Abstract
Hydrochloric acid (HCl) is produced in parietal cells of gastric epithelium by a H+–K+ pump. Protons are secreted into the gastric lumen in exchange for K+ by the action of the H+–K+-ATPase. Luminal K+ is essential for the operation of the pump and is thought to be supplied by unidentified K+ channels localized at the apical membrane of parietal cells. In this study, we showed that histamine- and carbachol-induced acid secretion from isolated parietal cells monitored by intracellular accumulation of aminopyrine was depressed by Ba2+, an inhibitor of inwardly rectifying K+ channels. Among members of the inwardly rectifying K+ channel family, we found with reverse transcriptase-polymerase chain reaction analyses that Kir4.1, Kir4.2 and Kir7.1 were expressed in rat gastric mucosa. With immunohistochemical analyses, Kir4.1 was found to be expressed in gastric parietal cells and localized specifically at their apical membrane. The current flowing through Kir4.1 channel expressed in HEK293T cells was not affected by reduction of extracellular pH from 7.4 to 3. These results suggest that Kir4.1 may be involved in the K+ recycling pathway in the apical membrane which is required for activation of the H+–K+ pump in gastric parietal cells.
Gastric acid is indispensable for sterilization of food and water, activation of pepsinogen, and maintenance of the activity of pepsin. It is produced by H+–K+-ATPase (or H+–K+ pump) in parietal cells (also called oxyntic cells) of gastric epithelium. When H+–K+-ATPase hydrolyzes one ATP molecule, it exports one H+ ion and imports one K+ ion in exchange. Acid secretion of both frog gastric mucosa (Sanders et al. 1973) and microsome isolated from rabbit gastric mucosa (Wolosin & Forte, 1981; Reenstra & Forte, 1990) is highly dependent on the extracellular K+ ions and thus, luminal extracellular K+ ions are supposed to be essential for activation of H+–K+-ATPase (Wolosin & Forte, 1981; Reenstra & Forte, 1990). In the resting condition, H+–K+-ATPases exist in the tubulovesicles of parietal cells (Smolka et al. 1983). The membrane of the tubulovesicle is impermeable to K+ ions, which prevents H+–K+-ATPase activity in the vesicles (Wolosin & Forte, 1981). Hormones such as histamine, acetylcholine, and gastrin cause fusion of tubulovesicles with the apical membrane of parietal cells, which results in elongation of microvilli and up to 10-fold expansion of the apical membrane area (Helander & Hirschowitz, 1972). The H+–K+-ATPases in the fused tubulovesicles are thus exposed to luminal fluid which contains K+ ions and so the ATPases are activated. To maintain the activity of the H+–K+-ATPases, it has been postulated that K+ channels at the apical membrane of parietal cells may supply K+ ions to the luminal extracellular fluid. Although it was recently proposed that the KCNQ1 channel is involved in acid secretion of gastric parietal cells (Grahammer et al. 2001), the properties of K+ channels have not yet been fully studied.
In this study, it was found that proton secretion, assessed by the accumulation of aminopyrine in isolated parietal cells, was suppressed by Ba2+, a non-specific blocker of inwardly rectifying K+ (Kir) channels. Using reverse transcriptase-polymerase chain reaction analyses we found that, of the members of the Kir family, Kir4.1, Kir4.2 and Kir7.1 were expressed in rat gastric mucosa. We further found using immunohistochemical techniques that Kir4.1 but neither Kir4.2 nor Kir7.1 was expressed in parietal cells of rat stomach. The immunogold electron microscopy clearly showed that Kir4.1 was specifically localized at the apical membrane of parietal cells. The Kir4.1 channel current expressed in HEK293T cells was unaffected by the reduction of external pH to 3. These results suggest that Kir4.1 may be involved in the K+ recycling pathway at the apical membrane of parietal cells that maintains the H+–K+-ATPase activity.
METHODS
Animals
The animal experiments were performed following the guidelines of the Animal Usage Committee of Osaka University Medical School. Male Wistar rats (Nippon Doubutsu, Kyoto, Japan) weighing 200–250 g and male ICR mice (8 weeks old; Japan SLC, Shizuoka, Japan) were used in this study.
Measurement of [14C]aminopyrine accumulation in intact parietal cells
Male Wistar rats were deeply anaesthetized with pentobarbital sodium (50 mg kg−1i.p.) and then stunned by a blow on the head and bled via the carotid arteries. Stomachs were removed and placed in a bathing solution of the following composition (mm): 137 NaCl, 2.7 KCl, 1.8 CaCl2, 1.1 MgCl2, 0.42 NaH2PO4, 11.9 NaHCO3 and 5.6 glucose. The contents of excised stomachs were gently flushed out with the bathing solution and were distended with Dulbecco's modified Eagle's medium (DMEM) (Nikken, Kyoto, Japan) containing 20 mm Hepes, pH 7.4, 2 mm EDTA, 30 mg ml−1 dextran (Mr 60 000–90 000), 0.1 mg ml−1 soybean trypsin inhibitor and 1000 units ml−1 pronase and incubated in the same medium without pronase at 37 °C for two periods of 30 min. A harvesting period of 30 min followed each 30 min of incubation. To harvest cells, stomachs were stirred in DMEM containing 20 mm Hepes, pH 7.4, and 30 mg ml−1 bovine serum albumin using a magnetic stirrer at room temperature. The suspension of isolated cells was then filtered through nylon mesh (150 μm pore size) and centrifuged at 100 g for 5 min. Cells were resuspended in DMEM containing 36 ml (100 ml)−1 isotonic Percoll, 12 mm Hepes, pH 7.4, 0.6 mg ml−1 bovine serum albumin, 0.3 mm dithiothreitol, and 1.9 mm EGTA, and the mixture was centrifuged for 13 min at 30 000 g (4 °C). A white band of parietal cells in the upper portion of the centrifuge tube was collected. Isolated parietal cells were washed twice with DMEM containing 10 mm Hepes, pH 7.4, and then incubated in DMEM containing 10 % fetal calf serum, 8 μg ml−1 insulin, and 10 nm hydrocortisone for 2 h at 37 °C. Immunohistochemical staining with anti-β subunit of H+–K+-ATPase antibody indicated that around 80 % of isolated cells in the preparation were parietal cells. Stimulation of parietal cells was quantified using the aminopyrine uptake assay. In this assay, the accumulated [14C]aminopyrine in isolated parietal cells is in parallel with the level of H+–K+-ATPase activity (Soll, 1980). Parietal cells were washed in 137 mm NaCl, 1 mm KCl, 0.4 mm NaH2PO4, 11.9 mm NaHCO3, 1.8 mm CaCl2, 11.1 mm glucose, 2 mm isoleucine, 20 mm Hepes, pH 7.4 (modified Tyrode solution) and suspended in the same solution containing 1 μm [14C]aminopyrine (New England Nuclear, Boston, MA, USA) and 0.1 mm isobutylmethylxanthine. Cells were incubated in a 24-well dish (2 × 105 cells per well) and BaCl2 (0.1 or 1 mm) was added to the cells 15 min before the addition of agonists. Parietal cells were stimulated with 0.1 mm histamine and 10 μm carbachol in a final volume of 0.4 ml for 30 min at 37 °C. After stimulation, 0.3 ml of the cell suspension was layered over 0.9 ml of modified Tyrode solution and spun down in a microfuge at 6000 g for 10 s. The supernatant was removed. Tubes were recentrifuged briefly and the remaining supernatant was aspirated with a pipette. The pellet was then dissolved in 0.3 ml of 10 % sodium hypochlorite and centrifuged. Radioactivity of the supernatant (0.2 ml) was determined with a scintillation counter. Values obtained from unstimulated parietal cells were subtracted from those of stimulated cells. Data were represented as a percentage of control values which were determined without BaCl2. Data are shown as mean ± s.d. of four samples. Similar results were obtained in at least two independent experiments.
Reverse transcriptase-polymerase chain reaction (RT-PCR) analysis
Total RNAs from adult rat gastric mucosa were isolated with TRIZOL reagent (Life Technologies, Grand Island, NY, USA) and reverse-transcribed with the oligo(dT)12–18 primer using SuperScript II RT (Life Technologies), according to the manufacturer's instructions. The cDNAs were amplified by PCR with LA-Taq polymerase (Takara, Tokyo, Japan). We designed specific primers for fifteen Kir channel subtypes, and glyceraldehyde-3-phosphate-dehydrogenase (GAPDH). Primer sequences are indicated as follows:
Rat Kir1.1: 5′-GTATGTCGTAGCGTATGTTCATA-3′ (446–468) 5′-TGGTCAATAATGTGGTAGATCGT-3′ (948–970)
Rat Kir2.1: 5′-TCATGGTGGTATTCCAGTCAATC-3′ (456–497) 5′-GAATCTGATTGGCCAGATATGAA-3′ (939–971)
Rat Kir2.2: 5′-TGCATCATTGACTCCTTCATGAT-3′ (571–593) 5′-TCATCAATCTCGTGCAAGATGGT-3′ (868–890)
Rat Kir2.3: 5′-ACTCCTTCATGATTGGTACTATC-3′ (491–513) 5′-TAGTCCACCTTGTAGTGACTCTT-3′ (976–988)
Rat Kir2.4: 5′-TAAGTGACCTGTTCACCACATGT-3′ (780–802) 5′-CAATCTCATGCACAATGGTAATG-3′ (1372–1394)
Rat Kir3.1: 5′-AAATGTGTACGTATGCCTAA-3′ (2651–2670) 5′-AACCACCCTTTTTATTCTAA-3′ (2682–3000)
Rat Kir3.2: 5′-TTTTATTCTCCATAGAGACA-3′ (914–933) 5′-TGTGAGAATTCCTCAAGTCCCC-3′ (1159–1180)
Rat Kir3.3: 5′-CCAGTGTCCCGAGGGCATCG-3′ (611–630) 5′-GGCCTCCACCATGCCCTCGA-3′ (1048–1067)
Rat Kir3.4: 5′-TTCAGAGTCATTACAGAGAAGTG-3′ (510–532) 5′-AGGAGCTCACCATTCCTCTTCAT-3′ (1108–1130)
Rat Kir4.1: 5′-GTAGACACAGCCTCTGATAGCC-3′ (836–857) 5′-AGCAGGTGTGAACTCGTAGC-3′ (1041–1060)
Human Kir4.2: 5′-TGTTCGCTGCCACTTTTGTGATG-3′ (276–298) 5′-GTTCCGGTGATGAAGATCTCAAT-3′ (536–558)
Rat Kir6.1: 5′-TCTTCTCCATCGAGGTTC-3′ (401–418) 5′-TCTCCTTCTGGCGTCGTGG-3′ (701–722)
Rat Kir6.2: 5′-ATGCTGTCCCGAAAGGGCAT-3′ (4–23) 5′-TTGGTGCCCTCTCCG-3′ (309–323)
Rat Kir7.1: 5′-TCTCCTTCTCTCTGGAGACACAA-3′ (329–351) 5′-GTTGGATGTTCAGGAACACTCTT-3′ (958–980)
Rat GAPDH: 5′-GGCTGCCTTCTCTTGTGACAA-3′ (84–104) 5′-CGCTCCTGGAGGAT-3′ (263–282)
PCR amplification was performed for 30 cycles at 94 °C for 1 min, at 55 °C for 1 min, and then 72 °C for 1 min followed by 72 °C for 8 min. Amplified DNA fragments were separated in a 0.8 % agarose gel. Subsequently, fragments were sequenced with an automatic sequencer (A-381, The Perkin-Elmer Corp., Foster City, CA, USA) after thymine adenine cloning (Invitrogen, San Diego, CA, USA).
Antibody
Rabbit anti-Kir4.1 (anti-KAB-2C2) and anti-Kir7.1 (anti-Kir7.1–1H1) antibodies were used as described previously (Ito et al. 1996; Kusaka et al. 2001, respectively). Polyclonal antisera against mouse Kir4.2 and monoclonal antisera against the β subunit of H+–K+-ATPase and β-catenin were purchased from Alomone Labs (Jerusalem, Israel), Affinity BioReagents Inc. (Golden, CO, USA) and Transduction Laboratories (Lexington, KY, USA), respectively.
Immunocytochemistry
For immunocytochemical study, we used male Wistar rats and male ICR mice which were fasted overnight and then allowed to eat freely for 1 h before fixation. We always confirmed the presence of a bolus of food in the stomachs of the rats and mice. Rats and mice were deeply anaesthetized with pentobarbital sodium (50 mg kg−1i.p.), and stomachs were fixed by transcardiac perfusion as described previously (Nagelhus et al. 1998; Fujita et al. 1999). For light microscopic immunohistochemistry, the sections were visualized with fluorescein isothiocyanate (FITC)-labelled anti-rabbit IgG (EY Laboratories, San Mateo, CA, USA) and Texas Red-labelled anti-mouse IgG (Protos Immunoresearch, San Francisco, CA, USA), and examined with a confocal microscope (MRC-1024, Bio-Rad, Hertfordshire, UK). For electron microscopic immunocytochemistry, rat stomachs were fixed with ‘pH shift protocol’ (Nagelhus et al. 1998; Fujita et al. 1999). Ultrathin sections were cut with a Reichert ultramicrotome, mounted on nickel grids and processed for immunogold cytochemistry as described previously (Matsubara et al. 1996; Nagelhus et al. 1998; Fujita et al. 1999). Finally, the sections were counterstained and examined with a HITACHI 7100-α electron microscope.
Electrophysiological recordings
Rat Kir4.1 cDNA was subcloned into the expression vector, pcDNA3 (Invitrogen) and transfected with LipofectAMINE Plus Reagent (Gibco BRL) into HEK293T cells as described previously (Horio et al. 1997). Two days after transfection, the cells were used for electrophysiological recording in the whole-cell and inside-out patch configurations. Currents of HEK293T cells transfected with rat Kir4.1 were measured at room temperature using a patch-clamp amplifier (EPC-7, List, Darmstadt, Germany). The tips of the patch electrodes were coated with Sylgard (Dow Corning) and fire polished. The tip resistance of the electrodes was 3–5 MΩ. For analysis, data were reproduced, low pass-filtered at 1 kHz (−3 dB) by an 8-pole Bessel filter (Frequency Devices, Haverhill, MA, USA), sampled at 5 kHz, and analysed off-line on a computer (PowerMac G3, Apple Computer Inc., Cupertino, CA, USA) using a commercially available software (Patch Analyst Pro, MT Corporation, Nishinomiya, Hyogo, Japan). The bathing and pipette solutions for whole-cell recording contained (mm): 130 NaCl, 20 KCl, 1.8 CaCl2, 0.53 MgCl2, 5 Hepes-NaOH, pH 7.4, and 50 KCl, 3 K2ATP, 1 MgCl2, 1 CaCl2, 100 Hepes potassium salt-KOH, pH 7.4, respectively, and the internal and pipette solutions for inside-out recording contained (mm): 50 KCl, 5 EGTA, and 100 Hepes (pH 7.4 adjusted by adding KOH). To prepare external or internal solutions with pH lower than 7, Mes was used instead of Hepes. The pH of solutions was adjusted to the desired values by adding NaOH for external solution or KOH for internal and pipette solutions.
RESULTS
Effects of K+ channel blockers on secretion of gastric acid
We first examined pharmacologically the possibility that some of inwardly rectifying K+ (Kir) channels are involved in secretion of gastric acid. Isolated parietal cells were stimulated by histamine and carbachol, and accumulation of aminopyrine in the cells, which represents acid secretion (Soll, 1980), was assayed (Fig. 1A). When Ba2+, a non-selective blocker of Kir channels (Takumi et al. 1995; Tanemoto et al. 2000; Neusch et al. 2001), was added to the incubation medium, the accumulation of aminopyrine was decreased to 54.8 ± 17.3 % and 24.7 ± 15.0 % (n = 4 each) of the control in the presence of 0.1 mm and 1 mm Ba2+, respectively. These results suggest that the K+ channels sensitive to Ba2+ ions may play important roles in acid secretion of parietal cells. Because 0.1 mm Ba2+ suppressed the acid secretion to around half (Fig. 1A), some of the Kir channels may be candidates for these K+ channels.
Figure 1. [14C]aminopyrine accumulation was inhibited by Ba2+.
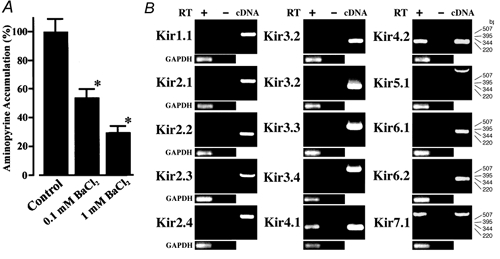
A, isolated parietal cells were enriched and incubated in a medium containing 1 μm [14C]aminopyrine in a 24-well dish (2 × 105 cells per well) and BaCl2 was added to the cells 15 min before the addition of agonists. Parietal cells were stimulated by 0.1 mm histamine and 10 μm carbachol and incubated for 30 min. Radioactivity of [14C]aminopyrine absorbed by parietal cells was assayed. Data are represented as a percentage of control values. Data are means ± s.d. of four samples. Similar results were obtained in at least two independent experiments. * Significant difference from the control, Student's t test (P < 0.01). B, RT-PCR analyses in rat gastric mucosa. PCR products were resolved by agarose gel electrophoresis and detected by staining with ethidium bromide. Gastric mucosa expressed mRNAs of Kir4.1, Kir4.2 and Kir7.1. As an internal standard, amplified fragments of glyceraldehyde-3-phosphate-dehydrogenase (GAPDH) mRNA were detected in experiments of all subunits. No DNA fragments were amplified with the template without treatment with reverse transcriptase (RT-). Numbers to the right of the gels indicate the size markers in base pairs.
RT-PCR analyses and immunohistochemistry in gastric mucosa
Fifteen members have been identified so far in the mammalian Kir family (Isomoto et al. 1997; Topert et al. 1998; Doring et al. 1998; Krapivinsky et al. 1998). To identify which Kir subunits are expressed in gastric mucosa, the RT-PCR analysis of rat gastric mucosa total RNA was performed using specific sets of primers for fifteen Kir subunits: Kir1.1, Kir2.1–2.4, Kir3.1–3.4, Kir4.1–4.2, Kir5.1, Kir6.1–6.2, Kir7.1 (Fig. 1B). Each set of primers amplified its specific DNA fragment when cDNA of each Kir subunit was used as a template (Fig. 1B, cDNA). We found that three Kir subunits, Kir4.1, Kir4.2 and Kir7.1 were expressed in rat gastric mucosa (Fig. 1B). No fragments were amplified with the template without reverse transcriptase treatment. The nucleotide sequences of these three amplified DNA fragments were confirmed by sequencing.
Next, we examined the distributions of Kir4.1, Kir4.2 and Kir7.1 in the stomach by immunohistochemistry using polyclonal antibodies specific to Kir4.1, Kir4.2 and Kir7.1. Specific immunoreactivity of Kir4.1 was detected in many cells of rat gastric epithelium (green in Fig. 2A). The Kir4.1 immunoreactivity was not detected when the antibody was preincubated with antigenic peptide (data not shown). When the same preparation was co-immunostained with the antibody to the β subunit of H+–K+-ATPase (Fig. 2B), prominent yellow signals were developed in all Kir4.1-positive cells in the merged image (Fig. 2C). Therefore, Kir4.1 immunoreactivity existed specifically in parietal cells of the gastric epithelium. We next examined the Kir4.2 immunoreactivity in a mouse stomach preparation, because only the anti-mouse Kir4.2 was available. The Kir4.2 immunoreactivity was predominantly detected in surface mucous cells of mice gastric mucosa and not in parietal cells (not shown). It was previously reported that the expression of Kir4.2 mRNA was detected in gastric epithelium of mouse embryos (Thiery et al. 2000) and that cultured glandular stomach epithelial cells isolated from fetal rat differentiated into surface mucous cells in primary culture (Fukamachi et al. 1994). Thus, Kir4.2 may be expressed in surface mucous cells of adult and fetus mice. On the other hand, the immunoreactivity of Kir7.1 was detected in neural regions of the gastric mucosa but not in the gastric epithelium at all (not shown). Therefore, of the various Kir channels so far identified, only Kir4.1 was expressed in gastric parietal cells.
Figure 2. Immunohistochemical analysis of Kir4.1 in rat gastric mucosa.
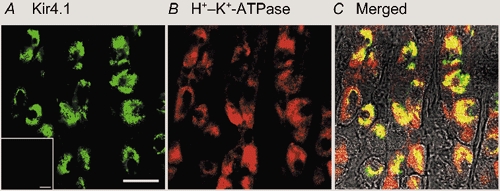
Double-staining of Kir4.1 and H+–K+-ATPase (β subunit) in stomach. A, Kir4.1 (green); B, H+–K+-ATPase (red); C, double exposures of both images. Scale bar, 10 μm.
Within each parietal cell, the immunoreactivities of Kir4.1 and the β subunit of H+–K+-ATPase did not completely overlap. In the merged image (Fig. 2C), the yellow signal and a small amount of green signal were detected mainly in the centre portion of each cell, and a large portion of red signal remained at the peripheral regions. Therefore, it is suggested that Kir4.1 and H+–K+-ATPase may co-exist at the central portion of parietal cells, while at the peripheral regions only H+–K+-ATPase may exist.
Immunogold electron microscopic examination of Kir4.1-immunoreactivity in parietal cells
To determine the precise localization of Kir4.1 channels in the gastric parietal cells, we performed immunogold electron microscopy using anti-KAB-2C2 antibody. Parietal cells could easily be identified by their shape, intracellular tubulovesicles, and apical microvilli (Sedar & Friedman 1961; Adkins et al. 1967; Helander & Hirschowitz 1972). Immunogold particles for Kir4.1 proteins were detected exclusively on the microvilli at the apical membrane of parietal cells, and not in intracellular tubulovesicles or at the basolateral membrane (Fig. 3Aa-c). The labelling for Kir4.1 was not detected when the antibody was preincubated with antigenic peptide (data not shown). On the other hand, abundant gold particles for immunoreactivity of the β subunit of H+–K+-ATPase were detected in microvilli as well as tubulovesicles (Fig. 3Ba and b), but not at the basolateral membrane (Fig. 3Bc), as previously reported (Jones et al. 1991). Specific localization of Kir4.1 at the apical membrane of parietal cells suggests that Kir4.1 may participate in the K+-recycling pathway involved in activation of the H+–K+-ATPase localized in the tubulovesicles when these are fused to the apical membrane.
Figure 3. Immunogold electron microscopy of Kir4.1 in the stomach.
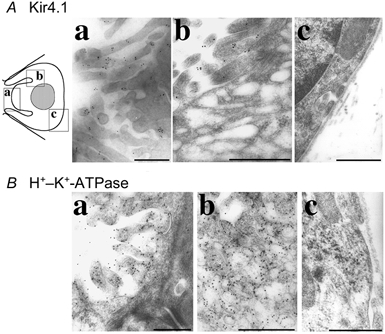
Ultra-thin sections were stained with anti-KAB-2C2 antibody following anti-rabbit IgG coupled to colloidal gold particles (A) and monoclonal anti-β subunit of H+–K+-ATPase antibody following anti-mouse IgG coupled to colloidal gold particles (B). Electron microscopic images were obtained of the regions indicated in the diagram. Microvilli of parietal cells (Aa and Ba) were stained with both antibodies. Tubulovesicles were stained with anti-β subunit of H+–K+-ATPase antibody (Bb), but not with anti-Kir4.1 antibody (Ab). Kir4.1 and β subunit of H+–K+-ATPase were not expressed on basal membrane (Ac and Bc). Scale bars, 0.5 μm.
Effect of extracellular acidification on the channel activity of Kir4.1
We next examined the effect of acidified external solution on the activity of the Kir4.1 channel expressed in HEK293T cells. A ramp voltage step (1 s in duration) from −100 to +30 mV was applied every 5 s. The holding potential was −50 mV. The bathing solution contained 20 mm [K+]o. The pH of external bathing solutions (pHo) was varied from 7.4 to 6.0, 5.0, 4.0 and 3.0. The Kir4.1 current was unaffected by the acidified external solutions (Fig. 4A and B, n = 5). The cell membrane, however, became leaky after ∼20 s perfusion of pHo 3.0-solution (Fig. 4A). Thus, the effects of pHo below 3 could not be examined. In contrast to the effects of acidified extracellular solution, the Kir4.1 channel activity was easily depressed by acidification of the internal solution. The channel activity decreased even when the pH of the internal solution (pHi) was reduced from 7.4 to 6.5. It disappeared at pHi < 5.5 (Fig. 4C), as reported previously (Shuck et al. 1997; Yang & Jiang 1999; Tanemoto et al. 2000; Tucker et al. 2000). These results indicate that the Kir4.1 channel activity was sensitive to protons from the internal side but resistant to them from external side. This feature of Kir4.1 may be consistent with a function as the K+-recycling pathway for H+–K+-ATPase at the apical membrane of parietal cells.
Figure 4. Effect of extracellular pH on the current of Kir4.1-expressing HEK293T cells.
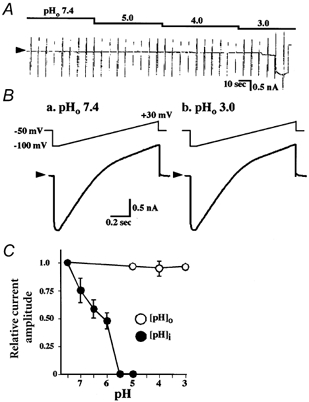
A and B, whole-cell currents of HEK293T cells transfected with Kir4.1 cDNA. Typical traces of three separate experiments. Voltage ramps (1000 ms in duration), from −100 to +30 mV were applied from a holding potential of −50 mV in 20 mm [K+]o solution. The external solutions of different pH (pHo) were applied as indicated. C, the current amplitude at −100 mV at different pHo levels is plotted relative to the value at pHo 7.4. Comparison between pHo and internal pH (pHi) effects. The effects of pHi were examined in inside-out macro-patches from Kir4.1 expressing HEK293T cells with a ramp ranging from −100 to +40 mV, 1000 ms in duration, from a holding potential of 0 mV, in 150 mm [K+] internal solution at pHi 5 to 7.4. Data are expressed as means ± s.e.m.
DISCUSSION
The major findings in this study are as follows. (1) Sub-millimolar concentrations of Ba2+, a non-specific inhibitor of Kir channels (Takumi et al. 1995; Tanemoto et al. 2000; Neusch et al. 2001), suppressed the accumulation of aminopyrine in isolated parietal cells. (2) Kir4.1 was localized specifically at the apical microvilli of gastric parietal cells. (3) Kir4.1 current was unaffected by external acidification at least up to pH 3.
Gastric parietal cells secrete protons (H+) via H+–K+-ATPase which is localized at the apical membrane and on the tubulovesicles fused to the apical membrane. The concentration of HCl in the secreted solution is 0.16 m, which is isotonic with plasma fluid and has a pH is as low as 0.8, while the pH of the cytoplasm of the parietal cells is ∼7.3 (Hersey & Sachs, 1995). The extrusion of H+ from the parietal cells against its concentration gradient by H+–K+-ATPase requires not only intracellular ATP but also extracellular K+ ions (Wolosin & Forte, 1981). Previously, Reenstra & Forte (1990) have characterized K+ and Cl− conductive pathways in apical membrane vesicles of stimulated parietal cells. The pH gradient formation in apical membrane vesicles, which can be interpreted as acid secretion, was dependent on the existence of K+ and Cl− in the extracellular solution and was also inhibited by Ba2+ ions and Cl− channel inhibitors. The inhibition by Ba2+ was reversed by an increase in the concentration of K+ in the extracellular solution. Theses studies clearly demonstrated that the acid secretion by the H+–K+-ATPase required both K+ and Cl− conductive pathways. In this study, it was confirmed in isolated parietal cells that sub-millimolar concentrations of Ba2+ suppressed the secretion of H+ ions (Fig. 1A). Therefore, it is suggested that some of the Kir channels may be involved in K+-recycling to maintain the activity of H+–K+-ATPase at the apical membrane of parietal cells. Actually, RT-PCR examination suggested that Kir4.1, Kir4.2 and Kir7.1 were expressed in the gastric mucosa (Fig. 1B). Among them, the Kir4.1 channel protein was immunohistochemically identified as being expressed in parietal cells (Fig. 2).
It is known, however, that in addition to Kir channels Ba2+, especially at millimolar concentrations, can block some of K+ channels including Ca2+-activated K+ (Diaz et al. 1996) and two-pore K+ channels (Lesage et al. 1996; Kim et al. 1998). In this study the aminopyrine accumulation was only reduced to about half by 0.1 mm Ba2+ and 1 mm Ba2+ was required for almost complete inhibition (Fig. 1A). Therefore, Ba2+-sensitive K+ channels other than Kir may also participate in the K+-recycling pathway. Actually, it was recently reported that KCNQ1 (also called KvLQT1) is expressed in parietal cells and that KCNQ1 inhibitor 293B inhibits acid secretion from parietal cells (Grahammer et al. 2001).
High-resolution immunogold electron microscopic study showed that Kir4.1-immunolabelling was specifically localized at the apical microvilli of parietal cells in the rat gastric mucosa (Fig. 3). This specific subcellular localization supports the notion that Kir4.1 may be at least one of the K+ channels which supply K+ ions to H+–K+-ATPases. Furthermore, while Kir4.1 channel activity was shown to be sensitive to intracellular acidification and completely blocked at pHi < 5.5 (Shuck et al. 1997; Yang & Jiang 1999; Tanemoto et al. 2000; Tucker et al. 2000), the channel expressed in HEK293T cells was unaffected by the external acidification up to pH 3.0 (Fig. 4). Because the HEK293T cells themselves became leaky after ∼40 s of perfusion with the bathing solution of pH 3, the exact sensitivity of Kir4.1 channel activity to external acidification was hard to determine. However, it can be at least concluded that the channel activity of Kir4.1 is very resistant to protons from the external side. Thus, it seems likely that Kir4.1 channels localized at the apical microvilli of parietal cells can continue to supply K+ ions to the H+–K+-ATPase even in an acidified environment.
It has been indicated that the Kir4.1 channel plays two functional roles in various tissues: i.e. K+-buffering and K+-recycling actions. The K+-buffering action of Kir4.1 has been proposed in brain astrocytes (Higashi et al. 2001), in retinal Müller cells (Ishii et al. 1997) and also in retinal pigmented epithelial cells (Kusaka et al. 2001). In these tissues, Kir4.1 channels are localized at certain specific domains on the cell membrane and may mediate intrusion and/or extrusion of K+ ions across the cell membrane, and thus be responsible for transportation of K+ ions in the tissues: e.g. from the synaptic sites to peri-vascular sites through some of astrocytes in the brain (Higashi et al. 2001). As for the K+-recycling action, Kir4.1 channels were found at the same site as Na+-K+-ATPase at the basolateral membrane of renal distal convoluted tubular epithelial cells (Ito et al. 1996). The Kir4.1 channels may thus support activity of the active ion transporting pump by supplying K+ ions to its extracellular site. This study showed for the first time that Kir4.1 channels may participate in K+-recycling for another active ion transporting pump, i.e. H+–K+-ATPase, by localizing at the apical membrane of parietal cells.
The subcellular localization of Kir4.1 channel proteins on the cell membrane thus seems to play an essential role in determining the functional roles of the channel in various types of cell. Therefore, it is important to clarify the molecular mechanisms responsible for control of the subcellular localization of the channel proteins. We previously reported that Kir4.1 could interact through the sequence Ser-Asn-Val in its C-terminus with anchoring proteins containing PSD-95/Dlg/ZO-1 (PDZ) domains, such as PSD-95 and SAP97 (Horio et al. 1997). A novel PDZ domain-containing anchoring protein, CIPP, was isolated from brain using the yeast two hybrid method (Kurschner et al. 1998). Therefore, PDZ domain-containing anchoring proteins might be involved in physiological control of Kir4.1-localization. In retinal Müller cells, the clustered distribution of Kir4.1 on the cell surface membrane was suggested to be controlled by SAP97 (Horio et al. 1997), which is the subject of extracellular matrix regulation (Ishii et al. 1997). This mechanism, however, can be divergent among cells. In parietal cells, SAP97 was reported to be localized at the basolateral membrane (Jons et al. 1999). Thus, this anchoring protein may not be involved in the control of Kir4.1 in parietal cells. The mechanisms for co-localization of Kir4.1 channels with Na+-K+-ATPase at the basolateral membrane of renal tubular epithelial cells have not been examined at all so far. Further studies are clearly needed to clarify the regulatory mechanism of the subcellular localization of Kir4.1 in various cells.
In parietal cells, Na+-K+-ATPase is localized at the basolateral membrane (Dunbar et al. 1998). For this active ion transporting pump, some of K+ channels may potentially function as the K+-recycling pathway. Thus, it is also necessary to identify the K+ channel in order to elucidate the roles of K+ ion channels in the control of parietal cell function.
Acknowledgments
We thank Dr Ian Findlay (University of Tours, Tours, France) for his critical reading of this manuscript, Ms Mari Imanishi and Ms Kadue Takahashi for their technical assistance, and Ms Keiko Tsuji for the secretarial work. This work was supported by grants, ‘Grant-in-Aid for Scientific Research on Priority Areas (B)’ from the Ministry of Education, Culture, Sports and Science of Japan, from the Research for the Future Program of the Japan Society for the Promotion of Science (96L00302), from the Human Frontier Science Program (RG0158/1997-B) and from The Ryoichi Naito Foundation for Medical Research.
Supplementary material
The online version of this paper http://www.jphysiol.org/cgi/content/full/540/1/85 contains two supplementary colour figures.
REFERENCES
- Adkins RB, Jr, Ende N, Gobbel WG., Jr A correlation of parietal cell activity with ultrastructural alterations. Surgery. 1967;62:1059–1069. [PubMed] [Google Scholar]
- Diaz F, Wallner M, Stefani E, Toro L, Latorre R. Interaction of internal Ba2+ with a cloned Ca(2+)-dependent K+ (hslo) channel from smooth muscle. Journal of General Physiology. 1996;107:399–407. doi: 10.1085/jgp.107.3.399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doring F, Derst C, Wischmeyer E, Karschin C, Schneggenburger R, Daut J, Karschin A. The epithelial inward rectifier channel Kir7. 1 displays unusual K+ permeation properties. Journal of Neuroscience. 1998;18:8625–8636. doi: 10.1523/JNEUROSCI.18-21-08625.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunbar LA, Courtois-Coutry N, Roush DL, Muth TR, Gottardi CJ, Rajendran V, Geibel J, Kashgarian M, Caplan MJ. Sorting of P-type ATPases in polarized epithelial cells. Acta Physiologica Scandinavica Supplement. 1998;643:289–295. [PubMed] [Google Scholar]
- Fujita A, Horio Y, Nielsen S, Nagelhus EA, Hata F, Ottersen OP, Kurachi Y. High-resolution immunogold cytochemistry indicates that AQP4 is concentrated along the basal membrane of parietal cell in rat stomach. FEBS Letters. 1999;459:305–309. doi: 10.1016/s0014-5793(99)01256-9. [DOI] [PubMed] [Google Scholar]
- Fukamachi H, Ichinose M, Ishihama S, Tsukada S, Yasugi S, Shiokawa K, Furihata C, Yonezawa S, Miki K. Fetal rat glandular stomach epithelial cells differentiate into surface mucous cells which express cathepsin E in the absence of mesenchymal cells in primary culture. Differentiation. 1994;56:83–89. doi: 10.1046/j.1432-0436.1994.56120083.x. [DOI] [PubMed] [Google Scholar]
- Grahammer F, Herling AW, Lang HJ, Schmitt-Gräff A, Wittekindt OH, Nitschke R, Bleich M, Barhanin J, Warth R. The cardiac K+ channel KCNQ1 is essential for gastric acid secretion. Gastroenterology. 2001;120:1363–1371. doi: 10.1053/gast.2001.24053. [DOI] [PubMed] [Google Scholar]
- Helander HF, Hirschowitz BI. Quantitative ultrastructural studies on gastric parietal cells. Gastroenterology. 1972;63:951–961. [PubMed] [Google Scholar]
- Hersey SJ, Sachs G. Gastric acid secretion. Physiological Reviews. 1995;75:155–189. doi: 10.1152/physrev.1995.75.1.155. [DOI] [PubMed] [Google Scholar]
- Higashi K, Fujita A, Inanobe A, Tanemoto M, Doi K, Kubo T, Kurachi Y. An inwardly rectifying K+ channel, Kir4. 1, expressed in astrocytes surrounds synapses and blood vessels in brain. American Journal of Physiology. 2001;281:C922–931. doi: 10.1152/ajpcell.2001.281.3.C922. [DOI] [PubMed] [Google Scholar]
- Horio Y, Hibino H, Inanobe A, Yamada M, Ishii M, Tada Y, Satoh E, Hata Y, Takai Y, Kurachi Y. Clustering and enhanced activity of an inwardly rectifying potassium channel, Kir4. 1, by an anchoring protein, PSD-95/SAP90. Journal of Biological Chemistry. 1997;272:12885–12888. doi: 10.1074/jbc.272.20.12885. [DOI] [PubMed] [Google Scholar]
- Ishii M, Horio Y, Tada Y, Hibino H, Inanobe A, Ito M, Yamada M, Gotow T, Uchiyama Y, Kurachi Y. Expression and clustered distribution of an inwardly rectifying potassium channel, KAB-2/Kir4. 1, on mammalian retinal Müller cell membrane; Their regulation by inulin and laminin signals. Journal of Neuroscience. 1997;17:7725–7735. doi: 10.1523/JNEUROSCI.17-20-07725.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isomoto S, Kondo C, Kurachi Y. Inwardly rectifying potassium channels: their molecular heterogeneity and function. Japanese Journal of Physiology. 1997;47:11–39. doi: 10.2170/jjphysiol.47.11. [DOI] [PubMed] [Google Scholar]
- Ito M, Inanobe A, Horio Y, Hibino H, Isomoto S, Ito H, Mori K, Tonosaki A, Tomoike H, Kurachi Y. Immunolocalization of an inwardly rectifying K+ channel, KAB-2 (Kir4. 1), in the basolateral membrane of renal distal tubular epithelia. FEBS Letters. 1996;388:11–15. doi: 10.1016/0014-5793(96)00502-9. [DOI] [PubMed] [Google Scholar]
- Jones CM, Toh B-H, Pettitt JM, Martinelli TM, Humphris DC, Callaghan JM, Goldkorn I, Mu F-T, Gleeson PA. Monoclonal antibodies specific for the core protein of the β-subunit of the gastric proton pump (H+/K+ ATPase) European Journal of Biochemistry. 1991;197:49–59. doi: 10.1111/j.1432-1033.1991.tb15881.x. [DOI] [PubMed] [Google Scholar]
- Jons T, Heim HK, Kistner U, Ahnert-Hilger G. SAP 97 is a potential candidate for basolateral fixation of ezrin in parietal cells. Histochem. Cell Biology. 1999;111:313–318. doi: 10.1007/s004180050362. [DOI] [PubMed] [Google Scholar]
- Kim D, Fujita A, Horio Y, Kurachi Y. Cloning and functional expression of a novel cardiac two-pore background K+ channel (cTBAK-1) Circulation Research. 1998;82:513–518. doi: 10.1161/01.res.82.4.513. [DOI] [PubMed] [Google Scholar]
- Kurschner C, Mermelstein PG, Holden WT, Surmeier DJ. CIPP, a novel multivalent PDZ domain protein, selectively interacts with Kir4. 0 family members, NMDA receptor subunits, neurexins, and neuroligins. Molecular and Cellular Neurosciences. 1998;11:161–172. doi: 10.1006/mcne.1998.0679. [DOI] [PubMed] [Google Scholar]
- Kusaka S, Inanobe A, Fujita A, Makino Y, Tanemoto M, Matsushita K, Tano Y, Kurachi Y. Functional Kir7. 1-channels localized at the root of apical processes in rat retinal pigment epithelium. Journal of Physiology. 2001;531:27–36. doi: 10.1111/j.1469-7793.2001.0027j.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krapivinsky G, Medina I, Eng L, Krapivinsky L, Yang Y, Clapham DE. A novel inward rectifier K+ channel with unique pore properties. Neuron. 1998;20:995–1005. doi: 10.1016/s0896-6273(00)80480-8. [DOI] [PubMed] [Google Scholar]
- Lesage F, Guillemare E, Fink M, Duprat F, Lazdunski M, Romey G, Barhanin J. TWIK-1, a ubiquitous human weakly inward rectifying K+ channel with a novel structure. EMBO Journal. 1996;15:1004–1011. [PMC free article] [PubMed] [Google Scholar]
- Matsubara A, Laake JH, Davanger S, Usami S, Ottersen OP. Organization of AMPA receptor subunits at a glutamate synapse: a quantitative immunogold analysis of hair cell synapses in the rat organ of Corti. Journal of Neuroscience. 1996;16:4457–4467. doi: 10.1523/JNEUROSCI.16-14-04457.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagelhus EA, Veruki ML, Torp R, Haug F-M, Laake JH, Nielsen S, Agre P, Ottersen OP. Aquaporin-4 water channel protein in the rat retina and optic nerve: polarized expression in Müller cells and fibrous astrocytes. Journal of Neuroscience. 1998;18:2506–2519. doi: 10.1523/JNEUROSCI.18-07-02506.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neusch C, Rozengurt N, Jacobs RE, Lester HA, Kofuji P. Kir4. 1 potassium channel subunit is crucial for oligodendrocyte development and in vivo myelination. Journal of Neuroscience. 2001;21:5429–5438. doi: 10.1523/JNEUROSCI.21-15-05429.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reenstra WW, Forte JG. Characterization of K+ and Cl− conductances in apical membrane vesicles from stimulated rabbit oxyntic cells. American Journal of Physiology. 1990;259:G850–858. doi: 10.1152/ajpgi.1990.259.5.G850. [DOI] [PubMed] [Google Scholar]
- Sanders SS, Noyes DH, Spangler SG, Rehm WS. Demonstration of a barium-potassium antagonism on lumen side of in vitro frog stomach. American Journal of Physiology. 1973;224:1254–1259. doi: 10.1152/ajplegacy.1973.224.6.1254. [DOI] [PubMed] [Google Scholar]
- Sedar AW, Friedman M. Correlation of fine structure of gastric parietal cell (dog) with functional activity of the stomach. Journal of Biophysical and Biochemical Cytology. 1961;11:349–363. doi: 10.1083/jcb.11.2.349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shuck ME, Piser TM, Bock JH, Slightom JL, Lee KS, Bienkowski MJ. Cloning and characterization of two K+ inward rectifier (Kir) 1. 1 potassium channel homologs from human kidney (Kir1.2 and Kir1.3) Journal of Biological Chemistry. 1997;272:586–593. doi: 10.1074/jbc.272.1.586. [DOI] [PubMed] [Google Scholar]
- Smolka A, Helander HF, Sachs G. Monoclonal antibodies against gastric H++K+ ATPase. American Journal of Physiology. 1983;245:G589–596. doi: 10.1152/ajpgi.1983.245.4.G589. [DOI] [PubMed] [Google Scholar]
- Soll AH. Secretagogue stimulation of [14C]aminopyrine accumulation by isolated canine parietal cells. American Journal of Physiology. 1980;238:G366–375. doi: 10.1152/ajpgi.1980.238.4.G366. [DOI] [PubMed] [Google Scholar]
- Takumi T, Ishii T, Horio Y, Morishige K, Takahashi N, Yamada M, Yamashita T, Kiyama H, Sohmiya K, Nakanishi S, Kurachi Y. A novel ATP-dependent inward rectifier potassium channel expressed predominantly in glial cells. Journal of Biological Chemistry. 1995;270:16339–16346. doi: 10.1074/jbc.270.27.16339. [DOI] [PubMed] [Google Scholar]
- Tanemoto M, Kittaka N, Inanobe A, Kurachi Y. In vivo formation of a proton sensitive K+ channel by heteromeric subunit assembly of Kir5. 1 with Kir4.1. Journal of Physiology. 2000;525:587–592. doi: 10.1111/j.1469-7793.2000.00587.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiery E, Gosset P, Damotte D, Delezoide A-L, de Saint-Sauveur N, Vayssettes C, Créau N. Developmentally regulated expression of the murine ortholog of the potassium channel KIR4. 2 (KCNJ15) Mechanisms of Development. 2000;95:313–316. doi: 10.1016/s0925-4773(00)00364-6. [DOI] [PubMed] [Google Scholar]
- Topert C, Doring F, Wischmeyer E, Karschin C, Brockhaus J, Ballanyi K, Derst C, Karschin A. Kir2. 4: a novel K+ inward rectifier channel associated with motoneurons of cranial nerve nuclei. Journal of Neuroscience. 1998;18:4096–5105. doi: 10.1523/JNEUROSCI.18-11-04096.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tucker SJ, Imbrici P, Salvatore L, D'Adamo MC, Pessia M. pH dependence of the inwardly rectifying potassium channel, Kir5. 1, and localization in renal tubular epithelia. Journal of Biological Chemistry. 2000;275:16404–16407. doi: 10.1074/jbc.C000127200. [DOI] [PubMed] [Google Scholar]
- Wolosin JM, Forte JG. Functional differences between K+-ATPase rich membrane isolated from resting or stimulated fundic mucosa. FEBS Letters. 1981;125:208–212. doi: 10.1016/0014-5793(81)80720-x. [DOI] [PubMed] [Google Scholar]
- Yang Z, Jiang C. Opposite effects pH on open-state probability and single channel conductance of Kir4. 1 channels. Journal of Physiology. 1999;520:921–927. doi: 10.1111/j.1469-7793.1999.00921.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


