Abstract
Nitric oxide (NO) synthesis by prepositus hypoglossi (PH) neurons is necessary for the normal performance of horizontal eye movements. We have previously shown that unilateral injections of NO synthase (NOS) inhibitors into the PH nucleus of alert cats produce velocity imbalance without alteration of the eye position control, both during spontaneous eye movements and the vestibulo-ocular reflex (VOR). This NO effect is exerted on the dorsal PH neuropil, whose fibres increase their cGMP content when stimulated by NO. In an attempt to determine whether NO acts by modulation of a specific neurotransmission system, we have now compared the oculomotor effects of NOS inhibition with those produced by local blockade of glutamatergic, GABAergic or glycinergic receptors in the PH nucleus of alert cats. Both glutamatergic antagonists used, 2-amino-5-phosphonovaleric acid (APV) and 2,3-dihydro-6-nitro-7-sulphamoyl-benzo quinoxaline (NBQX), induced a nystagmus contralateral to that observed upon NOS inhibition, and caused exponential eye position drift. In contrast, bicuculline and strychnine induced eye velocity alterations similar to those produced by NOS inhibitors, suggesting that NO oculomotor effects were due to facilitation of some inhibitory input to the PH nucleus. To investigate the anatomical location of the putative NO target neurons, the retrograde tracer Fast Blue was injected in one PH nucleus, and the brainstem sections containing Fast Blue-positive neurons were stained with double immunohistochemistry for NO-sensitive cGMP and glutamic acid decarboxylase. GABAergic neurons projecting to the PH nucleus and containing NO-sensitive cGMP were found almost exclusively in the ipsilateral medial vestibular nucleus and marginal zone. The results suggest that the nitrergic PH neurons control their own firing rate by a NO-mediated facilitation of GABAergic afferents from the ipsilateral medial vestibular nucleus. This self-control mechanism could play an important role in the maintenance of the vestibular balance necessary to generate a stable and adequate eye position signal.
Eye movements in the horizontal plane are controlled by the lateral and medial recti muscles that are driven by motoneurons in the abducens and oculomotor nuclei, respectively. Internuclear neurons in the abducens nucleus project to the contralateral oculomotor nucleus and are responsible for conjugate eye movements. Due to this synaptic arrangement, the abducens nucleus is the final output for horizontal eye movements. The discharge of the abducens motoneurons consists of bursts of spikes proportional to the eye velocity for ipsilateral rapid eye movements and tonic discharge rates proportional to the eye position during periods of gaze-holding (Fuchs & Luschei, 1970; Henn & Cohen, 1973; Delgado-García et al. 1986; de la Cruz et al. 1990).
Both abducens nuclei are functionally organized in a push-pull mode and the premotor ocular system follows the same organization. Afferents to the abducens nucleus are arranged as a triple system of reciprocal excitatory and inhibitory inputs (Escudero & Delgado-García, 1988). Ipsilateral excitatory (Kaneko et al. 1981; Strassman et al. 1986a) and contralateral inhibitory (Hikosaka et al. 1978; Yoshida et al. 1982; Strassman et al. 1986b) reticular burst neurons in the pontomedullary reticular formation are the main source of velocity commands for saccadic eye movements and fast phases of the vestibular and optokinetic nystagmus. Ipsilateral inhibitory and contralateral excitatory vestibular neurons (Baker et al. 1969; Hikosaka et al. 1980; McCrea et al. 1980; Berthoz et al. 1989; Escudero et al. 1992) transmit velocity signals during displacements of the head. Finally, the ipsilateral excitatory and contralateral inhibitory prepositus hypoglossi (PH) neurons (Escudero & Delgado-García, 1988; Spencer et al. 1989; Escudero et al. 1992) convey to the abducens neurons eye position signals for different eye movements (López-Barneo et al. 1982; Cheron et al. 1986a, b; Cannon & Robinson, 1987; Cheron & Godaux, 1987; Delgado-García et al. 1989; Escudero et al. 1992; Fukushima et al. 1992; McFarland & Fuchs, 1992; Kaneko, 1997). In accordance with the idea that the generation of position signals requires the mathematical integration of the velocity signals (Robinson, 1968,1975), the PH nucleus receives information from the above-mentioned structures conveying velocity signals to the abducens nucleus, that is, the pontomedullary reticular formation and the vestibular nuclei (McCrea & Baker, 1985).
Previously, we have reported that the PH nucleus contains a large number of neurons which express neuronal nitric oxide synthase (NOS I), and that the physiological production of nitric oxide (NO) in this nucleus is necessary for the correct execution of eye movements in the alert cat (Moreno-López et al. 1996, 1998). Unilateral injections of NOS inhibitors in the PH nucleus induce a nystagmus whose slow phases are linear and directed contralaterally to the injected side. During the vestibulo-ocular reflex (VOR), a velocity imbalance toward the contralateral side appears, without alteration of the gain or phase lead. All these results indicate that NO produced by PH neurons is involved in the processing of pure velocity signals. On the other hand, local administration of NO donors produces velocity imbalances directed to the injected side for both spontaneous and vestibular-induced eye movements, together with alterations of the position signals during spontaneous eye movements. The effects of NO donors can be mimicked by a cell permeable cyclic GMP (cGMP) analogue, suggesting that NO effects in the PH nucleus are mediated by activation of soluble guanylyl cyclase. Anatomical identification of NO-sensitive cGMP-producing structures in the PH nucleus indicated that the target of NO is probably a cGMP immunoreactive (cGMP-ir) neuropil in the dorsal part of the nucleus (Moreno-López et al. 1998).
The aim of the present study was to characterize more precisely the mechanism of action of NO in eye movement control, using two different approaches. First, the oculomotor effects derived from inhibition of NOS activity in the PH nucleus were compared with those produced by the blockade of different neurotransmitter receptors involved in synaptic signalling within this nucleus. Second, the neuronal targets of NO were investigated by injecting a retrograde tracer in the PH nucleus. We have identified NO-sensitive GABAergic neurons located in the medial vestibular nucleus (MVN) and projecting to the ipsilateral PH nucleus, which may be responsible for the physiological effects of NO in the oculomotor system.
METHODS
Subjects
Seven female adult cats (2.5–3.5 kg) of European and Abyssinian strains, obtained from an authorized supplier (Iffa-Credo, L'Arbresle, France), were used as experimental subjects. Acute and chronic experiments were performed in accordance with the European Union directive 609/86/CEE and with Spanish legislation (RD 233/89) on the use of laboratory animals.
Surgical procedures
Five cats were prepared for chronic recording of eye movements and for microinjection of pharmacological substances into the PH nucleus, as previously described (Moreno-López et al. 1996). Briefly, animals were anaesthetized with sodium pentobarbitone (35 mg kg−1i.p.) following a protective injection of atropine (0.5 mg kg−1i.m.) aimed at preventing vagal reflexes. Under aseptic conditions, the cats were implanted bilaterally with Teflon-coated stainless-steel coils (250 μm) sutured to the scleral margin of the eye (Fuchs & Robinson, 1966). In the same surgical act, a 4 × 4 mm hole was drilled through the occipital bone to allow access to the posterior brainstem via the cerebellum. Bipolar silver stimulating electrodes were implanted bilaterally on the VIth nerve at its exit from the brainstem (stereotaxic coordinates L = 3.5 and P = 1, according to Berman, 1968). The final location of the stimulating electrode was adjusted to evoke the maximum abducting eye movement with the minimum electrical stimulation (50 μs, cathodic square pulses of < 0.1 mA of current intensity). A head-holding system, consisting of three bolts cemented to the skull perpendicular to the stereotaxic plane, was also implanted. Eye coils and stimulating electrodes were connected to a socket attached to the holding system. Field potential and unitary activity were recorded with glass micropipettes of 2–6 MΩ of electrode resistance. Further details of this type of chronic preparation have been reported elsewhere (Delgado-García et al. 1986; Escudero et al. 1992). The animals received post-operative systemic treatment with antibiotics, and anti-inflammatory and analgesic drugs. During the complete experimental period, antibiotics, local anesthetics and corticoids were topically applied to the eyes and the cranial window.
Physiological experiments
One to two weeks later, when there was total recovery from surgery, experiments were carried out in the alert cat once every 2–4 days, for 2–3 h per day, for a maximum of 4–8 weeks. During the experimental sessions, the animal was lightly restrained by elastic bandages and the head was fixed (21 deg nose down) to the recording table by means of the head-holding system. Under these conditions, the heart and respiratory rates were not different from those recorded when holding the animals in a neutral situation, indicating that they did not suffer any stress or discomfort.
A glass micropipette was advanced through the cerebellum toward either the left or the right abducens nucleus, which was identified by the recording of the antidromic field potential induced by electric stimulation of the ipsilateral VIth nerve (Fig. 1A). The PH nucleus was found in the same parasagittal plane and posterior to the abducens nucleus, just below the floor of the fourth ventricle. All injections were restricted to the rostral third of the PH nucleus, 1–1.5 mm posterior to the location where the antidromic field potential was recorded (Fig. 1B). The correct position of the micropipette was confirmed by recording the characteristic firing discharge of PH neurons during spontaneous and vestibular-induced eye movements (Escudero et al. 1992). Injections were performed by means of glass micropipettes with tip diameters of 7–8 μm, filled with the corresponding drug dissolved in phosphate buffer, 0.1 m, pH 7.4, and sterilized by filtration through a 22 μm pore filter. Air pulses (1 kg cm−2, 1 s) were applied with an air pressure device connected to the injection micropipette to deliver 40–45 nl pulse−1.
Figure 1. Experimental design and injection sites.
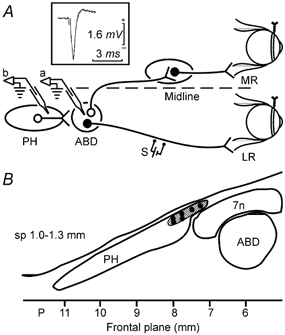
A, schematic representation of the animal preparation. Teflon-coated stainless-steel coils were fixed to the scleral margin of both eyes. A stimulating electrode (S) was implanted on the VIth nerve to retrogradely stimulate motoneurons in the abducens nucleus (ABD). A glass micropipette was introduced in the abducens nucleus (a) where its position was confirmed by the antidromic field potential recording (inset). After withdrawal, the pipette was introduced in the prepositus hypoglossi nucleus (PH, b) for drug injection; LR, lateral rectus muscle; MR, medial rectus muscle. B, schematic mapping of the injection sites in the rostral PH. Six representative injection sites are shown. A total of 17 drug injections were performed within the hatched area, in a saggital plane (sp) between 1.0 and 1.3 mm from midline and a frontal plane between 7 and 8 mm posterior to bregma. 7n, facial nerve.
Spontaneous eye movements were continuously recorded in complete darkness, and occasionally in the light, with the micropipette in place both before and after injections. Eye movements were calibrated at the beginning of each experimental session by rotating (± 10 deg) the magnetic field frame about both the horizontal and vertical planes. The horizontal VOR was elicited by sinusoidal rotation around the vertical axis at 0.1 and 1 Hz. The amplitude of the table movement was adjusted in order to keep maximal angular head velocity constant at 30 deg s−1 for both frequencies. Eye movements during VOR were recorded in the dark both before and after drug injections. Field and unitary electrical activities and head and eye position were stored using an eight-channel video tape recording system and fed into a computer for off-line analysis. Eye and head position signals were sampled at 500 Hz.
At the end of the experimental period, animals were anaesthetized with sodium pentobarbitone (50 mg kg−1i.p.) and transcardially perfused with saline and 4 % paraformaldehyde. In two animals, biotin dextran amine was injected in the PH nucleus before perfusion, using the procedure described for drug injections. The brainstems were used for the anatomical identification of the injection sites and for additional morphological studies.
Analysis of the data
Spontaneous eye movements in darkness consist of saccades separated by periods of gaze-holding. Drug injections in the PH nucleus produced nystagmus with either straight or curved slow phases separated by quick resetting movements. The significance of these two types of eye movement alteration has been well established (Cannon & Robinson, 1987; Godaux et al. 1993; Mettens et al. 1994a, b, c; Godaux & Cheron, 1996; Moreno-López et al. 1998). Velocity imbalance, such as that produced when the tonic inputs from both vestibular nuclei are not identical, produces nystagmus with straight slow phases. This is different from a failure in oculomotor integration which produces gaze-holding impairment and causes exponential drifts towards a central position in the post-saccadic periods. The consequence of a dual alteration of both velocity and gaze-holding is a nystagmus with curved slow phases. Analysis of the slow phases was performed during the 3 min period of maximum effect for each injection, and immediately before vestibular stimulation. Slow phases with duration greater than 0.5 s were fitted separately, by the least-squares method, to linear and exponential equations, and were considered to be linear or exponential when more than 80 % of the analysed phases had a correlation coefficient > 0.99 or > 0.90, respectively. When exponential slow phases were present, their time constant was calculated as an indicator of the severity of the integration deficit. To avoid underestimation due to concomitant velocity imbalance during nystagmus, time constants were calculated from the first derivative of eye position (eye velocity).
During VOR, the eye movement response was defined by three parameters: velocity imbalance, reflex gain, and phase lead. The velocity imbalance was measured as the mean slow eye velocity and expressed in degrees per second. The reflex gain was calculated as the ratio between the peak-to-peak amplitude of slow eye velocity versus the peak-to-peak amplitude of head velocity. To analyse the head and slow eye velocity, a computer program was developed. For each cycle, the sinusoidal function of the head velocity was calculated by fitting a periodic function (trigonometric polynomial) by the least-squares method (Batschelet, 1981). This sine wave was adjusted by cursors to the eye velocity signal. The points of the eye velocity signal that were in the range ± 10 % with respect to the reference sine wave were selected, and the rest, corresponding to the quick phases, were ignored. Parameters of the resulting sine wave were calculated as indicated for head velocity. The phase lead was quantified as the temporal shift between the eye and the head position for each hemicycle and then averaged for each complete cycle. This method avoids the shift produced in the eye position by the velocity imbalance, which is of the same magnitude and opposite sign for each hemicycle. Data from phase shifts were expressed in degrees. Each parameter was measured for at least 10 cycles at 0.1 Hz and 30 cycles at 1 Hz.
Results are presented as means ± s.e.m., except where otherwise indicated. Comparisons within one experiment (for example, when VOR gains were compared in a large number of cycles before and after drug injection) were performed using the Student's t test. Comparisons between two groups of experiments were performed by the non-parametric test of Mann-Whitney. A probability < 0.05 was considered significant.
Fast Blue microinjections
To identify neurons projecting to the PH nucleus, injections of the retrograde tracer Fast Blue were performed in the rostral third of this nucleus in two cats. The animals were anaesthetized as described above. A stimulating electrode was implanted in one lateral rectus muscle and a micropipette was introduced in the PH nucleus, after localization of the abducens nucleus by recording of the antidromic field potential, essentially as described for functional experiments, with the exception that the whole procedure was performed in the same surgical act. Injections were performed by means of glass micropipettes with tip diameters of 50 μm, filled with 1 % Fast Blue in 0.1 M phosphate buffer, pH 7.4. Two air pulses (1 kg cm−2, 1 s) were applied with an air pressure device connected to the injection micropipette to deliver a total of 300–400 nl.
Histological studies
Five days after the Fast Blue injections, the two animals were anaesthetized with ketamine (35 mg kg−1i.m.) and xylidine-dihydrothiazine (1 mg kg−1i.m.) and perfused through the ascending aorta with a physiological solution (composition (mm): 120 NaCl, 2 KCl, 2 CaCl2, 26 NaHCO3, 1.2 KH2PO4, 1.2 MgSO4, and 11 glucose) at 37 °C and bubbled with 95 % O2 and 5 % CO2, containing the NO donor sodium nitroprusside (SNP, 10 mm) and the phosphodiesterase inhibitor 3-isobutylmethylxantine (IBMX, 1 mm), for 5 min at 200 ml min−1. The aim of this treatment, originally described by Southam & Garthwaite (1993), was to increase cGMP in those cells expressing NO-sensitive soluble guanylyl cyclase. Immediately afterwards, the animals were perfused with 4 % paraformaldehyde in 0.1 m phosphate buffer, pH 7.4 at 4 °C. The brains were then removed and the brainstems post-fixed for 2 h and cryoprotected by incubation for 2 days with 30 % sucrose at 4 °C. Coronal 40 μm sections were obtained with a freezing microtome.
Brain sections were treated with 2 % hydrogen peroxide in 60 % methanol to block endogenous peroxidase, and incubated for 24 h at room temperature with a 1:4000 dilution of an antibody raised in sheep against a cGMP-paraformaldehyde-bovine thyroglobulin complex (Tanaka et al. 1997), kindly provided by Dr de Vente (Maastrich, Netherlands), and a rabbit polyclonal antibody against GAD67 (Chemicon; 1:1000). Afterwards, the tissue was incubated for 2 h at room temperature in the dark with an anti-sheep IgG labelled with Cy3 (Jackson, 1:400) and a biotin-conjugated anti-rabbit IgG (Jackson, 1:500). The brain sections were mounted in Vectashield and observed under fluorescence microscopy for Fast Blue and Cy3 labelling. Different fields were captured with a DP10 digital camera and analysed with the image analysis software Microimage from Olympus. Thereafter, biotin coupled to the secondary antibody labelling GAD67 was revealed by the avidin-biotin-peroxidase method using an ABC kit (Vector Laboratories, Burlingame, CA, USA). Peroxidase was made visible by incubation with a solution containing 0.05 % 3,3′-diaminobenzidine and 0.003 % hydrogen peroxide in 0.05 m Tris, pH 7.6, for 10 min. Finally, the tissue was mounted on gelatin-coated slides, dehydrated, cleared in xylene, and coverslipped in DePeX. The fields that had been captured under fluorescence microscopy were further analysed with a light microscope to identify GABAergic neurons. No immunostaining was observed when the primary antibodies were omitted. No significant cGMP-ir was found in the structures described when staining was performed under the same conditions in animals perfused with IBMX, but without SNP (Moreno-López et al. 2001).
RESULTS
Oculomotor effects induced by the blockade of NOS activity in the PH nucleus
Unilateral inhibition of NOS activity in the PH nucleus induced a nystagmus whose slow phases were linear and directed contralaterally to the injected side. Alterations were restricted to movements in the horizontal plane, whereas the vertical component of the eye movement was unaffected. Figure 2 shows spontaneous eye movements under control conditions and after microinjection of the NOS inhibitor NΩ-nitro-l-arginine methyl ester (l-NAME) in the right PH nucleus of an alert cat. Sinusoidal vestibular stimulation at 0.1 and 1 Hz after l-NAME injection induced a VOR with gain and phase similar to those observed in control animals, but with a velocity imbalance in a direction contralateral to the injected side (Fig. 3 and Fig. 6). These results are similar to those previously reported (Moreno-López et al. 1998) and are shown here for purposes of the comparison with the effects of neurotransmitter receptor antagonists. The effects of l-NAME were not due to local changes in pressure or osmolarity, since eye movements remained unaltered after injection of the inactive stereoisomer d-NAME (Moreno-López et al. 1996).
Figure 2. Effects of unilateral injections of the NOS inhibitor l-NAME, or the glutamatergic receptor antagonists APV and NBQX in the PH nucleus of an alert cat during spontaneous eye movements.
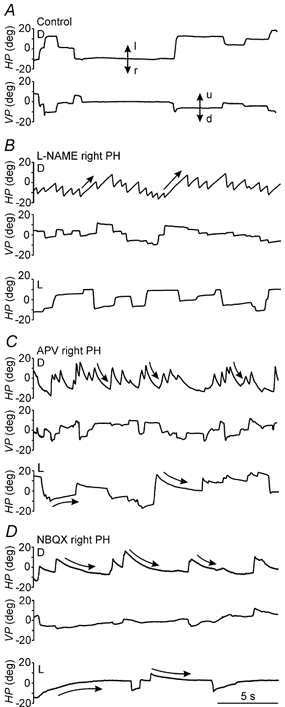
Recordings of eye position in the horizontal and vertical planes (HP and VP, respectively) under control conditions and after injections of the indicated drugs in the right PH nucleus, in either darkness (D) or light (L). Doses and times after injection were as follows: l-NAME, 24 nmol, 2 min (D) and 5 min (L); APV, 7 nmol, 22 min (D) and 21 min (L); and NBQX, 1 nmol, 6 min (D) and 4 min (L). Eye position is plotted in degrees (deg). Vertical arrows indicate movement direction: l, left; r, right; u, up; d, down. Straight and curved arrows indicate linear and exponential slow phases, respectively. Slow phases for l-NAME were best fitted to a linear equation, whereas slow phases for APV and NBQX were best fitted to an exponential equation.
Figure 3. Effects of unilateral injections of l-NAME or glutamatergic antagonists in the PH nucleus of an alert cat during sinusoidal vestibular stimulation in darkness.
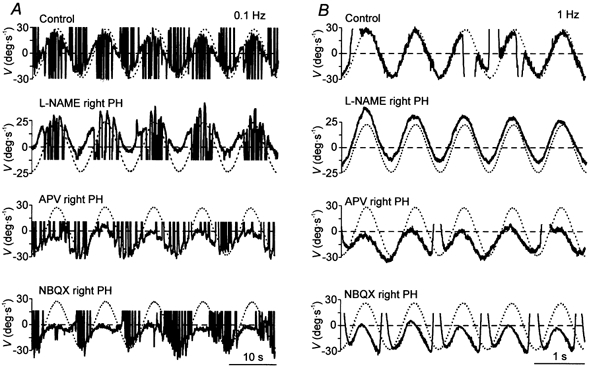
Representative recording of eye (continuous line) and head (dotted line) velocity (V) during VOR induction by turntable rotation at 0.1 (A) and 1 Hz (B) under control conditions and after injections of the indicated drugs in the right PH nucleus. The head velocity curve has been inverted to facilitate comparison with the eye velocity curve. Movement direction as indicated in Fig. 2.
Figure 6. Velocity imbalance, gain, and phase lead of the VOR after local administration of the indicated drugs in the PH nucleus.
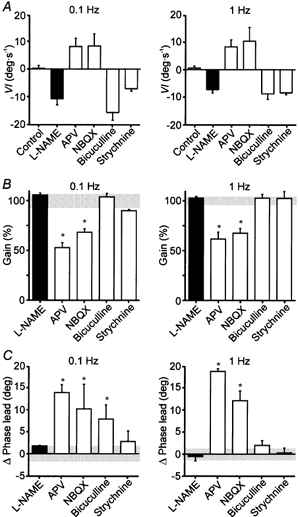
A, velocity imbalance (VI) during VOR induction by rotation of the head at 0.1 and 1 Hz. Positive values represent velocity imbalance toward the injected side and negative values toward the contralateral side. B, gain of the VOR induced by rotation of the head at 0.1 and 1 Hz after injection of the different drugs. Gain is expressed as percentage of the control value for each experiment. Grey horizontal bar, 100 % ± s.d. of control values. C, changes in phase lead during the VOR induced by rotation of the head at 0.1 and 1 Hz produced by injection of the different drugs. Values are expressed in degrees (deg). Grey horizontal bar, 0 ± s.d. of control values. Values are expressed as means ± s.e.m. *P < 0.05 compared with control values, using the non-parametric Mann-Whitney U test. n = 5 for l-NAME; n = 3 for the other drugs.
Oculomotor effects induced by local injections of glutamatergic antagonists in the PH nucleus
Unilateral microinjections of the NMDA antagonist 2-amino-5-phosphonovaleric acid (APV) (3–7 nmol) or the AMPA/kainate antagonist 2,3-dihydro-6-nitro-7-sulphamoyl-benzo quinoxaline (NBQX) (0.5–1.5 nmol) in the PH nucleus induced a nystagmus with curved slow phases directed ipsilaterally to the injected side, when the animal was in darkness (Fig. 2). The mean time constants of exponential curves fitted to the slow phases (r > 0.90) during the maximum effects of APV and NBQX injections were 0.63 ± 0.06 and 0.88 ± 0.11 s (mean ± s.e.m., n = 3), respectively. These results reveal a velocity imbalance, as indicated by the nystagmus, and a failure in the generation of the eye position signal, as indicated by the curved slow phases. In light, the nystagmus was absent, and the eyes moved to a central position in the orbit after each saccade (Fig. 2), indicating a gaze-holding deficit. The vertical component of eye movements remained unchanged. The amplitude of eye movements in any direction was not affected by the drug injections. During sinusoidal vestibular stimulation at 0.1 and 1 Hz, both APV and NBQX induced similar effects on the performance of the VOR (Fig. 3 and Fig. 6). In both cases, a velocity imbalance ipsilateral to the injected side, a strong decrease in gain, and an increase in phase lead were present (Fig. 6). Table 1 shows for each individual injection the mean variation values of gain and phase lead during VOR, together with the mean time constant of the post-saccadic drift during spontaneous eye movements immediately before vestibular stimulation.
Table 1.
Effect of individual glutamatergic-antagonist injections on vestibulo-ocular reflex (VOR) gain and phase lead, and on the time constant of post-saccadic drifts during spontaneous eye movements
| VOR (0.1 Hz) | VOR(1 Hz) | ||||
|---|---|---|---|---|---|
| Drug | ΔGain† | ΔPhase lead ‡ (deg) | ΔGain† | ΔPhase lead ‡ (deg) | Spontaneous eye movements Time constant § (s) |
| APV | −0.38* | 14.5* | −0.34* | −18.0* | 1.53 ± 0.40 |
| 0.37* | 10.5* | −0.27* | 19.8* | 1.47 ± 0.74 | |
| −0.46* | 16.6* | −0.45* | 18.3* | 0.77 ± 0.22 | |
| NBQX | −0.23* | 0.5 | −0.38* | 10.9* | 2.59 ± 0.39 |
| −0.38* | 10.0* | −0.23* | 9.1* | 4.32 ± 1.40 | |
| −0.23* | 20.1* | −0.33* | 16.4* | 1.12 ± 0.41 | |
Values represents changes in VOR gain afte drug injection. Absolute gain values in control conditions were 0.86 ± 0.07 for 0.1 Hz and 0.93 ± 0.04 for 1 Hz, n = 6.
Values represent changes in VOR phase lead after drug injection. Absolute phase lead values in control were 9.6 ± 2.1 for 0.1 Hz and 5.1 ± 1.4 for 1 Hz, n = 6.
Mean ± s.d. of the time constants calculated immediately before VOR induction.
P < 0.05 when gain and phase leads before and after injection were compared.
Oculomotor effects induced by local injections of GABAergic and glycinergic antagonists in the PH nucleus
Local blockade of GABAergic type A receptors by bicuculline (0.5–2.0 nmol) or glycinergic receptors by strychnine (3.3–9.8 nmol) in the PH nucleus induced similar effects (Fig. 4). In darkness, spontaneous eye movements in the horizontal plane were characterized by a nystagmus with slow phases directed contralaterally to the injected side. The slow phases of the nystagmus were ramp like, with a best fit to a linear equation (r > 0.99). Under light conditions, when the nystagmus was strongly attenuated, no evident signs of gaze-holding deficit were observed. The amplitude of eye movements was unchanged. No significant alterations were detected in the vertical component of spontaneous eye movements. These results indicate that the local inhibition of GABAergic or glycinergic neurotransmission in the PH nucleus induces velocity imbalance without apparent changes in the generation of the eye position signals during spontaneous eye movements.
Figure 4. Effects of unilateral injections of the GABAA receptor antagonist bicuculline and the glycine receptor antagonist strychnine, in the PH nucleus of an alert cat during spontaneous eye movements.
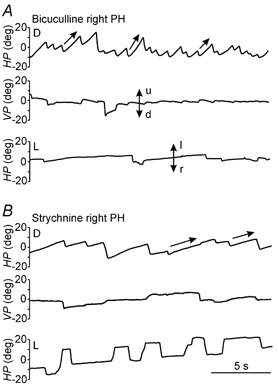
Recordings of eye position in the horizontal (HP) and vertical (VP) planes after injections of the indicated drugs in the right PH nucleus, in either darkness (D) or light (L). Doses and times after injection were as follows: bicuculline, 1 nmol, 15 min (D) and 18 min (L); strychnine, 3.3 nmol, 8 min (D) and 9 min (L). Eye position is plotted in degrees (deg). Movement direction as indicated in Fig. 2. Slow phases for bicuculline and strychnine were best fitted to a linear equation, similar to slow phases after l-NAME injections.
During sinusoidal vestibular stimulation (Fig. 5 and Fig. 6), bicuculline and strychnine, induced velocity imbalance contralateral to the injected side (Fig. 6A) without modification of the gain (Fig. 6B) or phase (Fig. 6C) of the VOR. Bicuculline produced a small increase in phase lead at 0.1 Hz, but this disappeared when stimulated at 1 Hz. Table 2 shows the mean values of gain and phase lead variation during VOR for individual injections of bicuculline and strychnine in comparison with those produced by l-NAME.
Figure 5. Effects of unilateral injections of GABAA and glycine receptor antagonists in the prepositus hypoglossi (PH) nucleus of an alert cat during sinusoidal vestibular stimulation in darkness.
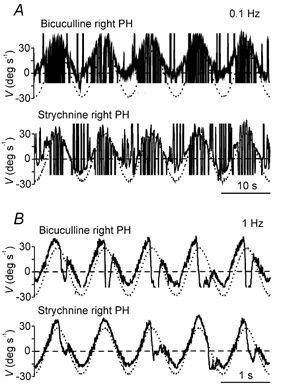
Representative recording of eye (continuous line) and head (dotted line) velocity (V) during VOR induction by turntable rotation at 0.1 (A) and 1 Hz (B) after injections of the indicated drugs in the right PH nucleus. The head velocity curve has been inverted to facilitate comparison with the eye velocity curve. Movement direction as indicated in Fig. 2.
Table 2.
Effect of individual injections of a NOS inhibitor and of inhibitor neurotransmitter receptor antagonists on vestibulo-ocular reflex (VOR) gain and phase lead
| VOR(0.1 Hz) | VOR(1 Hz) | |||
|---|---|---|---|---|
| Drug | ΔGain† | ΔPhase lead ‡ (deg) | ΔGain† | ΔPhase lead ‡ (deg) |
| l-NAME | 0.06 | 2.7 | −0.03 | −3.8 |
| 0.08* | 2.7 | 0.03 | 1.6 | |
| 0.03 | 0.6 | 0.06 | −1.0 | |
| −0.01 | 3.1 | 0.03 | −0.9 | |
| 0.03 | 0.2 | 0.01 | 1.4 | |
| Bicuculline | −0.02 | 1.4 | −0.03 | 3.4 |
| 0.01 | 11.2* | 0.00 | −0.1 | |
| 0.08* | 11.0* | 0.09 | 2.7 | |
| Strychnine | −0.10* | 7.5* | −0.08 | 2.4 |
| −0.07 | −0.6 | 0.10 | −1.1 | |
| 0.10* | 1.4 | 0.0 | −0.4 | |
Values represents changes in VOR gain afte drug injection. Absolute gain values in control conditions were 0.82 ± 0.04 for 0.1 Hz and 0.96 ± 0.05 for 1 Hz, n = 6.
Values represent changes in VOR phase lead after drug injection. Absolute phase lead values in control conditions were 9.7 ± 1.4 for 0.1 Hz and 6.0 ± 1.1 for 1 Hz, n = 6.
P < 0.05 when gain ad phase leads before and after injection were compared.
Therefore, both qualitative and quantitative results obtained from unilateral injections of inhibitory neurotransmitter antagonists resulted in very similar oculomotor effects to those induced by the diminution of endogenous NO in the PH nucleus.
Anatomical identification of neuronal targets for NO produced in the PH nucleus
To be identified as targets for the NO synthesized by nitrergic neurons in the PH nucleus, neurons should fulfil three requirements. First, they have to increase their cGMP content when stimulated by NO because the described NO effects on eye movements are mediated by activation of soluble guanylyl cyclase (Moreno-López et al. 1996). Second, they have to project to the PH nucleus, to expose their processes and terminals to the local action of NO. Third, they should synthesize an inhibitory neurotransmitter. To identify such neurons, microinjections of the fluorescent retrograde marker Fast Blue were performed unilaterally in the PH nucleus of two cats (Fig. 7A). Five days later, and immediately before fixation, the animals were perfused with a physiological solution containing the NO donor SNP, to massively activate soluble guanylyl cyclase and increase cGMP concentration in NO-sensitive neurons. Brainstem sections obtained from these animals were then double-processed for cGMP and GAD immunohistochemistry. Fig. 7B-D shows an example of a brainstem section through the rostral MVN in which a fast blue-labelled, cGMP-ir and GAD-ir neuron was made visible. Quantitative analysis indicated that most of the triple-marked neurons were located in the ipsilateral MVN, and a few of them were also identified in the marginal zone (Table 3). Within the MVN, the labelled cells were preferentially located in the rostral and medial part of the nucleus (Fig. 7E). Some scattered neurons were found along the paramedial reticular formation and the inferior vestibular nucleus in only one cat (not shown). Within the MVN there was a high degree of co-localization between cGMP-ir and GAD-ir (65.5 % of the 543 cGMP-ir neurons counted were GABAergic); however, the population of cGMP-ir cells projecting to the PH nucleus was comparatively more enriched in GAD-ir neurons (85.7 % of 52 FB-cGMP-ir neurons were GABAergic).
Figure 7. Co-localization of cyclic GMP and glutamic acid decarboxylase in neurons in the medial vestibular nucleus projecting to the ipsilateral prepositus hypoglossi nucleus.
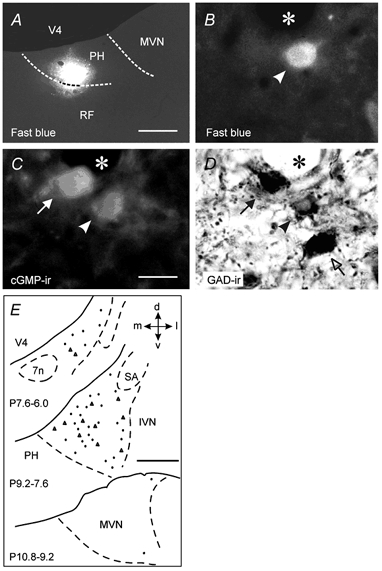
A, coronal section showing the Fast Blue injection area in the PH nucleus of one cat. B-D, photomicrographs showing a neuron (arrowhead) triple labelled with Fast Blue, cyclic GMP (cGMP-ir) and glutamic acid decarboxylase (GAD-ir) in the medial vestibular nucleus (MVN) ipsilateral to the injection side. A neuron positive for GAD-ir and cGMP-ir (filled arrow) and another one positive only for GAD-ir (open arrow) are also shown. The asterisk indicates a blood vessel. E, distribution of the neurons triple labelled with Fast Blue, cGMP-ir and GAD-ir in the MVN ipsilateral to the PH nucleus injected with Fast Blue. Each section contains the neurons found in the indicated frontal plane intervals. Data correspond to cats 36 (triangles) and 37 (dots). Calibration bars: A, 500 μm; B-D, 25 μm; E, 1 mm. 7n, facial nerve; d, dorsal; IVN, inferior vestibular nucleus; l, lateral; m, medial; RF, reticular formation; SA, stria acustica; v, ventral; V4, fourth ventricle.
Table 3.
Number of GABAergic neurons among those which produce cGMP in response to NO and project to the PH nucleus, in different brainstem structures related to eye movements
| FB cells counted | FB + CGMP-ir | FB + CGMP-ir + GAD | |||||
|---|---|---|---|---|---|---|---|
| Structure | Cat | Ipsilateral | Contralated | Ipsilateral | Contralateral | Ipsilateral | Contralateral |
| IVN | 36(2) | 33 | 22 | 4 | 5 | 3 | 2 |
| 37(2) | 32 | 20 | 4 | 2 | 0 | 0 | |
| MZ | 36(2) | 115 | 11 | 7 | 4 | 7 | 2 |
| 37(2) | 106 | 26 | 11 | 3 | 5 | 2 | |
| MVN | 36(2) | 209 | 88 | 15 | 6 | 12 | 4 |
| 37(3) | 308 | 200 | 37 | 5 | 33 | 2 | |
Animals were injected with the retrograde tracer Fast Blue (FB) in one PH nucleus. Five days later, brainstem sections were stained for cGMP (cGMP-ir) and glutamic acid decarboxylase (GAD) immunohistochemistry, as described in Methods. Five series of 40 μm brainstem setions were obtained from each cat. The number of series counted for each nucleus in each cat is shown in parenthesis. IVN, inferior vestibular nucleus; MVN, medial vestibular nucleus; MZ, marginal zone. Injection in cat 37 was small and circumscribed to the rostral part of the PH nucleus, whereas injection in cat 36 was slightly larger and affected the most medial border of the MVN.
DISCUSSION
This study shows that NO produced by nitrergic neurons in the cat PH nucleus modulates eye velocity signals during both spontaneous eye movements and VOR by facilitation of inhibitory neurotransmission within the PH nucleus. The most likely candidates as targets for this NO action are the GABAergic NO-responsive neurons located in the MVN which project to the ipsilateral PH nucleus.
NO facilitates inhibitory neurotransmission in the PH nucleus
In an attempt to investigate whether NO produced by PH neurons may act by either facilitation or inhibition of a specific neurotransmission system, the functional alterations produced by local inhibition of NOS in the PH nucleus of alert cats were compared with those observed after injections in the same location of antagonists of the excitatory neurotransmitter glutamate and the inhibitory neurotransmitters GABA and glycine, which are largely present in the oculomotor system (Precht et al. 1973; Spencer et al. 1989; Holstein et al. 1996).
Analysis of the functional data indicated that NOS inhibitors behaved identically to specific GABAA and glycine receptor antagonists in two ways. First, all of them exclusively affected velocity signals without significant alteration of gaze-holding, VOR gain or phase lead. Secondly, the velocity imbalance was directed in all cases contralaterally to the injected side, i.e. it was directed to the side where either NO, GABA or glycine were more effective. Thus, the effects of NO in the PH nucleus could be fully explained by a facilitatory action on inhibitory neurotransmission, probably by inducing release of GABA and/or glycine, since NO has been shown to produce neurotransmitter release in different preparations (Montague et al. 1994).
On the other hand, the consequences of blocking glutamatergic transmission, either through NMDA or non-NMDA receptors, were different from those of NOS inhibition in several aspects. There was a velocity imbalance in spontaneous eye movements in the dark and in VOR, but in the opposite direction, i.e. ipsilateral to the injected side. Furthermore, spontaneous eye movements revealed additional impairment in gaze-holding and there were changes in VOR gain and phase lead, indicating an alteration of the position signals for both kinds of movements, that was never manifested after l-NAME injections.
The opposite direction of the velocity imbalance produced by NOS inhibitors and glutamatergic receptor blockade raised the possibility that NO may act by inhibition of glutamate release in the PH nucleus. If this were true, administration of NO donors in the PH nucleus would produce the same effects as APV or NBQX. Previous results (Moreno-López et al. 1998) have shown that, although this is the case for spontaneous eye movements, NO donors did not modify the position signals during VOR. This disparity suggests that the mechanisms by which glutamate and NO participate in the generation of position signals are independent, and probably exerted on different structures. More specifically, we have proposed that the marginal zone might have been the target area for the reported effect of exogenous NO on eye position (Moreno-López et al. 1998).
Glutamate, but not inhibitory neurotransmitters, participates in the generation of the position signal in the rostral pole of the PH nucleus
The consequences of local blockade in the PH nucleus of either excitatory or inhibitory neurotransmitter receptors have been previously reported by various investigators. Blockade of NMDA, kainate or AMPA glutamatergic receptors in monkeys (Arnold et al. 1999) and cats (Mettens et al. 1994a) produced spontaneous eye movement alterations similar to those described in the present study, except for NBQX, which did not affect eye position signals in cats (Mettens et al. 1994a). However, the results obtained following pharmacological modification of the inhibitory neurotransmission systems reveal a large variability, which makes it difficult to understand comprehensively how neurotransmitters work in the generation of velocity and position signals in the PH nucleus. Thus, strychnine, which in our experiments caused velocity imbalance, has been reported to produce either velocity imbalance, gaze-holding deficit, or no effect on eye movements (Mettens et al. 1994b; Arnold et al. 1999). Furthermore, Arnold et al. (1999) have shown that bicuculline produced gaze-holding deficits when administered in the PH nucleus of monkeys, whereas in the present study, similar doses did not produce signs of eye position alteration in the cat, except for an increase in phase lead when VOR was induced at 0.1 Hz. This variability in functional response could be due to the fact that the PH nucleus is elongated in the rostro-caudal axis, and different sites of injection may affect neurons with different properties if they are heterogeneously distributed. In this context, we have shown that the oculomotor effects of NOS inhibition were only apparent when l-NAME was injected in the rostral third of the PH nucleus, but not when it was administered in a posterior plane (Moreno-López et al. 1996). Also, our previous unitary recording observations, that neurons showing similar responses were frequently found in the same tract (Escudero et al. 1992), support the idea that PH neurons with similar signalling properties (head/eye velocity and/or eye position) may be clustered in specific locations.
In the present study, we have tried to minimize the variability of the responses by administering all drugs within the rostral part of the PH nucleus, the region in which l-NAME affected eye movements (Moreno-López et al. 1996). Since the same vehicle and injection volume were used for all the drugs, it is reasonable to think that the diffusion volume was very similar in all cases. Also, the lack of alteration in the vertical component of eye movements indicated that the diffusion was restricted to the region where neurons controlling horizontal eye movements are located. Furthermore, the exploration within the same experimental condition of both spontaneous eye movements and VOR, and the identical effect (velocity and/or position signal alteration) observed on both kinds of eye movements for each antagonist tested, strengthen the reliability of the results. Therefore, we conclude that in the anterior pole of the PH nucleus glutamatergic transmission is essential for the processing of both velocity and position signals, whereas inhibitory neurotransmission modulates only eye velocity commands.
Identification of neuronal targets for NO produced by PH neurons
The pure velocity effects of endogenous NO in the PH nucleus are probably exerted on nerve terminals conveying inputs from the MVN. From a functional point of view, it is well known that the PH nucleus receives inhibitory and excitatory tonic velocity signals from the ipsi- and contralateral MVN, respectively (Baker & Berthoz, 1975), and that unilateral alterations of these inputs result in velocity imbalances (Godaux et al. 1993), identical to those observed after NOS inhibition or inhibitory transmission blockade. In addition, the morphological identification of NO targets by cGMP immunohistochemistry showed that the PH nucleus did not contain cGMP-ir neuronal cell bodies, thus ruling out any NO effect on intranuclear interneurons or projection neurons. On the contrary, cGMP-ir neuropil was observed in a position dorsal to the region enriched in nitrergic neurons (Moreno-López et al. 1998). The cGMP-containing fibres belong necessarily to neurons located outside of, and projecting to, the PH nucleus. Neurons projecting to the PH nucleus and increasing their cGMP content in response to NO have been identified in different brainstem structures, among which the MVN contained the largest number (Moreno-López et al. 2001).
We now show that the majority (65 %) of the NO-sensitive neurons in the MVN nucleus were GABAergic. Furthermore, among the MVN neurons projecting to the PH nucleus, a larger proportion of NO-sensitive neurons was found in the ipsilateral side, where the inhibitory input to the PH nucleus originates. Finally, a large percentage (> 85 %) of these cGMP-ir neurons which projected to the PH nucleus were GABAergic. These results provide the morphological identification of the NO targets neurons, and explain the functional findings that in the PH nucleus endogenous NO may act by facilitating inhibitory neurotransmission, and by modulating the MVN inputs conveying velocity signals.
We cannot disregard the possibility that NO in the PH nucleus may also act on terminals from the GABAergic Purkinje cells in the cerebellar flocculus, since previous anatomical studies have shown that these neurons contain soluble guanylyl cyclase (Ariano et al. 1982; Southam & Garthwaite, 1993), and project to the PH nucleus (McCrea & Baker, 1985). However, the functional significance of this projection remains to be established. Floccular lesions produce nystagmus and integration deficits (Flandrin et al. 1983; Fukushima et al. 1992), but these effects are probably due to the disruption of the more extensive direct connections between the flocculus and the vestibular nuclei.
Taken together, the functional and anatomical results strongly indicate that at least part of the NO action in the PH nucleus is exerted by facilitation of GABAergic inputs arriving to this nucleus from the ipsilateral MVN. What is the physiological significance of the NO produced by PH neurons in eye movement control? In the absence of head rotation, each PH nucleus receives balanced excitatory inputs from the contralateral MVN, and inhibitory inputs from the ipsilateral MVN. When the head rotates, one PH nucleus receives increased excitatory and decreased inhibitory inputs, resulting in net activation, whereas the other nucleus behaves exactly in the opposite manner. If, by any chance, PH neurons receive only increased excitatory signals in an inadequate context (e.g. no head movement), a velocity imbalance would appear in the absence of the corresponding stimulus. In these situations, activation of NOS by the increased intracytoplasmic Ca2+ derived from glutamatergic stimulation of NMDA receptors (Bredt & Snyder, 1989; Fedele & Raiteri, 1999) would facilitate the inhibitory inputs and thus restore the velocity balance necessary for the correct performance of eye movements. This mechanism may explain the precise balance control which is necessary to avoid integration of the resting noise signals and correctly generate a stable and adequate eye position, a function that has been attributed to the PH nucleus for horizontal eye movements.
Acknowledgments
Supported by grants 97/2054 and 00/1080 from the Fondo de Investigación Sanitaria and PM98–0011 from the Ministerio de Educación y Cultura, Spain. We thank Dr de Vente for kindly providing the anti-cGMP antibody.
REFERENCES
- Ariano MA, Lewicki JA, Brandwein JH, Murad F. Immunohistochemical localization of guanylate cyclase within neurons of rat brain. Proceedings of the National Academy of Sciences of the USA. 1982;79:1316–1320. doi: 10.1073/pnas.79.4.1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnold DB, Robinson DA, Leigh RJ. Nystagmus induced by pharmacological inactivation of the brainstem ocular motor integrator in monkey. Vision Research. 1999;39:4286–4295. doi: 10.1016/s0042-6989(99)00142-x. [DOI] [PubMed] [Google Scholar]
- Baker R, Berthoz A. Is the prepositus hypoglossi nucleus the source of another vestibulo-ocular pathway? Brain Research. 1975;86:121–127. doi: 10.1016/0006-8993(75)90643-5. [DOI] [PubMed] [Google Scholar]
- Baker R, Mano N, Shimazu H. Postsynaptic potentials in abducens motoneurons induced by vestibular stimulation. Brain Research. 1969;15:577–580. doi: 10.1016/0006-8993(69)90189-9. [DOI] [PubMed] [Google Scholar]
- Batschelet E. Circular Statistics in Biology. Academic Press; 1981. [Google Scholar]
- Berman AL. The Brain Stem of the Cat: a Cytoarchitectonic Atlas with Stereotaxic Coordinates. Madison, WI, USA: The University of Wisconsin Press; 1968. [Google Scholar]
- Berthoz A, Droulez J, Vidal PP, Yoshida K. Neural correlates of horizontal VOR cancelation during rapid eye movements in the cat. Journal of Physiology. 1989;419:717–751. doi: 10.1113/jphysiol.1989.sp017895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bredt DS, Snyder SH. Nitric oxide mediates glutamate-linked enhancement of cGMP levels in the cerebellum. Proceedings of the National Academy of Sciences of the USA. 1989;86:9030–9033. doi: 10.1073/pnas.86.22.9030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannon SC, Robinson DA. Loss of the neural integrator of the oculomotor system from brain stem lesions in monkey. Journal of Neurophysiology. 1987;57:1383–1409. doi: 10.1152/jn.1987.57.5.1383. [DOI] [PubMed] [Google Scholar]
- Cheron G, Gillis P, Godaux E. Lesion in the cat prepositus complex: effects on the optokinetic system. Journal of Physiology. 1986a;372:95–111. doi: 10.1113/jphysiol.1986.sp015999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheron G, Godaux E. Disabling of the oculomotor neural integrator by kainic acid injections in the prepositus vestibular complex of the cat. Journal of Physiology. 1987;394:267–290. doi: 10.1113/jphysiol.1987.sp016870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheron G, Godaux E, Laune JM, Vanderkeler B. Lesions in the cat prepositus complex: effects on the vestibulo-ocular reflex and saccades. Journal of Physiology. 1986b;372:75–94. doi: 10.1113/jphysiol.1986.sp015998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de la Cruz RR, Escudero M, Delgado-García JM. Behavior of medial rectus motoneurons in the alert cat. European Journal of Neuroscience. 1990;1:288–295. doi: 10.1111/j.1460-9568.1989.tb00796.x. [DOI] [PubMed] [Google Scholar]
- Delgado-García JM, del Pozo F, Baker R. Behavior of neurons in the abducens nucleus of the alert cat. I. Motoneurons. Neuroscience. 1986;17:929–952. doi: 10.1016/0306-4522(86)90072-2. [DOI] [PubMed] [Google Scholar]
- Delgado-García JM, Vidal PP, Gómez C, Berthoz A. A neurophysiological study of prepositus hypoglossi neurons projecting to oculomotor and preoculomotor nuclei in the alert cat. Neuroscience. 1989;29:291–307. doi: 10.1016/0306-4522(89)90058-4. [DOI] [PubMed] [Google Scholar]
- Escudero M, de la Cruz RR, Delgado-García JM. A physiological study of vestibular and prepositus hypoglossi neurones projecting to the abducens nucleus in the alert cat. Journal of Physiology. 1992;458:539–560. doi: 10.1113/jphysiol.1992.sp019433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Escudero M, Delgado-García JM. Behavior of reticular, vestibular and prepositus neurons terminating in the abducens nucleus of the alert cat. Experimental Brain Research. 1988;71:218–222. doi: 10.1007/BF00247538. [DOI] [PubMed] [Google Scholar]
- Fedele E, Raiteri M. In vivo studies of cerebral glutamate receptor/NO/cGMP pathway. Progress in Neurobiology. 1999;58:89–120. doi: 10.1016/s0301-0082(98)00077-x. [DOI] [PubMed] [Google Scholar]
- Flandrin JM, Courjon JH, Jeannerod M, Schmid R. Effects of unilateral flocculus lesions on vestibulo-ocular responses in the cat. Neuroscience. 1983;8:809–817. doi: 10.1016/0306-4522(83)90012-x. [DOI] [PubMed] [Google Scholar]
- Fuchs AF, Luschei ES. Firing patterns of abducens neurons of alert monkeys in relationship to horizontal eye movement. Journal of Neurophysiology. 1970;33:382–392. doi: 10.1152/jn.1970.33.3.382. [DOI] [PubMed] [Google Scholar]
- Fuchs AF, Robinson DA. A method for measuring horizontal and vertical eye movement chronically in the monkey. Journal of Applied Physiology. 1966;21:1068–1070. doi: 10.1152/jappl.1966.21.3.1068. [DOI] [PubMed] [Google Scholar]
- Fukushima K, Kaneko CRS, Fuchs AF. The neuronal substrate of integration in the oculomotor system. Progress in Neurobiology. 1992;39:609–639. doi: 10.1016/0301-0082(92)90016-8. [DOI] [PubMed] [Google Scholar]
- Godaux E, Cheron G. The hypothesis of the uniqueness of the oculomotor neural integrator: direct experimental evidences in the cat. Journal of Physiology. 1996;492:517–527. doi: 10.1113/jphysiol.1996.sp021326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godaux E, Mettens P, Cheron G. Differential effect of injections of kainic acid into the prepositus and the vestibular nuclei of the cat. Journal of Physiology. 1993;472:459–482. doi: 10.1113/jphysiol.1993.sp019956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henn V, Cohen B. Quantitative analysis of activity in eye muscle motoneurons during saccadic eye movements and position of fixation. Journal of Neurophysiology. 1973;36:115–126. doi: 10.1152/jn.1973.36.1.115. [DOI] [PubMed] [Google Scholar]
- Hikosaka O, Igusa Y, Imai H. Inhibitory connections of nystagmus-related reticular burst neurons in the abducens, prepositus hypoglossi and vestibular nuclei in the cat. Experimental Brain Research. 1980;39:301–311. doi: 10.1007/BF00237119. [DOI] [PubMed] [Google Scholar]
- Hikosaka O, Igusa Y, Nakao S, Shimazu H. Direct inhibitory synaptic linkage of pontomedullary reticular burst neurons with abducens motoneurons in the cat. Experimental Brain Research. 1978;33:337–352. doi: 10.1007/BF00235558. [DOI] [PubMed] [Google Scholar]
- Holstein GR, Martinelli GP, Degen JW, Cohen B. GABAergic neurons in the primate vestibular nuclei. Annals of the New York Academy of Sciences. 1996;781:443–457. doi: 10.1111/j.1749-6632.1996.tb15719.x. [DOI] [PubMed] [Google Scholar]
- Kaneko CRS. Eye movement deficits following ibotenic acid lesions of the nucleus prepositus hypoglossi in monkeys. I. Saccades and fixations. Journal of Neurophysiology. 1997;78:1753–1768. doi: 10.1152/jn.1997.78.4.1753. [DOI] [PubMed] [Google Scholar]
- Kaneko CRS, Evinger C, Fuchs AF. Role of cat pontine burst neurons in generation of saccadic eye movements. Journal of Neurophysiology. 1981;46:387–408. doi: 10.1152/jn.1981.46.3.387. [DOI] [PubMed] [Google Scholar]
- López-Barneo J, Darlot C, Berthoz A, Baker R. Neuronal activity in prepositus nucleus correlated with eye movement in the alert cat. Journal of Neurophysiology. 1982;47:329–352. doi: 10.1152/jn.1982.47.2.329. [DOI] [PubMed] [Google Scholar]
- McCrea RA, Baker R. Anatomical connections of the nucleus prepositus of the cat. Journal of Comparative Neurology. 1985;237:377–407. doi: 10.1002/cne.902370308. [DOI] [PubMed] [Google Scholar]
- McCrea RA, Yoshida K, Berthoz A, Baker R. Eye movements related activity and morphology of second order vestibular neurons terminating in the cat abducens nucleus. Experimental Brain Research. 1980;40:468–473. doi: 10.1007/BF00236156. [DOI] [PubMed] [Google Scholar]
- McFarland JL, Fuchs AF. Discharge patterns in nucleus prepositus hypoglossi and adjacent medial vestibular nucleus during horizontal eye movements in behaving macaques. Journal of Neurophysiology. 1992;68:319–332. doi: 10.1152/jn.1992.68.1.319. [DOI] [PubMed] [Google Scholar]
- Mettens P, Cheron G, Godaux E. NMDA receptors are involved in temporal integration in the oculomotor system of the cat. NeuroReport. 1994a;5:1333–1336. [PubMed] [Google Scholar]
- Mettens P, Cheron G, Godaux E. Role of the vestibular commisure in gaze-holding in the cat: a pharmacological evaluation. NeuroReport. 1994b;5:1421–1424. doi: 10.1097/00001756-199407000-00004. [DOI] [PubMed] [Google Scholar]
- Mettens P, Godaux E, Cheron G, Galiana HL. Effect of muscimol microinjection into the prepositus hypoglossi and the medial vestibular nuclei on cat eye movements. Journal of Neurophysiology. 1994c;72:785–802. doi: 10.1152/jn.1994.72.2.785. [DOI] [PubMed] [Google Scholar]
- Montague PR, Gancayco CD, Winn MJ, Marchase RB, Friedlander MJ. Role of NO production in NMDA receptor-mediated neurotransmitter release in cerebral cortex. Science. 1994;263:973–977. doi: 10.1126/science.7508638. [DOI] [PubMed] [Google Scholar]
- Moreno-López B, Escudero M, Delgado-García JM, Estrada C. Nitric oxide production by brain stem neurons is required for normal performance of eye movements in alert animals. Neuron. 1996;17:739–745. doi: 10.1016/s0896-6273(00)80205-6. [DOI] [PubMed] [Google Scholar]
- Moreno-López B, Escudero M, de Vente J, Estrada C. Morphological identification of nitric oxide sources and targets in the cat oculomotor system. Journal of Comparative Neurology. 2001;435:311–324. doi: 10.1002/cne.1032. [DOI] [PubMed] [Google Scholar]
- Moreno-López B, Estrada C, Escudero M. Mechanisms of action and targets of nitric oxide in the oculomotor system. Journal of Neuroscience. 1998;18:10672–10679. doi: 10.1523/JNEUROSCI.18-24-10672.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Precht W, Schwindt PC, Baker R. Removal of vestibular commisural inhibition by antagonists of GABA and glycine. Brain Research. 1973;62:222–226. doi: 10.1016/0006-8993(73)90631-8. [DOI] [PubMed] [Google Scholar]
- Robinson DA. Eye movement control in primates. Science. 1968;161:1219–1224. doi: 10.1126/science.161.3847.1219. [DOI] [PubMed] [Google Scholar]
- Robinson DA. Oculomotor control signals. In: Lennerstrand G, Bach-y-Rita P, editors. Basic Mechanisms of Ocular Motility and Their Clinical Implications. Oxford: Pergamon Press; 1975. pp. 337–374. [Google Scholar]
- Southam E, Garthwaite J. The nitric oxide-cyclic GMP signalling pathway in the rat brain. Neuropharmacology. 1993;32:1267–1277. doi: 10.1016/0028-3908(93)90021-t. [DOI] [PubMed] [Google Scholar]
- Spencer RF, Wehthold RJ, Baker R. Evidence for glycine as an inhibitory neurotransmitter of vestibular, reticular and prepositus hypoglossi neurons that project to the cat abducens nucleus. Journal of Neuroscience. 1989;9:2718–2736. doi: 10.1523/JNEUROSCI.09-08-02718.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strassman A, Highstein SM, Mccrea RA. Anatomy and physiology of saccadic burst neurons in the alert squirrel monkey. I. Excitatory burst neurons. Journal of Comparative Neurology. 1986a;249:337–357. doi: 10.1002/cne.902490303. [DOI] [PubMed] [Google Scholar]
- Strassman A, Highstein SM, Mccrea RA. Anatomy and physiology of saccadic burst neurons in the alert squirrel monkey. II. Inhibitory burst neurons. Journal of Comparative Neurology. 1986b;249:358–380. doi: 10.1002/cne.902490304. [DOI] [PubMed] [Google Scholar]
- Tanaka J, van Markerink Ittersum M, Steinbusch HWM, de Vente J. Nitric oxide-mediated cGMP synthesis in oligodendrocytes in the developing rat brain. Glia. 1997;19:286–297. [PubMed] [Google Scholar]
- Yoshida K, McCrea R, Berthoz A, Vidal PP. Morphological and physiological characteristics of inhibitory burst neurons controlling horizontal rapid eye movements in the alert cat. Journal of Neurophysiology. 1982;48:761–784. doi: 10.1152/jn.1982.48.3.761. [DOI] [PubMed] [Google Scholar]


