Abstract
In contrast to the conventional theory, the external and internal intercostal muscles show marked rostrocaudal gradients in their actions on the lung. We hypothesized that these gradients are the result of a non-uniform coupling between the ribs and the lung. Rib displacements (Xr) and the changes in airway opening pressure (Pa,o) were thus measured in anaesthetized, pancuronium-treated, supine dogs while loads were applied in the cranial direction to individual pairs of odd-numbered ribs and in the caudal direction to individual pairs of even-numbered ribs. During cranial loading, Xr induced by a given load increased gradually with increasing rib number. The decrease in Pa,o also increased from the third to the fifth rib pair but then decreased markedly to the eleventh pair. A similar pattern was observed during caudal loading, although Xr and ΔPa,o were smaller. These results were then combined to calculate the net Xr and the net ΔPa,o that a hypothetical intercostal muscle lying parallel to the longitudinal body axis would produce in different interspaces. The net Xr was cranial in all interspaces. However, whereas the net ΔPa,o was negative in the cranial interspaces, it was positive in the caudal interspaces. These observations confirm that the coupling between the ribs and the lung varies from the top to the base of the ribcage. This coupling confers to both the external and the internal intercostal muscles an inspiratory action on the lung in the cranial interspaces and an expiratory action in the caudal interspaces.
The actions of the external and internal interosseous intercostal muscles are conventionally regarded according to the theory proposed 250 years ago by Hamberger (1749). According to this theory, the fibres of the external intercostals slope obliquely caudad and ventrally from the rib above to the rib below, and so their lower insertion is further from the centre of rotation of the ribs (i.e. the costo-vertebral articulations) than their upper insertion. Consequently, when this muscle contracts in a single interspace and applies equal and opposite forces at both insertions, the torque acting on the lower rib, which tends to raise it, is greater than that acting on the upper rib, which tends to lower it. The net effect of the muscle, therefore, would be to raise the ribs and to inflate the lung. In contrast, the fibres of the internal interosseous intercostals slope obliquely caudad and dorsally from the rib above to the rib below so that their lower insertion is closer to the centre of rotation of the ribs than the upper one. As a result, the net effect of their contraction would be to lower the ribs and to deflate the lung.
This theory, however, has not been verified, and recent measurements of the changes in length of the canine external and internal intercostal muscles during passive inflation have led to the conclusion that the actions of these muscles on the lung vary markedly depending on their location (De Troyer et al. 1999). Specifically, the external intercostals in the dorsal portion of the rostral interspaces have a large inspiratory effect on the lung, but this effect decreases rapidly towards the base of the ribcage and towards the costochondral junctions. As a result, the muscles in the ventral portion of the caudal interspaces have a clear-cut expiratory, rather than inspiratory, effect on the lung. The internal interosseous intercostals in the caudal interspaces have an even larger expiratory effect on the lung, but this effect decreases in the cranial and the ventral direction such that the muscles in the most cranial interspaces have a small inspiratory effect on the lung. The dorsoventral gradients in respiratory effects were attributed to the fact that the ribcage is a three-dimensional, rather than a two-dimensional structure as assumed by Hamberger (1749), but the mechanism of the rostrocaudal gradients was not readily identified. Moreover, some aspects of these gradients appear paradoxical to the earlier observation that in the dog, activation of the external or internal intercostal muscle in a single rostral or caudal interspace invariably produces a net inspiratory rib displacement (De Troyer et al. 1983, 1985; Ninane et al. 1991). We speculated, therefore, that the coupling between the ribs and the lung is not uniform (De Troyer et al. 1999), and the present studies were designed to test this hypothesis.
METHODS
The studies were carried out on six adult mongrel dogs (19–32 kg), as approved by the Animal Ethics and Welfare Committee of the Brussels School of Medicine. The animals were anaesthetized with pentobarbitone sodium (initial dose, 30 mg kg−1i.v.), placed in the supine posture, and intubated with a cuffed endotracheal tube. A venous cannula was inserted in the forelimb to give maintenance doses of anaesthetic (3–5 mg kg−1 h−1i.v.) and a catheter was inserted in the right femoral artery to monitor blood pressure and heart rate, after which the ribcage and intercostal muscles were exposed on both sides of the chest from the first to the twelfth rib by reflection of the skin and the superficial muscle layers. Hooks were then screwed into the third right and left bony ribs, 1–2 cm lateral to the costochondral junctions. A long inextensible thread was attached to each hook and led cranially, parallel to the longitudinal body axis of the animal, over a pulley placed at the head of the table, and it was connected to a small basket in which weights could be placed later. An additional hook was screwed into the third right rib in the mid-axillary line and connected to a linear displacement transducer (Schaevitz Eng., Pennsauken, NJ, USA) to measure the craniocaudal (axial) rib displacement (De Troyer & Kelly, 1982), and a differential pressure transducer (Validyne Corp., Northridge, CA, USA) was connected to a side port of the endotracheal tube to measure airway opening pressure (Pa,o).
Fifteen minutes after instrumentation, the animal was injected with a neuromuscular blocking agent (2 mg pancuronium i.v.) and ventilated mechanically. After calibration of the displacement transducer, the ventilation was stopped and the chest wall was allowed to relax to equilibrium. The endotracheal tube was occluded, and 200 g lead balls were placed in both baskets attached to the third ribs such that the load in each basket was increased by 0.2 kg increments from 0.2 to 1.0 kg. Three runs of loading were performed in each animal. The hook-basket system and the displacement transducer were then transferred to the fifth rib pair, and three runs of loading were obtained. The procedure was subsequently repeated for the seventh, ninth and eleventh rib pair, after which the hooks and the displacement transducer were attached to the second rib pair. In this case, however, the thread-basket system was led caudally over pulleys positioned at the foot of the table, such that rib loading was made in the caudal direction. As for the odd-numbered rib pairs, three runs of (caudal) loading were performed. A similar procedure was used to load the fourth, sixth, eighth and tenth rib pair.
Blood pressure and heart rate were monitored during the course of the experiments and no changes occurred. Also, the pupils in each animal remained constricted and unresponsive to light, thus indicating a deep level of anaesthesia. At the conclusion of the measurements, the animals were given an overdose (30–40 mg kg−1) of anaesthetic.
Data analysis
For each rib pair in each individual animal, the axial rib displacements (Xr) and the changes in Pa,o induced by each load (force, F) were averaged over the three runs; by convention, Xr was assigned a positive sign when displacement was in the cranial direction and a negative sign when displacement was in the caudal direction. The relationships thus obtained between F and Xr and between F and ΔPa,o were then calculated by using linear regression techniques (coefficient of correlation, r between 0.975 and 0.999), and the slopes of these relationships (ΔXr/F and ΔPa,o/F) were averaged over the animal group. These data are presented as means ± s.e.m. Comparisons between the slopes for the different rib pairs were made by analysis of variance (ANOVA) with repeated measures and multiple comparison testing of the mean values was performed, when appropriate, using Student-Newman-Keul's tests. The criterion for statistical significance was taken as P < 0.05.
RESULTS
The records of rib displacement and ΔPa,o obtained during (cranial) loading of the fifth rib pair and during (caudal) loading of the fourth rib pair in a representative animal are shown in Fig. 1, and Fig. 2 displays the relationships between force and displacement and between force and ΔPa,o for all the ribs in the same animal. In each animal, cranial loading of a given rib pair induced a progressive cranial displacement of the ribs and a progressive fall in Pa,o (Fig. 1A), and caudal loading of a given rib pair elicited a caudal rib displacement and a rise in Pa,o (Fig. 1B). The magnitudes of these changes, however, were different for the different rib pairs. Thus the rib displacement induced by a given cranially oriented force increased continuously with increasing rib number (Fig. 2A), but the fall in Pa,o increased from the third to the fifth rib pair and then decreased gradually to the eleventh pair (Fig. 2B). Similarly, whereas the rib displacement produced by a given caudally oriented force increased from the second to the tenth rib pair (Fig. 2C), the rise in Pa,o increased to the sixth pair and then decreased to the tenth (Fig. 2D).
Figure 1. Traces of axial rib displacement (X rib) and airway opening pressure (Pa,o) during rib loading.
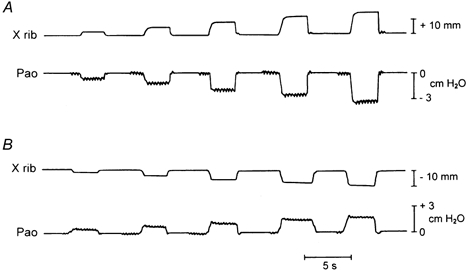
Records obtained during cranial loading of the fifth rib pair (A) and during caudal loading of the fourth rib pair (B) in a representative animal. The load in each run was increased by 0.2 kg increments from 0.2 to 1.0 kg; each load was applied while the endotracheal tube was occluded.
Figure 2. Relationships between force, rib displacement (Xr) and airway opening pressure (Pa,o) during rib loading.
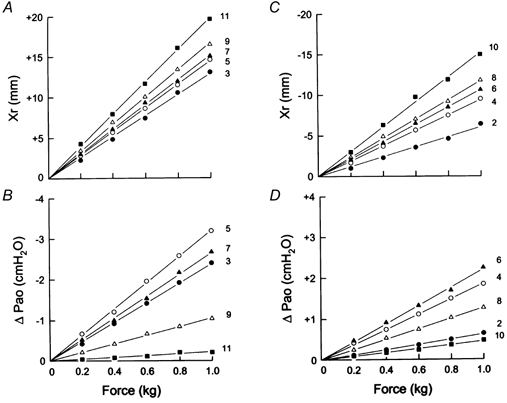
A and B show the relationships between force and Xr (A) and between force and ΔPa,o (B) during cranial loading of the different odd-numbered rib pairs in the same animal as in Fig. 1. C and D show the same relationships during caudal loading of the even-numbered rib pairs. In each panel, the numbers on the right refer to the ribs to which force was applied. Note that all these relationships are linear. Note also that the axial rib displacement induced by a given load increases gradually with increasing rib number (A and C). In contrast, the fall in Pa,o during cranial loading increases from the third to the fifth rib pair but then decreases progressively to the eleventh pair (B); similarly, the rise in Pa,o during caudal loading increases from the second to only the sixth rib pair (D).
The slopes of the relationships, Xr/F and ΔPa,o/F, for the different rib pairs in the six animals are shown in Fig. 3. Although Xr/F (Fig. 3A) increased progressively with increasing rib number for both cranial and caudal loading (P < 0.001), Xr/F during cranial loading of any given rib pair was greater than that during caudal loading of the pairs immediately above and below (P < 0.005). As a result, the line through the data for cranial loading lay above the line for caudal loading. The magnitude of ΔPa,o/F (Fig. 3B) during cranial loading of the third, fifth and seventh rib pair was also much greater than that during caudal loading of the pairs immediately above and below, but ΔPa,o/F during cranial loading of the ninth and eleventh rib pair was smaller than that during caudal loading of the pairs above. The values of ΔPa,o/F and Xr/F were then combined to calculate ΔPa,o/Xr (i.e. the change in Pa,o produced by a given rib displacement) for the different ribs. As shown in Fig. 4, the values of ΔPa,o/Xr for cranial and caudal loading lay along a single line, increasing gradually from the second to the fifth rib (P < 0.001) and declining markedly from the seventh to the eleventh rib (P < 0.001).
Figure 3. Slopes of the relationships between force and rib displacement and between force and airway opening pressure for the different ribs.
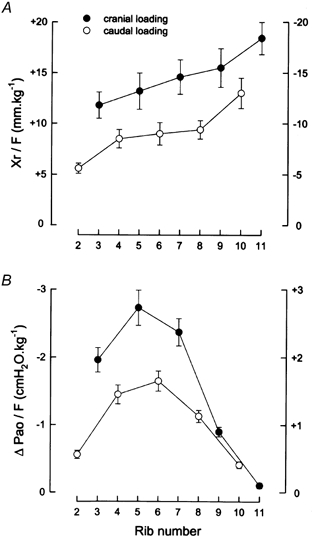
The data in A are the mean ± s.e.m. values of axial rib displacement per unit force (Xr/F) obtained from six animals during cranial loading of the odd-numbered rib pairs (filled circles) and during caudal loading of the even-numbered rib pairs (open circles). Positive values indicate displacements in the cranial direction and negative values indicate displacements in the caudal direction. The data in B are the corresponding mean ± s.e.m. values of ΔPa,o per unit force.
Figure 4. Coupling between rib displacement and lung volume.

The data shown are the mean ± s.e.m. values of the changes in airway opening pressure (Pa,o) per unit rib displacement (Xr) obtained for the different ribs in six animals. The filled circles correspond to the values obtained during cranial loading of the odd-numbered rib pairs; the open circles correspond to the values obtained during caudal loading of the even-numbered rib pairs.
When an intercostal muscle contracts alone in a single interspace, it produces equal and opposite forces on the two ribs to which it is attached. By combining the data obtained during cranial loading of the odd-numbered ribs and during caudal loading of the even-numbered ribs, we therefore calculated the net Xr and the net ΔPa,o that an intercostal muscle force of 1 kg would induce in each even-numbered interspace in each animal. The results of the calculations in the six animals are summarized in Fig. 5. The calculated net Xr was definitely cranial for all interspaces. However, the calculated net ΔPa,o was highly dependent on interspace number (P < 0.001). Whereas this net ΔPa,o averaged −1.41 ± 0.15 cmH2O in the second interspace, it was only −0.72 ± 0.12 cmH2O in the sixth interspace (P < 0.001) and was reversed to +0.23 ± 0.04 and +0.31 ± 0.03 cmH2O in the eighth and tenth interspace, respectively (P < 0.001).
Figure 5. Computed effects of intercostal muscle force in different interspaces.

The values in A are the calculated net rib displacements (ΔXr) produced by intercostal muscles lying parallel to the longitudinal axis of the body and developing a force of 1 kg on the adjacent ribs. The values in B are the calculated net changes in Pa,o corresponding to these rib displacements. These values were calculated from the data shown in Fig. 3A and B, respectively.
DISCUSSION
The first important result of this study is the observation that for a given cranially or caudally oriented force, rib displacement increases monotonically with increasing rib number. If rib compliances in the cranial and caudal directions were equal, one would therefore expect that a cranially oriented force acting on a particular rib would produce a larger rib displacement than if the same, but caudally oriented, force acted on the rib immediately above. Rib compliances in the cranial and caudal directions, however, are not equal. In agreement with our previous findings (De Troyer et al. 1983, 1985), the displacements produced by cranially oriented forces in our animals were greater than the displacements produced by caudally oriented forces (Fig. 3A). As a result of these two effects, in each interspace, the net rib displacement produced by a hypothetical intercostal muscle lying parallel to the longitudinal axis of the body is clearly cranial (Fig. 5A).
The theory of Hamberger (1749) cannot explain this result, and this is due to the fact that the model on which the theory is based contains a number of simplifications. The model is planar, and the ribs are modelled as straight bars rotating around axes that lie perpendicular to the plane of the ribs. Moreover, the ribs in the model are linked firmly to each other by the sternum, and this ventral linkage imposes the constraint that the displacements of all the ribs are equal. The model also assumes that the ribs are equally compliant in the cranial and the caudal direction. Therefore, according to the theory, a net cranial displacement of the ribs can only be produced by muscle fibres having the orientation of the external intercostals. Fibres with the orientation of the internal intercostals can only produce a net caudal displacement of the ribs, and fibres lying parallel to the longitudinal body axis cause no net rib displacement at all. In contrast, the present findings suggest, in agreement with the previous observations of De Troyer et al. (1983, 1985) and Ninane et al. (1991), that both the external and the internal interosseous intercostals would produce a net cranial displacement of the ribs.
The second important result of this study is the demonstration that the coupling between rib displacement and lung volume varies from the top to the base of the ribcage (Fig. 4). Specifically, the coupling increases with increasing rib number in the cranial half of the cage, whereas in the caudal half, it decreases markedly and continuously with increasing rib number. This phenomenon is not addressed in the theory of Hamberger (1749); the theory simply assumes that a cranial displacement of the ribs causes an increase in lung volume and that a caudal displacement of the ribs causes a decrease in lung volume. The differential coupling described herein, however, is an important aspect of the mechanism of intercostal muscle action.
In the cranial half of the ribcage, ΔPa,o/Xr increases with increasing rib number for both cranial and caudal rib displacements. As a result, the fall in Pa,o caused by the cranial displacement of a particular rib is larger than the rise in Pa,o caused by the same, but caudal, displacement of the rib above. In addition, as previously pointed out (see above), cranially oriented forces applied to a particular rib produce larger rib displacements than caudally oriented forces applied to the rib above. For these two reasons, a hypothetical intercostal muscle lying parallel to the longitudinal body axis in a cranial interspace has, therefore, a clear-cut inspiratory action on the lung (Fig. 5B). In contrast, in the caudal half of the ribcage, the coupling between the ribs and the lung is such that the fall in Pa,o caused by the cranial displacement of a particular rib is much smaller than the rise in Pa,o produced by the same, but caudal, displacement of the rib above. Therefore, although this large difference is partly compensated for by the smaller caudal compliance of the rib above, the net effect of an intercostal muscle lying parallel to the longitudinal body axis in a caudal interspace is expiratory to the lung (Fig. 5B). These data are fully consistent with our recent conclusion, based on the application of the Maxwell reciprocity theorem, that the effect of the intercostal muscles on the lung changes from inspiratory to expiratory as one goes from the top to the base of the ribcage (De Troyer et al. 1999). These data also provide a solution to the apparent paradox that the intercostal muscles in the caudal interspaces cause a net cranial displacement of the ribs on which they insert but have an expiratory effect on the lung.
We do not mean to imply that the orientations of the muscle fibres play no role in determining the actions of the intercostal muscles on the lung. Thus, although both the external and the internal interosseous intercostals would produce a net cranial displacement of the ribs on which they insert, the magnitude of this net displacement should be greater for the external intercostals than the internal intercostals. The orientations of the muscle fibres should also modulate the effects of the muscles on the lung. For a given muscle mass, the external intercostal in a cranial interspace would have a larger inspiratory effect on the lung than the internal intercostal, and the external intercostal in a caudal interspace would have a smaller expiratory effect on the lung than the internal intercostal.
The critical question remains as to what is the mechanism of the non-uniform coupling between rib displacement and lung volume. We have no data to answer this question, but it seems reasonable to speculate that the change in lung volume produced by the displacement of a particular rib is directly related to the area of the lung subtended by the rib. Previous studies of the geometry of the ribs have shown that in the dog, the radii of the ribs in the cranial half of the ribcage increase gradually with increasing rib number (Margulies et al. 1989). In this half of the ribcage, therefore, the area of the lung subtended by a particular rib should be greater than that subtended by the rib above. The radii of ribs 7–10 continue to increase with increasing rib number, but unlike the more cranial ribs, these ribs are only in part apposed to the lung. At resting end-expiration, the most caudal ribs are even entirely apposed to the abdomen through the diaphragm (Mead, 1979). Consequently, a cranial displacement of these ribs results primarily in an expansion of the ventral abdominal wall and a fall in abdominal pressure. This fall also induces a fall in Pa,o through the (passive) caudal displacement of the diaphragm, but because of the elastance of the diaphragm, this fall in Pa,o is smaller than the fall in abdominal pressure. On this basis, it would therefore be expected that the fall in Pa,o due to a given cranial displacement of the most caudal ribs would be smaller than that produced by the same cranial displacement of the cranial ribs.
The idea that the non-uniform coupling between the ribs and the lung is primarily related to the area of the lung subtended by the different ribs further implies that this coupling would be affected by a number of variables. The zone of apposition of the diaphragm to the ribcage in particular is well-known to decrease when lung volume is increased by passive inflation or by a contraction of the diaphragm (Mead, 1979; Mead & Loring, 1982); it would also decrease when a supine animal is moved to the prone posture with the abdomen pendant. In these conditions, therefore, the area of the lung subtended by ribs 7, 8 and perhaps 9 should increase, leading to a rise in the corresponding values of ΔPa,o/F (Fig. 3B). As a result, the peak in the curve of ΔPa,o/Xr (Fig. 4) would extend to higher rib numbers, and the descent on the caudal side of the peak would be steeper. Consequently, the expiratory action of the intercostal muscles in the most caudal interspaces would be greater. In addition, as lung volume is passively increased above functional residual capacity, the difference between cranial and caudal rib compliance disappears (De Troyer et al. 1985). At very high lung volumes, the directional dependence of rib compliance is even reversed, being greater for caudal displacements than cranial displacements. This alteration would further augment the expiratory effect of the intercostal muscles in the caudal interspaces and reduce the inspiratory effect of the muscles in the cranial half of the ribcage.
Acknowledgments
This study was supported in part by a grant (3.4521.01) from the Fonds National de la Recherche Scientifique (FNRS-Belgium).
REFERENCES
- De Troyer A, Kelly S. Chest wall mechanics in dogs with acute diaphragm paralysis. Journal of Applied Physiology. 1982;53:373–379. doi: 10.1152/jappl.1982.53.2.373. [DOI] [PubMed] [Google Scholar]
- De Troyer A, Kelly S, Macklem PT, Zin WA. Mechanics of intercostal space and actions of external and internal intercostal muscles. Journal of Clinical Investigation. 1985;75:850–857. doi: 10.1172/JCI111782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Troyer A, Kelly S, Zin WA. Mechanical action of the intercostal muscles on the ribs. Science. 1983;220:87–88. doi: 10.1126/science.6828883. [DOI] [PubMed] [Google Scholar]
- De Troyer A, Legrand A, Wilson TA. Respiratory mechanical advantage of the canine external and internal intercostal muscles. Journal of Physiology. 1999;518:283–289. doi: 10.1111/j.1469-7793.1999.0283r.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamberger GE. De Respirationis Mechanismo et usu Genuino. Iena: 1749. [Google Scholar]
- Margulies SS, Rodarte JR, Hoffman EA. Geometry and kinematics of dog ribs. Journal of Physiology. 1989;67:707–712. doi: 10.1152/jappl.1989.67.2.707. [DOI] [PubMed] [Google Scholar]
- Mead J. Functional significance of the area of apposition of diaphragm to rib cage. American Review of Respiratory Disease. 1979;119:31–32. doi: 10.1164/arrd.1979.119.2P2.31. [DOI] [PubMed] [Google Scholar]
- Mead J, Loring SH. Analysis of volume displacement and length changes of the diaphragm during breathing. Journal of Applied Physiology. 1982;53:750–755. doi: 10.1152/jappl.1982.53.3.750. [DOI] [PubMed] [Google Scholar]
- Ninane V, Gorini M, Estenne M. Action of intercostal muscles on the lung in dogs. Journal of Applied Physiology. 1991;70:2388–2394. doi: 10.1152/jappl.1991.70.6.2388. [DOI] [PubMed] [Google Scholar]


