Abstract
Late-gestation blockade of vasoactive intestinal polypeptide (VIP) activity in pregnant mice produces discrete morphological abnormalities in the somatosensory cortex of offspring. We investigated the functional implications of this lesion on the behavioural arousal response to moderate hypoxia. Pregnant mice received twice-daily injections of 200 μl saline (control), or saline + 50 μg VIP antagonist (anti-VIP) on embryonic days 17 and 18. Offspring were studied unrestrained at 6–7 weeks after birth, in a bias-flow whole-body plethysmograph during behavioural quiet sleep. Arousal was defined by movement (MVT) lasting >1 s. Hypoxic ventilatory (HVR) and arousal responses were measured during a 5 min exposure to 10 % O2-3 % CO2 (hypoxia); peripheral chemoreflex drive was estimated by transient hyperoxia administered at rest and end-hypoxia (Dejours-type test). MVTs increased in all mice during hypoxia, but in anti-VIP mice: (a) MVT onset was delayed (174 ± 90 vs. 108 ± 59 s from the start of hypoxia, anti-VIP vs. control; P = 0.008); and (b) MVTs were less frequent, and total MVT time in hypoxia was less (8 ± 7 vs. 15 ± 9 %; P = 0.03). The HVR, and peripheral drive at rest and end-hypoxia were comparable in control and anti-VIP mice. In conclusion, a significant arousal deficit was evident in anti-VIP mice. This was not associated with obviously deranged peripheral or brainstem-mediated responses to hypoxia during sleep. This may signal a general deficit in the way hypoxic distress is monitored and processed, and arousal initiated and sustained in these mice.
Acute hypoxia disrupts the depth and duration of sleep, increasing the frequency of arousals and proportion of wakefulness, and reducing or even suppressing completely slow wave (quiet) and rapid eye movement (active) sleep (Pappenheimer, 1977; Baker & McGinty, 1979). The shift towards waking activity patterns is accompanied by powerful reflex cardiorespiratory and motor activation, and is a strategy to improve oxygen supply. Arousal is initiated by the peripheral arterial chemoreceptors (the hypoxic sensors), which trigger, via pathways arising in the brainstem and hypothalamus, diffuse cortical activation, and the cascade of arousal-related events (Bowes et al. 1981; Yardley & Hilton, 1986; Steriade et al. 1993). This is an important defence against cardiorespiratory failure: a delayed, impaired, or poorly co-ordinated arousal response may exacerbate hypoxaemia, and increase morbidity and mortality (Phillipson & Sullivan, 1978; Hunt, 1989; Berry & Gleeson, 1997).
Damage to the arterial chemoreceptors and/or brainstem cardiorespiratory centres, or physiological stresses such as repetitive hypoxia, sleep fragmentation, or certain drugs, may depress arousability to hypoxia (Bowes et al. 1981; Fewell & Konduri, 1989; Filiano & Kinney, 1992; Hafström et al. 2000). Physiological deficits may also be due to damage sustained before birth (Barker, 1998), although linking a functional deficit with a specific antenatal abnormality can be difficult if neurological damage is widespread and debilitating. In this study we have used a pharmacological agent to produce a relatively circumscribed antenatal lesion. Analysing the resulting behavioural changes may help elucidate the functional consequences of subtle changes in neural connectivity.
Maternal administration of a specific antagonist of the neurotransmitter-neuromodulator vasoactive intestinal polypeptide (anti-VIP) late in gestation disturbs the neural architecture in the sensorimotor cortex of offspring mice. This lesion is not associated with gross physical or behavioural abnormalities (Zupan et al. 2000). Since arousal from sleep is the result of brainstem-mediated activation of the cortex and associated relay centres, lesions in the cortex could conceivably impair the capacity to pattern and propagate arousal. We have examined whether the latency or patterning of the hypoxic arousal response was impaired in adolescent mice following late-gestation VIP blockade. Our findings indicate that seemingly innocuous and discrete abnormalities in neural development may result in long-term ‘reprogramming’ of the hypoxic arousal reflex.
METHODS
Animals
Ethical approval for these studies was granted by the French Ministère de l'Agriculture et de la Forêt, and all procedures conformed with the guidelines of the Institut National de la Santé et de la Recherche Médicale (INSERM). Swiss mice were housed in a quiet room under a 12 h light-dark cycle, and were provided with water and dry food pellets ad libitum. The day of conception (embryonic day (E) 0) was estimated by the presence of a vaginal plug. Equal numbers of pregnant dams were assigned to experimental and control groups. At 08.00–09.00 and 18.00–19.00 h on E17 and E18, dams in the experimental group received an intraperitoneal injection of 200 μl phosphate-buffered saline (PBS) containing 50 μg of a specific VIP antagonist (a neurotensin 1–6, VIP 7–28 hybrid; Gozes et al. 1991). Control dams were treated in the same way but received only PBS. Pups which issued from these animals were sexed and separated at 4 weeks old, prior to reaching sexual maturity, and were studied at 6–7 weeks old (inclusive). Mice were killed by cervical dislocation at the end of the experiment.
Measurement of ventilation
Ventilation and the ventilatory responses to transient hyperoxia and hypoxia were measured using whole-body flow-through plethysmography, as described previously (Dauger et al. 1998). We reduced the volume of the measurement and reference chambers of the plethysmograph by ∼75 % in this study in order to improve the washout of gases within the measurement chamber (the chamber containing the mouse). In this configuration, a sudden reduction in the fractional concentration of O2 (FI,O2) of the gas flowing through the plethysmograph reached 90 % of its steady-state value within 2 min. Measurement and reference chambers were connected to a differential pressure transducer (range, ±0.1 mbar, EFFA, Asnières, France) for the measurement of respiratory volumes and times. Gas was sampled at 100 ml min−1 from a port at the bottom of the measurement chamber (i.e. at the level of the mouse) for the continuous measurement of the fractional concentration of O2 and CO2 (FI,O2 and FI,CO2, Arelco CO2/O2 analyser, Fontenay-sous-Bois, France). The delay in response of the gas analyser following a sudden change in FI,O2 at the sampling port ( = gas sample transit time + paramagnetic cell response time) was estimated to be 5 s. Volume calibration was performed at the beginning of each study by injecting 50 μl of air into the measurement chamber with a precision syringe (Hamilton Bonaduz AG, Switzerland). Analog voltage signals from the pressure transducer and gas analyser were bandwidth filtered (0.4–15 Hz at −3 db), converted to digital signals at 100 Hz (MacAdios A/D 12 bit converter, GW Instruments, Somerville, MA, USA), and were displayed in real time and stored for later analysis using data aquisition, analysis and presentation software (Superscope II; GW Instruments).
Protocol
Mice were unrestrained in the plethysmograph, and were left to explore the device, groom etc. for as long as was necessary for sleep to ensue. Recording commenced at sleep onset, and mice cycled spontaneously between different behavioural states. Continuous notes were kept regarding the position and movements of each mouse, as observed through the plethysmograph. These ‘on-line’ observations were used (a) to identify the various phases of the sleep cycle to ensure that tests were commenced during defined sleep, and (b) to assist in later (‘off-line’) scoring of behavioural state.
The test protocol consisted of two phases (Fig. 1). During phase 1 (duration, 25 min) the plethysmograph was perfused with air. No tests were administered during the initial 5 min, to permit sleep to consolidate. Subsequently, three or four brief pulses of O2 (Dejours, 1962) were added to the plethysmograph at 5 min intervals to test hyperoxic responses (chamber FI,O2 increased to ∼48 % within 20 s and returned to 21 % prior to repeating the test). At the end of phase 1, we commenced phase 2 (duration, 6 min). Here we measured the ventilatory and arousal responses to hypoxia (10 % O2+ 3 % CO2 in N2). The mixture was mildly hypercapnic to moderate any change in arterial partial pressure of carbon dioxide (Pa,CO2) during hyperpnoea, since this may depress ventilation (Pepelko & Dixon, 1975). Tests comprised 1 min baseline (air), followed by 5 min hypoxia, then 100 % O2. All gases were premixed commercially, and fed directly to the plethysmograph without prior warming or humidification. We assumed body temperature was constant at 37 °C; the measurement chamber was maintained within the thermoneutral range for young, adult mice (26–28 °C; Cassin, 1963).
Figure 1. Experimental protocol.
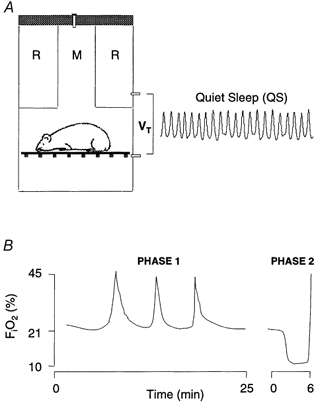
A, tidal volume (VT) was calculated from pressure differences between measurement (M) and reference (R) chambers of a bias-flow, body plethysmograph. Mice were unrestrained, and cycled spontaneously between various activity states. B, respiratory stimuli were administered during behavioural QS. During phase 1, three to four brief pulses of 100 % O2 were added, over a period of 25 min, to the air stream perfusing both chambers. During phase 2, a 1 min control period (air) was followed by 5 min hypoxia, then 100 % O2.
Data analysis
Behavioural state
Sleep state was scored ‘off-line’ by an experienced sleep investigator (G.C.) who reviewed all the recordings and study notes en bloc, unaware of gender, treatment group, or when tests were started. State was scored using standard behavioural criteria (Gramsbergen et al. 1970), and an epoch length of 5 s. Briefly, mice were deemed to be either awake (W: eyes open, gross body movements, such as crawling, grooming etc.), in quiet sleep (QS: eyes closed, recumbent with limbs adducted, regular respiratory rhythm, absence of movements except for periodic sighs and intermittent brief startles), or in active sleep (AS: recumbent, eyes closed, irregular respiratory pattern, frequent twitches of the whiskers, ears and extremities; eye movements were not used since it was difficult to see these through the plethysmograph). If uncertain, state was recorded as indeterminate (IN). Sleep onset was taken to be the first period of > 3 min continuous QS. An arousal was defined as any movement (MVT) of > 1 s duration as determined from the pressure artefact (loss of respiratory waveform). Epochs which consisted of > 50 % movement artefact (below) were scored as W, otherwise as the prevailing state. Scored epochs were used to calculate sleep efficiency (SEF) during hypoxia:
Ventilation
Recordings were scrutinised off-line, and parts of the record where breaths were not clearly evident (i.e. MVTs) were excised. Data files consisted of breath-by-breath arrays of tidal volume (VT), inspiratory (TI), expiratory (TE) and total (TTOT) breath time, and minute ventilation (V̇E = VT× 1000/(TI+TE)). Augmented breaths (sighs) were defined as breaths with VT greater than twice the mean VT of the five preceding breaths.
Ventilatory responses to hyperoxia
Only hyperoxic tests not disturbed by a MVT or sigh within 30 s preceding the rise in FI,O2 were analysed. We compared breath-by-breath V̇E, VT and TTOT during the 20 s preceding (control period), and the initial 20 s of hyperoxia. The ‘Dejours effect’ (ΔV̇E-DJ) was the minimum of the 10 breath moving average during the initial 20 s hyperoxia, expressed as a percentage of control; the mean value (all tests, each mouse) was calculated. We took into account the delay in response of the gas analyser when calculating ΔV̇E-DJ. Baseline ventilation (air, QS, phase 1) was calculated from all prehyperoxic test control periods.
Hypoxic ventilatory response (HVR)
MeanV̇E, VT and TTOT for each half-minute preceding and following the switch to hypoxia were calculated; data were expressed as a percentage of control (control was taken as the 60 s preceding the switch to hypoxia). Since arousal modulates breathing (Sullivan, 1980), it was necessary to take account of the differences in arousability of control and anti-VIP mice when comparing HVRs. To do this, we used arbitrary SEF cut-offs to match tests according to whether sleep during hypoxia remained relatively stable (SEF > 90 %, ‘quiet’ mice), or was unstable/fragmented (SEF < 90 %, ‘agitated’ mice). Hyperoxic transitions at end-hypoxia were analysed as described above for phase 1 hyperoxic tests (control = mean V̇E during final 20 s hypoxia; ΔV̇E-DJ = minimum 10-point moving average during the initial 20 s hyperoxia). Only transitions undisturbed by a MVT or sigh during the preceding 30 s, and which took place during QS, were analysed.
Statistics
Statistical analyses were performed using Superanova (Abacus Concepts, Inc., Berkeley, CA, USA). Baseline data (Table 1), and MVTs during hypoxia were analysed by ANOVA, with gender and treatment group the between-subject factors, and the variable of interest as the dependent variable. Hyperoxic responses and the HVR were compared using repeated-measures ANOVA to assess the effects of gender, treatment group, FI,O2 (hyperoxia), and exposure time and SEF (hypoxia). Significant interactions were followed by post hoc pairwise comparisons. Fisher's exact test was used to determine whether the proportion of tests categorized by SEF (i.e. ‘quiet’vs.‘agitated’ mice) was similar across treatment groups. Data are presented as means ±sd. in the text and table, and (for clarity) means ±, s.e.m. in the figures. A P value of < 0.05 signified a statistical difference.
Table 1.
Baseline data
| Control mice | Anti-VIP mice | ||||
|---|---|---|---|---|---|
| Male | Female | Male | Female | P (ANOVA) | |
| n | 14 | 8 | 11 | 10 | — |
| Body weight (g) | 29 ± 4 | 24 ± 2 | 26 ± 5 | 23 ± 3 | 0.0006 |
| Delay to sleep onset (min) | 75 ± 35 | 113 ± 34 | 70 ± 23 | 91 ± 30 | 0.003 |
| TI (ms) | 169 ± 17 | 187 ± 19 | 181 ± 31 | 178 ± 32 | 0.37 |
| TTOT (ms) | 362 ± 33 | 381 ± 36 | 377 ± 43 | 367 ± 61 | 0.76 |
| VT (μl g−1) | 5.9 ± 1 | 7.3 ± 0.5 | 5.8 ± 0.6 | 6.6 ± 0.7 | 0.001 |
| V̇E (μl s−1 g−1) | 17 ± 3 | 20 ± 3 | 16 ± 3 | 19 ± 3 | 0.004 |
The number of mice studied (n), together with body weights and the delay to sleep onset (first period of ≥ 3 min continuous QS) are indicated. Baseline ventilation in air (phase 1) is also shown. TI and TTOT, inspiratory and total breath times, respectively; VT and V̇E, tidal volume and minute ventilation, respectively. Significant gender differences are indicated in the final column; treatment group values are not significantly different from control values. Data are means ±s.d.
RESULTS
Litter size and pup survival rates were similar across groups. There were no fundamental differences between control and anti-VIP mice at rest: females were consistently smaller than males, took longer to establish sleep, and had a slightly greater baseline VT and V̇E (Table 1). Behaviourally, anti-VIP mice were unremarkable: exploratory behaviour appeared normal, as was the delay to sleep onset; behavioural correlates of QS and AS were easily identified in both groups of mice (Fig. 2).
Figure 2. Behavioural features of murine sleep-wakefulness.
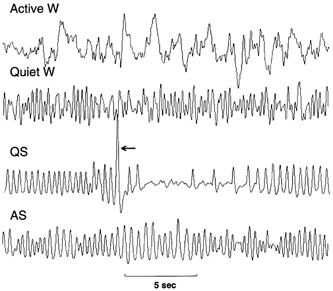
Wakefulness (W), quiet sleep (QS) and active sleep (AS) were identified from the breathing rhythm and pattern of body movements. Sequential extracts from a 25 min recording are shown. Initially mice explored the plethysmograph (Active W). After several hours, relative quiescence and grooming ensued (Quiet W). This always preceded the onset of QS; sighs (arrow) were characteristic of QS. The florid phase of AS was evident from breathing irregularities and large scale deflections (artifacts) due to twitches of the head, body and extremities.
Attenuated hypoxic arousal response in anti-VIP mice
Hypoxia normally increases arousal time and decreases sleep quality and quantity (Pappenheimer, 1977). We analysed MVTs to determine whether hypoxic arousal was different in anti-VIP mice. Gender was not a significant factor in any of these analyses, so only group data are presented. MVTs during hypoxia were of two general types: (a) intermittent or self-limiting, followed by a resumption of sleep (Fig. 3A); (b) frequent and repetitive, interrupted by brief bouts of marked hyperpnoea, with eyes open or partially open (Fig. 3B). Intermittent MVTs occurred principally during the initial minute of hypoxia (falling FI,O2), and repetitive MVTs from mid-test onwards. The onset of repetitive MVTs appeared to signal a shift from deeper to lighter-stage sleep and W.
Figure 3. Movement arousals (MVTs).
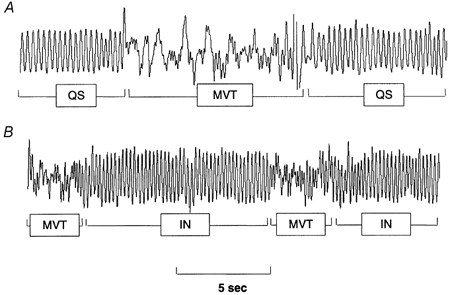
Hypoxia increased the frequency and duration of MVTs, as identified by artefact (loss of respiratory waveform). A, during the initial minute of hypoxia, MVTs were generally intermittent and self-limiting. B, towards the hypoxic nadir, they tended to become more frequent and repetitive, signalling a shift away from deeper-stage QS; periods between repetitive MVTs were scored as indeterminate (IN).
Hypoxic arousal in anti-VIP mice was unusual in several respects. The latency to first arousal was delayed (174 ± 90 vs. 108 ± 59 s from hypoxic onset; anti-VIP vs. control; P = 0.009), and occurred at a lower FI,O2 (Fig. 4A). This suggests that the arousal threshold was higher in anti-VIP mice. The time-dependent increase in MVTs during hypoxia was less in anti-VIP mice (P = 0.003; Fig. 4B), as was total MVT time (8 ± 7 vs. 15 ± 9 % of each test; anti-VIP vs. control; P = 0.03). The frequency rather than duration of MVTs was reduced (Fig. 4C and D), suggesting that anti-VIP mice may sustain a deficit in initiating (frequency) rather than propagating (duration) MVTs.
Figure 4. Arousal response to hypoxia.
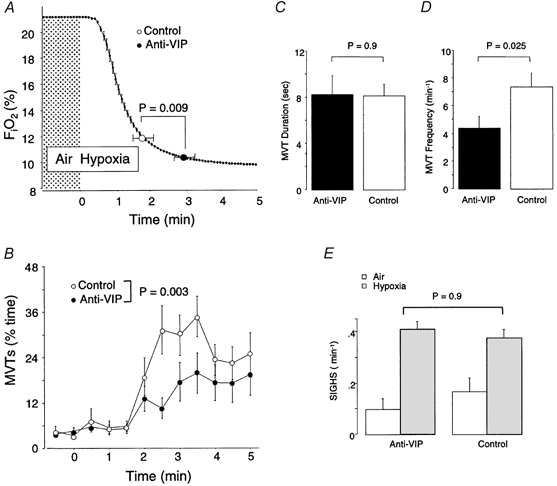
A, time course of the fall in FI,O2 (switch to hypoxia at 0 min), and the point of 1st arousal; note arousal latency was significantly delayed in anti-VIP mice. B, the increase in MVTs over time was also less in anti-VIP mice. C and D, overall, MVTs during hypoxia were less frequent (D) but of similar duration (C) in anti-VIP mice. E, hypoxia provoked a comparable increase in the frequency of sighs in both groups of mice.
Hypoxia normally increases the frequency of augmented breaths (sighs); these breaths help maintain gas exchange and lung stability (Cherniack et al. 1981). The hypoxia-provoked increase in sigh frequency was similar across groups (P = 0.9; Fig. 4E), suggesting that the brainstem-mediated, inspiratory-augmenting (sigh) reflex was functionally intact in anti-VIP mice.
Hyperoxic responses are normal in anti-VIP mice
Physiological denervation of the peripheral chemoreceptors by hyperoxia normally causes ventilation to fall transiently; the extent of fall indirectly measures the strength of peripheral chemoreflex respiratory drive (Dejours, 1962). We used this technique to test whether peripheral chemoreflex function was normal in anti-VIP mice (Fig. 5A and B).
Figure 5. Ventilatory response to hyperoxia.
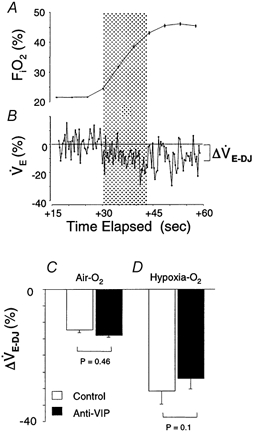
A shows the mean hyperoxic stimulus delivered during Dejours-type tests. Note that an O2 pulse at time = 0 caused a delayed rise in FI,O2 due to system dead space. B shows a sample breath-by-breath response; the response nadir (‘Dejours effect’, ΔV̇E-DJ) during the initial 20 s hyperoxia (shaded area) is shown approximately. C and D, comparison of mean ΔV̇E-DJ for hyperoxic tests administered in air and at end-hypoxia; there were no differences between groups (ANOVA).
We analysed 89/137 air-hyperoxia tests (39 anti-VIP vs. 50 control) and 8/43 hypoxia-hyperoxia tests (4 per group); the remaining tests were disturbed by MVTs or a sigh. Hyperoxia in background air provoked a comparable, 12–14 % fall in V̇E from both groups of mice (Fig. 5C), due principally to a fall in breathing rate (increased TTOT: +13 ± 15 vs.+13 ± 18 %, control vs. anti-VIP). Peripheral drive, whilst greater at end-hypoxia, was similar between groups (Fig. 5D), due to a comparable fall in VT (−12 ± 15 vs. −10 ± 16 %, P = 0.78) and rate (TTOT: +39 ± 51 vs.+25 ± 23 %, P = 0.12). These data suggest that peripheral chemoreflex function was intact in anti-VIP mice.
Normal ventilatory responsiveness in anti-VIP mice
Arousal is normally closely coupled to the increased rate and depth of breathing provoked by hypoxia (Gleeson et al. 1990). Here we determined whether the arousal deficit in anti-VIP mice was secondary to an attenuted HVR. Time-dependent changes in V̇E were influenced by the vigour of arousal (i.e. SEF) during hypoxia, but the effects were comparable for control and anti-VIP mice (P = 0.11; Fig. 6). Whilst V̇E plateaued once FI,O2 stabilized in ‘quiet’ mice (Fig. 6A and C), a progressive hyperpnoea was observed in ‘agitated’ mice (Fig. 6B and D). V̇E at end-hypoxia was consequently greater in ‘agitated’ mice, regardless of treatment group (V̇E = +128 ± 46 vs.+81 ± 25 %, ‘agitated’vs.‘quiet’ mice, P = 0.0004; Fig. 6C and D). MVT profiles of ‘quiet’ and ‘agitated’ mice demonstrate convincingly that sleep prevailed during the former tests, and arousal in the latter (Fig. 6A and B). These data illustrate that in mice, as in other species, the HVR is attenuated during sleep, and augmented by arousal. Sleep during hypoxia was better maintained in anti-VIP mice: mean SEF was greater (83 ± 17 vs. 65 ± 20 % for control mice; P = 0.02), and a smaller proportion of these mice were classified as ‘agitated’ (7/21 anti-VIP vs. 16/22 control mice; P = 0.017). Gender was not a significant factor in any of these analyses.
Figure 6. Hypoxic ventilatory response (HVR).
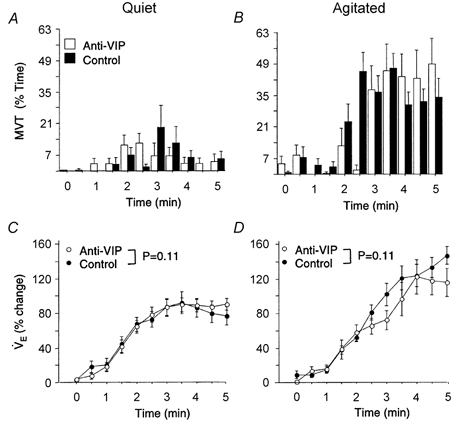
The effect of arousal on the HVR is shown by comparing MVT (A and B) and corresponding HVR (C and D) profiles of ‘quiet’ (sleep efficiency > 90 %) vs.‘agitated’ mice (sleep efficiency < 90 %). The plateau in HVR of quiet mice (coincident with the hypoxic nadir) suggests that sleep prevailed during these tests (C). Note the greater HVR of agitated mice (D). HVR profiles matched by sleep efficiency were comparable between groups (P = 0.11; ANOVA), indicating normal hypoxic responsiveness in anti-VIP mice.
DISCUSSION
VIP blockade attenuated arousal in adolescent mice, but did alter subcortical manifestations of hypoxic responsiveness. The deficit may be in the processing of sensory input by arousal (including cortical) mechanisms. Interrupting the actions of neurotrophic factors when neuronal connections are being refined may thus result in long-lasting ‘reprogramming’ of critical defence reflexes.
Absence of gross abnormalities at rest
A standard behavioural analysis of sleep indicated that, at rest, anti-VIP mice did not sleep poorly, nor was their sleep cycle obviously deranged or absent, as occurs with major brain lesions. This suggests that the neural substrates regulating sleep are basically intact in these animals. Other measures of brain function (e.g. the electroencephalogram) are needed to confirm this, and to establish whether VIP blockade alters the electrogenic maturation of the brain, or the fine-structure of the sleep cycle (Schaub et al. 1998).
Facilitation of movement is abnormal in anti-VIP mice
Our data reveal a fundamental change in the patterning of movements under stress following gestational VIP blockade. Movement is an important functional measure of arousal (Mograss et al. 1994). Specific movements (e.g. head-turning and neck flexion) aid recovery by improving airflow; afferent, sympathetic and endocrine activity provoked by movement also facilitates ongoing arousal (Gardner & Grossman, 1975; Harper et al. 2000). This contributes to the progressive escalation in arousal intensity (agitation, excitation) seen in control mice, and other mammals during hypoxia (Pappenheimer, 1977; Baker & McGinty, 1979). Arousal was less frequent and generally poorly sustained in anti-VIP mice, as shown by the predominance of ‘quiet’ behaviour. This may reflect a general reduction in the underlying periodicity of CNS excitation during sleep in these animals, and/or failure of afferent feedback to sustain and facilitate ongoing movement and arousal (Gardner & Grossman, 1975). We could not tell whether this was partly due to reduced quality (range, speed, extent of displacement) of movements in anti-VIP mice. Movement-evoked CNS excitation is a major mechanism promoting recovery from cardiorespiratory failure (Harper et al. 2000). Deficits in the patterning or propagation of movement during sleep may delay recovery and prolong hypoxia, increasing the risk of attendant CNS and ventilatory depression (Bissonnette, 2000).
Dissociation of cortical and sub-cortical responsiveness to hypoxic distress
Deficits in arousal are often associated with insensitivity to hypoxia, indicating that the hypoxic sensors or brainstem respiratory centres are damaged (Hunt et al. 1981; Filiano & Kinney, 1992). There was no evidence of this in anti-VIP mice. Hyperoxic responses, a widely used indirect test of carotid body function (Katz-Salamon & Lagercrantz, 1994; Kline et al. 1998), and the HVR and the hypoxia-provoked increase in sighs - which reflects brainstem patterning of efferent drive (Cherniack et al. 1981; Cohen & Henderson-Smart, 1996) - were all appropriate in anti-VIP mice. Thus, although VIP is co-located in these structures (Kusakabe et al. 1998; Poncet et al. 1994), functional responsiveness to hypoxia appeared to be intact following late-gestation VIP blockade. The HVR does not unequivocally demonstrate normal hypoxic sensitivity, since systemic adjustments which affect the V̇E-Pa,O2 relationship, e.g. hypoxic hypometabolism, may be different in anti-VIP mice (Frappell et al. 1992). Sex hormones are also known to influence hypoxic responsiveness (Mortola & Saiki, 1996), but we found no significant gender differences between HVRs or arousal responses in these mice during sleep.
Although the carotid bodies are vital in initiating the changes which ultimately provoke arousal (Bowes et al. 1981; Ryan et al. 1983), it is the hyperpnoea (i.e. increased rate and depth of breathing) provoked by hypoxia which ultimately triggers arousal (Gleeson et al. 1990). This indicates that peripheral sensory input (e.g. from effort and load receptors in the airway, chest and lungs) is a critical component of the arousal trigger. We observed an uncoupling between effort (normal HVR) and arousal (delayed and depressed) in anti-VIP mice, suggesting that peripheral sensatory input, or the CNS response to peripheral input, was attenuated. Cortical lesions have previously been described in these mice (Zupan et al. 2000). This, together with the absence of deranged chemoreflexes, suggests that higher order processing deficits may partially underpin the arousal deficit observed in these mice. Since VIP is normally present throughout the body - including the cells and nerves of the respiratory system (Verastegui et al. 1997) - it is conceivable that VIP blockade could also attenuate arousability by altering, in some way, the flow of peripheral sensory input.
A possible cellular basis for the arousal deficit
Cortical damage sustained following VIP blockade may provide clues to the origin of the associated arousal deficit (Zupan et al. 2000). Brainstem centres suppress sleep and dramatically enhance forebrain activity (arousal) by modulating the release of various neurotransmitters. By altering neuronal ion conductances and membrane potentials, this facilitates synaptic transmission and excitability (Steriade et al. 1993). Altered neuronal connectivity, ion channel structure, or expression of neuronal receptors following antenatal VIP blockade may alter the sequence or timing of these events. This may impair excitatory transmission, or enhance inhibitory gating of input, delaying the transition from sleep to arousal (Steriade et al. 1993). Specific abnormalities in anti-VIP mice, e.g. altered N-methyl-d-aspartate (NMDA) receptor expression, may impair the potentiation and propagation of excitatory input by cortical circuits (Steriade & McCarley, 1990). Damage to intrinsic peptidergic expression or regulation may also affect cortical excitability (Bayraktar et al. 2000). Cortical damage may be a marker for more widespread damage affecting other nuclei and transmitter systems involved in arousal. This may include the carotid body and brainstem cardiorespiratory centres; changes in cytoarchitecture or endogenous VIP expression may occur in these structures following VIP blockade, even though functional deficits were not revealed by the testing protocol we used.
Developmental implications
Factors which delay awakening may be particularly serious when vulnerability to rapid and profound hypoxaemia is greatest, i.e. in newborn and developing organisms. Depression of arousal mechanisms delays recovery from spontaneous apnoea, exacerbating hypoxaemia. This may disturb growth and development, further exacerbate sleep-disordered breathing, and contribute to respiratory failure and sudden death during sleep (Hunt, 1989). Our data suggest that one way this may occur is via antenatal ‘reprogramming’ of the underlying neural substrates. Here we studied only adolescent mice. Longitudinal studies using this model may establish whether significant deficits are also present at earlier, more vulnerable stages of development, as well as the extent to which underlying deficits of this sort increase the susceptibility to external modifiers of arousability.
Acknowledgments
This work was supported by INSERM and the National Health and Medical Research Council of Australia.
REFERENCES
- Baker TL, McGinty DJ. Sleep-waking patterns in hypoxic kittens. Developmental Psychobiology. 1979;12:561–575. doi: 10.1002/dev.420120606. [DOI] [PubMed] [Google Scholar]
- Barker DJ. In utero programming of chronic disease. Clinical Science. 1998;95:115–128. [PubMed] [Google Scholar]
- Bayraktar T, Welker E, Freund TF, Zilles K, Staiger JF. Neurons immunoreactive for vasoactive intestinal polypeptide in the rat primary somatosensory cortex:morphology and spatial relationship to barrel-related columns. Journal of Comparative Neurology. 2000;420:291–304. [PubMed] [Google Scholar]
- Berry RB, Gleeson K. Respiratory arousal from sleep: mechanisms and significance. Sleep. 1997;20:654–675. doi: 10.1093/sleep/20.8.654. [DOI] [PubMed] [Google Scholar]
- Bissonnette JM. Mechanisms regulating hypoxic respiratory depression during fetal and postnatal life. American Journal of Physiology - Regulatory, Integrative and Comparative Physiology. 2000;278:R1391–1400. doi: 10.1152/ajpregu.2000.278.6.R1391. [DOI] [PubMed] [Google Scholar]
- Bowes G, Townsend ER, Kozar LF, Bromley SM, Phillipson EA. Effect of carotid body denervation on arousal response to hypoxia in sleeping dogs. Journal of Applied Physiology. 1981;51:40–45. doi: 10.1152/jappl.1981.51.1.40. [DOI] [PubMed] [Google Scholar]
- Cassin S. Critical oxygen tensions in newborn, young and adult mice. American Journal of Physiology. 1963;205:325–330. doi: 10.1152/ajplegacy.1963.205.2.325. [DOI] [PubMed] [Google Scholar]
- Cherniack NS, Von Euler C, Glogowska M, Honma I. Characteristics and rate of occurrence of spontaneous and provoked augmented breaths. Acta Physiologica Scandinavica. 1981;111:349–360. doi: 10.1111/j.1748-1716.1981.tb06747.x. [DOI] [PubMed] [Google Scholar]
- Cohen G, Henderson-Smart DJ. The characteristics and frequency of augmented breaths during CO2-induced hyperpnoea of newborn infants. Journal of Physiology. 1996;490:551–557. doi: 10.1113/jphysiol.1996.sp021166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dauger S, Nsegebe E, Vardon G, Gaultier C, Gallego J. The effects of restraint on ventilatory responses to hypercapnia and hypoxia in adult mice. Respiration Physiology. 1998;112:215–225. doi: 10.1016/s0034-5687(98)00027-9. [DOI] [PubMed] [Google Scholar]
- Dejours P. Chemoreceptors in breathing. Physiological Reviews. 1962;42:335–358. doi: 10.1152/physrev.1962.42.3.335. [DOI] [PubMed] [Google Scholar]
- Fewell JE, Konduri GG. Influence of repeated exposure to rapidly developing hypoxaemia on the arousal and cardiopulmonary response to rapidly developing hypoxaemia in lambs. Journal of Developmental Physiology. 1989;11:77–82. [PubMed] [Google Scholar]
- Filiano JJ, Kinney HC. Arcuate nucleus hypoplasia in the sudden infant death syndrome. Journal of Neuropathology and Experimental Neurology. 1992;51:394–403. doi: 10.1097/00005072-199207000-00002. [DOI] [PubMed] [Google Scholar]
- Frappell P, Lanthier C, Baudinette RV, Mortola JP. Metabolism and ventilation in acute hypoxia: a comparative analysis in small mammalian species. American Journal of Physiology. 1992;262:R1040–1046. doi: 10.1152/ajpregu.1992.262.6.R1040. [DOI] [PubMed] [Google Scholar]
- Gardner R, Grossman WI. Normal motor patterns in sleep in man. In: Weitzman ED, editor. Advances in Sleep Research. New York: Spectrum Publications; 1975. pp. 67–69. [Google Scholar]
- Gleeson K, Zwillich CW, White DP. The influence of increasing ventilatory effort on arousal from sleep. American Review of Respiratory Disease. 1990;142:295–300. doi: 10.1164/ajrccm/142.2.295. [DOI] [PubMed] [Google Scholar]
- Gozes I, McCune SK, Jacobson L, Warren D, Moody TW, Fridkin M, Brenneman DE. An antagonist to vasoactive intestinal polypeptide affects cellular functions in the central nervous system. Journal of Pharmacology and Experimental Therapeutics. 1991;257:959–966. [PubMed] [Google Scholar]
- Gramsbergen A, Schwartze P, Prechtl HF. The postnatal development of behavioral states in the rat. Developmental Psychobiology. 1970;3:267–280. doi: 10.1002/dev.420030407. [DOI] [PubMed] [Google Scholar]
- Hafström O, Milerad J, Asokan N, Poole HSD, Sundell HW. Nicotine delays arousal during hypoxemia in lambs. Pediatric Research. 2000;47:646–652. doi: 10.1203/00006450-200005000-00015. [DOI] [PubMed] [Google Scholar]
- Harper RM, Woo MA, Alger JR. Visualization of sleep influences on cerebeller and brainstem cardiac and respiratory control mechanisms. Brain Research Bulletin. 2000;53:125–131. doi: 10.1016/s0361-9230(00)00317-8. [DOI] [PubMed] [Google Scholar]
- Hunt CE. Impaired arousal from sleep: relationship to sudden infant death syndrome. Journal of Perinatology. 1989;9:184–187. [PubMed] [Google Scholar]
- Hunt CE, McCulloch K, Brouillette RT. Diminished hypoxic ventilatory response in near-miss sudden infant death syndrome. Journal of Applied Physiology. 1981;50:1313–1317. doi: 10.1152/jappl.1981.50.6.1313. [DOI] [PubMed] [Google Scholar]
- Katz-Salamon M, Lagercrantz H. Hypoxic ventilatory defence in very preterm infants: attenuation after long-term oxygen treatment. Archives of Disease in Childhood. 1994;70:F90–95. doi: 10.1136/fn.70.2.f90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kline DD, Yang T, Huang PL, Prabhakar NR. Altered respiratory responses to hypoxia in mutant mice deficient in neuronal nitric oxide synthase. Journal of Physiology. 1998;511:273–287. doi: 10.1111/j.1469-7793.1998.273bi.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kusakabe T, Hayashida Y, Matsuda H, Gono Y, Powell FL, Ellisman MH, Kawakami T, Takenaka T. Hypoxic adaptation of the peptidergic innervation in the rat carotid body. Brain Research. 1998;806:165–174. doi: 10.1016/s0006-8993(98)00742-2. [DOI] [PubMed] [Google Scholar]
- Mograss MA, Ducharme FM, Brouillette RT. Movement/arousals. Description, classification, and relationship to sleep apnea in children. American Review of Respiratory and Critical Care Medicine. 1994;150:1690–1696. doi: 10.1164/ajrccm.150.6.7952634. [DOI] [PubMed] [Google Scholar]
- Mortola JP, Saiki C. Ventilatory response to hypoxia in rats: gender differences. Respiration Physiology. 1996;106:21–34. doi: 10.1016/0034-5687(96)00064-3. [DOI] [PubMed] [Google Scholar]
- Pappenheimer JR. Sleep and respiration of rats during hypoxia. Journal of Physiology. 1977;266:191–207. doi: 10.1113/jphysiol.1977.sp011763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paspalas CD, Papadopoulos GC. Noradrenergic innervation of peptidergic interneurons in the rat visual cortex. Cerebral Cortex. 1999;9:844–853. doi: 10.1093/cercor/9.8.844. [DOI] [PubMed] [Google Scholar]
- Pepelko WE, Dixon GA. Arterial blood gases in conscious rats exposed to hypoxia, hypercapnia, or both. Journal of Applied Physiology. 1975;38:581–587. doi: 10.1152/jappl.1975.38.4.581. [DOI] [PubMed] [Google Scholar]
- Phillipson EA, Sullivan CE. Arousal: the forgotton response to respiratory stimuli. American Review of Respiratory Disease. 1978;118:807–809. doi: 10.1164/arrd.1978.118.5.807. [DOI] [PubMed] [Google Scholar]
- Poncet L, Denoroy L, Dalmaz Y, Péquignot JM, Jouvet M. Chronic hypoxia affects peripheral and central vasoactive intestinal peptide-like immunoreactivity in the rat. Neuroscience Letters. 1994;176:1–4. doi: 10.1016/0304-3940(94)90856-7. [DOI] [PubMed] [Google Scholar]
- Ryan AT, Ward DA, Megirian D. Sleep-waking patterns of intact and carotid sinus nerve-transected rats during hypoxic CO2 breathing. Experimental Neurology. 1983;80:337–348. doi: 10.1016/0014-4886(83)90287-x. [DOI] [PubMed] [Google Scholar]
- Schaub CD, Tankersley C, Schwartz AR, Smith PL, Robotham JL, O'Donnell CP. Effect of sleep/wake state on arterial blood pressure in genetically identical mice. Journal of Applied Physiology. 1998;85:366–371. doi: 10.1152/jappl.1998.85.1.366. [DOI] [PubMed] [Google Scholar]
- Steriade M, McCarley RW. Brainstem Control of Wakefulness and Sleep. New York: Plenum Press; 1990. pp. 163–201. [Google Scholar]
- Steriade M, McCormick DA, Sejnowski TJ. Thalamocortical oscillations in the sleeping and aroused brain. Science. 1993;262:679–685. doi: 10.1126/science.8235588. [DOI] [PubMed] [Google Scholar]
- Sullivan CE. Breathing in sleep. In: Orem J, Barnes D, editors. Physiology in Sleep. New York: Academic Press; 1980. pp. 213–271. [Google Scholar]
- Verastegui C, Fernandez-Vivero J, Prada A, Rodriguez F, Romero A, Gonzalez-moreno M, De Castro JM. Presence and distribution of 5HT, VIP, NPY, and SP-immunoreactive structures in adult mouse lung. Histology and Histopathology. 1997;12:909–918. [PubMed] [Google Scholar]
- Yardley CP, Hilton SM. The hypothalamic and brainstem areas from which the cardiovascular and behavioral components of the defence reaction are elicited in the rat. Journal of the Autonomic Nervous System. 1986;15:227–244. doi: 10.1016/0165-1838(86)90066-4. [DOI] [PubMed] [Google Scholar]
- Zupan V, Nehlig A, Evrard P, Gressens P. Prenatal blockade of vasoactive intestinal polypeptide alters cell death and synaptic equipment in the murine neocortex. Pediatric Research. 2000;47:53–63. doi: 10.1203/00006450-200001000-00012. [DOI] [PubMed] [Google Scholar]


