Abstract
In man, epidemiological studies have shown that low birth weight (BW) is associated with an increased risk of cardiovascular disease in later life. In this study, the long-term consequences of variations in natural BW on basal cardiovascular function were investigated in pigs at 3 months of postnatal age. Low (< 1.41 kg; n = 20) and high (> 1.52 kg; n = 20) BW Large White piglets were selected from a total of 12 litters for study at 3 months of age. Basal mean arterial pressure (MAP) and heart rate (HR) were recorded for ∼30 min using standard recording equipment and basal arterial blood samples were taken for hormone analyses. Concentrations of angiotensin-converting enzyme (ACE) were also measured in kidney, lung and plasma. Basal MAP, but not HR, in 3-month-old pigs was significantly inversely related to BW and positively related to the ratio of head length to BW. Postnatal growth rate of low BW pigs was slower than that of high BW pigs such that low BW piglets remained significantly smaller at 3 months of age. There were no differences in basal plasma adrenaline or cortisol concentrations between low and high BW pigs. However, basal plasma noradrenaline concentrations were significantly elevated in low BW compared to high BW pigs. Renal and pulmonary ACE levels were significantly reduced in low BW compared to high BW pigs. These data show that basal MAP in 3-month-old pigs is negatively associated with BW and positively correlated to disproportionate size at birth. This effect was associated with an increase in basal plasma noradrenaline concentrations.
Epidemiological studies in man have shown that poor fetal growth resulting in low birth weight (BW) is associated with an increased risk of hypertension and cardiovascular disease in adult life (Barker et al. 1989). The association between low BW and cardiovascular, and other diseases such as non-insulin-dependent diabetes mellitus, has now been described in several human populations of different ages, sex and ethnic origin (see Law & Shiell, 1996). Anthropomorphic measurements have shown that it is disproportionate fetal growth leading to a low ponderal index (weight × length−3) or reduced head circumference at birth, that particularly predisposes an individual to adult hypertension (Barker et al. 1993). In addition, poor growth in the first year of life, followed by rapid weight gain, further increases the risk of coronary heart disease in later life (Eriksson et al. 2001). While there are few data relating maternal nutritional intake to specific patterns of fetal growth in humans, disproportionate fetal growth is often associated with poor nutrition in utero, in animals, particularly during late gestation (see Widdowson & McCance, 1975). These studies have led to the hypothesis that some adult diseases may originate in early life as the result of tissue programming by environmental influences acting at critical periods of development (Widdowson & McCance, 1975).
Prenatal nutritional programming of adult hypertension has now been reported in rats, guinea-pigs and sheep using a range of experimental techniques including manipulation of maternal dietary intake, uterine blood flow or placental size number of experimental animals (Persson & Jansson, 1992; Langley, 1999; Hoet & Hanson, 1999). These studies showed that undernutrition in utero can lead to elevated postnatal blood pressure (BP) both in association with low BW and when BW is within the normal range (Hoet & Hanson, 1999). In rats, this elevated mean arterial blood pressure (MAP) was linked to increased activity of the renin-angiotensin system (RAS; Langley, 1999). However, more detailed examination of the aetiology of adult hypertension induced by intrauterine undernutrition has been constrained by the small size of the rat pup.
The pig may be a more suitable model than rodents and sheep for investigating the mechanisms underlying the association between natural variations in BW and elevated BP in adult life. Piglets are large enough to study physiologically from birth and, unlike ruminants, their postnatal nutrient intake can be regulated precisely after weaning. In addition, BW varies 2- to 3-fold amongst littermates in normally fed sows (Bauer et al. 1998), which provides a naturally occurring form of growth retardation with less genetic variation than is seen in man and other monotocous species.
Hence, the aims of this study were to: (1) determine the long-term consequences on basal cardiovascular function of natural variations in intrauterine growth in pigs at 3 months of postnatal age; and (2) investigate possible mechanisms underlying the association between BW and postnatal MAP by measuring basal concentrations of vasoactive hormones and tissue and plasma angiotensin-converting enzyme (ACE).
METHODS
Animals
Pure-bred Large White pigs were obtained from sows allowed to farrow normally at term (115 ± 1 days). Twelve litters from eight sows were used in this study (average litter size 11 ± 1). Sows were fed a standard diet (15 % protein; 12.6 MJ kg−1 digestible energy; ABN, Oundle Rd, Woodstone, Peterborough, UK) for at least 4 weeks before conception (2 kg day−1) and during gestation and lactation (2.5–3 kg day−1) according to standard guidelines (Agricultural and Food Research Council, 1990). Water was provided ad libitum. Piglets had access to straw bedding and infrared heat lamps from birth until weaning at 4–5 weeks of age. Weaner piglets were housed in groups and fed ad libitum on a standard pig weaner diet (creep food with small pellet size, 20 % protein; H & C Beart Ltd, Brighton Mill, Kings Lynn, UK) until studies were performed at ∼3 months of age. From this time, pigs were housed individually, but adjacent to their littermates, and fed according to their body weight (0.5 kg per 30 kg body weight twice a day at 08.00 h and 17.00 h) such that nutritional requirements were satisfied according to standard guidelines (Agricultural and Food Research Council, 1990).
At birth and at weekly intervals until 4 weeks of age, all piglets in each litter were weighed and the following morphometric measurements were made: head length (snout to between ears), crown rump length (between ears to base of tail; CRL) and abdominal circumference (AC). The average BW of all piglets born in the 12 litters used in this study was 1.46 ± 0.02 kg (n = 131) and the 95 % confidence interval of the mean was 1.41–1.52 kg. After determination of BW, pigs were assigned to one of two groups, such that those with BW lower than the 95 % confidence interval of the mean were defined as ‘low BW’ pigs (< 1.41 kg at birth) and those with BW higher than the 95 % confidence interval of the mean BW were defined as ‘high BW’ pigs (> 1.52 kg at birth). The range of birth weights in the low BW group was 0.8–1.4 kg (total n = 20) and in the high BW group was 1.7–2.4 kg (total n = 20). It was not possible to obtain all data from all animals: the number of observations for each experimental data set is indicated in each table or figure.
Surgical procedures
At 9–10 weeks of age, before the morning feed, selected pigs were tranquilised with azaperone (Janssen Pharmaceuticals Ltd, Oxford, UK; 5 mg kg−1i.m. for pigs > 20 kg) or diazepam (Phoenix Pharmaceuticals Ltd, Gloucester, UK; 2 mg kg−1i.m. for pigs < 20 kg), each in combination with ketamine (Fort Dodge Animal Health Ltd, Southampton, UK; 10 mg kg−1i.m.) and then anaesthetised with halothane (3–6 % in O2). Catheters were inserted into the dorsal aorta and vena cava via the femoral vessels and were exteriorised via a small incision on the animal's back. Pigs were kept in protective coats made of elastic tubing (Tubigrip, Seton Healthcare Group, Oldham, UK) to protect the catheters. Antibiotic treatment was administered on the day of surgery (Depocillin; procaine benzylpenicillin, 15 mg kg−1; Depocillin Mycofarm Ltd, Cambridge, UK and Duphatrim; trimethoprim, 2.5 mg kg−1 with sulphadiazine, 12.5 mg kg−1; Fort Dodge Animal Health Ltd; i.m.), for 3 days following surgery and every 2–3 days thereafter (Duphatrim alone, i.v.). The normal feeding regime was restored immediately after recovery from surgery.
Experimental procedures
Between 10.00–12.00 h at least 2 days following surgery, basal MAP and heart rate (HR) were recorded for approximately 30 min using standard recording equipment. Recording began at least 30 min after moving the pigs to the recording pen to ensure basal conditions. For each animal, at least six measurements were taken at regular intervals during the recording period and the results for MAP and HR were averaged.
Immediately prior to starting the recording, arterial blood samples were taken for the measurement of basal cortisol concentrations (2 ml collected into chilled EDTA tubes) and catecholamine concentrations (1 ml collected into chilled heparinised tubes containing EGTA (5 μmol (ml blood)−1) and glutathione (40 μmol (ml blood)−1)). Where possible, up to three basal blood samples were collected from each animal over several days, during similar experimental conditions (including the day of recording) and the hormone concentration results were averaged. All blood samples were centrifuged immediately for 5 min at 4 °C and the plasma was stored at −20 °C (samples for cortisol analysis) or −80 °C (samples for catecholamine analysis).
All procedures were carried out in accordance with the regulations of the UK Home Office Animals (Scientific Procedures) Act, 1986.
Tissue collection
At the end of the experimental period, tissues were collected from 31 animals (low BW, n = 14; high BW, n = 17) after the administration of a lethal dose of sodium pentobarbitone. One adrenal gland was collected and weighed. Samples of lung and kidney were collected, frozen immediately in liquid nitrogen and stored at −80 °C for analysis of tissue ACE. Prior to euthanasia, a blood sample (2 ml) was taken into heparinised tubes for the analysis of plasma ACE.
Biochemical analyses
Endocrine measurements
Plasma cortisol concentrations were measured by radioimmunoassay as previously described (Silver & Fowden, 1983). The inter-assay coefficient of variation for the cortisol assay was 10 % and the minimum detectable dose was 0.4 ng ml−1. Plasma catecholamine (adrenaline and noradrenaline) concentrations were determined by high pressure liquid chromatography (HPLC) using electrochemical detection (Silver et al. 1982). Samples were prepared by absorption of 250 μl of plasma onto acid-washed alumina and 20 μl aliquots of the 100 μl perchloric acid elutes were injected onto the column. Dihydroxybenzylamine was added as the internal standard to each plasma sample before absorption. Recovery ranged from 63–97 % and all catecholamine values were corrected for their respective recovery. The inter-assay coefficients of variation for noradrenaline and adrenaline were 6.2 and 7.3 %, respectively, and the minimum detectable dose was 10 pg ml−1.
Tissue and plasma ACE measurements
Tissue and plasma ACE concentrations were determined by a spectrophotometric enzyme assay originally described by Hurst & Lovell-Smith (1981) and Raimbach & Thomas (1990) which was modified for ovine and porcine tissues and plasma as previously described (Forhead et al. 2000). The assay used hippuryl-histidyl-leucine as a substrate and addition of 1 m captopril to the incubation mixture abolished the production of hippurate. The inter-assay coefficient of variation was < 10 %. Tissue ACE concentration was expressed as nmoles of hippurate generated per minute per microgram of protein and plasma ACE concentration was expressed as units per litre of plasma, where 1 unit equals 1 μmol of hippurate generated in 1 min. Tissue protein content was measured by the Lowry method (Lowry et al. 1951).
Statistical analyses
All results are expressed as mean ± standard error of the mean (s.e.m.). The relationships between two factors were tested using linear regression analysis (SigmaStat Statistical Software version 2.0, SPSS Inc., Chicago, IL, USA). Partial correlation analysis was used to determine which of two independent variables was the strongest predictor of a dependent variable. Student's unpaired t tests were used to identify significant differences between two factors. For all statistical tests, significance was accepted when P < 0.05.
RESULTS
Postnatal growth
Morphometric measurements at birth and at 3 months of age in low and high BW pigs are shown in Table 1. Current weight at 3 months of age (CW) was significantly (P < 0.001) correlated to BW (Fig. 1). There was also a significant relationship between the head length:body weight ratio at 3 months and at birth (R = 0.43, P < 0.05). However, ponderal index, body mass index, head length:CRL and head length:AC ratios measured at 3 months were not significantly related to those measured at birth. The overall postnatal growth rate from birth until 3 months of age was significantly (P < 0.001) less in low BW than in high BW pigs (Table 1). During suckling (0–1 month), the fractional growth rate (weight gained per day per starting weight) was significantly (P < 0.05) greater in low than in high BW pigs, but during the post-weaning period of the study (1–3 months), the relative increase in body weight was significantly (P < 0.05) reduced in low compared to high BW pigs (Table 1). At 3 months of age, low BW pigs remained significantly (P < 0.01) smaller in body weight and in body mass index than their high BW littermates, despite their initially higher fractional growth rate (Table 1). The ratios of head length:body weight and head length:AC also remained significantly (P < 0.01) greater in low BW pigs than in their larger littermates (Table 1).
Table 1.
Body weights, morphometric ratios (at birth and at 3 months of age) and postnatal growth rates in low and high BW pigs
| Correlation coefficients (R) | ||||
|---|---|---|---|---|
| Low BW | High BW | MAP | HR | |
| At birth | ||||
| Birth weight (kg) | 1.11 ± 0.04 †† | 1.87 ± 0.04 | −0.60 † | −0.34 |
| Ponderal index (kg m−3) | 69.3 ± 1.9 | 74.8 ± 2.9 | −0.01 | +0.09 |
| Body mass index (kg m−2) | 17.4 ± 0.4 †† | 21.8 ± 0.6 | −0.37 | −0.17 |
| Head length:BW (cm kg−1) | 8.72 ± 0.40 †† | 5.59 ± 0.14 | +0.65 † | +0.38 |
| Head length:CRL | 0.376 ± 0.011 | 0.359 ± 0.011 | +0.43 | +0.26 |
| Head length:AC | 0.435 ± 0.016 | 0.399 ± 0.010 | +0.29 | +0.31 |
| At 3 months | ||||
| Current weight (kg) | 23.00 ± 2.14 ** | 39.55 ± 3.26 | −0.29 | −0.10 |
| Ponderal index (kg m−3) | 52.9 ± 3.6 | 60.7 ± 1.5 | −0.33 | −0.47 |
| Body mass index (kg m−2) | 38.5 ± 3.1 ** | 50.6 ± 2.6 | −0.27 | −0.48 |
| Head length:CW (cm kg−1) | 1.17 ± 0.19 ** | 0.65 ± 0.07 | −0.05 | +0.35 |
| Head length:CRL | 0.282 ± 0.010 | 0.284 ± 0.008 | −0.44 | −0.46 |
| Head length:AC | 0.332 ± 0.014 * | 0.297 ± 0.010 | +0.23 | +0.19 |
| Adrenal:CW (g kg−1) | 0.076 ± 0.010 ** | 0.046 ± 0.004 | +0.61 | +0.79 † |
| Growth rate 0–3 months (kg day−1) | 0.266 ± 0.025 †† | 0.454 ± 0.036 | −0.29 | −0.11 |
| Fractional growth rate 0–1 months (kg day−1 kg−1) | 0.172 ± 0.009 * | 0.141 ± 0.011 | +0.38 | +0.14 |
| Fractional growth rate 1–3 months (kg day−1 kg−1) | 0.041 ± 0.004 * | 0.054 ± 0.004 | +0.29 | +0.26 |
Values are means ±s.e.m.R represents the correlation coefficient between MAP or HR and each body weight, morphometric or growth rate variable. BW, birth weight; CRL, crown rump length; AC, abdominal circumference; CW, current weight. For weight and measurement data: low BW, n = 20; high BW, n = 20. For correlations: MAP, n = 18; HR, n = 25. Comparisons between low and high BW (unpaired t test) statistical significance of correlation coefficients
P < 0.05
P < 0.01
P < 0.005
P < 0.001.
Figure 1. Body weight at 3 months.
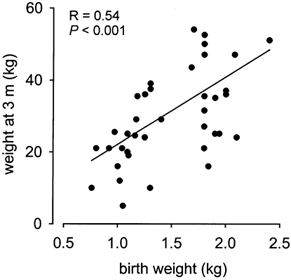
The relationship between body weight at 3 months (m) of age (CW) and birth weight (BW) in pigs. CW = 3.3 + (17.6 × BW).
Absolute adrenal weights at 3 months of age were not significantly different between low BW (1.48 ± 0.13 g) and high BW (1.65 ± 0.14 g) pigs. However, the ratio of adrenal:CW was significantly (P < 0.01) greater in low BW than in high BW pigs (Table 1). There were significant negative correlations between the adrenal:CW ratio and both BW (P < 0.01) and CW (P < 0.001), and a positive (P < 0.05) relationship with the head length:BW ratio (Table 2). The adrenal:CW ratio was also significantly (P < 0.001) negatively related to the ponderal index and body mass index measured at 3 months and positively correlated to the head length:AC (P < 0.05) and head length:CW (P < 0.001) ratios at 3 months. Growth rates (0–1 and 0–3 months; P < 0.001) and the fractional growth rate (1–3 months; P < 0.05) were negatively associated with the adrenal:CW ratio (Table 2).
Table 2.
Basal plasma adrenaline, noradrenaline, cortisol and angiotensin converting enzyme (ACE) concentrations, tissue ACE levels and the adrenal:body weight ratio
| Correlation coefficients (R) | |||||||
|---|---|---|---|---|---|---|---|
| Adrenaline | Noradr. | Cortisol | Plasma ACE | Renal ACE | Pulmonary ACE | Adrenal: CW | |
| At birth | |||||||
| Birth weight | +0.18 | −0.48 * | −0.22 | +0.13 | +0.45 | +0.52 * | −0.54 † |
| Ponderal index | +0.08 | −0.12 | +0.06 | +0.29 | −0.25 | −0.08 | +0.11 |
| Body mass index | +0.19 | −0.31 | −0.10 | +0.27 | +0.03 | +0.20 | −0.24 |
| Head length:BW | −0.06 | +0.55 ** | +0.21 | +0.12 | −0.60 ** | −0.56 * | +0.48 * |
| Head length:CRL | +0.20 | +0.35 | +0.21 | +0.43 | −0.41 | −0.30 | +0.22 |
| Head length:AC | +0.16 | +0.32 | +0.12 | +0.46 * | −0.59 * | −0.46 | +0.12 |
| At 3 months | |||||||
| Current weight | −0.43 * | −0.66 †† | −0.29 | +0.04 | +0.79 †† | +0.44 | −0.69 †† |
| Ponderal index | −0.27 | +0.13 | +0.37 | +0.40 | +0.55 * | +0.38 | −0.81 †† |
| Body mass index | −0.55 * | −0.37 | −0.01 | +0.28 | +0.68 † | +0.28 | −0.81 †† |
| Head length:CW | +0.44 | +0.43 | +0.23 | −0.34 | −0.68 † | −0.49 * | +0.91 †† |
| Head length:CRL | +0.13 | +0.17 | +0.56 * | +0.21 | −0.10 | −0.23 | −0.07 |
| Head length:AC | −0.02 | +0.07 | +0.57 * | −0.11 | −0.60 * | −0.38 | +0.52 * |
| Adrenal:CW | +0.13 | +0.19 | +0.08 | 0.54 * | −0.69 † | −0.46 | — |
| Growth rate 0-3 months | −0.41 | −0.58 † | −0.18 | −0.01 | +0.79 †† | +0.54 * | −0.67 †† |
| Fractional growth rate 0-1 months | −0.32 | +0.03 | +0.05 | −0.15 | +0.45 | −0.11 | −0.21 |
| Fractional growth rate 1-3 months | −0.59 †† | −0.55 ** | −0.04 | +0.32 | +0.40 | +0.48 | −0.51 * |
| MAP | −0.10 | −0.26 | −0.03 | −0.71 | +0.56 | −0.81 | +0.61 |
| HR | −0.48 * | −0.13 | −0.10 | −0.63 | −0.17 | −0.41 | +0.79 † |
R represents the correlation coefficient between each variable and each body weight, morphometric or growth rate variable. Noradr., noradrenaline; BW, birth weight; CRL, crown rump length; AC, abdominal circumference; CW, current weight. For catecholamine data, n = 22; cortisol, n = 30; plasma ACE, n = 19; renal ACE, n = 18; pulmonary ACE, n = 16; and adrenal:CW, n = 24. Statistical significance of correlation coefficients
P < 0.05
P < 0.01
P < 0.005
P < 0.001.
Basal MAP and HR
The average MAP in low BW pigs at 3 months of age was not significantly (P = 0.1) different from that in high BW pigs (Fig. 2A). However, there was a significant (P < 0.005) negative correlation between basal MAP at 3 months of age and BW (Fig. 3A). Basal MAP at 3 months of age was also significantly (P < 0.005) negatively correlated to the head length:BW ratio (Fig. 3B), but there were no significant relationships between MAP and the ponderal index, body mass index or the ratios of head length:CRL and head length:AC at birth (Table 1). Basal MAP was not significantly correlated to CW, to any of the other morphometric measurements at 3 months, or to the postnatal growth rates (Table 1). There was no significant association between basal MAP and the adrenal:CW ratio (P = 0.08; Table 1). In order to clarify whether MAP was best determined by BW or the head length:BW ratio, partial correlation analysis of both BW and head length:BW with MAP was performed. This analysis revealed that the head length:BW ratio was the strongest (P < 0.005) predictor of basal MAP at 3 months of age.
Figure 2. Basal mean arterial pressure (MAP) and heart rate (HR).
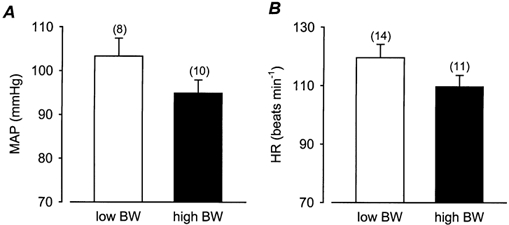
Basal MAP (A) and HR (B) in low and high BW pigs at 3 months of age. Numbers in parentheses represent the number of observations.
Figure 3. Basal mean arterial pressure (MAP) and heart rate (HR).
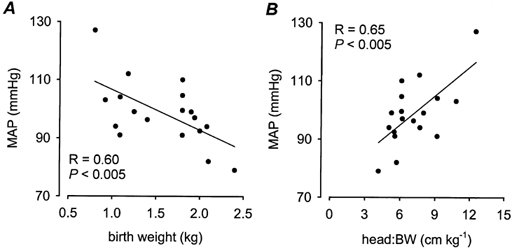
The relationships between MAP and birth weight (BW; A) and the head length:BW ratio (B). A, MAP = 120.5 - (13.8 × BW); B, MAP = 75.2 + (3.3 × head length:BW).
Basal HR was not significantly different between low and high BW pigs at 3 months of age (Fig. 2B) and was not significantly correlated to BW (P = 0.1), head length:BW, CW or to any morphometric measurements at birth or at 3 months (Table 1). Basal HR was significantly positively (P < 0.005) correlated to the adrenal:CW ratio at 3 months of age (Table 1). There was no significant relationship between basal MAP and HR at 3 months of age.
Basal hormone concentrations
Basal plasma adrenaline, noradrenaline and cortisol concentrations in low and high BW pigs at 3 months of age are shown in Fig. 4. Basal noradrenaline concentrations in low BW pigs were significantly (P < 0.05) elevated compared to high BW pigs (Fig. 4). Basal noradrenaline concentrations were significantly (P < 0.05) negatively correlated to BW and CW, and positively correlated to the head length:BW ratio (Table 2). Basal noradrenaline concentrations were also significantly (P < 0.01) negatively correlated to the overall growth rate (0–3 months) and the fractional growth rate between 1 and 3 months of age (Table 2). Basal noradrenaline concentrations were not significantly correlated to basal MAP or HR or to the adrenal:CW ratio (Table 2).
Figure 4. Basal catecholamine and cortisol concentrations.
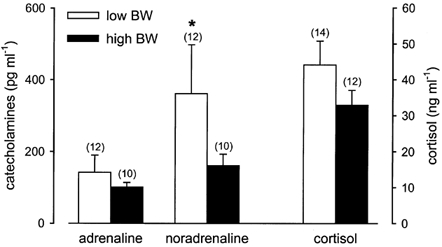
Basal adrenaline, noradrenaline and cortisol concentrations in low and high BW pigs at 3 months of age. Numbers in parentheses represent the number of observations. Low vs. high BW (unpaired t test): *P < 0.05.
There were no significant differences in basal adrenaline concentrations between low and high BW pigs (Fig. 4). However, adrenaline levels were significantly (P < 0.05) negatively correlated to CW, body mass index at 3 months and the fractional growth rate (1–3 months; Table 2). Basal adrenaline concentrations were not significantly correlated to basal MAP or the adrenal:CW ratio but there was a significant (P < 0.05) negative correlation between adrenaline concentrations and basal HR (Table 2).
Basal cortisol concentrations were not significantly different between low and high BW pigs (Fig. 4) and were not correlated to either BW, CW or the adrenal:CW ratio, but were significantly (P < 0.05) positively correlated to the head length:CRL and head length:AC ratios measured at 3 months of age (Table 2). Neither basal MAP or HR were significantly correlated to basal cortisol concentrations (Table 2).
Tissue and plasma ACE concentrations
Concentrations of ACE in both the kidney and lung of low BW pigs at 3 months of age were significantly (P < 0.05) reduced compared to high BW pigs (Fig. 5). Renal ACE concentrations were weakly related to BW (P = 0.06) and were significantly (P < 0.001) positively correlated to CW (Table 2). The ratios of head length:AC and head length:body weight at birth and at 3 months were significantly (P < 0.05) negatively related to renal ACE concentrations (Fig. 6A and B). In addition, there were significant positive associations between renal ACE concentrations and the ponderal (P < 0.05) and body mass (P < 0.005) indices at 3 months, and the growth rate between birth and 3 months (P < 0.001). Renal ACE concentrations were also significantly (P < 0.005) negatively related to the adrenal:CW ratio at 3 months of age (Table 2). Pulmonary ACE concentrations were significantly (P < 0.05) positively associated with BW and the overall growth rate (0–3 months) and were significantly (P < 0.05) negatively related to the head length:body weight ratio at birth and at 3 months (Fig. 6C and D). There were no significant differences in plasma ACE concentrations in low BW (112.7 ± 12.3 units l−1) and high BW (122.9 ± 8.1 units l−1) pigs at 3 months of age. However, plasma ACE concentrations at 3 months of age were significantly (P < 0.05) positively associated with the head length:AC ratio at birth and the adrenal:CW ratio at 3 months of age (Table 2). There were no significant correlations between renal, pulmonary or plasma ACE concentrations with either basal MAP or HR at 3 months of age (Table 2).
Figure 5. Renal and pulmonary angiotensin-converting enzyme (ACE).
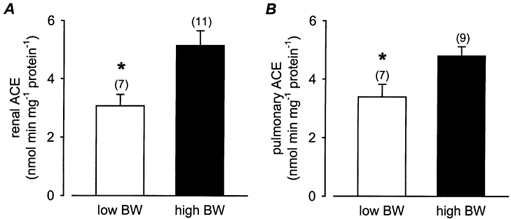
Renal (A) and pulmonary (B) ACE activity in low and high BW pigs at 3 months of age. Numbers in parentheses represent the number of observations. Low vs. high BW (unpaired t test): *P < 0.05.
Figure 6. Renal and pulmonary angiotensin-converting enzyme (ACE).
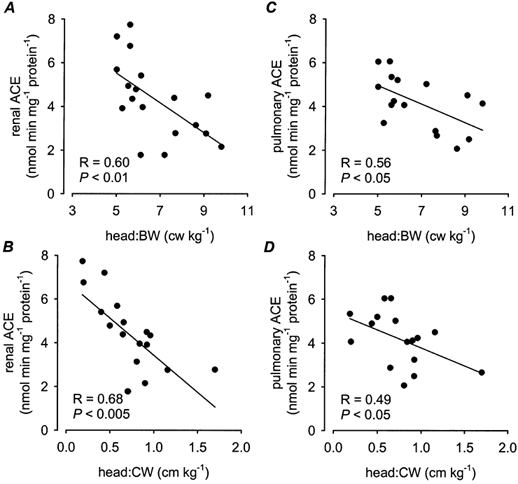
The relationships between renal ACE (A and B) and lung ACE (C and D) and the ratios of head length:birth weight (BW) and head length:current weight (CW). A, ACE = 8.9 - (0.7 × head length:BW); B, ACE = 6.8 - (3.4 × head length:CW); C, ACE = 7.1 - (0.4 × head length:BW); D, ACE = 5.4 - (1.6 × head length:CW).
DISCUSSION
This study has shown for the first time that low BW, within the natural range of birth weights in pigs, is negatively correlated with blood pressure in later life. Importantly, current weight and body proportions were not significantly related to the basal MAP recorded at 3 months of age. These findings are in agreement with epidemiological studies of natural variations in BW in human populations (Barker et al. 1989) and with studies in which low BW has been induced experimentally in other species (Persson & Jansson, 1992; Langley, 1999; Hoet & Hanson, 1999).
In humans, disproportionate body size at birth, as indicated by a low ponderal index, is an important risk factor for cardiovascular disease (Barker et al. 1993). This type of growth retardation in utero is often indicative of undernutrition in late gestation. In pigs, low BW, particularly of the ‘runt’ piglet, is related to a decreased nutrient supply as a result of reduced placental size and transport capacity (Finch et al. 2000). Runt piglets are asymmetrically growth retarded, as measured by an increased brain to liver ratio at birth (Bauer et al. 1998), and in the current study basal MAP was best predicted by an increased head length:BW ratio. Therefore, disproportionate, rather than symmetrical, growth retardation positively correlates with blood pressure in pigs, as occurs in human epidemiological studies (Barker et al. 1993).
The mechanisms whereby fetal nutrient restriction, natural or experimentally induced, programmes the subsequent development of hypertension remain unclear; however, changes in the sympathetic nervous system (SNS), hypothalamo-pituitary adrenal (HPA) axis, RAS, and development of the kidneys and vasculature have all been implicated (see Barker, 1998). Increased plasma concentrations and/or tissue contents of noradrenaline have been measured in fetuses during intrauterine growth retardation in a number of species (Divers et al. 1981; Hiraoka et al. 1991; Gagnon et al. 1994; Simonetta et al. 1997; Ruijtenbeek et al. 2000). In the chick embryo, this is associated with an increased density of sympathetic innervation in the arteries and heart (Ruijtenbeek et al. 2000). In growth retarded rats, the increased SNS activity observed in utero persists after birth for at least 3–4 months (Shaul et al. 1989; Jansson & Lambert, 1999). In humans, low BW has been associated with a high resting pulse rate, an indicator of increased basal SNS activity, at 50 years of age (Phillips & Barker, 1997). In the current study, while HR was not elevated, circulating noradrenaline concentrations were increased in 3-month-old low BW pigs, an effect also associated with poor growth during the first 3 months postnatally. The negative relationship between BW and postnatal blood pressure in pigs may therefore be due increases in sympathetic activity or innnervation sustained in utero.
In human populations, there is evidence that low BW is associated with increased adrenocortical function in 9 year old children (Clark et al. 1996) and with elevated fasting plasma cortisol concentrations and blood pressure in adult life (Phillips et al. 1998). In the current study, there were no overall mean differences in cortisol levels between low and high BW pigs at 3 months of age, but disproportionate body shape (thin or short in relation to head length) at 3 months of age, not at birth, was positively correlated to basal cortisol concentrations. This finding may indicate that adrenal, or HPA axis function, is altered in animals that show poor postnatal growth. Indeed, adrenal weight, in relation to body size, was significantly greater in low BW than high BW pigs in the current study. The adrenal:CW ratio was also greatest in those pigs that were disproportionate in body shape both at birth and at 3 months and that showed poor postnatal growth. Previous studies have shown that low BW in pigs is associated with elevated basal cortisol concentrations and an increased adrenal to body weight ratio in the first week of life (Wise et al. 1991; Klemcke et al. 1993). The current observations suggest that these very early changes in adrenal size may persist until 3 months of age and, in combination with increased HPA axis activity, may contribute to the negative association between MAP and BW in pigs.
Increased activity of the RAS has also been implicated in the development of hypertension associated with poor fetal growth. Pulmonary ACE activity is increased in the hypertensive offspring of rats protein-deprived during pregnancy (Langley, 1999), and early administration of the ACE inhibitor captopril prevents the development of postnatal hypertension in this model (Sherman & Langley-Evans, 1998). However, in the current study, both renal and pulmonary ACE concentrations were significantly lower in low BW pigs than in their high BW littermates. Tissue ACE levels were also negatively related to disproportionate shape at birth and 3 months of age as well as with poor growth postnatally. In contrast, high plasma ACE concentrations were positively correlated to thinness at birth, although there were no mean differences in plasma ACE levels between low and high BW pigs. Although the role of plasma ACE in cardiovascular function is unclear (Esther et al. 1997), the positive association between plasma ACE concentrations and thinness at birth, despite low tissue ACE activity in low BW pigs, may contribute to the negative association between blood pressure and BW in 3-month-old pigs. In common with other species (see Guron & Friberg, 2000), renal ACE is essential for the normal development of porcine kidneys in early neonatal life. In pigs, nephrogenesis is completed postnatally at around 3 weeks of age (Friis, 1980), and administration of an ACE inhibitor during early postnatal life leads to permanent changes in renal structure and function and to a reduction in body weight that persists until at least 8 weeks of age (Guron et al. 1998). Hence, compromised growth in utero, compounded by poor early postnatal growth when nephrogenesis is not yet complete may programme tissue ACE content in pigs and, thereby, affect renal development and function with long-term adverse consequences for the regulation of basal blood pressure.
In the current study, the negative correlation between basal MAP and BW and the lack of correlation between HR and BW at 3 months suggests that poor growth and low birth weight may alter not only the mechanisms involved in the long-term maintenance of basal blood pressure, but also development of baroreceptor function in utero that could persist into postnatal life. In sheep, the ontogenic increase in arterial baroreflex set-point (Blanco et al. 1988) parallels the fetal prepartum cortisol surge, and fetal treatment with the synthetic glucocorticoid dexamethasone leads to persistent elevations in resting MAP and a right-ward shift in the baroreflex set-point (Fletcher et al. 2000). Since low body weight is associated with high cortisol levels in fetal pigs (Klemcke & Christenson, 1997), the apparent resetting of baroreceptor function in the low BW pigs at 3 months of age may reflect the persisting effects of increased glucocorticoid exposure in utero. Indeed, overexposure to glucocorticoids before birth has been shown to cause adult hypertension in rats and sheep and may account, in part, for the programming effects of prenatal undernutrition (Fowden & Forhead, 2000; Seckl et al. 2000).
In conclusion, this study has shown that basal MAP in pigs is negatively associated with birth weight and positively correlated to disproportionate size at birth. This effect was observed within the natural range of birth weights in pigs and may have involved fetal programming of several systems known to regulate basal blood pressure, including the sympathetic nervous system activity, hypothalamo-pituitary adrenal function, the renin-angiotensin system and/or baroreceptor reflexes. The pig is therefore a useful model for investigating the intrauterine origins of abnormal cardiovascular function in adulthood.
Acknowledgments
This work is supported by The Wellcome Trust. We are grateful to Paul Hughes for surgical assistance, Sue Nichols and Vicky Johnson for care of the animals and Malcolm Bloomfield for technical assistance.
REFERENCES
- Agricultural and Food Research Council. Technical Committee on Responses to Nutrients, Report No 4: Nutrient requirements of sows and boars. Nutrition Abstracts and Reviews. Series B: Livestock and Feeding. 1990;60:383–406. [Google Scholar]
- Barker DJ. In utero programming of chronic disease. Clinical Science. 1998;95:115–128. [PubMed] [Google Scholar]
- Barker DJ, Osmond C, Golding J, Kuh D, Wadsworth ME. Growth in utero, blood pressure in childhood and adult life, and mortality from cardiovascular disease. British Medical Journal. 1989;298:564–567. doi: 10.1136/bmj.298.6673.564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker DJ, Osmond C, Simmonds SJ, Wield GA. The relation of small head circumference and thinness at birth to death from cardiovascular disease in adult life. British Medical Journal. 1993;306:422–426. doi: 10.1136/bmj.306.6875.422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer R, Walter B, Hoppe A, Gaser E, Lampe V, Kauf E, Zwiener U. Body weight distribution and organ size in newborn swine (Sus scrofa domestica) - a study describing an animal model for asymmetrical intrauterine growth retardation. Experimental Toxicology and Pathology. 1998;50:59–65. doi: 10.1016/S0940-2993(98)80071-7. [DOI] [PubMed] [Google Scholar]
- Blanco CE, Dawes GS, Hanson MA, McCooke HB. Carotid baroreceptors in fetal and new-born sheep. Pediatric Research. 1988;24:342–346. doi: 10.1203/00006450-198809000-00014. [DOI] [PubMed] [Google Scholar]
- Clark PM, Hindmarsh PC, Shiell AW, Law CM, Honour JW, Barker DJ. Size at birth and adrenocortical function in childhood. Clinical Endocrinology. 1996;45:721–726. doi: 10.1046/j.1365-2265.1996.8560864.x. [DOI] [PubMed] [Google Scholar]
- Divers WA, Wilkes MM, Babaknia A, Hill LM, Quilligan EJ, Yen SS. Amniotic fluid catecholamines and metabolites in intrauterine growth retardation. American Journal of Obstetrics and Gynecology. 1981;141:608–610. doi: 10.1016/s0002-9378(15)33298-1. [DOI] [PubMed] [Google Scholar]
- Eriksson JG, Forsen T, Tuomilehto J, Osmond C, Barker DJP. Early growth and coronary heart disease in later life: longitudinal study. British Medical Journal. 2001;322:949–953. doi: 10.1136/bmj.322.7292.949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esther CR, Marino EM, Howard TE, Machaud A, Corvol P, Capecchi MR, Bernstein KE. The critical role of tissue angiotensin-converting enzyme as revealed by gene targeting in mice. Journal of Clinical Investigation. 1997;99:2375–2385. doi: 10.1172/JCI119419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finch AM, Ashworth CJ, Page KR, McArdle HJ. Gestational changes in leucine transport across porcine placentas supplying littermates of different sizes. Journal of Physiology. 2000;528P:26–27P. [Google Scholar]
- Fletcher AJW, Gardner DS, Fowden AL, Giussani DA. Persistent resetting of cardiac baroreflex function following treatment of fetal sheep with dexamethasone. Journal of Physiology. 2000;528P:107P. [Google Scholar]
- Forhead A, Gillespie C, Fowden A. Role of cortisol in the ontogenic control of pulmonary and renal angiotensin-converting enzyme in fetal sheep near term. Journal of Physiology. 2000;526:409–416. doi: 10.1111/j.1469-7793.2000.00409.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fowden AL, Forhead AJ. The role of hormones in intrauterine development. In: Barker DJP, editor. Fetal Origins of Cardiovascular and Lung Disease. New York: Marcel Dekker Inc.; 2000. pp. 199–228. [Google Scholar]
- Friis C. Postnatal development of the pig kidney: ultrastucture of the glomerulus and the proximal tubule. Journal of Anatomy. 1980;130:513–526. [PMC free article] [PubMed] [Google Scholar]
- Gagnon R, Challis J, Johnston L, Fraher L. Fetal endocrine responses to chronic placental embolization in the late-gestation ovine fetus. American Journal of Obstetrics and Gynecology. 1994;170:929–938. doi: 10.1016/s0002-9378(94)70309-4. [DOI] [PubMed] [Google Scholar]
- Guron G, Friberg P. An intact renin-angiotensin system is a prerequisite for normal renal development. Journal of Hypertension. 2000;18:123–137. doi: 10.1097/00004872-200018020-00001. [DOI] [PubMed] [Google Scholar]
- Guron G, Sundelin B, Wickman A, Friberg P. Angiotensin-converting enzyme inhibition in piglets induces persistent renal abnormalities. Clinical and Experimental Pharmacology and Physiology. 1998;25:88–91. doi: 10.1111/j.1440-1681.1998.tb02182.x. [DOI] [PubMed] [Google Scholar]
- Hiraoka T, Kudo T, Kishimoto Y. Catecholamines in experimentally growth-retarded rat fetus. Asia and Oceania Journal of Obstetrics and Gynaecology. 1991;17:341–348. doi: 10.1111/j.1447-0756.1991.tb00284.x. [DOI] [PubMed] [Google Scholar]
- Hoet JJ, Hanson MA. Intrauterine nutrition: its importance during critical periods for cardiovascular and endocrine development. Journal of Physiology. 1999;514:617–627. doi: 10.1111/j.1469-7793.1999.617ad.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurst PL, Lovell-Smith CJ. Optimized assay for serum angiotensin-converting enzyme activity. Clinical Chemistry. 1981;27:2048–2052. [PubMed] [Google Scholar]
- Jansson T, Lambert GW. Effect of intrauterine growth restriction on blood pressure, glucose tolerance and sympathetic nervous system activity in the rat at 3–4 months of age. Journal of Hypertension. 1999;17:1239–1248. doi: 10.1097/00004872-199917090-00002. [DOI] [PubMed] [Google Scholar]
- Klemcke HG, Christenson RK. Porcine fetal and maternal adrenocorticotropic hormone and corticosteroid concentrations during gestation and their relation to fetal size. Biology of Reproduction. 1997;57:99–106. doi: 10.1095/biolreprod57.1.99. [DOI] [PubMed] [Google Scholar]
- Klemcke HG, Lunstra DD, Brown-Borg HM, Borg KE, Christenson RK. Association between low birth weight and increased adrenocortical function in neonatal pigs. Journal of Animal Science. 1993;71:1010–1018. doi: 10.2527/1993.7141010x. [DOI] [PubMed] [Google Scholar]
- Langley SC. Impact of maternal diet on the renin-angiotensin system in the rat. In: O'Brien PMS, Wheeler T, Barker DJP, editors. Fetal Programming: Influences on Development of Disease in Later Life. London: RCOG Press; 1999. pp. 374–388. [Google Scholar]
- Law CM, Shiell AW. Is blood pressure inversely related to birth weight? The strength of evidence from a systematic review of the literature. Journal of Hypertension. 1996;14:935–941. [PubMed] [Google Scholar]
- Lowry O, Rosenbrough N, Farr A, Randall R. Protein measurement with folin-phenol reagent. Journal of Biological Chemistry. 1951;193:267–275. [PubMed] [Google Scholar]
- Persson E, Jansson T. Low birth weight is associated with elevated adult blood pressure in the chronically catheterized guinea-pig. Acta Physiologica Scandinavica. 1992;145:195–196. doi: 10.1111/j.1748-1716.1992.tb09356.x. [DOI] [PubMed] [Google Scholar]
- Phillips DI, Barker DJ. Association between low birth weight and high resting pulse in adult life: is the sympathetic nervous system involved in programming the insulin resistance syndrome. Diabetic Medicine. 1997;14:673–677. doi: 10.1002/(SICI)1096-9136(199708)14:8<673::AID-DIA458>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- Phillips DL, Barker DJ, Fall CH, Seckl JR. Elevated plasmacortisol concentrations: a link between low birth weight and theinsulin resistance syndrome? Journal of Clinical EndocrinologyMetabolism. 1998;83:757–760. doi: 10.1210/jcem.83.3.4634. [DOI] [PubMed] [Google Scholar]
- Raimbach SJ, Thomas AL. Renin and angiotensinconvertingenzyme concentrations in the fetal and neonatalguinea-pig. Journal of Physiology. 1990;423:441–451. doi: 10.1113/jphysiol.1990.sp018032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruijtenbeek K, Le Noble FA, Janssen GM, Kessels CG, Fazzi GE, Blanco CE, De Mey JG. Chronic hypoxia stimulates periarterial sympathetic nerve development in chicken embryo. Circulation. 2000;102:2892–2897. doi: 10.1161/01.cir.102.23.2892. [DOI] [PubMed] [Google Scholar]
- Seckl JR, Cleasby M, Nyirenda MJ. Glucocorticoids, 11β-hydroxysteroid dehydrogenase, and fetal programming. Kidney International. 2000;57:1412–1417. doi: 10.1046/j.1523-1755.2000.00984.x. [DOI] [PubMed] [Google Scholar]
- Shaul PW, Cha CJ, Oh W. Neonatal sympathoadrenal response to acute hypoxia: impairment after experimental intrauterine growth retardation. Pediatric Research. 1989;25:466–472. doi: 10.1203/00006450-198905000-00008. [DOI] [PubMed] [Google Scholar]
- Sherman RC, Langley-Evans SC. Early administration of angiotensin-converting enzyme inhibitor captopril, prevents the development of hypertension programmed by intrauterine exposure to a maternal low-protein diet in the rat. Clinical Science. 1998;94:373–381. doi: 10.1042/cs0940373. [DOI] [PubMed] [Google Scholar]
- Silver M, Barnes RJ, Comline RS, Burton GJ. Placental blood flow: some fetal and maternal cardiovascular adjustments during gestation. Journal of Reproduction and Fertility Supplement. 1982;31:139–160. [PubMed] [Google Scholar]
- Silver M, Fowden AL. Fetal and maternal endocrine changes during the induction of parturition with PGF analogue, clopostenol, in chronically catheterized sows and fetuses. Journal of Developmental Physiology. 1983;5:307–321. [PubMed] [Google Scholar]
- Simonetta G, Rourke AK, Owens JA, Robinson JS, McMillen IC. Impact of placental restriction on the development of the sympathoadrenal system. Pediatric Research. 1997;42:805–811. doi: 10.1203/00006450-199712000-00015. [DOI] [PubMed] [Google Scholar]
- Widdowson EM, McCance RA. A review: new thoughts on growth. Pediatric Research. 1975;9:154–156. doi: 10.1203/00006450-197503000-00010. [DOI] [PubMed] [Google Scholar]
- Wise T, Stone RT, Vernon MW. Relationships of serum estriol, cortisol and albumin concentrations with pig weight at 110 days of gestation and at birth. Biology of the Neonate. 1991;59:114–119. doi: 10.1159/000243331. [DOI] [PubMed] [Google Scholar]


