Abstract
Postprandial hypotension occurs frequently in older people and may lead to syncope and falls. Some recent studies suggest that the magnitude of the postprandial fall in blood pressure (BP) is influenced by the rate of gastric emptying. The aim of this study was, therefore, to determine whether the fall in blood pressure induced by intraduodenal glucose is influenced by the rate of nutrient delivery into the small intestine, bypassing the effects of gastric emptying. Eight healthy elderly subjects (four male and four female, age 70.3 ± 3.4 years) were studied on two separate days, in double-blind, randomised order. Glucose was infused intraduodenally at a rate of either 1 or 3 kcal min−1, for 60 min, (0–60 min) followed by 0.9 % saline for a further 60 min (60–120 min). Blood pressure and heart rate were recorded at baseline and every 3 min during the study. Blood glucose and plasma insulin were also determined. Only the 3 kcal min−1 infusion caused a significant fall in systolic (P < 0.001) and diastolic (P < 0.0001) blood pressure and an increase in the heart rate (P < 0.0001). The rises in blood glucose (P < 0.01) and plasma insulin (P < 0.05) concentrations were greater during the 3 kcal min−1 infusion. We conclude that in healthy older subjects, the magnitude of the fall in blood pressure and increase in heart rate induced by intraduodenal glucose infusion is dependent on the rate of nutrient delivery into the small intestine. These results may have relevance to the treatment of postprandial hypotension.
Postprandial hypotension, defined as a fall in systolic blood pressure of ≥ 20 mmHg, occurring within 2 h of a meal (Jansen & Lipsitz, 1995), is now recognised to be an important clinical problem, predisposing to syncope and falls (Hines et al. 1981; Jansen & Hoefnagels, 1991; Mathias, 1991; Jansen & Lipsitz, 1995). Those most at risk include older people, and patients with autonomic neuropathy; the latter is most commonly seen in association with diabetes mellitus (Mathias et al. 1989a; Jansen & Hoefnagels, 1991; Mathias, 1991; Jansen et al. 1995). Postprandial hypotension occurs in 30–40 % of nursing home residents and is more common than orthostatic hypotension (Jansen & Lipsitz, 1995).
Despite its high prevalence and clinical significance, the pathophysiology of postprandial hypotension is poorly understood, and approaches to treatment are largely based on anecdotal observations (Jansen & Lipsitz, 1995). The magnitude of the postprandial fall in blood pressure is known to be dependent on meal composition; ingestion of carbohydrate, particularly glucose, has the greatest effect (Potter et al. 1989), with only minor changes occurring after protein or fat ingestion. Accordingly, it has been suggested that a reduction in dietary carbohydrate content may be of benefit in the treatment of postprandial hypotension (Jansen & Lipsitz, 1995). Moreover, in adults with autonomic failure, an increase in meal frequency (without any change in total carbohydrate or energy intake) so that the amount of carbohydrate in each meal was reduced, decreases postprandial hypotension (Puvi-Rajasingham & Mathias, 1996). Given that the postprandial fall in blood pressure is almost immediately evident, with a maximum response at 30–60 min (Jansen & Lipsitz, 1995), and that intravenous glucose has little, if any, effect on blood pressure (Jansen & Hoefnagels, 1987), it appears that the interaction of carbohydrate with receptors in the gastrointestinal tract is pivotal to the blood pressure response.
Our recent studies suggest that the magnitude of the postprandial fall in blood pressure may be dependent on the rate of carbohydrate delivery to the small intestine (Jones et al. 1998, 2001); the implications of this are that slowing of gastric emptying and/or small intestinal carbohydrate absorption may prove to be therapeutically useful. In patients with type 2 diabetes mellitus, we observed that the magnitude of the fall in systolic blood pressure after ingestion of a drink containing 75 g glucose was greater when gastric emptying was relatively more rapid (Jones et al. 1998). A limitation of this study was the cross-sectional design. In a subsequent study of healthy older subjects, the addition of guar gum to a drink containing 50 g glucose slowed gastric emptying and glucose absorption, and this was associated with a substantial attenuation of the fall in systolic blood pressure (Jones et al. 2001). Moreover, postprandial blood pressure was inversely related to glucose absorption (Jones et al. 2001). While these observations (Jones et al. 1998, 2001) establish a role for the rate of small intestinal nutrient absorption as a determinant of the postprandial fall in blood pressure, it should be recognised that both ‘gastric’ and ‘small intestinal’ factors may have contributed to this effect. For example, the slowing of gastric emptying by guar reflects an increase in small intestinal feedback mechanisms that slow gastric emptying, as well as the more direct effects of an increase in the viscosity of gastric contents (Meyer et al. 1988). By acting as a barrier to glucose absorption in the small intestine, guar allows the exposure of glucose to a greater length of small intestine, possibly including the distal small intestine (Meyer et al.). Accordingly, in considering the mechanisms mediating the observed effect of guar in attenuating the fall in blood pressure after oral glucose, a distinction cannot be made between the potential effects of slowing of the rate of nutrient entry into the small intestine (i.e. retardation of gastric emptying) and those of slower glucose absorption from the lumen of the small intestine. The effects of infusion of nutrients directly into the small intestine (i.e. bypassing ‘gastric’ factors) has hitherto not been evaluated.
The purpose of this study was to evaluate the hypothesis that the effect of intraduodenal infusion of glucose on blood pressure in older subjects is related to the rate of glucose delivery.
METHODS
Subjects
Eight healthy older subjects (four male and four female) with a mean age of 70.3 ± 3.4 years, and a body mass index (BMI) of 23.6 ± 0.78 kg m−2 were recruited by advertisement. All were non-smokers, and none had a history of gastrointestinal disease or surgery, diabetes mellitus, significant respiratory or cardiac disease, alcohol abuse or epilepsy. No subject was taking medication known to influence either blood pressure or gastrointestinal function.
The protocol was approved by the Research Ethics Committee of the Royal Adelaide Hospital, and each subject gave written, informed consent prior to the commencement of the study. All experiments were carried out in accordance with the Declaration of Helsinki.
Protocol
Each subject underwent paired studies, separated by an interval of 7 days (range 5–9 days). The two studies were performed in randomised order. On each study day, the subject attended the laboratory at 09.00 h after a 12 h fast. On arrival, a silicone rubber manometric assembly (diameter 4 mm) was inserted into the stomach via an anaesthetised nostril. The assembly included an infusion channel with a port located ∼10 cm distal to the pylorus; two other channels, positioned in the antrum and duodenum, respectively, were perfused with normal saline (Heddle et al. 1988). The tip of the tube was allowed to pass into the duodenum by peristalsis, which took between 20 and 120 min. A saline-filled, reference electrode (20 gauge intravenous cannula) was inserted subcutaneously into the subject's forearm to enable measurement of the antroduodenal transmucosal potential difference (TMPD) in order to position the catheter accurately across the pylorus. This technique has been used previously (Heddle et al. 1988). An intravenous cannula was positioned in the right antecubital vein for blood sampling, and an automated blood pressure cuff (Dinamap, Johnson & Johnson, Tampa, FL, USA) was placed on the opposite arm. The subject was then allowed to rest comfortably in the recumbent position. At time = 0 min, an intraduodenal infusion of either 25 % glucose (Baxter Health Care, Old Toongabbie, NSW, Australia) or a mixture of 4.9 % saline and 25 % glucose in a ratio of 2:1 (i.e. equiosmotic), was infused at a rate of 3 ml min−1 for 60 min resulting in an energy delivery of 3 kcal min−1 and 1 kcal min−1, respectively. Following the glucose infusions, 0.9 % saline was infused intraduodenally for a further 60 min (t = 120 min). Blood pressure was recorded at regular intervals throughout the study. Cardiovascular autonomic function (Ewing & Clarke, 1982; Piha, 1991) was evaluated on one of the study days, about 60 min after the manometric assembly had been removed.
Measurements
Blood pressure and heart rate
Blood pressure (systolic, diastolic, mean arterial) and heart rate were measured immediately before commencement of the intraduodenal infusion and then at 3 min intervals for the duration of the study (120 min).
Blood glucose and plasma insulin
Venous blood samples (5 ml) were obtained immediately before commencement of the intraduodenal infusion and then at 15, 30, 45, 60, 75, 90, 105 and 120 min. Blood glucose concentrations were determined immediately using a portable blood glucose meter (Medisense Companion 2, Medisense Inc., Waltham, MA, USA). The accuracy of these measurements has been confirmed previously using the standardised hexokinase technique (Horowitz et al. 1996). Plasma was stored at −70 °C for analysis of insulin using a commercially available kit (Abbott Laboratories, Japan; intra-assay coefficients of variation were 4 % at 8.3 μU ml−1, 2.9 % at 40.4 μU ml−1 and 2.5 % at 121.7 μU ml−1) (Horowitz et al. 1996). Plasma insulin concentrations were measured on the samples collected at baseline, 15, 30, 45 and 60 min.
Cardiovascular autonomic nerve function
Autonomic nerve function was evaluated using standardised cardiovascular reflex tests (Ewing & Clarke, 1982); parasympathetic function was evaluated by the variation (R-R interval) of the heart rate during deep breathing and the response to standing (‘30:15′ ratio). Sympathetic function was assessed by the systolic blood pressure response to standing. Each of the test results was scored, according to age-adjusted criteria, as 0 = normal, 1 = borderline and 2 = abnormal for a total maximum score of six. A score ≥ 3 was considered to indicate autonomic dysfunction (Ewing & Clarke, 1982; Horowitz et al. 1987).
Statistical analysis
Data were evaluated using repeated measures analysis of variance (ANOVA) and are presented as means ±s.e.m. unless otherwise stated. All parameters were analysed from 0–60 min, as the maximum fall in blood pressure in response to a meal is usually evident between 30 and 60 min postprandially (Jansen & Lipsitz, 1995) and the glucose infusion was stopped at 60 min. Contrasts were used to examine point-by-point comparisons (changes in blood pressure and heart rate from baseline at 3, 6, 9, 15, 30 and 60 min). A P value < 0.05 was considered significant in all analyses.
RESULTS
All of the studies were well tolerated. In all cases the three tests of cardiovascular autonomic nerve function were within the normal age-adjusted range (R-R variation in response to deep breathing: 11.3 ± 2.7 breaths min−1, ‘30:15′ ratio: 1.12 ± 0.05, fall in systolic blood pressure in response to standing: 5.5 ± 1.8). No subject had definite evidence of autonomic neuropathy; the mean score for autonomic dysfunction was 0.5 ± 0.2.
Blood pressure and heart rate
There were no significant differences in baseline blood pressures between the two study days (P > 0.05). There was a fall in systolic blood pressure between 0 and 60 min during the 3 kcal min−1 glucose infusion (F(1,20) = 2.6, P < 0.001), but not during the 1 kcal min−1 infusion (F(1,20) = 0.7, P = 0.8) (Fig. 1 and Fig. 2). The fall in systolic blood pressure during the 3 kcal min−1 infusion was first evident at 15 min (F(1,20) = 4.1, P = 0.04). Similarly, between 0 and 60 min, during the 3 kcal min−1 infusion, diastolic blood pressure (F(1,20) = 3.1, P = 0.0001) and mean arterial blood pressure (F(1,20) = 3.5, P = 0.0001) fell, but did not change during the 1 kcal min−1 infusion (F(1,20) = 0.9, P = 0.6, F(1,20) = 0.929, P = 0.55, respectively). Heart rate increased between 0 and 60 min during both infusions (F(1,20) = 5.992, P = 0.0001), first evident at 15 min (F(1,20) = 8.3, P = 0.005), but the magnitude of the increase was greater during the 3 kcal min−1 infusion compared with the 1 kcal min−1 infusion (F(1,20) = 2.699, P = 0.0004) (Fig. 1). Between 60 and 120 min (i.e. after cessation of the glucose infusion), there was a trend towards an increase in systolic blood pressure on the 3 kcal min−1 infusion day, though this did not reach statistical significance (F(1,20) = 1.4, P = 0.14).
Figure 1. Effect of intraduodenal glucose infusion at a rate of either 1 kcal min−1 (•) or 3 kcal min−1 (▵) on systolic blood pressure (top graph), diastolic blood pressure (middle graph) and heart rate (bottom graph).
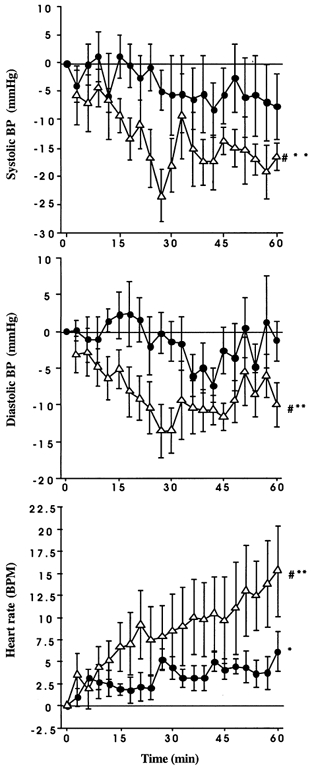
Data are presented as changes from baseline and are means ±s.e.m. Significant differences: *change from baseline for 1 kcal min−1; **change from baseline for 3 kcal min−1 and #3 kcal min−1vs. 1 kcal min−1 over time.
Figure 2. Effect of intraduodenal glucose infusion at a rate of either 1 kcal min−1 (left) or 3 kcal min−1 (right) on systolic blood pressure.
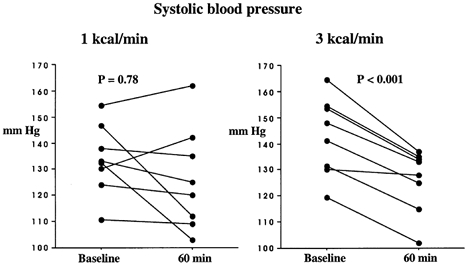
Individual raw data are presented at 0 and 60 min.
Blood glucose and plasma insulin concentration
On both study days, the intraduodenal glucose infusion was associated with increases in blood glucose (F(1,7) = 33.28, P = 0.0001) and plasma insulin (F(1,4) = 11.636, P = 0.0001); for both blood glucose (F(1,7) = 2.694, P = 0.014) and plasma insulin (F(1,4) = 7.324, P = 0.0004), the magnitude of the rise was greater with the 3 kcal min−1 infusion and evident from 45 min for blood glucose (F(1,7) = 9.9, P = 0.002) and at 30 min for plasma insulin (F(1,4) = 6.544, P = 0.01) (Fig. 3).
Figure 3. Effect of intraduodenal glucose infusion at a rate of either 1 kcal min−1 (•) or 3 kcal min−1 (▵) on blood glucose (left) and plasma insulin (right) concentrations (absolute values).
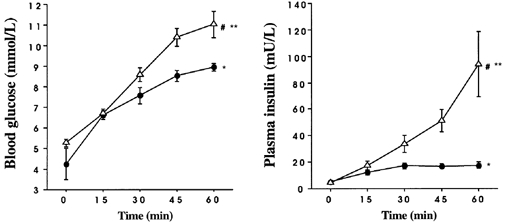
Data are means ±s.e.m. Significant differences: *change from baseline for 1 kcal min−1; ** change from baseline for 3 kcal min−1 and #3 kcal min−1vs. 1 kcal min−1 over time.
DISCUSSION
Our study establishes for the first time that the magnitude of the fall in blood pressure and the rise in heart rate in response to intraduodenal glucose infusion in healthy older subjects is influenced by the rate of nutrient delivery.
A number of potential mechanisms may account for the fall in blood pressure after a meal and in response to intraduodenal glucose. It has been suggested that pooling of blood in the splanchnic veins, leading to a reduction in cardiac output, contributes to the development of postprandial hypotension (Mathias, 1991; Jansen & Lipsitz, 1995). Following a meal, splanchnic blood volume increases by some 20 %, and the magnitude of this change is greatest after high carbohydrate meals. Although the increase in superior mesenteric arterial blood flow after a high carbohydrate meal is comparable in young and elderly patients (Lipsitz et al. 1993), there is evidence that the sympathetic response to meal-induced vasodilatation is impaired in the elderly and may be of aetiological importance (Lipsitz et al. 1983, 1986).
The effects of food on blood pressure appear to relate primarily to the glucose content; oral fructose, xylose, protein and fat have relatively little, if any, effect (Mathias et al. 1989b). Insulin has been implicated in the aetiology of postprandial hypotension (Kearney et al. 1998), and in our study, the rise in plasma insulin was predictably greater in response to the 3 kcal min−1 infusion. However, while insulin has vasodilatory properties, observations that a fall in blood pressure in response to oral glucose occurs in patients with type 1 diabetes who are, by definition, insulin-dependent (Stevens et al. 1991) and that intravenous glucose has little effect on blood pressure in non-diabetics (Jansen & Hoefnagels, 1987), argue against a major role.
Differences in the cardiovascular responses to the two intraduodenal glucose infusions were evident by 15 min, and at this time the rises in blood glucose and plasma insulin were relatively modest. These observations support the concept that gut peptides other than insulin, or the presence of glucose itself in the small intestine, may be important in mediating these effects. Small intestinal glucose stimulates the release of the ‘incretin’ hormones glucagon-like peptide-1 (GLP-1) and gastric inhibitory polypeptide (GIP) (Schirra et al. 1996), but neither appears to have substantial cardiovascular effects. In contrast, there is evidence from a cross-sectional study that calcitonin gene-related peptide (CGRP) may play a role (Edwards et al. 1996). It should also be recognised that while plasma levels of peptides with vasodilatory properties, including the neurotransmitter vasoactive intestinal polypeptide (VIP), do not change following ingestion of carbohydrate (Mathias et al. 1989a), their potential role should not be discounted, as in patients with autonomic failure the hypotensive response to vasodepressor agents is known to be enhanced (Mathias et al. 1977). Both the slowing of gastric emptying and suppression of appetite by small intestinal glucose infusion are related to the length of small intestine exposed to glucose (Lin et al. 1989; Lavin & Read, 1995; Meyer et al. 1998). It would not be suprising if this also applied to the cardiovascular effects of oral glucose. The mechanisms by which the length of small intestine exposed to glucose influences gastric emptying and satiety are poorly defined (Lin et al. 1989; Meyer et al. 1998), but it is of interest that neurones sensitive to glucose have been identified in the enteric nervous system (Liu et al. 1999).
While an impaired sympathetic response may contribute to the fall in blood pressure following oral or intraduodenal carbohydrate, measurement of plasma catecholamine levels, while of interest, may not accurately reflect sympathetic nervous system activation at the level of the vasculature (Kearney et al. 1998). A more appropriate method of assessing this may be the measurement of muscle nerve sympathetic activity (MSA) by microneurography (Fagius & Berne, 1994). Measurement of the effect of intraduodenal glucose infusion on splanchnic blood flow would also be of interest.
Pharmacological options for the treatment of postprandial hypotension are limited. Frusemide therapy should be withdrawn where appropriate (van Kraaij et al. 1999); the somatostatin analogue octreotide has been shown to be effective, but is both impractical and expensive (Jansen et al. 1989); caffeine may be beneficial (Onrot et al. 1985). Hence, there is considerable interest in potential non-pharmacological approaches to treatment. It has recently been demonstrated in patients with autonomic failure (and also to a lesser degree in healthy older subjects) that ingestion of water has a profound pressor effect (Jordan et al. 2000). This is thought to result from increased sympathetic activity (Scott et al. 2001) and impaired baroreflex buffering, triggered by gastric distention. Meal size is known to influence the cardiovascular response to food (Sidery et al. 1993), and moreover, a reduction in the carbohydrate content and volume of meals is of benefit in the treatment of postprandial hypotension (Jansen & Lipsitz, 1995). It has, however, been assumed that the effects of meal size and carbohydrate content reflect more prolonged gastric emptying as a result of increased meal volume, i.e. the stomach initially contains more carbohydrate and empties this into the small intestine over a longer period of time. Our observations suggest an alternative, albeit complementary, hypothesis that could account for these observations, i.e. that the effects of increased meal size and carbohydrate content reflect the rate of caloric delivery to the small intestine, particularly initially.
Gastric emptying of liquids is known to be influenced by propulsive forces generated by intragastric volume and gravity (Burn-Murdoch et al. 1980; Anvari et al. 1995) as well as feedback from receptors in the small intestinal lumen (Lin et al. 1989). Thus, increasing the volume of a liquid meal is associated with more rapid gastric emptying (Brener et al. 1983), even when the liquid contains nutrient (Hunt et al. 1965). We have recently reported that the rate of energy delivery to the small intestine is also greater with increased size of a solid meal (Doran et al. 1998). Gastric emptying is also influenced by posture, which may potentially affect blood pressure (Hunt et al. 1965; Anvari et al. 1995). A number of studies have evaluated gastric emptying of glucose in healthy subjects (mainly using scintigraphic or intubation techniques) and shown that this approximates an overall linear pattern at a rate of 1–3 kcal min−1, after an initial emptying phase that may be slightly faster, or slower, than the subsequent rate (Horowitz et al. 1996), and hence, the rationale for the rates of intraduodenal glucose infusion used in our study. It should, however, be recognised that gastric emptying, is predominately pulsatile rather than continuous, and that there is substantial variation in the characteristics (volume and duration) of individual flow pulses (Malbert & Mathis, 1994; Hausken et al. 1998).
In conclusion, our study has shown that the rate of delivery of glucose into the small intestine plays an important role in postprandial fall of blood pressure and increase in heart rate. Although this study relates to healthy older subjects, the observations have potential relevance to the treatment of postprandial hypotension in other groups. While it may be appropriate to reduce the carbohydrate content of individual meals and favour foods of low glycaemic index over those with a high glycaemic index (MacDonald, 1999), treatment strategies should perhaps also be specifically directed at slowing the rate of delivery of carbohydrate to the small intestine, e.g. by modifying the timing and order of ingestion of solid and liquid components of a meal. This is analogous to the observed slowing of alcohol absorption when alcohol is consumed after a solid meal when compared to the fasted state (Horowitz et al. 1989) and the improvement in postprandial glycaemia when fat is added to a carbohydrate meal (Cunningham & Read, 1989).
Acknowledgments
This study was supported by the National Health and Medical Research Council of Australia and the Royal Adelaide Hospital.
REFERENCES
- Anvari M, Horowitz M, Fraser R, Maddox A, Myers A, Dent J, Jamieson G. Effects of posture on gastric emptying of non-nutrient liquids and antropyloroduodenal motility. American Journal of Physiology. 1995;268:G868–871. doi: 10.1152/ajpgi.1995.268.5.G868. [DOI] [PubMed] [Google Scholar]
- Brener P, Hendrix TR, McHugh PR. Regulation of gastric emptying of glucose. Gastroenterology. 1983;85:76–82. [PubMed] [Google Scholar]
- Burn-Murdoch R, Fisher MA, Hunt JN. Does lying on the right side increase the rate of gastric emptying? Journal of Physiology. 1980;302:395–398. doi: 10.1113/jphysiol.1980.sp013251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham KM, Read NW. The effect of incorporating fat into different components of a meal on gastric emptying and postprandial blood glucose and insulin responses. British Journal of Nutrition. 1989;61:285–290. doi: 10.1079/bjn19890116. [DOI] [PubMed] [Google Scholar]
- Doran S, Jones KL, Andrews JM, Horowitz M. Effets of meal volume and posture on gastric emptying of solids and appetite. American Journal of Physiology. 1998;275:R1712–1718. doi: 10.1152/ajpregu.1998.275.5.R1712. [DOI] [PubMed] [Google Scholar]
- Edwards BJ, Perry HM, iii, Kaiser FE, Morley JE, Kraenzle D, Stevenson R, Kreutter D. Relationship of age and calcitonin gene-related peptide to postprandial hypotension. Mechanisms of Ageing and Development. 1996;87:61–73. doi: 10.1016/0047-6374(96)01688-0. [DOI] [PubMed] [Google Scholar]
- Ewing DJ, Clarke BF. Diagnosis and management of diabetic autonomic neuropathy. British Medical Journal. 1982;285:916–918. doi: 10.1136/bmj.285.6346.916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fagius J, Berne C. Increase in muscle nerve sympathetic activity in humans after food intake. Clinical Science. 1994;86:159–167. doi: 10.1042/cs0860159. [DOI] [PubMed] [Google Scholar]
- Hausken T, Gilja OH, Undeland KA, Berstad A. Timing of postprandial dyspeptic symptoms and transpyloric passage of gastric contents. Scandanavian Journal of Gastroenterology. 1998;33:822–827. doi: 10.1080/00365529850171477. [DOI] [PubMed] [Google Scholar]
- Heddle R, Dent J, Toouli J, Read NW. Topography and measurement of pyloric pressure and tone in humans. American Journal of Physiology. 1988;255:G490–497. doi: 10.1152/ajpgi.1988.255.4.G490. [DOI] [PubMed] [Google Scholar]
- Hines S, Houston M, Robertson D. The clinical spectrum of autonomic dysfunction. American Journal of Medicine. 1981;70:1091–1096. doi: 10.1016/0002-9343(81)90878-0. [DOI] [PubMed] [Google Scholar]
- Horowitz M, Cunningham KM, Wishart J, Jones KL, Read NW. The effect of short-term dietary supplementation with glucose on gastric emptying of glucose and fructose and oral glucose tolerance in normal subjects. Diabetologia. 1996;39:481–486. doi: 10.1007/BF00400681. [DOI] [PubMed] [Google Scholar]
- Horowitz M, Maddox A, Bochner M, Wishart J, Bratasiuk R, Collins P, Shearman D. Relationships between gastric emptying of solid and liquid meals and alcohol absorption. American Journal of Physiology. 1989;257:G291–298. doi: 10.1152/ajpgi.1989.257.2.G291. [DOI] [PubMed] [Google Scholar]
- Horowitz M, Maddox A, Harding PE, Maddern GJ, Chatterton BE, Wishart J, Shearman DJC. Effect of cisapride on gastric emptying in insulin-dependent diabetes mellitus. Gastroenterology. 1987;92:1899–1907. doi: 10.1016/0016-5085(87)90622-6. [DOI] [PubMed] [Google Scholar]
- Hunt JM, Knox M, Oginski A. The effect of gravity on gastric emptying of various test meals. Journal of Physiology. 1965;178:92–97. doi: 10.1113/jphysiol.1965.sp007616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jansen R W M M, Connelly CM, Kelley-Gagnon MM, Parker JA, Lipsitz LA. Postprandial hypotension in elderly patients with unexplained syncope. Archives of Internal Medicine. 1995;155:945–952. [PubMed] [Google Scholar]
- Jansen R W M M, Hoefnagels WH. Influence of oral and intravenous glucose loading on blood pressure in normotensive and hypertensive elderly subjects. Journal of Hypertension. 1987;5(suppl. 5):S501–503. [Google Scholar]
- Jansen R W M M, Hoefnagels WH. Hormonal mechanisms of postprandial hypotension. Journal of the American Geriatric Society. 1991;39:1201–1207. doi: 10.1111/j.1532-5415.1991.tb03575.x. [DOI] [PubMed] [Google Scholar]
- Jansen R W M M, Lipsitz LA. Postprandial hypotension: epidemiology, pathophysiology, and clinical management. Annals of Internal Medicine. 1995;122:286–295. doi: 10.7326/0003-4819-122-4-199502150-00009. [DOI] [PubMed] [Google Scholar]
- Jansen R W M M, Peeters TL, Lenders JW, van Lier HJ, v‘tLaar A, Hoefnagels WH. Somatostatin analog octreotide (SMS 201–995) prevents the decrease in blood pressure after oral glucose loading in the elderly. Journal of Clinical Endocrinology and Metabolism. 1989;68:752–756. doi: 10.1210/jcem-68-4-752. [DOI] [PubMed] [Google Scholar]
- Jones KL, Macintosh C, Su Y-C, Wells F, Chapman IM, Tonkin A, Horowitz M. Guar gum reduces postprandial hypotension in the elderly. Journal of the American Geriatric Society. 2001;49:162–167. doi: 10.1046/j.1532-5415.2001.49037.x. [DOI] [PubMed] [Google Scholar]
- Jones KL, Tonkin A, Horowitz M, Wishart J, Carney BI, Guha S, Green L. The rate of gastric emptying is a determinant of postprandial hypotension in non-insulin diabetes mellitus. Clinical Science. 1998;94:65–70. doi: 10.1042/cs0940065. [DOI] [PubMed] [Google Scholar]
- Jordan J, Shannon JR, Black BK, Ali Y, Farley M, Costa F, Diedrich A, Robertson RM, Biaggioni I, Robertson D. The pressor response to water drinking in humans: a sympathetic reflex? Circulation. 2000;101:504–509. doi: 10.1161/01.cir.101.5.504. [DOI] [PubMed] [Google Scholar]
- Kearney MT, Alan DM, Cowley AJ, Stubbs TA, Evans A, MacDonald IA. Depressor action of insulin on skeletal muscle vasculature: a novel mechanism ofr postprandial hypotension in the elderly. Journal of the American College of Cardiology. 1998;31:209–216. doi: 10.1016/s0735-1097(97)00451-8. [DOI] [PubMed] [Google Scholar]
- Lavin J, Read NW. The effect on hunger and satiety of slowing the absorption of glucose: relationship with gastric emptying and postprandial blood glucose and insulin responses. Appetite. 1995;25:89–96. doi: 10.1006/appe.1995.0043. [DOI] [PubMed] [Google Scholar]
- Lin HC, Doty JE, Reedy TJ, Meyer JH. Inhibition of gastric emptying by glucose depends on length of intestine exposed to nutrients. American Journal of Physiology. 1989;256:6404–6411. doi: 10.1152/ajpgi.1989.256.2.G404. [DOI] [PubMed] [Google Scholar]
- Lipsitz LA, Nyquist RPJ, Wei JY, Rowe JW. Postprandial reduction in blood pressure in the elderly. New England Journal of Medicine. 1983;309:81–83. doi: 10.1056/NEJM198307143090205. [DOI] [PubMed] [Google Scholar]
- Lipsitz LA, Pluchino FC, Wei JY, Minaker KL, Rowe JW. Cardiovascular and norepinephrine responses after meal consumption in elderly (older than 75 years) persons with postprandial hypotension. American Journal of Cardiology. 1986;58:810–815. doi: 10.1016/0002-9149(86)90359-0. [DOI] [PubMed] [Google Scholar]
- Lipsitz LA, Ryan SM, Parker JA, Freeman R, Wei JY, Goldberger AL. Haemodynamic and autonomic nervous system responses to mixed meal ingestion in healthy young and old subjects and dysautonomic patients with postprandial hypotension. Circulation. 1993;87:391–400. doi: 10.1161/01.cir.87.2.391. [DOI] [PubMed] [Google Scholar]
- Liu M, Seino S, Kirchgessner AL. Identification and characterisation of glucoresponsive neurons in the enteric nervous system. Journal of Neuroscience. 1999;19:10305–10317. doi: 10.1523/JNEUROSCI.19-23-10305.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDonald IA. Carbohydrate as a nutrient in adults: range of acceptable intakes. European Journal of Clinical Nutrition. 1999;53:S101–106. doi: 10.1038/sj.ejcn.1600750. [DOI] [PubMed] [Google Scholar]
- Malbert CH, Mathis CJ. Antropyloric modulation of transpyloric flow of liquids in pigs. Gastroenterology. 1994;107:37–46. doi: 10.1016/0016-5085(94)90058-2. [DOI] [PubMed] [Google Scholar]
- Mathias CJ. Postprandial hypotension: pathophysiological mechanisms and clinical implications in different disorders. Hypertension. 1991;18:694–704. doi: 10.1161/01.hyp.18.5.694. [DOI] [PubMed] [Google Scholar]
- Mathias CJ, Cdaosta DF, Fosbraey P, Bannister R, Wood SM, Bloom SR. Cardiovascular, biochemical and hormonal changes during food-induced hypotension in chronic autonomic failure. Journal of Neurological Sciences. 1989a;94:255–269. doi: 10.1016/0022-510x(89)90235-9. [DOI] [PubMed] [Google Scholar]
- Mathias CJ, da Costa DF, McIntosh CM, Fosbraey P, Bannister R, Wood SM, Bloom SR. Differential blood pressure and hormonal effects after glucose and xylose ingestion in chronic autonomic failure. Clinical Science. 1989b;77:85–92. doi: 10.1042/cs0770085. [DOI] [PubMed] [Google Scholar]
- Mathias CJ, Matthews JB, Spalding JM. Postural changes in plasma renin activity and response to vasoactive drugs in a case of Shy-Drager syndrome. Journal of Neurology, Neuroscience and Psychiatry. 1977;2:147–156. doi: 10.1136/jnnp.40.2.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer JG, Gu YG, Jehn D, Taylor IL. Intragastric vs. intraintestinal viscous polymers and glucose tolerance after liquid meals of glucose. American Journal of Clinical Nutrition. 1988;48:260–266. doi: 10.1093/ajcn/48.2.260. [DOI] [PubMed] [Google Scholar]
- Meyer JH, Tabrizi Y, Dimaso N, Hlinka M, Raybould HE. Length of intestinal contact on nutrient-driven satiety. American Journal of Physiology. 1998;275:R1308–1319. doi: 10.1152/ajpregu.1998.275.4.R1308. [DOI] [PubMed] [Google Scholar]
- Onrot J, Goldberg MR, Biaggioni I, Hollister AS, Kincaid D, Robertson D. Hemodynamic and humoral effects of caffeine in autonomic failure - therapeutic implications for postprandial hypotension. New England Journal of Medicine. 1985;313:549–554. doi: 10.1056/NEJM198508293130905. [DOI] [PubMed] [Google Scholar]
- Piha SJ. Cardiovascular autonomic reflex tests: normal responses and age-related reference values. Clinical Physiology. 1991;11:277–290. doi: 10.1111/j.1475-097x.1991.tb00459.x. [DOI] [PubMed] [Google Scholar]
- Potter JF, Heseltine D, Hartley G, Matthews J, MacDonald IA, Janes OF. Effects of meal composition on the postprandial blood pressure, catecholamine and insulin changes in elderly subjects. Clinical Science. 1989;77:265–272. doi: 10.1042/cs0770265. [DOI] [PubMed] [Google Scholar]
- Puvi-Rajasingham S, Mathias CJ. Effect of meal size on postprandial blood pressure and on postural hypotension in primary autonomic failure. Clinical Autonomic Research. 1996;6:111–114. doi: 10.1007/BF02291232. [DOI] [PubMed] [Google Scholar]
- Schirra J, Katschinski M, Weidmann C, Schafer T, Wank U, Arnold R, Goke B. Gastric emptying and release of incretin hormones after glucose ingestion in humans. Journal of Clinical Investigation. 1996;97:92–103. doi: 10.1172/JCI118411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott EM, Greenwood JP, Gilbey SG, Stoker JB, Mary D A S G. Water ingestion increases sympathetic vasoconstrictor discharge in normal humans subjects. Clinical Science. 2001;100:335–342. [PubMed] [Google Scholar]
- Sidery MB, Cowley AJ, MacDonald IA. Cardiovascular responses to a high-fat and a high-carbohydrate meal in healthy elderly subjects. Clinical Science. 1993;84:263–270. doi: 10.1042/cs0840263. [DOI] [PubMed] [Google Scholar]
- Stevens MJ, Edmonds ME, Mathias CJ, Watkins PJ. Disabling postural hypotension complicating diabetic autonomic neuropathy. Diabetic Medicine. 1991;8:870–874. doi: 10.1111/j.1464-5491.1991.tb02127.x. [DOI] [PubMed] [Google Scholar]
- van Kraij DJ, Jansen RW, Bouwels LH, Hoefnagels WH. Furosemide withdrawal improves postprandial hypotension in elderly patients with heart failure and preserved left ventricular systolic function. Archives of Internal Medicine. 1999;159:1599–1605. doi: 10.1001/archinte.159.14.1599. [DOI] [PubMed] [Google Scholar]


