistry. 1996;271:24610–24616. doi: 10.1074/jbc.271.40.24610. [DOI] [PubMed] [Google Scholar]
- Blanco G, Mercer RW. Isozymes of the Na-K-ATPase: Heterogeneity in structure, diversity in function. American Journal of Physiology. 1998;44:F633–F650. doi: 10.1152/ajprenal.1998.275.5.F633. [DOI] [PubMed] [Google Scholar]
- Blostein R. Proton-activated rubidium transport catalyzed by the sodium pump. Journal of Biological Chemistry. 1985;260:829–833. [PubMed] [Google Scholar]
- Blostein R, Harvey WJ. Na+, K+-pump stoichiometry and coupling in inside-out vesicles from red blood cell membranes. Methods Enzymology. 1989;173:377–380. doi: 10.1016/s0076-6879(89)73025-1. [DOI] [PubMed] [Google Scholar]
- Cameron R, Klein L, Shyjian AW, Rakic P, Levenson R. Neurons and astroglia express distinct subsets of Na,K-ATPase α and β subunits. Molecular Brain Research. 1994;21:333–343. doi: 10.1016/0169-328x(94)90264-x. [DOI] [PubMed] [Google Scholar]
- Canessa CM, Horisberger J-D, Louvard D, Rossier BC. Mutation of a cysteine in the first transmembrane segment of Na-K-ATPase α subunit confers ouabain resistance. EMBO Journal. 1992;11:1681–1687. doi: 10.1002/j.1460-2075.1992.tb05218.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canessa CM, Horisberger J-D, Rossier BC. Functional cloning of the epithelial sodium channel: relation with genes involved in neurodegeneration. Nature. 1993;361:467–470. doi: 10.1038/361467a0. [DOI] [PubMed] [Google Scholar]
- Canessa CM, Schild L, Buell G, Thorens B, Gautshi Y, Horisberger J-D, Rossier BC. The amiloride-sensitive epithelial sodium channel is made of three homologous subunits. Nature. 1994;367:463–467. doi: 10.1038/367463a0. [DOI] [PubMed] [Google Scholar]
- Crambert G, Hasler U, Beggah AT, Yu CL, Modyanov NN, Horisberger J-D, Lelievre L, Geering K. Transport and pharmacological properties of nine different human Na,K-ATPase isozymes. Journal of Biological Chemistry. 2000;275:1976–1986. doi: 10.1074/jbc.275.3.1976. [DOI] [PubMed] [Google Scholar]
- De Weer P. Cellular sodium-potassium transport. In: Seldin DW, Giebisch G, editors. The Kidney: Physiology and Pathophysiology. New York: Raven; 1985. pp. 31–48. [Google Scholar]
- Duquette P-P, Bissonnette P, Lapointe J-Y. Local osmotic gradients drive the water flux associated with Na+/glucose cotransport. Proceedings of the National Academy of Sciences of the USA. 2001;98:3796–3801. doi: 10.1073/pnas.071245198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Efthymiadis A, Rettinger J, Schwarz W. Inward-directed current generated by the Na+,K+ pump in Na+- and K+-free medium. Cell Biology International. 1993;17:1107–1116. doi: 10.1006/cbir.1993.1043. [DOI] [PubMed] [Google Scholar]
- Gadsby DC, Nakao M. Steady-state current-voltage relationship of the Na/K pump in guinea pig ventricular myocytes. Journal of General Physiology. 1989;94:511–537. doi: 10.1085/jgp.94.3.511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasler U, Wang X, Crambert G, Beguin P, Jaisser F, Horisberger J-D, Geering K. Role of β-subunit domains in the assembly, stable expression, intracellular routing and functional properties of Na,K-ATPase. Journal of Biological Chemistry. 1998;273:30826–30835. doi: 10.1074/jbc.273.46.30826. [DOI] [PubMed] [Google Scholar]
- Horisberger J-D, Jaunin P, Good PJ, Rossier BC, Geering K. Coexpression of α1 with putative β3 subunits results in functional Na-K-pumps in Xenopus oocytes. Proceedings of the National Academy of Sciences of the USA. 1991;88:8397–8400. doi: 10.1073/pnas.88.19.8397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaisser F, Jaunin P, Geering K, Rossier BC, Horisberger J-D. Modulation of the Na,K-pump function by the β-subunit isoforms. Journal of General Physiology. 1994;103:605–623. doi: 10.1085/jgp.103.4.605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaunin P, Horisberger J-D, Richter K, Good PJ, Rossier BC, Geering K. Processing, intracellular transport and functional expression of endogenous and exogenous α-β3 Na,K-ATPase complexes in Xenopus oocytes. Journal of Biological Chemistry. 1992;267:577–585. [PubMed] [Google Scholar]
- Jewell EA, Lingrel JB. Comparison of the substrate dependence properties of the rat Na, K-ATPase α1, α2, and α3 isoforms expressed in HeLa cells. Journal of Biological Chemistry. 1991;266:16925–16930. [PubMed] [Google Scholar]
- Munzer JS, Daly SE, Jewell-Motz EA, Lingrel JB, Blostein R. Tissue- and isoform-specific kinetic behavior of the Na, K-ATPase. Journal of Biological Chemistry. 1994;269:16668–16676. [PubMed] [Google Scholar]
- Nakao M, Gadsby DC. [Na] and [K] dependence of the Na/K pump current-voltage relationship in guinea pig ventricular myocytes. Journal of General Physiology. 1989;94:539–565. doi: 10.1085/jgp.94.3.539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peluffo RD, Argüello JM, Berlin JR. The role of Na,K-ATPase alpha subunit Serine 775 and Glutamate 779 in determining the extracellular K+ and membrane potential-dependent properties of the Na,K-pump. Journal of General Physiology. 2000;116:47–59. doi: 10.1085/jgp.116.1.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peluffo RD, Berlin JR. Electrogenic K+ transport by the Na+-K+ pump in rat cardiac ventricular myocytes. Journal of Physiology. 1997;501:33–40. doi: 10.1111/j.1469-7793.1997.033bo.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polvani C, Blostein R. Protons as substitutes for sodium and potassium in the sodium pump reaction. Journal of Biological Chemistry. 1988;263:16757–16763. [PubMed] [Google Scholar]
- Polvani C, Blostein R. Effects of cytoplasmic sodium concentration on the electrogenicity of the sodium pump. Journal of Biological Chemistry. 1989;264:15182–15185. [PubMed] [Google Scholar]
- Rakowski RF, Gadsby DC, De Weer P. Voltage dependence of the Na/K pump. Journal of Membrane Biology. 1997;155:105–112. doi: 10.1007/s002329900162. [DOI] [PubMed] [Google Scholar]
- Rakowski RF, Paxson CL. Voltage dependence of Na/K pump current in Xenopus oocytes. Journal of Membrane Biology. 1988;106:173–182. doi: 10.1007/BF01871399. [DOI] [PubMed] [Google Scholar]
- Rakowski RF, Vasilets LA, Latona J, Schwarz W. A negative slope in the current-voltage relationship of the Na+/K+ Pump in Xenopus oocytes produced by reduction of external [K+] Journal of Membrane Biology. 1991;121:177–187. doi: 10.1007/BF01870531. [DOI] [PubMed] [Google Scholar]
- Sagar A, Rakowski RF. Access channel model for the voltage dependence of the forward-running Na+/K+ pump. Journal of General Physiology. 1994;103:869–894. doi: 10.1085/jgp.103.5.869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schweigert B, Lafaire AV, Schwarz W. Voltage dependence of the Na-K ATPase: measurements of ouabain-dependent membrane current and ouabain binding in oocytes of Xenopus laevis. Pflügers Archiv. 1988;412:579–588. doi: 10.1007/BF00583758. [DOI] [PubMed] [Google Scholar]
- Segall L, Daly SE, Blostein R. Mechanistic basis for kinetic differences between the rat α1, α2, and α3 isoforms of the Na,K-ATPase. Journal of Biological Chemistry. 2001;276:31535–31541. doi: 10.1074/jbc.M103720200. [DOI] [PubMed] [Google Scholar]
- Shyjian AW, Ceña V, Klein DC, Levenson R. Differential expression and enzymatic properties of the Na+,K+-ATPase α3 isoenzyme in rat pineal glands. Proceedings of the National Academy of Sciences of the USA. 1990;87:1178–1182. doi: 10.1073/pnas.87.3.1178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sweadner KJ. Isozymes of the Na+/K+-ATPase. Biochimica et Biophysica Acta. 1989;988:185–220. doi: 10.1016/0304-4157(89)90019-1. [DOI] [PubMed] [Google Scholar]
- Sweadner KJ. Overlapping and diverse distribution of Na-K ATPase isozymes in neurons and glia. Canadian Journal of Physiology and Pharmacology. 1992;70(Suppl.):S255–S259. doi: 10.1139/y92-269. [DOI] [PubMed] [Google Scholar]
- Therien AG, Nestor NB, Ball WJ, Blostein R. Tissue-specific versus isoform-specific differences in cation activation kinetics of the Na,K-ATPase. Journal of Biological Chemistry. 1996;271:7104–7112. doi: 10.1074/jbc.271.12.7104. [DOI] [PubMed] [Google Scholar]
- Vasilets LA, Ohta T, Noguchi S, Kawamura M, Schwarz W. Voltage-dependent inhibition of the sodium pump by external sodium: Species differences and possible role of the N- terminus of the α-subunit. European Biophysical Journal. 1993;21:433–443. doi: 10.1007/BF00185871. [DOI] [PubMed] [Google Scholar]
- Vasilets LA, Omay HS, Ohta T, Noguchi S, Kawamura M, Schwarz W. Stimulation of the Na+/K+ pump by external [K+] is regulated by voltage-dependent gating. Journal of Biological Chemistry. 1991;266:16285–16288. [PubMed] [Google Scholar]
- Wang X, Horisberger J-D. A conformation of the Na,K-pump is permeable to proton. American Journal of Physiology. 1995;37:C590–595. doi: 10.1152/ajpcell.1995.268.3.C590. [DOI] [PubMed] [Google Scholar]
- Wang X, Jaisser F, Horisberger J-D. Role in cation translocation of the N-terminus of the α-subunit of the Na+,K+-pump of Bufo. Journal of Physiology. 1996;491:579–594. doi: 10.1113/jphysiol.1996.sp021241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Gao J, Mathias RT, Cohen IS, Sun X, Baldo GJ. Alpha-adrenergic effects on Na+-K+ pump current in guinea-pig ventricular myocytes. Journal of Physiology. 1998;509:117–128. doi: 10.1111/j.1469-7793.1998.117bo.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wetzel RK, Sweadner KJ. Immunocytochemical localization of NaK-ATPase isoforms in the rat and mouse ocular ciliary epithelium. Investigative Ophthalmology and Visual Science. 2001;42:763–769. [PubMed] [Google Scholar]
- Wuddel I, Apell H-J. Electrogenicity of the sodium transport pathway in the Na,K-ATPase probed by charge-pulse experiments. Biophysical Journal. 1995;69:909–921. doi: 10.1016/S0006-3495(95)79965-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zahler R, Brines M, Kashgarian M, Benz EJ, JR, Gilmore-Hebert M. The cardiac conduction system in the rat expresses the α2 and α3 isoforms of the Na+,K+-ATPase. Proceedings of the National Academy of Sciences of the USA. 1992;89:99–103. doi: 10.1073/pnas.89.1.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zahler R, Zhang ZT, Manor M, Boron WF. Sodium kinetics of Na,K-ATPase α isoforms in intact transfected Hela cells. Journal of General Physiology. 1997;110:201–213. doi: 10.1085/jgp.110.2.201. [DOI] [PMC free article] [PubMed] [Google Scholar]


