Abstract
In Rana catesbeiana the upper airways are used for two distinct yet highly coordinated ventilatory behaviours: buccal ventilation and lung inflation cycles. How these behaviours are generated and coordinated is unknown. The purpose of this study was to identify putative rhythmogenic brainstem loci involved in these ventilatory behaviours. We surveyed the isolated postmetamorphic brainstem to determine sites where local depolarization, produced by microinjecting the non-NMDA glutamate receptor agonist, AMPA, augmented the ventilatory motor patterns. Two sites were identified: a caudal site, at the level of cranial nerve (CN) X, where AMPA injections caused increased buccal burst frequency but abolished lung bursts, and a rostral site, between the levels of CN VIII and IX, where injections increased the frequency of both types of ventilatory bursts. These two sites were further examined using GABA microinjections to locally inhibit cells. GABA injected into the caudal site suppressed the buccal rhythm but the lung rhythm continued, albeit at a different frequency. When GABA was injected into the rostral site the lung bursts were abolished but the buccal rhythm continued. When the two sites were physically separated by transection, both rostral and caudal brainstem sections were capable of rhythmogenesis. The results suggest the respiratory network within the amphibian brainstem is composed of at least two distinct but interacting oscillators, the buccal and lung oscillators. These putative oscillators may provide a promising experimental model for studying coupled oscillators in vertebrates.
Coupled neuronal oscillators have been surmised in the nervous system, both to bind sensory information during processing and to coordinate complex behaviours such as locomotion and feeding (e.g. Grillner et al. 1995; Steriade, 1997; Zhong et al. 1997; Skinner & Mulloney, 1998; Smith & Gilbey, 2000; Wilson & Kleinhaus, 2000). The coordination of ventilation with other rhythmic behaviours such as locomotion, vocalization, sucking and hiccups suggests that coupling of oscillators may also be an important theme in central respiratory control (e.g. Newsom Davis, 1970; Funk et al. 1992; Schmidt, 1992; Niizeki et al. 1996; Suthers et al. 1999; Nakamura et al. 1999).
To better understand the role of multiple brainstem oscillators in controlling ventilation we have turned to a late-stage postmetamorphic tadpole brainstem preparation that produces two distinct ventilatory motor patterns (McLean et al. 1995a; Torgerson et al. 1998). These motor patterns correspond to buccal and lung ventilation (Kogo et al. 1994; Gdovin et al. 1998). Buccal ventilation, a remnant of gill ventilation, consists of tidal ventilation of the buccal cavity by alternate contractions of buccal depressor and elevator muscles. Powerful contractions of these muscles are a key component of the buccal-force-pump, which ventilates the lung (West & Jones, 1975). Lung ventilation occurs phase-locked to buccal ventilation in vivo (N. Kimura, personal communication) and can occur in episodes (Kinkead & Milsom, 1994). How the brainstem of the tadpole generates these behaviours is largely unknown and no model has been proposed.
The isolated, superfused tadpole brainstem preparation is oxygenated throughout, being small in size and having a low metabolic rate (Torgerson et al. 1997a; Wilson et al.1999a). Under the experimental conditions we have described previously, the brainstem will generate robust ventilatory motor patterns similar to those characterized in vivo (Sakakibara, 1984; McLean et al. 1995a; Torgerson et al. 1998; Gdovin et al. 1998). These motor patterns consist almost entirely of lung and buccal ventilatory bursts. Several lines of evidence suggest that distinct circuits produce these motor patterns. Pharmacological manipulations demonstrate that the buccal bursts are dependent on GABAA- and glycine-mediated postsynaptic inhibition, whereas lung bursts appears to be generated by a different mechanism (Galante et al. 1996). Similarly, buccal and lung bursts differ in their sensitivity to GABAB-receptor agonists (Straus et al. 2000) and reduced Cl− (K. Vasilakos, unpublished observations). Finally, hypercapnic challenge in preparations from postmetamorphic animals causes lung burst frequency to increase but has no effect on buccal frequency (Torgerson et al. 1997b). Transection studies have so far been suggestive but ultimately inconclusive in determining whether lung and buccal rhythm generating circuits are spatially separated. In premetamorphic animals, transection studies indicate that the only region of the brainstem capable of rhythmogenesis resides caudal to CN IX (Gdovin et al. 1999; Torgerson et al. 2001b). In postmetamorphic animals, brainstem sections were capable of rhythmogenesis as long as they included the region between CN VII and IX, suggesting that this region alone was essential for rhythmogenesis (Torgerson et al. 2001b). Thus, transection studies to date have failed to demonstrate the presence of multiple rhythmogenic brainstem sites in the same animal. Here we report the results of a drug microinjection and transection study. Our rationale was to use drug microinjections to identify important sites for rhythmogenesis and then use transection to determine whether rhythmogenesis persisted after the sites were physically separated. Similar techniques have been used previously to identify brain regions important for respiration and other behaviours (e.g. Smith et al. 1991; Coles & Dick, 1996; Ramirez et al. 1998; Solomon et al. 1999; McCrimmon et al. 2000; Sirota et al. 2000). For the drug microinjection study we used small volumes of AMPA and GABA which probably have local and transient effects on neuronal activity (excitation and inhibition, respectively) without affecting axons of passage. The results of the microinjections were consistent with there being two distinct rhythmogenic sites for the generation of buccal and lung bursts. When the brainstem was transected between the two putative rhythmogenic sites, both sections were capable of rhythm generation. Based on available data, we formulate a model to explain how the complex motor pattern might be generated. (Some of the results presented have been published previously in abstract form, Wilson et al. 1999b).
METHODS
Superfused isolated brainstem preparation
Postmetamorphic Rana catesbeiana (5–15 g) were obtained from a commercial suppliers (Charles D. Sullivan, Nashville, TN, USA) and maintained in aerated water at room temperature prior to use. Stages 22–25 (Taylor & Kollros, 1946) were used for experiments. Experimental protocols were in accordance with The Canadian Council on Animal Care guidelines and approved by the University of Calgary Animal Care Committee. Animals were first anaesthetized by placing in ice-cold pond water containing tricaine methane sulphonate (1:10 000) until unresponsive to tail pinch and then decerebrated. During dissection, brainstems were superfused with oxygenated mock cerebral-spinal fluid (CSF) (mm): NaCl, 104; KCl, 4; MgCl2, 1.4; d-glucose, 10; NaHCO3, 25; CaCl2 2.4; pH ∼9. The preparation consisted of the isolated brainstem (caudal to CN III) and rostral spinal cord (transected caudal to SN II) with the choroid plexus, dura and ventral arachnoid removed. Whole brainstem preparations were used for experiments in which the brainstem was surveyed for sufficient sites, whereas sagittal hemisections were used in experiments that included necessity testing. Hemisections were prepared using a razor blade before the brainstems were removed from the cranium. All experiments were performed in a recirculating superfusion chamber (Wilson et al. 1999a). The recirculation mechanism was driven by gas (98 % O2:∼2 % CO2) which also equilibrated the superfusate (pH ∼7.8). This chamber minimizes the unstirred layer around the brainstem facilitating gas exchange into and out of the tissue. To ensure injected drugs did not accumulate in the recirculating fluid during the course of the experiment, the fluid in the dish (∼3 ml) was exchanged at a rate of 5–7 ml min−1 with fresh mock CSF from an external tonometer (equilibrated with the same gas used to drive the recirculation mechanism). Excess superfusate was removed by suction from a small well adjoining the recording compartment. The pH in the chamber was monitored either directly or by monitoring the pH in the tonometer and was maintained by adjusting the fractional concentration of CO2 in the gas.
Electrophysiology
Motor patterns corresponding to gill and lung ventilation were recorded from the roots of CN V and SN II using extracellular glass suction electrodes. Extracellular signals were amplified (× 10 000) and filtered (100 Hz-1 kHz) using high gain, differential AC amplifiers (model 1700, A-M Systems Inc., Everett, WA, USA), fed to a moving averager (time constant: 50 ms; SagaTech, Calgary, Alberta, Canada) digitized at 100 Hz (TL-2–80, Axon Instruments, Union City, CA, USA) and archived as computer files (AxoTape software, Axon Instruments). Analysis, including burst triggered averaging, was performed off line using commercially available software (AxoGraph 3.5 software, Axon Instruments).
Drug injections
Micropipettes were made from four-barrel glass capillary tubing, each barrel having an internal diameter of 1 mm (Friedrich & Dimmock, NJ, USA). The capillary tubes were drawn to a fine point using a micropipette puller (Sutter Instruments, CA, USA). The tip of the pulled glass was broken manually under a dissection microscope to give a tip diameter of ∼10 μm per barrel. Micropipettes were partially filled with mock CSF, equilibrated with the same gas mixture used to equilibrate the mock CSF superfusing the preparation, containing no drug (control), 2–10 μm AMPA or 1 mm GABA. In some experiments the tips of two of the barrels were filled with a solution saturated with the fluorescent lipophilic marker, DiI (Molecular Probes, Inc., Eugene, OR, USA). The micropipette was mounted on a nanostepper (SPI, Heidelburg, Germany), connected to a four-channel picospritzer (General Valve Corp., NJ, USA) and positioned over the surface of the ventral medulla. During injections, the meniscus of the fluid in the barrel of the micropipette was observed using a dissection microscope mounted horizontally (× 50, OPMI 1-F, Zeiss). Typically, injections were performed using a pressure of 25–75 p.s.i. applied for 50–200 ms, resulting in small injection volumes. The volume of each injection was sufficient to produce a drop in the meniscus, but too small to measure reliably. We estimated the volume of such injections to be ∼2 nl based on the measured displacement of the meniscus after multiple injections within tissue. We calculate that a 2 nl injection would produce a cavitational sphere with a diameter of 156 μm.
Protocol for identifying sites that promote rhythmogenesis
AMPA injections (10 μm, ∼2 nl) were made at 300, 600, 900 and 1200 μm below the ventral surface at the locations illustrated in Fig. 2. Two injections were made at each site, drug followed by control, with injections spaced 4 min apart. Areas identified in the systematic survey in which AMPA injections produced increases in either buccal or lung burst frequency (referred to here as ‘buccal’ and ‘lung’ areas, respectively) were targeted to determine whether they were required for rhythmogenesis. Rhythm promoting sites were located by using one barrel of the multi-barrel micropipette to inject AMPA. Subsequently, trains of GABA injections from a different barrel were used to test whether the site was required for rhythmogeneis. Trains were 1–2 min in duration and were followed by 4 min recovery. Individual injections within the train were spaced 3–4 s apart. A single buccal and/or lung site was tested in any given animal. To ensure that the response of a site to an injection was independent of the phase of the rhythm generator, multiple tests were made at each site. Data from multiple tests at any given site within a preparation were summarized by averaging. These average values were used in subsequent analysis.
Figure 2. Range of responses to AMPA injections.

A, the rostral-caudal axis of the isolated brainstem was surveyed for sites where local excitation produced by ∼2 nl injections of 10 μm AMPA produced reliable increases in either the buccal or the lung rhythm. Injections were made 300, 600, 900 and 1200 μm below the ventral surface at 9 locations along the rostral-caudal axis as illustrated (ventral face is uppermost, with rostral at the top; dashed line depicts midline). B, traces from stage 24 tadpole showing examples of responses to injections 600 μm below the ventral surface (pH 7.53). At several sites AMPA inhibited both rhythms or caused long lasting burst discharges (apneusis). However, only injections 300 or 600 μm below the ventral surface between CN VIII and CN IX (Locations 4 and 5, rostral site) reliably increased the frequency of lung-like bursts. Augmentation of buccal burst frequency occurred with injections 600 μm below the ventral surface at the level of CN X (Locations 6 and 7, caudal site). Control injections in which AMPA was absent had no effect. Injections were separated by 4 or more minutes.
Analysis of drug injection data
The motor pattern corresponding to lung and buccal ventilation was identified and assessed according to published criteria (Torgerson et al. 1998; Gdovin et al. 1998). We discarded preparations that failed to produce lung and buccal ventilatory bursts within 2 h of dissection. We found that under the experimental conditions and using the mock CSF described above the motor patterns of the remainder of the preparations were only rarely interrupted by the large amplitude, long duration ‘non-respiratory’ bursts (> 5 s) described by others (Reid & Milsom, 1998). Thus, they were not analysed. The frequent occurrence of the long duration bursts described by others (Reid et al. 2000; i.e. one long duration burst for every lung burst) probably results from differences in experimental conditions, including the bicarbonate concentration of the perfusate and the superfusion technique. Note that in our case we used a bicarbonate concentration similar to that found in vivo (Just et al. 1973; Stinner & Hartzler, 2000) and an efficient superfusion system as measured by the absence of an unstirred layer around the tissue (Wilson et al. 1999a). Buccal burst frequency was calculated during periods of consecutive buccal bursts when lung bursts were absent. Unless stated otherwise, overall lung burst frequency was calculated from the number of bursts per unit time, whether or not buccal bursts were present. Overall frequency of events was converted to mean inter-peak interval (IPI) to facilitate comparisons with duration of apnoeas. We define a ‘lung episode’ as two or more consecutive lung bursts without buccal burst in between.
Histology
In some experiments, ∼6 nl of DiI solution (3 mg ml−1DiI in dimethyl sulfoxide) was injected into rhythm promoting sites to better define their transverse location. Brainstems were fixed overnight in ice cold 4 % paraformaldehyde, embedded in 30 % sucrose and cryo-sectioned. Transverse sections (50 μm) containing DiI-labelled areas were photographed with an epifluorescent dissection microscope fitted with rhodamine filters (× 20 magnification; Stemi SV 6; Carl Zeiss Canada Ltd, Ontario). Some of the photographs were then scaled slightly along the dorsal-ventral axis, normalizing the width of slices at the midline. Traces of the slices were aligned using the midline as a common reference and overlaid so the labelled regions could be compared.
Transections
Brainstems were transected within the cranium at the level of CN IX using a razor blade. The rostral and caudal sections were then dissected and superfused simultaneously in the same chamber. Recordings were made from cranial and spinal nerves as above. Bursts with a width of < 2 s and an amplitude twice that of the noise were quantified in the same way as lung bursts (described above). Bursts of longer duration were not analysed. In two preparations, the caudal section produced regular bursts that had a poor signal to noise ratio. In order to determine the frequency of these bursts, integrated recordings were autocorrelated and the frequency components analysed using a Fast Fourier Transform. In the other preparations, the caudal section failed to produce spontaneous rhythmic discharges. In these cases, bath applied AMPA (∼1 μm) was used in an attempt to compensate for the possible loss of tonic excitation.
Statistics
Data are given as means ±s.e.m. and n refers to the number of preparations. Differences in means were evaluated using either paired t tests or analysis of variance for repeated measures (ANOVA). When ANOVA was used, if the difference reached significance (P < 0.05), a post hoc comparison was performed using the Student-Newman-Keuls (SNK) test.
RESULTS
Motor output of the isolated tadpole brainstem preparation
The intact isolated brainstem preparation of the postmetamorphic tadpole produced a robust respiratory rhythm similar to that described in adult frogs (Sakakibara, 1984; Kogo et al. 1994; McLean et al. 1995a). The motor pattern consisted of rhythmic bursts from cranial nerves composed almost entirely of short duration (< 2 s) bursts of either small or large amplitude, corresponding to buccal and lung ventilation, respectively (Fig. 1A; Kogo et al. 1994; Gdovin et al. 1998). The relationship between the buccal and lung bursts was complex. Lung burst triggered averaging (Fig. 1B and C) demonstrated that, for a given preparation, the first lung burst of a lung burst episode occurred at a consistent point in the buccal cycle (Fig. 1B). Similarly, the buccal burst rhythm recommenced predictably following the last lung burst in an episode (Fig. 1C). These data suggest coupling between circuits generating the buccal and lung bursts. However, the inter-burst interval of consecutive lung bursts during a lung burst episode (1.09 ± 0.08 s, n = 6, pH 7.7, stage 25) was significantly less than that of both the buccal bursts preceding the episode (1.25 ± 0.10 s; SNK Method: q = 6.84, P < 0.005, n = 6) and those that followed (1.23 ± 0.17 s; SNK Method: q = 5.91, P < 0.005, n = 6).
Figure 1. Lung and gill ventilatory motor patterns of isolated superfused brainstem from a postmetamorphic (stage 25) tadpole.

A, integrated recordings from cranial (CN) and spinal (SN) nerves obtained with extracellular suction electrodes. Boxed region in the left panel shown time expanded on right. Two distinct motor patterns are apparent, large and small amplitude bursts corresponding to lung and buccal ventilation bursts, respectively. Lung bursts tend to occur in episodes (as shown) but can also occur individually. Note the larger amplitude and faster frequency of buccal bursts after the lung burst episode. B and C, lung burst triggered average of integrated recording from CN VII. 70 traces averaged for each panel, with trigger points indicated by arrows. Dotted lines show ± 2 s.e.m. Note that the first lung bursts in an episode occur at the same point in the buccal cycle (B) and that the buccal cycle continues predictably from the last lung burst in an episode (C). Similar results were obtained from 5 other preparations.
Systematic survey for rhythm promoting sites
To determine approximately where within the postmetamorphic tadpole brainstem the rhythm generator responsible for these two motor patterns might be located, we conducted a systematic AMPA microinjection survey in six animals to identify areas where local activation of excitatory non-NMDA glutamate receptors increased the frequency of either rhythm. We assumed that such an increase was indicative of either a rhythm generating site or a site that provides excitatory modulatory inputs (Ramirez et al. 1998). Injections of 10 μm AMPA nearly always produced a change in the output of respiratory nerves, whereas control injections (CSF alone) had no effect. Responses to AMPA varied from site to site as illustrated in Fig. 2 and summarized in Fig. 3. Responses included cessation of rhythms (apnoea, e.g. Fig. 2, Location 1), long lasting bursts (apneusis, e.g. Fig. 2, Location 8) and increases in ventilatory burst frequency (e.g. Fig. 2, Locations 3–6). Areas that produced apnoea were commonly found in the rostral brainstem (around CN V) but were not limited to this region. Of particular interest in the current study were injections made in two regions, 300–900 μm below the ventral surface: (1) a region caudal to CN VIII and rostral to CN XI that initially increased lung burst frequency (Fig. 2 and Fig. 3, Locations 4–5) and (2) another region at the level of CN X that increased both buccal burst amplitude and frequency (Fig. 2 and Fig. 3, Locations 6–7). These responses, however, were often accompanied with large aberrant bursts, occurring shortly after the increase in ventilatory burst frequency. In subsequent experiments, we targeted these regions to more precisely define their role in generating the lung and buccal motor patterns.
Figure 3. Summary of AMPA injection survey.
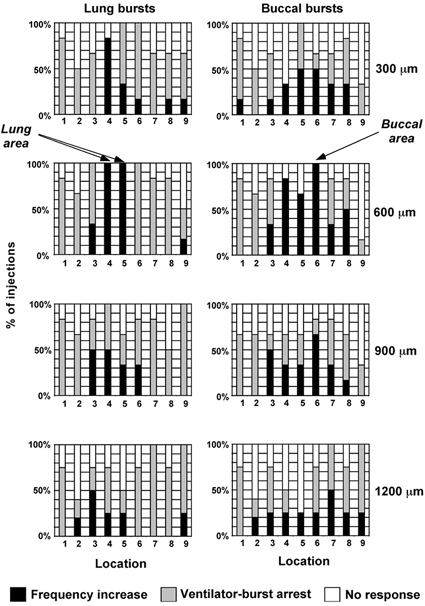
Locations (abscissa) refer to injection sites illustrated in Fig. 2A. Injections were sorted according to depth (rows) and analysed according to affect on both lung bursts (left column) and buccal bursts (right column). Effects on ventilation were catagorised into three groups: no response (open bars), frequency increase (black bars) and ventilatory burst arrest (grey bars). The ventilatory burst arrest category included injections that resulted in apnoea or tonic discharge. Data from 195 AMPA injections (one injection per site per animal) pooled from 6 animals. Note that injections 600 μm deep at location 6 increased the frequency of buccal bursts reliably but arrested lung bursts, whereas injections at the same depth at locations 4 and 5 accelerated the frequency of both burst types. pH 7.7 ± 0.2.
Determining whether rhythm promoting sites are required for rhythmogenesis
Previously, we have shown that hemisected brainstems are capable of producing ventilatory motor patterns (McClean et al. 1995b) and, in pilot experiments, qualitatively similar results were obtained with 5 μm AMPA injections irrespective of whether the injections were made in the intact or hemisected preparation. Therefore, to avoid the redundancy problem associated with bilaterally iterated rhythm generating circuits (McLean et al. 1995b) the following injections were performed on hemi-sections. We first located areas that promoted rhythmogenesis using ∼2 nl injections of 1–5 μm AMPA from a multi-barrel pipette in which half the barrels were filled with 5 mm GABA. Using the lower concentrations of AMPA, two very discrete areas within the regions described above were identified. In the rostral region, AMPA injections 300–600 μm below the ventral surface, just caudal and lateral to the root of CN VI, increased lung frequency by 149 ± 33 % (Fig. 4A and B; paired t test: t = 4.07, P < 0.01, n = 6) and increased the frequency of subsequent buccal bursts by 15 ± 5 % (Fig. 4A and C; paired t test: t = 3.34, P < 0.05, n = 6). We define this rostral site as the ‘lung area’. In the caudal region, injections of 1–5 μm AMPA, 300–600 μm below the ventral surface at the level of the most posterior root of CN X, augmented buccal burst frequency (Fig. 4D and F; paired t test: t = 2.81, P < 0.05, n = 6). These injections also strongly inhibited lung bursts (i.e. caused apnoea; see Fig. 4D inset and 4E; paired t test: t = −5.78, P < 0.01, n = 6). We define this caudal site as the ‘buccal area’.
Figure 4. Two ventilatory burst promoting sites in hemisected preparations.

A, injection of 1 μm AMPA into the rostral area, 300 μm below the ventral surface just caudal and lateral to where CN VI exits the brainstem (close to location 4 in Fig. 2A) revealed a discrete site that produced increases in both lung and buccal frequency. We define this site as the ‘lung area’. pH 7.63; stage 25. B and C, summary data showing effect of AMPA injections in the lung area, on lung and buccal bursts, respectively. D, injection of 1 μm AMPA into the caudal area, 600 μm below the ventral surface at the level of the most posterior root of CN X (approximately at location 7 in Fig. 2A) produced an increase in the frequency of the buccal rhythm, but arrested lung bursts (boxed region in inset shown expanded below). We define this site as the ‘buccal area’. pH 7.41; stage 25. E and F, summary data showing effect of AMPA injections in the buccal area, on lung and buccal bursts, respectively. Data in B, C, E and F were from 6 hemisected preparations (stage 24–25, pH 7.5 ± 0.1) with injections of 1–5 μm AMPA. Column and bars show means ±s.e.m. Inject volumes were ∼2 nl. IPI, inter peak interval.
Having identified either the lung or buccal area with AMPA injections, 5 mm GABA was injected from a different barrel in a train of pulses (∼2 nl per pulse, one pulse every ∼3 s for 1–2 min). This GABA concentration was 5 % of that required to abolish the gill and lung bursts in cranial nerves of the isolated brainstem of metamorphic tadpoles, when bath applied (Galante et al. 1996). Using GABA to determine if these areas are required for rhythmogenesis assumes: (1) a widespread distribution of GABA receptors within the brainstem and (2) that GABA is inhibitory (see Discussion).
Lung bursts were eliminated during a train of GABA injections into the lung area (Fig. 5A), with the apnoea continuing after the train of injections had stopped (Fig. 5A and B; SNK test: q = 13.78, P < 0.001, n = 5). However, the buccal rhythm continued and the frequency of the buccal bursts during the GABA injections did not differ significantly from that before (Fig. 5C; paired t test: t = 0.63, P = 0.56, n = 5).
Figure 5. Lung and buccal areas were critical for lung and buccal bursts respectively in hemisected preparations (stage 24–25).

A, a train of GABA injections (5 mm, ∼2 nl every 2–3 s) into the lung area (close to location 4 in Fig. 2A) abolished lung bursts for the duration of the train, but buccal bursts persisted. B and C, summary data from 5 preparations showing effects of GABA injections into the lung area (train duration indicated by open bar in B) on lung and buccal bursts, respectively. D, a similar train of GABA injections into the buccal area (close to location 7 in Fig. 2A) abolished buccal bursts for the duration of the train. The train of injections disrupted lung bursts by causing an initial short duration apnoea. While the lung bursts returned for the remainder of the train, their frequency was reduced compared to before the injection. E and F, summary data from 6 preparations showing effects of GABA injections into the buccal area (train duration indicated by open bar in F) on lung and buccal bursts respectively. Data in A and D were from a stage 24 tadpole, pH 7.75 and 7.43, respectively. In the top traces, the train of upward deflections indicates the duration of GABA injections. For B, C, E and F the pH was 7.52 ± 0.1.
During GABA injections into the buccal area, buccal bursts were eliminated (Fig. 5D and F). On the initiation of injections, a short lung burst apnoea ensued (comparison of IPI before injection with duration of apnoea, SNK test: q = 4.60, P < 0.05, n = 6), which lasted 8.0 ± 1.6 s (Fig. 5E). Importantly, lung bursts returned despite subsequent GABA pulses. Lung burst frequency between the end of the apnoea and the end of the injection train did not differ significantly from that preceding the train (SNK test: q = 0.91, P = 0.91, n = 6). However, we noted that some injections produced a pronounced increase in frequency, whereas others produced a pronounced decrease (e.g. Fig. 5D).
Transverse location of lung and buccal areas
A separate set of experiments was performed to more precisely define the location of the lung and buccal areas in the transverse plane. Five lung and five buccal areas were identified in different animals using ∼2 nl AMPA injections (as described above) and labelled using another barrel of the same pipette to injected ∼6 nl of a solution containing the lipophilic fluorescent dye, DiI. Brainstems were fixed and cryosectioned and the location of DiI-labelled sites was compared with anatomical landmarks (Adli et al. 1999). The lung area was coincident with the reticularis parvocellularis, whereas the buccal area was coincident with the reticularis dorsalis (Fig. 6).
Figure 6. Lung and buccal areas in transverse sections.

A, horizontal lines illustrate level of transverse slices containing lung and buccal areas in different animals identified using ∼2 nl AMPA injections (see legend of Fig. 4 for details) and labelled with ∼6 nl DiI injections from different barrels of a multi-barrel pipette. B and C, outlines of 5 transverse slices from different brainstems containing the lung and buccal areas respectively. Outlines were aligned at the midline and scaled slighty along the dorsal-ventral axis for comparison. Only the DiI-labelled side of transverse sections are shown (left). Nearest anatomical landmarks are illustrated on the right (Adli et al. 1999). Vent, ventricle; RPc, reticularis parvocellularis; RPgl, reticularis paragigantocellularis lateralis; SO, superior olive; RD, reticularis dorsalis.
Rhythmogenesis after transection between lung and buccal areas
Based on the AMPA and GABA pharmocological tests described above, one cannot distinguish between a rhythm generating site or a rhythm modulatory site (e.g. Ramirez et al. 1998). To determine the likelihood as to whether the two sites identified above might constitute distinct neuronal oscillators for lung and buccal bursts we performed a set of transection experiments in which the two sites were physically separated.
When brainstems were transected at the level of CN IX, both the rostral section containing the lung area and the caudal section containing the buccal area were capable of rhythmogenesis (Fig. 7). Bursts in the rostral section occurred spontaneously in all preparations (n = 7). The frequency of bursts in this section increased from 3.2 ± 1.4 to 8.1 ± 2.0 bursts min−1 when the pH was lowered from 7.8 to 7.4 (paired t test: t = 2.61; P < 0.05, n = 7). In the intact postmetamorphic tadpole brainstem preparation, CO2-H+ chemosensitivity is a unique characteristic of lung bursts (Torgerson et al. 1997b). Rhythmogenesis in the caudal section occurred spontaneously in 2 of 7 preparations. Two types of bursts were present: (1) large amplitude infrequent bursts, which occurred irregularly and had a duration of tens of seconds, which was too long to be considered respiratory (+ in Fig. 7A); (2) small amplitude bursts having a frequency of 0.5–1 Hz (Fig. 7B), which is within the range of buccal bursts produced by the intact brainstem (Torgerson et al. 1998). Burst trigger averaging indicated that these bursts were similar in shape to the buccal bursts in SN II of the intact brainstem (Fig. 7C). In four of the preparations, bursts in the caudal section were produced (21.7 ± 4.2 min−1) when 1 μm AMPA was added to the superfusate. In the remaining caudal section, 1 μm AMPA caused tonic discharge.
Figure 7. Transections between lung and buccal areas suggest brainstem contains two rhythmogenic sites.

Brainstems were transected within the cranium at the level of CN IX and the two sections transferred to the recording chamber. Integrated recordings from CN VII and SN II were used to monitor activity of the rostral and caudal sections, respectively. In two of seven preparations both rostral and caudal sections were rhythmogenic. Data from one of these two preparations are shown. A, the frequency of bursts produced by the rostral section increased during hypercapnia, a unique characteristic of lung bursts in the intact brainstem. The caudal section produced two bursts types, infrequent large amplitude bursts (+) with a duration > 10 s (too long to be considered respiratory), and frequent small amplitude bursts which cannot be resolved from the noise at this time scale. B, power spectrum of recordings from rostral (CN VII) and caudal (SN II) sections. The small amplitude bursts in the caudal section had a frequency of 0.7 Hz, similar to that of buccal bursts (Torgerson et al. 1998). Frequently occurring bursts comparable to buccal bursts were not present in the rostral section. C, comparison of lung (CN VII) and buccal (SN II) bursts produced by the intact brainstem (left two panels, upper and lower traces respectively) with the bursts produced in the rostral (CN VII) and caudal (SN II) brainstem sections following transection (right two panels). Each trace shows average of 25 bursts (continuous line) ±s.e.m. (dotted lines). Note the similarity in shape between buccal bursts recorded in SN II of the intact brainstem preparation (bottom left) with the bursts recorded in SN II of the caudal section following transection (bottom right).
DISCUSSION
This study revealed two anatomically distinct areas important for generating ventilatory rhythms: a ‘lung area’ just caudal and lateral to the root of CN VI and a ‘buccal area’ at the level of the posterior root of CNX. AMPA injections into the lung area increased the frequency of both lung and buccal bursts, whereas GABA injections abolished only lung bursts. AMPA injections into the buccal area increased buccal burst frequency but inhibited lung bursts, whereas GABA injections abolished buccal bursts but spared the lung bursts. These results suggest that the lung and buccal areas are critical for the lung and buccal rhythms, respectively. The buccal area appears not to be critical for lung bursts and the lung area appears not to be critical for buccal bursts. However, interaction between these two areas appears to be important in generating the normal ventilatory motor pattern.
Pharmacological tests to identify sites important for rhythmogenesis
We used micro-injection of drugs to manipulate neuronal activity locally within the brainstem. While this approach offers reversibility, it requires assumptions as to the affects of the drugs used. In the current study we assumed that AMPA and GABA injections caused local excitation and inhibition, respectively. We choose AMPA and GABA because glutamate and GABA receptors are fairly ubiquitous within the brainstem and because AMPA excites (e.g. Greer et al. 1991; Bissonnette et al. 1997; Ge & Feldman, 1998; Whitney et al. 2000) and GABA inhibits (e.g. Koshiya & Guyenet, 1996; St Jacques & St John, 1999) important elements of the respiratory system. During early development GABA is excitatory owing to a relatively high Cl− reversal potential (e.g. Luhmann & Prince, 1991; Rivera et al. 1999; Ritter & Zhang, 2000). If this were the case in the postmetamorphic tadpole, then our results would have to be interpreted differently. However, in postmetamorphic tadpoles, developmental changes in the Cl− reversal potential are complete, as demonstrated by the presence of postsynaptic Cl−-mediated inhibition of respiratory neurons in metamorphic tadpoles (Liao et al. 1996). That GABA is probably inhibitory in the stages we used is further indicated by the fact that the frequency of buccal and lung bursts produced by the isolated brainstem of metamorphic tadpoles diminishes with increasing concentrations of bath-applied GABA (Galante et al. 1996).
Comparison with a previous injection study in frogs
McLean et al. (1995b) identified two regions in the adult frog medulla that modulate lung burst frequency: a rostral region where glutamate injections (100 μm, 10 nl) increased the frequency of lung bursts and a caudal region around CN X where glutamate arrested the lung rhythm. McLean et al. (1995b) also showed that GABA (100 mm, 10 nl) or lidocaine (lignocaine) injections (1 %, 10 nl) into the rostral region abolished lung bursts, whereas lung bursts persisted when lidocaine or GABA was injected into the caudal region. Our results are entirely consistent with these findings. The rostral and caudal regions encompass the buccal and lung areas we describe and the effects on lung frequency of AMPA and GABA injections into the lung and buccal areas mirror those for glutamate and GABA, as conveyed by McLean et al. (1995b). However, the current drug injection study extends the results of McLean in two important ways: firstly, the smaller injection volumes and lower drug concentrations allowed us to identify discrete sites within the larger regions described by McLean et al. (1995b). Secondly, by using postmetamorphic tadpoles our preparations produced robust lung and buccal bursts and, thus, we were able to determine for the first time the effects of injections on buccal burst generation in addition to lung bursts. The results are consistent with the existence of two distinct oscillators, each possessing independent rhythmogenic capability. The apparent interaction between these putative oscillators was used to formulate our current two-oscillator model of rhythm generation (see below).
Brainstem transections
One interpretation of the drug injection data is that the areas identified herein constitute distinct oscillators. However, another possibility is that the areas constitute ‘upstream’ modulatory sites (Ramirez et al. 1998) that project to a single oscillator, switching it between lung and buccal burst generating states. Testing an area for its ability to promote rhythmogenesis and determining whether the area is required for rhythmogenesis cannot distinguish between an oscillator and an upstream modulatory site. Consequently, we performed a set of transection experiments to physically separate the two sites. Overall, our results indicate that rostral and caudal brainstem sections from the same animal are capable of rhythmogenesis.
The results are consistent with the lung oscillator being rostral of the buccal oscillator, in that the occurrence of bursts in the rostral section, but not the caudal section, increased dramatically during hypercapnia. In the intact in vitro brainstem preparation this property is unique to lung burst (Torgerson et al. 1997b). We also note that the frequency and shape of the bursts in the caudal section is similar to that of buccal bursts produced by the intact brainstem.
Thus, we consider the most parsimonious interpretation of the available data is that lung and buccal ventilation in frogs is generated by two coupled but spatially distinct brainstem oscillators (see Fig. 8). The possibility remains, however, that the rostral and caudal areas identified constitute modulatory sites upstream of the rhythm generator, owing to the inherent limitation of transection experiments. These limitations include: (1) the possibility that transection removes inputs that normally suppress any rhythmogenesis capability; and (2) the difficulty of assigning function to bursts produced after transection. The chemosensitivity of the rhythm produced by the rostral section is highly indicative of lung bursts. However, while the frequency of the caudal rhythm resembles that of buccal bursts in the intact brainstem, our current lack of knowledge regarding the properties of the buccal rhythm prevents a more definitive identification.
Figure 8. Two-oscillator model for bimodal ventilation.

The model consists of a bilaterally iterated circuit consisting of lung and buccal oscillators. We propose the buccal oscillator is active continuously and phasically inhibits the lung oscillator (line with filled circle), and the lung oscillator, when active, excites the buccal oscillator (line with bar). We speculate that these oscillators produce the ventilatory motor pattern as follows: during buccal ventilation, lung oscillator interneurons remain subthreshold but receive phasic inhibition from the buccal oscillator. An unknown set of events or mechanisms (either intrinsic to the two oscillators or involving areas in the rostral brainstem; Kinkead et al. 1997) raise the lung oscillator interneurons above threshold for generating a burst of action potentials. Once initiated, the lung oscillator drives the buccal oscillator, increasing the bursting frequency of the buccal oscillator. Phasic inhibition from the buccal oscillator back to the lung oscillator assists and accelerates subsequent depolarization of the lung oscillator interneurons by activating channels involved in post inhibitory rebound. This ‘recurrent excitation’ between the buccal and lung oscillators continues for several cycles, maintaining the high frequency of lung bursts that occur during lung inflation cycles (see Fig. 1). Through synaptic fatigue, inhibition or loss of tonic excitation the lung oscillator interneurons stop firing bursts, ending the lung episode. Residual excitation of the buccal oscillator then fades rapidly and the endogenous frequency of the buccal rhythm is restored. Current evidence suggests that rhythmogenesis of the buccal oscillator requires postsynaptic inhibition between buccal interneurons, whereas the lung oscillator does not (Galante et al. 1996).
We found no direct evidence for additional brainstem oscillators involved in ventilation. However, we cannot rule out the possibility that additional oscillators may have been missed (e.g. Schmidt, 1992; Kinkead et al. 1997). A further caveat is that, while the data suggest that the lung and buccal oscillators are spatially discrete, it remains possible that some parts of the oscillators may overlap. For example, the buccal oscillator may encompass the lung area, but the most rostral part of the buccal oscillator may not be sufficient for independent buccal rhythmogenesis. Similarly, lung oscillator neurons may be present in the buccal area, but their activity when isolated from inputs from more rostral components of the lung oscillator might be overwhelmed by local inhibition from caudal buccal neurons.
Coupling between oscillators
Whatever the spatial arrangement of the oscillators, they must be tightly coupled to account for the coordination of lung and buccal bursts apparent in Fig. 1. The results of the drug microinjection experiments provided clues to what form this coupling might take. When the buccal rhythm was accelerated by injecting AMPA into the buccal area, lung bursts were abolished (Fig. 4D inset and 4E) suggesting inhibitory inputs from the buccal oscillator onto the lung oscillator. We speculate that these inhibitory inputs are phasic and responsible for the coordination of lung bursts with the buccal rhythm (Fig. 1B and C). This hypothesis is consistent with available intracellular recording data from lung neurons (Kogo & Remmers, 1994; Wilson et al. 1999a). For example, Wilson et al. (1999a) found a neuron that appears to receive phasic subthreshold synaptic inputs during the buccal rhythm that makes the likelihood of firing spikes dependent on the phase of the buccal rhythm. That inputs from the buccal oscillator contribute to the lung burst generating mechanism is corroborated by the effects of injecting GABA into the buccal area. For example, a short duration lung-burst apnoea always ensued following arrest of the buccal rhythm, suggesting that the buccal rhythm contributes to short-term rhythmogenesis of the lung oscillator (Fig. 5E). In several (but not all) cases the frequency of lung bursts following the apnoea differed from that before and after the period of GABA injections (e.g. Fig. 5D). This suggests that, in some cases, inputs from the buccal oscillator may also help set the frequency of the lung oscillator over longer periods. Coupling between oscillators affecting both coordination and frequency is a common feature of other motor systems (Panchin et al. 1995; Russo & Hounsgaard, 1996; Smith, 1997; Arshavsky et al. 1998).
When AMPA was injected into the lung area, lung and subsequent buccal burst frequency increased, suggesting that the lung oscillator can provide a facilitatory input to the buccal oscillator. During spontaneous activity this facilitation may be short lived because (1) arresting the lung rhythm by injecting GABA into the lung area had no effect on buccal burst frequency and (2) we only rarely observed acceleration of buccal bursts after a lung episode (e.g. Fig. 1A). In the model illustrated in Fig. 8, short-lived excitation from the lung oscillator onto the buccal oscillator, and the subsequent increase in the frequency of phasic inhibitory feedback, is important in ensuring that the frequency of lung bursts during a lung burst episode exceed the frequency of the buccal motor pattern.
The two oscillator central pattern generator (CPG) in the context of the whole animal
The isolated brainstem preparation allows the study of the CPG for buccal and lung ventilation in the absence of sensory feedback. However, sensory feedback has a powerful role in respiration (e.g. Kinkead et al. 1994; Kinkead & Milsom, 1994, 1997). In intact frogs, for example, buccal ventilation after a lung episode is reduced compared with that preceding the episode, yet in vitro the exact opposite is sometimes observed (West & Burggren, 1983). One explanation for these differences is that, in vivo, drive from peripheral respiratory chemoreceptors is attenuated and inhibition from lung mechanoreceptors is augmented after the lung episode. Determining how these inputs modulate the output of, or compete with connections within, the CPG to generate the richness of the ventilatory motor pattern seen in vivo will be an important area of future research.
Are multiple oscillators involved in respiratory rhythmogenesis in other tetrapods?
Brainstem transection studies of the embryonic chick (Fortin et al. 1995, 1999) and embryonic mouse (Abadie et al. 2000) suggest multiple brainstem oscillators play a role in the production of primordial respiratory-like motor patterns. In neonatal and adult animals, interactions between respiration and other rhythmic behaviours suggest coupled oscillators within the brainstem are not just a developmental phenomenon (e.g. Nakamura et al. 1999). However, whether spatially distinct multiple oscillators are involved in mammalian respiratory rhythmogenesis per se is unclear. Slices of the medulla containing the pre-Bötzinger complex (PreBötzC) can produce a variety of rhythmic motor patterns in the presence of 9 mm KCl (Lieske et al. 2000) and, in anaesthetized animals, the PreBötzC is critical for eupnogenesis (e.g. Ramirez et al. 1998; Solomon et al. 1999). However, in unanaesthetized animals apnoea resulting from lesioning the PreBötzC and the surrounding regions of the ventral respiratory group could be reversed by stimulating peripheral respiratory chemoreceptors (St Jacques & St John, 1999). These results suggest that other sites may be involved in respiratory rhythmogenesis, at least in unanaesthetized animals. While such sites remain unidentified, it should be noted that the rostral pons in unanaesthetized animals is also critical for eupnogenesis (St John, 1987; Morrison et al. 1994; Fung & St John, 1995). Furthermore, some data suggest that the pons itself may be capable of rhythmogenesis, although this idea remains unsubstantiated (St John, 1996).
Comparison of the lung area with the mammalian PreBötzinger Complex
In amphibia accumulating experimental data point to fundamental differences between the mechanism of lung and buccal burst generation. For example, buccal bursts are dependent on chloride-mediated postsynaptic inhibition whereas lung bursts continue in the presence of moderate concentrations of GABA, a cocktail of strychnine and bicucculine (Galante et al. 1996) or low Cl− mock CSF (K. Vasilakos, unpublished observation). These results demonstrate that Cl− dependent network interactions are vital for the generation of buccal bursts, but not lung bursts. How lung bursts are generated is unknown.
One possibility is that lung bursts may be produced by network-mediated rhythmogenesis not involving Cl− (Feldman & Smith, 1989; Chub & O'Donovan, 1998). Network models in which cell-cell interactions are essential for rhythmogenesis have been proposed for respiratory rhythm generation in mammals, but invariably the essential cell-cell interactions involve postsynaptic Cl− mediated inhibition (Ramirez et al. 1997; Busselberg et al. 2001; Richter & Spyer, 2001). Thus, if the lung bursts are produced by network interactions, the mechanism is distinct from that proposed in the network models of mammalian breathing. Perhaps a more likely possibility is that the mechanism involves neurons with ‘pacemaker-like’ properties, comparable to those described in the mammalian PreBötzC slice preparation (Johnson et al. 1994; Galante et al. 1996). If this is the case, then eupnoea in the frog is the product of interactions between a network driven oscillator (the buccal oscillator) and a second oscillator driven by the pacemaker properties of individual neurons (the lung oscillator). Interestingly, this scheme is very similar to the ‘hybrid-pacemaker’ model of mammalian respiratory rhythmogenesis (Smith, 1995; Funk & Feldman, 1995).
An intriguing question arising from our data is whether the lung area and the PreBötzC may be homologous structures. While the data currently available are insufficient to give any definitive answer to this question, we note that: (1) lung burst generation in tadpoles and rhythmogenesis in slices containing the PreBötzC are independent of chloride-mediated postsynaptic inhibition (Galante et al. 1996; Shao & Feldman, 1997); (2) the lung area and the PreBötzC are situated in the ventral lateral medulla between the VIIth and IXth motor nuclei (Fig. 6) and (c) both sites appear to be CO2-H+ chemosensitive (Solomon et al. 1999; Torgerson et al. 2001a).
Conclusion
We identified two brainstem sites, essential for lung and buccal bursts. Transections between these sites results in rhythmogenic sections, supporting pharmacological data suggesting that the motor patterns for lung and buccal ventilation may be generated by distinct neuronal oscillators. Using our data, we develop a simple model of the neuronal architecture underlying bimodal ventilation in amphibia. While speculative, we consider this model to be an important and necessary step in elucidating the mechanisms responsible for the respiratory rhythm in this system.
Acknowledgments
R.J.A.W. was supported by the Alberta Heritage Foundation for Medical Research and the Parker B. Francis Foundation for Pulmonary Research. J.E.R. and K.V. were supported by the MRC.
REFERENCES
- Abadie V, Champagnat J, Fortin G. Branchiomotor activities in mouse embryo. NeuroReport. 2000;11:141–145. doi: 10.1097/00001756-200001170-00028. [DOI] [PubMed] [Google Scholar]
- Adli DSH, Stuesse SL, Cruce WLR. Immunohistochemistry and spinal projections of the reticular formation in the northern leopard frog, Rana pipiens. Journal of Comparative Neurology. 1999;404:387–407. [PubMed] [Google Scholar]
- Arshavsky YI, Deliagina TG, Orlovsky GN, Panchin YV, Popova LB, Sadreyev RI. Analysis of the central pattern generator for swimming in the mollusk Clione. Annals of the New York Academy of Sciences. 1998;860:51–69. doi: 10.1111/j.1749-6632.1998.tb09038.x. [DOI] [PubMed] [Google Scholar]
- Bissonnette JM, Hohimer AR, Knopp SJ. Non-NMDA receptors modulate respiratory drive in fetal sheep. Journal of Physiology. 1997;501:415–423. doi: 10.1111/j.1469-7793.1997.415bn.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busselberg D, Bischoff AM, Paton JFR, Richter DW. Reorganisation of respiratory network activity after loss of glycinergic inhibition. Pflügers Archiv. 2001;441:444–449. doi: 10.1007/s004240000453. [DOI] [PubMed] [Google Scholar]
- Chub N, O'Donovan MJ. Blockade and recovery of spontaneous rhythmic activity after application of neurotransmitter antagonists to spinal networks of the chick embryo. Journal of Neuroscience. 1998;18:294–306. doi: 10.1523/JNEUROSCI.18-01-00294.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coles SK, Dick TE. Neurones in the ventrolateral pons are required for post-hypoxic frequency decline in rats. Journal of Physiology. 1996;497:79–94. doi: 10.1113/jphysiol.1996.sp021751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldman JL, Smith JC. Cellular mechanisms underlying modulation of breathing pattern in mammals. Annals of the New York Academy of Sciences. 1989;563:114–130. doi: 10.1111/j.1749-6632.1989.tb42194.x. [DOI] [PubMed] [Google Scholar]
- Fortin G, Jungbluth S, Lumsden A, Champagnat J. Segmental specification of GABAergic inhibition during development of hindbrain neural networks. Nature Neuroscience. 1999;2:873–877. doi: 10.1038/13172. [DOI] [PubMed] [Google Scholar]
- Fortin G, Kato F, Lumsden A, Champagnat J. Rhythm generation in the segmented hindbrain of chick embryos. Journal of Physiology. 1995;486:735–744. doi: 10.1113/jphysiol.1995.sp020849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fung ML, St John WM. The functional expression of a pontine pneumotaxic centre in neonatal rats. Journal of Physiology. 1995;489:579–591. doi: 10.1113/jphysiol.1995.sp021074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funk GD, Feldman JL. Generation of respiratory rhythm and pattern in mammals: insights from developmental studies. Current Opinions in Neurobiology. 1995;5:778–785. doi: 10.1016/0959-4388(95)80106-5. [DOI] [PubMed] [Google Scholar]
- Funk GD, Steeves JD, Milsom WK. Coordination of wingbeat and respiration in birds. II. ‘Fictive’ flight. Journal of Applied Physiology. 1992;73:1025–1033. doi: 10.1152/jappl.1992.73.3.1025. [DOI] [PubMed] [Google Scholar]
- Galante RJ, Kubin L, Fishman AP, Pack AI. Role of chloride-mediated inhibition in respiratory rhythmogenesis in an in vitro brainstem of tadpole, Rana catesbeiana. Journal of Physiology. 1996;492:545–558. doi: 10.1113/jphysiol.1996.sp021328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gdovin MJ, Torgerson CS, Remmers JE. Neurorespiratory pattern of gill and lung ventilation in the decerebrate spontaneously breathing tadpole. Respiratory Physiology. 1998;113:135–146. doi: 10.1016/s0034-5687(98)00061-9. [DOI] [PubMed] [Google Scholar]
- Gdovin MJ, Torgerson CS, Remmers JE. The fictively breathing tadpole brainstem preparation as a model for the development of respiratory pattern generation and central chemoreception. Comparative Biochemistry and Physiology A. 1999;124:275–286. doi: 10.1016/s1095-6433(99)00116-6. [DOI] [PubMed] [Google Scholar]
- Ge Q, Feldman JL. AMPA receptor activation and phosphatase inhibition affect neonatal rat respiratory rhythm generation. Journal of Physiology. 1998;509:255–266. doi: 10.1111/j.1469-7793.1998.255bo.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greer JJ, Smith JC, Feldman JL. Role of excitatory amino acids in the generation and transmission of respiratory drive in neonatal rat. Journal of Physiology. 1991;437:727–749. doi: 10.1113/jphysiol.1991.sp018622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grillner S, Deliagina T, Ekeberg O, el Manira A, Hill RH, Lansner A, Orlovsky GN, Wallen P. Neural networks that co-ordinate locomotion and body orientation in lamprey. Trends in Neurosciences. 1995;18:270–279. [PubMed] [Google Scholar]
- Johnson SM, Smith JC, Funk GD, Feldman JL. Pacemaker behavior of respiratory neurons in medullary slices from neonatal rat. Journal of Neurophysiology. 1994;72:2598–2608. doi: 10.1152/jn.1994.72.6.2598. [DOI] [PubMed] [Google Scholar]
- Just JJ, Gatz RN, Crawford EC., Jr Changes in respiratory functions during metamorphosis of the bullfrog, Rana catesbeiana. Respiratory Physiology. 1973;17:276–282. doi: 10.1016/0034-5687(73)90002-9. [DOI] [PubMed] [Google Scholar]
- Kinkead R, Filmyer WG, Mitchell GS, Milsom WK. Vagal input enhances responsiveness of respiratory discharge to central changes in pH/CO2 in bullfrogs. Journal of Applied Physiology. 1994;77:2048–2051. doi: 10.1152/jappl.1994.77.4.2048. [DOI] [PubMed] [Google Scholar]
- Kinkead R, Harris MB, Milsom WK. The role of the nucleus isthmi in respiratory pattern formation in bullfrogs. Journal of Experimental Biology. 1997;200:1781–1793. doi: 10.1242/jeb.200.12.1781. [DOI] [PubMed] [Google Scholar]
- Kinkead R, Milsom WK. Chemoreceptors and control of episodic breathing in the bullfrog (Rana catesbeiana) Respiratory Physiology. 1994;95:81–98. doi: 10.1016/0034-5687(94)90049-3. [DOI] [PubMed] [Google Scholar]
- Kinkead R, Milsom WK. Role of pulmonary stretch receptor feedback in control of episodic breathing in the bullfrog. American Journal of Physiology. 1997;272R:497–508. doi: 10.1152/ajpregu.1997.272.2.R497. [DOI] [PubMed] [Google Scholar]
- Kogo N, Perry SF, Remmers JE. Neural organization of the ventilatory activity in the frog, Rana catesbeiana I. Journal of Neurobiology. 1994;25:1067–1079. doi: 10.1002/neu.480250904. [DOI] [PubMed] [Google Scholar]
- Kogo N, Remmers JE. Neural organization of the ventilatory activity in the frog, Rana catesbeiana II. Journal of Neurobiology. 1994;25:1080–1094. doi: 10.1002/neu.480250905. [DOI] [PubMed] [Google Scholar]
- Koshiya N, Guyenet PG. Tonic sympathetic chemoreflex after blockade of respiratory rhythmogenesis in the rat. Journal of Physiology. 1996;491:859–869. doi: 10.1113/jphysiol.1996.sp021263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao G-S, Kubin L, Galante RJ, Fishman AP, Pack AI. Respiratory activity in the facial nucleus in an in vitro brainstem of tadpole, Rana catesbeiana. Journal of Physiology. 1996;492:529–544. doi: 10.1113/jphysiol.1996.sp021327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieske SP, Thoby-Brisson M, Telgkamp P, Ramirez JM. Reconfiguration of the neural network controlling multiple breathing patterns: eupnea, sighs and gasps. Nature Neuroscience. 2000;3:600–607. doi: 10.1038/75776. [DOI] [PubMed] [Google Scholar]
- Luhmann HJ, Prince DA. Postnatal maturation of the GABAergic system in rat neocortex. Journal of Neurophysiology. 1991;65:247–263. doi: 10.1152/jn.1991.65.2.247. [DOI] [PubMed] [Google Scholar]
- McCrimmon DR, Monnier A, Hayashi F, Zuperku EJ. Pattern formation and rhythm generation in the ventral respiratory group. Clinical Experimental Pharmacology and Physiology. 2000;27:126–131. doi: 10.1046/j.1440-1681.2000.03193.x. [DOI] [PubMed] [Google Scholar]
- McLean HA, Kimura N, Kogo N, Perry SF, Remmers JE. Fictive respiratory rhythm in the isolated brainstem of frogs. Journal of Comparative Physiology A. 1995a;176:703–13. doi: 10.1007/BF00192499. [DOI] [PubMed] [Google Scholar]
- McLean HA, Perry SF, Remmers JE. Two regions in the isolated brainstem of the frog that modulate respiratory-related activity. Journal of Comparative Physiology A. 1995b;177:135–144. doi: 10.1007/BF00225094. [DOI] [PubMed] [Google Scholar]
- Morrison SF, Cravo SL, Wilfehrt HM. Pontine lesions produce apneusis in the rat. Brain Research. 1994;652:83–86. doi: 10.1016/0006-8993(94)90320-4. [DOI] [PubMed] [Google Scholar]
- Nakamura Y, Katakura N, Nakajima M. Generation of rhythmical ingestive activities of the trigeminal, facial, and hypoglossal motoneurons in in vitro CNS preparations isolated from rats and mice. Journal of Medical Dentistry Science. 1999;46:63–73. [PubMed] [Google Scholar]
- Newsom Davis J. An experimental study of hiccup. Brain. 1970;93:851–872. doi: 10.1093/brain/93.4.851. [DOI] [PubMed] [Google Scholar]
- Niizeki K, Kawahara K, Miyamoto Y. Cardiac, respiratory, and locomotor coordination during walking in humans. Folia Primatologica. 1996;66:226–239. doi: 10.1159/000157197. [DOI] [PubMed] [Google Scholar]
- Panchin YV, Sadreyev RI, Arshavsky YI. Control of locomotion in marine mollusc Clione limacina. X. Effects of acetylcholine antagonists. Experimental Brain Research. 1995;106:135–144. doi: 10.1007/BF00241363. [DOI] [PubMed] [Google Scholar]
- Ramirez JM, Schwarzacher SW, Pierrefiche O, Olivera BM, Richter DW. Selective lesioning of the cat pre-Bötzinger complex in vivo eliminates breathing but not gasping. Journal of Physiology. 1998;507:895–907. doi: 10.1111/j.1469-7793.1998.895bs.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramirez JM, Telgkamp P, Elsen FP, Quellmalz UJ, Richter DW. Respiratory rhythm generation in mammals: synaptic and membrane properties. Respiratory Physiology. 1997;110:71–85. doi: 10.1016/s0034-5687(97)00074-1. [DOI] [PubMed] [Google Scholar]
- Reid SG, Meier JT, Milsom WK. The influence of descending inputs on breathing pattern formation in the isolated bullfrog brainstem-spinal cord. Respiratory Physiology. 2000;120:197–211. doi: 10.1016/s0034-5687(99)00117-6. [DOI] [PubMed] [Google Scholar]
- Reid SG, Milsom WK. Respiratory pattern formation in the isolated bullfrog (Rana catesbeiana) brainstem-spinal cord. Respiratory Physiology. 1998;114:239–255. doi: 10.1016/s0034-5687(98)00091-7. [DOI] [PubMed] [Google Scholar]
- Richter DW, Spyer KM. Studying rhythmogenesis of breathing: comparison of in vivo and in vitro models. Trends in Neurosciences. 2001;24:464–472. doi: 10.1016/s0166-2236(00)01867-1. [DOI] [PubMed] [Google Scholar]
- Ritter B, Zhang W. Early postnatal maturation of GABAA-mediated inhibition in the brainstem respiratory rhythm-generating network of the mouse. European Journal of Neuroscience. 2000;12:2975–2984. doi: 10.1046/j.1460-9568.2000.00152.x. [DOI] [PubMed] [Google Scholar]
- Rivera C, Voipio J, Payne JA, Ruusuvuori E, Lahtinen H, Lamsa K, Pirvola U, Saarma M, Kaila K. The K+/Cl− co-transporter KCC2 renders GABA hyperpolarizing during neuronal maturation. Nature. 1999;397:251–255. doi: 10.1038/16697. [DOI] [PubMed] [Google Scholar]
- Russo RE, Hounsgaard J. Burst-generating neurones in the dorsal horn in an in vitro preparation of the turtle spinal cord. Journal of Physiology. 1996;493:55–66. doi: 10.1113/jphysiol.1996.sp021364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- St Jacques R, St John WM. Transient, reversible apnoea following ablation of the pre-Botzinger complex in rats. Journal of Physiology. 1999;520:303–314. doi: 10.1111/j.1469-7793.1999.00303.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- St John WM. Pneumotaxic mechanisms influence phrenic, hypoglossal, and trigeminal activities. Experimental Neurology. 1987;97:301–314. doi: 10.1016/0014-4886(87)90091-4. [DOI] [PubMed] [Google Scholar]
- St John W. Medullary regions for neurogenesis of gasping: noeud vital or noeuds vitals. Journal of Applied Physiology. 1996;81:1865–1877. doi: 10.1152/jappl.1996.81.5.1865. [DOI] [PubMed] [Google Scholar]
- Sakakibara Y. The pattern of respiratory nerve activity in the bullfrog. Japanese Journal of Physiology. 1984;34:269–282. doi: 10.2170/jjphysiol.34.269. [DOI] [PubMed] [Google Scholar]
- Schmidt RS. Neural correlates of frog calling: production by two semi-independent generators. Behavioural and Brain Research. 1992;50:17–30. doi: 10.1016/s0166-4328(05)80284-0. [DOI] [PubMed] [Google Scholar]
- Shao XM, Feldman JL. Respiratory rhythm generation and synaptic inhibition of expiratory neurons in pre-Bötzinger complex: differential roles of glycinergic and GABAergic neural transmission. Journal of Neurophysiology. 1997;77:1853–1860. doi: 10.1152/jn.1997.77.4.1853. [DOI] [PubMed] [Google Scholar]
- Sirota MG, Di Prisco GV, Dubuc R. Stimulation of the mesencephalic locomotor region elicits controlled swimming in semi-intact lampreys. European Journal of Neuroscience. 2000;12:4081–4092. doi: 10.1046/j.1460-9568.2000.00301.x. [DOI] [PubMed] [Google Scholar]
- Skinner FK, Mulloney B. Intersegmental coordination in invertebrates and vertebrates. Current Opinion in Neurobiology. 1998;8:725–732. doi: 10.1016/s0959-4388(98)80114-1. [DOI] [PubMed] [Google Scholar]
- Smith JC. New computational models of the respiratory oscillator in mammals. Advances in Experimental and Medical Biology. 1995;393:7–13. doi: 10.1007/978-1-4615-1933-1_2. [DOI] [PubMed] [Google Scholar]
- Smith JC. Integration of cellular and network mechanisms in mammalian oscillatory motor circuits; insights from the respiratory oscillator. In: Stein PSG, Grillner S, Selverston AI, Stuart DG, editors. Neurons, Networks and Motor Behaviors. MA, USA: MIT Press; 1997. pp. 97–104. [Google Scholar]
- Smith JC, Ellenberger HH, Ballanyi K, Richter DW, Feldman JL. Pre-Bötzinger complex: a brainstem region that may generate respiratory rhythm in mammals. Science. 1991;254:726–729. doi: 10.1126/science.1683005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith JE, Gilbey MP. Coherent rhythmic discharges in sympathetic nerves supplying thermoregulatory circulations in the rat. Journal of Physiology. 2000;523:449–457. doi: 10.1111/j.1469-7793.2000.00449.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solomon IC, Edelman NH, Neubauer JA. Patterns of phrenic motor output evoked by chemical stimulation of neurons located in the pre-Botzinger complex in vivo. Journal of Neurophysiology. 1999;81:1150–1161. doi: 10.1152/jn.1999.81.3.1150. [DOI] [PubMed] [Google Scholar]
- Steriade M. Synchronized activities of coupled oscillators in the cerebral cortex and thalamus at different levels of vigilance. Cerebral Cortex. 1997;7:583–604. doi: 10.1093/cercor/7.6.583. [DOI] [PubMed] [Google Scholar]
- Stinner JN, Hartzler LK. Effect of temperature on pH and electrolyte concentration in air-breathing ectotherms. Journal of Experimental Biology. 2000;203:2065–2074. doi: 10.1242/jeb.203.13.2065. [DOI] [PubMed] [Google Scholar]
- Straus C, Wilson RJA, Tezenas du Montcel S, Remmers JE. Baclofen eliminates cluster lung breathing of the tadpole brainstem, in vitro. Neuroscience Letters. 2000;292:13–16. doi: 10.1016/s0304-3940(00)01422-1. [DOI] [PubMed] [Google Scholar]
- Suthers RA, Goller F, Pytte C. The neuromuscular control of birdsong. Philosophical Transactions of the Royal Society. 1999;354B:927–939. doi: 10.1098/rstb.1999.0444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor AC, Kollros J. Stages in the normal development of Rana pipiens larvae. Anatomical Record. 1946;94:7–24. doi: 10.1002/ar.1090940103. [DOI] [PubMed] [Google Scholar]
- Torgerson CS, Gdovin MJ, Brandt R, Remmers JE. Location of central respiratory chemoreceptors in the developing tadpole. American Journal of Physiology - Regulatory. Integrative and Comparative Physiology. 2001a;280R:921–928. doi: 10.1152/ajpregu.2001.280.4.R921. [DOI] [PubMed] [Google Scholar]
- Torgerson CS, Gdovin MJ, Kogo N, Remmers JE. Depth profiles of pH and PO2 in the in vitro brainstem preparation of the tadpole Rana catesbeiana. Respiratory Physiology. 1997a;108:205–213. doi: 10.1016/s0034-5687(97)00027-3. [DOI] [PubMed] [Google Scholar]
- Torgerson CS, Gdovin MJ, Remmers JE. Ontogeny of central chemoreception during fictive gill and lung ventilation in an in vitro brainstem preparation of Rana catesbeiana. Journal of Experimental Biology. 1997b;200:2063–2072. doi: 10.1242/jeb.200.15.2063. [DOI] [PubMed] [Google Scholar]
- Torgerson CS, Gdovin MJ, Remmers JE. Fictive gill and lung ventilation in the pre- and postmetamorphic tadpole brain stem. Journal of Neurophysiology. 1998;80:2015–2022. doi: 10.1152/jn.1998.80.4.2015. [DOI] [PubMed] [Google Scholar]
- Torgerson CS, Gdovin MJ, Remmers JE. Sites of respiratory rhythmogenesis during development in the tadpole. American Journal of Physiology - Regulatory. Integrative and Comparative Physiology. 2001b;280R:913–920. doi: 10.1152/ajpregu.2001.280.4.R913. [DOI] [PubMed] [Google Scholar]
- West NH, Burggren WW. Reflex interactions between aerial and aquatic gas exchange organs in larval bullfrogs. American Journal of Physiology. 1983;244R:770–777. doi: 10.1152/ajpregu.1983.244.6.R770. [DOI] [PubMed] [Google Scholar]
- West NH, Jones DR. Breathing movements in the frog Rana pipiens. I. The mechanical events associated with lung and buccal ventilation. Canadian Journal of Zoology. 1975;53:332–344. doi: 10.1139/z75-042. [DOI] [PubMed] [Google Scholar]
- Whitney GM, Ohtake PJ, Simakajornboon N, Xue YD, Gozal D. AMPA glutamate receptors and respiratory control in the developing rat: anatomic and pharmacological aspects. American Journal of Physiology - Regulatory. Integrative and Comparative Physiology. 2000;278R:520–528. doi: 10.1152/ajpregu.2000.278.2.R520. [DOI] [PubMed] [Google Scholar]
- Wilson RJA, Kleinhaus AL. Segmental control of midbody peristalsis during the consummatory phase of feeding in the medicinal leech, Hirudo medicinalis. Behavioral Neuroscience. 2000;114:635–646. [PubMed] [Google Scholar]
- Wilson RJA, Straus C, Harris MB, Remmers JE. A coupled oscillator model for the neuronal control of respiration in tadpoles. Society for Neuroscience Abstracts. 1999b;29:422. [Google Scholar]
- Wilson RJA, Straus C, Remmers JE. Efficacy of a low volume recirculating superfusion chamber for long term administration of expensive drugs and dyes. Journal of Neuroscience Methods. 1999a;87:175–184. doi: 10.1016/s0165-0270(99)00005-9. [DOI] [PubMed] [Google Scholar]
- Zhong S, Zhou SY, Gebber GL, Barman SM. Coupled oscillators account for the slow rhythms in sympathetic nerve discharge and phrenic nerve activity. American Journal of Physiology. 1997;272R:1314–1324. doi: 10.1152/ajpregu.1997.272.4.R1314. [DOI] [PubMed] [Google Scholar]


