Abstract
The Ca2+-activated Cl− current (ICl(Ca)) has been identified in atrial, Purkinje and ventricular cells, where it plays a substantial role in phase-1 repolarization and delayed after-depolarizations. In sinoatrial (SA) node cells, however, the presence and functional role of ICl(Ca) is unknown. In the present study we address this issue using perforated patch-clamp methodology and computer simulations. Single SA node cells were enzymatically isolated from rabbit hearts. ICl(Ca) was measured, using the perforated patch-clamp technique, as the current sensitive to the anion blocker 4,4′-diisothiocyanostilbene-2,2′-disulphonic acid (DIDS). Voltage clamp experiments demonstrate the presence of ICl(Ca) in one third of the spontaneously active SA node cells. The current was transient outward with a bell-shaped current-voltage relationship. Adrenoceptor stimulation with 1 μm noradrenaline doubled the ICl(Ca) density. Action potential clamp measurements demonstrate that ICl(Ca) is activate late during the action potential upstroke. Current clamp experiments show, both in the absence and presence of 1 μm noradrenaline, that blockade of ICl(Ca) increases the action potential overshoot and duration, measured at 20 % repolarization. However, intrinsic interbeat interval, upstroke velocity, diastolic depolarization rate and the action potential duration measured at 50 and 90 % repolarization were not affected. Our experimental data are supported by computer simulations, which additionally demonstrate that ICl(Ca) has a limited role in pacemaker synchronization or action potential conduction. In conclusion, ICl(Ca) is present in one third of SA node cells and is activated during the pacemaker cycle. However, ICl(Ca) does not modulate intrinsic interbeat interval, pacemaker synchronization or action potential conduction.
Atrial, Purkinje and ventricular myocytes from different species have been demonstrated to express a Ca2+-activated Cl− current (ICl(Ca)) (for reviews, see Sorota, 1999; Hume et al. 2000). ICl(Ca) is the 4-aminopyridine insensitive part of the transient outward current and is also termed Ito2. ICl(Ca) is primarily activated by Ca2+ ions released from the sarcoplasmic reticulum (Sipido et al. 1993; Kawano et al. 1995). The current does not follow the time course of the intracellular Ca2+ transients, but reaches a peak within 10–20 ms and then inactivates in the following 100 ms (Zygmunt & Gibbons, 1991, 1992; Sipido et al. 1993). It is postulated that this transient behaviour may be due to an intrinsic Ca2+-dependent inactivation of ICl(Ca) or may be due to the time course of local, subsarcolemmal Ca2+ gradients (Sipido et al. 1993; Zygmunt, 1994; Kawano et al. 1995).
The reversal potential for Cl− ions (ECl) in cardiomyocytes is approximately −50 mV (Sorota, 1999). Therefore, activation of ICl(Ca) has the potential, in theory, to generate an inwardly directed, depolarizing current at resting membrane potentials, whereas it generates an outwardly directed, repolarizing current during phase-1 and phase-2 of the action potential. Indeed, it has been demonstrated experimentally that ICl(Ca) strongly influences phase-1 repolarization of atrial (Wang et al. 1995) and ventricular action potentials (Hiraoka & Kawano, 1989; Kawano & Hiraoka, 1991), especially under conditions of adrenoceptor stimulation (Verkerk et al. 2001). Moreover, it has been demonstrated that ICl(Ca) contributes to the potentially arrhythmogenic delayed after-depolarizations in Purkinje and ventricular cells (Zygmunt et al. 1998; Verkerk et al. 2000). These findings indicate that ICl(Ca) plays a substantial role in atrial, Purkinje and ventricular electrophysiology.
The presence of ICl(Ca) in sinoatrial (SA) node cells is unknown. In SA node tissue, the sarcoplasmic reticulum is relatively sparse compared with atrial cells (Masson-Pévet et al. 1978). Nevertheless, cultured and freshly isolated mammalian SA node cells show clear intracellular Ca2+ transients (Li et al. 1997; Huser et al. 2000; Rigg et al. 2000; Bogdanov et al. 2001) as do amphibian pacemaker cells (Ju & Allen, 1999, 2000), indicating that the substrate for activating ICl(Ca) in SA node cells is present. The present study was designed to assess the presence and functional role of ICl(Ca) in single SA node cells of rabbit. We report that ICl(Ca) is present in one third of the rabbit SA node cells. Action potential clamp measurements show that ICl(Ca) is activated during the normal pacemaker cycle late during the action potential upstroke. Blockade of ICl(Ca) results in an increase of the action potential overshoot. Noradrenaline (1 μm) doubled the size of ICl(Ca), but even under such conditions, ICl(Ca) did not change the firing rate. We incorporated ICl(Ca) into our previously published model of a rabbit SA node cell (Wilders et al. 1991), and used this model to assess the functional role of ICl(Ca) in pacemaker activity. The computer simulations show that ICl(Ca) has minimal effects on synchronization of SA nodal cells and action potential conduction between SA nodal and atrial cells.
METHODS
Cell preparation
All experiments were carried out in accordance with guidelines of the local Institutional Animal Care and Use Committee. Single SA node cells were isolated from rabbit hearts by an enzymatic dissociation procedure as described by Verheijck et al. (1995). The hearts were obtained from New Zealand White rabbits (body weight: 3.0–3.5 kg), which were anaesthetized with a 1 ml kg−1 intramuscular injection of Hypnorm (10 mg ml−1 fluanisone and 0.315 mg ml−1 fentanyl citrate; Jansen Pharmaceutics, Tilburg, The Netherlands). Small aliquots of cell suspension were put in a recording chamber on the stage of an inverted microscope. Cells were allowed to adhere for 5 min after which perfusion with Tyrode solution (36 ± 0.5 °C) was started. The Tyrode solution contained (mm): NaCl 140, KCl 5.4, CaCl2 1.8, MgCl2 1.0, glucose 5.5 and Hepes 5.0 (pH was adjusted to 7.4 with NaOH). Cells were dissociated from the entire SA node region and represent a mixed population of cells showing heterogeneity in shape (Denyer & Brown, 1990; Verheijck et al. 1998a). For our experiments, we selected spindle and elongated spindle-like cells displaying regular contractions.
Recording procedures
Membrane potentials and membrane currents were recorded using the amphotericin perforated-patch technique (Horn & Marty, 1988) to prevent run down of membrane currents by dilution of intracellular components. Patch pipettes were pulled from borosilicate glass and heat polished. The pipette solution contained (mm): potassium gluconate 125, KCl 20, amphotericin 2.2 and Hepes 10 (pH was adjusted to 7.2 with KOH). The potential between pipette and bath solution was adjusted to zero before a high-resistance seal was formed. All potentials were off-line corrected for the estimated 13 mV liquid junction potential. Membrane currents and potentials were filtered on-line with a cutoff frequency of 1 kHz, and digitized by a 12-bit analog-to-digital converter (NB-MIO-16, National Instruments Co. Austin, TX, USA) with a sampling frequency of 2 kHz. Data were stored and analysed by custom software.
Current-clamp experiments
To characterize action potentials, several action potential parameters were determined: action potential duration at 20, 50 and 100 % repolarization (APD20, APD50, and APD100, respectively), maximal diastolic potential (MDP), action potential overshoot, cycle length and maximum upstroke velocity (dV/dtmax). Diastolic depolarization rate (DDR) was measured over the 50 ms time interval starting at the MDP + 1 mV. MDP + 1 mV was used rather than MDP because the time at which the MDP + 1 mV was reached could be determined more reliably than the time at which the MDP was reached. Cell capacitance (Cm) was estimated from the change in slope of the membrane potential (Δ(dVm/dt)) upon 100 ms hyperpolarizing current pulses of 25 to 50 pA (ΔIm), which were applied shortly after the action potential had reached its MDP. Cell capacitance was calculated as Cm = ΔIm / (Δ(dVm/dt), and ranged between 18 and 110 pF with a mean value of 51 ± 5.8 pF (n = 40; mean ± s.e.m.).
Voltage clamp experiments
The L-type Ca2+ current (ICa,L) and the delayed rectifier current (IK) were elicited by series of depolarizing voltage clamp steps of 500 ms duration to membrane potentials ranging from −40 to +70 mV, with 10 mV increments. Voltage steps were applied once every 2 s from a holding potential of −40 mV. We defined ICa,L as inward peak current at the beginning of a depolarizing voltage clamp step (Ipeak). IK was defined as the quasi steady-state current at the end of the depolarizing voltage clamp steps (Iqss). Ipeak and Iqss are largely due to ICa,L and IK, respectively, but other currents might also contribute to these currents. The hyperpolarizing-activated current (If) was elicited by series of hyperpolarizing voltage clamp steps of 500 ms duration to membrane potentials ranging from −40 to −100 mV, with 10 mV decrements. Voltage clamp steps were applied once every 2 s from a holding potential of −40 mV. We defined If as the difference between the current at the end and start of the hyperpolarizing voltage clamp step. In addition, tail currents of the delayed rectifier current and If were analysed. As tail currents predominantly express deactivation of a current, they are expressed relative to the current level at the holding potential, i.e. −40 mV. The Ca2+-activated Cl− outward current (ICl(Ca)) was elicited by a series of depolarizing voltage clamp steps of 500 ms duration to membrane potentials ranging from −40 to +70 mV, with 10 mV increments. Voltage steps were applied once every 2 s from a holding potential of −40 mV. ICl(Ca) was defined as the transient outward current sensitive to 4,4′diisothiocyanatostilbene-2,2′-disulphonic acid (DIDS; Sigma Chemical Co). DIDS was freshly prepared as a 0.5 m stock solution in DMSO (Merck) and kept in the dark. It was diluted in Tyrode solution for use at a final concentration of 0.2 mm. The action potential clamp technique, as introduced in SA node cells by Doerr et al. (1989), was used to determine the time course of the ICl(Ca) during the pacemaking cycle. Therefore, transmembrane potentials were recorded during stable spontaneous pacemaker activity (current clamp conditions), digitized at 10 kHz, and stored. One representative action potential waveform was selected and used as the command signal in voltage clamp conditions to drive the potential in the same cell. Continuous pacemaker activity was obtained by repeatedly applying the same waveform to the cell. At least 20 consecutive waveforms were applied in order to reach stable electrical activity. The membrane currents were recorded under control conditions, in the presence of 0.2 mm DIDS and after drug washout. All currents were normalized for cell size by dividing current amplitude by Cm. Adrenoceptor stimulation was induced by application of 1 μm noradrenaline (Centrafarm. Etten-Leur, The Netherlands).
Computer simulations
We used computer simulations to investigate the role of ICl(Ca) in pacemaker activity. In these simulations, the membrane potential (Vm) of a rabbit SA nodal cell was calculated from our detailed mathematical SA node model cell (Wilders et al. 1991), into which we incorporated equations for ICl(Ca). The formulation of ICl(Ca) was based on a model study of ICl(Ca) in atrial cells (Gomis-Tena & Saiz, 1999a, 1999b) and is simulated as a [Ca2+]i-dependent conductance with (Vm-dependent) outward rectification through:
| (1) |
where R, T and F are universal gas constant, absolute temperature and Faraday constant, respectively, and where:
| (2) |
and
| (3) |
represent the [Ca2+]i dependence and Vm dependence, respectively. Since our model does not discriminate between free and bound intracellular calcium, the [Ca2+]i in eqn (2) is total intracellular calcium concentration. According to Gomis-Tena & Saiz (1999a, b), the intracellular and extracellular chloride concentrations, [Cl−]i and [Cl−]o, are set to the Lindblad et al. (1996) values of 30 and 132 mm, respectively.
Following the approach of Gomis-Tena & Saiz (1999a, b), ICl(Ca) is thus the product of: (a) the driving force for chloride ions, described by the Goldmann-Hodgkin-Katz equation, (b) the [Ca2+]i dependence, tentatively described by a Hill-type equation with parameters Km and nh, and (c) the Vm dependence as is demonstrated in a variety of excitable and non-excitable cells using physiological [Cl−]i (for reviews, see Sorota, 1999; Hume et al. 2000). Quantitative experimental data on the [Ca2+]i dependence of ICl(Ca), however, are absent. In our computer simulations the binding constant Km and exponent nh (in eqn (2)) were set to 5 mm and 3, respectively. Using these two parameter settings, both the experimentally observed current-voltage relationship and the moment of activation during an action potential could be well fitted (see Figs 2, 6 and 7). In addition, the permeability PCl(Ca) (in eqn (1)) was set on 0.33 μL s−1 to match the density of ICl(Ca) with our own experimental data (see Fig. 2 and Fig. 7). Thus some parameter values were not based on experimental data but were chosen because they allow a proper fit of our own experimental data.
Figure 2. Presence of the Ca2+-activated Cl− current (ICl(Ca)) in SA node cells.
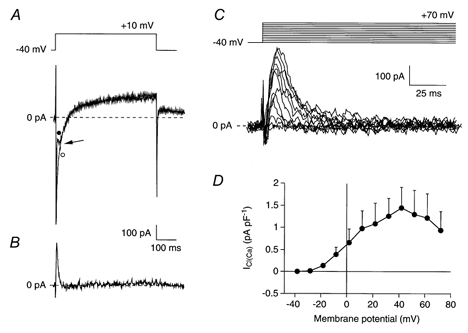
A, superimposed current traces of an SA node cell (Cm, 110 pF) elicited by voltage steps from −40 to +10 mV in absence (•) and presence (○) of 0.2 mm DIDS. Arrow indicates an outwardly directed transient current, which is superimposed on the inward peak current. This transient current is blocked by DIDS. B, DIDS-sensitive ICl(Ca) current obtained by digital subtraction of the two current traces of panel A. C, ICl(Ca) measured as the DIDS-sensitive current at potentials between −40 and +70 mV in the same cell as shown in panels A and B. The current activates around −20 mV and the peak amplitude increases upon further depolarization to +40 mV. At more depolarized potentials, the peak amplitude of ICl(Ca) decreases. D, average I-V relationship of ICl(Ca) in 7 cells.
Figure 6. Action potential clamp recordings of ICl(Ca) in an SA node cell.
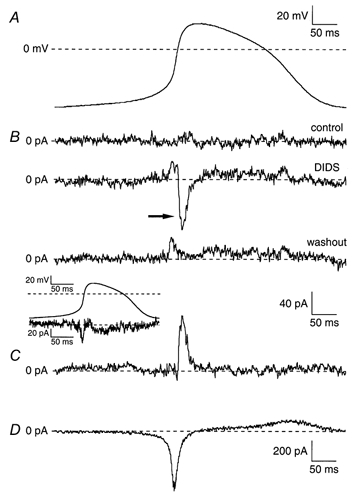
A, complete cycle of pacemaker activity of an SA node cell (Cm, 81 pF) recorded under current clamp conditions. B, currents recorded during reapplied pacemaker activity under voltage clamp conditions (‘action potential clamp’) in absence of DIDS (upper panel), in presence of 0.2 mm DIDS (middle panel), and upon wash out of the drug (lower panel). Inset shows current obtained by digitally subtracting the current trace recorded under wash out conditions from the one recorded under control conditions. C, DIDS-sensitive current ICl(Ca) obtained by digital subtraction of compensation currents in presence and after wash out of 0.2 mm DIDS. D, reconstructed current (Im) flow during the cycle of pacemaker activity. Current was calculated by Im = -Cm × dV/dt, where Cm is the membrane capacity of the cell. Note the different current scale.
Figure 7. Computer simulations of ICl(Ca) under voltage clamp and current clamp conditions.
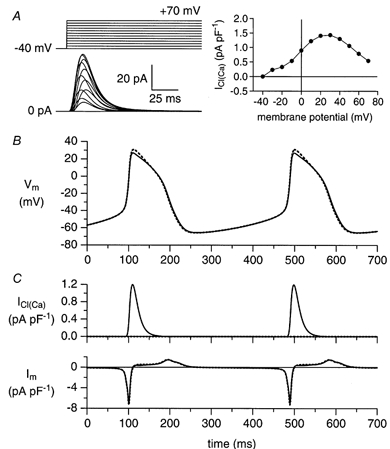
A, ICl(Ca) in response to voltage clamp steps as indicated (left) and associated I-V relationship (right). B, free-running membrane potential (Vm) of the Wilders et al. (1991) rabbit SA nodal cell model with and without ICl(Ca) (continuous and dashed lines, respectively). C, time course of ICl(Ca) and net membrane current (Im) during the spontaneous activity of panel B.
For numerical integration of differential equations we applied a simple and efficient Euler scheme with a fixed time step of 5 μs. All software was compiled as a 32-bit Windows application using Compaq Visual Fortran 6.5 and run on a 667-MHz Alpha processor workstation (Microway Screamer).
Statistics
Results are expressed as means ± s.e.m. Data on action potential characteristics were obtained from 10 consecutive action potentials and averaged. Using statistical analysis software of Microsoft® Excel 98, statistical significance was determined by one-way or two-way analysis of variance (ANOVA) combined with Student's t test for paired or unpaired observations. A probability value of P < 0.05 was considered significant.
RESULTS
Sensitivity of SA node cation membrane currents to DIDS
In previous studies in atrial, Purkinje and ventricular cells, ICl(Ca) was defined as the current sensitive to DIDS (Zygmunt & Gibbons, 1991, 1992; Sipido et al. 1993). In a first series of experiments, we tested the sensitivity to 0.2 mm DIDS of the major cationic membrane currents, i.e., If, ICa,L (defined as the inward peak current at the beginning of a voltage clamp step, Ipeak), and IK (defined as quasi steady-state current at the end of a voltage clamp step, Iqss) in SA node cells. For Fig. 1, to avoid interference with the DIDS-sensitive ICl(Ca), we only used cells lacking ICl(Ca) (see below). Figure 1A shows current traces in the absence and presence of DIDS recorded upon voltage clamp steps to −100, 0 and +50 mV, from a holding potential of −40 mV. Figure 1, B-D, shows the average I-V relationship of If, Ipeak, and Iqss of 10 SA node cells in the absence (•) and presence (○) of 0.2 mm DIDS. Current traces as well as the I-V relationships before and during the administration of 0.2 mm DIDS almost completely overlap. Therefore we conclude that DIDS does not significantly affect If, Ipeak (predominantly reflecting ICa,L), or Iqss (predominantly reflecting IK).
Figure 1. Effects of 0.2 mm DIDS on cationic membrane currents in sinoatrial (SA) node cells.
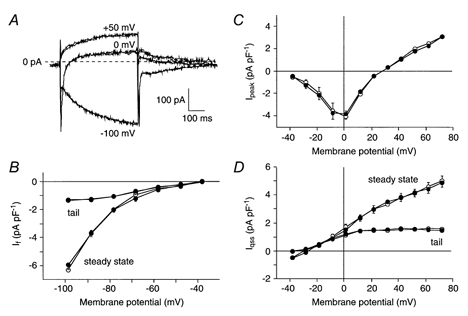
A, superimposed current traces of an SA node cell (Cm, 57 pF) elicited by 500 ms voltage steps from −40 mV to −100, 0, and +50 mV in absence and presence of DIDS. B-D, average current-voltage (I-V) relationship of the hyperpolarization-activated current (If, B), the inward peak current at the beginning of a voltage clamp step (Ipeak, C), and the quasi steady-state current at the end of a voltage clamp step (Iqss, D) in 10 cells in the absence (•) and presence (○) of DIDS. Both quasi steady-state and tail currents of Iqss and If are shown.
Presence of ICl(Ca) in SA node cells
In a second series of experiments, we tested the presence of ICl(Ca) in 21 single SA node cells. We applied a series of depolarizing voltage clamp steps from a holding potential of −40 mV to membrane potentials ranging from −40 to +70 mV in the absence and presence of 0.2 mm DIDS and recorded the resulting family of currents. Figure 2A shows two current traces elicited upon stepping to +10 mV recorded before (•) and during (○) the administration of 0.2 mm DIDS. Before administration, Ipeak was accompanied by a transient outwardly directed current component (see arrow), which was blocked during the administration of DIDS. By digitally subtracting the two current traces, the DIDS-sensitive ICl(Ca) current was obtained (Fig. 2B). In 7 out of 21 cells, we observed such a DIDS-sensitive current. Figure 2C shows the current traces of this DIDS-sensitive current recorded between −40 and +70 mV with 10 mV increments on an expanded time scale. The current is transient and outwardly directed, with characteristics similar to those of ICl(Ca) in atrial, Purkinje and ventricular cells (for reviews, see Sorota, 1999; Hume et al. 2000). Figure 2D shows the average I-V relationship of ICl(Ca) in these 7 SA node cells. The I-V relationship was bell shaped with an activation threshold around −20 mV and a maximum near +40 mV.
Our results demonstrate that ICl(Ca) is present in one third of the cells tested. It might be argued that cells which display ICl(Ca) are not nodal but atrial cells since we have previously described that atrial cells are present in the SA node region (Verheijck et al. 1998a). Figure 3A shows an example of the electrical activity of a cell with ICl(Ca) (○) and one without ICl(Ca) (•). The two cells clearly show similar electrical activity. Table 1 contrasts action potential parameters of the 7 SA node cells with ICl(Ca) and the 14 SA node cells without ICl(Ca). No difference in any action potential parameter could be detected between both groups of cells. However, a flattening of the top of the action potential was consistently present in cells including ICl(Ca) (see Fig. 3A). Figs 3B-D shows that the average I-V relationships of If, Ipeak, and Iqss of SA node cells with ICl(Ca) (○) and without ICl(Ca) (•) also do not differ from each other.
Figure 3. Electrical characteristics of SA node cells with and without ICl(Ca).
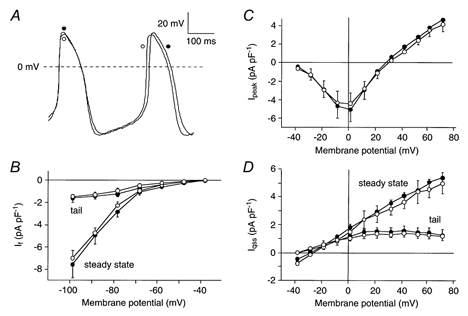
A, typical pacemaker activity of an SA node cell with ICl(Ca) (○; Cm, 29 pF) and an SA node cell without ICl(Ca) (•; Cm, 62 pF). B-D, average I-V relationship of If (B), Ipeak (C), and Iqss (D) of 7 cells with (•) and 14 cells without (○) ICl(Ca). Both quasi steady-state and tail currents of Iqss and If are shown.
Table 1.
Action potential characteristics of SA node cells with and without ICl(Ca)
| No ICl(Ca) (n = 14) | With ICl(Ca) (n = 7) | |
|---|---|---|
| APD20 (ms) | 63 ± 11 | 70 ± 7 |
| APD50 (ms) | 92 ± 12 | 102 ± 10 |
| APD100 (ms) | 167 ± 17 | 171 ± 18 |
| MDP (mV) | −61.0 ± 1.6 | −60.4 ± 3.2 |
| DDR (mVs−1) | 92 ± 12 | 91 ± 18 |
| Overshoot (mV) | 23.3 ± 1.6 | 20.9 ± 2.9 |
| dV/dtmax (V s−1) | 5.9 ± 1.3 | 5.1 ± 1.5 |
| Cycle length (ms) | 316 ± 31 | 314 ± 38 |
| Capacitance (pF) | 49 ± 6 | 47 ± 10 |
Data are means ± s.e.m. APD20, APD50 and APD100,action potential duration at 20, 50 and 100% repolarization, respectively. MDP, maximal diastolic potential; DDR,diastolic depolarization rate; Overshoot,action potential overshoot and dV/dtmax, maximum upstroke velocity.
Effect of ICl(Ca) blockade on electrical activity of SA nodal myocytes
Our voltage clamp experiments show that ICl(Ca) is present in one third of the regularly beating single SA node cells of rabbit. Recently, it was demonstrated that the increase in subsarcolemmal intracellular Ca2+ concentration in spontaneously active cells occurred concomitantly with the late phase of diastolic depolarization (Huser et al. 2000; Rigg et al. 2000; Bogdanov et al. 2001). This implies that ICl(Ca) may already be activated during the late phase of the diastolic depolarization and thus can play a role in regulating pacemaker activity. The selective action of DIDS on ICl(Ca) enabled us to evaluate the contribution of ICl(Ca) to spontaneous electrical activity.
The effects of DIDS on SA node action potentials were investigated on the 7 cells including ICl(Ca). Figure 4 shows a typical example of an SA node action potential recorded in the absence (•) and presence (○) of 0.2 mm DIDS. DIDS predominantly increased the action potential overshoot and prolonged the APD20. Table 2 summarizes the effect of 0.2 mm DIDS on action potential parameters. DIDS significantly increased the action potential overshoot and prolonged APD20, without affecting dV/dtmax, DDR, MDP, APD50, APD100 or cycle length. Thus, ICl(Ca) modulates the action potential overshoot of SA node cells, but it does not play a significant role in beating rate regulation under normal conditions.
Figure 4. Effects of blockade of ICl(Ca) in SA node cells.
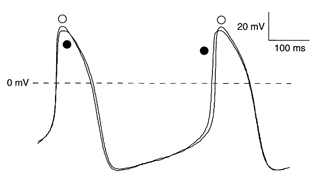
Typical pacemaker activity of a ICl(Ca) expressing SA node cell (Cm, 29 pF) in absence (•) and presence (○) of 0.2 mm DIDS.
Table 2.
Effect of 0.2 mm DIDS on action potential characteristics of SA node cells expressing ICl(Ca) (n = 7)
| Control | DIDS | |
|---|---|---|
| APD20 (ms) | 70 ± 7 | 73 ± 7* |
| APD50 (ms) | 102 ± 10 | 104 ± 9 |
| APD100 (ms) | 171 ± 18 | 172 ± 19 |
| MDP (mV) | −60.4 ± 3.2 | −60.3 ± 3.5 |
| DDR (mV s−1) | 91 ± 18 | 105 ± 25 |
| Overshoot (mV) | 20.9 ± 2.9 | 22.8 ± 3.3* |
| dV/dtmax (V s−1) | 5.1 ± 1.5 | 5.1 ± 1.4 |
| Cycle length (ms) | 314 ± 38 | 316 ± 43 |
Data are means ± s.e.m.. APD20, APD50, and APD100, action potention duration at 20, 50, and 100% repolarization, respectively. MDP, maximal diastolic potential; DDR, diastolic depolarization rate; Overshoot, action potential overshoot and dV/dtmax, maximum upstroke velocity.
P < 0.05.
Role of ICl(Ca) on electrical activity during adrenergic stimulation
It is well known that the calcium content of the SR is increased during adrenoceptor stimulation, mainly as a consequence of an increase in ICa,L (Osterrieder et al. 1982) and an increase in the loading of the SR (Wolska et al. 1996). It has been demonstrated that sympathetic stimulation with isoprenaline increased the amplitude of Ca2+ transient by about 75 % in guinea-pig SA node cells (Rigg et al. 2000) and by about 80 % in amphibian pacemaker cells (Bramich & Cousins, 1999; Ju & Allen, 1999). It is conceivable that this enhanced filling and subsequent release of Ca2+ from the SR during adrenergic stimulation may enhance ICl(Ca) and thereby increase its role in SA node electrophysiology.
In an additional series of experiments, we determined the effect of adrenoceptor stimulation on the density of ICl(Ca). Therefore, we compared the ICl(Ca) already measured in the 7 cells under control conditions with ICl(Ca) in another set of cells in presence of 1 μm noradrenaline. In 6 out of 16 cells, ICl(Ca) was observed in the presence of noradrenaline. The percentage of cells showing ICl(Ca) thus was comparable in the absence (33.3 %, n = 21) and presence (37.5 %, n = 16) of noradrenaline. This suggests that adrenoceptor stimulation could not ‘wake up’ ICl(Ca) in cells that had no apparent current at baseline. In 5 out of these 6 cells, the voltage clamp experiments could be completed before the cells showed any signs of run down. Figure 5A shows the mean I-V relationship of ICl(Ca) under control conditions (•) and in presence of noradrenaline (○). Both I-V relationships are bell shaped with an activation threshold around −20 mV and a peak close to +40 mV. However, density of ICl(Ca) is about doubled in the presence of noradrenaline.
Figure 5. Adrenoceptor stimulation and ICl(Ca) in SA node cells.
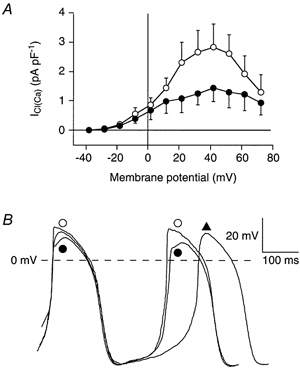
A, average I-V relationship of ICl(Ca) in absence (n = 7; •) and presence of 1 μm noradrenaline (n = 5; ○). B, typical pacemaker activity of a ICl(Ca) expressing SA node cell (Cm, 36 pF) under control conditions (▴), in the presence of 1 μm noradrenaline (•), and in the presence of both 1 μm noradrenaline and 0.2 mm DIDS (○).
In the same group of cells, we measured whether ICl(Ca) may influence pacemaker activity upon adrenoceptor stimulation. The effects of DIDS on SA node action potentials parameters in the presence of noradrenaline were investigated in the 6 cells expressing ICl(Ca). Figure 5B shows a typical example of SA node action potentials recorded under control conditions (▴), in the presence of noradrenaline (•), and in the presence of noradrenaline and 0.2 mm DIDS (○). Table 3 summarizes the average action potential parameters under these three conditions. In the SA node cells expressing ICl(Ca), noradrenaline induces the well-known positive chronotropic effect (for review, see Irisawa et al. 1993; Boyett et al. 2000). In addition, the cycle length and action potential overshoot were significantly decreased, while dV/dtmax and DDR were significantly increased. In the presence of noradrenaline, blockade of ICl(Ca) with DIDS significantly increased action potential overshoot and APD20, without changing the other action potential characteristics. Our experiments thus show that adrenoceptor stimulation increased ICl(Ca) density and thereby enhanced its modulating role on the action potential overshoot. However, even under conditions of potentiated ICl(Ca) density, this current does not play a significant role in controlling beating rate.
Table 3.
Effects of 0.2 mm DIDS on action potential characteristics of SA node cells expressing ICl(Ca) (n = 6) in the presence of adrenergic receptor stimulation by 1 μM noradrenaline
| Control | Norad. | Norad. + DIDS | |
|---|---|---|---|
| APD20 (ms) | 72 ± 7 | 61 ± 4* | 70 ± 5† |
| APD50 (ms) | 105 ± 11 | 94 ± 7 | 100 ± 6 |
| APD100 (ms) | 160 ± 11 | 153 ± 5 | 155 ± 5 |
| MDP (mV) | −62.0 ± 1.2 | −61.4 ± 2.0 | −61.6 ± 1.5 |
| DDR (mV s−1) | 91 ± 17 | 117 ± 17* | 118 ± 17 |
| Overshoot (mV) | 19.3 ± 1.2 | 16.4 ± 1.3* | 21.9 ± 1.6† |
| dV/dtmax (V s−1) | 5.4 ± 0.6 | 6.8 ± 0.4* | 6.6 ± 0.3 |
| Cycle length (ms) | 339 ± 16 | 267 ± 24* | 270 ± 19 |
Data are means ± s.e.m.. APD20, APD50, and APD100, action potential duration at 20, 50, and 100 % repolarization, respectively. MDP, maximal diastolic potential; DDR, diastolic depolarization rate; Overshoot, action potential overshoot; and dV/dtmax, maximum upstroke velocity, Norad., noradrenaline.
P < 0.05 noradrenaline vs. control
P < 0.05 noradrenaline + DIDS vs. noradrenaline.
Action potential clamp and DIDS sensitive current during electrical activity
The above experiments provide evidence that ICl(Ca) is active during spontaneous activity, but plays no role in regulating the intrinsic cycle length. Also, the above experiments suggest that ICl(Ca) is activated late during the fast action potential depolarization. Pharmacological blockade of individual currents provides useful information, but the changes in membrane potential induced by blockade of a single current may, in turn, affect other currents (Zaza et al. 1997), resulting in an overlap of primary and secondary effects of blockade, thus confusing the interpretation of results. Therefore, we carried out a final series of experiments in which we used the action potential clamp technique as introduced in SA node cells by Doerr et al. (1989) to avoid these problems. In these experiments, the time course of the DIDS-sensitive ICl(Ca) during the pacemaking cycle is measured while clamping the membrane potential with action potential waveforms recorded from the same cell under control conditions.
Figure 6 shows a typical example of an action potential clamp experiment. Figure 6A shows the membrane potential recorded during stable spontaneous pacemaking activity (current clamp conditions) in a single cell under control conditions. This waveform was used as the command signal to drive the membrane potential of the same cell in the voltage clamp mode. Figure 6B shows the membrane current recorded under action potential clamp conditions during control conditions (upper panel), in presence of 0.2 mm DIDS (middle panel), and after wash out of the drug (lower panel). The total membrane current recorded under control conditions was close to zero (Fig. 6B, top), indicating that there is no difference in the intrinsic and the applied voltage waveform. The current recorded after wash out of DIDS was also close to zero, except for a short period during the action potential upstroke and shortly thereafter (Fig. 6B, bottom). Apparently, a difference in intrinsic and the applied voltage occurs after wash out, which is reflected by a small compensation current. The inset shows the current obtained by digitally subtracting the current trace recorded after wash out of DIDS from the one recorded during control conditions. The resulting current shows similarities in shape with the nifedipine-sensitive current, ICa,L, as recorded by Zaza and coworkers (1997) during action potential clamp conditions. The current amplitude in our experiments, however, is much smaller than reported by Zaza et al. (1997), suggesting that the small compensation current during the wash out conditions is due to slight run down of ICa,L. An additional compensation current was observed in the presence of DIDS (Fig. 6B, middle panel, arrow). This DIDS-induced compensation current provides a mirror image of the contribution of the blocked component, i.e. ICl(Ca), to the normal pacemaking cycle. Figure 6C shows ICl(Ca), which was obtained by digitally subtracting the current trace recorded in presence of DIDS from the one recorded after wash out of the drug. ICl(Ca) measured using the action potential clamp technique is a transient outward current that becomes apparent near +5 mV and reaches a peak value of about 80 pA in the final phase of the action potential upstroke. The contribution of ICl(Ca) to the current generating the action potential waveform is limited to the final phase of the upstroke and the early plateau phase. Figure 6D shows the reconstructed net membrane current (Im = - Cm dV/dt) of the action potential shown in Panel A. Note that ICl(Ca) is active in the phase when Im declined from its large inward peak of about −600 pA to values near about −100 pA.
Using the action potential clamp technique, we observed ICl(Ca) in 4 out of 13 (30.8 %) single SA node cells tested, which agrees with our conventional voltage clamp experiments that show that about one third of the SA node cells exhibit ICl(Ca). The current was active late during the upstroke and early plateau phase. The average density of peak ICl(Ca) measured during the action potential clamp measurements is 1.16 ± 0.19 pA pF−1 (n = 4), which is comparable with the current density measured during the conventional voltage clamp steps (Fig. 2D).
Role of ICl(Ca) in pacemaker activity
Our experiments demonstrate that ICl(Ca) is active late during the upstroke and early during the plateau phase of the action potential, resulting in a flattening of the top of the action potential in SA node cells. In atrial and ventricular cells, it was demonstrated that such reduction of action potential overshoot has implications for action potential conduction, especially under conditions of decreased intercellular coupling (Joyner et al. 1996; Wang et al. 2000; Huelsing et al. 2001). As a consequence of the reduced height of the early plateau phase of the action potential, the driving force for intercellular coupling current through gap junctions was found to be reduced, thereby increasing the minimum value of intercellular coupling conductance (Gc) required for successful action potential transfer (Joyner et al. 1996; Wang et al. 2000; Huelsing et al. 2001). To assess the functional role of ICl(Ca) in pacemaker activity, we carried out computer simulations using our previously published model of a rabbit SA node cell (Wilders et al. 1991), into which we incorporated the Gomis-Tena & Saiz (1999a, b)ICl(Ca) equations which are as set out in Methods.
Validation of ICl(Ca)
In a first set of simulations, we validated our model description of ICl(Ca) by comparing the model-generated ICl(Ca) with experimental data, both under voltage clamp and current clamp conditions. Figure 7A (left) shows the model ICl(Ca) in response to voltage clamp steps from a holding potential of-40 mV to test potentials ranging between −40 and +70 mV. We obtained current traces quite similar to those obtained with the same protocol in our experiments (Fig. 2C) by setting the model parameters Km and nh (eqn (2)) to 5 mm and 3, respectively. The model I-V relationship (Fig. 7A, right) is also quite similar to the one obtained experimentally (Fig. 2D). The value of the one remaining model parameter, i.e. PCl(Ca) (eqn (1)), was chosen such that the maximum current density in the model I-V relationship (1.43 pA pF−1) was similar to the average maximum density that we observed experimentally (1.44 ± 0.45 pA pF−1, n = 7). The differences in amplitude between the current traces of Fig. 2C and Fig. 7A (left) are largely due to the difference in size between the real cell (110 pF) and the model cell (32 pF).
Next, we carried out current clamp simulations with our SA nodal cell model, with the same ICl(Ca) equations and parameters as used in the voltage clamp simulations, to test to which extent the results of our current clamp and action potential clamp experiments could be reproduced. Figure 7B shows the spontaneous activity of the model cell with and without ICl(Ca) (continuous and dashed lines, respectively). Like the experimental case (Fig. 4), the most prominent effect of ‘blocking’ ICl(Ca) is a 4 mV increase in action potential overshoot. If we increase PCl(Ca) (eqn (1)) by a factor of 2, thus doubling ICl(Ca), as occurs during adrenergic stimulation (Fig. 5A), and then study the effects of ‘blocking’ ICl(Ca), the most prominent effect is a somewhat larger increase in action potential overshoot (7 mV), in accordance with the experimental observations (Fig. 5B). Figure 7C shows ICl(Ca) and the net membrane current (Im) during the spontaneous activity of Fig. 7B. The time course of those currents resembles that observed experimentally in the action potential clamp experiment of Fig. 6. Also, with a value of 1.20 pA pF−1, the peak amplitude of ICl(Ca) in the simulations compares well with the experimentally observed value of 1.16 ± 0.19 pA pF−1 (n = 4).
Effects of ICl(Ca) on pacemaker synchronization
Subsequently, we tested the importance of ICl(Ca) for pacemaker synchronization between SA node cells, which is a prerequisite for normal action potential initiation in the SA node. Previously, we demonstrated that pacemaker synchronization in pairs of SA node cells at low Gc results mainly from the phase resetting effects of the action potential of one cell on the depolarization phase of the other cell, while at higher coupling conductances, the tonic, diastolic interaction prevails (Wilders et al. 1996; Verheijck et al. 1998b). Consequently, we hypothesized that ICl(Ca) may play a role in pacemaker synchronization under conditions of decreased intercellular coupling. To address this issue, we electrically coupled two model rabbit SA nodal cells via a variable Gc and estimated the critical value of Gc, i.e. the minimal value of Gc required for action potential synchronization. The critical Gc was determined in absence of ICl(Ca) and after incorporation of ICl(Ca) into the SA nodal cell model at distinct (difference in) cycle lengths. The cycle length was varied by changing the maximum conductance of If. As we reported previously (Wilders et al. 1996), critical Gc increases almost linearly with increasing difference in cycle length. The critical Gc in presence of ICl(Ca), however, did not differ in any significant way from the critical Gc in absence of ICl(Ca) (data not shown). Also, at higher values of Gc, ICl(Ca) had minimal effects on synchronization of the model cells. These results suggest that ICl(Ca) has a limited role in pacemaker synchronization.
Effects of ICl(Ca) on action potential conduction
In a final set of simulations, we assessed the importance of ICl(Ca) for action potential conduction between SA nodal and atrial cells. Previously, we demonstrated that our model rabbit SA nodal cell drives a real rabbit atrial cell at Gc > 0.55 nS (Joyner et al. 1998). In our simulations, we coupled the model SA node cell to a model rabbit atrial cell (Lindblad et al. 1996) and studied the critical Gc at which the SA node cell could successfully pace and drive the atrial cell. This critical Gc was determined in absence of ICl(Ca) and after incorporation of ICl(Ca) into the SA nodal cell model. The critical Gc in presence of ICl(Ca) did not differ significantly from the critical Gc in absence of ICl(Ca) (data not shown). Also at higher values of Gc, ICl(Ca) had minimal effects on action potential transfer between the model cells. These results suggest that ICl(Ca) has a limited role in action potential conduction between SA nodal and atrial cells.
DISCUSSION
In this paper we employed patch-clamp methodology and computer simulations to investigate the presence and functional role of ICl(Ca) in single SA node cells of rabbit. Using the amphotericin-permeabilized patch clamp technique, we found a transient ICl(Ca) in one third of the cells. The I-V relationship of ICl(Ca) is bell shaped with an activation threshold around −20 mV, and a maximum close to +40 mV. Using the action potential clamp technique, we demonstrate that ICl(Ca) is active late during the upstroke and early during the plateau phase of the action potential. Adrenoceptor stimulation with noradrenaline doubles ICl(Ca), which resulted in a decrease of the action potential overshoot. Both under control conditions and upon adrenoceptor stimulation, blockade of ICl(Ca) caused an increase in action potential overshoot and APD20, but did not otherwise affect pacemaker activity. Our computer simulations demonstrate that ICl(Ca) has a neither a role in pacemaker synchronization nor in action potential conduction.
Selective effect of DIDS on ICl(Ca)
In the present study, we used 0.2 mm DIDS as a tool to demonstrate the presence of ICl(Ca) and to study the functional role of this current in SA node cells. DIDS, however, is not a specific blocker for the Ca2+-activated Cl− conductance, but blocks also the swelling-activated Cl− current (ICl(swell)) and carriers involved in pHi regulation, i.e. the Na+-HCO3− cotransporter and the Cl−-HCO3− exchanger (Leem & Vaughan-Jones, 1998; Hume et al. 2000).
We cannot exclude the possibility that ICl(swell) also contributes to our DIDS-sensitive current, but we presume that the role of ICl(swell) is limited for the following reasons. Firstly, we found a transient DIDS-sensitive current with a bell shaped I-V relation. These characteristics compare to those of ICl(Ca) rather than to the characteristics of a swelling-activated Cl− conductance, which is time independent and exhibits outward rectification (for review, see Vandenberg et al. 1996; Sorota, 1999; Hume et al. 2000). Secondly, the ability to observe a transient DIDS-sensitive current immediately after formation of the whole-cell perforated-patch configuration suggests that the observed current is unrelated to ICl(swell), which develops over time (Vandenberg et al. 1996). Thirdly, ICl(swell) was reported to be insensitive to β-receptor stimulation in SA node cells (Hagiwara et al. 1992), while in our experiments the DIDS-sensitive current was doubled in the presence of noradrenaline.
In our experiments, we expect no influences of DIDS on pHi. We used a Hepes-buffered solution where pHi is regulated mainly by DIDS-insensitive Na+-H+ exchange (Buckler et al. 1990; Leem & Vaughan-Jones, 1998). This is supported by the absence of changes in cycle length, while both alakalinization and acidification alter significantly the frequency of spontaneous activity in rabbit sinoatrial node (Satoh & Seyama, 1986).
Recently, it was demonstrated that DIDS (10 μm) inhibits the ICa,L and IK in colonic myocytes (Dick et al. 1999). We found no indications that the main cation currents in SA node cells were significantly changed by as much as 0.2 mm DIDS (Fig. 1). Our findings agree with observations made in atrial and ventricular myocytes of dog, rabbit and sheep where the drug failed to influence the ICa,L, the IK, the 4-aminopyridine sensitive transient outward current, the inward rectifier current, or the action potential when ICl(Ca) was already blocked by 20 mm internal EGTA (Zygmunt & Gibbons, 1992; Zygmunt, 1994; Kawano et al. 1995; Zygmunt et al. 1997; Verkerk et al. 2001). In addition, no effects of DIDS on [Ca2+]i-transients were found (Sipido et al. 1993; Verkerk et al. 2001), further indicating that Ca2+-modulated membrane currents other than ICl(Ca) do not play a role in the effects of the drug on action potential configuration. Thus in our experiments, the effects of DIDS on the action potentials and membrane currents seem entirely attributable to blockade of ICl(Ca).
Presence of ICl(Ca) in SA node cells
It has been demonstrated that SA node cells contain at least two distinct Cl− currents. Stretch-activated anion currents were observed in single rabbit SA node cells (Hagiwara et al. 1992; Arai et al. 1996; Lei & Kohl, 1998; Kohl et al. 1999). Moreover, an angiotensin-II-activated chloride current was found in rabbit SA node cells suggesting that SA node cells contain protein kinase-C-sensitive Cl− channels (Bescond et al. 1994). In our experiments, we additionally demonstrate that ICl(Ca) is present in one third of the rabbit SA node cells (Figs 2, 5 and 6). It is unknown why ICl(Ca) is non-uniformly distributed among the SA node cells. All tested cells displayed regular contractions indicating that Ca2+ release from the SR, and thus the substrate for activating ICl(Ca), was significantly present.
The non-uniform distribution of ICl(Ca) may be related to the inhomogeneity of function and structure of the SA node (for review, see Bouman & Jongsma, 1995; Boyett et al. 2000). Functionally, a distinction has been made in the SA node between the site of earliest activation, i.e. the primary pacemaker area, and a group of nodal fibres that are triggered to excitation, i.e. the subsidiary pacemaker area. Action potentials in the primary pacemaker area have a lower amplitude, dV/dtmax, MDP and a higher DDR and action potential duration compared with those in the subsidiary pacemaker area (Bouman & Jongsma, 1995; Boyett et al. 2000). In our study, action potential characteristics did not differ significantly between isolated SA node cells with and without ICl(Ca) (Table 1). We therefore suggest that the role of pacemaker cell type in the non-uniform distribution of ICl(Ca) is limited. Structurally, Boyett and coworkers found a cell size-dependent variation in densities of various cation currents in isolated SA node cells (for review, see Boyett et al. 2000), although this is not a consistent finding (Wilders et al. 1996; Verheijck et al. 1998a; Wu et al. 2001; Mangoni & Nargeot, 2001). In the present study, we did not observe a cell size-dependent variation in density of the anion current, ICl(Ca). The cell capacitance did not differ significantly between SA node cells with and without ICl(Ca) (Table 1). We therefore suggest that the role of cell size in the non-uniformly distributed ICl(Ca) is limited. Recently, Wu et al. (2001) found a morphology-dependent variation in densities of a cation current in isolated SA node cells. They observed a smaller If in spindle cells compared with spider-like cells. In the present study, we only used spindle-like cells. Thus, a possible role of morphological differences in the non-uniform distribution of ICl(Ca) was excluded.
In our experiments, the pipette solution did not contain sodium. Sipido et al. (1997) and Faber & Rudy (2000) have demonstrated that this may have implications for the amplitude of Ca2+ transients. Sipido et al. (1997) performed experiments with low (0 mm) or high [Na+]i (20 mm) and found in guinea-pig ventricular cells that Ca2+ transients were smaller using low [Na+]i. Comparable findings were made by Faber & Rudy (2000) using a detailed theoretical model of a guinea-pig ventricular cell. In our experiments, a lower Ca2+ transient may have led to a smaller current density of ICl(Ca). However, we think that the absence of [Na+]i is not responsible for the heterogeneity of presence of ICl(Ca) in SA node cells. All cells showed clear contractions, and upregulation of [Ca2+]i transient by 1 μm noradrenaline could not activate ICl(Ca) in cells that had no apparent current at baseline. Moreover, we have used a similar pipette solution in previous studies on sheep Purkinje and ventricular cells (Verkerk et al. 2000, 2001), and found that ICl(Ca) was present in all cells tested.
It has been suggested, based on cell size-dependent variation, that the expression of Ca2+-handling proteins of the ryanodine receptor declines from the periphery to the centre of rabbit SA node cells (Musa et al. 1999). This suggests that intracellular Ca2+ regulation may vary between different parts of the SA node. In a recent report by the same group, it was found that intracellular Ca2+ transients as well as diastolic Ca2+ concentration depend on SA nodal cell size (Lancaster et al. 2001). It is tempting to speculate that the non-uniform distribution of ICl(Ca) in SA node cells is due to heterogeneity of intracellular Ca2+ regulation within the SA node. Further studies are required to clarify this issue.
Functional role of ICl(Ca) in SA node
Recently, it has become clear that Ca2+ released from the SR plays an important role in regulating pacemaker activity in mammalian SA node, probably by modulating membrane currents and exchangers (Hata et al. 1996; Li et al. 1997; Satoh, 1997; Rigg et al. 2000; Bogdanov et al. 2001). In the present study, our action potential clamp measurements demonstrate that Ca2+-activated Cl− current only is present late during the upstroke of the action potential and early during the plateau phase (Fig. 6). Both this transient nature of the current and its time of occurrence during the pacemaker cycle suggest that ICl(Ca) plays a role in modulation of the action potential overshoot. This hypothesis was supported by the finding that blockade of the current increased the action potential overshoot and APD20 significantly (Fig. 4, Table 2), which agrees with findings in atrial and ventricular cells (Wang et al. 1995; Hiraoka & Kawano, 1989; Kawano & Hiraoka, 1991). We found that the modulating role of ICl(Ca) on the action potential overshoot was increased during adrenoceptor stimulation (Fig. 5B, Table 3), most likely due to the potentiated ICl(Ca) (Fig. 5A). In dog, rabbit and sheep ventricular cells, ICl(Ca) also increases during adrenoceptor stimulation (Tseng & Hoffmann 1989; Zygmunt & Gibbons, 1991; Verkerk et al. 2001) and it was demonstrated that such potentiation prevents drastic action potential prolongation (Verkerk et al. 2001). In our experiments, however, blockade of ICl(Ca) failed to significantly affect APD50 or APD100, both under control conditions and upon adrenoceptor stimulation (Fig. 4 and Fig. 5, Tables 2 and 3). Nor did we observe significant effects of ICl(Ca) blockade on dV/dtmax, cycle length, and DDR under control conditions and adrenoceptor stimulation (Fig. 4 and Fig. 5, Tables 2 and 3). Our experiments thus in addition, show that even under conditions of enhanced ICl(Ca) density, this current does not play a significant role in controlling beating rate in single SA node cells.
In our study, DIDS caused a flattening of the top of the SA node action potential, which is more pronounced during adrenoceptor stimulation (Fig. 4 and Fig. 5, Tables 2 and 3). In atrial and ventricular cells, it was demonstrated that such reduction of action potential overshoot has implications for action potential conduction, especially under conditions of decreased cellular coupling (Joyner et al. 1996; Wang et al. 2000; Huelsing et al. 2001). Our computer simulations, however, neither revealed an important role of ICl(Ca) in pacemaker synchronization of SA node cells nor in action potential transfer between SA node and atrial cells.
Conclusions
We found an ICl(Ca) in single SA node cells which modulates importantly the action potential overshoot. However, we did not find a clear physiological importance of ICl(Ca) for the generation or conduction of the SA nodal action potential.
Acknowledgments
This work was partly supported by the Academic Medical Center, University of Amsterdam, The Netherlands, and by the Research Council for Earth and Life Sciences (ALW) with financial aid from the Netherlands Organization for Scientific Research (NWO) through grants 805–06.154 and 805–06.155.
REFERENCES
- Arai A, Kodama I, Toyama J. Roles of Cl− channels and Ca2+ mobilization in stretch-induced increase of SA node pacemaker activity. American Journal of Physiology. 1996;270:H1726–1735. doi: 10.1152/ajpheart.1996.270.5.H1726. [DOI] [PubMed] [Google Scholar]
- Bescond J, Bois P, Petit-Jacques J, Lenfant J. Characterization of an angiotensin-II-activated chloride current in rabbit sino-atrial cells. Journal of Membrane Biology. 1994;140:153–161. doi: 10.1007/BF00232903. [DOI] [PubMed] [Google Scholar]
- Bogdanov KY, Vinogradova TM, Lakatta E. Sinoatrial nodal cell ryanodine receptor and Na+- Ca2+ exchanger. Circulation Research. 2001;88:1254–1258. doi: 10.1161/hh1201.092095. [DOI] [PubMed] [Google Scholar]
- Bouman LN, Jongsma HJ. The sino-atrial node: structure, inhomogeneity and intercellular interaction. In: Huizinga JD, editor. Pacemaker Activity and Intercellular Communication. Florida, USA: CRC Press, Inc.; 1995. pp. 37–49. [Google Scholar]
- Boyett MR, Honjo M, Kodama I. The sinoatrial node, a heterogeneous pacemaker structure. Cardiovascular Research. 2000;47:658–687. doi: 10.1016/s0008-6363(00)00135-8. [DOI] [PubMed] [Google Scholar]
- Bramich NJ, Cousins HM. Effects of sympathetic nerve stimulation on membrane potential, [Ca2+]i, and force in toad sinus venosus node. American Journal of Physiology. 1999;276:H115–128. doi: 10.1152/ajpheart.1999.276.1.H115. [DOI] [PubMed] [Google Scholar]
- Buckler KJ, Denyer JC, Vaughan-Jones RD, Brown HF. Intracellular pH regulation in rabbit isolated sino-atrial node cells. Journal of Physiology. 1990;426P:22–22P. [Google Scholar]
- Denyer JC, Brown HF. Rabbit sino-atrial node cells: isolation and electrophysiological properties. Journal of Physiology. 1990;428:405–424. doi: 10.1113/jphysiol.1990.sp018219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dick GM, Kong ID, Sanders KM. Effects of anion channel antagonists in canine colonic myocytes: comparative pharmacology of Cl−, Ca2+ and K+ currents. British Journal of Pharmacology. 1999;127:1819–1831. doi: 10.1038/sj.bjp.0702730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doerr T, Denger R, Trautwein W. Calcium currents in single SA nodal cells of the rabbit heart studied with action potential clamp. Pflügers Archiv. 1989;413:599–603. doi: 10.1007/BF00581808. [DOI] [PubMed] [Google Scholar]
- Faber GM, Rudy Y. Action potential and contractility changes in [Na+]i overloaded cardiac myocytes: a simulation study. Biophysical Journal. 2000;78:2392–2404. doi: 10.1016/S0006-3495(00)76783-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomis-Tena J, Saiz J. Computers in Cardiology. Vol. 26. IEEE Computer Society; 1999a. Role of Ca-activated Cl currents in the heart: a computer model; pp. 109–112. [Google Scholar]
- Gomis-Tena J, Saiz FJ. Proceedings of the First Joint BMES/EMBS Conference. IEEE Engineering in Medicine and Biology Society; 1999b. Role of calcium-activated chloride currents in heart in patological conditions: a computer model; 143 pp. [Google Scholar]
- Hagiwara N, Masuda H, Shoda M, Irisawa H. Stretch-activated anion currents of rabbit cardiac myocytes. Journal of Physiology. 1992;456:285–302. doi: 10.1113/jphysiol.1992.sp019337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hata T, Noda T, Nishimura M, Watanabe Y. The role of Ca2+ release from the sarcoplasmic reticulum in the regulation of the sinoatrial node automaticity. Heart and Vessels. 1996;11:234–241. doi: 10.1007/BF01746203. [DOI] [PubMed] [Google Scholar]
- Hiraoka M, Kawano S. Calcium-sensitive and insensitive transient outward currents in rabbit ventricular myocytes. Journal of Physiology. 1989;410:187–212. doi: 10.1113/jphysiol.1989.sp017528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horn M, Marty H. Muscarinic activation of ionic currents measured by a new whole-cell recording method. Journal of General Physiology. 1988;92:145–159. doi: 10.1085/jgp.92.2.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huelsing DJ, Pollard AE, Spitzer KW. Transient outward current modulates discontinuous conduction in rabbit ventricular cell pairs. Cardiovascular Research. 2001;49:779–789. doi: 10.1016/s0008-6363(00)00300-x. [DOI] [PubMed] [Google Scholar]
- Hume JR, Duan D, Collier ML, Yamazaki J, Horowitz B. Anion transport in heart. Physiological Reviews. 2000;80:31–81. doi: 10.1152/physrev.2000.80.1.31. [DOI] [PubMed] [Google Scholar]
- Huser J, Blatter LA, Lipsius SL. Intracellular Ca2+ release contributes to automaticity in cat atrial pacemaker cells. Journal of Physiology. 2000;524:415–422. doi: 10.1111/j.1469-7793.2000.00415.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irisawa H, Brown HF, Giles W. Cardiac pacemaking in the sinoatrial node. Physiological Reviews. 1993;73:197–227. doi: 10.1152/physrev.1993.73.1.197. [DOI] [PubMed] [Google Scholar]
- Joyner RW, Kumar R, Golod DA, Wilders R, Jongsma HJ, Verheijck EE, Bouman LN, Goolsby WN, van Ginneken ACG. Electrical interactions between a rabbit atrial cell and a nodal cell model. American Journal of Physiology. 1998;274:H2152–2162. doi: 10.1152/ajpheart.1998.274.6.H2152. [DOI] [PubMed] [Google Scholar]
- Joyner RW, Kumar R, Wilders R, Jongsma HJ, Verheijck EE, Golod DA, van Ginneken ACG, Wagner MB, Goolsby WN. Modulating L-type calcium current affects discontinuous cardiac action potential conduction. Biophysical Journal. 1996;71:237–245. doi: 10.1016/S0006-3495(96)79220-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ju Y-K, Allen DG. How does beta-adrenergic stimulation increase the heart rate? The role of intracellular Ca2+ release in amphibian pacemaker cells. Journal of Physiology. 1999;516:793–804. doi: 10.1111/j.1469-7793.1999.0793u.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ju Y-K, Allen DG. The mechanisms of sarcoplasmic reticulum Ca2+ release in toad pacemaker cells. Journal of Physiology. 2000;525:695–705. doi: 10.1111/j.1469-7793.2000.t01-1-00695.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawano S, Hiraoka M. Transient outward currents and action potential alternations in rabbit ventricular myocytes. Journal of Molecular and Cellular Cardiology. 1991;23:681–693. doi: 10.1016/0022-2828(91)90978-u. [DOI] [PubMed] [Google Scholar]
- Kawano S, Hirayama Y, Hiraoka M. Activation mechanisms of Ca2+-sensitive transient outward current in rabbit ventricular myocytes. Journal of Physiology. 1995;486:593–604. doi: 10.1113/jphysiol.1995.sp020837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohl P, Hunter P, Noble D. Stretch-induced changes in heart rate and rhythm: clinical observations, experiments and mathematical models. Progress in Biophysics and Molecular Biology. 1999;71:91–138. doi: 10.1016/s0079-6107(98)00038-8. [DOI] [PubMed] [Google Scholar]
- Lancaster MK, Jones SA, Harrison SM, Boyett MR. Differences in the intracellular Ca2+ transient within the rabbit sinoatrial node. Journal of Physiology. 2001;533.P:30–30P. doi: 10.1113/jphysiol.2003.057372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leem C-H, Vaughan-Jones RD. Sarcolemmal mechanisms for pHi recovery from alkalosis in the guinea-pig ventricular myocyte. Journal of Physiology. 1998;509:487–496. doi: 10.1111/j.1469-7793.1998.487bn.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lei M, Kohl P. Swelling-induced decrease in spontaneous pacemaker activity of rabbit isolated sino-atrial node cells. Acta Physiologica Scandinavica. 1998;164:1–12. doi: 10.1046/j.1365-201X.1998.00390.x. [DOI] [PubMed] [Google Scholar]
- Li J, Qu J, Nathan RD. Ionic basis of ryanodine's negative chronotropic effect on pacemaker cells isolated from the sinoatrial node. American Journal of Physiology. 1997;273:H2481–2489. doi: 10.1152/ajpheart.1997.273.5.H2481. [DOI] [PubMed] [Google Scholar]
- Lindblad DS, Murphey CR, Clark JW, Giles WR. A model of the action potential and underlying membrane currents in a rabbit atrial cell. American Journal of Physiology. 1996;271:H1666–1696. doi: 10.1152/ajpheart.1996.271.4.H1666. [DOI] [PubMed] [Google Scholar]
- Mangoni ME, Nargeot J. Properties of the hyperpolarization activated current (If) in isolated mouse sino-atrial node cells. Cardiovascular Research. 2001;52:51–64. doi: 10.1016/s0008-6363(01)00370-4. [DOI] [PubMed] [Google Scholar]
- Masson-Pévet M, Bleeker WK, Mackaay AJC, Gros D, Bouman LN. Utrastructural and functional aspects of the rabbit sinoatrial node. In: Bonke FIM, editor. The Sinus Node. The Hague, Boston, London: Martinus Nijhoff; 1978. pp. 195–211. [Google Scholar]
- Musa H, Lei M, Dobrzynski H, Honjo H, Henderson Z, Kodama I, Boyett MR. Heterogeneous expression of the ryanodine receptor in the rabbit sinoatrial node. Journal of Physiology. 1999;521.P:31–31P. [Google Scholar]
- Osterrieder W, Brum G, Hescheler J, Trautwein W, Flockerzi V, Hofmann F. Injections of subunits of cyclic AMP-dependent protein kinase into cardiac myocytes modulates Ca2+ current. Nature. 1982;298:576–578. doi: 10.1038/298576a0. [DOI] [PubMed] [Google Scholar]
- Rigg L, Heath BM, Cui Y, Terrar DA. Localisation and functional significance of ryanodine receptors during β-adrenoceptor stimulation in the guinea-pig sino-atrial node. Cardiovascular Research. 2000;48:254–264. doi: 10.1016/s0008-6363(00)00153-x. [DOI] [PubMed] [Google Scholar]
- Satoh H. Electrophysiological actions of ryanodine on single rabbit sinoatrial nodal cells. General Pharmacology. 1997;28:31–38. doi: 10.1016/s0306-3623(96)00182-6. [DOI] [PubMed] [Google Scholar]
- Satoh H, Seyama I. On the mechanism by which changes in extracellular pH affect the electrical activity of the rabbit sino-atrial node. Journal of Physiology. 1986;381:181–191. doi: 10.1113/jphysiol.1986.sp016321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sipido KR, Callewaert G, Carmeliet E. [Ca2+]i transients and [Ca2+]i-dependent chloride currents in single Purkinje cells from rabbit heart. Journal of Physiology. 1993;468:641–667. doi: 10.1113/jphysiol.1993.sp019793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sipido KR, Maes M, Van de Werf F. Low efficiency of Ca2+ entry through the Na+-Ca2+ exchanger as trigger for Ca2+ release from the sarcoplasmic reticulum. Circulation Research. 1997;81:1034–1044. doi: 10.1161/01.res.81.6.1034. [DOI] [PubMed] [Google Scholar]
- Sorota S. Insights into the structure, distribution and function of the cardiac chloride channels. Cardiovascular Research. 1999;42:361–376. doi: 10.1016/s0008-6363(99)00039-5. [DOI] [PubMed] [Google Scholar]
- Tseng G-N, Hoffmann BF. Two components of transient outward current in canine ventricular myocytes. Circulation Research. 1989;64:633–647. doi: 10.1161/01.res.64.4.633. [DOI] [PubMed] [Google Scholar]
- Vandenberg JI, Rees SA, Wright AR, Powell T. Cell swelling and ion transport pathways in cardiac myocytes. Cardiovascular Research. 1996;32:85–97. [PubMed] [Google Scholar]
- Verheijck EE, van Ginneken ACG, Bourier J, Bouman LN. Effects of delayed rectifier current blockade by E-4031 on impulse generation in single sinoatrial nodal myocytes of the rabbit. Circulation Research. 1995;76:607–617. doi: 10.1161/01.res.76.4.607. [DOI] [PubMed] [Google Scholar]
- Verheijck EE, Wessels A, van Ginneken ACG, Bourier J, Markman MWM, Vermeulen JLM, de Bakker JMT, Lamers WH, Opthof T, Bouman LN. Distribution of atrial and nodal cell within the rabbit sinoatrial node. Circulation. 1998a;97:1623–1631. doi: 10.1161/01.cir.97.16.1623. [DOI] [PubMed] [Google Scholar]
- Verheijck EE, Wilders R, Joyner RW, Golod DA, Kumar R, Jongsma HJ, Bouman LN, van Ginneken ACG. Pacemaker synchronization of electrically coupled rabbit sinoatrial node cells. Journal of General Physiology. 1998b;111:95–112. doi: 10.1085/jgp.111.1.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verkerk AO, Schumacher CA, van Ginneken ACG, Veldkamp MW, Ravesloot JH. Role of Ca2+-activated Cl− current in ventricular action potentials of sheep during adrenoceptor stimulation. Experimental Physiology. 2001;86:151–159. doi: 10.1113/eph8602113. [DOI] [PubMed] [Google Scholar]
- Verkerk AO, Veldkamp MW, Bouman LN, van Ginneken ACG. Calcium-activated Cl− current contributes to delayed afterdepolarizations in single Purkinje and ventricular myocytes. Circulation. 2000;101:2639–2644. doi: 10.1161/01.cir.101.22.2639. [DOI] [PubMed] [Google Scholar]
- Wang YG, Wagner MB, Kumar R, Goolsby WN, Joyner RW. Fast pacing facilitates discontinuous action potential propagation between rabbit atrial cells. American Journal of Physiology - Heart and Circulatory Physiology. 2000;279:H2095–2103. doi: 10.1152/ajpheart.2000.279.5.H2095. [DOI] [PubMed] [Google Scholar]
- Wang Z, Fermini B, Feng J, Nattel S. Role of chloride currents in repolarizing rabbit atrial myocytes. American Journal of Physiology. 1995;268:H1992–2002. doi: 10.1152/ajpheart.1995.268.5.H1992. [DOI] [PubMed] [Google Scholar]
- Wilders R, Jongsma HJ, van Ginneken ACG. Pacemaker activity of the rabbit sinoatrial node: a comparison ofmathematical models. Biophysical Journal. 1991;60:1202–1216. doi: 10.1016/S0006-3495(91)82155-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilders R, Verheijck EE, Kumar R, Goolsby WN, van Ginneken ACG, Joyner RW, Jongsma HJ. Model clamp and its application to synchronization of rabbit sinoatrial node cells. American Journal of Physiology. 1996;271:H2168–2182. doi: 10.1152/ajpheart.1996.271.5.H2168. [DOI] [PubMed] [Google Scholar]
- Wolska BM, Stojanovic MO, Luo W, Kranias EG, Solaro RJ. Effects of ablation of phospholamban on dynamics of cardiac myocytes contraction and intracellular Ca2+ American Journal of Physiology. 1996;271:C391–397. doi: 10.1152/ajpcell.1996.271.1.C391. [DOI] [PubMed] [Google Scholar]
- Wu J, Schuessler RB, Rodefeld MD, Saffitz JE, Boineau JP. Morphological and membrane characteristics of spider and spindle cells isolated from rabbit sinus node. American Journal of Physiology - Heart and Circulatory Physiology. 2001;280:H1232–1240. doi: 10.1152/ajpheart.2001.280.3.H1232. [DOI] [PubMed] [Google Scholar]
- Zaza A, Micheletti A, Brioschi A, Rocchetti M. Ionic currents during sustained pacemaker activity in rabbit sinoatrialmyocytes. Journal of Physiology. 1997;505:667–688. doi: 10.1111/j.1469-7793.1997.677ba.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zygmunt AC. Intracellular calcium activates a chloride current in canine ventricular myocytes. American Journal of Physiology. 1994;267:H1984–1995. doi: 10.1152/ajpheart.1994.267.5.H1984. [DOI] [PubMed] [Google Scholar]
- Zygmunt AC, Gibbons WR. Calcium-activated chloride current in rabbit ventricular myocytes. Circulation Research. 1991;68:424–437. doi: 10.1161/01.res.68.2.424. [DOI] [PubMed] [Google Scholar]
- Zygmunt AC, Gibbons WR. Properties of the calcium-activated chloride current in heart. Journal of General Physiology. 1992;99:391–414. doi: 10.1085/jgp.99.3.391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zygmunt AC, Goodrow RJ, Weigel CM. INaCa and ICl(Ca) contribute to isoproterenol-induced delayed afterdepolarizations in midmyocardial cells. American Journal of Physiology. 1998;275:H1979–1992. doi: 10.1152/ajpheart.1998.275.6.H1979. [DOI] [PubMed] [Google Scholar]
- Zygmunt AC, Robitelle DC, Eddlestone GT. Ito1 dictates behaviour of ICl(Ca) during early repolarization of canine ventricle. American Journal of Physiology. 1997;273:H1096–1106. doi: 10.1152/ajpheart.1997.273.3.H1096. [DOI] [PubMed] [Google Scholar]


