Abstract
We tested the effect of repetitive transcranial magnetic stimulation (rTMS) over the motor cortex on the size of transcortical stretch and mixed nerve reflexes. Fourteen healthy subjects were investigated using either 25 min of 1 Hz rTMS or 30 min of 0.1 Hz rTMS paired with electrical stimulation of the motor point of the first dorsal interosseous muscle (FDI). Following treatment, we measured the effect on the size of: (1) EMG responses evoked in FDI by transcranial magnetic stimulation (MEPs), (2) somatosensory evoked potentials (SEPs) evoked by ulnar nerve stimulation, and (3) transcortical stretch or electrically elicited reflexes. rTMS at 1 Hz reduced the amplitude of both MEPs and long latency reflexes by 20–30 % for about 10 min after the end of stimulation. Short latency reflexes were unaffected. SEPs were not studied, as it has been shown previously that they are also suppressed. rTMS at 0.1 Hz paired with motor point stimulation (interstimulus interval of 25 ms) increased the amplitude of the MEP and the cortical components of the SEP (N20/P25 and later peaks) for up to 10 min. Long latency reflexes were facilitated with the same time course. We conclude that rTMS over the motor cortex either alone or in conjunction with peripheral inputs can decrease or increase the excitability of the sensory and motor cortex for short periods after the end of stimulation. These changes affect not only MEPs and SEPs but also EMG responses to more ‘natural’ inputs involved in transcortical stretch reflexes.
Repetitive transcranial magnetic stimulation (rTMS) over the motor cortex is known to produce changes in cortical excitability that outlast the period of stimulation by several minutes (Pascual-Leone et al. 1994; Chen et al. 1997; Maeda et al. 2000; Muellbacher et al. 2000; Touge et al. 2001). In most experiments, the effects of rTMS have been tested by measuring the amplitude of an EMG response (MEP) evoked by a single standard TMS pulse in resting subjects. The effects of rTMS at a cortical and spinal level can be factored out by a combination of monosynaptic (H reflex) testing, anodal electrical stimulation of the cortex (Day et al. 1989) and transmastoid stimulation of the descending pathways at the level of the brainstem (Ugawa et al. 1991). In general, rTMS at low frequency (< 5 Hz) reduces cortical excitability whereas at higher frequencies excitability is increased (Maeda et al. 2000; but see lack of effect of 1 Hz rTMS on excitability in Siebner et al. 1999b). However, it should be noted that, at least for short trains of rTMS, the intensity of the conditioning stimulation as well as its frequency is an important factor in determining the sign of the after-effect (Modugno et al. 2001). The duration of the after-effect depends on the length of time that rTMS is applied; for example, Touge et al. (2001) found that 10 min of motor cortex 1 Hz rTMS of motor cortex at 90 % of relaxed threshold suppressed EMG responses for about 10 min whereas 25 min of stimulation suppressed responses for about 30 min.
Several groups have tried to modulate the effects of rTMS by pairing cortical stimuli with somatosensory input from the periphery. Ziemann et al. (1998) found that facilitation of the biceps MEP during and after ischaemic anaesthesia of the forearm was increased by concurrent 0.1 Hz rTMS of the biceps motor area. Recently, Stefan et al. (2000) and Ridding & Taylor (2001) enhanced corticospinal excitability by pairing rTMS at 0.1 Hz with electrical stimulation of muscle afferents at the motor point. rTMS alone at this frequency had no effect on corticospinal excitability. They suggested that this type of effect might be similar to the associative long-term plasticity that has been reported in animal experiments.
Although MEPs are a convenient way of measuring corticospinal excitability, the synchronised discharge that is produced by TMS is a rather non-physiological way of testing the motor cortex. If rTMS is to be useful as a therapeutic intervention (e.g. George et al. 1999), then it is important to be able to demonstrate that it can produce lasting effects on behaviour as well as on MEPs. At present, data from healthy subjects are equivocal. Muellbacher et al. (2000) found no change in the amplitude and velocity of finger and thumb movements after 15 min rTMS sufficient to reduce the amplitude of MEPs for 30 min. In contrast, Boroojerdi et al. (2000) found that 15 min of 1 Hz rTMS over the primary visual cortex at an intensity that was just at the threshold for evoking visual phosphenes could increase this threshold. However, the increase was small and equal to just under 3 % of stimulator output. Schlaghecken et al. (2001) noted a similar small effect on the motor system. In an abstract, they reported that 1 Hz rTMS over the motor cortex could increase choice reaction times by about 4 %. Data from patients with neurological disorders are more positive, with clear changes in writing performance being found after rTMS over the motor cortex in patients with writer's cramp (Siebner et al. 1999b) and increases in movement speed reported in patients with Parkinson's disease (Siebner et al. 1999a). It may be that a behavioural correlate of rTMS is easier to demonstrate when basal performance is compromised.
One of the problems of trying to identify a more physiological motor effect of rTMS in healthy subjects is the intrinsic variability of volitional movements. If rTMS has only a small effect on cortical excitability then large numbers of subjects would be needed to reveal this effect. In the present paper we have tried to overcome that problem by using a reflex, rather than a volitional response, to test for effects of rTMS on motor behaviour. We reasoned that this would be less variable than a voluntary reaction, and hence a more sensitive probe of motor function. The reflex used was the long latency stretch reflex (LLSR) of the flexor pollicis longus (FPL) muscle (Marsden et al. 1976), which is produced by extension of the interphalangeal joint of the thumb. After some debate about its origin in the 1980s this response is now accepted to be due to activity in a transcortical reflex pathway that involves transmission through the primary motor cortex (Rothwell, 1990). As such, its size should reflect changes in cortical excitability produced by rTMS. One of the advantages of using this reflex is that it is possible to contrast its behaviour with that of the short latency stretch reflex (SLSR) in the same muscle, which uses only a spinal reflex pathway. If rTMS affects the LLSR, but not the SLSR, then we can conclude that its main action is at the cortical and not the spinal level.
In the present study, the experiments involved two types of conditioning in order to produce opposite effects on MEPs. We then investigated whether this conditioning had the same effects on LLSR in FPL. The two types of conditioning comprised 1 Hz stimulation over the motor cortex, to give a suppressive effect on MEPs, and 0.1 Hz rTMS paired with motor point stimulation of the first dorsal interosseous muscle (FDI) to give a facilitatory effect on MEPs. We also tested whether the effects of rTMS on cortical somatosensory processing might contribute to changes in the LLSR. Enomoto et al. (2001) noted that 1 Hz rTMS over the motor cortex, but not at a site 3 cm posterior to it, could reduce the amplitude of cortical somatosensory evoked potentials (SEPs) produced by electrical stimulation of the median nerve. If there were a similar effect on the input from muscle stretch then it could contribute to any change that we observed. Finally, control experiments were conducted on the mixed nerve reflex in the first dorsal interosseous muscle (Deuschl & Eisen, 1999). The long latency component of this response (known as LLR II to distinguish it from the spinal component, LLR I) also involves a transcortical reflex pathway (Farmer et al. 1990). Because the reflex is evoked by electrical stimulation of a peripheral nerve, it can be compared more directly with the SEP than the natural stretch input used to evoke the LLSR.
METHODS
Subjects
Fourteen healthy volunteers (6 males, 8 females; mean age, 29.2 years; range, 19–42 years; all right-handed) were recruited. All subjects gave informed oral consent to the studies, which were approved by the local ethical committee and conformed to the requirements of the Declaration of Helsinki. The subjects had neither a psychiatric medical history nor contraindications to TMS (Wassermann, 1998).
Experimental design
Subjects were seated in a comfortable reclining chair so that the whole body including both arms was at rest. In nine subjects, repeated measurements of the stretch reflex in the right flexor pollicis longus (FPL) muscle and the MEPs in the right first dorsal interosseous (FDI) muscle were performed. For determination of baseline, two measurements for the stretch reflex made 10 min apart, and a single measurement of the mean MEP in relaxed muscle, were made before rTMS. Five trains of 1 Hz rTMS each lasting 5 min and separated by an interval of 1 min (see Touge et al. 2001) were applied over the left area of the primary motor cortex that stimulates the right hand (MIHAND). Post-rTMS corticospinal excitability was investigated immediately (0 min) and 10, 20 and 30 min after completion of conditioning.
Another nine subjects, four of whom also participated in the first experiment, received 30 min of conditioning in which just suprathreshold motor point stimulation of the right FDI muscle was paired every 10 s with 0.1 Hz rTMS over the left MIHAND. As above, two baseline measurements of the stretch reflex in FPL and a single measurement of the mean MEP in the relaxed right FDI were made before rTMS conditioning. These measurements were then repeated immediately (0 min) and 10, 20 and 30 min after completion of conditioning.
The same nine subjects were studied again on a different day, with the same protocol, except that the stretch reflex was replaced by the electrically elicited LLR I and LLR II reflex in the FDI. Finally, six of the same nine subjects were studied on a third occasion in which reflex evaluation was replaced by evaluation of SEPs induced by electrical stimulation of the right or left ulnar nerve at the wrist.
Repetitive transcranial magnetic stimulation
Five trains of 1 Hz rTMS, each lasting 5 min and separated by 1 min, were applied over the left MIHAND at an intensity of 95 % of resting threshold using a Magstim rapid stimulator (The Magstim Co., Dyfed, UK) and a figure-of-eight-shaped coil with an outer winding diameter of 9 cm (Magstim Co.). The magnetic stimulus had a biphasic waveform with a pulse width of about 300 μs. During the first phase of the stimulus, the current in the centre of the coil flowed towards the handle. The coil was held tangentially to the skull with the handle pointing postero-laterally at 45 deg. A total of 1500 magnetic stimuli were applied during the rTMS session.
Motor point stimulation of FDI paired with rTMS
We employed the method described by Ridding & Taylor (2001). In this, electrical stimulation is applied over the motor point of the right FDI muscle (500 ms of 10 Hz stimulation at just above motor threshold intensity and with a pulse width of 1 ms) and paired with single pulse TMS over the left MIHAND at 105 % of resting threshold given 25 ms after the start of motor point stimulation. Each pairing is repeated every 10 s for a total of 30 min. In this experiment, TMS was delivered using a monopolar High Power Magstim 200 machine and a figure-of-eight-shaped coil with an outer winding diameter of 9 cm. Initial current in the coil flowed in an anterior to posterior direction over the hand area.
Measurement of MEPs
Subjects were instructed to keep their hands still and as relaxed as possible. Motor threshold (MT) was determined with the target muscle at rest, and was defined as the minimum stimulus intensity that evoked MEPs with an amplitude of at least 50 μV in five out of ten successive stimuli. Corticospinal excitability was evaluated using suprathreshold stimulation, with the intensity adjusted to produce an MEP of 0.5–1.0 mV peak-to-peak amplitude (mean, 110 % MT; range, 105–120 %). Sixteen MEPs, elicited at 0.2 Hz, were measured and averaged at each time point studied. The stimuli were delivered using a monopolar High Power Magstim 200 machine and a figure-of-eight-shaped coil with an outer winding diameter of 9 cm. Magnetic stimuli were given over the left MIHAND, i.e. the scalp position from which TMS induced MEPs of maximal amplitude in the right target hand muscle. The coil current during the rising phase of the magnetic field flowed towards the handle. EMG electrodes were placed over the belly of the right FDI muscle, with the reference electrode over the first interphalangeal joint of the index finger. Signals were amplified and band-pass filtered (10 Hz to 1 kHz) by a Digitimer D150 amplifier (Digitimer Ltd, Herts, UK) and stored at a sampling rate of 5 kHz on a personal computer for off-line analysis (Sigavg software, Cambridge Electric Design, Cambridge, UK). The peak-to-peak amplitude of each MEP was measured and then averaged at each time point.
Stretch reflexes
Stretch reflexes were elicited in the right FPL muscle. Subjects sat with their semi-pronated forearm supported before them on a table, and with the thumb pad resting on the lever arm of a small torque motor (Printed Motor type G9M4H). The interphalangeal joint of the thumb was aligned with the axis of rotation of the motor shaft and the proximal phalanx was clamped so that movement was limited to the distal phalanx. The starting position of the thumb was at approximately 30 deg flexion at the interphalangeal joint. Subjects held their thumb at a constant position with reference to an oscilloscope display before them, against a steady standing torque of 0.06 N m. Two sizes of stretch, referred to as small and large disturbances, were intermixed and given at random intervals between 4 and 5.5 s. The torque was increased by 0.2 N m for small disturbances or 0.3 N m for large disturbances for a 50 ms period. Stretches were applied in 50 % of trials at random, and visual feedback was removed for the duration of the disturbance. Control trials in which there was no increase in torque were also recorded. Joint angular position (from an infinite resolution Bourns 2 in diameter servopotentiometer mounted on the motor shaft), joint velocity (by analog differentiation of the position signal) and rectified surface EMG (from Ag-AgCl electrodes placed over the muscle belly) were recorded. The EMG was amplified by a Digitimer D150 amplifier (Digitimer Ltd) with band-pass filters set at 10 Hz and 1 kHz. All data were collected at a sampling rate of 5 kHz over a 250 ms period beginning 50 ms before each stretch. An average of 15 trials were carried out for each stretch size and data were stored on a personal computer for off-line analysis (Sigavg software).
Reflexes produced by electrical nerve stimulation
As described by Deuschl & Eisen (1999), reflexes were elicited in the contracted right FDI muscle by electrical stimulation of the ulnar nerve at the wrist. Subjects sat with their pronated forearm supported before them on a table. Subjects made an isometric contraction of the FDI to approximately 40 % of maximum by abducting the distal phalanx of the index finger against a force transducer with reference to a visual display before them. The ulnar nerve was stimulated at motor threshold intensity using surface electrodes with the cathode proximal to the anode (stimulus duration, 1.0 ms; random rate from 0.9 to 1.1 Hz; constant current source). Rectified surface EMG (from Ag-AgCl electrodes placed over the muscle belly) was recorded. The EMG was amplified by a Digitimer D150 amplifier with band-pass filters set at 10 Hz and 1 kHz. Data were collected at a sampling rate of 5 kHz over a 250 ms period beginning 50 ms before each stretch. An average of 256 trials per subject were collected and data were stored on a personal computer for off-line analysis (Sigavg software).
The reflexes following the stretch reflex or electrical nerve stimulation were measured by visual inspection of average records of full-wave rectified EMG activity on the computer display. The end of the short latency and beginning of the long latency reflex were determined by an abrupt increase in the average surface rectified EMG at a latency of between 45 and 55 ms. First, the duration of the short and long latency components of the EMG responses was estimated. Then, the size of the reflexes was taken as the integral of the rectified EMG activity, measured by the computer within the same interval. Finally, this size was expressed as a percentage of control levels of EMG activity extrapolated from the first 50 ms of the sweep, before stretch was delivered.
Somatosensory evoked potentials
SEPs were recorded following motor threshold electrical stimulation of the right or left ulnar nerve at the wrist at 1 Hz (Mauguiere et al. 1999). The intensity of stimulation was checked throughout the course of the experiment by monitoring the evoked EMG response in the FDI muscle. SEPs were recorded from scalp electrodes 2 cm posterior to C3 (parietal component) and 5 cm anterior to C3 (frontal component) referred to the contralateral earlobe. Recordings were made with a band-pass of 5 Hz to 3 kHz using a Digitimer D150 amplifier. All data were collected at a sampling rate of 5 kHz over a 100 ms period beginning 50 ms before each stimulus. An average of 256 trials per subject were carried out and data were stored on a personal computer for off-line analysis (Sigavg software). SEP components were measured peak-to-peak from averaged records.
Data analysis
All measurements were made off-line on a Dell Optiplex GX1 (Dell Computer, Texas, USA) using Nucusor software (MRC-HMBU, Institute of Neurology, University College of London; http://www.ion.ucl.ac.uk). Measurements made after rTMS conditioning were expressed as a percentage of the mean pre-conditioning control values.
All data were analysed using the General Linear Model repeated measures ANOVA in SPSS. Main factors of Time (before and at intervals after rTMS conditioning) and Component (short or long latency stretch or electrical reflexes or components of the SEP) were common to all analyses. Stretch reflexes also had an additional component of stretch size (Disturbance), and SEP had an additional component of Side of stimulation that was included in initial three-way ANOVAs. Conditional on a significant F value, post hoc tests were performed. Values were considered statistically significant when P < 0.05, after making a Fisher's PLSD correction for multiple comparisons.
RESULTS
No subjects experienced any side effects from the stimulation.
Stretch reflex studies
Figure 1 shows typical examples of average responses to the larger of the two stretch sizes before and after rTMS conditioning trains. Stretch evoked a reflex response that had a short and a long latency component (SLSR and LLSR) with an onset of about 25 and 45 ms after stretch onset, respectively (see also Marsden et al. 1976). Conditioning trains of 1 Hz rTMS appeared to decrease the amplitude of the LLSR while having no effect on the SLSR. In contrast, 0.1 Hz rTMS plus motor point stimulation increased the LLSR, again with no effect on the SLSR. The graphs in Fig. 1 show the mean data from all subjects, with the size of the SLSR and LLSR expressed as a percentage of their size in the first control period. Data from both sizes of stretch are plotted as well as the time course of effects on MEPs elicited in the relaxed FDI muscle.
Figure 1. Effect of suppressive (left) or facilitatory (right) conditioning with rTMS on FPL stretch reflexes and on MEPs in the FDI muscle.
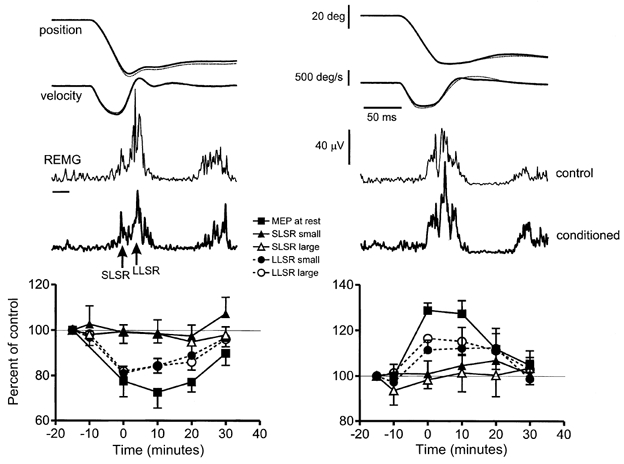
Suppressive conditioning was 25 min of 1 Hz rTMS at 95 % resting threshold; facilitatory conditioning was 30 min of 0.1 Hz rTMS at the same intensity paired with motor point stimulation of the FDI muscle 25 ms earlier. The upper panels show average (of 15 trials each) stretch reflex data produced by a large stretch to FPL in one subject; lower graphs summarise data (means ± s.e.m.) from 9 subjects. Two sets of records are superimposed in the raw data: thin dotted lines represent the control response elicited before any rTMS conditioning, thick continuous lines are the responses immediately after conditioning. The short (SLSR) and long (LLSR) latency components of the stretch reflex are indicated in the rectified EMG from the FPL muscle. The small horizontal bar to the left of the EMG represents baseline. The graphs at the bottom summarise the change in size of the SLSR and LLSR (100 % = pre-rTMS value) to a small and large stretch given to FPL. In addition the change in size of the MEP evoked by a single TMS pulse in FDI is plotted. Note that the SLSR did not change in size after rTMS, but that the MEP and the LLSR decreased after suppressive conditioning and increased after facilitatory conditioning.
In order to check that the stretches given to the thumb were the same before and after rTMS, we measured the maximum velocity of movement (Vmax), and the position of the thumb 25 and 50 ms (P25 and P50, respectively) after the onset of stretch (see Tables 1 and 2). The mechanical effect of any reflex response to the displacement would be expected to occur after 50 ms (Rothwell et al. 1983) so that these measures give an estimate of the mechanical parameters of the stretch itself. A two-way ANOVA of this data with Trajectory (Vmax, P25 and P50) and Time as main factors showed no effect of time for either amplitude of stretch, or for either form of rTMS, indicating that the stretches given to the terminal phalanx of the thumb were the same throughout the experiment.
Table 1.
Similarity of stretch profiles before and after conditioning with 1 Hz rTMS
| P25 (deg) | P50 (deg) | Vmax (deg s−1) | ||||
|---|---|---|---|---|---|---|
| Time | Small | Large | Small | Large | Small | Large |
| Before 1 | 9.4 ± 0.9 | 20.1 ± 1.6 | 19.6 ± 2.3 | 50.8 ± 4.7 | 520 ± 54 | 1161 ± 77 |
| Before 2 | 9.3 ± 1.0 | 21.4 ± 1.5 | 21.0 ± 2.5 | 51.1 ± 4.1 | 520 ± 58 | 1207 ± 49 |
| 0 min | 8.9 ± 0.8 | 20.0 ± 1.6 | 18.8 ± 1.9 | 48.9 ± 4.5 | 485 ± 47 | 1113 ± 78 |
| 10 min | 9.3 ± 0.7 | 20.6 ± 1.2 | 19.3 ± 1.5 | 47.8 ± 3.1 | 507 ± 44 | 1172 ± 66 |
| 20 min | 9.0 ± 0.7 | 19.8 ± 1.1 | 18.7 ± 1.8 | 48.3 ± 3.3 | 494 ± 45 | 1101 ± 62 |
| 30 min | 9.1 ± 0.9 | 20.8 ± 1.5 | 20.1 ± 2.5 | 49.8 ± 5.5 | 528 ± 59 | 1151 ± 77 |
P25 and P50 are the angular positions of the interphalangeal joint 25 and 50 ms, respectively, after stretch onset; Vmax is the maximum velocity of joint rotation. Before 1 and 2, baseline measurements made before rTMS. The two columns of data (Small and Large) refer to the two sizes of stretch that were applied. Data are means ± s.e.m. from 9 subjects.
Table 2.
Similarity of stretch profiles before and after conditioning with 0.1 Hz rTMS paired with motor point stimulation
| P25 (deg) | P50 (deg) | Vmax (deg s−1) | ||||
|---|---|---|---|---|---|---|
| Time | Small | Large | Small | Large | Small | Large |
| Before 1 | 9.1 ± 0.5 | 19.4 ± 1.3 | 17.7 ± 1.5 | 39.8 ± 6.4 | 517 ± 36 | 1212 ± 39 |
| Before 2 | 9.2 ± 0.4 | 19.7 ± 1.1 | 18.9 ± 1.7 | 40.0 ± 6.4 | 529 ± 34 | 1246 ± 21 |
| 0 min | 9.2 ± 0.6 | 20.3 ± 1.3 | 19.9 ± 2.2 | 40.3 ± 6.6 | 527 ± 44 | 1238 ± 41 |
| 10 min | 9.2 ± 0.5 | 19.4 ± 0.9 | 18.2 ± 1.5 | 39.4 ± 5.5 | 533 ± 40 | 1205 ± 36 |
| 20 min | 9.1 ± 1.1 | 19.4 ± 2.4 | 19.4 ± 2.6 | 38.8 ± 7.5 | 513 ± 35 | 1224 ± 37 |
| 30 min | 9.4 ± 0.3 | 19.2 ± 1.3 | 18.2 ± 1.3 | 39.8 ± 5.7 | 493 ± 29 | 1198 ± 36 |
Abbreviations as in Table 1. Data are means ± s.e.m. from 9 subjects.
In the next stage of the analysis we compared the effect of rTMS on the amplitude of the SLSR and LLSR evoked by the large and small stretch in a three-way analysis. This was followed by a comparison of the behaviour of the LLSR and MEP in a two-way analysis.
1 Hz rTMS conditioning
The three-way repeated measures ANOVA showed a significant main effect of Time (F5,40 = 3.7; P < 0.01), and a significant interaction Time × Component (F5,40 = 3.3; P < 0.05), indicating that rTMS had a different effect on the short and long latency components of the stretch reflex. The absence of a significant three-way interaction (Time × Component × Stretch size) suggests that the conditioning effect of rTMS was the same for both sizes of stretch. A two-way ANOVA on the data for the short and long latency components separately showed that there was a main effect of Time on the LLSR (F5,40 = 11.0; P < 0.001) but not on the SLSR. Post hoc tests showed that there was significant suppression of the LLSR at 0, 10 and 20 min after the end of rTMS for both small and large stretches. The two-way ANOVA comparing the time course of effects on LLSR and MEP showed no significant Time × Response type interaction, suggesting that the effect of rTMS was the same on both responses.
0.1 Hz rTMS with paired motor point stimulation
The three-way repeated measures ANOVA showed a significant main effect of Time (F5,40 = 3.1; P < 0.01), and a significant interaction Time × Component (F5,40 = 2.4; P < 0.05), indicating that rTMS had a different effect on the short and long latency components of the stretch reflex. A two-way ANOVA on the data for the short and long latency components separately showed that there was a main effect of Time on the LLSR (F5,40 = 3.4; P lt; 0.05) but not on the SLSR. Post hoc tests showed that there was significant facilitation of the LLSR immediately after the end of rTMS for the large stretch. A separate ANOVA comparing the effect of rTMS on LLSR and MEP showed no significant interaction Time × Response type, suggesting that the effect of rTMS was the same on both responses.
Somatosensory evoked potentials
Enomoto et al. (2001) have already noted that SEPs from median nerve stimulation are reduced by 1 Hz rTMS over the motor cortex. It is possible therefore that reduced excitability in the sensory input of the transcortical loop could have contributed to the decrease in LLSR that we observed after 1 Hz rTMS. To test this idea more thoroughly, we examined whether our second form of conditioning (0.1 Hz rTMS of left sensorimotor cortex plus motor point stimulation of the right FDI) would have the opposite effect. Ulnar stimulation was used to evoke the SEP for direct comparison with data from the final series of experiments below.
The left panel of Fig. 2 illustrates a typical SEP recorded from an electrode over C3 (10–20 system: linked ear reference) following right ulnar stimulation. The stimulus artefact has been removed and the record began 14 ms after the stimulus was given (P14). The N20, P25 and N33 peaks are all clearly visible. Two records are superimposed, from immediately before and after conditioning. The amplitudes of the N20/P25 and the P25/N33 components were larger after conditioning.
Figure 2. Effect of facilitatory rTMS conditioning on SEPs evoked by stimulation of the contralateral and ipsilateral ulnar nerve at the wrist.
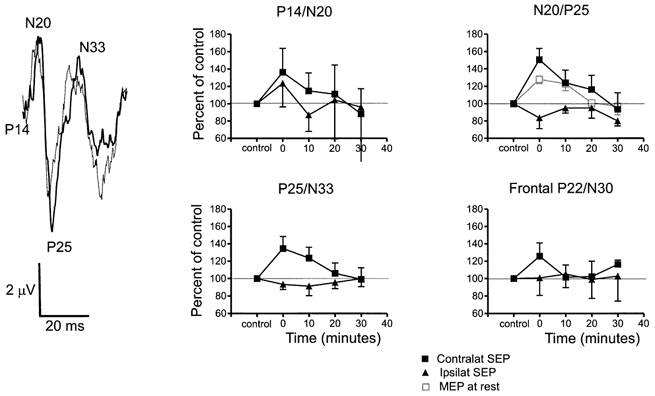
Facilitatory conditioning was 0.1 Hz rTMS at 95 % resting threshold paired with motor point stimulation over the right FDI. The left panel shows an example of an average (of 256 trials) SEP following right ulnar nerve stimulation in one subject, recorded over the left sensory cortex. Two traces are superimposed: the thin line represents the response before conditioning, the thick line shows the response after conditioning. The stimulus artefact has been removed and the traces start 12 ms after the nerve stimulus. The N20/P25 and P25/N33 components were increased after conditioning. The graphs on the right summarise the data from all 9 subjects (means ± s.e.m.) for SEPs recorded simultaneously from both hemispheres. The amplitude of each component of the response is expressed as a percentage of the pre-conditioning control (100 %). Three of the graphs show the amplitude of the parietal SEP components (P14/N20, N20/P25, P25/N33); the fourth shows the amplitude of the P22/N30 component recorded from frontal leads. Note the increase in amplitude of all contralateral components except P14/N20 after conditioning. There was no effect on ipsilateral components produced by stimulation of the left ulnar nerve. The effect of the conditioning on the MEP in the FDI of the same subjects is shown by the open symbols in the graph of N20/P25.
The graphs in Fig. 2 illustrate the mean data from all subjects, with the amplitude of the various components normalised to that measured in the pre-conditioning control period. The data contrast the effect on SEPs from right and left ulnar nerve. In addition to the components shown in the raw data trace we also measured the amplitude of the P22/N30 component that occurs in more frontal leads (5 cm anterior to C3). For comparison, the graph of the N20/P25 component also includes the time course of the conditioning effect on MEPs in relaxed FDI in the same subjects.
A three-way repeated measures ANOVA on the data with Time, SEP component and Side of stimulation as main factors yielded a significant Time × Side of stimulation interaction (F4,50 = 2.5; P < 0.05). This shows that the conditioning stimulation had a different effect on the SEPs from right and left sides. A separate two-way ANOVA on the data from right and left SEPs with Time and SEP component as main factors only showed a main effect of Time on SEPs from right stimulation (F4,25 = 3.1; P < 0.05). Post hoc tests indicated that there was no effect of rTMS on the P14/N20 component from either arm. However, later components of the SEPs from the right arm were facilitated at 0 (N20/P25, P25/N33 and P22/N30) and 10 min (N20/P25 and P25/N33) after the end of conditioning whereas left SEPs were unaffected at all intervals. We conclude that 0.1 Hz rTMS over the left motor cortex paired with motor point stimulation of the right FDI can enhance the SEPs evoked from the right but not from the left ulnar nerve. In a final analysis we used a two-way ANOVA to compare the time course of rTMS conditioning on the N20/P25 component of the SEP and the MEP in the FDI muscle (see Fig. 2). The absence of any significant interaction term (Time × Type of response) indicates that the time course was similar for both types of response.
Electrically elicited transcortical reflexes
The experiments above and those by Enomoto et al. (2001) on SEPs suggest that changes in sensory transmission to cortex produced by rTMS conditioning could contribute to the effects on LLSR amplitude. However, SEPs are evoked by an electrically elicited synchronous input to the CNS, whereas LLSRs are produced by a natural and more sustained asynchronous input. Our final experiment was designed to test whether long latency reflexes elicited by a synchronous input would behave in the same way as LLSRs after rTMS conditioning.
The upper panel of Fig. 3 illustrates the typical pattern of reflex response in the FDI to single pulse electrical stimulation of the ulnar nerve at the wrist. A large M-wave occurs after the stimulus artefact, and this is followed by the short and long latency reflex responses (LLR I and LLR II) at about 30 and 50 ms after the stimulus. The record is a superimposition of the response from one subject recorded immediately before and after conditioning with 0.1 Hz rTMS paired with motor point stimulation. There was a small increase in the size of LLR II after rTMS but no effect on LLR I. The mean data from all subjects are plotted in the lower panel of Fig. 3, together with the effect of conditioning on the MEP in the same muscle at rest.
Figure 3. Effect of facilitatory rTMS conditioning on the amplitude of electrically elicited reflexes and MEPs in the FDI muscle.
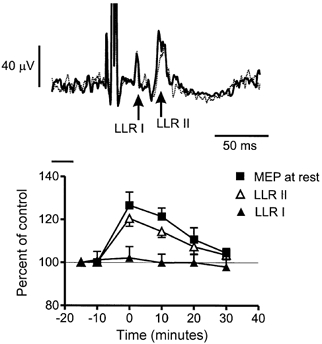
Facilitatory conditioning was 0.1 Hz rTMS at 95 % resting threshold paired with motor point stimulation over the FDI. The upper panel shows an example of an average (of 256 trials) reflex in one subject. Two traces are superimposed: the thin dotted line represents the response before conditioning, the thick continuous line shows the response after conditioning. The short (LLR I) and long (LLR II) components of the response are indicated. The short horizontal bar represents the basal level of EMG. The LLR II component was increased by the conditioning protocol. The lower panel summarises the mean data (± s.e.m.) from 9 subjects. The size of the reflex responses is expressed as a percentage of the pre-conditioning control. LLR I was unchanged, whereas LLR II was facilitated. For comparison, the size of the MEP evoked at rest in the same muscle is shown in the same format.
A two-way repeated measures ANOVA showed a significant main effect of Time (F5,16 = 5.9; P lt; 0.0001) and a significant Time × Component interaction (F5,16 = 4.4; P lt; 0.001), indicating that the effect of rTMS plus peripheral conditioning was different on LLR I and LLR II. Post hoc tests showed that LLR II was increased in amplitude at 0 and 10 min after the end of the train whereas LLR I was unaffected at any interval. A second two-way ANOVA comparing the effect on LLR II and MEP showed a significant main effect of Time (F5,16 = 5.9; P lt; 0.001) but no significant Time × Response type interaction. This suggests that the effect of the conditioning trains was the same on both responses. We conclude that transcortical reflexes elicited by a synchronous electrical nerve volley are conditioned by rTMS in the same way as natural stretch reflexes.
DISCUSSION
In the present paper we have: (1) confirmed previous observations (see Chen et al. 1997; Stefan et al. 2000) that rTMS of the motor cortex can affect the amplitude of MEPs evoked in relaxed hand muscles for several minutes after the end of stimulation. Thus, 25 min of 1 Hz rTMS at 95 % resting motor threshold over the motor cortex suppressed the MEPs in FDI for 20 min, whereas MEPs were enhanced for a similar period after 30 min of 0.1 Hz rTMS paired with FDI motor point stimulation. (2) We have extended the observations of Enomoto et al. (2001) on the effect of rTMS on cortical SEPs by showing that SEPs are increased for about 10 min by 30 min of 0.1 Hz rTMS paired with motor point stimulation. (3) Finally, we have shown that that the amplitude of the transcortical stretch reflex in the FPL muscle is modulated in the same way and with the same time course as both the MEP and SEP, whereas the spinal stretch reflex is unaffected. We conclude that conditioning with rTMS can affect cortical processing of ‘natural’ physiological inputs.
Changes in the MEP
The data show that it is possible to either increase or decrease the amplitude of MEPs evoked in relaxed muscles by appropriate combinations of rTMS and rTMS/afferent input. The magnitude and time course of the effects we describe in the FDI muscle are similar to those described by others in different hand muscles (e.g. abductor pollicis brevis; Chen et al. 1997; Stefan et al. 2000), and in previous work on FDI (Touge et al. 2001). The mechanism of the effect is not known, but many authors have drawn attention to the possibility that processes such as long term depression and potentiation may be involved (Chen et al. 1997). However, Touge et al. (2001) recently showed that although 1 Hz rTMS suppressed MEPs evoked in relaxed muscle, it had no effect when MEPs were tested during voluntary contraction. They argued that since the same cortical elements are stimulated by TMS whether subjects are active or relaxed, changes in synaptic efficacy should also have been observable in both states. They concluded that changes in MEP amplitude were more likely to reflect changes in levels of excitability at rest rather than changes in the strength of cortical synapses.
Changes in the SEP
Enomoto et al. (2001) applied 1 Hz rTMS at an intensity similar to that used here to condition the LLSR, and found that it decreased the amplitude of the early cortical components of the median nerve SEP without changing the baseline to peak amplitude of the initial N20. Since the latter potential is usually assumed to represent arrival of the sensory volley at the primary sensory cortex, they presumed that the rTMS had affected later processing (P25 and later potentials) of the input rather than any subcortical transmission. Stimulation 2–3 cm anterior (premotor cortex) or posterior (sensory cortex) had no effect at the same intensity, and Enomoto et al. (2001) concluded that the effect on sensory processing was due to a cortico-cortical effect from motor to sensory cortex. However, they could not exclude the possibility of a spread of stimulation from the motor cortex site to adjacent postcentral cortex.
In the present experiments we extended this finding to show that 0.1 Hz rTMS paired with motor point conditioning could increase SEPs in parallel to its effect on the MEP and LLSR. Again, we found no obvious effects on the early P14/N20 component of the potential, whereas there was a clear effect on later events. We therefore presume that the mechanism was similar to that proposed by Enomoto et al. (2001). We also showed that rTMS affected the amplitude of the frontal P22/N30 component, which may reflect direct input to precentral areas of cortex (e.g. Valeriani et al. 1997). The implication from these studies is that rTMS conditioning not only changes the excitability of the motor cortex but may also change the responsiveness of the sensory cortex to afferent input. In the context of the present experiments, such effects could contribute to the overall changes we measured in transcortical reflexes. They may also have to be taken into account when interpreting behavioural studies of movement control after rTMS.
Changes in transcortical reflexes
Both the MEP and the SEP are produced by synchronous neural discharges that test the excitability of the motor and sensory pathways in a non-physiological manner. We therefore employed a more ‘natural’ input from muscle stretch to test the effect of rTMS conditioning on an asynchronous physiological input to the CNS. In the final experiment we also evoked long latency reflexes in FDI using electrical stimulation of the ulnar nerve to test whether these responses also behaved in a manner that could be predicted from changes in cortical excitability. The long latency reflexes evoked by either technique are thought to involve a transcortical relay from somatosensory to motor cortex (Rothwell, 1990; Deuschl & Eisen, 1999). For example, the onset latency of the LLSR in FPL is about 45 ms, which is around 5 ms longer than the minimum conduction delay from muscle spindle to cortex and back to muscle; the onset of LLR II may be 55 ms, which is more than 10 ms longer than the minimum conduction delay from FDI to cortex and back again. One explanation for this extra delay is that extra time is consumed as a result of synaptic relays between cortical areas (see Marsden et al. 1983). This idea is also consistent with the results of studies in patients with cortical lesions (Marsden et al. 1977).
rTMS conditioning produced changes in the long latency components of both reflexes but did not affect the short latency response. Since the latter is thought to be of spinal origin, it seems likely that the changes in the long latency component were due to changes in excitability in supraspinal structures, probably in the cerebral cortex. Interestingly, we found that 0.1 Hz rTMS paired with motor point stimulation of the FDI had the same effect on the LLSR in FPL as on the MEP in FDI. Stefan et al. (2000) and Ridding & Taylor (2001) have emphasised that when they used MEP testing, such paired conditioning was spatially specific to the muscle being stimulated, with little or no effect in muscles at a distance. In the present experiments we stimulated FDI, but obtained clear effects in the distant FPL. This might have been due to the fact that FPL acts synergistically with FDI in precision grip tasks, and therefore shares some of the same cortical fields conditioned by the repetitive paired inputs. The experiments with the LLSR II evoked by single pulse electrical stimulation of the ulnar nerve in FDI confirmed that paired rTMS conditioning also increased transcortical reflexes elicited in the target muscle.
Relation between effects on SEP, MEP and long latency reflexes
The fact that the sign and time course of the conditioning effects were similar in all three cases suggests strongly that changes in the amplitude of long latency reflexes reflect changes in excitability of both sensory and motor cortices. There are, however, two problems with this explanation. First, the effect on the LLSR was never larger than the effect on the SEP or the MEP alone. If motor and sensory cortex are both involved in the transcortical responses, one might have expected a much stronger effect on the LLSR. The second problem is that Touge et al. (2001) found that MEPs were no longer affected by rTMS when tested in contracting muscles rather than at rest. Since long latency reflexes are always evoked in contracting muscle, then why should rTMS conditioning change them?
One possibility is that the effects of rTMS on reflexes depend on changes in excitability of both sensory and motor cortex, whilst voluntary contraction only abolishes the effect on responses mediated via the motor cortex. If reflexes are affected by the excitability of both sensory and motor cortex, then they may still be suppressed during contraction. Another explanation is that MEPs and transcortical reflexes test different subpopulations of the corticospinal system. These could be, for example, fast and slow conducting pyramidal fibres or even (since TMS tends to activate corticospinal neurones trans-synaptically) different sets of inputs to the same pyramidal fibres. Maertens de Noordhout et al. (1992) advanced a similar proposal in their study of transcortical cutaneous reflexes. They found that MEPs were suppressed relative to baseline at the same time as the onset of the excitatory transcortical response. If different populations of neurones were involved, then voluntary contraction could affect rTMS conditioning of MEPs but not LLSRs.
Conclusions
The present data show that rTMS conditioning has clear effects on the processing of natural inputs evoked by muscle stretch. By implication, they suggest that such procedures might also produce behavioural effects on voluntary movement (see Schlaghecken et al. 2001). Since rTMS over motor cortex appears to have effects on the excitability of sensory as well as motor cortex, both may contribute to changes in transcortical reflexes as well as voluntary movement. Finally, although voluntary contraction reduces or abolishes the effect of rTMS conditioning on the MEP, effects on the LLSR are preserved, indicating that changes in MEP amplitude do not always correlate with behavioural effects.
Acknowledgments
We would like to thank Mr P. Asselman for his assistance with the equipment used in these experiments. The work was supported by the Medical Research Council.
REFERENCES
- Boroojerdi B, Prager A, Muellbacher W, Cohen LG. Reduction of human visual cortex excitability using 1 Hz transcranial magnetic stimulation. Neurology. 2000;54:1529–1531. doi: 10.1212/wnl.54.7.1529. [DOI] [PubMed] [Google Scholar]
- Chen R, Classen J, Gerloff C, Celnik P, Wassermann EM, Hallett M, Cohen LG. Depression of motor cortex excitability by low-frequency transcranial magnetic stimulation. Neurology. 1997;48:1398–1403. doi: 10.1212/wnl.48.5.1398. [DOI] [PubMed] [Google Scholar]
- Day BL, Dressler D, Maertens de Noordhout A, Marsden CD, Nakashima K, Rothwell JC, Thompson PD. Electric and magnetic stimulation of human motor cortex: surface EMG and single motor unit responses. Journal of Physiology. 1989;412:449–473. doi: 10.1113/jphysiol.1989.sp017626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deuschl G, Eisen A. Long-latency reflexes following electrical nerve stimulation. The International Federation of Clinical Neurophysiology. Electroencephalography and Clinical Neurophysiology Supplement. 1999;52:263–268. [PubMed] [Google Scholar]
- Enomoto H, Ugawa Y, Kanazawa I. Decreased sensory cortical excitability after 1 Hz rTMS over the ipsilateral primary motor cortex. Clinical Neurophysiology. 2001;112:2154–2158. doi: 10.1016/s1388-2457(01)00667-8. [DOI] [PubMed] [Google Scholar]
- Farmer SF, Ingram DA, Stephens JA. Mirror movement studied in a patient with Klippel-Feil syndrome. Journal of Physiology. 1990;428:467–484. doi: 10.1113/jphysiol.1990.sp018222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- George MS, Lisanby SH, Sackheim HA. Transcranial magnetic stimulation. Applications in neuropsychiatry. Archives of General Psychiatry. 1999;56:300–311. doi: 10.1001/archpsyc.56.4.300. [DOI] [PubMed] [Google Scholar]
- Maeda F, Keenan JP, Tormos JM, Topka H, Pascual-Leone A. Modulation of corticospinal excitability by repetitive transcranial magnetic stimulation. Clinical Neurophysiology. 2000;111:800–805. doi: 10.1016/s1388-2457(99)00323-5. [DOI] [PubMed] [Google Scholar]
- Maertens de Noordhout A, Rothwell JC, Day BL, Dressler D, Nakashima K, Thompson PD, Marsden CD. Effect of digital nerve stimuli on responses to electrical or magnetic stimulation of the human brain. Journal of Physiology. 1992;447:535–548. doi: 10.1113/jphysiol.1992.sp019016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsden CD, Merton PA, Morton HB. Stretch reflex and servo action in a variety of human muscles. Journal of Physiology. 1976;259:531–560. doi: 10.1113/jphysiol.1976.sp011481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsden CD, Merton PA, Morton HB, Adam J. The effect of lesions of the sensorimotor cortex and the capsular pathways on servo responses from the human long thumb flexor. Brain. 1977;100:503–526. doi: 10.1093/brain/100.3.503. [DOI] [PubMed] [Google Scholar]
- Marsden CD, Rothwell JC, Day BL. Long latency automatic responses to muscle stretch in man: origin and function. Advances in Neurology. 1983;39:509–540. [PubMed] [Google Scholar]
- Mauguiere F, Allison T, Babiloni C, Buchner H, Eisen AA, Goodin DS, Jones SJ, Kakigi R, Matsuoka S, Nuwer M, Rossini PM, Shibasaki H. Somatosensory evoked potentials. The International Federation of Clinical Neurophysiology. Electroencephalography and Clinical Neurophysiology Supplement. 1999;52:79–90. [PubMed] [Google Scholar]
- Modugno N, Nakamura Y, Mackinnon C, Filipovic SR, Bestmann S, Berardelli A, Rothwell JC. Motor cortex excitability following short trains of repetitive magnetic stimuli. Experimental Brain Research. 2001;140:453–459. doi: 10.1007/s002210100843. [DOI] [PubMed] [Google Scholar]
- Muellbacher W, Ziemann U, Boroojerdi B, Hallett M. Effects of low-frequency transcranial magnetic stimulation on motor excitability and basic motor behavior. Clinical Neurophysiology. 2000;111:1002–1007. doi: 10.1016/s1388-2457(00)00284-4. [DOI] [PubMed] [Google Scholar]
- Pascual-Leone A, Valls-Sole J, Wassermann EM, Hallett M. Responses to rapid-rate transcranial magnetic stimulation of the human motor cortex. Brain. 1994;117:847–858. doi: 10.1093/brain/117.4.847. [DOI] [PubMed] [Google Scholar]
- Ridding MC, Taylor J. Mechanisms of motor-evoked potential facilitation following prolonged dual peripheral and central stimulation in humans. Journal of Physiology. 2001;537:623–632. doi: 10.1111/j.1469-7793.2001.00623.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothwell JC. Long latency reflexes of human arm muscles in health and disease. Electroencephalography and Clinical Neurophysiology Supplement. 1990;41:251–263. doi: 10.1016/b978-0-444-81352-7.50030-3. [DOI] [PubMed] [Google Scholar]
- Rothwell JC, Obeso JA, Traub MM, Marsden CD. The behaviour of the long-latency stretch reflex in patients with Parkinson's disease. Journal of Neurology Neurosurgery and Psychiatry. 1983;46:35–44. doi: 10.1136/jnnp.46.1.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlaghecken F, Munchau A, Bloem B, Rothwell JC, Eimer M. Efects of premotor cortex repetitive transcranial magnetic stimulation (rTMS) on reaction times in a masked priming task. Human Brain Mapping Abstracts. 2001:1252. [Google Scholar]
- Siebner HR, Mentschel C, Auer C, Conrad B. Repetitive transcranial magnetic stimulation has a beneficial effect on bradykinesia in Parkinson's disease. NeuroReport. 1999a;10:589–594. doi: 10.1097/00001756-199902250-00027. [DOI] [PubMed] [Google Scholar]
- Siebner HR, Tormos JM, Ceballos-Baumann AO, Auer C, Catala MD, Conrad B, Pascual-Leone A. Low-frequency repetitive transcranial magnetic stimulation of the motor cortex in writer's cramp. Neurology. 1999b;52:529–537. doi: 10.1212/wnl.52.3.529. [DOI] [PubMed] [Google Scholar]
- Stefan K, Kunesch E, Cohen LG, Benecke R, Classen J. Induction of plasticity in the human motor cortex by paired associative stimulation. Brain. 2000;123:572–584. doi: 10.1093/brain/123.3.572. [DOI] [PubMed] [Google Scholar]
- Touge T, Gerschlager W, Brown P, Rothwell JC. Are the after-effects of low frequency rTMS on motor cortex excitability due to changes in the efficacy of cortical synapses? Clinical Neurophysiology. 2001;112:2138–2145. doi: 10.1016/s1388-2457(01)00651-4. [DOI] [PubMed] [Google Scholar]
- Ugawa Y, Day BL, Rothwell JC, Thompson PD, Merton PA, Marsden CD. Modulation of motor cortical excitability by electrical stimulation over the cerebellum in man. Journal of Physiology. 1991;441:57–72. doi: 10.1113/jphysiol.1991.sp018738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valeriani M, Restuccia D, Di Lazzaro V, Le Pera D, Scerrati M, Tonali P, Mauguiere F. Giant central N20-P22 with normal area 3b N20-P20: an argument in favour of an area 3a generator of early median nerve cortical SEPs? Electroencephalography and Clinical Neurophysiology. 1997;104:60–67. doi: 10.1016/s0168-5597(96)96660-5. [DOI] [PubMed] [Google Scholar]
- Wassermann EM. Risk and safety of repetitive transcranial megnetic stimulation: report and guidelines. Electroencephalography and Clinical Neurophysiology. 1998;108:1–16. doi: 10.1016/s0168-5597(97)00096-8. [DOI] [PubMed] [Google Scholar]
- Ziemann U, Corwell B, Cohen LG. Modulation of plasticity in human motor cortex after forearm ischemic nerve block. Journal of Neuroscience. 1998;18:1115–1123. doi: 10.1523/JNEUROSCI.18-03-01115.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]


