Abstract
Rhythm generation in mature respiratory networks is influenced strongly by synaptic inhibition. In early neonates, GABAA-receptor- and glycine-receptor-mediated inhibition is not present, thus the question arises as to whether GABAB-receptor-mediated inhibition plays an important role. Using brainstem slices of neonatal mice (postnatal day, P0-P15), we analysed the role of GABAB-mediated modulation of GABA and glycine synaptic transmission in the respiratory network. Blockade of GABA uptake by nipecotic acid (0.25–2 mm) reduced the respiratory frequency. This reduction was prevented by the selective GABAB receptor antagonist CGP55845A (CGP) alone at P0-P3, but by bicuculline as well as CGP at P7-P15. Blockade of GABAB receptors by CGP increased the respiratory frequency at P0-P3, whereas it caused a reduction of frequency in older animals. The effect of CGP on respiratory frequency was diminished in the presence of bicuculline and strychnine in older but not in younger animals. The relative contribution of GABAB-receptor-mediated pre- and postsynaptic modulation was examined by analysing the effect of GABAB receptors on spontaneous and miniature IPSCs. In younger animals (P0-P3), the GABAB receptor agonist baclofen had no detectable effect on IPSC frequency, but caused a significant decrease in the amplitude. In older animals (P7-P15), baclofen decreased both the frequency and amplitude of spontaneous and miniature IPSCs. These results demonstrate that GABAB-receptor-mediated postsynaptic modulation plays an important role in the respiratory network from P0 on. GABAB-receptor-mediated presynaptic modulation develops with a longer postnatal latency, and becomes predominant within the first postnatal week.
In the respiratory and locomotor circuits of adult animals, rhythmic motor patterns result from alternating membrane depolarisation and hyperpolarisation. Depolarisation results from activation of AMPA/kainate and N-methyl-d-aspartate (NMDA) receptors, whereas periodic membrane hyperpolarisation occurs in association with the activation of postsynaptic GABAA and glycine receptors and the influx of chloride ions through ligand-gated ion channels (Grillner et al. 1995; Marder & Calabrese, 1996; Richter et al. 1999). Rhythmically active neurones in several regions of the adult medullary respiratory network, including the pre-Bötzinger complex (PBC), exhibit periodic chloride-dependent, GABAA-receptor- and glycine-receptor-activated membrane hyperpolarisation (Richter et al. 2000). Functional GABAA receptors as well as glycine receptors in the adult PBC may be essential for respiratory rhythmogenesis, because microinjections of bicuculline and strychnine in this region abolish rhythm in pentobarbital-anaesthetised cats (Pierrefiche et al. 1998).
The synaptic processes responsible for rhythm generation in immature respiratory networks have not been studied as extensively as in the adult, and at present not a great deal is known about how rhythmogenesis evolves and matures during postnatal development (Sillar et al. 1992; Ballanyi et al. 1999; Zhang et al. 1999). The PBC is essential for respiratory rhythmogenesis in neonatal rats (Smith et al. 1991), as it is in adult animals. From this complex, axons project to other respiratory-related regions of the medulla (Ellenberger & Feldman, 1990; Ellenberger et al. 1990). In addition, GABAA and glycine receptors are present in the brainstem and spinal cord of neonatal animals. However, blockade of both GABAA and glycine receptors in the in vitro brainstem preparation of the neonatal rodent containing the PBC does not significantly affect rhythmic burst discharges, but rather the treatment leads to additional interburst firing (Feldman & Smith, 1989; Onimaru et al. 1990; Ramirez et al. 1996; Rekling & Feldman, 1998; Ritter & Zhang, 2000). Also, a recent investigation in this laboratory showed that the equilibrium potential of chloride ions in PBC neurones of mice at postnatal days P0-P4 is more depolarising than the resting membrane potential, and that glycine-receptor- and GABAA-receptor-mediated inhibition is not present (Ritter & Zhang, 2000). Towards the end of the first postnatal week, at a time when functional chloride-mediated inhibition appears, blockade of GABAA and glycine receptors abolishes the respiratory rhythm and evokes seizure-like activity (Brockhaus & Ballanyi, 1998; Ritter & Zhang, 2000). These findings indicate that receptors other than GABAA and glycine receptors are involved in respiratory rhythm generation from birth to P4. At later stages of development, the latter assume a predominant role in rhythmogenesis.
GABAB receptors mediate pre- and postsynaptic inhibition by decreasing membrane calcium conductance and increasing potassium conductance (Misgeld et al. 1995). There is a general consensus that GABAB receptors are functional in the respiratory networks of adult animals (Lalley, 1986; Livingston & Berger, 1989; Lipski et al. 1990; Pierrefiche et al. 1993; Hey et al. 1995). However, reports to date have not shown clearly that GABAB receptors play a significant role in respiratory rhythm generation in neonatal animals. In the present study, we investigated whether GABAB-receptor-mediated pre- and postsynaptic modulation is a major factor in respiratory rhythmogenesis during the first postnatal days of neonatal mice. The issues we address in the present investigation were: the relative importance of GABAB receptors in the modulation of respiratory rhythm during the first few postnatal days, and the relative contribution of GABAB-receptor-mediated pre- and postsynaptic modulation during postnatal development in the respiratory network.
Parts of this study have been presented previously in abstract form (Zhang et al. 1999).
METHODS
Preparation and general procedures
The procedures for preparing rhythmically active brainstem slices have been described in detail previously (Zhang et al. 1999). Male or female mice (NMRI mice: P0-P15) were deeply anaesthetised with ether, then the brain and upper cervical spinal cord were isolated in ice-cold artificial cerebrospinal fluid (ACSF) bubbled with Carbogen (95 % O2 and 5 % CO2). After removing the cerebellum and forebrain, coronal sections of the brainstem were cut with a slicer in a rostral-to-caudal direction until the nucleus ambiguus and inferior olive were seen at the rostral boundary of the PBC. Thereafter, a single slice (700 μm) containing the PBC, hypoglossal motor nucleus (NXII) and NXII nerve rootlets was cut, immediately transferred into a recording chamber and submerged under a stream of ACSF (28–30 °C; flow rate 10–15 ml min−1). After a stabilisation period of 15 min, the concentration of potassium in the ACSF was raised from 3 mm to 8 mm over a period of 20 min (Smith et al. 1991).
All experimental procedures were in accordance with the ethics committee guideline of Bezirksregierung Braunschweig.
Recording and data analysis
Rhythmic inspiratory phase discharges in the motor output of the brainstem respiratory network were monitored from NXII nerve rootlets with a suction electrode (Ramirez et al. 1996). Raw nerve discharge was amplified (20 000 ×) and filtered (high pass, 1.5 kHz; low pass, 250 Hz) for recording. The activity was rectified, low-pass filtered and integrated (Paynter filter, time constant τ = 20–30 ms) to obtain moving averages of burst activity (∫NXII).
Whole-cell recordings were obtained from the somata of PBC inspiratory neurones located close to the surface of the slice (about 20–50 μm). Recording electrodes (tip size ∼ 2 μm, resistance 4–6 MΩ) were prepared by pulling borosilicate glass micropipettes (GC150–10F, Clark Electromedical Instruments, UK) on a multistage puller (P87, Sutter, USA). The junction potential of the patch electrodes was on average 2.1 ± 0.34 mV and was corrected before approaching neurones. PBC neurones were patched with the aid of a microscope (Axioscope, Zeiss, Germany) equipped with an infrared contrast enhancement system (C2400, Hamamatsu Photonics, Enfield, Middlesex, UK). Patch electrodes were connected to an Axopatch 200 amplifier (Axon Instruments, USA). After the electrodes contacted neurones, seal resistances of up to 2 GΩ were typically formed. Once a giga-seal was established, the whole-cell configuration was obtained by gentle suction. At least 80 % of the serial resistance was compensated. Generally, no significant age-related differences in series resistance or leakage currents were observed. The signals were filtered by a four-pole Bessel filter set at a corner frequency of 2 kHz and digitised at a sampling rate of 5 kHz using a DigiData 1200 interface (Axon Instruments). Neurones within the PBC showing rhythmic burst activity that was temporally related to the discharge of the NXII rootlet are referred to as inspiratory PBC neurones (cf. Fig. 4), while neurones within the PBC in the vicinity of the inspiratory neurones but that exhibited no synchronised rhythmic activity are referred to as unidentified PBC neurones. The rhythmic bursting activity of inspiratory neurones was recorded in current-clamp mode (Fig. 4).
Figure 4. Effect of GABAB receptor blockade on the membrane potential and discharge properties of inspiratory neurones at different postnatal ages.
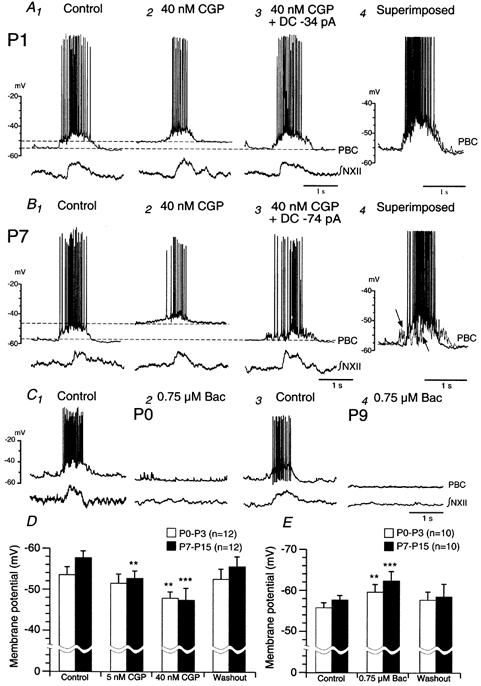
A and B, sample recordings in current-clamp mode (pre-Bötzinger complex, PBC) illustrating the effect of CGP on the membrane potential and discharge properties of inspiratory PBC neurones in P1 (A) and P7 (B) slice preparations. Lower traces are integrals of rhythmic discharges from NXII (∫NXII). Recordings show depolarisation of the membrane potential and shortening of burst duration elicited by CGP. Injections of hyperpolarising DC current (A3, B3) reversed the effects on membrane potential and burst duration. In the P7 slice, injections of hyperpolarising DC current did not reverse the reduction in the depolarising synaptic drive potential (arrows in B4). Traces A1, A3 and B1, B3 are superimposed in A4 and B4, respectively. C, sample recordings obtained in current-clamp mode illustrating that baclofen (0.75 μm) hyperpolarises the membrane potential and abolishes rhythmic bursting in inspiratory neurones in P0 (C1,2) and P9 (C3,4) slice preparations. D, bar graph summarising the effects of CGP on membrane potential in inspiratory PBC neurones from P0-P3 (open bars, n = 12) and P7-P15 (filled bars, n = 12) slices. E, bar graph summarising the effects of baclofen on membrane potential in inspiratory PBC neurones from P0-P3 (open bars, n = 10) and P7-P15 (filled bars, n = 10) slice preparations.
Experimental protocol
GABAB-receptor-mediated effects on spontaneous and miniature IPSCs (sIPSCs and mIPSCs, respectively) were tested in the presence of the non-NMDA-receptor blocker 6-cyano-7-nitroquinoxaline-2,3-dione (CNQX; 20 μm) and the NMDA-receptor blocker d-2-amino-5-phosphonopentanoate (AP5; 20 μm) in voltage-clamp mode (holding potential −70 mV, Figs 5–6). In all experiments testing IPSCs, the chloride reversal potential was close to 0 mV (cf. below). Therefore we observed all chloride-mediated IPSCs as inward currents (Figs 5–6). For testing whether the tested IPSCs were GABAA-receptor- and glycine-receptor-mediated, bicuculline (2 μm) and strychnine (2 μm) were added to the solution at the end of some experiments (cf. Results). Both sIPSCs and mIPSCs were recorded for 3 min, during which up to 300 events were collected. To monitor changes in input resistance, current responses to 10 mV voltage steps (20 ms) from a holding potential of −70 mV were recorded before every stimuli.
Figure 5. Effects of baclofen on the interval and amplitude of spontaneous IPSCs (sIPSCs) of inspiratory and unidentified PBC neurones at different postnatal stages.
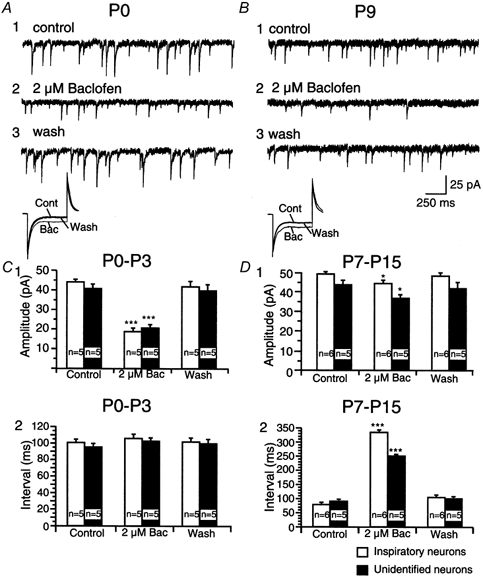
A and B, voltage-clamp recordings of sIPSCs of an inspiratory PBC neurone in the presence of 6-cyano-7-nitroquinoxaline-2,3-dione (CNQX) and d-2-amino-5-phosphonopentanoate (AP5) at P1 (A) and P9 (B), before application (1), in the presence (2) and after application of baclofen (2 μm, 3). The holding potential was −70 mV. Insets in A and B show changes in the input resistance of corresponding neurones. C and D, bar graphs summarising the effect of baclofen (Bac) on the amplitudes (1) and interval (2) of sIPSCs of inspiratory (open bars) and unidentified (filled bars) neurones in P0-P3 (C) and P7-P15 (D) slice preparations. The n values denote the number of animals tested.
Figure 6. Effects of baclofen on the interval and amplitude of miniature IPSCs (mIPSCs) at different postnatal stages.
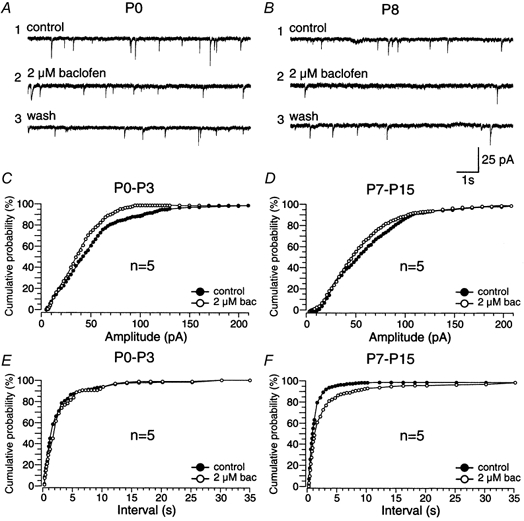
A and B, voltage-clamp recordings of mIPSCs of inspiratory PBC neurones in the presence of CNQX (20 μm), AP5 (20 μm) and TTX (1 μm) at P0 (A) and P8 (B) before (1), in the presence (2) and after application of baclofen (2 μm, 3). The holding potential was −70 mV. C and D, cumulative amplitude histograms before and after the application of baclofen in P0-P3 (P≤ 0.01, n = 5) and P7-P15 (P≤ 0.05, n = 5) slices. E and F, cumulative interval histograms before and after the application of baclofen in P0-P3 (n.s., n = 5) and P7-P15 (P≤ 0.01, n = 5) slice preparations.
Solution and drugs
Experiments were carried out in an ACSF containing (mm): 118 NaCl, 8 KCl, 1.5 CaCl2, 1 MgCl2, 25 NaHCO3, 1 NaH2PO4, 5 glucose equilibrated with Carbogen at 27–29 °C (pH 7.4). Patch electrodes were filled with a solution containing (mm): 140 KCl, 1 CaCl2, 10 EGTA, 2 MgCl2, 4 Na3ATP, 0.5 Na3GTP, 10 Hepes (adjusted to pH 7.3 with KOH) for IPSC experiments (Fig. 5 and Fig. 6), while potassium gluconate was used for measuring inspiratory burst activity (Fig. 4). (±)-Baclofen (RBI, UK) was prepared as a stock solution and added to the bath solution to achieve the desired concentration (0.5–10 μm). To block GABA uptake, nipecotic acid (0.25–2 mm, Tocris) was added to the bath solution. CGP55845A (5–40 nm) was kindly donated by Novartis (Basel, Switzerland). In the following text, CGP 55845A is referred to as CGP. All other chemicals were purchased from Sigma (USA).
Analysis and statistics
Statistical data are expressed as means ±s.e.m.The statistical significance of differences between averaged rhythmic frequency before and after drug applications was assessed with paired Student t tests (InStat, GraphPad Software, USA). The threshold concentration of a drug is defined as the concentration at which the drug-induced change is more than 5 % and the effect is statistically significant. The statistical significance of sIPSCs and mIPSCs before and after drug applications was tested in each experiment with the Kolmogorov-Smirnow test (MiniAnalysis, Synaptosoft, USA, cf. also Ropert et al. 1990). The level of stastistical significance was set at P≤ 0.05. (*P≤ 0.05, **P≤ 0.01, ***P≤ 0.001, n.s. = not significant). The significance of any change was tested between the control and the first drug application, between two consecutive drug applications, or between the last drug application and washout.
RESULTS
Endogenously released GABA slows respiratory burst activity by activating mainly GABAB receptors in younger neonates, while it activates GABAA as well as GABAB receptors in older ones
To examine the functional roles of endogenously activated GABAA and GABAB receptors in modulating respiratory rhythmic activity, we measured NXII inspiratory discharges after increasing GABA concentrations at synaptic sites with the GABA uptake inhibitor nipecotic acid (Krogsgaard-Larsen, 1980). Five concentrations of nipecotic acid (0.25, 0.5, 0.75, 1 and 2 mm) were tested and the measurements were made after 10 min of incubation for each concentration. Thereafter, the GABAA and GABAB receptors were blocked with bicuculline (2 μm) and CGP (40 nm), respectively.
Elevating endogenous GABA levels with nipecotic acid depressed the respiratory burst frequency at all ages. The degree of depression was concentration-dependent, however it was comparatively greater in preparations from more mature neonates (P7-P15, Fig. 1). In P0-P3 preparations (n = 12, Fig. 1C), the threshold concentration was 0.5 mm nipecotic acid. Burst discharge frequency was reduced to 70.1 ± 3.32 % of control levels at 0.5 mm, 60.7 ± 3.03 % at 0.75 mm, 50.4 ± 2.63 % at 1 mm and 25.6 ± 3.32 % at 2 mm.
Figure 1. Depression of rhythmic activity by endogenous GABA is reversed by GABAB receptor blockade in younger neonates, and by GABAA and GABAB receptor blockade in older ones.
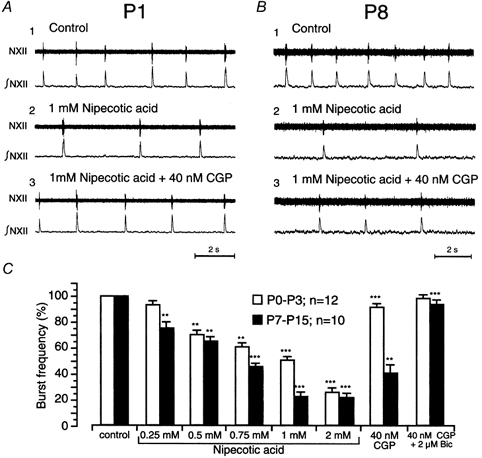
A and B, recordings of raw (hypoglossal motor nucleus, NXII) and integrated (∫NXII) rhythmic activity in slices from postnatal day (P)1 and P8 slices under control conditions (A1), in the presence of nipecotic acid (1 mm; A2) and additional CGP55845A (CGP, 40 nm) (A3). C, bar graph summarising the concentration-dependent depression of rhythmic activity elicited by nipecotic acid, and its reversibility in the presence of CGP and bicuculline (Bic) in younger (P0-P3, n = 12) and older (P7-P15, n = 10) slice preparations. In this and other graphs, the error bars represent s.e.m. The n-values indicate number of animals tested and the asterisks denote the level of statistical significance: *P≤ 0.05, **P≤ 0.01, ***P≤ 0.001.
In P7-P15 preparations (n = 10, Fig. 1C), the threshold concentration was 0.25 mm nipecotic acid. Burst discharge frequency was reduced to 75 ± 4.74 % of control levels at 0.25 mm, 65 ± 3.16 % at 0.5 mm, 45.4 ± 2.47 % at 0.75 mm, 22.3 ± 3.2 % at 1 mm and 20.8 ± 2.56 % at 2 mm. Nipecotic acid never abolished rhythmic activity, even when the slices were incubated in 2 mm for 120 min (n = 15, P0-P10, data not shown).
In P0-P3 preparations, blockade of GABAB receptors by 40 nm CGP restored burst frequency from 25.6 ± 3.32 % (2 mm nipecotic acid) to 91.6 ± 2.6 % of control values (P≤ 0.001, n = 12, Fig. 1A, C). The GABAA receptor antagonist bicuculline (2 μm) produced only a small, statistically insignificant increase in frequency when applied after CGP (to 98.5 ± 2.6 %, Fig. 1C). This is also true if 2 μm bicuculline was applied in the presence of 5 nm CGP (P0-P3, n = 5, data not shown). Higher concentrations of bicuculline did not change the burst frequency, but evoked seizure-like burst activity (data not shown, cf. also Brockhaus et al. 1998; Ritter & Zhang 2000).
In older neonates (P7-P15), CGP was less effective in reversing the nipecotic-acid-induced depression, from 20.8 ± 2.56 % to 40.5 ± 6.32 % of control levels (Fig. 1B). However, bicuculline was appreciably more effective on P7–15 slices than on P0-P3 slices. The nipecotic-acid-induced depression of respiratory frequency remaining after CGP treatment was nearly abolished (discharge frequency restored to 93.8 ± 3.29 % of control; Fig. 1C).
These experiments indicate that at least two significant changes related to GABA-mediated modulation occur during postnatal maturation of the respiratory network: sensitivity to GABAergic inhibition increases, and there is a switching of the receptor-mediated inhibitory processes from predominately GABAB to GABAA and GABAB.
Burst frequency is depressed after blocking GABAB receptors in older neonatal preparations
The effects on rhythmic discharges of blocking GABAB receptors in the absence of other treatments were analysed in brainstem slices at different 9postnatal ages (P0-P15). With maturation, not only sensitivity but also the effect of GABAB receptor blockade with CGP changed (Fig. 2).
Figure 2. Effects of GABAB receptor blockade on burst frequency at different ages.
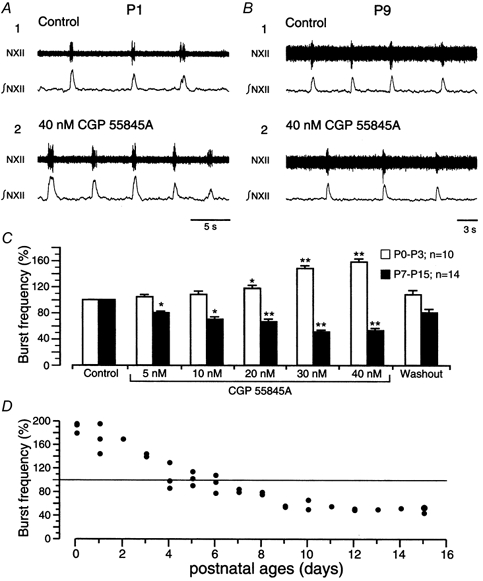
A and B, examples of age-dependent effects of CGP (40 nm) on burst discharge frequency in P1 (A) and P9 (B) slice preparations. C, bar graph summarising the concentration-dependent effects of CGP in P0-P3 (open bars, n = 10) and P7-P15 (filled bars, n = 14) slice preparations. The threshold concentration of CGP was 20 nm in P0-P3 preparations and 5 nm in P7-P15 preparations. D, plot showing reversal of the CGP (40 nm) effect from excitatory to depressant over days P4-P6. Each symbol represents one experiment carried out on one individual slice obtained from a mouse at the indicated age.
In slices from P0-P3 mice, 20–40 nm CGP increased the frequency of rhythmic NXII nerve discharges (n = 10, Figs 2A-D, 3C). In P4-P6 preparations, 40 nm CGP increased burst activity in three slices, whereas frequency decreased in five others (Fig. 2D), and in all P7-P15 preparations (n = 14) burst frequency was decreased by 5–40 nm CGP (Figs 2B-D, 3C). The switch of the effect of 40 nm CGP as a function of age is illustrated in Fig. 2D (each data point represents one individual experiment). Significant effects occurred within 2 min in all age groups. Recovery (≥ 75 %) was obtained within 10 min of washout (Fig. 2C).
Figure 3. Effect of blocking GABAB receptors on burst frequency during blockade of GABAA and glycine receptors.
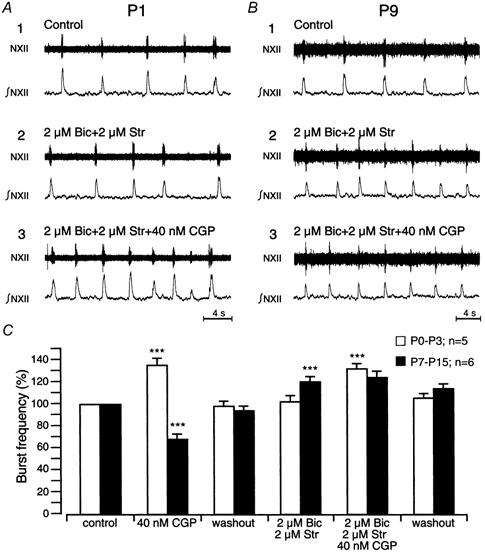
A and B, example of rhythmic discharges showing the effect of CGP (40 nm) in the presence of 5 μm bicuculline (Bic) and 5 μm strychnine (Str) in P1 (A) and P9 (B) slice preparations. C, bar graph summarising the effects of CGP (40 nm) in the presence of both drugs in P0-P3 (open bars, n = 5) and P7-P15 (filled bars, n = 6) slice preparations. Note that CGP alone had a contrasting effect on burst frequency (cf. Fig. 2).
CGP-induced depression of rhythmic burst discharges is prevented by blockade of GABAA and glycine receptors in older preparations
In P0-P3 preparations (n = 5), blocking GABAA and glycine receptors with bicuculline (2 μm) and strychnine (2 μm) had no significant effects (Fig. 3A, C). However, 40 nm CGP still increased respiratory burst frequency in the presence of bicuculline and strychnine (Fig. 3A, C).
In P7-P15 preparations (n = 6), bicuculline (2 μm) and strychnine (2 μm) significantly increased burst frequency (Fig. 3B, C) and blocked the effect of CGP (40 nm) on burst frequency (Fig. 3B, C). In all experiments, partial recovery of the drug effect was obtained within 10 min of washout in all slices (Fig. 3C).
Effects of GABAB receptor blockade on membrane potential and discharge properties of PBC neurones intensify with maturation
The effects of blocking GABAB receptors with CGP on the membrane potential and discharge properties of 24 inspiratory neurones in P0-P15 slices were analysed. Under control conditions, membrane potential ranged from −48 to −55 mV without significant difference at different postnatal ages (n = 24). In neurones of P0-P3 preparations (n = 12) 5 nm CGP had no significant effect on the membrane potential, whereas 40 nm CGP significantly depolarised the membrane potential (5.4 ± 0.35 mV, Fig. 4A, D) and reduced burst duration (20 ± 3.4 %), but did not significantly alter the amplitude of the depolarising potential (Fig. 4A).
Neurones of P7-P15 mice exhibited greater sensibility to CGP. Bath application of 5 nm CGP depolarised the membrane potential by 5.1 ± 0.45 mV, while 40 nm CGP depolarised the membrane potential by 9.8 ± 0.65 mV (Fig. 4D) and reduced the amplitude of depolarising potentials (n = 12, arrows in Fig. 4B).
Return of the membrane potential to control levels by applying hyperpolarising DC current largely reversed the reduction of burst duration by CGP in animals of all ages (Fig. 4A3, B3), but not the depression of the initial depolarising drive potential in the older neonates (arrow heads in Fig. 4B4).
In addition, 0.75 μm baclofen significantly hyperpolarised the membrane potential (3.6 ± 0.9 mV, Fig. 4E) and abolished the burst (Fig. 4C2) in P0-P3 slices, while it hyperpolarised the membrane potential by 5.1 ± 1.9 mV (Fig. 4E) and abolished the burst (Fig. 4C4) in P7-P15 slices. Thus, the present findings suggest that at least part of the GABAB-mediated effect is due to direct effects of post- or extrasynaptic GABAB receptors, or to tonic inhibition of the excitatory drive to the respiratory network.
GABAB effects on sIPSCs during postnatal development
The effect of the GABAB receptor agonist baclofen (2 μm) on sIPSCs were tested in 11 inspiratory neurones and in 10 unidentified PBC neurones located near to inspiratory neurones. sIPSCs were recorded after blocking glutamatergic EPSCs with 20 μm CNQX and 20 μm AP5, which also abolished rhythmic burst activity in individual neurones and in NXII nerve rootlets.
In P0-P3 inspiratory neurones, baclofen significantly decreased the amplitude of sIPSCs from 43.8 ± 3.0 pA to 18.7 ± 4.0 pA (P≤ 0.01, n = 5, Fig. 5C1) and decreased the input resistance without affecting the interval of sIPSCs (Fig. 5C2), the results for unidentified neurones being similar (Fig. 5C2). In P7-P15 inspiratory neurones, baclofen decreased the amplitude of sIPSCs from 48.7 ± 3.0 pA to 44.0 ± 4.0 pA (P≤ 0.05, n = 6, Fig. 5B, D1) and decreased the input resistance and increased the sIPSC intervals from 78.6 ± 4.47 ms to 334 ± 5.37 ms (n = 6, P≤ 0.01, Fig. 5B, D2). The results for unidentified neurones were similar (Fig. 5B, D2). The effects of baclofen were partially reversible after washout of at least 15 min. The effects of baclofen were blocked if the slice was pre-incubated with CGP for 3 min (n = 5, data not shown). In all experiments, the remaining sIPSCs could be blocked by bicuculline (2 μm) and strychnine (2 μm).
Thus, application of baclofen exerted an effect on the amplitude and input resistance of both bicuculline- and strychnine-sensitive sIPSCs, due to tonic activation of potassium conductance. No difference in the effect of baclofen on sIPSCs could be found between inspiratory and unidentified PBC neurones.
GABAB effects on mIPSCs during postnatal development
The effect of baclofen-induced GABAB receptor activation on mIPSCs were examined in 10 inspiratory and 12 unidentified PBC neurones in the presence of TTX (1 μm). In P0-P3 slices, baclofen (2 μm) reduced mIPSC amplitude in inspiratory neurones, as indicated by a shift to the left of the cumulative amplitude histogram (n = 5, P≤ 0.01, Fig. 6A, C). In contrast, there was no significant change in mIPSC interval (cumulative interval histogram, n = 5, n.s., Fig. 6E). In unidentified PBC neurones, baclofen (2 μm) also decreased the amplitude of mIPSCs (n = 4, P≤ 0.001; data not shown) and had no effect on the interval of mIPSCs (n = 4, n.s., data not shown).
In P7-P15 slices, baclofen (2 μm) increased the interval of mIPSCs in inspiratory neurones, as indicated by the shift of the cumulative interval histogram towards longer intervals (n = 5, P≤ 0.005, Fig. 6B, F), and reduced the mIPSC amplitude (n = 5, P≤ 0.05), as shown by a shift to the left of the cumulative amplitude histogram (Fig. 6D). In unidentified PBC neurones, baclofen (2 μm) decreased the amplitude and increased the interval of mIPSCs (n = 5, P≤ 0.001; data not shown). In all experiments, the remaining mIPSCs could be blocked by bicuculline (2 μm) and strychnine (2 μm).
Taken together, the results suggest that GABAB-receptor-mediated postsynaptic modulation is operational from the first postnatal day in the medullar respiratory network, whereas presynaptic GABAB-receptor-mediated modulation of GABA and/or glycine release becomes significant towards the end of the first postnatal week.
DISCUSSION
This investigation has revealed three major, temporally ordered changes in GABAergic inhibition during postnatal maturation of the respiratory network in neonatal mice. During the first postnatal days (P0-P3), GABAB receptors effectively modulate respiratory rhythm postsynaptically (i.e. through direct effects on inspiratory PBC neurones). Then, as maturation proceeds, GABAB receptors located at the presynaptic terminals of interneurones to inspiratory PBC neurones assume a more predominant role in shaping respiratory rhythmicity. The third change involves the emergence of GABAA-receptor- and glycine-receptor-mediated effects in older animals (P7-P15), which assume a major role in the inhibitory modulation of respiratory rhythm.
Early postnatal switching of functional inhibitory receptor predominance from GABAB receptors to GABAA and glycine receptors
We found in the present study that GABAergic inhibitory modulation of rhythmic generation is operational from the first postnatal day, and from P0-P3, function exclusively through activation of GABAB receptors (Figs 1–3). At this stage, functional depolarising GABAA and glycine receptors are present, although they seem to play no significant role in rhythm modulation (Figs 1–3; cf. also Ritter & Zhang, 2000). GABAB receptors continue to mediate the depression of respiratory rhythmic discharges in older animals (P7-P15, Fig. 2), but between P3 and P7, additional inhibition mediated by GABAA and glycine receptors become operational and are even more effective than GABAB receptors in depressing rhythmic activity in the in vitro brainstem preparation (Figs 1, 3). The functional switching of inhibitory receptor predominance from GABAB to GABAA and glycine receptors between P3 and P7 coincides with the postnatal appearance of chloride-mediated hyperpolarisation in the mouse respiratory network (Ritter & Zhang, 2000). In the PBC neurones of P0-P3 slices, selective activation of GABAA receptors with muscimol evokes chloride-mediated membrane depolarisation because the chloride reversal potential is more depolarising than the membrane potential (Ritter & Zhang, 2000). Such a postnatal maturational process seems to be species-dependent, since chloride-dependent depolarisation of respiratory neurones has not been reported in the neonatal cat (Lawson et al. 1992) or in the neonatal rat (Shao & Feldman, 1997; Brockhaus & Ballanyi, 1998). Our data are supported by other studies in which it has been shown that baclofen-induced activation of GABAB receptors in hippocampal neurones of neonatal rats elicits synaptic inhibition in the presence of chloride-mediated excitation (Cherubini et al. 1991; Gaiarsa et al. 1995).
Mechanisms underlying the GABAB-receptor-mediated modulation of respiratory rhythm
Activation of GABAB receptors is known to induce inwardly rectified potassium currents, which hyperpolarise the membrane potential towards the potassium equilibrium potential (Johnson et al. 1996; O'Callaghan et al. 1996). This would explain why in the present investigation, blockade of GABAB receptors by CGP depolarised the membrane potential, while baclofen hyperpolarised the membrane potential and abolished the burst activity of inspiratory neurones during ongoing rhythmic activity (Fig. 4). Fritschy et al. (1999) have shown that most of the GABAB receptors in rat are localised on extrasynaptic sites, and this is also true for mouse brainstem (B. Ritter & W. Zhang, unpublished observation). Bath application of baclofen (Figs 5, 6) and incubation with nipecotic acid (Fig. 1), thus, activates all GABAB receptors, including the extrasynaptic GABAB receptors, leading to an increase in whole-cell potassium conductance, which would shunt other synaptic PSCs. In addition, blockade of GABAB-receptor-mediated suppression of calcium-activated potassium conductance can explain why CGP shortened burst duration (Fig. 4).
In P0-P3 slice preparations, CGP increased the respiratory frequency (Fig. 3) in the absence of both GABAA-receptor- and glycine-receptor-mediated inhibition. We attribute these findings to GABAB-receptor-mediated, GABAA- and glycine-independent postsynaptic effects. In addition, these and the effects of CGP in more mature slices (Figs 1–3) point to tonic GABAB-mediated modulation by depressing excitatory synaptic transmission within the respiratory network of neonatal mice. Medullary respiratory neurones in the neonatal rat also seem to be subject to this type of inhibition (Brockhaus & Ballanyi, 1998).
Age-related changes in GABAB-receptor-mediated modulation of the neonatal respiratory network
It has been shown that in P0-P3 slices, tonic GABAB receptor-mediated postsynaptic hyperpolarisation of inspiratory neurones leads to a reduction in respiratory frequency (Johnson et al. 1996; Brockhaus & Ballanyi, 1998), whereas opposite effects were elicited by blockade of GABAB receptor through CGP (Fig. 2). The relevant GABAB receptors seem to have been on the postsynaptic membrane, because: (1) baclofen depressed sIPSC and mIPSC amplitudes without changing their frequency (Figs 5, 6), (2) baclofen decreased the input resistance of both inspiratory and unidentified neurones, indicating an increase in the membrane conductance of postsynaptic neurones (Fig. 5), and (3) activation of GABAB receptors only affects the L-type, not the N-type calcium currents in younger mice (P0-P3, Zhang et al. 1999), the latter being involved in transmitter release.
In more mature slices (P7-P15), CGP decreased burst frequency, while at the same time depolarising the membrane potential (Fig. 2, 4). Moreover, in the presence of bicuculline and strychnine, CGP had no significant effect on burst frequency (Fig. 3). We attribute these findings to blockade of presynaptic GABAB receptor-mediated modulation, which reduced the tonic release of GABA and/or glycine that caused an acceleration of the respiratory frequency (Fig. 2). This interpretation is supported because baclofen selectively depressed the frequency of both sIPSCs and mIPSCs (Figs 5, 6). This is also supported by earlier study in which it is reported that in PBC neurones of these more mature preparations, activation of GABAB receptors also decreases the N-type calcium currents (P5-P15, Zhang et al. 1999) involved in transmitter release.
It is well established that neurotransmitter release can be depressed by activation of presynaptic GABAB receptors. In particular, it has been demonstrated that GABA in the synaptic cleft also activates presynaptic GABAB receptors, which provides for a continuous regulation of GABA release (autoreceptor, see review by Misgeld et al. 1995). The present results demonstrate in a functional respiratory rhythm-generating network that such GABAB-receptor-mediated regulation plays a functional role in rhythm modulation in older animals, as the CGP effect on burst frequency was abolished in the presence of bicuculline and strychnine (Fig. 3).
Higher concentrations of the GABA uptake blocker nipecotic acid were required to depress rhythmic activity in younger neonates (P0-P3, cf. Fig. 1). A dilution of GABA in a larger extracellular space in immature tissue (Lehmenkuhler et al. 1993) could explain the low sensitivity to nipecotic acid. An additional factor may stem from the lower sensitivity and/or reactivity of GABAB receptors in the earlier stages of development. This is also consistent with our previous finding that the threshold for modulation of voltage-activated calcium currents by baclofen is higher in younger neonatal preparations (Zhang et al. 1999). The lower sensitivity in the early postnatal stage may be related to lower GABAB receptor binding affinity to GABA (Malitschek et al. 1998), or to the differential expression of different subtypes of the GABAB receptor (Fritschy et al. 1999; Mohler & Fritschy, 1999).
Differential ontogenesis of presynaptic and postsynaptic GABAB-receptor-mediated modulation
There are at least two possibilities that might account for the absence of functional GABAB-mediated presynaptic modulation in the respiratory neurones of P0-P3 slice preparations in the present investigation. First, a distinct subtype of GABAB receptor is absent. It was recently shown that expression of the splice variant GABAB1a is preponderant at birth, while the splice variant GABAB1b is predominant in the adult brain (Fritschy et al. 1999). However, whether the different variants have a preferred pre- or postsynaptic localisation is not yet known. A second explanation is that functional coupling between GABAB receptors and the N- and P/Q-type calcium channels is not yet adequately established (cf. discussions in Misgeld et al. 1995). This possibility is supported by an earlier study in this laboratory showing that in PBC neurones of P0-P3 slice preparations, activation of GABAB receptors had no effect on N-type calcium channels, but caused a reduction of L-type calcium channels (Zhang et al. 1999), even if L- and N-type calcium currents are present in respiratory neurones (Elsen & Ramirez, 1998.). The fact that this conclusion applies to both inspiratory and unidentified PBC neurones indicates that the delayed appearance of functional presynaptic GABAB receptors might be a general phenomenon in brainstem regions that are not limited to a certain type of respiratory neurone.
The appearance of functional pre- and postsynaptic GABAB-receptor-mediated inhibition depends upon the stage of postnatal maturation and varies among brain regions (Caillard et al. 1998). In the adult vertebrate central nervous system, activation of GABAB receptors mediates slow postsynaptic inhibitory potentials and presynaptically modulates neurotransmitter release (Misgeld et al. 1995). In neonates, GABAB-receptor-mediated presynaptic modulation has been reported to be functional at early stages of development in the neocortex (Fukuda et al. 1993) and hippocampus (Harrison et al. 1988; Gaiarsa et al. 1995), while postsynaptic GABAB-receptor-mediated inhibition appears later and does not reach adult levels before the end of the first postnatal week in the rat visual cortex (Luhmann & Prince, 1991) and the hippocampus (Gaiarsa et al. 1995). On the other hand, although functional presynaptic GABAB receptors are present, they do not operate using endogenously released GABA before the end of the first postnatal week in rat hippocampus (Caillard et al. 1998).
Physiological implications
The respiratory network must operate efficiently to support life immediately after birth, and both excitatory and inhibitory synapses must be functional in the neuronal network in order to drive effective rhythmic respiratory movements (Ramirez et al. 1997; Richter et al. 1999). In the respiratory network of the neonatal mouse during early postnatal development, postsynaptic GABAB-receptor-mediated modulation partly compensates for the absence of GABAA-receptor- and glycine-receptor-mediated inhibition when chloride-mediated inhibition has not yet emerged (Ritter & Zhang, 2000). With maturation of the rhythm-generating network and the emergence of chloride-mediated inhibition, GABAB receptors assume a modulatory role at pre- and postsynaptic sites.
Acknowledgments
We thank Drs S. Hülsmann, P. Lalley, D. Parker and D. W. Richter for discussions and critical reading of this manuscript, to Mrs C. Bartje and A. Herdlitschke for skilful technical assistance and to Novartis for generously supplying CGP55845A. We also thank Deutsche Forschungsgemeinschaft (SFB 406) for generous support.
REFERENCES
- Ballanyi K, Onimaru H, Homma I. Respiratory network function in the isolated brainstem-spinal cord of newborn rats. Progress in Neurobiology. 1999;59:583–634. doi: 10.1016/s0301-0082(99)00009-x. [DOI] [PubMed] [Google Scholar]
- Brockhaus J, Ballanyi K. Synaptic inhibition in the isolated respiratory network of neonatal rats. European Journal of Neuroscience. 1998;10:3823–3839. doi: 10.1046/j.1460-9568.1998.00396.x. [DOI] [PubMed] [Google Scholar]
- Caillard O, McLean HA, Ben-Ari Y, Gaiarsa JL. Ontogenesis of presynaptic GABAB receptor-mediated inhibition in the CA3 region of the rat hippocampus. Journal of Neurophysiology. 1998;79:1341–1348. doi: 10.1152/jn.1998.79.3.1341. [DOI] [PubMed] [Google Scholar]
- Cherubini E, Gaiarsa JL, Ben-Ari Y. GABA: an excitatory transmitter in early postnatal life. Trends in Neurosciences. 1991;14:515–519. doi: 10.1016/0166-2236(91)90003-d. [DOI] [PubMed] [Google Scholar]
- Ellenberger HH, Feldman JL. Subnuclear organization of the lateral tegmental field of the rat. I: Nucleus ambiguus and ventral respiratory group. Journal of Comparative Neurology. 1990;294:202–211. doi: 10.1002/cne.902940205. [DOI] [PubMed] [Google Scholar]
- Ellenberger HH, Feldman JL, Zhan WZ. Subnuclear organization of the lateral tegmental field of the rat. II: Catecholamine neurons and ventral respiratory group. Journal of Comparative Neurology. 1990;294:212–222. doi: 10.1002/cne.902940206. [DOI] [PubMed] [Google Scholar]
- Elsen FP, Ramirez JM. Calcium currents of rhythmic neurons recorded in the isolated respiratory network of neonatal mice. Journal of Neuroscience. 1998;18:10652–10662. doi: 10.1523/JNEUROSCI.18-24-10652.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldman JL, Smith JC. Cellular mechanisms underlying modulation of breathing pattern in mammals. Annals of the New York Academy of Sciences. 1989;563:114–130. doi: 10.1111/j.1749-6632.1989.tb42194.x. [DOI] [PubMed] [Google Scholar]
- Fritschy JM, Meskenaite V, Weinmann O, Honer M, Benke D, Mohler H. GABAB-receptor splice variants GB1a and GB1b in rat brain: developmental regulation, cellular distribution and extrasynaptic localization. European Journal of Neuroscience. 1999;11:761–768. doi: 10.1046/j.1460-9568.1999.00481.x. [DOI] [PubMed] [Google Scholar]
- Fukuda A, Mody I, Prince DA. Differential ontogenesis of presynaptic and postsynaptic GABAB inhibition in rat somatosensory cortex. Journal of Neurophysiology. 1993;70:448–452. doi: 10.1152/jn.1993.70.1.448. [DOI] [PubMed] [Google Scholar]
- Gaiarsa JL, McLean H, Congar P, Leinekugel X, Khazipov R, Tseeb V, Ben-Ari Y. Postnatal maturation of gamma-aminobutyric acidA and B-mediated inhibition in the CA3 hippocampal region of the rat. Journal of Neurobiology. 1995;26:339–349. doi: 10.1002/neu.480260306. [DOI] [PubMed] [Google Scholar]
- Gaiarsa JL, Tseeb V, Ben-Ari Y. Postnatal development of pre- and postsynaptic GABAB-mediated inhibitions in the CA3 hippocampal region of the rat. Journal of Neurophysiology. 1995;73:246–255. doi: 10.1152/jn.1995.73.1.246. [DOI] [PubMed] [Google Scholar]
- Grillner S, Deliagina T, Ekeberg O, el Manira A, Hill RH, Lansner A, Orlovsky GN, Wallén P. Neural networks that co-ordinate locomotion and body orientation in lamprey. Trends in Neurosciences. 1995;18:270–279. [PubMed] [Google Scholar]
- Harrison NL, Lange GD, Barker JL. (-)-Baclofen activates presynaptic GABAB receptors on GABAergic inhibitory neurons from embryonic rat hippocampus. Neuroscience Letters. 1988;85:105–109. doi: 10.1016/0304-3940(88)90437-5. [DOI] [PubMed] [Google Scholar]
- Hey JA, Mingo G, Bolser DC, Kreutner W, Krobatsch D, Chapman RW. Respiratory effects of baclofen and 3-aminopropylphosphinic acid in guinea-pigs. British Journal of Pharmacology. 1995;114:735–738. doi: 10.1111/j.1476-5381.1995.tb13265.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson SM, Smith JC, Feldman JL. Modulation of respiratory rhythm in vitro: role of Gi/o protein-mediated mechanisms. Journal of Applied Physiology. 1996;80:2120–2133. doi: 10.1152/jappl.1996.80.6.2120. [DOI] [PubMed] [Google Scholar]
- Krogsgaard-Larsen P. Inhibitors of the GABA uptake systems. Molecular and Cellular Biochemistry. 1980;31:105–121. doi: 10.1007/BF00240816. [DOI] [PubMed] [Google Scholar]
- Lalley PM. Effects of baclofen and gamma-aminobutyric acid on different types of medullary respiratory neurons. Brain Research. 1986;376:392–395. doi: 10.1016/0006-8993(86)90206-4. [DOI] [PubMed] [Google Scholar]
- Lawson EE, Schwarzacher SW, Richter DW. Postnatal development of the medullary respiratory network of cat. Proceedings of the 20th Göttingen Neurobiology Conference. 1992;1:69. [Google Scholar]
- Lehmenkuhler A, Sykova E, Svoboda J, Zilles K, Nicholson C. Extracellular space parameters in the rat neocortex and subcortical white matter during postnatal development determined by diffusion analysis. Neuroscience. 1993;55:339–351. doi: 10.1016/0306-4522(93)90503-8. [DOI] [PubMed] [Google Scholar]
- Lipski J, Waldvogel HJ, Pilowsky P, Jiang C. GABA-immunoreactive boutons make synapses with inspiratory neurons of the dorsal respiratory group. Brain Research. 1990;529:309–314. doi: 10.1016/0006-8993(90)90842-y. [DOI] [PubMed] [Google Scholar]
- Livingston CA, Berger AJ. Immunocytochemical localization of GABA in neurons projecting to the ventrolateral nucleus of the solitary tract. Brain Research. 1989;494:143–150. doi: 10.1016/0006-8993(89)90153-4. [DOI] [PubMed] [Google Scholar]
- Luhmann HJ, Prince DA. Postnatal maturation of the GABAergic system in rat neocortex. Journal of Neurophysiology. 1991;65:247–263. doi: 10.1152/jn.1991.65.2.247. [DOI] [PubMed] [Google Scholar]
- Malitschek B, Ruegg D, Heid J, Kaupmann K, Bittiger H, Frostl W, Bettler B, Kuhn R. Developmental changes of agonist affinity at GABABR1 receptor variants in rat brain. Molecular and Cellular Neurosciences. 1998;12:56–64. doi: 10.1006/mcne.1998.0698. [DOI] [PubMed] [Google Scholar]
- Marder E, Calabrese RL. Principles of rhythmic motor pattern generation. Physiological Reviews. 1996;76:687–717. doi: 10.1152/physrev.1996.76.3.687. [DOI] [PubMed] [Google Scholar]
- Misgeld U, Bijak M, Jarolimek W. A physiological role for GABAB receptors and the effects of baclofen in the mammalian central nervous system. Progress in Neurobiology. 1995;46:423–462. doi: 10.1016/0301-0082(95)00012-k. [DOI] [PubMed] [Google Scholar]
- Mohler H, Fritschy JM. GABAB receptors make it to the top - as dimers. Trends in Pharmacological Sciences. 1999;20:87–89. doi: 10.1016/s0165-6147(99)01323-1. [DOI] [PubMed] [Google Scholar]
- Onimaru H, Arata A, Homma I. Inhibitory synaptic inputs to the respiratory rhythm generator in the medulla isolated from newborn rats. Pflügers Archiv. 1990;417:425–432. doi: 10.1007/BF00370663. [DOI] [PubMed] [Google Scholar]
- O'Callaghan J, Jarolimek W, Lewen A, Misgeld U. (-)-Baclofen-induced and constitutively active inwardly rectifying potassium conductances in cultured rat midbrain neurons. Pflügers Archiv. 1996;433:49–57. doi: 10.1007/s004240050247. [DOI] [PubMed] [Google Scholar]
- Pierrefiche O, Foutz AS, Denavit SaubieM. Effects of GABAB receptor agonists and antagonists on the bulbar respiratory network in cat. Brain Research. 1993;605:77–84. doi: 10.1016/0006-8993(93)91358-y. [DOI] [PubMed] [Google Scholar]
- Pierrefiche O, Schwarzacher SW, Bischoff AM, Richter DW. Blockade of synaptic inhibition within the pre-Botzinger complex in the cat suppresses respiratory rhythm generation in vivo. Journal of Physiology. 1998;509:245–254. doi: 10.1111/j.1469-7793.1998.245bo.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramirez JM, Quellmalz UJA, Richter DW. Postnatal changes in the mammalian respiratory network as revealed by the transverse brainstem slice of mice. Journal of Physiology. 1996;491:799–812. doi: 10.1113/jphysiol.1996.sp021258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramirez JM, Telgkamp P, Elsen FP, Quellmalz UJ, Richter DW. Respiratory rhythm generation in mammals: synaptic and membrane properties. Respiration Physiology. 1997;110:71–85. doi: 10.1016/s0034-5687(97)00074-1. [DOI] [PubMed] [Google Scholar]
- Rekling JC, Feldman JL. PreBotzinger complex and pacemaker neurons: hypothesized site and kernel for respiratory rhythm generation. Annual Review of Physiology. 1998;60:385–405. doi: 10.1146/annurev.physiol.60.1.385. [DOI] [PubMed] [Google Scholar]
- Richter DW, Ballanyi K, Lalley PM. Mechanisms of respiratory rhythm generation and their disturbance. In: Cherniack NS, et al., editors. Rehabilitationof the Patient with Respiratory Disease. New York: McGraw-Hill, Inc.; 1999. pp. 53–68. [Google Scholar]
- Richter DW, Mironov SL, Busselberg D, Lalley PM, Bischoff AM, Wilken B. Respiratory rhythm generation: plasticity of a neuronal network. The Neuroscientist. 2000;6:181–198. [Google Scholar]
- Richter DW, Schmidt-Garcon P, Pierrefiche O, Bischoff AM, Lalley PM. Neurotransmitters and neuromodulators controlling the hypoxic respiratory response in anaesthetized cats. Journal of Physiology. 1999;514:567–578. doi: 10.1111/j.1469-7793.1999.567ae.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritter B, Zhang W. Early postnatal maturation of GABAA-mediated inhibition in the brainstem respiratory rhythm-generating network of the mouse. European Journal of Neuroscience. 2000;12:2975–2984. doi: 10.1046/j.1460-9568.2000.00152.x. [DOI] [PubMed] [Google Scholar]
- Ropert N, Miles R, Korn H. Characteristics of miniature inhibitory postsynaptic currents in CA1 pyramidal neurones of rat hippocampus. Journal of Physiology. 1990;428:707–722. doi: 10.1113/jphysiol.1990.sp018236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shao XM, Feldman JL. Respiratory rhythm generation and synaptic inhibition of expiratory neurons in pre-Botzinger complex: differential roles of glycinergic and GABAergic neural transmission. Journal of Neurophysiology. 1997;77:1853–1860. doi: 10.1152/jn.1997.77.4.1853. [DOI] [PubMed] [Google Scholar]
- Sillar KT, Simmers AJ, Wedderburn JF. The post-embryonic development of cell properties and synaptic drive underlying locomotor rhythm generation in Xenopus larvae. Proceedings of the Royal Society B. 1992;249:65–70. doi: 10.1098/rspb.1992.0084. [DOI] [PubMed] [Google Scholar]
- Smith JC, Ellenberger HH, Ballanyi K, Richter DW, Feldman JL. Pre-Botzinger complex: a brainstem region that may generate respiratory rhythm in mammals. Science. 1991;254:726–729. doi: 10.1126/science.1683005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W, Barnbrock A, Eicke A, Ritter B. The changing role of GABAB-mediated modulation in a developing respiratory rhythm-generating network of mouse. Proceedings of the Göttingen Conference of the German Neuroscience Society. 1999;1:14. [Google Scholar]
- Zhang W, Elsen F, Barnbrok A, Richter DW. Postnatal development of GABAB receptor-mediated modulation of voltage-activated Ca2+ currents in mouse brain stem neurones. European Journal of Neuroscience. 1999;11:2332–2342. doi: 10.1046/j.1460-9568.1999.00655.x. [DOI] [PubMed] [Google Scholar]


