K+ channels form the most diverse family of ion channels with more than 70 genes cloned in humans. They can be broadly subdivided into three structural classes made of two, four or six transmembrane segments (TMS) (Patel & Honoré, 2001a). All K+ channels can be recognized by the presence of a conserved motif called the P domain (the pore-forming region), which is part of the K+ conduction pathway. The two TMS and six TMS classes contain a single P domain while the class of four TMS subunits contains two P domains in tandem. Functional K+ channels are tetramers of pore-forming subunits for the two and six TMS classes and probably dimers in the case of the four TMS class (Fig. 1).
Figure 1. TREK channels are opened by a variety of physical (stretch, acidosis and heat) and chemical stimuli (polyunsaturated fatty acids, lysophospholipids and volatile anaesthetics) and are regulated by phosphorylation.
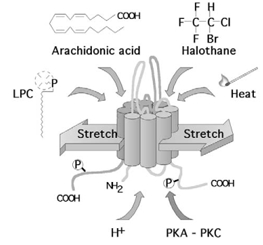
4TMS/2P subunits are believed to dimerize to form functional K+ channels. TREK channels and their functional homologue, the Aplysia S-type K+ channel, have been implicated in learning and memory. At the pathophysiological level, cell swelling, intracellular acidosis, along with the release of cellular lipids, will contribute to the opening of TREK and TRAAK channels during brain ischaemia and could represent an important neuroprotective switch.
The family of mammalian 4TMS/2P K+ channel subunits has increased to 14 members (Patel & Honoré, 2001a). These subunits share the same structural motif, but low sequence identity is found outside the P domains. TREK-1 (KCNK2), TREK-2 (KCNK10) and TRAAK (KCNK4) subunits share the closest sequence identity ranging from 63 to 78 %, and form a structural as well as a functional subgroup. Human TREK channels are highly expressed in the central and peripheral nervous systems, but are absent from the heart. TREK channels are opened by a variety of physical and chemical stimuli (Patel & Honoré, 2001a).
TREK channel activity is elicited by increasing mechanical pressure applied to the cell membrane (Patel & Honoré, 2001a; Patel et al. 2001). At the whole-cell level, TREK-1 is modulated by cellular volume. Acidosis converts TREK mechano-gated channels into constitutively active channels. Finally, heat gradually and reversibly opens TREK-1 with an exceptional Q10 value of 7. Deletional analysis demonstrates that the carboxy terminus, but not the amino terminus and the extracellular M1P1 loop, is critical for activation of TREK-1 by stretch, intracellular acidosis and heat.
TREK channels are reversibly opened by anionic polyunsaturated fatty acids including arachidonic acid. The activation is either directly on the channel protein or via a membrane effect (Patel et al. 2001). Lysophospholipids containing large polar heads are additional potent openers. TREK channels are the target for volatile general anaesthetics including halothane and isoflurane (Patel & Honoré, 2001b). Opening of these channels by inhalational anaesthetics induces cell hyperpolarization and may contribute to general anaesthesia (Patel & Honoré, 2001b). Chemical activation of TREK-1 is also critically dependent on the carboxy terminal domain (Patel & Honoré, 2001a,b; Patel et al. 2001).
TREK channels are additionally regulated by the neurotransmitter/cAMP/PKA pathway (Patel & Honoré, 2001a). For instance, opening of TREK-1 by lipids is reversed by protein kinase A stimulation. Protein kinase A-mediated phosphorylation of Ser333 in the carboxy terminus mediates TREK-1 closing. TREK-1 activity is also inhibited by the protein kinase C pathway, although the phosphorylation site remains to be identified.
The biophysical and pharmacological properties of TREK channels resemble those of the Aplysia S-type K+ channel (Belardetti & Siegelbaum, 1988; Patel & Honoré, 2001a) (Fig. 1). The S channel is responsible for the control of presynaptic facilitation of transmitter release that underlies behavioural sensitization, a simple form of learning and memory. Considering that TREK channels are highly expressed in the hippocampus and the cerebral cortex, they may play a significant role in cognition (Patel & Honoré, 2001a).
In this issue of The Journal of Physiology, Gu et al. identify two splice variants of TREK-2 differing in their 5′ untranslated region and in the coding region for the first 17 amino acids. These splice variants are differentially expressed in the brain and the kidney. Interestingly, the brain splice variant gives 2.5-fold larger currents than the kidney splice variant when transfected in human embryonic kidney cells. These results suggest a novel role for the amino terminus of TREK-2 in regulating targeting to the cell surface, channel turnover and/or interaction with auxiliary proteins. Cell-specific alternative splicing may thus be important to tune the functional expression of the 2P domain K+ channels.
References
- Belardetti F, Siegelbaum SA. Trends in Neurosciences. 1988;11:232–238. doi: 10.1016/0166-2236(88)90132-4. [DOI] [PubMed] [Google Scholar]
- Gu W, Schlichthchlichthörlouml;rl G, Hirsch JR, Engels H, Karschin C, Karschin A, Derst C, Steinlein OK, Daut J. Journal of Physiology. 2002;539:657–668. doi: 10.1113/jphysiol.2001.013432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel AJ, Honoronoré E. Trends in Neurosciences. 2001a;24:339–346. doi: 10.1016/s0166-2236(00)01810-5. [DOI] [PubMed] [Google Scholar]
- Patel AJ, Honoronoré E. Anesthesiology. 2001b;95:1013–1021. doi: 10.1097/00000542-200110000-00034. [DOI] [PubMed] [Google Scholar]
- Patel AJ, Lazdunski M, Honoronoré E. Current Opinion in Cell Biology. 2001;13:422–428. doi: 10.1016/s0955-0674(00)00231-3. [DOI] [PubMed] [Google Scholar]


