Abstract
Previous reports showed that sympathetic stimulation affects the activity of muscle spindle afferents (MSAs). The aim of the present work is to study the characteristics of sympathetic modulation of MSA response to stretch: (i) on the dynamic and static components of the stretch response, and (ii) on group Ia and II MSAs to evaluate potentially different effects. In anaesthetised rabbits, the peripheral stump of the cervical sympathetic nerve (CSN) was stimulated at 10 impulses s−1 for 45–90 s. The responses of single MSAs to trapezoidal displacement of the mandible were recorded from the mesencephalic trigeminal nucleus. The following characteristic parameters were determined from averaged trapezoidal responses: initial frequency (IF), peak frequency at the end of the ramp (PF), and static index (SI). From these, other parameters were derived: dynamic index (DI = PF - SI), dynamic difference (DD = PF - IF) and static difference (SD = SI - IF). The effects of CSN stimulation were also evaluated during changes in the state of intrafusal muscle fibre contraction induced by succinylcholine and curare. In a population of 124 MSAs, 106 units (85.4 %) were affected by sympathetic stimulation. In general, while changes in resting discharge varied among different units (Ia vs. II) and experimental conditions (curarised vs. non-curarised), ranging from enhancement to strong depression of firing, the amplitude of the response to muscle stretches consistently decreased. This was confirmed and detailed in a quantitative analysis performed on 49 muscle spindle afferents. In both the non-curarised (23 units) and curarised (26 units) condition, stimulation of the CSN reduced the response amplitude in terms of DD and SD, but hardly affected DI. The effects were equally present in both Ia and II units; they were shown to be independent from gamma drive and intrafusal muscle tone and not secondary to muscle hypoxia. Sympathetic action on the resting discharge (IF) was less consistent. In the non-curarised condition, IF decreased in most Ia units, while in II units decreases and increases occurred equally often. In the curarised condition, IF in group II units mostly increased. The results have important functional implications on the control of motor function in a state of ‘high’ sympathetic activity, like excessive stress, as well as in certain pathological conditions such as sympathetically maintained pain.
Traditionally, the muscle spindle has been considered as a muscle receptor measuring muscle length and changes in muscle length, whose behaviour is controlled by the fusimotor innervation. However, a century ago, Ruffini (1898) had already suggested the existence of sympathetic innervation of the muscle spindle, leading to the possibility of an additional sympathetic modulation of muscle spindle afferent discharge. If this held true, it would have far-reaching consequences. First, it would closely link the autonomic and the somatosensory motor systems at the receptor level. Second, it would enable the autonomic system to influence all those functions assigned to muscle spindles, i.e. motor reflex functions, coordination and proprioception. Third, it could expand the understanding and functional interpretation of motor and proprioceptive dysfunction under conditions of diminished or enhanced sympathetic activity, e.g. during excessive stress and certain syndromes such as sympathetically maintained pain (e.g. Schwartzman & Kerrigan, 1990).
Indeed, more recent work on cat hindlimb muscles (Santini & Ibata, 1971; Ballard, 1978; Barker & Saito, 1981) confirmed that sympathetic fibres penetrate into the muscle spindle capsule and that adrenergic terminals are distributed within the capsule lamellar layers, inside the spindle capsule (within the periaxial space, adjacent to sensory endings), as well as in ‘neuroeffective association’ with bag and chain intrafusal muscle fibres in the polar regions, placed apart from intrafusal blood vessels. Very recent work carried out using specific antibodies shows that the noradrenaline co-transmitter neuropeptide Y (NPY) is present in a large number of muscle spindles in human lumbrical muscles (L. E. Thornell, personal communication).
Whilst the morphological data on sympathetic spindle innervation have not been disputed, the mechanisms of sympathetic modulation of muscle spindle afferent discharge and its functional relevance have been. The effects were sometimes considered to be secondary to vasoconstriction (Eldred et al. 1960). By contrast, Hunt (1960) and more recent authors (Francini et al. 1978; Grassi et al. 1993a,b; Matsuo et al. 1995; Grassi et al. 1996) have established that the sympathetic system exerts direct effects on muscle spindles in cat hindlimb as well as in rat and rabbit jaw-elevator muscles. However, Hunt et al. (1982) although allowing for direct sympathetic action showed that the effects in cat hindlimb muscles were small, and thus attributed minor functional relevance to them. More recent work on jaw muscles showed instead that sympathetic activation exerts a powerful depressant action on spindle afferent discharge, and on jaw jerk and tonic vibration reflexes (Grassi et al. 1993A,b; Matsuo et al. 1995; Passatore et al. 1996a). In addition, a preliminary report shows the existence of a powerful sympathetically induced depressant action on spindle afferent activity in the trapezius muscle in cats (Thunberg et al. 2000). However, no systematic attempt has been made so far to investigate the characteristics of sympathetic modulation of the stretch response of muscle spindle afferents (MSAs), as well as the possibility that sympathetic effects may be different on group Ia and II MSAs.
The present work expands previous work on rabbit jaw-closing muscles in specifically addressing the issue of sympathetic effects on changes in static and dynamic sensitivity of MSAs and potentially different effects on group Ia and II MSAs. To this aim, trapezoidal muscle stretches were applied to jaw-closing muscles during stimulation of the cervical sympathetic nerve (CSN), and a number of indices characterising the spindle response in terms of static and dynamic sensitivity were computed (see Taylor et al. 1992a). A preliminary report of these data has been presented (Passatore et al. 1997).
METHODS
All experimental procedures were performed in accordance with the regulations for the welfare of animals and the approval of the local Ethical Committee.
The experiments were performed on 22 rabbits (weight 3–4 kg), anaesthetised with urethane (dose 1.2 mg kg−1i.v.). Full surgical anaesthesia was maintained by injecting additional doses of urethane (0.4 mg kg−1), when needed, through a cannulated femoral vein. The CSN was dissected free from the surrounding tissue and the depressor nerve, cut low in the neck, and its peripheral stump was placed in a cylindrical cuff containing the stimulation electrodes (see Grassi et al. 1993b). The effectiveness of sympathetic stimulation was evaluated by monitoring blood flow changes in muscle-cutaneous tissues of the cheek through an inductive proximity sensor (Selet Sensor Mod. 18/5 OC, Turin, Italy) working as a ‘surface plethysmograph’ (Roatta et al. 1996). The skull was fixed in a stereotaxic frame through screws implanted in the frontal bone, and craniotomy was performed to enable recording of MSA activity from the mesencephalic trigeminal nucleus (MTN). Respiratory movements were monitored through an additional inductive proximity sensor detecting the displacement of a target placed on the thoracic wall. Blood pressure and rectal temperature were monitored, and the latter was maintained at 38 °C through an electric blanket regulated by feedback from a rectal probe (Harvard, England). During the course of the experiment, most of the animals were injected with a neuromuscular blocking agent (tubocurarine, Wellcome, 0.3 mg kg−1i.v.) and artificially ventilated so as to maintain the end-tidal CO2 concentration at the pre-curarisation level measured by ossicapnometer (Engstrom Eliza Duo, Sweden). Therefore, MSA responses to sympathetic stimulation were collected in both non-curarised and curarised conditions. In five non-curarised animals succinylcholine (200–400 mg kg−1, i.v.) was administered with the aim of increasing the resting discharge and stretch sensitivity of spindle afferents. In these trials great care was taken in the transition between the assisted ventilation, utilised while the drug was effective, and the spontaneous respiratory regime, which is particularly critical in rabbits. In fact, in rabbits - at variance with other animal species - hyperventilation does not stop spontaneous respiratory efforts (M. Passatore, unpublished observations), the imminent danger being the possibility of inducing disturbances in pulmonary circulation with a risk of developing pulmonary oedema if the rabbit is allowed to even briefly breathe ‘against the pump’. For this reason, succinylcholine (SCh) injection was performed only once in the same animal. To check whether neuromuscular blockade by SCh had occurred, the response to stimulation of the tibialis nerve was monitored on the extensor movements of the paw.
Tungsten microelectrodes (3–5 MΩ) were used to record from single MSAs in the mesencephalic trigeminal nucleus. This nucleus contains the cell bodies of two types of neurons, i.e. muscle spindle first-order afferents of ipsilateral jaw-closing muscles and mechanoreceptor afferents of ipsilateral maxillary and mandibular teeth, while there is no evidence for representation of temporomandibular joint receptors or of tendon organs innervating jaw muscles and stretch receptors located in extra-ocular muscles (Manni et al. 1966; Cody et al. 1972; Passatore et al. 1983). Afferent units were identified as MSAs on the basis of their response to gentle surface pressure applied with a rod on different areas of the masseter and temporalis muscles and their response to other mechanical stimuli. These stimuli consisted of high-frequency vibrations and trapezoidal stretches delivered through a servo-controlled puller (Cambridge Technology Inc., MA, USA) connected with a screw to the mandibular symphysis. The puller transmits a rotary movement to the mandible through an 84 mm arm connected with a screw to the mandibular symphysis, this arm length closely approximating the rotation radius of the mandibular symphysis with respect to the mandibular joint in rabbits weighing 3–4 kg. The mechanical stimuli were programmed on a PC that delivered to the puller the analog signal, corresponding to desired angular displacement, through a digital-to-analog converter. The puller's internal transducers in turn provided analog signals indicating actual angular displacement and resisting torque of the mandible, which were first converted to length displacement and resisting force at the level of the symphysis and then recorded on magnetic tape along with MSA afferent activity. Due to the complex anatomical configuration of the jaw elevator muscles, different parts of them undergo different magnitudes of lengthening during a given movement of the mandible. Keeping the amplitude of stretch constant would therefore not produce comparable stretches to all spindles. For this reason, the amplitudes of both trapezoidal stimuli and vibrations were adjusted for each recorded unit in a range of 1–3 mm and of 5–60 mm peak-to-peak, respectively, these amplitudes indicating the displacement of the mandible at the symphysis level. In different trials trapezoidal stretches were used with a stretch velocity ranging between 5 and 20 mm s−1, a plateau lasting 5 s and the cycle repeated every 15 s. Only trials in which a stretch velocity of 10 mm s−1 (rise time of 100–300 ms, depending on the amplitude) was used were later selected for the analysis of the parameters characterising the response to stretch.
The distinction between primary and secondary muscle spindle afferents was based on their variability in baseline discharge and their pattern of response to trapezoidal stretch and vibration at 170 Hz delivered to the mandible (Matthews, 1972; Luschei & Goldberg, 1981). In particular the following aspects were considered to qualify the unit as primary: (i) high variability in the resting discharge, (ii) high dynamic sensitivity, (iii) abrupt silencing of the discharge during passive shortening, and (iv) ability to be ‘driven’ by vibration. Secondary afferents essentially lack these properties.
Conduction velocity measurements were not considered reliable because of the short conduction distance and the lack of bimodality in the fibre diameter spectrum (Smith et al. 1968; Inoue et al. 1981; Morimoto et al. 1982). Succinylcholine was not systematically used to identify the muscle spindle afferents due to constraints of its application in rabbits as detailed above. The peripheral stump of the CSN was stimulated at 10 impulses s−1 for 60–90 s, using rectangular electrical pulses (6–10 V, 0.5 ms). Occasionally, stimulus frequencies of 3 and 5 impulses s−1 and stimulation lasting up to 4 min were tested.
In order to enable the recovery of the CSN and the restoration of control MSA activity after a period of stimulation, an interval of at least 20 min was allowed before the electrical stimulation was repeated.
MSA discharge was recorded throughout CSN stimulation, continuously displayed on a polygraph (Gould Electronique, ES 1000, France) and stored on magnetic tape for further off-line analysis. A window discriminator (WPI mod. 121, New Haven, CT, USA) was used to convert single MSA spikes into digital pulses that were then fed both into an integrator with a time constant of 100–500 ms (Digitimer Ltd, NL 703, UK) to be recorded on the polygraph and to a digital acquisition board (AT-DIO-32F, National Instruments, TX, USA) installed on a PC. The digital signal was used to trigger the digital acquisition from a 24 bit, 10 kHz timer. Processing software developed under the LABView (National Instruments) programming environment was used to compute the instantaneous discharge frequency of the recorded MSA and for further processing.
The acquisition of a digital trigger synchronous with the beginning of trapezoidal stimulus allowed for precise identification and averaging of the afferent responses. For each trial the effects of sympathetic stimulation were evaluated from the comparison of the average responses obtained in the pre-stimulation period (control) and the stimulation period. Averages were computed from two to six successive ramp responses in the form of cycle histograms (see Fig. 2, right side) with a bin width of 25 or 50 ms. The criterion for selecting the sequence of responses to be averaged during sympathetic stimulation was the following. They were selected, when possible, among those exhibiting the most stable effect (e.g. see Fig. 2). Whenever the unit fell silent and showed only small or no responses to muscle stretch, averaging was performed of the stretch responses preceding that phase. While this procedure does not capture the maximal effect of sympathetic stimulation, it allows the changes in the different parameters characterising the response of MSAs to muscle length changes to be traced.
Figure 2. Typical examples of the effect of sympathetic stimulation at 10 impulses s−1 on a group Ia (A) and a group II (B) MSA located in the masseter muscle, while trapezoidal stretches were delivered by lowering the mandible.
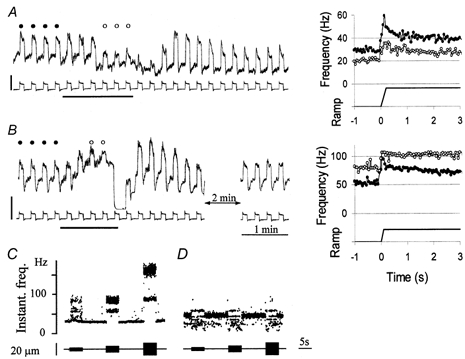
Note that different amplitudes of stretch were chosen for the two units, larger for the Ia unit innervating a spindle located in a posterior portion of the masseter muscle, smaller for the group II unit innervating a spindle located in the very anterior portion of the muscle. The left side shows records of afferent activity (upper traces in A and B), integrated with a 200 ms time constant, and the tension signal detected by the puller (lower traces in A and B; calibration: 200 g), providing a stimulus reference. The heavy horizontal bars indicate CSN stimulation. On the right side are shown averages of MSA responses to a number of consecutive trapezoidal stretches, selected before (control: •) and during sympathetic stimulation (○); the traces below are the corresponding length signals: stretch rate 10 mm s−1, amplitude 2 mm for A, 1 mm for B. The stretch responses selected for averaging are marked with the same symbols on the left-hand traces. The parameters characterising the muscle spindle responses were determined from the averages on the right. C and D, responses to 170 Hz vibration at different amplitudes of the same Ia and II units shown in A and B, respectively.
Selected parameters characterising the average ramp response were computed as follows (see also Fig. 1 and Fig. 2 and Taylor et al. 1992b): (i) initial frequency (IF; spontaneous discharge frequency averaged from the 0.5 s preceding the ramp stretch); (ii) peak frequency (PF; maximum frequency at the end of the ramp stretch); (iii) static index (SI; normally defined as the frequency at 0.5 s after the end of the ramp stretch, and here computed as the average firing frequency in the interval 0.5–1 s after the end of the ramp stretch, to increase stability of the estimate); (iv) dynamic index (DI = PF-SI); (v) dynamic difference (DD = PF-IF); (vi) static difference (SD = SI-IF). The changes in the different parameters from control to sympathetic stimulation were estimated for each MSA unit. Average changes were then computed separately for group Ia and II afferents, and for curarised and non-curarised groups. The significance of these changes was evaluated by means of Student's paired t test (two tailed), the significance level being set at P = 0.05.
Figure 1. Scheme illustrating the parameters describing spindle afferent discharge during ramp stretch, as formalised by Taylor et al. (1992b).

The muscle stretch signal is represented by the fine line on top, while the heavy line below reproduces a theoretical averaged instantaneous frequency curve. The descriptive parameters are: initial frequency (IF), dynamic difference (DD), dynamic index (DI), static difference (SD), static index (SI) and peak frequency (PF). Note that both IF and SI are based on average firing rates within 0.5 s intervals (see text).
In order to make explicit the distribution of the results, the effects were additionally classified descriptively as increase (I), decrease (D) or unaffected (U, if changes were below 5 % of the control value).
In three rabbits, the carotid glomus was bilaterally denervated by sectioning the Hering nerve. In five rabbits, it was checked whether the actions induced by sympathetic stimulation on MSA discharge could be secondary to hypoxaemia due to concomitant vasoconstriction. To this end, the common carotid artery was occluded unilaterally or bilaterally, and CSN stimulation was tested during occlusion.
At the end of the experiments, all animals were killed with an i.v. injection of a lethal dose (2 g kg−1) of urethane.
RESULTS
A total of 124 muscle spindle afferents innervating the masseter and temporalis muscles were evaluated as to whether, upon sympathetic stimulation, they exhibited changes in resting discharge and/or in the amplitude of modulation in the discharge frequency during trapezoidal stretches. Only units that displayed a significant change (> 5 %) in at least one of these parameters were considered ‘responsive’ to sympathetic stimulation, leaving 106 units (85.4 %). The analysis of the effects of the sympathetic stimulation on the different parameters characterising the stretch response (IF, DD, SD, DI, see Methods) was performed on 49 of these units. The other units were excluded because of lower recording quality or different stretch velocities employed or unreliable identification of MSA type. However, recordings from these units qualitatively supported the more rigorous analysis. In all trials, the effectiveness of sympathetic stimulation was routinely checked by evaluating the vasomotor response in muscle and cutaneous tissues of the cheek through an inductive proximity sensor (see Methods). Trials in which the vasomotor response appeared clearly weakened were not considered. Nevertheless, the percentage of responsive units was probably underestimated since it cannot be excluded that sympathetic nerves could have been partially damaged on some occasions. All units were classified according to the criteria described in Methods as either primary (group Ia) or secondary (group II) spindle afferents. However, a few of them did not comply with all the listed criteria, in which case their response to vibration was used as a discriminator. The data were collected from both anaesthetised non-curarised preparations and anaesthetised curarised preparations and were then grouped into four distinct sets: Ia and II non-curarised, Ia and II curarised. In addition, the effect of sympathetic stimulation on five units was tested concomitantly with injection of succinylcholine (SCh), a depolarising blocker of neuromuscular transmission producing transient excitation of intrafusal fibres.
In general, sympathetic stimulation at 10 impulses s−1 lasting 60–90 s induced changes in resting discharge, which varied among different groups of units and experimental conditions, ranging from enhancement to strong depression of firing, which in some cases ceased. The amplitude of the response to muscle length changes exhibited a rather consistent decrease in all groups (Fig. 2A and B). This depressant effect appeared within 10–45 s from the stimulus onset and outlasted the stimulation, its strength and duration being dependent on the duration of the stimulation. Both resting discharge and response amplitude returned to the pre-stimulation levels at times ranging between 30 s and 4 min, a rather constant feature being a transient rebound increase of the response amplitude just after the end of sympathetic stimulation (Fig. 2). The above effects were often preceded by a transient increase in both basal discharge rate and response amplitude. This increase occurred with a few seconds latency from the beginning of the stimulation and varied in magnitude and duration among different trials, its duration ranging between 5 and 30 s. Since this effect was always paralleled by a modest respiratory activation, i.e. a small increase in tidal volume occasionally accompanied by an increase in respiratory frequency, we suspected that this transient excitatory phase could be secondary to the concomitant sympathetically induced activation of chemoreceptors located in the carotid glomi. In order to support this interpretation, experiments were performed on three animals in which the carotid glomi had been bilaterally denervated. In these experiments, CSN stimulation produced neither respiratory activation nor the initial transient increase in basal discharge and amplitude of response to muscle stretch. Therefore, this initial portion of the sympathetic effect was not considered a direct action on muscle spindles and was not subjected to the routine analysis. Thus the samples to be analysed were usually collected starting 30 s after the onset of stimulation, for the following minute.
Effects of sympathetic stimulation on group Ia and II MSAs in non-curarised preparations
Figure 2 shows typical examples of the action exerted by CSN stimulation at 10 impulses s−1 on the responses of group Ia (A) and II (B) MSAs to trapezoidal stretches. CSN stimulation induced in the Ia unit (Fig. 2A) a transient increase, followed by a decrease in both basal discharge rate and response to muscle length changes. The amplitude of the stretch response progressively decreased through the second half of the stimulation and outlasted it. Both parameters returned to the pre-stimulation levels about 2 to 3 min after the end of stimulation. Quantitative analysis, comparing stretch responses before and during sympathetic stimulation, is shown on the right side of Fig. 2A. Averages of four individual pre-stimulation stretch responses (labelled by filled circles above the stretch responses on the left) are given as the line of filled circles on the right, and averages of three individual responses during stimulation (labelled by open circles above the responses on the left) are given as the line of open circles on the right. The resting discharge (IF) decreased by 28 % and the stretch response decreased in terms of reductions in DD (52 %), SD (41 %), and DI (60 %). In the group II afferent (Fig. 2B), CSN stimulation induced a reduction in stretch amplitude similar to that in the group Ia unit, as shown by a decrease in DD (38 %), SD (10 %) and DI (75 %). This indicates a clear-cut reduction in both dynamic and static sensitivity to stretch. At variance with the Ia unit, the IF of the group II unit exhibited a considerable increase (44 %).
Table 1 summarises the effects of 10 impulses s−1 CSN stimulation on the resting discharge (IF) and the other parameters in both group Ia and II units. The stimulation induced a decrease in the resting discharge (IF) of 71 % of the Ia units, while decreases and increases were about equally represented in group II units. DD decreased in 64 % of the group Ia units and in almost 90 % of the group II units (see the row ‘Distribution of effects’ in Table 1). SD, too, consistently decreased in both groups. DI often decreased during sympathetic stimulation. However, this effect did not reach significance, either in group Ia or in group II units. Hence, sympathetic stimulation clearly reduced the stretch sensitivity of both group Ia and II units. In particular, the static sensitivity was reduced, while the velocity sensitivity as measured by DI was, on average, less affected.
Table 1.
Summary of the effect of sympathetic stimulation on la and ll muscle spindle afferent discharge during trapezoidal stretches, in non-curarised and curarised rabbits
| Non-curarised | Curarised | |||||
|---|---|---|---|---|---|---|
| Ia (n = 14) | II (n = 9) | Ia (n = 11) | II (n = 15) | |||
| IF | Control (±s.d.) (Hz) | 22.8 ± 18.3 | 42.2 ± 13.6 | 1.0 ± 2.7 | 36.1 ± 15.9 | |
| Mean change (±s.d.) (Hz) | −7.5 ± 10.8* | −4.8 ± 23.3 | 9.1 ± 25.2 | 12.7 ± 12.9** | ||
| % change of control | −32.9 | −11.4 | 920 | 35.2 | ||
| Distribution of effects | U | 1 | 1 | 8 | 4 | |
| I | 3 | 4 | 2 | 10 | ||
| D | 10 | 4 | 1 | 1 | ||
| DD | Control (±s.d.) (Hz) | 98.4 ± 45.0 | 50.3 ± 18.5 | 74.8 ± 19.8 | 35.3 ± 13.7 | |
| Mean change (±s.d.) (Hz) | −16.2 ± 16.2** | −12.4 ± 7.9** | −14.5 ± 22.0* | −8.2 ± 9.5** | ||
| % change of control | −16.5 | −24.6 | −19.4 | −23.2 | ||
| Distribution of effects | U | 5 | 1 | 6 | 3 | |
| I | 0 | 0 | 0 | 1 | ||
| D | 9 | 8 | 5 | 9 | ||
| SD | Control (±s.d.) (Hz) | 35.8 ± 15.7 | 35.8 ± 21.2 | 33.6 ± 10.9 | 17.5 ± 8.1 | |
| Mean change (±s.d.) (Hz) | −12.6 ± 11.7** | −10.5 ± 11.5* | −12.5 ± 16.4* | −3.7 ± 5.5* | ||
| % change of control | −35.2 | −29.3 | −37.2 | −21.1 | ||
| DI | Control (±s.d.) (Hz) | 62.6 ± 40.9 | 14.5 ± 12.4 | 41.3 ± 18.3 | 17.8 ± 6.6 | |
| Mean change (±s.d.) (Hz) | −3.6 ± 12.8 | −1.9 ± 8.1 | −2.1 ± 18.6 | −4.5 ± 5.6** | ||
| % change of control | −5.7 | −13.1 | −5.1 | −25.3 | ||
IF, initial frequency; DD, dynamic difference; SD, static difference, DI, dynamic index. For IF and DD, observed distribution of effects is indicated by the number of units which exhibited increase (I), decrease (D) or remained unchanged (U, if vaaariability is below 5 %)
P < 0.01
P < 0.05.
The responses of MSAs to lower-frequency sympathetic stimulation were tested occasionally. Stimulation at 5 impulses s−1 proved effective in most cases, even though to a lesser degree than the routinely applied stimulation at 10 impulses s−1 (Fig. 3). Stimulation at 3 impulses s−1 often produced a small effect. In some of these trials, the low-frequency stimulation exclusively reduced the amplitude of the response to muscle stretch while leaving the resting discharge unaltered.
Figure 3. Two comparisons of the effects of CSN stimulation at 10 and 5 impulses s−1 on the responses of a group Ia (A and B) and a group II (C and D) spindle afferent to trapezoidal muscle stretches.
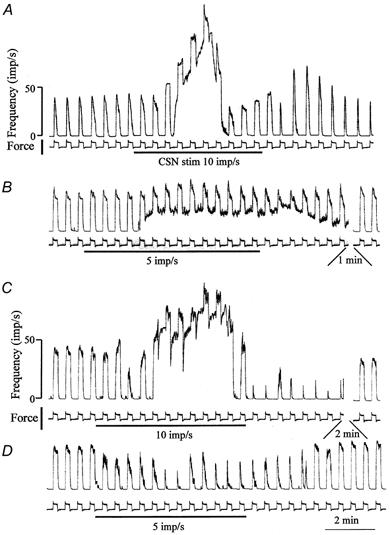
Time constant used for integration: 500 ms; CSN stimulation signalled by horizontal bars; force calibration: 200 g. Stretch amplitude: 2 mm for A and B and 1 mm for C and D.
Effects of sympathetic stimulation on group Ia and II MSAs in curarised preparations
Curare is known to abolish resting discharge in primary afferents (Matthews, 1972). The instantaneous frequency of discharge was followed in a number of Ia units while the effect of curare was taking place and, indeed, disappearance of the resting discharge (IF = 0) was observed, possibly accompanied by a disappearance of the static response to the trapezoidal stimulus (SD = 0). All Ia afferents collected in the curarised preparation lacked a resting discharge (but those that also exhibited SD = 0 were excluded from the analysis). Therefore, SD and DD should be considered with care since the lack of a reference level IF makes these parameters less accurate indicators of receptor sensitivity and makes them sensitive to variation in bias as well.
The effect of CSN stimulation at 10 impulses s−1 on group Ia and II MSAs in curarised preparations is summarised in Table 1. Except for two units exhibiting an increase in IF, the majority of Ia afferents remained silent in resting conditions during sympathetic stimulation, while the effect of stimulation on the stretch response was, on average, still present, as evidenced by the significant decrease in DD and SD values (19 and 37 %, respectively). These effects are compatible with those observed in the non-curarised group. In most group II units, sympathetic stimulation induced a significant increase in IF (+35 %) and a decrease in stretch sensitivity, as evidenced by significant decreases in DD, SD and DI.
In summary, the depressant action exerted by sympathetic activation on muscle spindle stretch sensitivity is not modified by curarisation, while the occurrence of increases in the resting discharge of group II units is higher in curarised (66 % of units) than in non-curarised preparations (44 % of units).
Effects of sympathetic stimulation on group Ia and II MSAs under succinylcholine
Succinylcholine (SCh) is known to induce a transient excitation of muscle spindles, ascribed to contracture of bag1 and bag2 intrafusal muscle fibres, which increases the resting discharge and stretch sensitivity of spindle afferents (Gladden, 1976; Boyd, 1985; Taylor et al. 1992b; Proske & Carr, 1995). Five MSAs were recorded during concomitant injection of SCh and sympathetic stimulation. We focused on group Ia afferents to check how the opposite effects produced by these two stimuli combine in the same unit. Figure 4 shows an example. Figure 4A displays the usual depressant action of sympathetic stimulation on spindle discharge. Figure 4B shows the usual excitation of the spindle afferent, with increases in all computed parameters, IF, DD, SD, DI. CSN stimulation during the maximum effect of SCh induced a depressant action (Fig. 4C) which was very similar to that observed during sympathetic stimulation alone (Fig. 4A). Hence the sympathetic depressant action clearly predominated, rather than summing algebraically with the SCh effect.
Figure 4. Effects of sympathetic stimulation alone (A), of SCh injection alone (B) and of combined SCh injection and sympathetic stimulation (C) on the discharge of a Ia MSA located in the masseter muscle.
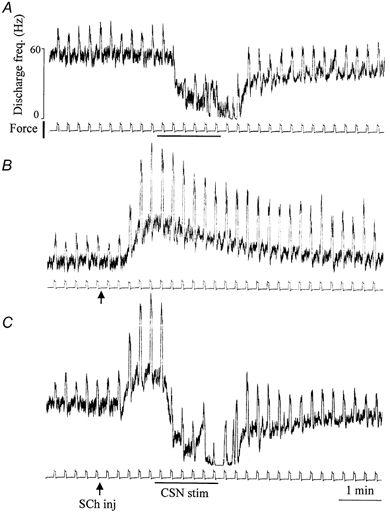
Below each trial is the tension signal detected by the puller (calibration: 200 g); stretch amplitude: 2 mm. Sympathetic stimulation at 10 impulses s−1, lasting 90 s, is marked by horizontal bars. SCh administration 200 μg kg−1i.v. is marked by the arrows. Time constant used for integration: 500 ms.
Tests for vasomotor effects
We checked in three rabbits whether the above described effects of CSN stimulation could be secondary to changes in muscle blood flow. To this end, we recorded the discharge from 12 MSAs, five group Ia and seven group II, while unilateral or bilateral occlusions of the common carotid artery were performed, to induce a large sudden reduction or abolition of blood flow in jaw muscles. In 11 out of 12 units recorded from the ipsilateral MTN, unilateral occlusion lasting up to 6 min did not induce any depression in the discharge. In one unit, the afferent discharge exhibited frequency fluctuations during the occlusion, which, however, were very different from the strong decrease in discharge induced by CSN stimulation on the same unit. To block any possible blood flow compensation from the contralateral side, the effect of bilateral common carotid artery occlusion lasting 3 min was tested on the discharge of four MSAs. It proved ineffective in all of them.
On six units, CSN stimulation lasting 90 s was performed during hypoxia induced by common carotid occlusion. The effects of two sympathetic stimulations were compared on the same MSA, one performed before and the other 2 min after starting a 6–8 min unilateral common carotid artery occlusion. The two stimulations produced very similar effects, i.e. neither the magnitude nor the time course of the effects of sympathetic stimulation was any different. In particular, the MSA discharge returned to control values after the end of sympathetic stimulation with a similar time course in both conditions, i.e. also in the trial in which the artery was still occluded.
DISCUSSION
The present study addressed the effects of sympathetic stimulation on the activity of rabbit jaw-elevator muscle spindles in terms of their resting discharge and responses to trapezoidal stretches.
In non-curarised rabbits, sympathetic stimulation consistently reduced or completely suppressed the response of MSA to muscle length changes in a high percentage of units, as expressed in a decrease in DD and SD, while DI changed little. These effects were present in both group Ia and II units. Unlike the sympathetic action on the stretch response, the influence exerted on the resting discharge was not consistent. Decreases were induced in most group Ia units, while increases and decreases were equally represented in group II units. When resting discharge and stretch sensitivity were increased by SCh injection, sympathetic stimulation still had a powerful depressant action, overcoming the SCh-induced excitation.
Effectiveness of sympathetic stimulation
In the present study, sympathetic stimulation proved effective on 87 % of the MSAs tested. To our knowledge, the only previous studies that attempted to estimate the proportion of sympathetically innervated muscle spindles have been performed by Barker's and Matsuo's groups. Barker & Saito (1981) showed that the proportion of spindles receiving autonomic innervation ranged from 65 % in lumbrical muscles to 8 % in peroneus brevis muscle. In particular, only 30 % of the spindles in deep masseter muscle of rats were reported to receive adrenergic innervation (Barker & Saed, 1987). Our data on rabbit jaw elevator muscles indicate a considerably higher percentage of spindles responding to sympathetic stimulation, and match fairly well with the data of morphological studies by Matsuo et al. (1995) who reported 68 % of sympathetically innervated spindles in the rat masseter muscle. But such comparisons must be made with caution because the results may differ because of different methods, species and muscle differences.
The results of this work are in accordance with the results of previous studies in which the action of sympathetic stimulation was tested in rabbits on jaw jerk and tonic vibration reflex in jaw elevator muscles, evaluated on the basis of EMG activity and developed tension (Grassi et al. 1993a,b). In these studies, sympathetic stimulation was reported to induce, after a transient increase in the reflex, a powerful depression lasting throughout the stimulation. These effects parallel in sign and time course the corresponding changes in stretch sensitivity observed in the behaviour of the MSA units in this experimental series. Matsuo et al. (1995) reported in rats a strong depression in the discharge rate of unidentified spindle afferents, as well as in jaw jerk reflex, during sympathetic stimulation.
At apparent variance with the present results are previous findings of consistent increases in the basal discharge of unidentified MSAs (Passatore et al. 1985). However, this study was performed on curarised rabbits, and therefore it is highly probable that mainly group II units were selected which in this condition exhibit spontaneous discharge, while the majority of group Ia units are silent. Correspondingly, in the experiments performed on curarised animals in the present experimental series, the large majority of spindle afferents exhibiting spontaneous discharge belonged to group II, most of which responded to sympathetic stimulation with an increase in resting discharge.
The relevance of the effects observed depends of course on the extent to which the stimulation frequency adopted is representative of physiological values, i.e. those occurring under conditions of physical or emotional stress. In cats, sympathetic excitation, at least for vasomotor fibres to skeletal muscles, as indirectly assessed during graded exercise and superimposed graded sympathetic nerve activation, has been reported as being 2, 6 and somewhat above 10 impulses s−1 during ‘light’, ‘moderate’ and ‘heavy’ exercise, respectively (Folkow, 1951; Maspers et al. 1991). In the present paper, sympathetic effects were obtained with 10 impulses s−1 stimulation, while 5 and occasionally 3 impulses s−1 stimulation was also effective, although to a lesser degree. This suggests that the sympathetic system may modulate MSA activity under physiological conditions.
Possible sites of action
There has been no general agreement in the past on whether sympathetically induced changes in MSA activity are independent of concomitant blood flow changes in skeletal muscles. However, some old and more recent studies support the view that they are (Francini et al. 1978; Grassi et al. 1993a,b; Matsuo et al. 1995), on the basis of data obtained in experiments specifically designed to clarify this matter. The present work shows that prolonged muscle hypoxia does not change the MSA discharge nor the magnitude and time course of the depressant effect of sympathetic stimulation on MSA discharge. The same conclusion was reached by Grassi et al. (1993a,b), who performed similar trials, i.e. combining occlusion of blood supply and sympathetic stimulation, while recording the tension developed by jaw jerks and the tendon vibration reflex (TVR) in jaw elevator muscles of rabbits. Also, in recent work performed on jaw muscles of rats, Matsuo et al. (1995) clearly state that the effects of sympathetic stimulation are not dependent on vasoconstriction. They directly measured the blood flow in both anterior and posterior parts of the masseter muscle and showed that the sympathetically induced effects on muscle spindle afferents, as well as on EMG activity, were very stable in the face of large changes in blood flow obtained with various sympathetic stimulation protocols. Finally, preliminary results from experiments studying the effect of CSN stimulation on MSAs of cat trapezius muscle rule out the involvement of vascular actions in the recorded responses (Hellstrom et al. 2001).
It is also unlikely that the sympathetic action is mediated by changes in blood flow in the mesencephalic areas where the somata of the MSAs are located, since sympathetic stimulation at 10–30 Hz elicited flow reductions below 5 % (Deriu et al. 1996; Passatore et al. 1996b).
Sympathetic actions on muscle spindles could be exerted at various sites: (1) pre-junctional γ-fibre terminals; (2) post-junctional regions of intrafusal muscle fibres; (3) extra-junctional regions of intrafusal fibres; (4) sensory endings of group Ia and II afferents; (5) encoder site.
Sites 1 and 2 appear to be excluded by (i) the consistent reduction in stretch sensitivity, irrespective of the presence and extent of gamma tone; (ii) the increase in resting discharge, particularly of group II afferents, in animals treated with tubocurarine. On the other hand, sites 3 to 5 might all be involved, which would explain the variability of spindle responses to sympathetic inputs. For instance, if sympathetic action depolarised sensory afferents (sites 4 and 5), increases in resting discharge as observed in some units would be explained. Indeed, depolarisations induced by blocking the Na+-K+ pump entail a sequence of increase in discharge followed by silencing very similar to the sequence seen with sympathetic stimulation (Fisher, 2000). If sympathetic action at site 3 depressed the tone of intrafusal muscle fibres, the decreased resting discharge of some units and the decreased stretch sensitivity of all units would be accounted for. In addition, sympathetic actions might be differentiated as to type of intrafusal fibre and/or related receptor terminal. That is, sympathetic stimulation might have different effects on bag1, bag2 and chain fibres and the sensory endings on them. This might account not only for the variability of sympathetic effects on the resting discharge, but also for the different effects on the different stretch response parameters. Finally, some variability could result from different pharmacological dynamics of noradrenaline and its co-transmitter neuropeptide Y (NPY). NPY has been found to be partially responsible for the sympathetically induced reduction of the stretch reflex in rabbit jaw elevator muscles (Grassi et al. 1996) and to be co-released from sympathetic terminals within muscle spindles of human lumbrical muscles (L.-E. Thornell, personal communication). Further studies are needed to clarify the mechanisms of the sympathetic action on MSA sensitivity.
Functional relevance of sympathetic actions on muscle spindles
Signals from muscle spindles contribute to a number of body functions, such as spinal and supraspinal reflexes, control and co-ordination of ongoing movements, perception of position and movement of our body (kinaesthesia), and learning of stereotyped movements and motor skills (e.g. Nashner, 1976; Dietz, 1992; Davidoff; 1992; Gandevia & Burke, 1992; Pearson, 1993; Prochazka, 1996; Masuda et al. 1997). In addition, they contribute to the calibration of the body schema (awareness of the spatial dimensions of one's body) and to the calibration of visual and auditory localisation, in a frame of tight links between the different senses (Lackner & DiZio, 2000). Therefore, any system able to modulate these signals from spindles will affect those functions. In this context we want to stress the possible consequences of an increase in sympathetic activity on kinaesthesia and motor control, at least in those muscles whose spindle afferent discharge has already been proven to be affected by sympathetic stimulation, i.e. jaw and neck muscles in rabbits, rats and cats (Matsuo et al. 1995; Passatore et al. 1996b; Thunberg et al. 2000; Hellstrom et al. 2001).
An important implication of the present results is that an increase in sympathetic outflow depresses the feedback control of muscle length. The variable changes in resting discharge of MSAs should, with the same sign, alter skeleto-motoneuron excitability, which in turn would affect the muscle tone. This alone would affect the stretch reflex in terms of its threshold and gain (Houk & Rymer, 1981). Stretch reflex gain would be expected to be consistently lowered by the decrease in muscle spindle stretch responsiveness elicited by sympathetic activation, and this expectation is supported by reduced jaw jerk and tonic vibration reflexes in jaw elevator muscles under the same conditions (Grassi et al. 1993a,b). This gain reduction may be employed by the motor system in the case of rapidly executed movements to achieve stability and avoid oscillations (e.g. Rack, 1981; Capaday & Stein, 1986, 1987; Prochazka, 1989). Low feedback gain may be useful in a motor condition typically associated with sympathetic activation, such as the fight-or-flight reaction, in which precision and fine control of movements can be transiently sacrificed for stability and reliability of fast running or fighting movements. Otherwise, when motor tasks requiring precision and continuous proprioceptive feedback are performed under conditions of strong excitement and stress, the enhanced sympathetic outflow may detrimentally affect motor performance output and possibly cause inefficient muscle use. Such mechanisms might well underlie some cases of work-related myalgia that frequently develop under various types of psychosocial stress (Rissén et al. 2000; Sjøgaard et al. 2000; Waersted, 2000).
The stretch reflex may also be influenced more indirectly by sympathetic activation. Sensorimotor transmission, and thus also the gain and offset of the stretch reflex, is controlled by several CNS structures at various peripheral, spinal and supraspinal levels, as part of the central motor programme. The modulation of the reflex is aimed at adjusting its operational characteristics to suit a particular motor task or the context in which it is executed, such as prior instructions given to the subject, external perturbations, state of vigilance and emotional factors. The sympathetic action on muscle spindles may be one of the mechanisms involved in adjusting a motor act to the context in which it is executed, such as states of physical and emotional stress. Several reports provide evidence for a reduction in proprioceptive capacity under conditions of muscle fatigue, which is always associated with sympathetic activation (Seals & Victor, 1991). Under fatigue conditions, the position sense (Skinner et al. 1986; Sharpe & Miles, 1993; Carpenter et al. 1998) and the acuity of sense of movements (Pedersen et al. 1999) have been reported to be reduced.
As mentioned at the beginning of this section, muscle spindles are involved in further, more complex functions, and their modulation by sympathetic activity can thus be expected to affect these functions as well. Future work in these fields should address and take account of these influences.
Finally then, influences of the sympathetic system on muscle spindle activity should raise caution against any simplified interpretation of muscle spindle activity in awake, behaving animals and humans. Since in the awake condition sympathetic outflow is highly variable, depending on variable states of arousal and attention, and on emotional conditions, sympathetically induced changes in spindle afferent activity should be expected. This might confound attempts to indirectly infer the pattern of fusimotor outflow from the evaluation of spindle afferents discharge during voluntary or imposed movement. Of course this applies to those muscles whose spindle afferents have so far been shown, or will be in the future, to be significantly affected by sympathetic stimulation.
Acknowledgments
This work was supported by grants from the Italian MURST and from the European Community (CIND grant no. BMH4-CT95–052). Dr Silvestro Roatta gratefully acknowledges the partial support for this work by The Swedish Council for Work Life Research and Inga-Britt and Arne Lundbergs Forskningsstiftelse grants. The authors are indebted to Professor R. E. Poppele for his valuable suggestions for setting up the digital acquisition system. The authors express their gratitude to Mrs Luisella Milano for her valuable help in the surgery.
REFERENCES
- Ballard KJ. Typical sympathetic noradrenergic endings in a muscle spindle of the cat. Journal of Physiology. 1978;285:61–62P. [PubMed] [Google Scholar]
- Barker D, Saito M. Autonomic innervation of receptors and muscle fibres in cat skeletal muscle. Proceedings of the Royal Society B. 1981;212:317–332. doi: 10.1098/rspb.1981.0042. [DOI] [PubMed] [Google Scholar]
- Barker D, Saed H. Adrenergic innervation of rat jaw muscles. Journal of Physiology. 1987;391:114P. [Google Scholar]
- Boyd IA. Intrafusal muscle fibres in the cat and their motor control. In: Barnes WJP, Gladden MH, editors. Feedback and Motor Control in Invertebrates and Vertebrates. London: Croom Helm; 1985. pp. 123–144. [Google Scholar]
- Capaday C, Stein RB. Amplitude modulation of the soleus H-reflex in the human during walking and standing. Journal of Neuroscience. 1986;6:1308–1313. doi: 10.1523/JNEUROSCI.06-05-01308.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capaday C, Stein RB. Difference in the amplitude of the human soleus H reflex during walking and running. Journal of Physiology. 1987;392:513–522. doi: 10.1113/jphysiol.1987.sp016794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpenter JE, Blasier RB, Pellizzon GG. The effects of muscle fatigue on shoulder joint position sense. American Journal of Sports Medicine. 1998;26:262–265. doi: 10.1177/03635465980260021701. [DOI] [PubMed] [Google Scholar]
- Cody FWJ, Lee RWH, Taylor A. A functional analysis of the components of the mesencephalic nucleus of the fifth nerve in the cat. Journal of Physiology. 1972;226:249–261. doi: 10.1113/jphysiol.1972.sp009983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidoff RA. Skeletal muscle tone and the misunderstood stretch reflex. Neurology. 1992;42:951–963. doi: 10.1212/wnl.42.5.951. [DOI] [PubMed] [Google Scholar]
- Deriu F, Roatta S, Grassi C, Urciuoli R, Micieli G, Passatore M. Sympathetically-induced changes in microvascular cerebral blood and in the morphology of its low-frequency waves. Journal of the Autonomic Nervous System. 1996;59:66–74. doi: 10.1016/0165-1838(96)00008-2. [DOI] [PubMed] [Google Scholar]
- Dietz V. Human neuronal control of automatic functional movements: interaction between central programs and afferent input. Physiological Reviews. 1992;72:33–69. doi: 10.1152/physrev.1992.72.1.33. [DOI] [PubMed] [Google Scholar]
- Eldred E, Schnitzlein HN, Buchwald J. Response of muscle spindles to simulation of the sympathetic trunk. Experimental Neurology. 1960;2:13–25. doi: 10.1016/0014-4886(60)90044-3. [DOI] [PubMed] [Google Scholar]
- Fisher M. Effects of chlorobutanol on primary and secondary endings of isolated cat muscle spindles. Brain Research. 2000;854:106–121. doi: 10.1016/s0006-8993(99)02325-2. [DOI] [PubMed] [Google Scholar]
- Folkow B. Impulse frequency in sympathetic vasomotor fibres correlated to the release and elimination of the transmitter. Acta Physiologica Scandinavica. 1952;25:49–76. doi: 10.1111/j.1748-1716.1952.tb00858.x. [DOI] [PubMed] [Google Scholar]
- Francini F, Peruzzi P, Pizza L, Staderini G. Effects of sympathetic catecholamines (adrenaline and noradrenaline) injected intra arterially and of ischemia on the tonic vibration reflex (TVR) of the ankle extensor muscle in decerebrate cat. Bollettino della Società Italiana di Biologia Sperimentale. 1978;54:1357–1359. [PubMed] [Google Scholar]
- Gandevia SC, Burke D. Does the nervous system depend on kinaesthetic information to control natural limb movements? Behavioural Brain Sciences. 1992;15:614–632. [Google Scholar]
- Gladden MH. Structural features relative to the function of intrafusal muscle fibres in the cat. Progress in Brain Research. 1976;44:51–59. doi: 10.1016/S0079-6123(08)60722-0. [DOI] [PubMed] [Google Scholar]
- Grassi C, Deriu F, Artusio E, Passatore M. Modulation of the jaw jerk reflex by the sympathetic nervous system. Archives Italiennes de Biologie. 1993a;131:213–226. [PubMed] [Google Scholar]
- Grassi C, Deriu F, Passatore M. Effect of sympathetic nervous system activation on the tonic vibration reflex in rabbit jaw closing muscles. Journal of Physiology. 1993b;469:601–613. doi: 10.1113/jphysiol.1993.sp019832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grassi C, Deriu F, Roatta S, Santarelli R, Azzena GB, Passatore M. Sympathetic control of skeletal muscle function: possible co-operation between noradrenaline and neuropeptide Y in rabbit jaw muscles. Neuroscience Letters. 1996;212:204–208. doi: 10.1016/0304-3940(96)12835-4. [DOI] [PubMed] [Google Scholar]
- Hellstrom F, Thunberg J, Roatta S, Ljubisavljevic M, Passatore M, Johansson H. XXXIV International Congress of Physiological Sciences (IUPS), Aug. New Zealand: Christchurch; 2001. Discharge responses of muscle spindles to stimulation of the cervical sympathetic nerve in cat neck muscles; pp. 26–31. 2001. [Google Scholar]
- Houk JC, Rymer WZ. Neural control of muscle length and tension. In: Brookhart JM, Mountcastle VB, Brooks VB, Geiger SR, editors. Handbook of Physiology, section 1, The Nervous System, Motor Control. II. Bethesda, MD, USA: American Phsysiological Society; 1981. pp. 257–324. [Google Scholar]
- Hunt CC. The effect of sympathetic stimulation on mammalian muscle spindle. Journal of Physiology. 1960;151:332–341. doi: 10.1113/jphysiol.1960.sp006441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunt CC, Jami L, Laporte Y. Effects of stimulating the lumbar sympathetic trunk on cat hindlimb muscle spindles. Archives Italiennes de Biologie. 1982;120:371–384. [PubMed] [Google Scholar]
- Inoue H, Morimoto T, Kawamura Y. Response characteristics and classification of muscle spindles of the masseter muscle in the cat. Experimental Neurology. 1981;74:548–560. doi: 10.1016/0014-4886(81)90190-4. [DOI] [PubMed] [Google Scholar]
- Lackner JR, DiZio PA. Aspects of body self-calibration. Trends in Cognitive Sciences. 2000;4:279–288. doi: 10.1016/s1364-6613(00)01493-5. [DOI] [PubMed] [Google Scholar]
- Luschei ES, Goldberg LJ. Neural mechanisms of mandibular control: mastication and voluntary biting. In: Brookhart JM, Mountcastle VB, Brooks VB, Geiger SR, editors. Handbook of Physiology, section 1, The Nervous System, Motor Control. II. Bethesda, MD, USA: American Physiological Society; 1981. pp. 1237–1274. [Google Scholar]
- Manni E, Bortolami R, Desole C. Eye muscle proprioception and the semilunar ganglion. Experimental Neurology. 1966;16:226–236. doi: 10.1016/0014-4886(66)90101-4. [DOI] [PubMed] [Google Scholar]
- Maspers M, Ekelund U, Bjornberg J, Mellander S. Protective role of sympathetic nerve activity to exercising skeletal muscle in the regulation of capillary pressure and fluid filtration. Acta Physiologica Scandinavica. 1991;141:351–361. doi: 10.1111/j.1748-1716.1991.tb09091.x. [DOI] [PubMed] [Google Scholar]
- Masuda Y, Morimoto T, Hidaka O, Kato T, Matsuo R, Inoue T, Kobayashi M, Taylor A. Modulation of jaw muscle spindle discharge during mastication in the rabbit. Journal of Neurophysiology. 1997;77:2227–2231. doi: 10.1152/jn.1997.77.4.2227. [DOI] [PubMed] [Google Scholar]
- Matsuo R, Ikehara A, Nokubi T, Morimoto T. Inhibitory effect of sympathetic stimulation on activities of masseter muscle spindles and the jaw jerk reflex in rats. Journal of Physiology. 1995;483:239–250. doi: 10.1113/jphysiol.1995.sp020581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews PBC. Mammalian Muscle Receptors and their Central Actions. London: Edward Arnold; 1972. [Google Scholar]
- Morimoto T, Inoue H, Kawamura Y. Diameter spectra of sensory and motor fibers in nerves to jaw-closing and jaw-opening muscles in the cat. Japanese Journal of Physiology. 1982;32:171–182. doi: 10.2170/jjphysiol.32.171. [DOI] [PubMed] [Google Scholar]
- Nashner LM. Adapting reflexes controlling the human posture. Experimental Brain Research. 1976;26:59–72. doi: 10.1007/BF00235249. [DOI] [PubMed] [Google Scholar]
- Passatore M, Deriu F, Grassi C, Roatta S. A comparative study of changes operated by sympathetic nervous system activation on spindle afferent discharge and on tonic vibration reflex in rabbit jaw muscles. Journal of the Autonomic Nervous System. 1996a;57:163–167. doi: 10.1016/0165-1838(95)00074-7. [DOI] [PubMed] [Google Scholar]
- Passatore M, Deriu F, Roatta S, Grassi C, Micieli G. Effects of cervical sympathetic nerve stimulation on the cerebral microcirculation: possible clinical implications. Acta Neurobiologiae Experimentalis. 1996b;56:117–127. doi: 10.55782/ane-1996-1111. [DOI] [PubMed] [Google Scholar]
- Passatore M, Grassi C, Filippi GM. Sympathetically-induced development of tension in jaw muscles: the possible contraction of intrafusal muscle fibres. Pflügers Archiv. 1985;405:297–304. doi: 10.1007/BF00595681. [DOI] [PubMed] [Google Scholar]
- Passatore M, Lucchi ML, Filippi GM, Manni E, Bortolami R. Localization of neurons innervating masticatory muscle spindle and periodontal receptors in the mesencephalic trigeminal nucleus and their reflex actions. Archives Italiennes de Biologie. 1983;121:117–130. [PubMed] [Google Scholar]
- Passatore M, Roatta S, Deriu F, Grassi C. XXXIII International Congress of Physiological Sciences, St Petersburg. 1997. Sympathetic nervous system activation reduces muscle spindle sensitivity to ramp stretch, in rabbit jaw muscle; p. P70.06. [Google Scholar]
- Pearson KG. Common principles of motor control in vertebrates and invertebrates. Annual Review of Neuroscience. 1993;16:265–297. doi: 10.1146/annurev.ne.16.030193.001405. [DOI] [PubMed] [Google Scholar]
- Pedersen J, Lonn J, Hellstrom F, Djupsjobacka M, Johansson H. Localized muscle fatigue decreases the acuity of the movement sense in the human shoulder. Medicine and Science in Sports and Exercise. 1999;31:1047–1052. doi: 10.1097/00005768-199907000-00019. [DOI] [PubMed] [Google Scholar]
- Prochazka A. Sensorimotor gain control: a basic strategy of motor systems? Progress in Neurobiology. 1989;33:281–307. doi: 10.1016/0301-0082(89)90004-x. [DOI] [PubMed] [Google Scholar]
- Prochazka A. Proprioceptive feedback and movement regulation. In: Rowell L, Sheperd JT, editors. Handbook of Physiology, section 12, Exercise: Regulation and Integration of Multiple Systems. New York: Oxford University Press; 1996. pp. 89–127. [Google Scholar]
- Proske U, Carr RW. The sensitivity of mammalian muscle spindles to neuromuscular blocking agents. In: Taylor A, Gladden MH, Durbaba R, editors. Alpha and Gamma Motor Systems. New York and London: Plenum Press; 1995. pp. 261–266. [Google Scholar]
- Rack PMH. Limitations of somatosensory feedback in control of posture and movement. In: Brookhart JM, Mountcastle VB, Brooks VB, Geiger SR, editors. Handbook of Physiology, section 1, The Nervous System, Motor Control. II. Bethesda, MD, USA: American Physiological Society; 1981. pp. 229–256. [Google Scholar]
- Rissén D, Melin B, Sandsjo L, Dohns I, Lundberg U. Surface EMG and psychophysiological stress reactions in women during repetitive work. European Journal of Applied Physiology. 2000;83:215–222. doi: 10.1007/s004210000281. [DOI] [PubMed] [Google Scholar]
- Roatta S, Deriu F, Artusio E, Passatore M. A simple, non-invasive and inexpensive method for evaluating the displacement of local tissue surfaces: from vascular changes to muscle contraction. Clinical Physiology. 1996;16:83–94. doi: 10.1111/j.1475-097x.1996.tb00558.x. [DOI] [PubMed] [Google Scholar]
- Ruffini A. On the minute anatomy of the neuromuscular spindles of the cat, and on their physiological significance. Journal of Physiology. 1898;23:190–208. doi: 10.1113/jphysiol.1898.sp000723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santini M, Ibata Y. The fine structure of thin unmyelinated axons within muscle spindles. Brain Research. 1971;33:289–302. doi: 10.1016/0006-8993(71)90104-1. [DOI] [PubMed] [Google Scholar]
- Schwartzman RJ, Kerrigan J. The movement disorder of reflex sympathetic dystrophy. Neurology. 1990;40:57–61. doi: 10.1212/wnl.40.1.57. [DOI] [PubMed] [Google Scholar]
- Seals DR, Victor RG. Regulation of muscle sympathetic nerve activity during exercise in humans. Exercise and Sport Sciences Reviews. 1991;19:313–349. [PubMed] [Google Scholar]
- Sharpe MH, Miles TS. Position sense at the elbow after fatiguing contractions. Experimental Brain Research. 1993;94:179–182. doi: 10.1007/BF00230480. [DOI] [PubMed] [Google Scholar]
- Sjogaard G, Lundberg U, Kadefors R. The role of muscle activity and mental load in the development of pain and degenerative processes at the muscle cell level during computer work. European Journal of Applied Physiology. 2000;83:99–105. doi: 10.1007/s004210000285. [DOI] [PubMed] [Google Scholar]
- Skinner HB, Wyatt MP, Hodgdon JA, Conard DW, Barrack RL. Effect of fatigue on joint position sense of the knee. Journal of Orthopedic Research. 1986;4:112–118. doi: 10.1002/jor.1100040115. [DOI] [PubMed] [Google Scholar]
- Smith RD, Marcarian HQ, Niemer WT. Direct projections from the masseteric nerve to the mesencephalic nucleus. Journal of Comparative Neurology. 1968;133:495–502. doi: 10.1002/cne.901330408. [DOI] [PubMed] [Google Scholar]
- Taylor A, Durbaba R, Rodgers JF. The classification of afferents from muscle spindles of the jaw-closing muscles of the cat. Journal of Physiology. 1992a;456:609–628. doi: 10.1113/jphysiol.1992.sp019356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor A, Rodgers JF, Fowle AJ, Durbaba R. The effect of succinylcholine on cat gastrocnemius muscle spindle afferents of different types. Journal of Physiology. 1992b;456:629–644. doi: 10.1113/jphysiol.1992.sp019357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thunberg J, Hellström F, Ljubisavljevic M, Roatta S, Johansson H, Passatore M. Forum of European Neuroscience (FENS), June. UK: Brighton; 2000. The influence of cervical sympathetic nerve stimulation on muscle spindles in dorsal neck muscles in the cat; pp. 24–28. [Google Scholar]
- Waersted M. Human muscle activity related to non-biomechanical factors in the workplace. European Journal of Applied Physiology. 2000;83:151–158. doi: 10.1007/s004210000273. [DOI] [PubMed] [Google Scholar]


