Abstract
Although it is established that the fetus can successfully withstand a single, acute hypoxaemic challenge during gestation, little is known about what effects prevailing adverse intrauterine conditions might have on the fetal response to acute hypoxaemia. The aims of this study were therefore: (1) to characterise the effects of prevailing and sustained hypoxaemia, acidaemia or hypoglycaemia on the fetal cardiovascular responses to an episode of acute hypoxaemia; and (2) to determine the effects of these adverse intrauterine conditions on mechanisms mediating these cardiovascular responses. Thirty-three Welsh Mountain sheep fetuses were chronically instrumented (1–2 % halothane) between 117 and 125 days of gestation (term is ca 145 days) with amniotic and vascular catheters and with a transit-time flow probe around a femoral artery. The animals were divided retrospectively into four groups based upon post-surgical, sustained, basal blood oxygen (chronically hypoxaemic; Pa,O2, 17.3 ± 0.5 mmHg; n = 8), glucose (chronically hypoglycaemic; blood glucose, 0.49 ± 0.03 mmol l−1; n = 6) and acid-base (chronically acidaemic; pHa, 7.25 ± 0.01; n = 5) status. Values for compromised fetuses were −2 s.d. from a group of control (n = 14) fetuses. At 130 ± 4 days, a 1 h episode of acute, isocapnic hypoxaemia (9 % O2 in N2, to reduce carotid Pa,O2 to 12 ± 1 mmHg) was induced in all fetuses by reducing the maternal inspired O2 fraction (FI,O2). Fetal cardiovascular variables were recorded at 1 s intervals throughout the experimental protocol and arterial blood samples taken at appropriate intervals for biophysical (blood gases, glucose, lactate) and endocrine (catecholamines, vasopressin, cortisol, ACTH) measures. During acute hypoxaemia all fetuses elicited hypertension, bradycardia and femoral vasoconstriction. However, prevailing fetal compromise altered the cardiovascular and endocrine responses to a further episode of acute hypoxaemia, including: (1) enhanced pressor and femoral vasoconstriction; (2) greater increments in plasma noradrenaline and vasopressin during hypoxaemia; and (3) basal upward resetting of hypothalamic-pituitary-adrenal axis function. Only chronically hypoxaemic fetuses had significantly elevated basal concentrations of noradrenaline and enhanced chemoreflex function during acute hypoxaemia. These data show that prevailing adverse intrauterine conditions alter the capacity of the fetus to respond to a subsequent episode of acute hypoxaemia; however, the partial contributions of hypoxaemia, acidaemia or hypoglycaemia to mediating these responses can vary.
Fetal cardiovascular responses to alterations in intrauterine conditions, such as hypoxaemia, have been demonstrated since the 1940s by the pioneering work of Barcroft and Barron (Barcroft, 1946). Since then, it has become well established that the fetal cardiovascular responses to episodes of acute hypoxaemia are primarily: (1) transient bradycardia, (2) gradual hypertension and (3) peripheral vasoconstriction (for review see Giussani et al. 1994b). These responses serve to reduce oxygen consumption by the myocardium and to redistribute blood flow away from the periphery towards hypoxia-sensitive organs, such as the adrenals, the heart and the brain (Rudolph et al. 1981).
The mechanisms mediating the fetal cardiovascular responses to acute hypoxaemia involve neural, endocrine and local components (Giussani et al. 1994b; Green, 2001). At the onset of acute hypoxaemia the fetal bradycardia and peripheral vasoconstriction are triggered by a carotid chemoreflex (Giussani et al. 1993; Bartelds et al. 1993). Circulatory redistribution is maintained by a combination of local vasodilator effects of hypoxia on the fetal adrenals (Breslow et al. 1993), brain (Green et al. 1996) and heart (Reller et al. 1995) and vasoconstrictor effects of hormones, such as catecholamines (Jones et al. 1988), vasopressin (Perez et al. 1989), cortisol (Gardner et al. 2001b), angiotensin II (Green et al. 1998) and neuropeptide Y (Fletcher et al. 2000) on the peripheral circulation. The fetal metabolic response to acute hypoxaemia results in increased blood glucose and lactate concentrations (Jones, 1977).
Although it is established that the fetus can successfully withstand a single, acute hypoxaemic challenge during gestation, little is known about the effects prevailing adverse intrauterine conditions might have on these adaptive mechanisms. This is important since clinical evidence suggests that prolonged hypoxaemia in utero accompanies fetal growth retardation (Nicolaides et al. 1989) and that antenatal hypoxaemia may predispose the fetus to birth asphyxia with subsequent neurodevelopmental handicap (Creasy & Resnik, 1981; Blanco et al. 1984; Mann, 1986; Dijxhoorn et al. 1987; Hill, 1991). However, chronic hypoxaemia in the fetus rarely occurs in isolation and, in complicated pregnancies, is most often accompanied by concurrent acidaemia and/or hypoglycaemia (Nicolaides et al. 1989). The cardiovascular and metabolic effects each of these adverse intrauterine conditions have on the subsequent capacity of the fetus to respond to a further acute episode of hypoxaemia are, however, currently unknown.
Hence, the aims of this study were: (1) to characterise the effects of prevailing hypoxaemia, acidaemia or hypoglycaemia on the fetal cardiovascular, endocrine and metabolic responses to a superimposed episode of acute hypoxaemia; and (2) to determine the effects of prevailing adverse intrauterine conditions on neural and endocrine mechanisms mediating these responses.
METHODS
Surgical preparation
Thirty-three Welsh Mountain ewes of known gestational age were used in the study. All procedures were performed under the UK Animals (Scientific Procedures) Act, 1986. All animals were fasted for 24 h prior to surgery.
Surgery was performed under strict aseptic conditions between 117 and 125 days of gestation (dGA; term being ca 145 dGA) on ewes carrying either singleton (15 ewes) or twin (18 ewes) fetuses. For ewes carrying twin fetuses only one fetus was randomly chosen and instrumented. Anaesthesia was induced with sodium thiopentone (20 mg kg−1i.v. Intraval Sodium; Rhone Mérieux, Dublin, Ireland) and maintained with 1–2 % halothane in 50:50 O2/N2O. In brief, following midline abdominal and uterine incisions, the fetal head was exteriorised for insertion of carotid artery and jugular vein catheters (i.d., 0.86 mm; o.d., 1.52 mm; Critchly Electrical Products, NSW, Australia) with the tips of the catheters extended to the ascending aorta and superior vena cava, respectively. The catheters were plugged with sterile brass pins and the uterine incision closed in layers. The fetal hindlimbs were subsequently exteriorised through a second uterine incision for insertion of femoral artery (i.d., 0.86 mm; o.d., 1.52 mm) and femoral vein (i.d., 0.56 mm; o.d., 0.96 mm) catheters, which were extended into the descending aorta and inferior vena cava, respectively. Another catheter was anchored onto the fetal hindlimb for recording of the reference pressure in the amniotic cavity. A transit-time flow transducer (Transonics, NY, USA) was placed around the contralateral femoral artery for continuous measurement of femoral blood flow, which has been shown to provide a good continuous index of blood flow distribution to peripheral circulations (Giussani et al. 1996). The second uterine incision was closed in layers. A teflon catheter was placed in the maternal femoral artery and extended to the descending aorta. Antibiotics were administered to the fetus through the femoral vein (300 mg ampicillin; Penbritin, SmithKline Beecham Animal Health, Surrey, UK) and amniotic (300 mg ampicillin) catheters. All catheters were filled with heparinised saline (80 i.u. ml−1 heparin in 0.9 % NaCl) and plugged with brass pins. Then, together with the flow probe lead, the catheters were exteriorised through an incision in the maternal flank and housed in a pouch sutured to the maternal skin.
Post-operative care
Animals were housed in individual pens with access to hay and water ad libitum. Concentrates were fed twice daily (100 g; sheep nuts #6; H & C Beart Ltd, Kings Lynn, UK). All ewes received antibiotics (0.20–0.25 mg kg−1i.m. Depocillin; Mycofarm, Cambridge, UK) and analgesia (10–20 mg kg−1 oral phenylbutazone; Equipalozone paste, Arnolds Veterinary Products Ltd, Shropshire, UK) immediately after surgery and daily for 3 days. Patency of fetal vascular catheters was maintained by a slow continuous infusion of heparinised saline (25 i.u. ml−1 heparin at 0.1 ml h−1 in 0.9 % NaCl) containing antibiotic (1 mg ml−1 benzylpenicillin; Crystapen, Schering-Plough, Animal Health Division, Welwyn Garden City, UK).
Experimental procedure
The fetuses were divided retrospectively over a 5 year period in our laboratory into four experimental groups according to consistent basal blood gas, acid-base and blood glucose status for at least 6 days after surgery (Table 1). If basal carotid Pa,O2 was consistently ≤ 18 mmHg, the fetuses were designated as being chronically hypoxaemic. If basal arterial pH and acid-base excess (ABE) were consistently ≤ 7.29 and/or ≤ −2.5 mequiv l−1, respectively, the fetuses were designated as being chronically acidaemic. If basal arterial blood glucose concentration was consistently ≤ 0.60 mmol l−1, then the fetuses were designated as being chronically hypoglycaemic. The criteria for division of the fetuses into these groups were determined as one variable only being consistently −2 s.d. below the normal range in arterial Pa,O2 (mean ± s.e.m., 23.2 ± 0.5 mmHg; range, 18–28 mmHg), pH (7.34 ± 0.01; range, 7.29–7.38), ABE (1.3 ± 0.4 mequiv l−1; range, −2.5 to 5 mequiv l−1) or glucose (0.82 ± 0.02 mmol l−1; range, 0.60–1.04 mmol l−1) of a large population of fetuses in our laboratory. Using this classification, eight of the 33 fetuses were determined as being chronically hypoxaemic (HYPOX), five as being chronically acidaemic (AC) and six as being chronically hypoglycaemic (HG). The remaining fetuses were used as controls (n = 14), which were selected to be contemporaneous with the compromised fetuses to match for gestational age, degree of instrumentation and, where possible, number of fetuses, from a much larger cohort (n = 63) of normal animals studied over the same 5 year period. No fetus in any compromised group demonstrated more than one adverse intrauterine condition simultaneously.
Table 1.
Fetal arterial blood gas, acid–base and blood glucose status after surgery
| Day 1 | Day 2 | Day 3 | Day 4 | Day 5 | Day 6 | |
|---|---|---|---|---|---|---|
| pHa | ||||||
| Control | 7.36 ± 0.01 | 7.35 ± 0.01 | 7.35 ± 0.01 | 7.36 ± 0.0 | 7.36 ± 0.01 | 7.35 ± 0.01 |
| Hypoxaemic | 7.33 ± 0.01 | 7.34 ± 0.01 | 7.36 ± 0.01 | 7.35 ± 0.0 | 7.36 ± 0.01 | 7.37 ± 0.01 |
| Acidaemic | 7.37 ± 0.01 | 7.33 ± 0.01* | 7.33 ± 0.01* | 7.31 ± 0.02*† | 7.31 ± 0.02*†‡ | 7.28 ± 0.02*†‡ |
| Hypoglycaemic | 7.36 ± 0.01 | 7.35 ± 0.01 | 7.33 ± 0.01 | 7.35 ± 0.01 | 7.34 ± 0.01 | 7.33 ± 0.01 |
| Pa,CO2 (mmHg) | ||||||
| Control | 49.1 ± 1.1 | 50.0 ± 1.5 | 48.5 ± 1.3 | 48.5 ± 1.4 | 49.4 ± 1.1 | 52.0 ± 1.0* |
| Hypoxaemic | 52.5 ± 1.5 | 53.3 ± 1.1 | 52.1 ± 1.3 | 52.0 ± 1.3 | 53.0 ± 1.7 | 50.0 ± 1.3 |
| Acidaemic | 50.6 ± 1.4 | 51.0 ± 0.8 | 52.0 ± 1.5 | 51.0 ± 1.8 | 53.2 ± 1.4 | 50.8 ± 1.9 |
| Hypoglycaemic | 48.8 ± 1.0 | 50.6 ± 0.9 | 50.8 ± 0.6 | 50.5 ± 0.6 | 51.8 ± 0.9 | 49.6 ± 1.5 |
| Pa,O2 (mmHg) | ||||||
| Control | 26.2 ± 1.2 | 28.5 ± 2.4 | 26.5 ± 0.8 | 26.6 ± 1.6 | 25.5 ± 0.9 | 23.4 ± 1.1 |
| Hypoxaemic | 18.3 ± 1.8‡ | 18.0 ± 1.4‡ | 18.6 ± 1.5‡ | 18.8 ± 1.5‡ | 17.0 ± 1.2‡ | 17.3 ± 1.2‡ |
| Acidaemic | 21.6 ± 0.6 | 22.0 ± 0.8 | 22.8 ± 1.3 | 22.4 ± 2.0 | 23.0 ± 1.0 | 25.4 ± 2.0 |
| Hypoglycaemic | 24.0 ± 1.8 | 23.5 ± 2.1 | 24.0 ± 1.5 | 23.1 ± 1.3 | 22.5 ± 1.0 | 23.1 ± 1.2 |
| ABE (mequiv l−1) | ||||||
| Control | 2.0 ± 0.7 | 2.0 ± 0.9 | 0.6 ± 0.7 | 2.0 ± 0.7 | 2.2 ± 0.7 | 2.5 ± 0.7 |
| Hypoxaemic | 1.0 ± 1.2 | 2.1 ± 0.7 | 2.5 ± 0.4 | 2.1 ± 0.3 | 2.3 ± 0.4 | 2.8 ± 0.6 |
| Acidaemic | 2.4 ± 0.7 | 0.2 ± 0.8 | 0.4 ± 1.0 | 0.6 ± 0.6* | −0.8 ± 1.2* | −3.2 ± 1.7*‡ |
| Hypoglycaemic | 1.5 ± 1.2 | 1.6 ± 0.8 | 0.5 ± 1.0 | 1.0 ± 1.2 | −0.2 ± 0.9 | −0.6 ± 1.4 |
| Blood [glucose] (mmol l−1) | ||||||
| Control | 0.79 ± 0.11 | 0.68 ± 0.06 | 0.71 ± 0.07 | 0.78 ± 0.07 | 0.75 ± 0.07 | 0.71 ± 0.05 |
| Hypoxaemic | 0.78 ± 0.11 | 0.73 ± 0.12 | 0.65 ± 0.08 | 0.78 ± 0.23 | 0.89 ± 0.20 | 0.93 ± 0.16 |
| Acidaemic | 0.79 ± 0.11 | 0.67 ± 0.12 | 0.64 ± 0.06 | 0.65 ± 0.08 | 0.64 ± 0.09 | 0.69 ± 0.16 |
| Hypoglycaemic | 0.50 ± 0.05‡ | 0.48 ± 0.04‡ | 0.49 ± 0.02‡ | 0.47 ± 0.02‡ | 0.51 ± 0.07‡ | 0.46 ± 0.04‡ |
Fetal carotid arterial blood gas, acid-base and blood glucose status for 6 days post-surgery in control and chronically hypoxaemic (HYPOX), chronically acidaemic (AC) and chronically hypoglycaemic (HG) fetuses. Daily fetal blood samples were collected for measurement of blood gases between 08.30 and 10.00 h. Values are means ± s.e.m. for control (n = 14), HYPOX (n = 8), AC (n = 5) and HG (n = 6) fetuses. Statistical differences are:
P < 0.05, days 2–6 vs. day 1
P < 0.05, control vs. compromised (HYPOX or AC or HG) fetuses
P < 0.05, compromised vs. all other groups. Fetal blood gas values were corrected to 39.5°C. pHa, arterial pH;Pa,CO2, arterial partial pressure of CO2; Pa,O2, arterial partial pressure of O2, ABE, acid–base excess; blood [glucose], arterial blood glucose concentration.
All fetuses were subjected to one episode of acute hypoxaemia (at 130 ± 3 days, mean ± s.d., for control and AC fetuses; at 129 ± 4 days for HYPOX fetuses; and at 131 ± 4 days for HG fetuses). The protocol for acute fetal hypoxaemia involved a 3 h experiment consisting of 1 h of normoxia, 1 h of hypoxaemia and 1 h of recovery, as described previously (Gardner et al. 2001b). Briefly, a large, transparent, polythene bag was placed over the ewes’ head into which air was passed at a rate of ca 40 l min−1 for the first 1 h. Following this control period, fetal hypoxaemia was induced by changing the concentrations of gases breathed by the ewe to 9 % O2 in N2 with 2–3 % CO2. This mixture was designed to reduce fetal carotid Pa,O2 to ca 12 mmHg while maintaining Pa,CO2. Following the 1 h period of hypoxaemia, the ewe was returned to breathing air for the 1 h recovery period. At the end of the experimental protocol, the ewes and fetuses were humanely killed using a lethal dose of sodium pentobarbitone (200 mg kg−1iv. Pentoject; Animal Ltd, York, UK) and the positions of the implanted catheters and the flow probe were confirmed and fetal weights measured.
Measurements and calculations
Maternal (descending aorta) and fetal (carotid and descending aorta) arterial blood samples (0.4 ml) were drawn into sterile syringes daily for measurement of arterial blood gases, percentage saturation of O2 in haemoglobin, haemoglobin concentration and acid-base status using an ABL5 blood gas analyser and OSM2 haemoximeter (Radiometer, Copenhagen, Denmark) and blood glucose and lactate concentrations using an automated analyser (Yellow Springs 2300 Stat Plus glucose/lactate analyser; YSI, Farnborough, UK). During the acute hypoxaemia protocol, additional fetal arterial blood samples (4 ml) were collected at 15 and 45 min of normoxia, at 15 and 45 min of hypoxaemia and at 45 min of recovery for measurement of blood gases, acid-base status and glucose and lactate concentrations, and determination of plasma hormone concentrations. Measurements in maternal and fetal blood were corrected to 38 and 39.5 °C, respectively.
Fetal arterial blood oxygen content (Ca,O2; in mmol l−1) and oxygen delivery (O2,del; in μmol min−1) to the hindlimb were calculated using eqns (1) and (2), respectively:
| (1) |
| (2) |
where [Hb] (g dl−1) is the blood concentration of haemoglobin, SatHb is the percentage oxygen saturation of haemoglobin, femoral blood flow is measured in ml min−1, and where one molecule of Hb (MW 64 450) binds four molecules of oxygen. The contribution of oxygen dissolved in plasma is regarded as negligible (Owens et al. 1987).
Hormone analyses
Blood samples for hormone analyses were either collected into K+/EDTA-treated tubes (1.5 ml; ACTH, cortisol and vasopressin) or chilled EGTA- (5.0 μmol ml−1 blood) and glutathione (40 μmol ml−1 blood)-treated tubes (1 ml; catecholamines) and centrifuged immediately at 4000 r.p.m. for 4 min at 4 °C. Plasma samples were stored at −70 °C until analyses. All hormone analyses were completed within 2 months of plasma collection.
ACTH
Maternal and fetal plasma ACTH concentrations were measured using a commercially available double-antibody 125I radioimmunoassay (RIA) kit (Incstar Ltd, Wokingham, UK), as described previously (Gardner et al. 2001b). The lower limit of detection for the assay was between 10 and 25 pg ml−1 and the assay had < 0.01 % cross-reactivities for α-melanocyte-stimulating hormone, β-endorphin, β-lipotrophin, leucine enkephalin, methionine enkephalin, bombesin, calcitonin, parathyroid hormone, follicle-stimulating hormone, arginine vasopressin, oxytocin and substance P. The intra- and inter-assay coefficients of variation were 4 % and < 10 %, respectively.
Cortisol
Maternal and fetal plasma cortisol concentrations were measured by RIA validated for use in ovine plasma, as described previously (Fowden et al. 1993). The lower limit of detection for the assay was 1.0–1.5 ng ml−1 and cross-reactivity of the anti-serum at 50 % binding with other cortisol-related compounds was 0.5 % for cortisone, 2.3 % for corticosterone, 0.3 % for progesterone and 4.6 % for deoxycortisol. The intra- and inter-assay coefficients of variation were 5 % and 8 %, respectively.
Catecholamines
The plasma catecholamines, adrenaline and noradrenaline, were analysed by high performance liquid chromatography (HPLC) using electrochemical detection (Fowden et al. 1998). The samples were prepared by absorption of 250 μl of plasma onto acid-washed alumina and 20 μl aliquots of the 100 μl perchloric acid elutes were injected onto the column. Dihydroxy benzylamine was added as the internal standard to each plasma sample before absorption. The limit of sensitivity for the assay was 20 pg ml−1 for adrenaline and noradrenaline. The inter-assay coefficients of variation for adrenaline and noradrenaline were < 10 %.
Vasopressin
Plasma vasopressin concentrations were measured using a commercially available double-antibody RIA kit (Nichols Institute Diagnostics Ltd, Saffron Walden, Essex, UK) following separation from plasma proteins by methanol extraction and chromatography, as described previously (Gardner et al. 2001a). The lower detection limit of the assay was 0.75 pg ml−1. The inter-assay coefficients of variation for two plasma samples (2.71 and 5.55 pg ml−1) were 4.1 and 9.8 %, respectively (Giussani et al. 1994a).
Data collection and analyses
Fetal arterial blood pressure was corrected for amniotic pressure. Fetal femoral blood flow was measured with a T201 or T206 flow meter (Transonic Inc., NY, USA). Fetal heart rate was triggered from the flow pulse. Femoral vascular resistance was calculated according to Ohm's principle by dividing arterial blood pressure (corrected for amniotic pressure) by femoral blood flow (Giussani et al. 1993). Functional chemoreflex curves were constructed by plotting the fall in fetal arterial PO2 against the nadir in fetal heart rate or the maximal increase in fetal femoral vascular resistance (FVR) within the first 15 min of hypoxaemia. All analog signals for calibrated fetal cardiovascular data were recorded continuously throughout the study using a data acquisition system. All signals were digitised, displayed and subsequently stored at 1 s intervals on disk by custom software (NI-DAQ, National Instruments, Austin, Texas) running on a PC. Files were subsequently analysed using Microsoft Excel spreadsheets.
Statistical analyses
Values for all variables are expressed as means ± s.e.m. unless otherwise stated. Cardiovascular variables during the acute hypoxaemia protocol are expressed as minute averages. For these data, areas under the curve were constructed by changes from mean baseline and these were analysed by the summary measures method to focus the number of comparisons, as previously described in detail (Matthews et al. 1990; Giussani et al. 2001). In brief the 1 h period of hypoxaemia was divided into pre-determined intervals known to best describe the salient features of the fetal cardiovascular responses to this challenge: first 15 min (early hypoxaemia) and remaining 45 min (late hypoxaemia). Cardiovascular and all other measured variables were tested for normality of distribution and assessed for statistical significance using two-way ANOVA with repeated measures for the effect of time (baseline (1 h of normoxia) vs. early (first 15 min) or late (last 45 min) hypoxaemia or recovery) and group (control vs. HYPOX/AC/HG) and interactions between time and group, followed by the post hoc Tukey test or Student's t test for unpaired data (Sigma-Stat; SPSS Inc, Chicago, USA). A comparison between the slopes and intercepts of linear regression curves was conducted according to Armitage & Berry (1994). Multiple linear regression analyses were used to determine which independent variable best predicted the dependent variable. Where two or more independent variables were found to predict a dependent variable and co-linearity was present, partial correlation analysis was used to determine the relative contribution of each according to Snedecor (1946). For all comparisons statistical significance was accepted when P < 0.05.
RESULTS
Demography of the fetal groups
Fetal body weights were similar in all groups and averaged 2.54 ± 0.5 kg at post mortem (133 ± 3 dGA, mean ± s.d.). The control group comprised nine singleton (5 male, 4 female) and five twin (3 male, 2 female) fetuses. The HYPOX group comprised four singleton (2 male, 2 female) and four twin (3 male, 1 female) fetuses. The AC group comprised one singleton (1 female) and four twin (1 male, 3 female) fetuses. The HG group comprised one singleton (1 male) and five twin (3 male, 2 female) fetuses.
Maternal data
Values for basal arterial PO2, arterial haemoglobin concentration ([Hb]), saturation of haemoglobin (SatHb), pHa, acid-base excess (ABE), blood glucose and lactate concentrations were similar in all groups of ewes and were appropriate for Welsh Mountain ewes at this stage of gestation (combined group average for arterial PO2, 100 ± 4 mmHg; [Hb], 8 ± 2 g dl−1; SatHb, 94 ± 3 %; pH, 7.49 ± 0.01; ABE, 4.5 ± 1.5 mequiv l−1; blood glucose, 2.55 ± 0.30 mmol l−1; blood lactate, 0.34 ± 0.06 mmol l−1). In addition, basal values for ACTH, cortisol and vasopressin were similar in all groups of ewes (control: 37.7 ± 10.0 pg ml−1, 24.0 ± 6.5 ng ml−1 and 1.4 ± 0.3 pg ml−1, respectively; HYPOX: 62.1 ± 13.2 pg ml−1, 36.9 ± 15.0 ng ml−1 and 2.0 ± 0.9 pg ml−1; AC: 50.8 ± 12.5 pg ml−1, 29.6 ± 12.2 ng ml−1 and 2.2 ± 1.0 pg ml−1; HG: 40.1 ± 13.3 pg ml−1, 31.8 ± 9.2 ng ml−1 and 2.2 ± 1.0 pg ml−1). Maternal plasma concentrations of catecholamines were not measured in this study.
During acute hypoxaemia, maternal Pa,O2 and SatHb were reduced to a similar extent in control (to 41.0 ± 2.1 mmHg and 62.5 ± 3.9 %), HYPOX (to 42.7 ± 3.5 mmHg and 68.5 ± 2.5 %), AC (to 38.2 ± 2.7 mmHg and 58.8 ± 7.0 %) and HG (to 36.2 ± 2.0 mmHg and 50.4 ± 5.6 %) fetuses (all P < 0.05). All other maternal variables remained unchanged from basal values.
Fetal blood gas and metabolic status
Post-surgery
Blood gas, acid-base and blood glucose status on the first day after surgery were appropriate for control fetuses at this gestational age from our laboratory (Forhead et al. 1995; Fletcher et al. 2000; Gardner et al. 2001b) and remained unaltered between days 1 and 6 after surgery, with the exception of Pa,CO2, which was significantly elevated at day 6 relative to day 1 after surgery (Table 1). In contrast, HYPOX and HG fetuses were either hypoxaemic (significantly lower Pa,O2) or hypoglycaemic (significantly lower blood glucose), respectively, between days 1 and 6 after surgery and remained so until the end of the experimental protocol (P < 0.05 to all other groups; Table 1 and Fig. 1). AC fetuses developed acidaemia at 2 days after surgery and remained acidaemic thereafter until the end of the experimental protocol (Table 1).
Figure 1. Fetal arterial blood pressure and heart rate during acute hypoxaemia.
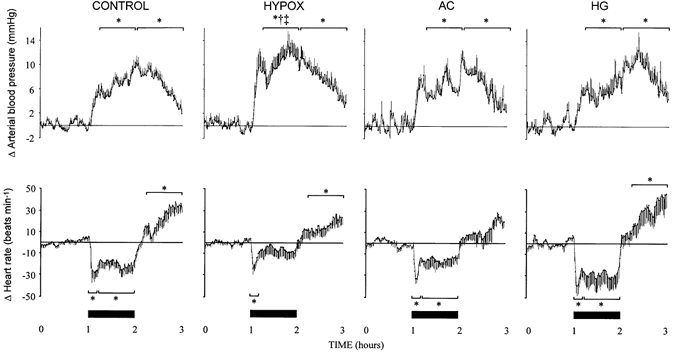
Data represent absolute changes from baseline for mean arterial blood pressure and heart rate during acute hypoxaemia in control (n = 14) and HYPOX (n = 8), AC (n = 5) or HG (n = 6) fetuses. Values are means ± s.e.m. of cardiovascular data (1 min average) for a baseline period (1 h normoxia), 1 h of hypoxaemia (filled bars) and 1 h of recovery. Statistical differences are: * P < 0.05, normoxia vs. early (first 15 min) or late (last 45 min) hypoxaemia or recovery; † P < 0.05, control vs. HYPOX fetuses; ‡ P < 0.05, HYPOX vs. HG fetuses. For details of groups refer to Table 1.
Acute hypoxaemic protocol
During baseline
In HYPOX fetuses, values for basal arterial PO2 and SatHb were significantly lower, and values for basal arterial [Hb] were significantly greater, than respective values in control fetuses (Tables 2 and 3). Consequently, Ca,O2 was not significantly different between control and HYPOX fetuses (Table 3). Basal acid-base status and blood glucose and lactate concentrations were also similar in control and HYPOX fetuses (Tables 2 and 3). In AC fetuses, values for basal arterial pH, ABE and blood lactate concentration were significantly lower relative to control fetuses (Tables 2 and 3). Values for all other variables were similar in AC and control fetuses. In HG fetuses, basal values for arterial pH, Pa,O2, SatHb, ABE and [Hb] were similar, but blood glucose and lactate concentrations were significantly lower, relative to control fetuses (Tables 2 and 3).
Table 2.
Fetal arterial blood gas and acid–base status during acute hypoxaemia
| Baseline | Acute hypoxaemia | Recovery | ||||
|---|---|---|---|---|---|---|
| N15 | N45 | H15 | H45 | R15 | R45 | |
| pHa | ||||||
| Control | 7.35 ± 0.01 | 7.34 ± 0.01 | 7.32 ± 0.01 | 7.27 ± 0.01* | 7.24 ± 0.02* | 7.29 ± 0.02* |
| Hypoxaemic | 7.36 ± 0.01 | 7.35 ± 0.01 | 7.31 ± 0.01 | 7.27 ± 0.01* | 7.23 ± 0.03* | 7.26 ± 0.02* |
| Acidaemic | 7.25 ± 0.01† | 7.25 ± 0.01† | 7.20 ± 0.01† | 7.12 ± 0.06*†‡ | 7.09 ± 0.05*†‡ | 7.15 ± 0.05*† |
| Hypoglycaemic | 7.33 ± 0.01 | 7.33 ± 0.01 | 7.29 ± 0.02 | 7.20 ± 0.06* | 7.17 ± 0.05* | 7.22 ± 0.05* |
| Pa,CO2 (mmHg) | ||||||
| Control | 53.2 ± 1.4 | 53.0 ± 1.8 | 52.8 ± 0.7 | 53.0 ± 1.3 | 48.6 ± 1.3* | 50.4 ± 1.6 |
| Hypoxaemic | 50.5 ± 1.5 | 52.8 ± 0.7 | 52.8 ± 1.4 | 50.8 ± 2.3 | 47.1 ± 3.6 | 51.3 ± 2.4 |
| Acidaemic | 52.8 ± 1.9 | 52.4 ± 1.6 | 53.8 ± 1.4 | 52.6 ± 2.7 | 50.2 ± 0.5 | 51.4 ± 0.4 |
| Hypoglycaemic | 50.2 ± 1.7 | 50.3 ± 1.4 | 48.5 ± 1.6 | 50.8 ± 2.7 | 49.6 ± 0.6 | 50.0 ± 1.0 |
| Pa,O2 (mmHg) | ||||||
| Control | 22.9 ± 1.0 | 22.0 ± 1.0 | 12.0 ± 0.8* | 12.4 ± 0.7* | 24.5 ± 2.2 | 21.5 ± 1.6 |
| Hypoxaemic | 17.3 ± 0.5† | 16.3 ± 1.0† | 10.4 ± 0.2* | 12.0 ± 0.4* | 19.3 ± 1.9†‡§ | 18.1 ± 0.6 |
| Acidaemic | 22.8 ± 1.3 | 22.2 ± 0.9 | 12.2± 0.4* | 12.8 ± 0.7* | 26.4 ± 2.3 | 23.6 ± 1.8 |
| Hypoglycaemic | 23.8 ± 1.3 | 23.0 ± 1.0 | 11.6 ± 0.6* | 13.0 ± 0.8* | 24.8 ± 2.4 | 23.6 ± 1.3 |
| SatHb (%) | ||||||
| Control | 64.6 ± 3.0 | 62.7 ± 3.4 | 33.3 ± 2.8* | 30.1 ± 3.7* | 62.3 ± 3.9 | 58.2 ± 4.4 |
| Hypoxaemic | 53.8 ± 1.7 † | 48.8 ±3.8† | 30.9 ± 1.6 | 32.6 ± 1.8* | 50.4 ± 2.8 | 44.1 ± 2.2 † |
| Acidaemic | 60.9 ± 3.9 | 61.1 ± 2.8 | 28.4 ± 3.4* | 28.8 ± 3.2* | 58.1 ± 5.2 | 54.8 ± 4.3 |
| Hypoglycaemic | 65.3 ± 4.0 | 65.4 ± 4.4 | 26.3 ± 4.1* | 26.2 ± 4.8* | 55.9 ± 5.9 | 56.5 ± 6.4 |
| ABE (mequiv l−1) | ||||||
| Control | 3.0 ± 0.5 | 2.2 ± 0.4 | 0.6 ± 0.6 | −3.5 ± 1.1* | −6.6 ± 1.5* | −2.8 ± 1.4* |
| Hypoxaemic | 2.1 ± 0.3 | 2.1 ± 0.3 | 0.0 ± 0.7 | −4.0 ± 1.4* | −6.5 ± 2.0* | −4.1 ± 2.2* |
| Acidaemic | −4.6 ± 1.4† | −4.8 ± 1.4† | −7.6 ± 1.1†‡ | −12.6 ± 3.0 *†‡ | −14.8 ± 2.8*†‡ | −10.4 ± 2.3*† |
| Hypoglycaemic | −0.1 ± 1.5 | −0.3 ± 1.2 | −3.0 ± 1.5 † | −8.6 ± 2.9* | −9.8 ± 3.1* | −6.6 ± 2.2 * |
Fetal carotid arterial blood gas and acid-base status during acute hypoxaemia in control and chronically hypoxaemic (HYPOX), chronically acidaemic (AC) and chronically hypoglycaemic (HG) fetuses. Fetal blood samples were collected for measurement of blood gases at 15 (N15) and 45 min (N45) of normoxia (baseline), at 15 (H15) and 45 min (H45) of hypoxaemia and at 15 (R15) and 45 min (R45) of recovery. Values are means ± s.e.m. for control (n = 14), HYPOX (n = 8), AC (n = 5) and HG (n = 6) fetuses. Statistical differences are:
P < 0.05, normoxia vs. hypoxaemia or recovery
P < 0.05, control vs.compromised (HYPOX or AC or HG) fetuses
P < 0.05, AC vs. HYPOX fetuses
P < 0.05, HYPOX vs. HG fetuses. Fetal blood gas values were corrected to 39.5 °C. SatHb, percentage oxygen saturation of haemoglobin.
Table 3.
Fetal metabolic status during hypoxaemia
| Baseline | Hypoxaemia | Recovery | ||||
|---|---|---|---|---|---|---|
| N15 | N45 | H15 | H45 | R15 | R45 | |
| [Glucose] (mmol l−1) | ||||||
| Control | 0.75 ± 0.05 | 0.76 ± 0.03 | 0.90 ± 0.05 | 1.12 ± 0.09* | 0.97 ± 0.12* | 0.80 ± 0.04 |
| Hypoxaemic | 0.87 ± 0.17 | 0.86 ± 0.16 | 1.18 ± 0.24* | 1.43 ± 0.23* | 1.23 ± 0.24* | 1.11 ± 0.25 |
| Acidaemic | 0.66 ± 0.07 | 0.76 ± 0.08 | 1.20 ± 0.08* | 1.48 ± 0.12* | 1.39 ± 0.24* | 1.14 ± 0.15* |
| Hypoglycaemic | 0.49 ± 0.03† | 0.56 ± 0.07† | 0.77 ± 0.07†‡ | 1.05 ± 0.01* | 1.07 ± 0.27* | 0.81 ± 0.19* |
| [Lactate[ (mmol l−1) | ||||||
| Control | 0.95 ± 0.06 | 0.96 ± 0.07 | 1.71 ± 0.17 | 3.66 ± 0.51* | 4.70 ± 0.69* | 3.70 ± 0.73* |
| Hypoxaemic | 1.08 ± 0.10 | 1.17 ± 0.11 | 2.28 ± 0.21 | 4.27 ± 0.63* | 5.08 ± 0.90* | 4.41 ± 0.86* |
| Acidaemic | 0.63 ± 0.08† | 0.73 ± 0.11† | 2.53 ± 0.15 | 4.65 ± 0.65* | 5.71 ± 1.27* | 5.55 ± 1.41* |
| Hypoglycaemic | 0.72 ± 0.07† | 0.77 ± 0.10 | 2.21 ± 0.32 | 4.88 ± 0.61* | 6.31 ± 0.92* | 5.25 ± 1.04* |
| Hb](g dl−1) | ||||||
| Control | 9.1 ± 0.9 | 8.7 ± 0.8 | 10.0 ± 0.7* | 9.8 ± 0.8* | 8.5 ± 0.9 | 8.6 ± 0.6 |
| Hypoxaemic | 11.2 ± 0.7† | 10.9 ± 0.6† | 11.7 ± 0.6 | 11.5 ± 0.6 | 9.0 ± 0.7* | 10.3 ± 0.7† |
| Acidaemic | 8.2 ± 0.4 | 8.1 ± 0.4 | 9.1 ± 0.3* | 9.2 ± 0.5* | 7.8 ± 0.4 | 8.1 ± 0.2 |
| Hypoglycaemic | 8.9 ± 0.7 | 8.7 ± 0.7 | 9.8 ± 0.6* | 9.3 ± 0.6 | 8.5 ± 0.5 | 8.8 ± 0.4 |
| Cao2(mmol1−1) | ||||||
| Control | 3.10 ± 0.28 | 3.16 ± 0.29 | 1.90 ± 0.23* | 1.71 ± 0.17* | 2.98 ± 0.24 | 2.70 ± 0.26* |
| Hypoxaemic | 3.72 ± 0.23 | 3.27 ± 0.21 | 2.26 ± 0.20* | 2.46 ± 0.20* | 3.07 ± 0.80* | 2.79 ± 0.17* |
| Acidaemic | 3.09 ± 0.19 | 3.07 ± 0.18 | 1.63 ± 0.23* | 1.66 ± 0.22* | 2.81 ± 0.30 | 2.78 ± 0.26 |
| Hypoglycaemic | 3.57 ± 0.33 | 3.34 ± 0.25 | 1.58 ± 0.24* | 1.67 ± 0.29* | 2.91 ± 0.34* | 3.05 ± 0.31* |
Fetal metabolic status during acute hypoxaemia in control and chronically hypoxaemic (HYPOX), chronically acidaemic (AC) and chronically hypoglycaemic (HG) fetuses. Fetal blood samples were collected for measurement of blood gases at 15 (N15) and 45min (N45) of normoxia (baseline), at 15 (H15) and 45min (H45) of hypoxaemia and at 15 (R15) and 45min (R45) of recovery. Values are means ± s.e.m. for control (n = 14), HYPOX (n = 8), AC (n = 5) and HG (n = 6) fetuses. Statistical differences are:
P < 0.05, normoxia vs. hypoxaemia or recovery
P < 0.05, control vs. compromised (HYPOX or AC or HG) fetuses
P < 0.05, HG vs. AC fetuses. Fetal blood gas values were corrected to 39.5 °C. [Glucose], arterial blood glucose concentration; [Lactate], arterial blood lactate concentration; [Hb], arterial concentration of haemoglobin; Ca,O2, arterial oxygen content.
During acute hypoxaemia
In all groups of fetuses, induction of acute hypoxaemia reduced fetal Pa,O2, SatHb and Ca,O2 to a similar level, without any change in Pa,CO2, and led to similar increments in blood glucose (control, 0.43 ± 0.09 mmol l−1; HYPOX, 0.56 ± 0.09 mmol l−1; AC, 0.76 ± 0.12 mmol l−1; HG, 0.52 ± 0.14 mmol l−1) and lactate (control, 3.74 ± 0.59 mmol l−1; HYPOX, 3.95 ± 0.95 mmol l−1; AC, 5.02 ± 1.28 mmol l−1; HG, 5.56 ± 0.96 mmol l−1) concentrations (Tables 2 and 3). Acute hypoxaemia led to significant reductions in arterial pH and ABE in all groups of fetuses; however, the magnitude of the decrements were significantly greater in AC (pHa: −0.13 ± 0.05; ABE: −7.9 ± 2.4 mequiv l−1) relative to control (pHa: −0.08 ± 0.01; ABE: −6.1 ± 1.0 mequiv l−1) fetuses (Table 2). In addition, an increase in arterial [Hb] occurred after 15 min of acute hypoxaemia in all groups, with the exception of HYPOX fetuses (Table 3).
During recovery
After 45 min recovery from acute hypoxaemia fetal arterial pH and ABE remained significantly lower relative to baseline in all fetuses; however, the decrements in both variables remained significantly greater in AC (pHa: −0.16 ± 0.05; ABE: −10.1 ± 2.3 mequiv l−1) relative to control (pHa: −0.10 ± 0.02; ABE: −8.6 ± 1.3 mequiv l−1) fetuses (Table 2). During recovery, fetal arterial PO2, SatHb and [Hb] returned towards basal levels in all fetuses, although in HYPOX, arterial PO2 and [Hb] remained significantly lower relative to control fetuses (Tables 2 and 3). In control and AC fetuses, Ca,O2 returned towards basal values, but remained lower throughout the recovery period in HYPOX and HG fetuses (Table 3).
Fetal cardiovascular variables
During baseline
Values for fetal arterial blood pressure and heart rate during baseline were similar in all groups (control, 49.0 ± 2.4 mmHg and 166 ± 3 beats min−1; HYPOX, 48.9 ± 3.9 mmHg and 167 ± 7 beats min−1; AC, 43.9 ± 2.6 mmHg and 162 ± 8.9 beats min−1; HG, 50.6 ± 3.4 mmHg and 156 ± 5 beats min−1, blood pressure and heart rate, respectively). However, values for femoral blood flow and femoral O2,del, in both AC (27.1 ± 1.6 ml min−1 and 74 ± 9 μmol min−1, respectively) and HG (29.5 ± 1.8 ml min−1 and 82 ± 10 μmol min−1) fetuses, were significantly lower than respective values in control (39.5 ± 2.3 ml min−1 and 116 ± 10 μmol min−1), but not HYPOX (32.3 ± 3.6 ml min−1 and 104 ± 12 μmol min−1) fetuses. Despite lower basal values for femoral blood flow in AC and HG fetuses, basal values for femoral vascular resistance were similar in all groups (control, 1.39 ± 0.10 mmHg (ml min−1)−1; HYPOX, 1.59 ± 0.09 mmHg (ml min−1)−1; AC, 1.71 ± 0.15 mmHg (ml min−1)−1; and HG, 1.67 ± 0.06 mmHg (ml min−1)−1).
During acute hypoxaemia
In all groups of fetuses studied, acute hypoxaemia led to significant hypertension, bradycardia and femoral vasoconstriction (Fig. 1 and Fig. 2). The pressor response to acute hypoxaemia was greater in HYPOX relative to control fetuses (Fig. 1). The initial reductions in heart rate at the onset of acute hypoxaemia were to a similar level in all groups, although heart rate returned towards baseline values in HYPOX fetuses during hypoxaemia, yet remained depressed, albeit at a lower level, throughout the challenge in all other groups (Fig. 1). In all fetuses, acute hypoxaemia reduced femoral blood flow and increased femoral vascular resistance for the duration of the challenge (Fig. 2). The increase in fetal femoral vascular resistance during hypoxaemia was, however, significantly greater in HYPOX, AC and HG relative to control (Fig. 2). The reduction in O2,del to the fetal hindlimb during hypoxaemia was similar between groups although lower deliveries in AC and HG (17 ± 6 and 12 ± 2 μmol min−1, respectively) were maintained relative to control and HYPOX (42 ± 8 and 28 ± 7 μmol min−1, respectively) fetuses.
Figure 2. Fetal femoral blood flow and vascular resistance during acute hypoxaemia.
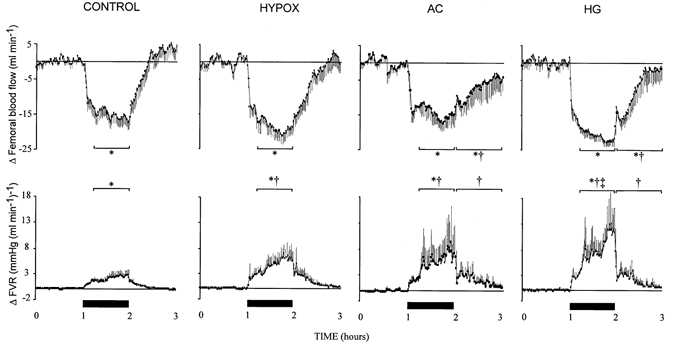
Data represent absolute changes from baseline for mean fetal femoral blood flow and vascular resistance (FVR) during acute hypoxaemia in control (n = 14) and HYPOX (n = 8), AC (n = 5) or HG (n = 6) fetuses. Values are means ± s.e.m. of cardiovascular data (1 min average) for a baseline period (1 h normoxia), 1 h of hypoxaemia (filled bars) and 1 h of recovery. Statistical differences are: * P < 0.05, normoxia vs. early (first 15 min) or late (last 45 min) hypoxaemia or recovery; † P < 0.05, control vs. compromised (HYPOX or AC or HG) fetuses; ‡ P < 0.05, HG vs. HYPOX fetuses. For details of groups refer to Table 1.
During recovery
Arterial blood pressure remained significantly elevated from baseline for the duration of the recovery period in all groups of fetuses (Fig. 1). All fetuses, with the exception of AC, exhibited significant tachycardia after cessation of acute hypoxaemia (Fig. 1). While femoral blood flow and vascular resistance returned towards basal levels during recovery in control and HYPOX fetuses, values for femoral blood flow remained significantly lower, and those for femoral vascular resistance significantly higher, in AC and HG fetuses (Fig. 2). During recovery, hindlimb O2,del was restored to baseline values in control fetuses but remained at a significantly lower level in HYPOX, AC and HG fetuses relative to baseline and to control fetuses (control, 103 ± 12 μmol min−1; HYPOX, 65 ± 15 μmol min−1; AC, 44 ± 16 μmol min−1; HG, 54 ± 15 μmol min−1, P < 0.05).
Functional chemoreflex activity during acute hypoxaemia
Acute hypoxaemia reduced carotid Pa,O2 to a similar level in all fetuses (Table 2). Whilst the absolute fall in Pa,O2 was similar in control, AC and HG fetuses (falls of 10 ± 3 mmHg), the decrement in Pa,O2 was significantly less in HYPOX (6 ± 2 mmHg) relative to control (12 ± 4 mmHg) fetuses (P < 0.05). Within the first 15 min of the onset of acute hypoxaemia, the fall in Pa,O2 was associated with a significant reduction in fetal heart rate (nadir of 38 ± 11 beats min−1) and a significant increase in femoral vascular resistance (maximum of 2.28 ± 0.86 mmHg (ml min−1)−1) in control fetuses. The nadir for fetal heart rate and the maximum increase in femoral vascular resistance during the first 15 min of hypoxaemia were of a similar magnitude in HYPOX, AC and HG fetuses relative to controls. Consequently, when individual values for heart rate and femoral vascular resistance were plotted against corresponding values for Pa,O2 during normoxia and the first 15 min of acute hypoxaemia, the slopes for all individual data points for the cardiac and femoral vascular chemoreflex function curves were similar in AC and HG fetuses, but the slopes of both cardiac and vasoconstrictor chemoreflex curves were enhanced in HYPOX fetuses, relative to control fetuses (Fig. 3).
Figure 3. Functional chemoreflex analysis during early hypoxaemia.
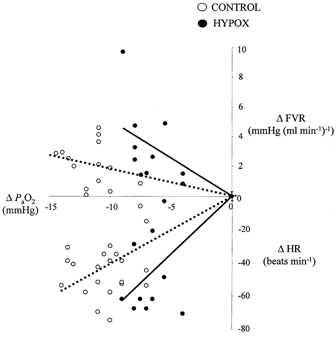
Values are paired individual data points for control (○, n = 14) and for chronically hypoxaemic (•, n = 8) fetuses. An analysis of chemoreceptor function was enabled by plotting the fall in carotid Pa,O2 against the maximal fall in fetal heart rate and the maximal increase in femoral vascular resistance within the first 15 min of acute hypoxaemia. The slopes of the relationships for all individual data points were significantly different between control and HYPOX fetuses for both the cardiac (y = 3.0016x + 0.5vs. y = 6.6483x + −0.3) and femoral (y = −0.1401x + 0.5vs. y = −0.4251x + −0.3) vascular chemoreflex function curves (P < 0.05, both cases).
Fetal endocrine responses to acute hypoxaemia
Catecholamines
In control fetuses, basal plasma concentrations of noradrenaline, adrenaline and of total catecholamines were 356 ± 65, 85 ± 12 and 502 ± 78 pg ml−1, respectively (Fig. 4). While basal plasma concentrations of catecholamines in AC and HG were similar (Fig. 4), basal concentrations of noradrenaline (1351 ± 276 pg ml−1) and of total catecholamines (1709 ± 341 pg ml−1) were significantly greater in HYPOX fetuses, relative to controls (P < 0.01, both cases). Acute hypoxaemia led to significant increases in the plasma concentration of noradrenaline and total catecholamines in all groups of fetuses and of plasma adrenaline in control, AC and HG, but not HYPOX, fetuses (Fig. 4). The increase in plasma noradrenaline and in total catecholamines during hypoxaemia was significantly greater in HYPOX, AC and HG fetuses relative to controls (Fig. 4). During recovery, values for noradrenaline, adrenaline and total catecholamines in all fetuses returned towards baseline values; however, the concentrations of noradrenaline and of total catecholamines remained significantly elevated from baseline in HYPOX relative to controls (Fig. 4).
Figure 4. Fetal plasma noradrenaline, adrenaline and total catecholamine concentrations during acute hypoxaemia.
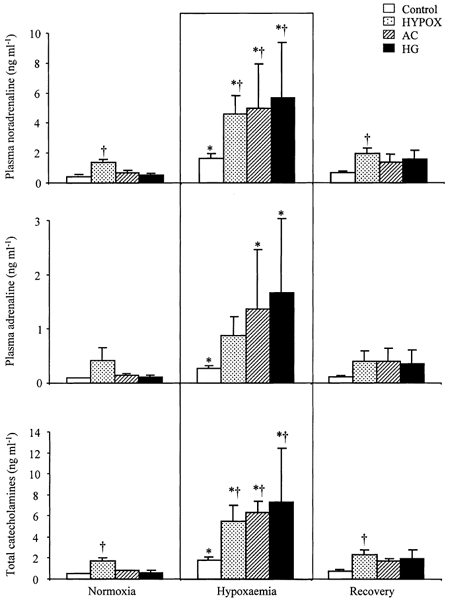
Values are means ± s.e.m. for control (□, n = 14), HYPOX ( , n = 7), AC (
, n = 7), AC ( , n = 6) and HG (▪, n = 5) fetuses for plasma noradrenaline, adrenaline and total catecholamine concentrations during normoxia, hypoxaemia and recovery. Statistical differences are: * P < 0.05, normoxia vs. hypoxaemia; † P < 0.05, control vs. HYPOX or AC or HG fetuses. For details of groups refer to Table 1.
, n = 6) and HG (▪, n = 5) fetuses for plasma noradrenaline, adrenaline and total catecholamine concentrations during normoxia, hypoxaemia and recovery. Statistical differences are: * P < 0.05, normoxia vs. hypoxaemia; † P < 0.05, control vs. HYPOX or AC or HG fetuses. For details of groups refer to Table 1.
Vasopressin
Basal plasma concentrations of vasopressin were similar in all groups of fetuses (controls, 3.0 ± 0.5 pg ml−1; HYPOX, 3.6 ± 1.3 pg ml−1; AC, 9.6 ± 3.1 pg ml−1; HG, 7.7 ± 2.5 pg ml−1; Fig. 5). Significant increases in plasma vasopressin occurred during acute hypoxaemia in all fetuses; however, the increases were significantly greater in AC and HG relative to control fetuses (Fig. 5). Furthermore, the magnitude of the increase in plasma vasopressin during acute hypoxaemia in AC fetuses was significantly greater relative to HYPOX fetuses (Fig. 5). During recovery, plasma vasopressin returned towards basal levels in control and HYPOX, but remained significantly elevated from baseline in AC and HG fetuses (Fig. 5).
Figure 5. Fetal plasma vasopressin concentration during acute hypoxaemia.
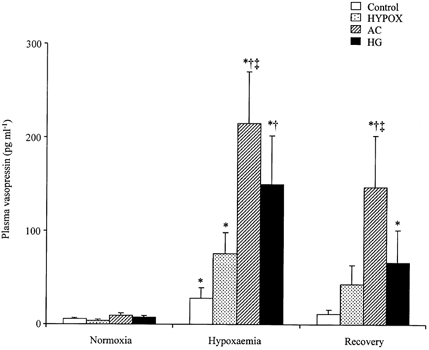
Values are means ± s.e.m. for control (□, n = 14), HYPOX ( , n = 7), AC (
, n = 7), AC ( , n = 6) and HG (▪, n = 5) fetuses for fetal plasma vasopressin concentration during normoxia, hypoxaemia and recovery. Statistical differences are: * P < 0.05, normoxia vs. hypoxaemia or recovery; † P < 0.05, control vs. AC or HG fetuses; ‡ P < 0.05, AC vs. HYPOX fetuses. For details of groups refer to Table 1.
, n = 6) and HG (▪, n = 5) fetuses for fetal plasma vasopressin concentration during normoxia, hypoxaemia and recovery. Statistical differences are: * P < 0.05, normoxia vs. hypoxaemia or recovery; † P < 0.05, control vs. AC or HG fetuses; ‡ P < 0.05, AC vs. HYPOX fetuses. For details of groups refer to Table 1.
ACTH and cortisol
Basal concentrations of fetal plasma ACTH and cortisol and the plasma ACTH and cortisol responses to hypoxaemia were not significantly different in HYPOX, AC and HG fetuses. Therefore, all data for ACTH and cortisol in these groups were combined to form a group of ‘compromised’ fetuses. Resting plasma ACTH and cortisol concentrations were significantly greater in compromised relative to control fetuses (Fig. 6). During acute hypoxaemia, despite similar increases in plasma ACTH, the increase in plasma cortisol was significantly greater in compromised fetuses relative to controls (Fig. 6). The increases in fetal plasma ACTH and cortisol concentration were maintained during the recovery period in both groups of fetuses relative to baseline; however, the plasma cortisol concentration remained significantly elevated in compromised fetuses relative to control, despite similar plasma ACTH concentrations (Fig. 6). Correlation of all paired plasma ACTH and cortisol concentration values during the experimental protocol showed that the intercept, but not the slope, of the linear relationship was significantly greater in compromised fetuses (y = 0.0550x + 42.12) relative to control fetuses (y = 0.0315x + 26.03) (Fig. 7).
Figure 6. Fetal plasma ACTH and cortisol concentrations during acute hypoxaemia.
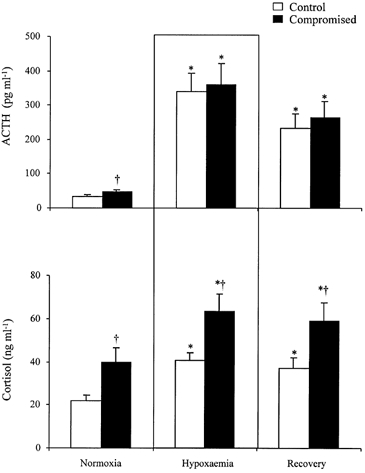
Values are means ± s.e.m. for control fetuses (□, n = 14) or compromised fetuses (combined data for HYPOX, AC and HG fetuses; ▪, n = 18) for plasma ACTH and cortisol concentrations during normoxia, hypoxaemia and recovery. Statistical differences are: * P < 0.05, normoxia vs. hypoxaemia or recovery; † P < 0.05, control vs. compromised fetuses.
Figure 7. Linear regression of fetal plasma ACTH and cortisol concentrations during acute hypoxaemia.
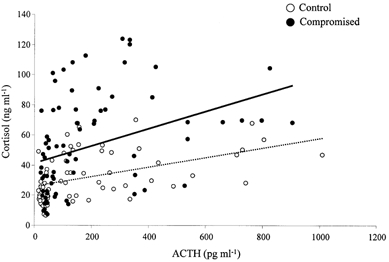
Linear regression for fetal plasma ACTH and cortisol in control fetuses (○; dotted line, n = 14) and compromised fetuses (combined data for HYPOX, AC and HG fetuses; •, continuous line, n = 18). Data points are for all paired ACTH and cortisol samples in individual fetuses obtained during acute hypoxaemia. There was a significant change in the intercept, but not the slope, of the relationship between plasma ACTH and cortisol in control and compromised fetuses (intercept: y = 0.0315x + 26.03vs. y = 0.0550x + 42.12, P < 0.05 for control and compromised fetuses, respectively).
Correlation analyses of values for femoral vascular resistance, blood gases, pH and plasma hormone concentrations
When values for the increases in femoral vascular resistance during acute hypoxaemia were combined for all groups, this combined increase could equally and independently be predicted by pHa or Pa,O2 (P < 0.001), although pHa (r = −0.70) appeared to be a greater determinant of changes in femoral vascular resistance than Pa,O2 (r = −0.24). In addition, the change in femoral vascular resistance during hypoxaemia in control and compromised fetuses could be predicted by plasma vasopressin (r = 0.70) and total plasma catecholamines (r = 0.51; P < 0.001, both cases). However, given the degree of co-linearity between vasopressin and total plasma catecholamines (r = 0.59, P < 0.001), partial correlation analyses indicated that vasopressin (P < 0.001) rather than total catecholamines (P = 0.052) was the best independent endocrine factor for predicting the fetal femoral vascular resistance response to acute hypoxaemia. Furthermore, there was a high degree of co-linearity between vasopressin and pHa (r = −0.74, P < 0.001) and partial correlation analyses revealed that the increase in fetal femoral vascular resistance during acute hypoxaemia could be equally predicted by either vasopressin (r = 0.36) or pHa (r = −0.42; P < 0.001, both cases).
DISCUSSION
In the present study, prevailing and sustained hypoxaemia, acidaemia or hypoglycaemia altered the cardiovascular responses to a further episode of acute hypoxaemia in fetal sheep during late gestation. Alterations in the cardiovascular responses to acute hypoxaemia in compromised fetuses included enhanced pressor and femoral vasoconstrictor responses, which were associated with greater circulating concentrations of plasma noradrenaline and vasopressin. All compromised fetal groups showed resetting of basal hypothalamic- pituitary-adrenal (HPA) function. However, only fetuses with prevailing hypoxaemia had significantly elevated basal concentrations of noradrenaline and enhanced chemoreflex function during acute hypoxaemia.
There has been a long-standing interest in the effects of chronic hypoxia on chemoreflex function. However, most of the available literature on this topic is on the effects of the chronic hypoxia of high altitude on ventilatory adaptation in adult life (Hanson, 1998). At sea level, it is known that acute episodes of hypoxia in adult life result in an increase in minute ventilation, a response which is known to be mediated by a carotid chemoreflex, because section of the carotid sinus nerves (Williams & Hanson, 1990) or removal of the carotid body (Chiocchio et al. 1984) prevent the hypoxic ventilatory drive. The effect of chronic hypoxia on this reflex is dependent on the duration of the challenge. For example, in the cat, while exposure to hypobaric hypoxia for 48 h sensitises (Vizek et al. 1987), prolonged exposure to hypoxia for several weeks or more desensitises the chemoreflex ventilatory response (Tatsumi et al. 1991). This desensitising effect on chemoreflex function of prolonged exposure to hypoxia is in agreement with the widely held view that residents at high altitude have blunted ventilatory responses to acute hypoxia.
In the fetus, acute hypoxia inhibits breathing movements (Boddy et al. 1974) but it produces well-described cardiovascular responses that have important carotid chemoreflex components. In fetal sheep, acute hypoxaemia elicits bradycardia and femoral vasoconstriction which are known to be triggered by a carotid chemoreflex, since section of the carotid sinus nerves prevents both responses within the first 15 min of the onset of the challenge (Giussani et al. 1993). In the present study, prevailing hypoxaemia in the sheep fetus sensitised both cardiac and vasoconstrictor chemoreflex responses. Combined, past and present data therefore suggest that exposure to hypoxia for days has a sensitising effect on cardiovascular chemoreflex function in fetal life and ventilatory chemoreflex function in postnatal life, while exposure to sustained hypoxia for a week or more switches the effect of hypoxia on chemoreflex function from sensitisation to desensitisation.
Sustained fetal hypoxaemia in complicated pregnancies rarely occurs in isolation but rather is accompanied by either acidaemia and/or hypoglycaemia (Nicolaides et al. 1989). Thus an additional objective of the present study was to determine the relative contributions of each of these adverse intrauterine conditions on the capacity of the fetus to respond to a further episode of acute hypoxaemia. Previous studies that have addressed the effects of prevailing adverse intrauterine conditions on the fetal cardiovascular response to acute hypoxaemia have induced adverse intrauterine conditions experimentally by reduction in placental size (carunclectomy; Robinson et al. 1983), by occlusion of uterine blood flow with a balloon catheter (Block et al. 1990b) or by embolisation of the umbilico-placental (Block et al. 1989) or utero-placental (Block et al. 1984) circulations. In addition, a series of studies have investigated the fetal cardiovascular responses to acute hypoxaemia in the fetal llama, a species that is genetically adapted to the chronic hypobaric hypoxia of life at high altitude (Giussani et al. 1996, 1999). However, both carunclectomy and embolisation are relatively severe challenges as indicated by the degree of associated growth retardation, morbidity and mortality (Robinson et al. 1979; Mallard et al. 1998) and carunclectomised fetuses become chronically hypoxaemic, polycythaemic, hypoglycaemic and lactacidaemic (Robinson et al. 1979).
Carunclectomised fetuses were shown to respond to acute hypoxaemia with only a transient bradycardia, with heart rate returning to basal levels within 15 min of the onset of the challenge, in contrast to control fetuses in which heart rate remained depressed for the duration of the challenge (Robinson et al. 1983). Similarly, in the present study, the duration of bradycardia during acute hypoxaemia was prolonged in control, chronically hypoglycaemic and acidaemic fetuses but was only transient in chronically hypoxaemic, polycythaemic fetuses. This suggests that in the carunclectomised fetus the pattern of the fetal heart rate response may be explained by prevailing hypoxaemia specifically, rather than by the prevailing hypoglycaemia and/or acidaemia (Robinson et al. 1983). Faster return of fetal heart rate towards basal values during acute hypoxaemia has been attributed to increased β-adrenergic stimulation of the heart, secondary to elevations in circulating concentrations of catecholamines in the fetal circulation (Parer, 1983). In this context, it is of interest that in the present study the increase in total plasma catecholamines during acute hypoxaemia was significantly enhanced in all groups of compromised fetuses, not just in the chronically hypoxaemic group.
The series of studies by Block et al. (1984, 1989, 1990a) reported that fetuses compromised in utero, by embolisation leading predominantly to hypoxaemia alone, exhibited a basal redistribution of blood flow towards the brain, heart and adrenal glands and that this basal circulatory compensation was accentuated during an episode of superimposed acute hypoxaemia. If acidaemia developed during superimposed acute hypoxaemia, the circulatory compensation was further exaggerated (Block et al. 1990a). These findings are in general agreement with those reported in the present study as the increase in femoral vascular resistance, a good index of redistribution of the fetal cardiac output away from the periphery (Giussani et al. 1996), was significantly enhanced during acute hypoxaemia in all groups of compromised fetuses. Similar conclusions were drawn from preliminary data obtained from one fetus which revealed an enhanced femoral vasoconstrictor response to acute hypoxaemia when superimposed upon chronic, partial compression of the maternal internal iliac artery (Bennet & Hanson, 1994). The faster return of femoral vascular resistance towards baseline during recovery from acute hypoxaemia in chronically hypoxaemic, relative to chronically acidaemic or hypoglycaemic, fetuses suggests a specific up-regulation of vasodilatory mechanisms, such as nitric oxide, by exposure to chronic hypoxaemia, as has been shown previously (Zhang et al. 1998).
In the present study, the enhanced femoral vasoconstrictor response to superimposed acute hypoxaemia was associated with marked elevations in plasma catecholamines and vasopressin in all groups of compromised fetuses. However, the increase in plasma vasopressin during acute hypoxaemia was greatest in the chronically acidaemic fetuses, and partial correlation analysis revealed that changes in fetal pHa rather than Pa,O2, and in fetal plasma vasopressin rather than total plasma catecholamines, during acute hypoxaemia appeared to be the greater determinants of fetal femoral vasoconstriction. In this regard, it is of interest to note that: (1) the llama fetus has enhanced femoral vasoconstrictor and plasma vasopressinergic responses to acute hypoxaemia relative to control sheep fetuses at comparable stages of gestation (Giussani et al. 1994b, 1996, 1999); (2) llama fetuses (Giussani et al. 1999) and chronically hypoxaemic, growth-retarded fetal sheep (Block et al. 1984) have a greater dependence on α-adrenergic mechanisms to survive episodes of acute hypoxaemia than control fetal sheep; and (3) the plasma vasopressin response to acute hypoxaemia in the sheep fetus is augmented by prevailing acidosis (Raff et al. 1991). Taken together, these findings suggest that adverse intrauterine conditions per se may enhance the capacity of the compromised fetus to redistribute blood flow away from the periphery to serve hypoxia-sensitive circulations, but the precise mechanisms which are recruited to mediate this enhanced response depend on the relative degree of, and interplay between, prevailing hypoxaemia, acidaemia or hypoglycaemia in complicated pregnancies.
It is becoming increasingly clear that fetal exposure to adverse intrauterine conditions induced experimentally by several techniques leads to elevations in basal fetal plasma noradrenaline, but not necessarily adrenaline, concentrations and resets basal HPA axis function (McMillen et al. 2001; Gardner et al. 2001b). Elevated basal fetal plasma noradrenaline concentrations occur after carunclectomy (Jones & Robinson 1983; Simonetta et al. 1997), embolisation of the fetal-placental circulations (Gagnon et al. 1994) and reductions in uterine (Hooper et al. 1990) and umbilical (Gardner et al. 2001a) blood flow. In the present study, prevailing hypoxaemia, but not acidaemia or hypoglycaemia, led to elevations in basal fetal plasma noradrenaline levels. This suggests that chronic hypoxaemia, rather than acidaemia or hypoglycaemia, may be a greater determinant of elevated basal fetal plasma noradrenaline concentrations during adverse intrauterine conditions. The mechanism mediating hypoxaemia-induced, sustained elevations in fetal plasma noradrenaline concentrations is unknown. However, Ruijtenbeek et al. (2000) demonstrated that in the chicken embryo chronic, moderate hypoxia led to increased noradrenaline content, sympathetic nerve fibre density and cocaine-sensitive neuronal uptake of noradrenaline in femoral arteries, findings which are consistent with increased numbers of sympathetic varicosities and enhanced neuronal capacity for the synthesis of noradrenaline. Although the extent to which elevated basal plasma noradrenaline concentration in the chronically hypoxaemic sheep fetus reflects enhanced neuronal spill-over rather than adrenal medullary output of the amine is unknown.
Exposure of the fetus to long-term intrauterine stress most often results in a resetting of the HPA axis, resulting in sustained elevations in basal plasma cortisol, despite only a transient increase in plasma ACTH concentration (Robinson et al. 1979; Bocking et al. 1986; Challis et al. 1989; Gagnon et al. 1994; Murotsuki et al. 1996). Furthermore, basal resetting of HPA axis function in the fetus in response to prolonged stress persists even after restoration of normal intrauterine conditions (Gardner et al. 2001b). However, interestingly, basal HPA axis sensitivity, rather than set-point, appears to be unaltered during adverse intrauterine conditions (Kerr et al. 1992; Harvey et al. 1993) or during stimulated acute stress superimposed on (Gagnon et al. 1997) or after (Gardner et al. 2001b) chronic adverse intrauterine conditions. In contrast to the differential effects of prevailing hypoxaemia, acidaemia or hypoglycaemia on basal plasma noradrenaline output and the increment in fetal plasma vasopressin during superimposed acute hypoxaemia, basal resetting of the HPA axis appears to be a general outcome during many forms of adverse intrauterine conditions. The mechanism(s) mediating sustained elevations in fetal plasma cortisol in the absence of significant elevations in fetal plasma ACTH during or following adverse intrauterine conditions remain unclear, but may involve increased adrenocortical steroidogenic capacity (Gardner et al. 2001b) or sensitivity (Edwards et al. 1986), altered transplacental cortisol transfer, changes in the ratio of bioactive:immunoreactive ACTH (Young et al. 1996) or increased action of an ACTH-independent steroidogenic factor such as vasoactive intestinal peptide (Bloom et al. 1987), corticotrophin-releasing hormone (Jones & Edwards, 1992) and the eicosanoid prostaglandin E2 (Young et al. 1996), since all have been shown to promote steroidogenesis in the absence of changes in circulating ACTH. At present, the more likely candidate would appear to be prostaglandin E2, since increased concentrations have been observed during prolonged adverse intrauterine conditions (Fowden et al. 1989; Hooper et al. 1990; Gagnon et al. 1997).
The reductions in pHa and acid-base excess (AC), Pa,O2 (HYPOX) and blood glucose (HG) were apparent on the first (HYPOX and HG) or second (AC) day after surgery. Whilst the hypoxaemia and hypoglycaemia could be due to slow recovery after surgery, the result of an acute compromise during surgery or altered maternal oxygenation or metabolic status, these are unlikely because: (1) the reductions in Pa,O2 and blood glucose were sustained; (2) maternal values for Pa,O2 and blood glucose were appropriate for control ewes at this stage of gestation; (3) poor post-surgical recovery is rare in our laboratory (< 5 %); and (4) control fetuses exposed to the same degree of surgical instrumentation had appropriate blood gas and glucose status immediately after surgery at an equivalent stage of gestation. The cause of the specific reduction in fetal pHa and acid-base excess despite appropriate blood lactate concentrations is not known, but is interesting. Arterial pH has been shown to alter independently from the concentration of circulating metabolic acids (Hooper et al. 1990; Gardner et al. 2001a,b), and was postulated to be related to changes in specific metabolically active hormone concentrations, in particular catecholamines and PGE2 (Hooper et al. 1990). While the present study suggests that catecholamines may not be important, a role for changes in PGE2 concentrations or the placental/renal handling of acid cannot be ruled out.
In the present study, the specific cause and actual duration of the adverse intrauterine conditions are not known. However, abnormal conditions in control fetuses result from a number of factors including low-grade subclinical infection, altered placental function and multiple pregnancy and a potential role for these on basal metabolic conditions or on the cardiovascular responses to acute hypoxaemia cannot be entirely excluded at present. Nevertheless, to date, there is no information in the literature on the partial contributions of different types of adverse intrauterine conditions (hypoxaemia or hypoglycaemia or acidaemia) on the capacity of the fetal cardiovascular and endocrine systems to respond to an acute stress that is similar to that encountered during labour and delivery (Rurak et al. 1997). Furthermore, the present study is the first to determine which neural and endocrine mechanisms involved in fetal responses to acute stress may be potentially affected after adaptation to chronic adverse intrauterine conditions. However, the present study indicates that if similar blood gas, acid-base or metabolic profiles are encountered in future physiological/ metabolic studies in fetal sheep then these fetuses should not be considered as appropriate controls and should be excluded from any further analyses or study.
Acknowledgments
The authors wish to acknowledge Mr Paul Hughes for his help during surgery and Mrs Sue Nicholls and Ms Victoria Johnson for the routine care of the animals used in this study. This work was supported by the British Heart Foundation, Tommys’ - the Baby Charity and the Department of Physiology, University of Cambridge. D.A.G. is currently a Fellow of the Lister Institute for Preventative Medicine.
REFERENCES
- Armitage P, Berry G. Statistical Methods in Medical Research. Oxford: Blackwell Scientific; 1994. Further analyses of straight-line data; pp. 292–305. [Google Scholar]
- Barcroft J. Researches on Prenatal Life. Oxford: Blackwells; 1946. The development of vascular reflexes; pp. 123–144. [Google Scholar]
- Bartelds B, Van Bel F, Teitel DF, Rudolph AM. Carotid, not aortic, chemoreceptors mediate the fetal cardiovascular response to acute hypoxemia in lambs. Pediatric Research. 1993;34:51–55. doi: 10.1203/00006450-199307000-00013. [DOI] [PubMed] [Google Scholar]
- Bennet L, Hanson MA. Intrauterine compromise: physiological consequences. In: Ward RHT, Smith SK, Donnai D, editors. Early Fetal Growth and Development. London: RCOG Press; 1994. pp. 363–370. [Google Scholar]
- Blanco CE, Dawes GS, Hanson MA, McCooke HB. The response to hypoxia of arterial chemoreceptors in fetal sheep and new-born lambs. Journal of Physiology. 1984;351:25–37. doi: 10.1113/jphysiol.1984.sp015229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Block BS, Llanos AJ, Creasy RK. Responses of the growth-retarded fetus to acute hypoxemia. American Journal of Obstetrics and Gynecology. 1984;148:878–885. doi: 10.1016/0002-9378(84)90529-5. [DOI] [PubMed] [Google Scholar]
- Block BS, Schlafer DH, Wentworth RA, Kreitzer LA, Nathanielsz PW. Intrauterine growth retardation and the circulatory responses to acute hypoxemia in fetal sheep. American Journal of Obstetrics and Gynecology. 1989;161:1576–1579. doi: 10.1016/0002-9378(89)90929-0. [DOI] [PubMed] [Google Scholar]
- Block BS, Schlafer DH, Wentworth RA, Kreitzer LA, Nathanielsz PW. Intrauterine asphyxia and the breakdown of physiologic circulatory compensation in fetal sheep. American Journal of Obstetrics and Gynecology. 1990a;162:1325–1331. doi: 10.1016/0002-9378(90)90046-a. [DOI] [PubMed] [Google Scholar]
- Block BS, Schlafer DH, Wentworth RA, Kreitzer LA, Nathanielsz PW. Regional blood flow distribution in fetal sheep with intrauterine growth retardation produced by decreased umbilical placental perfusion. Journal of Developmental Physiology. 1990b;13:81–85. [PubMed] [Google Scholar]
- Bloom SR, Edwards AV, Jones CT. Adrenal cortical responses to vasoactive intestinal peptide in conscious hypophysectomized calves. Journal of Physiology. 1987;391:441–450. doi: 10.1113/jphysiol.1987.sp016748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bocking AD, McMillen IC, Harding R, Thorburn GD. Effect of reduced uterine blood flow on fetal and maternal cortisol. Journal of Developmental Physiology. 1986;8:237–245. [PubMed] [Google Scholar]
- Boddy K, Dawes GS, Fisher R, Pinter S, Robinson JS. Foetal respiratory movements, electrocortical and cardiovascular responses to hypoxaemia and hypercapnia in sheep. Journal of Physiology. 1974;243:599–618. doi: 10.1113/jphysiol.1974.sp010768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breslow MJ, Tobin JR, Bredt DS, Ferris CD, Snyder SH, Traystman RJ. Nitric oxide as a regulator of adrenal blood flow. American Journal of Physiology. 1993;264:H464–469. doi: 10.1152/ajpheart.1993.264.2.H464. [DOI] [PubMed] [Google Scholar]
- Challis JR, Fraher L, Oosterhuis J, White SE, Bocking AD. Fetal and maternal endocrine responses to prolonged reductions in uterine blood flow in pregnant sheep. American Journal of Obstetrics and Gynecology. 1989;160:926–932. doi: 10.1016/0002-9378(89)90312-8. [DOI] [PubMed] [Google Scholar]
- Chiocchio SR, Hilton SM, Tramezzani JH, Willshaw P. Loss of peripheral chemoreflexes to hypoxia after carotid body removal in the rat. Respiration Physiology. 1984;57:235–246. doi: 10.1016/0034-5687(84)90096-3. [DOI] [PubMed] [Google Scholar]
- Creasy RK, Resnik R. Intrauterine fetal growth retardation. In: Milunsky A, Friedman EA, Gluck L, editors. Advances in Perinatal Medicine. New York: Plenum Book Medical Company; 1981. [Google Scholar]
- Dijxhoorn MJ, Visser GH, Touwen BC, Huisjes HJ. Apgar score, meconium and acidaemia at birth in small-for-gestational age infants born at term, and their relation to neonatal neurological morbidity. British Journal of Obstetrics and Gynaecology. 1987;94:873–879. doi: 10.1111/j.1471-0528.1987.tb03758.x. [DOI] [PubMed] [Google Scholar]
- Edwards AV, Jones CT, Bloom SR. Reduced adrenal cortical sensitivity to ACTH in lambs with cut splanchnic nerves. Journal of Endocrinology. 1986;110:81–85. doi: 10.1677/joe.0.1100081. [DOI] [PubMed] [Google Scholar]
- Fletcher AJ, Edwards CM, Gardner DS, Fowden AL, Giussani DA. Neuropeptide Y in the sheep fetus: effects of acute hypoxemia and dexamethasone during late gestation. Endocrinology. 2000;141:3976–3982. doi: 10.1210/endo.141.11.7770. [DOI] [PubMed] [Google Scholar]
- Forhead AJ, Fowden AL, Silver M, Hughes P, Broughton-Pipkin F, Sutherland MF. Haemodynamic responses to an angiotensin II receptor antagonist (GR 117289) in maternal and fetal sheep. Experimental Physiology. 1995;80:285–298. doi: 10.1113/expphysiol.1995.sp003848. [DOI] [PubMed] [Google Scholar]
- Fowden AL, Harding R, Ralph MM, Thorburn GD. Nutritional control of respiratory and other muscular activities in relation to plasma prostaglandin E in the fetal sheep. Journal of Developmental Physiology. 1989;11:253–262. [PubMed] [Google Scholar]
- Fowden AL, Mijovic J, Silver M. The effects of cortisol on hepatic and renal gluconeogenic enzyme activities in the sheep fetus during late gestation. Journal of Endocrinology. 1993;137:213–222. doi: 10.1677/joe.0.1370213. [DOI] [PubMed] [Google Scholar]
- Fowden AL, Mundy L, Silver M. Developmental regulation of glucogenesis in the sheep fetus during late gestation. Journal of Physiology. 1998;508:937–947. doi: 10.1111/j.1469-7793.1998.937bp.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gagnon R, Challis J, Johnston L, Fraher L. Fetal endocrine responses to chronic placental embolization in the late-gestation ovine fetus. American Journal of Obstetrics and Gynecology. 1994;170:929–938. doi: 10.1016/s0002-9378(94)70309-4. [DOI] [PubMed] [Google Scholar]
- Gagnon R, Murotsuki J, Challis JR, Fraher L, Richardson BS. Fetal sheep endocrine responses to sustained hypoxemic stress after chronic fetal placental embolization. American Journal of Physiology. 1997;272:E817–823. doi: 10.1152/ajpendo.1997.272.5.E817. [DOI] [PubMed] [Google Scholar]
- Gardner DS, Fletcher AJ, Fowden AL, Giussani DA. A novel method for controlled and reversible long term compression of the umbilical cord in fetal sheep. Journal of Physiology. 2001a;535:217–229. doi: 10.1111/j.1469-7793.2001.00217.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner DS, Fletcher AJ, Fowden AL, Giussani DA. Plasma adrenocorticotropin and cortisol concentrations during acute hypoxemia after a reversible period of adverse intrauterine conditions in the ovine fetus during late gestation. Endocrinology. 2001b;142:589–598. doi: 10.1210/endo.142.2.7980. [DOI] [PubMed] [Google Scholar]
- Giussani DA, Gardner DS, Cox DT, Fletcher AJ. Purinergic contribution to circulatory, metabolic, and adrenergic responses to acute hypoxemia in fetal sheep. American Journal of Physiology - Regulatory, Integrative and Comparative Physiology. 2001;280:R678–685. doi: 10.1152/ajpregu.2001.280.3.R678. [DOI] [PubMed] [Google Scholar]
- Giussani DA, McGarrigle HHG, Spencer JAD, Moore PJ, Bennet L, Hanson MA. Effect of carotid denervation on plasma vasopressin levels during acute hypoxia in the late-gestation sheep fetus. Journal of Physiology. 1994a;477:81–87. doi: 10.1113/jphysiol.1994.sp020173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giussani DA, Riquelme RA, Moraga FA, McGarrigle HH, Gaete CR, Sanhueza EM, Hanson MA, Llanos AJ. Chemoreflex and endocrine components of cardiovascular responses to acute hypoxemia in the llama fetus. American Journal of Physiology. 1996;271:R73–83. doi: 10.1152/ajpregu.1996.271.1.R73. [DOI] [PubMed] [Google Scholar]
- Giussani DA, Riquelme RA, Sanhueza EM, Hanson MA, Blanco CE, Llanos AJ. Adrenergic and vasopressinergic contributions to the cardiovascular response to acute hypoxaemia in the llama fetus. Journal of Physiology. 1999;515:233–241. doi: 10.1111/j.1469-7793.1999.233ad.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giussani DA, Spencer JAD, Hanson MA. Fetal cardiovascular reflex responses to hypoxaemia. Fetal and Maternal Medicine Review. 1994b;6:17–37. [Google Scholar]
- Giussani DA, Spencer JAD, Moore PJ, Bennet L, Hanson MA. Afferent and efferent components of the cardiovascular reflex responses to acute hypoxia in term fetal sheep. Journal of Physiology. 1993;461:431–449. doi: 10.1113/jphysiol.1993.sp019521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green LR. Programming of endocrine mechanisms of cardiovascular control and growth. Journal of the Society for Gynecological Investigation. 2001;8:57–68. [PubMed] [Google Scholar]
- Green LR, Bennet L, Hanson MA. The role of nitric oxide synthesis in cardiovascular responses to acute hypoxia in the late gestation sheep fetus. Journal of Physiology. 1996;497:271–277. doi: 10.1113/jphysiol.1996.sp021766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green LR, McGarrigle HH, Bennet L, Hanson MA. Angiotensin II and cardiovascular chemoreflex responses to acute hypoxia in late gestation fetal sheep. Journal of Physiology. 1998;507:857–867. doi: 10.1111/j.1469-7793.1998.857bs.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson MA. Role of chemoreceptors in effects of chronic hypoxia. Comparative Biochemistry and Physiology A: Molecular Integrative Physiology. 1998;119:695–703. doi: 10.1016/s1095-6433(98)01007-1. [DOI] [PubMed] [Google Scholar]
- Harvey LM, Gilbert RD, Longo LD, Ducsay CA. Changes in ovine fetal adrenocortical responsiveness after long-term hypoxemia. American Journal of Physiology. 1993;264:E741–747. doi: 10.1152/ajpendo.1993.264.5.E741. [DOI] [PubMed] [Google Scholar]
- Hill A. Current concepts of hypoxic-ischemic cerebral injury in the term newborn. Pediatric Neurology. 1991;7:317–325. doi: 10.1016/0887-8994(91)90060-x. [DOI] [PubMed] [Google Scholar]
- Hooper SB, Coulter CL, Deayton JM, Harding R, Thorburn GD. Fetal endocrine responses to prolonged hypoxemia in sheep. American Journal of Physiology. 1990;259:R703–708. doi: 10.1152/ajpregu.1990.259.4.R703. [DOI] [PubMed] [Google Scholar]
- Jones CT. The development of some metabolic responses to hypoxia in the foetal sheep. Journal of Physiology. 1977;265:743–762. doi: 10.1113/jphysiol.1977.sp011741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones CT, Edwards AV. The role of corticotrophin releasing factor in relation to the neural control of adrenal function in conscious calves. Journal of Physiology. 1992;447:489–500. doi: 10.1113/jphysiol.1992.sp019013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones CT, Robinson JS. Studies on experimental growth retardation in sheep. Plasma catecholamines in fetuses with small placenta. Journal of Developmental Physiology. 1983;5:77–87. [PubMed] [Google Scholar]
- Jones CT, Roebuck MM, Walker DW, Johnston BM. The role of the adrenal medulla and peripheral sympathetic nerves in the physiological responses of the fetal sheep to hypoxia. Journal of Developmental Physiology. 1988;10:17–36. [PubMed] [Google Scholar]
- Kerr DR, Castro MI, Valego NK, Rawashdeh NM, Rose JC. Corticotropin and cortisol responses to corticotropin-releasing factor in the chronically hypoxemic ovine fetus. American Journal of Obstetrics and Gynecology. 1992;167:1686–1690. doi: 10.1016/0002-9378(92)91762-y. [DOI] [PubMed] [Google Scholar]
- Mallard EC, Rees S, Stringer M, Cock ML, Harding R. Effects of chronic placental insufficiency on brain development in fetal sheep. Pediatric Research. 1998;43:262–270. doi: 10.1203/00006450-199802000-00018. [DOI] [PubMed] [Google Scholar]
- Mann LI. Pregnancy events and brain damage. American Journal of Obstetrics and Gynecology. 1986;155:6–9. doi: 10.1016/0002-9378(86)90066-9. [DOI] [PubMed] [Google Scholar]
- Matthews JN, Altman DG, Campbell MJ, Royston P. Analysis of serial measurements in medical research. British Medical Journal. 1990;300:230–235. doi: 10.1136/bmj.300.6719.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMillen IC, Adams MB, Ross JT, Coulter CL, Simonetta G, Owens JA, Robinson JS, Edwards LJ. Fetal growth restriction: adaptations and consequences. Reproduction. 2001;122:195–204. doi: 10.1530/rep.0.1220195. [DOI] [PubMed] [Google Scholar]
- Murotsuki J, Gagnon R, Matthews SG, Challis JR. Effects of long-term hypoxemia on pituitary-adrenal function in fetal sheep. American Journal of Physiology. 1996;271:E678–685. doi: 10.1152/ajpendo.1996.271.4.E678. [DOI] [PubMed] [Google Scholar]
- Nicolaides KH, Economides DL, Soothill PW. Blood gases, pH, and lactate in appropriate- and small-for-gestational-age fetuses. American Journal of Obstetrics and Gynecology. 1989;161:996–1001. doi: 10.1016/0002-9378(89)90770-9. [DOI] [PubMed] [Google Scholar]
- Owens JA, Falconer J, Robinson JS. Effect of restriction of placental growth on oxygen delivery to and consumption by the pregnant uterus and fetus. Journal of Developmental Physiology. 1987;9:137–150. [PubMed] [Google Scholar]
- Parer JT. The influence of beta-adrenergic activity on fetal heart rate and the umbilical circulation during hypoxia in fetal sheep. American Journal of Obstetrics and Gynecology. 1983;147:592–597. doi: 10.1016/0002-9378(83)90024-8. [DOI] [PubMed] [Google Scholar]
- Perez R, Espinoza M, Riquelme R, Parer JT, Llanos AJ. Arginine vasopressin mediates cardiovascular responses to hypoxemia in fetal sheep. American Journal of Physiology. 1989;256:R1011–1018. doi: 10.1152/ajpregu.1989.256.5.R1011. [DOI] [PubMed] [Google Scholar]
- Raff H, Kane CW, Wood CE. Arginine vasopressin responses to hypoxia and hypercapnia in late-gestation fetal sheep. American Journal of Physiology. 1991;260:R1077–1081. doi: 10.1152/ajpregu.1991.260.6.R1077. [DOI] [PubMed] [Google Scholar]
- Reller MD, Burson MA, Lohr JL, Morton MJ, Thornburg KL. Nitric oxide is an important determinant of coronary flow at rest and during hypoxemic stress in fetal lambs. American Journal of Physiology. 1995;269:H2074–2081. doi: 10.1152/ajpheart.1995.269.6.H2074. [DOI] [PubMed] [Google Scholar]
- Robinson JS, Jones CT, Kingston EJ. Studies on experimental growth retardation in sheep. The effects of maternal hypoxaemia. Journal of Developmental Physiology. 1983;5:89–100. [PubMed] [Google Scholar]
- Robinson JS, Kingston EJ, Jones CT, Thorburn GD. Studies on experimental growth retardation in sheep. The effect of removal of a endometrial caruncles on fetal size and metabolism. Journal of Developmental Physiology. 1979;1:379–398. [PubMed] [Google Scholar]
- Rudolph AM, Itskovitz J, Iwamoto H, Reuss ML, Heymann MA. Fetal cardiovascular responses to stress. Seminars in Perinatology. 1981;5:109–121. [PubMed] [Google Scholar]
- Ruijtenbeek K, Le Noble FA, Janssen GM, Kessels CG, Fazzi GE, Blanco CE, De Mey JG. Chronic hypoxia stimulates periarterial sympathetic nerve development in chicken embryo. Circulation. 2000;102:2892–2897. doi: 10.1161/01.cir.102.23.2892. [DOI] [PubMed] [Google Scholar]
- Rurak DW, Tan W, Riggs KW, Stobbs KE, Kwan E, Hall C. Circulatory and metabolic changes in fetal sheep during labour. Journal of the Society for Gynecological Investigation. 1997;4(suppl.):346A. [Google Scholar]
- Simonetta G, Rourke AK, Owens JA, Robinson JS, McMillen IC. Impact of placental restriction on the development of the sympathoadrenal system. Pediatric Research. 1997;42:805–811. doi: 10.1203/00006450-199712000-00015. [DOI] [PubMed] [Google Scholar]
- Snedecor GW. Statistical Methods. 4. Ames: Iowa State College Press; 1946. Multiple regression and covariance; pp. 340–373. [Google Scholar]
- Tatsumi K, Pickett CK, Weil JV. Attenuated carotid body hypoxic sensitivity after prolonged hypoxic exposure. Journal of Applied Physiology. 1991;70:748–755. doi: 10.1152/jappl.1991.70.2.748. [DOI] [PubMed] [Google Scholar]
- Vizek M, Pickett CK, Weil JV. Increased carotid body hypoxic sensitivity during acclimatization to hypobaric hypoxia. Journal of Applied Physiology. 1987;63:2403–2410. doi: 10.1152/jappl.1987.63.6.2403. [DOI] [PubMed] [Google Scholar]
- Williams BA, Hanson MA. Role of the carotid chemoreceptors in the respiratory response of newborn lambs to alternate pairs of breaths of air and a hypoxic gas. Journal of Developmental Physiology. 1990;13:157–164. [PubMed] [Google Scholar]
- Young IR, Deayton JM, Hollingworth SA, Thorburn GD. Continuous intrafetal infusion of prostaglandin E2 prematurely activates the hypothalamo-pituitary-adrenal axis and induces parturition in sheep. Endocrinology. 1996;137:2424–2431. doi: 10.1210/endo.137.6.8641195. [DOI] [PubMed] [Google Scholar]
- Zhang L, Xiao D, Bouslough DB. Long-term high-altitude hypoxia increases plasma nitrate levels in pregnant ewes and their fetuses. American Journal of Obstetrics and Gynecology. 1998;179:1594–1598. doi: 10.1016/s0002-9378(98)70031-6. [DOI] [PubMed] [Google Scholar]


