Abstract
In several types of neurons, firing is an intrinsic property produced by specific classes of ion channels. Low-voltage-activated T-type calcium channels (T-channels), which activate with small membrane depolarizations, can generate burst firing and pacemaker activity. Here we have investigated the specific contribution to neuronal excitability of cloned human T-channel subunits. Using HEK-293 cells transiently transfected with the human α1G (CaV3.1), α1H (CaV3.2) and α1I (CaV3.3) subunits, we describe significant differences among these isotypes in their biophysical properties, which are highlighted in action potential clamp studies. Firing activities occurring in cerebellar Purkinje neurons and in thalamocortical relay neurons used as voltage clamp waveforms revealed that α1G channels and, to a lesser extent, α1H channels produced large and transient currents, while currents related to α1I channels exhibited facilitation and produced a sustained calcium entry associated with the depolarizing after-potential interval. Using simulations of reticular and relay thalamic neuron activities, we show that α1I currents contributed to sustained electrical activities, while α1G and α1H currents generated short burst firing. Modelling experiments with the NEURON model further revealed that the α1G channel and α1I channel parameters best accounted for T-channel activities described in thalamocortical relay neurons and in reticular neurons, respectively. Altogether, the data provide evidence for a role of α1I channel in pacemaker activity and further demonstrate that each T-channel pore-forming subunit displays specific gating properties that account for its unique contribution to neuronal firing.
In the nervous system, information is encoded primarily by the number and the frequency of action potentials. In several types of neurons, firing of action potentials is thought to be an intrinsic property produced by specific ion channels (Llinas, 1988; Connors & Gutnick, 1990). The low-voltage-activated or T-type Ca2+ channels (T-channels), a subclass of voltage-gated Ca2+ channels, are able to activate from small depolarizations near the resting membrane potential of cells and can generate neuronal spontaneous firing and pacemaker activities (for review see Huguenard, 1996). In rat thalamic relay neurons, T-channels mediate low threshold spikes that are involved in rebound burst firing (Llinas & Jahnsen, 1982). In thalamus, T-channels are involved in slow-wave sleep (Steriade et al. 1993; McCormick & Bal, 1997) and in the pathogenesis of epilepsy (Tsakiridou et al. 1995; Huguenard, 1999). At this stage, further understanding of the functions and diseased states involving T-channels requires molecular investigations of the channel properties, now made possible by their cloning. Three genes encoding the T-channel pore subunits were identified and designated α1G (CaV3.1), α1H (CaV3.2) and α1I (CaV3.3) (Cribbs et al. 1998; Perez-Reyes et al. 1998; Klugbauer et al. 1999; Lee et al. 1999; Williams et al. 1999; Monteil et al. 2000a, 2000b; McRory et al. 2001). T-currents generated by the α1I subunit display slow kinetics that differ markedly from the α1G and α1H currents which share the typical signature of native neuronal T-currents (Klöckner et al. 1999; Monteil et al. 2000b; McRory et al. 2001; for review see Lacinova et al. 2000). Northern blot analysis has shown that α1G and α1H mRNA is widely expressed in various tissues and especially in the the central nervous system (CNS) (Cribbs et al. 1998; Monteil et al. 2000a), while the α1I mRNA is restricted to the CNS as well as to thyroid and adrenal glands (Lee et al. 1999; Monteil et al. 2000b). In situ hybridization experiments have indicated that the three isotypes can coexist in neuronal tissues such as amygdala or hippocampus, while in the rat cerebellum the α1G subunit is predominant in the same way as α1H in sensory ganglia. In rat thalamus, the α1G and α1I subunits are both present but exhibit distinct expression patterns with respect to the various nuclei (Talley et al. 1999). Because Purkinje neurons of the cerebellum and thalamic neurons display intrinsic firing either in short burst or in tonic/sustained mode, we have examined the specific role of T-channel isotypes in these patterns of activity. Taking advantage of the ability to express pure populations of recombinant T-channels together with the use of voltage clamp protocols mimicking these neuronal activities, we describe here the behaviour of the three human T-channel isotypes in neuronal excitability. These channel properties were modelled to delineate the contribution of each cloned T-channel in promoting firing patterns. This study indicates that the α1I currents are preferentially recruited during the depolarizing after-potential (DAP) and can generate sustained electrical activity, while the α1G and α1H currents promote short burst firing.
METHODS
Cell culture and transfection protocols
Human embryonic kidney cells (HEK-293 cell line; ATCC) were transfected as previously described (Chemin et al. 2001) with 0.3 μg of pBB14 plasmid encoding the reporter gene GFP (Brideau et al. 1998) and 2.7 μg of different pBK-CMV plasmid constructs that encode for α1G (α1G-a; Chemin et al. 2001), α1I (Monteil et al. 2000b) and α1H (HH7; Cribbs et al. 1998). Two to three days later, cells were harvested and plated at low confluence and electrophysiological recordings were performed between days 2 and 6 after transfection.
Electrophysiology
Macroscopic currents were recorded by the whole-cell patch clamp technique using an Axopatch 200B amplifier (Axon Instruments, CA, USA) at room temperature (∼25 °C) as previously described (Chemin et al. 2001). Extracellular solution contained (mm): 2 CaCl2, 160 TEACl and 10 Hepes (pH adjusted to 7.4 with TEAOH). Borosilicate glass pipettes have a typical resistance of 1–2 MΩ when filled with an internal solution containing (mm): 110 CsCl, 10 EGTA, 10 Hepes, 3 Mg-ATP and 0.6 GTP (pH adjusted to 7.2 with CsOH). For action potential clamp studies we have used: (i) a generic action potential (J. Pancrasio, Axon Instruments website); (ii) a regular train of spikes and a fast burst activity recorded in Purkinje neurons of the cerebellum (Raman & Bean, 1997), generously provided by Dr B. P. Bean (Harvard Medical School, Boston, MA, USA); and (iii) a firing activity typical of those measured on the thalamocortical relay (rTC) neurons generated by the NEURON model (Hines & Carnevale, 1997), described below. Records were filtered at 5 kHz. Cell capacitance was 12.9 ± 2.7 pF (n = 45). Series resistance (Rs) was 14.2 ± 0.6 MΩ (n = 45) and the voltage error factor before compensation was 0.026 ± 0.0016 (n = 45) according to the equation Vm = Vc(1 - Rs/(Rs + Rm)), where Vm is the membrane potential, Vc the voltage command and (Rs/(Rs + Rm)) the voltage error factor. Capacitance and Rs were compensated by 90–100 % using the whole-cell parameters of the Axopatch 200B amplifier. Leak and residual capacitive currents were subtracted using a P/-5 procedure for tail current recordings and action potential clamp experiments. Data were analysed as previously described (Chemin et al. 2001) using pCLAMP6 (Axon Instruments), Excel (Microsoft) and GraphPad Prism (GraphPad Inc.) software. One-way ANOVA combined with a Student-Newman-Keuls post hoc test was used to compare the different values, and differences were considered significant at P < 0.05. Results are presented as the means ± s.e.m., and n is the number of cells used.
Modelling
The impact of the expression of α1G, α1H and α1I channels on the firing of thalamocortical relay neurons and thalamic reticular neurons was estimated using the NEURON model (described in detail by Hines & Carnevale, 1997). This model was modified by Destexhe et al. (1998) for thalamocortical relay cells and by Destexhe et al. (1996) for the thalamic reticular neurons. Both models were downloaded from the model database at Yale University (http://senselab.med.yale.edu/senselab/neurondb/). The parameters used in our experiments were the ‘three-compartment model configuration of burst behaviour’ as described in detail by Destexhe et al. (1996). The electrophysiological properties of the α1G, α1H and α1I channels were modelled using Hodgkin-Huxley equations as described by Huguenard & McCormick (1992) and the values obtained for the various α1 isoforms were substituted for the corresponding values of native T-channels of thalamocortical relay cells (Huguenard & McCormick, 1992) or thalamic reticular neurons (Huguenard & Prince, 1992). All these values are presented in Table 1 except voltage-independent τ of activation, which is 0.8 ± 0.1 (n = 18), 1.34 ± 0.1 (n = 8) and 7.2 ± 0.8 ms (n = 18) for α1G, α1H and α1I channels, respectively. To match the voltage clamp data, the modelling experiments were performed at 28 °C. Firing was triggered by injecting the virtual soma with a 0.18 nA depolarizing current over 100 ms; the resulting activity and the Ca2+ entry via T-channels recorded in the soma are presented in Fig. 7 and Fig. 8. For voltage clamp experiments, a rTC neuronal firing pattern (using native T-channel parameters) was produced by a 0.3 nA current injection into the virtual soma over 700 ms, then converted into a pCLAMP stimulation file and further applied to the transfected HEK-293 cells.
Table 1.
Electrophysiological parameters and statistical comparison of the α1G, α1H and αI Ca2+ currents (2 mm external Ca2+) obtained in HEK-293 cells
| α1G | α1H | α1I | G vs. H | G vs. I | H vs. I | |
|---|---|---|---|---|---|---|
| Activation | ||||||
| V0.5 (mV) | −49.3 ± 0.7 (26) | −48.4 ± 1.2 (10) | −41.5 ± 1.1 (17) | n.s. | *** | *** |
| Slope (mV) | 4.6 ± 0.1 (26) | 5.2 ± 0.4 (10) | 6.2 ± 0.2 (17) | n.s. | *** | * |
| Inactivation | ||||||
| V0.5 (mV) | −74.2 ± 1.1 (8) | −75.6 ± 0.7 (19) | −69.8 ± 0.9 (17) | n.s. | ** | *** |
| Slope (mV) | 5.5 ± 0.3 (8) | 6.2 ± 0.2 (19) | 6.1 ± 0.1 (17) | *** | *** | n.s. |
| Activation kinetics | ||||||
| Rise −40 mV (ms) | 4.4 ± 0.2 (20) | 5.7 ± 0.2 (9) | 33.9 ± 1.4 (31) | * | *** | *** |
| Rise +10 mV (ms) | 1.2 ± 0.1 (18) | 2.1 ± 0.1 (8) | 10.5 ± 1.3 (18) | * | *** | *** |
| e-fold | 14.5 ± 1.2 (17) | 11.8 ± 0.8 (9) | 14.7 ± 0.8 (29) | n.s. | n.s. | n.s. |
| Inactivation kinetics | ||||||
| τ−40 mV (ms) | 18.8 ± 1.6 (15) | 23.4 ± 0.3 (22) | 122 ± 5 (30) | * | *** | *** |
| τ+10 mV (ms) | 12.6 ± 0.6 (16) | 18.2 ± 0.4 (20) | 84 ± 3 (32) | * | *** | *** |
| e-fold (mV) | 10.8 ± 0.9 (16) | 6.2 ± 0.6 (11) | 9.3 ± 0.6 (26) | ** | n.s. | ** |
| Deactivation kinetics | ||||||
| τ−100 mV (ms) | 2.6 ± 0.2 (9) | 3.6 ± 0.4 (14) | 1.12 ± 0.1 (31) | * | *** | *** |
| τ−70 mV (ms) | 6.2 ± 0.4 (9) | 8.5 ± 1.1 (14) | 2.1 ± 0.1 (30) | * | *** | *** |
| e-fold (mV) | 32.4 ± 2.6 (9) | 24.1 ± 1.6 (11) | 41 ± 2.6 (30) | n.s. | n.s. | *** |
| τ recovery (ms) | 137 ± 5 (12) | 448 ± 36 (7) | 260 ± 30 (18) | *** | ** | ** |
Values are expressed as means ± s.e.m. and n is the numbers of cells used. ‘Rise’, 10–90 % rise time. Statistical comparisons were done using a one-way ANOVA combined with a Student-Newman-Keuls post hoc test with
P < 0.05
P < 0.01
P < 0.001; n.s., not significant.
Figure 7. Simulated current clamp recording of thalamocortical relay neurons (rTC).
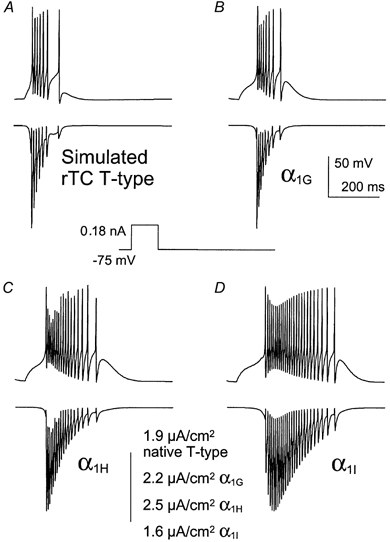
Cloned T-channel parameters (α1G (B), α1H (C) and α1I (D)) were substituted for the native Tcurrent (A) in the model and the resulting firing pattern and calcium entry via T-channels are presented. APs were elicited by a 0.18 nA current injected over 100 ms (see Methods).
Figure 8. Simulated current clamp recording of the reticular neurons of the thalamus (nRt).
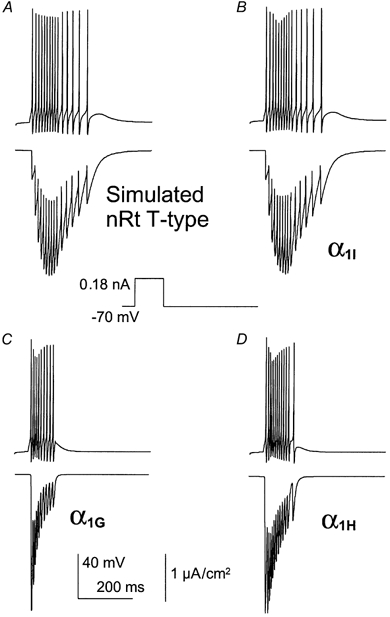
Cloned T-channel parameters (α1I (B), α1G (C) and α1H (D)) were substituted for the native T-current (A) in the model and the resulting firing pattern and calcium entry via T-channels are presented. APs were elicited by a 0.18 nA current injected over 100 ms (see Methods).
RESULTS
Biophysical properties of the three human cloned T-channels
The T-type Ca2+ currents generated by the cloned human α1G, α1H and α1I subunits were studied comparatively in HEK-293 cells. The three subunits produced robust Ca2+ currents, as illustrated in Fig. 1A. The current-voltage relationships (I-V curves) were normalized (Fig. 1B), revealing a 7 mV difference in the peak of the I-V curve of the α1I current. Steady-state activation curves were deduced from the I-V curves (Fig. 1C), which were fitted using a combined Boltzmann and linear Ohmic relationships as described previously (Chemin et al. 2001). Both steady-state activation and inactivation curves of the α1I current were moved towards positive voltages (7 and 6 mV, respectively), compared to the α1G and α1H currents (Table 1). As a consequence, a ∼5 mV positive shift of the window current component of α1I was observed. The three subunits also produced currents that were distinct in their kinetics. Besides the large kinetic differences between the α1G and α1I currents, it is important to note that the α1G subunits generated faster Ca2+ currents in both activation and inactivation kinetics compared to the α1H subunit (Fig. 2A and B, see also Table 1). Furthermore, the major difference between α1G and α1H channels was the recovery from short inactivation, which was significantly slower (3 times) for the α1H subunit, compared to the α1G subunit (Fig. 2C and Table 1). A specific electrophysiological feature of T-channels, compared to high-voltage-activated (HVA) Ca2+ channels, is their slow deactivation kinetics (Armstrong & Matteson, 1985). Again, significant differences were found among the three human isotypes (Fig. 3B), with α1I channels generating the fastest deactivation kinetics as illustrated in Fig. 3A while the α1H currents presented the slowest deactivation kinetics (Fig. 3B and Table 1). In each case, deactivating tail currents were fitted using a monoexponential function and the rate of deactivation was independent of the current amplitude (not shown), indicating further that accurate voltage control was obtained.
Figure 1.
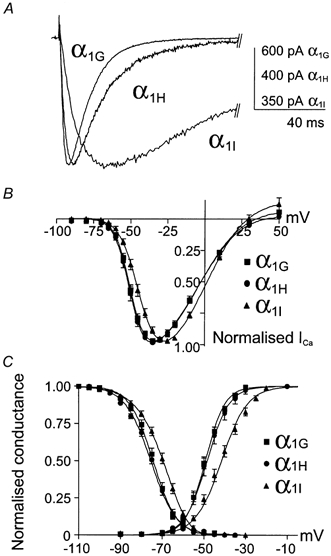
A, typical currents generated by the cloned human α1G, α1H and α1I channels. The traces correspond to the maximal currents elicited by a 100 ms test pulse (TP) from a holding potential (HP) of −110 mV. The voltage values of the TPs are −35 mV for the α1G and α1H subunits and −25 mV for the α1I subunit. B, current-voltage relationships (I-V curves) for the various α1 subunits. Note the positive shift of α1II-V curve. C, steady-state activation and inactivation curves. Steady-state inactivation curves were obtained by stepping the membrane potential at −30 mV from HPs ranging from −110 to −30 mV. The normalized peak current amplitude was plotted as a function of the HPs.
Figure 2.
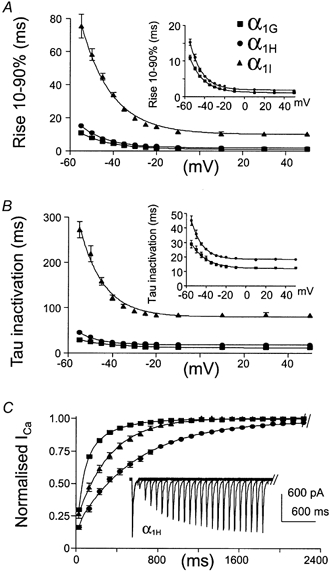
A, activation kinetics were presented as the time for the current to rise from 10 to 90 % (Rise 10–90 %) as function of the test potential. In order to better visualize the difference in activation kinetics between α1G and α1H currents, their corresponding rise times are presented in the inset. B, τ of inactivation of the various α1 isoforms as a function of voltage. Again, τ of inactivation of α1G and α1H currents is presented in the inset. C, recovery from short inactivation. As presented for α1H currents, recovery from short inactivation was measured using two −30 mV TPs lasting 100 ms which was applied from a HP of −110 mV of increasing duration.
Figure 3.
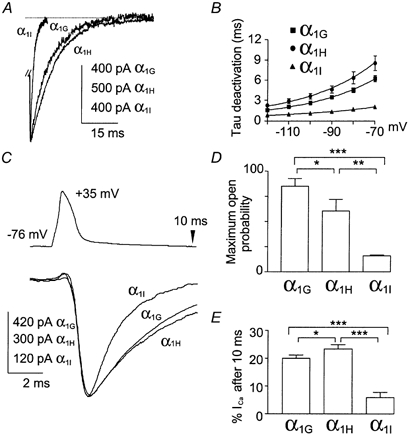
A, and B, deactivation kinetics. A, examples of normalized deactivating currents at −60 mV elicited after a −30 mV TP from an HP of −110 mV. For each subunit, the TP duration was adapted in order to trigger maximal deactivating currents (4 ms for α1G, 7 ms for α1H and 28 ms for α1I currents). B, plot of the deactivation kinetics as a function of the voltage. C, D and E, action potential clamp. C, normalized current for the various α1 isoforms elicited by an action potential (AP). D, maximum open probability of the channel isotypes during an AP. Channel maximum open probability during the AP was evaluated by calculating the ratio (r) of the maximal current amplitude induced by the AP to the maximal slope conductance (see I-V curve; Fig. 1A) recorded in the same cell. E, histogram of the percentage of the current amplitude remaining 10 ms after the triggering of the AP.
Single action potential clamp studies
Action potential (AP) clamp studies were then performed in order to investigate the specific behaviour of the three T-channel isotypes during neuronal activities. First, we evaluated the contributions of each cloned T-channel during a single AP (Fig. 3C). For the three channel isotypes, the onset of Ca2+ entry occurred during the repolarization phase of the AP. However, their behaviour during an AP stimulation was markedly distinct. The α1I subunit generated a rapidly inactivating current of small amplitude, revealing that this channel modestly contributed to Ca2+ entry during a single AP. In contrast, the α1G and α1H subunits generated larger and sustained currents (Fig. 3C). Channel maximum open probability during the AP was evaluated by calculating the ratio (r) of the maximal current amplitude induced by the AP to the maximal slope conductance from the I-V curve (see Fig. 1) recorded in the same cell. Maximum open probability (Fig. 3D) was larger for the α1G channels (r = 85.1 ± 7.4; n = 13) compared to the α1H channels (r = 60.4 ± 10.8; n = 12) and to the α1I channels (r = 16.1 ± 0.9; n = 11). In order to evaluate the Ca2+ entry duration triggered by the AP, we have calculated the percentage of current remaining 10 ms after the beginning of the AP (Fig. 3E). The Ca2+ entry duration, which reflects deactivation kinetics (Fig. 3B), was larger for α1G and α1H channels (percentage of current remaining: 19 ± 1 %, n = 13; and 23 ± 1 %, n = 12, respectively) compared to α1I channels (6 ± 2 %, n = 11). Overall, these data indicated that α1G and α1H channels produce larger and sustained Ca2+ entry during a single AP when compared to α1I channels.
Purkinje neuron and thalamocortical relay neuron action potential clamp studies
To further understand the role of the three T-channel isotypes in neuronal excitability, we performed AP clamp studies using several patterns of neuronal activity that occur in Purkinje neurons of the cerebellum (Fig. 4 and Fig. 5) and in relay neurons of the thalamus (Fig. 6). Although these neuronal activities were recorded in rats, we can predict that our approach is valid since the major T-current properties are conserved among rat and human T-channels (see Klöckner et al. 1999). We first applied a typical regular train of spikes (50 Hz) recorded in Purkinje cells (Raman & Bean, 1999a) that exhibited sustained spontaneous firing (Fig. 4A). In these neurons, APs are generated from a short DAP (18 ms). Several important differences could be observed among the Ca2+ currents produced by the three cloned T-channels. The α1G channels rapidly inactivated and only a very small current remained after the tenth spike (Fig. 4A and C). In contrast, the α1I channels generated a small and transient current during the first interspike interval (∼4 times less than the α1G and α1H channels), then increased during the next three spikes (Fig. 4A and C), suggesting an apparent facilitation of channel activity. After four to five spikes, slow inactivation overcame facilitation leading to a maintained Ca2+ entry during the entire train duration (Fig. 4B and C). The behaviour of the α1H channels was more complex and intermediate between α1G and α1I. The α1H channels contributed to a large depolarizing current during the entire train of stimulation (Fig. 4A and C) as a consequence of a robust first interspike interval current and slow inactivation, compared to the α1G channels. This difference between α1H and α1G currents was also found using more negative resting potentials. Similar to the result obtained for a holding potential (HP) of −70 mV (Fig. 4C), α1G currents decreased more rapidly and the ratio Imax last spike:Imax first spike of these currents was ∼3 times smaller than the α1H current ratio for a HP of −110 mV (n = 9, not shown). Interestingly, the α1H and α1I currents never inactivated completely during the interspike intervals (Fig. 4A), leading to a residual Ca2+ entry that was still observable at the end of the train of spikes (Fig. 4B). The presence of residual current at the steady state suggests that α1H and α1I currents could participate in sustained pacemaker activities. The second and the seventeenth inward current traces were scaled and superimposed (Fig. 4A, inset) to further demonstrate an adequate voltage control.
Figure 4. Behaviour of the three T-channel isotypes during tonic firing that occurred in Purkinje neurons.
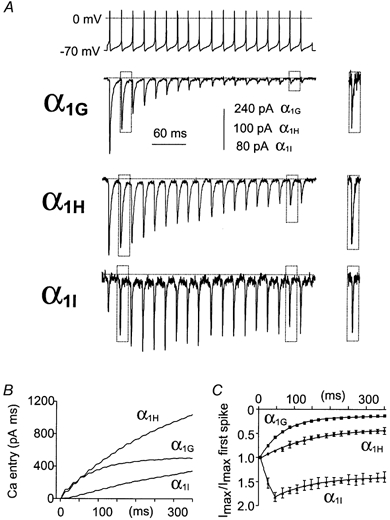
The top trace represents spontaneous activity of a Purkinje neuron which was used to perform AP clamp experiments on transfected cells. A, typical recorded currents for the α1G, α1H and α1I channels for cells expressing similar current density for I-V curves (see Fig. 1). Expanded and superimposed traces of inward currents triggering from the onset (second) to the end (seventeenth) of AP train stimulation are presented as an inset. B, calcium entry as a function of time for the same cells shown in A. In order to better compare the calcium entry between α1 subunits, we normalized it to the maximal slope conductance (from the I-V curve; see Fig. 1A) recorded in the same cell. C, normalized current amplitude for each spike as a function of time.
Figure 5. Purkinje neuron burst firing clamp.
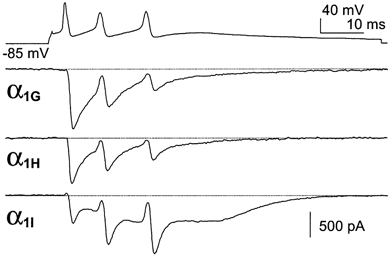
The top trace represents burst activity of a hyperpolarized Purkinje neuron which was used to perform AP clamp experiments on transfected cells. Typical α1G, α1H and α1I currents for cells expressing similar current density for I-V curves. Note the strong facilitation and the large calcium entry during the DAP for α1I currents.
Figure 6. Thalamocortical relay neuron activity clamp.
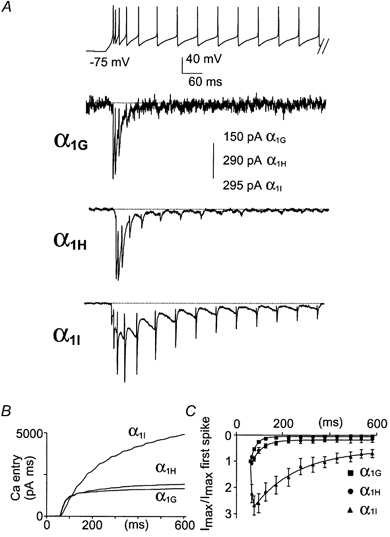
The top trace represents burst activity of a thalamocortical relay neuron which was used to perform AP clamp experiments on transfected cells. A, typical α1G, α1H and α1I currents for cells expressing similar current density for I-V curves. Note the facilitation and the rebound of the α1I currents during the DAP. B, calcium entry as a function of time for the same cells shown in A, normalized by the maximal slope conductance from the I-V curve (see Fig. 1A) recorded in the same cell. C, normalized current amplitude for each spike as a function of time.
We next performed AP clamp studies using patterns of burst activities. Figure 5 shows the behaviour of the three channel isotypes during short burst activities. When neurons from the Purkinje cell layer of the cerebellum are maintained hyperpolarized, they exhibit short and fast burst s(100 Hz) of a few APs, three on average as used in our experiments, upon injection of a depolarizing current (Raman & Bean, 1997; Fig. 5). In this case, we found that the α1G and α1H channels produced large inward currents that inactivated rapidly (Fig. 5). In contrast, the α1I channels generated a small inward current during the first spike, which strongly facilitated during the next APs. These experiments demonstrated that at a high frequency (100 Hz) the α1I currents still exhibited facilitation. Moreover, it is important to note that an increase of the α1I current amplitude was elicited during the DAP. We then performed voltage clamp analysis using a typical burst firing of the thalamocortical relay (rTC) neurons generated by the NEURON model (Fig. 6; see Methods). The rTC neurons have a resting membrane potential around −75 mV and respond to depolarizations by generating long-lasting burst activities from membrane potentials around −60 mV. As expected from the previous experiments, the α1G and α1H channels generated larger Ca2+ entry during the first spikes and then inactivated rapidly (Fig. 6A and B). No inward currents were observed after about six spikes for either α1G or α1H channels (Fig. 6C). In contrast, α1I channels produced large Ca2+ entry compared to the α1G and α1H channels (Fig. 6B), due to current facilitation and to a large ‘rebound’ inward current clearly associated with the DAP transition (Fig. 6A-C). It should be noted, however, that the α1H channels generated a small inward current that occurred during the DAP. Overall, these data suggest that α1I currents contribute to sustained neuronal activity, while α1G and α1H current profiles are more consistent with short burst firing.
Modelling experiments
Since action potential clamp studies do not provide dynamic information on the role of T-channels in shaping the neuronal firing patterns, we have performed simulations using the NEURON model (described in detail by Hines & Carnevale, 1997). This model was adapted for thalamocortical relay cells (Fig. 7) and for reticular cells of the thalamus (Fig. 8), as previously described (McCormick & Huguenard, 1992; Destexhe et al. 1996, 1998). In each simulation protocol, the parameters describing the native T-current were replaced with those of cloned T-channels. Figure 7A shows rTC simulation obtained with the native T-current parameters as described by McCormick & Huguenard (1992). To induce firing, a 100 ms pulse of 180 pA was injected into the virtual soma. This pulse does not trigger APs if T-channels are removed from the model environment (not shown). The parameters of the three cloned T-channels were then sequentially introduced in the model (Fig. 7B-D). The firing patterns predicted by the α1G channel parameters matched well those produced by the native T-channel parameters of the rTC neurons (Fig. 7A and B). Both native and α1G currents are able to depolarize the cells to approximately −60 mV during the 100 ms depolarizing pulse, with few APs (≤ 3) occurring after the stimulation. In addition, these two burst patterns exhibited similar strong frequency adaptation with a rapid decrease in the rate of APs. In both cases, this could be explained by fast current inactivation (see also Fig. 6). The latency to the first spike is nevertheless longer with α1G parameters. This is probably due to the more positive steady-state activation of α1G currents, compared to the native T-currents (V0.5 of −49 and −57 mV, respectively). In contrast, firing patterns predicted by α1H and α1I channel activity are markedly different from those produced by native T-currents. In both cases, there is a slower frequency adaptation and more importantly, α1H and α1I channels are both able to generate firing even after the end of the stimulation (Fig. 7C and D). As best exemplified with the α1I parameters, firing continued up to 200 ms after the end of the stimulation. Current facilitation was again observed with the α1I parameters (see also Fig. 6). In addition, the latency to the first AP obtained with the α1I parameters was the longest, probably due to its positive value for V0.5 of activation, as well as its slow activation kinetics. Overall, these results are in good agreement with the voltage clamp data described earlier and strongly suggest that the α1G channels are the dominant isotypes functionally expressed in the rTC neurons.
Figure 8 shows T-channel simulations using a thalamic reticular neuron environment. As previously described, firing was induced using a 100 ms pulse of 180 pA injected into the virtual soma. In contrast with thalamocortical relay neurons, firing was predicted to be persistent in reticular neurons (Fig. 8A). The α1I parameters best accounted for the firing pattern generated using the native T-current of reticular neurons (Fig. 8B; Huguenard & Prince, 1992). Indeed, the native T-current of reticular neurons showed facilitation during the firing activity, as shown for α1I channels both in voltage clamp and modelling experiments. In contrast, the α1G and α1H channels induced firing which stopped at the end of the stimulating pulse (Fig. 8C and D). Overall, simulation experiments indicated that the α1G and α1I currents might differentially participate in the firing pattern of rTC and reticular neurons of the thalamus and further suggest that α1I channels could promote sustained electrical activities.
DISCUSSION
This study demonstrates that the contribution of T-channels to neuronal excitability is related to isotype-specific properties. The properties of the three human T-channel α1 subunits cloned to date (α1G, α1H and α1I) were compared and we report significant differences among these isotypes in their biophysical properties, which are highlighted in action potential clamp studies. Modelling experiments further indicate a role of α1I channels in generating pacemaker activity. The functional signatures of the cloned T-channels described here should help both in understanding the diversity of native T-channels and in elucidating their specific physiological roles.
The specific properties of human cloned T-channels
The three T-channel α1 subunits exhibit distinct functional properties. Compared to α1G and α1H currents, α1I currents show a 6 mV positive shift in their steady-state properties. Because the window current component, a background Ca2+ current described in Chemin et al. (2000), results from the overlap of the activation and the inactivation curves, the α1I window current component should be evenly shifted. Such a difference could have major consequences on cell phenotype since the window current component of T-channels occurs near physiological resting potential and regulate basal levels of Ca2+ (Bijlenga et al. 2000; Chemin et al. 2000). In thalamocortical neurons, T-window currents contribute to enhanced firing through an intrinsic bistability-mediated phenomenon (Williams et al. 1997; Hughes et al. 1999). However, there is no evidence to date whether isotype-specific window currents could play distinct roles in neuronal physiology. The three human isotypes also display distinct kinetic properties. Cloned α1G and α1H channels promote fast gated currents typical of native T-currents (Carbone & Lux, 1984; Armstrong & Matteson, 1985; Nowycky et al. 1985; Monteil et al. 2000b), while α1I currents display slow activation and inactivation kinetics but faster deactivation. Nevertheless, α1H currents can be distinguished from α1G currents by their slower activation, inactivation and deactivation kinetics and more importantly by their slow recovery from short inactivation (see also Satin & Cribbs, 2000). These isotype-specific gating properties lead to distinct channel behaviour during neuronal activity. It should be noted, however, that splice variations can tune the properties of each isotype (Chemin et al. 2001). Nevertheless, the primary electrophysiological characteristics are isotype specific and conserved among species since the properties of the human cloned T-channels are in good agreement with those obsered in rat counterparts (Klöckner et al. 1999).
Activities of cloned T-channels during a single action potential
For T-channels, Ca2+ entry occurs mainly during the repolarization phase of the AP. For this reason, the rate of deactivation kinetics is important in tuning the current decay. Due to their slow deactivation kinetics, α1G and α1H channels produce a sustained current typical of that observed in native neurons using similar protocols (McCobb & Beam, 1991; Scroggs & Fox, 1992; Lambert et al. 1998). In contrast, the fast deactivation kinetics of α1I channels yields more transient currents during a single AP. Channel maximum open probability during an AP is also related to activation kinetics. Indeed, α1I channels that display slow activation kinetics are not fully activated during a single AP. Similarly, the reduced maximum open probability of α1H channels compared to α1G channels is likely to be due to slower activation kinetics since no significant difference exists in their steady-state properties. In contrast with the data obtained using square test pulses, our results indicate that α1G and α1H channels produce large and sustained Ca2+ currents during a single AP, while α1I channels generate small and fast inactivating Ca2+ currents. Reciprocally, activation of distinct T-channel populations may differentially influence the shaping of the AP.
Behaviour of cloned T-channels using cerebellum Purkinje neuron activities
The pacemaker activity occurring in Purkinje neurons of the cerebellum is generated by intrinsic properties of these neurons, which abundantly express T-channels (Gruol & Franklin, 1997; Raman & Bean, 1999a; Talley et al. 1999; Monteil et al. 2000a; Raman et al. 2000). Using neuronal activity of these neurons, we showed that α1G channels produce large and sustained currents during the first interspike intervals that rapidly decrease to a negligible Ca2+ entry during prolonged neuronal activity. This fast decrease of the α1G current could be explained by cumulative inactivation resulting from its slow deactivation and its fast inactivation kinetics (Kozlov et al. 1999; Serrano et al. 1999). The α1H channels are also preferentially recruited during short burst activities but generate persistent current during sustained firing, probably due to their slower inactivation kinetics compared to α1G channels. In contrast, the α1I currents that are tiny and transient during the first interspike interval exhibit apparent facilitation and slow inactivation leading to a sustained Ca2+ entry at the steady state. Facilitation that represents cumulative activation, or current summation, could be explained by the slow activation and inactivation kinetics of α1I currents. It is important to note that during fast burst activities, α1I channels generate a large Ca2+ current ‘rebound’ clearly associated with the DAP. Large current associated with the DAP is highly relevant because it probably favours the triggering of the next AP. The rebound of α1I currents during the DAP could be compared to the ‘resurgent’ sodium current, although it occurs from reopening of previously inactivated sodium channels, that promotes pacemaker activities in Purkinje neurons (Raman & Bean, 1997, 1999a,b).
Our results suggest that cloned α1G channels are not able to generate pacemaker activities in Purkinje neurons. Similar data were obtained in Purkinje neurons in which native T-current rapidly inactivates during the same train of spikes as used here (Raman & Bean, 1999a) suggesting that Purkinje T-currents are generated by α1G channels. Furthermore, these latter authors reported that the block of T-channels by cobalt or mibefradil does not prevent, but only decreases, the firing rate of Purkinje cells. Nevertheless, T-channels could possibly play a role in pacemaker activity after inhibitory synaptic input-induced hyperpolarization of Purkinje neurons (Raman & Bean, 1999a). In this case, deinactivated T-channels would generate a large current during the first interspike intervals and participate in the Ca2+ entry during short burst activity as observed in our α1G experiments.
Contribution of cloned T-channels to thalamic neuron excitability: action potential clamp and modelling studies
In thalamocortical relay (rTC) and thalamic reticular neurons (nRt), firing activity is proposed to be largely due to the interaction of two currents, the hyperpolarization-activated current, Ih, and the T-current (Jahnsen & Llinas, 1984; McCormick & Pape, 1990). T-current is thought to mediate APs and to control the frequency and time course of repetitive firing. The rTC neurons have a resting potential around −75 mV and respond to depolarization by rhythmic activity generated from a membrane potential around −60 mV (Contreras et al. 1993). This phenomenon, called bistability-mediated activity, is related to the T-window current component which maintains the membrane potential near −60 mV (Williams et al. 1997; Hughes et al. 1999). The voltage clamp data obtained using thalamic activity indicates that α1G and α1H channels produce currents that inactivate rapidly, while α1I currents facilitate and can be recorded during the entire stimulus. It should be noted that sustained α1I current, mainly associated with the DAP, persists even during the slower frequency phase of the burst. These results strongly suggest that α1I current is able to participate and to generate pacemaker activity in thalamic neurons.
The influence of each cloned T-channel on firing patterns was estimated in simulation experiments using the NEURON model adapted for rTC and nRt neurons (McCormick & Huguenard, 1992; Destexhe et al. 1996, 1998; Hines & Carnevale, 1997). The modelling experiments are in good agreement with the voltage clamp data. The α1G and α1H currents rapidly inactivate, while α1I currents display facilitation and slowly inactivate during the burst activity. More importantly, modelling experiments demonstrate that α1G currents induce firing activity of short duration while that generated by α1I currents (and to a lesser extent by α1H currents) is significantly more prolonged. Both firing pattern and Ca2+ entry induced by simulated α1G currents are similar to those induced by the native T-currents of rTC cells, while α1I currents better account for the native T-currents of nRt neurons. Interestingly, a recent report describing that α1I channels could be modulated by the γ2 subunit in HEK-293 cells (Green et al. 2001) indicates that further studies should investigate the physiological relevance of such a modulation in nRT neurons.
Concluding remarks
The data presented here allow us to ascribe distinct roles in firing activities to each T-channel isotype and complement well the previous observations made for native T-channels in a variety of neurons. Indeed, the critical role of α1G currents in generating burst firing in rTC neurons has just been demonstrated using mice lacking α1G channels (Kim et al. 2001). Using neuronal activity as waveforms and modelling studies, we illustrate that α1I channel properties are compatible with a role in sustained neuronal firing and in promoting pacemaker activities. The existence of native T-currents that resemble α1I currents is still a matter of debate. Zhuravleva et al. (2001) have reported that ‘slow’ and ‘fast’ components of T-currents can be recorded from thalamic neurons. These data are in good agreement with previous findings from nRt neurons in which slow T-currents participate in the generation of long-duration calcium-dependent spike bursts (Huguenard & Prince, 1992). The behavioural properties of cloned α1I channels described here can account for the T-channel properties in nRt, including long burst firing. Overall, these functional conclusions corroborate well in situ hybridization experiments that indicated that the α1G mRNA is predominantly expressed in the rTC nucleus and in cerebellum, while α1I mRNA is strongly expressed in the nRt nucleus, along with α1H mRNA (Talley et al. 1999). All together, the finding that T-channel isotypes differentially contribute to neuronal excitability strongly suggests that brain region specific expression of these channels plays an important role in the regulation of information processing in the nervous system.
Acknowledgments
This work was supported in part by CNRS, the Association pour la Recherche contre le Cancer (ARC), Association Française contre les myopathies (AFM). We thank M. Mangoni, S. Dubel, P. Fontanaud and V. Chevaleyre for helpful discussions and for critical reading of the manuscript and C. Barrére for help with cell cultures.
REFERENCES
- Armstrong CM, Matteson DR. Two distinct populations of calcium channels in a clonal line of pituitary cells. Science. 1985;227:65–67. doi: 10.1126/science.2578071. [DOI] [PubMed] [Google Scholar]
- Bijlenga P, Liu JH, Espinos E, Haenggeli CA, Fischer-Lougheed J, Bader CR, Bernheim L. T-type alpha 1H Ca2+ channels are involved in Ca2+ signaling during terminal differentiation (fusion) of human myoblasts. Proceedings of the National Academy of Sciences of the USA. 2000;97:7627–7632. doi: 10.1073/pnas.97.13.7627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brideau AD, Banfield BW, Enquist LW. The Us9 gene product of pseudorabies virus, an alphaherpes virus, is a phosphorylated, tail-anchored type II membrane protein. Journal of Virology. 1998;72:4560–4570. doi: 10.1128/jvi.72.6.4560-4570.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carbone E, Lux HD. A low voltage-activated, fully inactivating Ca channel in vertebrate sensory neurones. Nature. 1984;310:501–502. doi: 10.1038/310501a0. [DOI] [PubMed] [Google Scholar]
- Chemin J, Monteil A, Bourinet E, Nargeot J, Lory P. Alternatively spliced alpha1G (CaV3. 1) intracellular loops promote specific T-type Ca2+ channel gating properties. Biophysical Journal. 2001;80:1238–1250. doi: 10.1016/S0006-3495(01)76100-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chemin J, Monteil A, Briquaire C, Richard S, Perez-Reyes E, Nargeot J, Lory P. Overexpression of T-type calcium channels in HEK-293 cells increases intracellular calcium without affecting cellular proliferation. FEBS Letters. 2000;478:166–172. doi: 10.1016/s0014-5793(00)01832-9. [DOI] [PubMed] [Google Scholar]
- Connors BW, Gutnick MJ. Intrinsic firing patterns of diverse neocortical neurons. Trends in Neurosciences. 1990;13:99–104. doi: 10.1016/0166-2236(90)90185-d. [DOI] [PubMed] [Google Scholar]
- Contreras D, Curro-Dossi R, Steriade M. Electrophysiological properties of cat reticular thalamic neurones in vivo. Journal of Physiology. 1993;470:273–294. doi: 10.1113/jphysiol.1993.sp019858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cribbs LL, Lee JH, Yang J, Satin J, Zhang Y, Daud A, Barclay J, Williamson MP, Fox M, Rees M, Perez-Reyes E. Cloning and characterization of alpha1H from human heart, a member of the T-type Ca2+ channel gene family. Circulation Research. 1998;83:103–109. doi: 10.1161/01.res.83.1.103. [DOI] [PubMed] [Google Scholar]
- Destexhe A, Contreras D, Steriade M, Sejnowski TJ, Huguenard JR. In vivo, in vitro, and computational analysis of dendritic calcium currents in thalamic reticular neurons. Journal of Neuroscience. 1996;16:169–185. doi: 10.1523/JNEUROSCI.16-01-00169.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Destexhe A, Neubig M, Ulrich D, Huguenard J. Dendritic low-threshold calcium currents in thalamic relay cells. Journal of Neuroscience. 1998;18:3574–3588. doi: 10.1523/JNEUROSCI.18-10-03574.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green PJ, Warre R, Hayes PD, McNaughton NCL, Medhurst AD, Pangalos M, Duckworth DM, Randall AD. Kinetic modification of the α1I subunit-mediated T-type Ca2+ channel by a human neuronal Ca2+ channel γ subunit. Journal of Physiology. 2001;533:467–478. doi: 10.1111/j.1469-7793.2001.0467a.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruol DL, Franklin CL. Morphological and physiological differentiation of Purkinje neurons in cultures of rat cerebellum. Journal of Neuroscience. 1987;7:1271–1293. doi: 10.1523/JNEUROSCI.07-05-01271.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hines ML, Carnevale NT. The NEURON simulation environment. Neural Computation. 1997;9:1179–1209. doi: 10.1162/neco.1997.9.6.1179. [DOI] [PubMed] [Google Scholar]
- Hughes SW, Cope DW, Toth TI, Williams SR, Crunelli V. All thalamocortical neurones possess a T-type Ca2+ ‘window’ current that enables the expression of bistability-mediated activities. Journal of Physiology. 1999;517:805–815. doi: 10.1111/j.1469-7793.1999.0805s.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huguenard JR. Low-threshold calcium currents in central nervous system neurons. Annual Review of Physiology. 1996;58:329–348. doi: 10.1146/annurev.ph.58.030196.001553. [DOI] [PubMed] [Google Scholar]
- Huguenard JR. Neuronal circuitry of thalamocortical epilepsy and mechanisms of antiabsence drug action. Advances in Neurology. 1999;79:991–999. [PubMed] [Google Scholar]
- Huguenard JR, McCormick DA. Simulation of the currents involved in rhythmic oscillations in thalamic relay neurons. Journal of Neurophysiology. 1992;68:1373–1383. doi: 10.1152/jn.1992.68.4.1373. [DOI] [PubMed] [Google Scholar]
- Huguenard JR, Prince DA. A novel T-type current underlies prolonged Ca2+-dependent burst firing in GABAergic neurons of rat thalamic reticular nucleus. Journal of Neuroscience. 1992;12:3804–3817. doi: 10.1523/JNEUROSCI.12-10-03804.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jahnsen H, Llinas R. Ionic basis for the electro-responsiveness and oscillatory properties of guinea-pig thalamic neurones in vitro. Journal of Physiology. 1984;349:227–247. doi: 10.1113/jphysiol.1984.sp015154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim D, Song I, Keum S, Lee T, Jeong MJ, Kim SS, McEnery MW, Shi HS. Lack of burst firing of thalamocortical relay neurons and resistance to absence seizures in mice lacking a1G T-type Ca2+ channels. Neuron. 2001;31:35–45. doi: 10.1016/s0896-6273(01)00343-9. [DOI] [PubMed] [Google Scholar]
- Klöckner U, Lee JH, Cribbs LL, Daud A, Hescheler J, Pereverzev A, Perez-Reyes E, Schneider T. Comparison of the Ca2+ currents induced by expression of three cloned alpha1 subunits, alpha1G, alpha1H and alpha1I, of low-voltage-activated T-type Ca2+ channels. European Journal of Neuroscience. 1999;11:4171–4178. doi: 10.1046/j.1460-9568.1999.00849.x. [DOI] [PubMed] [Google Scholar]
- Klugbauer N, Marais E, Lacinova L, Hofmann F. A T-type calcium channel from mouse brain. Pflügers Archiv. 1999;437:710–715. doi: 10.1007/s004240050836. [DOI] [PubMed] [Google Scholar]
- Kozlov AS, McKenna F, Lee JH, Cribbs LL, Perez-Reyes E, Feltz A, Lambert RC. Distinct kinetics of cloned T-type Ca2+ channels lead to differential Ca2+ entry and frequency-dependence during mock action potentials. European Journal of Neuroscience. 1999;11:4149–4158. doi: 10.1046/j.1460-9568.1999.00841.x. [DOI] [PubMed] [Google Scholar]
- Lacinova L, Klugbauer N, Hofmann F. Low voltage activated calcium channels: from genes to function. General Physiology and Biophysics. 2000;19:121–136. [PubMed] [Google Scholar]
- Lambert RC, McKenna F, Maulet Y, Talley EM, Bayliss DA, Cribbs LL, Lee JH, Perez-Reyes E, Feltz A. Low-voltage-activated Ca2+ currents are generated by members of the CavT subunit family (alpha1G/H) in rat primary sensory neurons. Journal of Neuroscience. 1998;18:8605–8613. doi: 10.1523/JNEUROSCI.18-21-08605.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JH, Daud AN, Cribbs LL, Lacerda AE, Pereverzev A, Klöackner U, Schneider T, Perez-Reyes E. Cloning and expression of a novel member of the low voltage-activated T-type calcium channel family. Journal of Neuroscience. 1999;19:1912–1921. doi: 10.1523/JNEUROSCI.19-06-01912.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llinas R, Jahnsen H. Electrophysiology of mammalian thalamic neurones in vitro. Nature. 1982;297:406–408. doi: 10.1038/297406a0. [DOI] [PubMed] [Google Scholar]
- Llinas RR. The intrinsic electrophysiological properties of mammalian neurons: insights into central nervous system function. Science. 1988;242:1654–1664. doi: 10.1126/science.3059497. [DOI] [PubMed] [Google Scholar]
- McCobb DP, Beam KG. Action potential waveform voltage-clamp commands reveal striking differences in calcium entry via low and high voltage-activated calcium channels. Neuron. 1991;7:119–127. doi: 10.1016/0896-6273(91)90080-j. [DOI] [PubMed] [Google Scholar]
- McCormick DA, Bal T. Sleep and arousal: thalamocortical mechanisms. Annual Review of Neuroscience. 1997;20:185–215. doi: 10.1146/annurev.neuro.20.1.185. [DOI] [PubMed] [Google Scholar]
- McCormick DA, Huguenard JR. A model of the electrophysiological properties of thalamocortical relay neurons. Journal of Neurophysiology. 1992;68:1384–1400. doi: 10.1152/jn.1992.68.4.1384. [DOI] [PubMed] [Google Scholar]
- McCormick DA, Pape HC. Properties of a hyperpolarization-activated cation current and its role in rhythmic oscillation in thalamic relay neurones. Journal of Physiology. 1990;431:291–318. doi: 10.1113/jphysiol.1990.sp018331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McRory JE, Santi CM, Hamming KSC, Mezeyova J, Sutton KG, Baillie DL, Stea A, Snutch TP. Molecular and functional characterization of a family of rat brain T-type calcium channels. Journal of Biological Chemistry. 2001;276:3999–4011. doi: 10.1074/jbc.M008215200. [DOI] [PubMed] [Google Scholar]
- Monteil A, Chemin J, Bourinet E, Mennessier G, Lory P, Nargeot J. Molecular and functional properties of the human alpha1G subunit that forms T-type calcium channels. Journal of Biological Chemistry. 2000a;275:6090–6100. doi: 10.1074/jbc.275.9.6090. [DOI] [PubMed] [Google Scholar]
- Monteil A, Chemin J, Leuranguer V, Altier C, Mennessier G, Bourinet E, Lory P, Nargeot J. Specific properties of T-type calcium channels generated by the human alpha 1I subunit. Journal of Biological Chemistry. 2000b;275:16530–16535. doi: 10.1074/jbc.C000090200. [DOI] [PubMed] [Google Scholar]
- Nowycky MC, Fox AP, Tsien RW. Three types of neuronal calcium channel with different calcium agonist sensitivity. Nature. 1985;316:440–443. doi: 10.1038/316440a0. [DOI] [PubMed] [Google Scholar]
- Perez-Reyes E, Cribbs LL, Daud A, Lacerda AE, Barclay J, Williamson MP, Fox M, Rees M, Lee JH. Molecular characterization of a neuronal low-voltage-activated T-type calcium channel. Nature. 1998;391:896–900. doi: 10.1038/36110. [DOI] [PubMed] [Google Scholar]
- Raman IM, Bean BP. Resurgent sodium current and action potential formation in dissociated cerebellar Purkinje neurons. Journal of Neuroscience. 1997;17:4517–4126. doi: 10.1523/JNEUROSCI.17-12-04517.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raman IM, Bean BP. Ionic currents underlying spontaneous action potentials in isolated cerebellar Purkinje neurons. Journal of Neuroscience. 1999a;19:1663–1674. doi: 10.1523/JNEUROSCI.19-05-01663.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raman IM, Bean BP. Properties of sodium currents and action potential firing in isolated cerebellar Purkinje neurons. Annals of the New York Academy of Sciences. 1999b;868:93–96. doi: 10.1111/j.1749-6632.1999.tb11279.x. [DOI] [PubMed] [Google Scholar]
- Raman IM, Gustafson AE, Padgett D. Ionic currents and spontaneous firing in neurons isolated from the cerebellar nuclei. Journal of Neuroscience. 2000;20:9004–9016. doi: 10.1523/JNEUROSCI.20-24-09004.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satin J, Cribbs LL. Identification of a T-type Ca2+ channel isoform in murine atrial myocytes (AT-1 cells) Circulation Research. 2000;86:636–642. doi: 10.1161/01.res.86.6.636. [DOI] [PubMed] [Google Scholar]
- Scroggs RS, Fox AP. Multiple Ca2+ currents elicited by action potential waveforms in acutely isolated adult rat dorsal root ganglion neurons. Journal of Neuroscience. 1992;12:1789–1801. doi: 10.1523/JNEUROSCI.12-05-01789.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serrano JR, Perez-Reyes E, Jones SW. State-dependent inactivation of the alpha1G T-type calcium channel. Journal of General Physiology. 1999;114:185–201. doi: 10.1085/jgp.114.2.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steriade M, McCormick DA, Sejnowski TJ. Thalamocortical oscillations in the sleeping and aroused brain. Science. 1993;262:679–685. doi: 10.1126/science.8235588. [DOI] [PubMed] [Google Scholar]
- Talley EM, Cribbs LL, Lee JH, Daud A, Perez-Reyes E, Bayliss DA. Differential distribution of three members of a gene family encoding low voltage-activated (T-type) calcium channels. Journal of Neuroscience. 1999;19:1895–1911. doi: 10.1523/JNEUROSCI.19-06-01895.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsakiridou E, Bertollini L, deCurtis M, Avanzini G, Pape HC. Selective increase in T-type calcium conductance of reticular thalamic neurons in a rat model of absence epilepsy. Journal of Neuroscience. 1995;15:3110–3117. doi: 10.1523/JNEUROSCI.15-04-03110.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams ME, Washburn MS, Hans M, Urrutia A, Brust PF, Prodanovich P, Harpold MM, Stauderman KA. Structure and functional characterization of a novel human low-voltage activated calcium channel. Journal of Neurochemistry. 1999;72:791–799. doi: 10.1046/j.1471-4159.1999.0720791.x. [DOI] [PubMed] [Google Scholar]
- Williams SR, Toth TI, Turner JP, Hughes SW, Crunelli V. The ‘window’ component of the low threshold Ca2+ current produces input signal amplification and bistability in cat and rat thalamocortical neurones. Journal of Physiology. 1997;505:689–705. doi: 10.1111/j.1469-7793.1997.689ba.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhuravleva SO, Kostyuk PG, Shuba YM. Subtypes of low voltage-activated Ca2+ channels in laterodorsal thalamic neurons: possible localization and physiological roles. Pflügers Archiv. 2001;441:832–839. doi: 10.1007/s004240000490. [DOI] [PubMed] [Google Scholar]


