Abstract
To investigate the involvement of the medullary raphé in thermoregulatory vasomotor control, we chemically manipulated raphé neuronal activity while monitoring the tail vasomotor response to preoptic warming. For comparison, neuronal activity in the rostral ventrolateral medulla (RVLM) was manipulated in similar experiments. Injections of d,l-homocysteic acid (DLH; 0.5 mm, 0.3 μl) into a restricted region of the ventral medullary raphé suppressed the tail vasodilatation normally elicited by warming the preoptic area to 42 °C. DLH injection into the RVLM also suppressed the vasodilatation elicited by preoptic warming. Injection of bicuculline (0.5 mm, 0.3 μl) into the same raphé region suppressed the vasodilatation elicited by preoptic warming. Bicuculline injection into the RVLM did not suppress tail vasodilatation. These results suggest that neurones in both the medullary raphé and the RVLM are vasoconstrictor to the tail, but only those in the raphé receive inhibitory input from the preoptic area. That input might be direct and/or indirect (e.g. via the periaqueductal grey matter).
The preoptic area plays a key role in body temperature regulation (Kanosue et al. 1998). It integrates information about local brain temperature with the temperature of other body areas and sends appropriate efferent signals to the various thermoregulatory effector organs (Nagashima et al. 2000; Kanosue et al. 2001). It is well established that the preoptic area contains intrinsically thermosensitive neurones that may be warm-sensitive or cold-sensitive (Nakayama, 1985). Recent findings suggest, however, that both heat gain and heat loss mechanisms are controlled by signals that arise mainly from warm-sensitive neurones (Zhang et al. 1995; Kanosue et al. 2001).
In rats, non-evaporative heat loss occurs mainly through the tail (Gordon, 1990), the blood vessels in which vasodilate when the preoptic area is warmed (Carlisle & Laudenslager, 1979). The efferent signals for this response originate in warm-sensitive neurones, and the descending pathway passes through the medial forebrain bundle (Kanosue et al. 1994a). Two regions in the midbrain seem to participate in the tail vasomotor control: the ventrolateral part of the rostral periaqueductal grey (rPAG) and the ventral tegmental area (VTA; Zhang et al. 1997). Electrical or chemical stimulation in the rPAG causes tail vasodilatation. Stimulation of the VTA, however, suppresses the cutaneous vasodilatation otherwise elicited by preoptic warming. Warm-sensitive preoptic neurones may therefore send excitatory signals to the rPAG and perhaps also inhibitory signals to the VTA vasoconstrictor region. The relevant efferent neuronal projections from these two regions are not yet known.
Blood flow to the rat tail is determined by the level of activity in its sympathetic postganglionic vasoconstrictor fibres (O'Leary et al. 1985). These are supplied by preganglionic sympathetic neurones situated mainly in the intermediolateral cell column of the first and second lumbar segments (Rathner & McAllen, 1997; Smith & Gilbey, 1998). The site or sites of the premotor neurones that drive this pathway for thermoregulation are not yet established.
It is well accepted that the sympathetic premotor neurones in the rostral ventrolateral medulla (RVLM) are a major source of vasomotor drive to a range of tissues (Dampney, 1994). It has also been shown that electrical or chemical stimulation of the RVLM can decrease the surface temperature of the tail in hyperthermic, anaesthetized rats (Key & Wigfield, 1992). Recently, however, several lines of evidence have suggested that the medullary raphé is more important than the RVLM in the control of rat tail blood flow. First, when raphé neurones were activated chemically by glutamate microinjections, this caused a much greater increase in tail sympathetic nerve activity than did activation of neurones in the RVLM (Rathner & McAllen, 1999). Second, at the appropriate interval after injections into the wall of the tail artery of the transsynaptic retrograde tracer, pseudorabies virus, more sympathetic premotor neurones were found to be labelled in the medullary raphé nuclei than in the RVLM (Smith et al. 1998). Third, exposure of rats to cold was found to cause increased expression of Fos immunoreactivity, which was concentrated in the raphé (Bonaz & Taché, 1994).
In the present study we set out to investigate the role of the medullary raphé in tail vasomotor control during thermal stimulation of the preoptic area, and to compare it with the role played by the RVLM.
METHODS
Sixty-six adult male, specific pathogen-free crj-Wister rats (290–400 g; Charles River Japan, Osaka, Japan) were used in this study. Rats were individually housed at a room temperature of 22 °C with a light/dark schedule of 12 h/12 h and with free access to food and water. The experimental procedure was approved by the Animal Care Committee of Osaka University Faculty of Medicine.
The rat was anaesthetized with urethane (initially 1.4 g kg−1, i.p.). The depth of anaesthesia was assessed according to the criteria of Lumb & Jones (1984) and, if the first plane of stage 3 (surgical anaesthesia) was not reached, subsequent small doses of urethane (0.1 g kg−1, i.p.) were administered. Each rat was mounted in a stereotaxic apparatus according to the coordinate system of Paxinos & Watson (1986). An electrode-thermocouple assembly (Kanosue et al. 1991) was implanted either unilaterally or bilaterally, with the tip or tips located in the preoptic area (1.0–1.5 mm from the midline, between 0.5 mm anterior and 0.5 mm posterior to the bregma, and 9.0–9.5 mm below the skull surface). This assembly consisted of an insulated stainless steel tube (0.4 mm i.d., 0.6 mm o.d.) with a bared, sharp tip for thermal stimulation, and a copper-constantan thermocouple glued inside it for monitoring the temperature of the preoptic area (TPO). This assembly was fixed to the skull with dental cement. We have already confirmed that when the temperature measured at the assembly was increased by 4 °C, the temperature at sites 2 mm from the assembly rose less than 1 °C (Kanosue et al. 1990), and that warming the brain to 42 °C did not cause tissue damage (Kanosue et al. 1994b). We have also confirmed that this warming produces tail vasodilatation repeatedly and reproducibly, which indicates that the vasodilatation is not the result of irreversible tissue damage of the warmed area. Small holes were opened in the skull over the medullary raphé (0.0–0.5 mm lateral from the midline, and 9.3–11.6 mm posterior to bregma), or bilaterally over the RVLM (1.7–2.5 mm lateral from the midline, and 11.3–13.0 mm posterior to bregma) for the insertion of cannulae. A polyethylene catheter (filled with heparinized saline, 50 U ml−1) was placed into the right femoral artery to monitor arterial blood pressure and heart rate.
The anaesthetized rat was placed in a climatic chamber, and rectal temperature (Tre) was measured by a thermocouple placed 6 cm past the anal sphincter. Another thermocouple for measuring tail temperature (Ttail) was fixed to the lateral surface of the tail 10 cm from the base. For preoptic warming, radio frequency current (500 kHz; Lesion Generator RF-4; Radionics, Burlington, MA, USA) was passed between the tip of the electrode-thermocouple assembly and a needle electrode placed subcutaneously in the back. Ambient temperature (Ta) was kept at 28–30 °C throughout the experiment. Tre was maintained at 37–38.5 °C with a heating pad (10 cm × 10 cm) and a blanket. Recording was started after all the recorded parameters became stable.
One or two stainless steel cannulae (0.3 mm o.d., insulated up to 0.5 mm from the tip) were lowered into the brainstem (raphé and RVLM) through the prepared skull holes until the tips were 10.0–10.3 mm below the skull surface. Each cannula was connected by a polyethylene tube to a 10 μl microsyringe (Hamilton, Reno, NV, USA). Drugs for injection through the cannulae were dissolved immediately before each experiment.
Experiment 1: injection of d,l-homocysteic acid (DLH)
The preoptic area was first warmed to 42 °C to confirm that it elicited tail vasodilatation. The vasodilatation was detected as a rise in Ttail. The current was then turned off, after which the temperature of the vasodilated tail always promptly began to fall. When Ttail had returned to the pre-warming level, the preoptic area was again warmed to 42 °C. While Ttail was still rising, and had reached about 1 °C above the pre-warming level, 0.5 mm DLH (pH 7.4 in phosphate buffered saline; 0.3 μl) was injected into either the medullary raphé or into the RVLM ipsilateral to the preoptic thermode. Preoptic warming was then continued for at least a further 5 min after the injection. In some cases the same test was made with DLH injection into another site in the same animal.
Experiment 2: bicuculline injection
As in experiment 1, the preoptic warming was first confirmed to produce a rise in Ttail. Then 0.5 mm bicuculline (Sigma, in saline; 0.3 μl) was injected either into the medullary raphé, or bilaterally into the RVLM. One minute after the injection, the preoptic area was again warmed to 42 °C. In some cases a second test was made with bicuculline injections into another site in the same animal.
At the end of each experiment, a small lesion was made by passing DC current (1 mA, 0.5 s) through the cannula to mark the position of its tip. The anaesthetized rat was then killed by perfusing first with saline and then with 10 % formalin solution. The brain was removed and 40 μm frozen sections were made of the preoptic area and medulla. Sections were stained with Toluidine Blue, and the positions of the lesions were verified.
Student's paired t test was used to determine the significance of changes in blood pressure and heart rate before and after drug injection into the medulla. Data are given in the text as means ±s.e.m.
RESULTS
In experiments 1 and 2, the mean Tre during the experiment was 37.8 ± 0.1 °C and Ta was 29.3 ± 0.1 °C. The mean pre-warming TPO was 37.1 ± 0.1 °C. In the control condition without drug injection, Ttail always rose in response to preoptic warming, and fell after the warming was terminated (Fig. 1A and Fig. 2A). The preoptic area was warmed with either a ramp (Fig. 1 and Fig. 2) or a step (Fig. 4) profile, but since their effects were indistinguishable, the data from each are considered together.
Figure 1. Effect of d,l-homocysteic acid (DLH) injection into the medullary raphé on the rise in tail temperature (Ttail) elicited by preoptic warming.
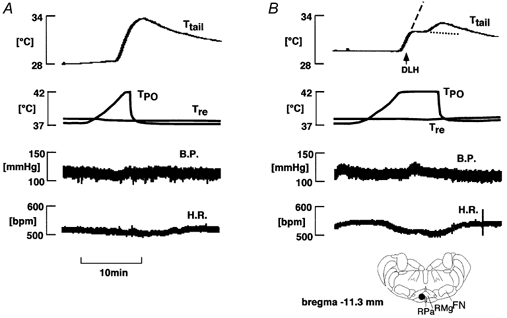
A, control (no injection); B, DLH injection. From top to bottom: Ttail, temperature of the preoptic area (TPO), rectal temperature (Tre), blood pressure (B.P.) and heart rate (H.R.). The arrow indicates DLH (0.5 mm, 0.3 μl) injection. The significance of the dashed and dotted lines in the top record in B is given in the text. The location of the cannula tip is indicated with a filled circle in the inset. FN, facial nucleus; RMg, raphé magnus nucleus; RPa, raphé pallidus nucleus.
Figure 2. Effect of DLH injection into the rostral ventrolateral medulla (RVLM) on the rise in Ttail elicited by preoptic warming.
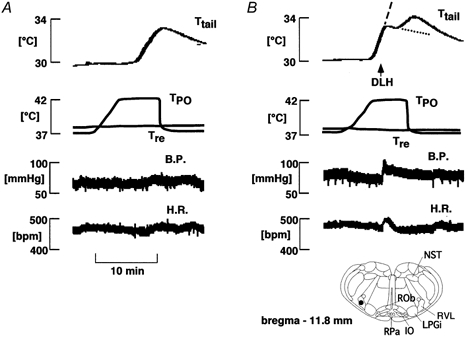
A, control (no injection); B, DLH injection. From top to bottom: Ttail, TPO, Tre, B.P. and H.R. The arrow indicates DLH (0.5 mm, 0.3 μl) injection ipsilateral to the side of preoptic warming. The significance of the dashed and dotted lines in the top record in B is given in the text. The location of the cannula tip is indicated with a filled circle in the inset. IO, inferior olive; LPGi, lateral gigantocellular nucleus; NST, nucleus of solitary tract; ROb, raphé obscurus nucleus; RVL, rostroventrolateral reticular nucleus
Figure 4. Effect of bicuculline injection into the medullary raphé on the increase in Ttail elicited by preoptic warming.
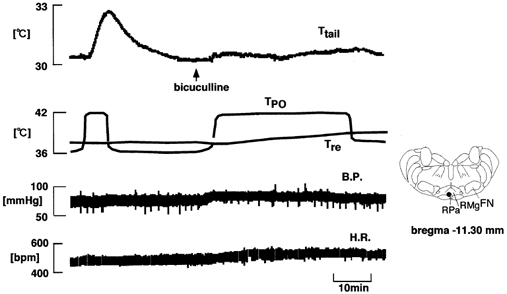
From top to bottom: Ttail, TPO, Tre, B.P. and H.R. Arrow indicates bicuculline (0.5 mm, 0.3 μl) injection. The location of the cannula tip is indicated with a filled circle in the inset.
DLH injection
Sixty-eight sites (33 in the raphé, 35 in the RVLM) were investigated for the effects of DLH injection on the tail vasomotor response to preoptic warming. In 27 rats two sites were tested; thus in total 41 rats were used in this series of experiments. DLH injection into 23 out of 33 sites in the medullary raphé suppressed the rise in Ttail elicited by preoptic warming. At 10 other raphé sites, DLH injection did not influence the rise in Ttail on preoptic warming. Figure 1B shows an example of how DLH injection into the raphé suppressed the rise in Ttail elicited by preoptic warming to 42 °C. Ttail started to increase in response to preoptic warming, but the rise in Ttail was then suppressed for about 10 min when DLH was injected into the raphé. Eventually, the rise in Ttail resumed until preoptic warming was terminated, after which it fell back to its baseline level. To evaluate the effect of DLH injection, the rate of Ttail rise was calculated. The steepest rate before DLH injection was designated as r1 (dashed line in Fig. 1B and Fig. 2B), and the slowest rate after the injection as r2 (dotted line in Fig. 1B and Fig. 2B). When r2 was negative, we took it as a ‘clear effect’. When r2 was positive but less than 60 % of r1, we took it as a ‘weaker effect’. DLH-effective sites were distributed in the ventral raphé nuclei, including the raphé magnus (RMg) and the raphé pallidus (RPa; Fig. 3). The ‘clear effect’ was seen when DLH was injected into a restricted caudal region of the RMg and RPa (Fig. 3). DLH injections into more rostral raphé regions had weaker effects.
Figure 3. Locations of cannula tips for applications of DLH.
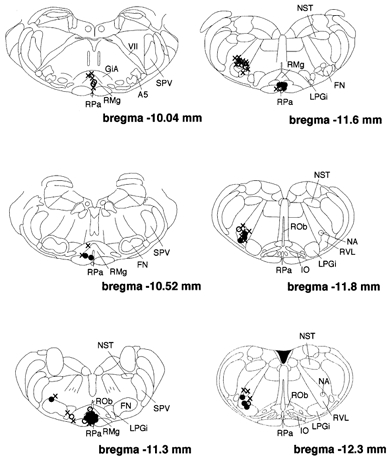
Filled circles show where DLH had clear effects on the increase in Ttail elicited by preoptic warming. Open circles show where DLH had weaker effects on that Ttail rise. Crosses show where the injections were ineffective. A5, A5 noradrenaline cells; GiA, gigantocellular reticular nucleus; IO, inferior olive; NA, nucleus ambiguus; SPV, spinal trigeminal tract; VII, facial nerve or its root.
Unilateral DLH injections were made into 35 RVLM sites. At seven of these sites, DLH injection had the clear effect of suppressing the rise in Ttail elicited by preoptic warming (Fig. 2). Those sites were located in the RVLM, ventral to the nucleus ambiguus (Fig. 3). At six other sites, DLH injections caused weaker effects, slowing the rate of rise of Ttail. At the remaining 21 sites, DLH injections had no influence on the response to preoptic warming.
Table 1 shows the mean blood pressure and heart rate before and after raphé and RVLM injections of DLH in those animals where there was a clear suppression of the rise in Ttail elicited by preoptic warming. Blood pressure increased significantly after effective DLH injections into both raphé and RVLM (P < 0.01). Effective DLH injections into the RVLM (P < 0.05), but not the raphé, caused significant increases in heart rate.
Table 1.
The change in blood pressure (B.P.) and heart rate (H.R.) after DLH injection that clearly suppressed Ttail rise elicited by preoptic warming as in Figs 1 and 2
| B.P. (mmHg) | H.R. (bpm) | ||||
|---|---|---|---|---|---|
| Injection site | n | Pre-injection | Post-injection | Pre-injection | Post-injection |
| Raphé | 17 | 83 ± 3 | 89 ± 2** | 468 ± 13 | 472 ± 15 |
| RVLM | 7 | 75 ± 7 | 86 ± 6** | 472 ± 9 | 487 ± 6* |
P < 0.01
P < 0.05, compared to pre-injection.
Bicuculline injections
The effects of raphé bicuculline injections on the tail vasodilator response to preoptic warming were tested at 18 sites. In five rats, two raphé sites were tested; thus a total of 13 rats was used. Figure 4 shows a representative example of an experiment of this type. First, preoptic warming produced a sharp rise in Ttail. After the injection of bicuculline into the raphé, the normal rise in Ttail was prevented, despite prolonged warming of the preoptic area. The rise in Ttail elicited by preoptic warming was suppressed by bicuculline injection into 11 out of the 17 tested sites: effective sites were restricted to the caudal part of the RMg and the RPa at the level of the facial nucleus (Fig. 6A). The bicuculline injections caused no significant change in either blood pressure or heart rate. Bicuculline injections into a further six sites, centred more rostrally in the raphé, failed to affect the rise in Ttail on preoptic warming.
Figure 6. Locations of cannula tips for applications of bicuculline.
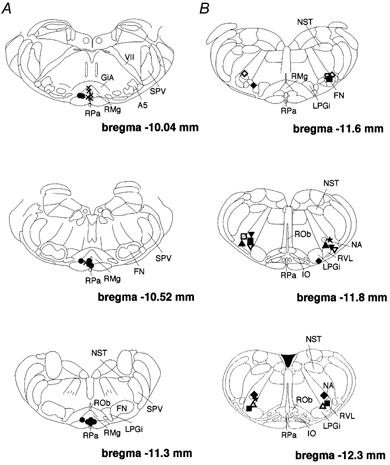
A, the medullary raphé. Filled circles show where bicuculline injection suppressed the rise in Ttail elicited by preoptic warming and crosses show where the injections were ineffective. B, the RVLM. Each symbol shows the injection site. The same symbols indicate the same animal.
The effects of bilateral bicuculline injections into the RVLM on the tail vasodilator response to preoptic warming were tested in 12 rats. Bicuculline injections into the RVLM always caused an increase in blood pressure from 92 ± 4 to 106 ± 5 mmHg without significant change in heart rate, and failed to prevent the rise in Ttail in response to preoptic warming in 11/12 cases (Fig. 5). The locations of RVLM injection sites are shown in Fig. 6B. In the one exceptional case, RVLM bicuculline injections (site indicated by stars in Fig. 6B) did prevent the rise in Ttail elicited by preoptic warming.
Figure 5. Effect of bicuculline injection into the bilateral RVLM on the increase in Ttail elicited by preoptic warming.
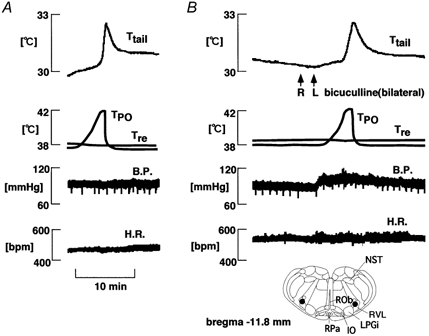
A, control (no injection); B, bicuculline injection. From top to bottom: Ttail, TPO, Tre, B.P. and H.R. Arrows indicate bicuculline (0.5 mm, 0.3 μl) injection. The locations of the cannula tips are indicated with filled circles in the inset.
DISCUSSION
The first finding of the present study is that DLH injections into either the medullary raphé or the RVLM were effective in suppressing the tail vasodilatation in response to preoptic warming. The suppression was accompanied by a rise, not a fall in blood pressure, so it could not have been a secondary effect of reduced perfusion pressure: it must have been caused by the constriction of tail blood vessels. Obtaining tail vasoconstriction in response to DLH injection means that the cell bodies of neurones mediating the response were located near the injection site, because DLH is not believed to affect fibres of passage (see Hilton & Redfern, 1986).
The magnitude of the effect of DLH injected into the medullary raphé, presumably reflecting the strength of vasoconstrictor drive that it evoked, varied with the rostrocaudal location of the injection. The most effective region included those parts of the RMg and the RPa located at the level of the facial nucleus. Injections made into more rostral sites were less effective. This location corresponds well with that found to activate tail sympathetic nerve activity in response to microinjections of glutamate (Rathner & McAllen, 1999). A recent study also suggests that the homologous region of the medullary raphé in rabbits mediates vasoconstriction of the ear (Blessing et al. 1999; Blessing & Nalivaiko, 2000).
In addition to the raphé, the present study found that DLH injection into the RVLM was effective in preventing thermal vasodilatation of rat tail vessels. Evidently this site could also drive vasoconstriction in the tail sufficient to offset the vasodilatator action of preoptic warming. This contrasts with the report that sympathetic activity to the tail artery was poorly driven from the RVLM, compared with from the raphé, in response to medullary glutamate injections (Rathner & McAllen, 1999). Others, however, have found that electrical or chemical stimulation of the RVLM can indeed affect the vasomotor supply to the rat's tail (Lovick, 1989; Key & Wigfield, 1992). It is possible that the most effective RVLM sites affecting tail vasoconstriction are located more caudally than those studied by Rathner & McAllen (1999; see Key & Wigfield, 1992 and Fig. 3). Alternatively or additionally, the relatively small increases in tail sympathetic nerve activity found by Rathner & McAllen (1999) after RVLM stimulation might have been sufficient to prevent thermal vasodilatation if tested under the present protocol. It has been shown in cats (McAllen & Dampney, 1989) and in rabbits (Ootsuka & Terui, 1997) that skin vasomotor responses can be elicited by neurones in the RVLM. Taking these findings together with the present evidence, it seems apparent that skin vasoconstrictor responses can be driven by neurones in the RVLM as well as by those in the raphé.
The ability of DLH injections to cause tail vasoconstriction does not necessarily mean, however, that the raphé or RVLM neurones in question transmit thermoregulatory signals from the preoptic area to the cutaneous sympathetic supply. It is equally possible that the DLH-evoked vasoconstrictor signal and the thermal vasodilator signal from the preoptic area could have travelled by separate, parallel pathways to the spinal sympathetic outflow. If the same bulbospinal pathway does transmit both signals, however, then the connection from the preoptic area to the raphé or RVLM neurones responsible should be inhibitory. This is because the vasodilatation elicited by preoptic warming is attributable to increased efferent signals from warm-sensitive preoptic neurones (Zhang et al. 1995). For this reason, we tested the effect of injecting the GABAA receptor antagonist, bicuculline, to block the action of this inhibitory transmitter on raphé or RVLM neurones. When bicuculline was injected into the region of the raphé where DLH injection was most effective, the vasodilatation in response to preoptic warming was suppressed. This supports the hypothesis that during preoptic warming, inhibitory signals would be transmitted to the vasoconstrictor neurones in the raphé. However, it should be mentioned that the concentration of bicuculline used in the present study was relatively high (0.5 mm), a concentration that could have affected not only GABA receptors but also nicotinic receptors (Rothlin et al. 1999). Thus, although it is most likely that bicuculline blocked an inhibitory GABAergic synapse, the involvement of cholinergic receptors cannot be ruled out. By contrast, in every case but one, bicuculline injections into the RVLM, even bilaterally, failed to suppress the rise in Ttail elicited by preoptic warming. This result provides little support for the hypothesis that tail skin vasoconstrictor neurones in the RVLM are controlled by inhibitory signals from the preoptic area.
If we accept that signals from preoptic warm-sensitive cells inhibit the skin vasoconstrictor neurones of the medullary raphé, it is uncertain whether this connection is direct or indirect. Anatomical studies have shown that there are direct projections from the preoptic area to the medullary raphé (Simerly & Swanson, 1988; Hermann et al. 1997; Murphy et al. 1999), although the physiological properties of those raphé-projecting preoptic neurones are not yet known. On the other hand, when stimulated electrically or chemically, neurones in the rPAG elicit tail vasodilatation (Zhang et al. 1997). It has been shown that many neurones throughout the rostrocaudal extent of the PAG have projections to the rostral ventral medulla (possibly including the area defined here, although this is unclear), and that many of these PAG-medullary neurones express Fos after electrical or chemical stimulation of the medial preoptic area (Rizvi et al. 1996). It is quite probable that there is an indirect pathway from the preoptic area to the raphé, involving a synaptic relay in the rPAG, for the control of the tail vasomotor supply. Both direct and indirect pathways from the preoptic area to the medullary raphé could therefore mediate the thermoregulatory control of tail vessels.
It was recently suggested that sympathetic premotor neurones for brown adipose tissue (BAT) are located in the RPa (Morrison, 1999; Morrison et al. 1999). Injection of bicuculline into the RPa caused a large, rapid rise in sympathetic nerve activity to the BAT. The area of RPa implicated in the control of BAT overlaps the raphé region where DLH injections were effective in preventing thermal vasodilatation in the present study. Skin vasoconstriction and BAT thermogenesis are both engaged as part of the thermoregulatory response against cold. It is also known that neurones in RPa express Fos in response to cold exposure (Morrison et al. 1999), and that spinally projecting neurones in the medullary raphé respond to mild, non-noxious cooling (Rathner et al. 2001). It may be significant, therefore, that the neurones controlling these two functions are located in this same small region. Although it is not yet clear whether the same neurones send signals to the BAT and tail blood vessels, the medullary raphé may serve as an ‘output centre’ for thermoregulation against cold. The identities of the neurones responsible for each, and the way in which they process relevant information for their respective functions, await future study.
In conclusion, tail vasoconstrictor neurones exist in the rostral medullary raphé, and receive inhibitory signals from preoptic area. This pathway may be direct and perhaps also indirect, via the rPAG. Tail vasoconstrictor neurones were also located in the RVLM, but this pathway is not under dominant inhibitory control from the preoptic area.
REFERENCES
- Blessing WW, Nalivaiko E. Regional blood flow and nociceptive stimuli in rabbits: patterning by medullary raphe, not ventrolateral medulla. Journal of Physiology. 2000;524:279–292. doi: 10.1111/j.1469-7793.2000.t01-2-00279.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blessing WW, Yu Y-H, Nalivaiko E. Raphe pallidus and parapyramidal neurons regulate ear pinna vascular conductance in the rabbit. Neuroscience Letters. 1999;270:33–36. doi: 10.1016/s0304-3940(99)00459-0. [DOI] [PubMed] [Google Scholar]
- Bonaz B, Taché Y. Induction of Fos immunoreactivity in the rat brain after cold-restraint induced gastric lesions and fecal excretion. Brain Research. 1994;652:56–64. doi: 10.1016/0006-8993(94)90316-6. [DOI] [PubMed] [Google Scholar]
- Carlisle H -J, Laudenslager ML. Observation on the thermoregulatory effects of preoptic warming in rats. Physiology and Behavior. 1979;23:723–732. doi: 10.1016/0031-9384(79)90166-5. [DOI] [PubMed] [Google Scholar]
- Dampney RAL. Functional organization of central pathways regulating the cardiovascular system. Physiological Reviews. 1994;74:323–364. doi: 10.1152/physrev.1994.74.2.323. [DOI] [PubMed] [Google Scholar]
- Gordon CJ. Thermal biology of the laboratory rat. Physiology and Behavior. 1990;47:963–991. doi: 10.1016/0031-9384(90)90025-y. [DOI] [PubMed] [Google Scholar]
- Hermann DK, Luppi P-H, Peyron C, Hinckel P, Jouvet M. Afferent projections to the rat nuclei raphe magnus, raphe pallidus and reticularis gigantocellularis pars α demonstrated by iontophoretic application of choleratoxin (subunit b) Journal of Chemical Neuroanatomy. 1997;13:1–21. doi: 10.1016/s0891-0618(97)00019-7. [DOI] [PubMed] [Google Scholar]
- Hilton SM, Redfern WS. A search for brain stem cell groups integrating the defence reaction in the rat. Journal of Physiology. 1986;378:213–228. doi: 10.1113/jphysiol.1986.sp016215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanosue K, Hosono T, Zhang Y -H, Chen X -M. Neuronal networks controlling thermoregulatory effectors. Progress in Brain Research. 1998;115:49–62. doi: 10.1016/s0079-6123(08)62029-4. [DOI] [PubMed] [Google Scholar]
- Kanosue K, Nakayama T, Tanaka H, Yanase M, Yasuda H. Modes of action of local hypothalamic and skin thermal stimulation on salivary secretion in rats. Journal of Physiology. 1990;424:459–471. doi: 10.1113/jphysiol.1990.sp018077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanosue K, Niwa K, Andrew PD, Yasuda H, Yanase M, Tanaka H, Matsumura K. Lateral distribution of hypothalamic signals controlling thermoregulatory vasomotor activity and shivering in rats. American Journal of Physiology. 1991;260R:485–493. doi: 10.1152/ajpregu.1991.260.3.R486. [DOI] [PubMed] [Google Scholar]
- Kanosue K, Yanase-Fujiwara M, Hosono T. Hypothalamic network for thermoregulatory vasomotor control. American Journal of Physiology. 1994a;267R:283–288. doi: 10.1152/ajpregu.1994.267.1.R283. [DOI] [PubMed] [Google Scholar]
- Kanosue K, Yoshida K, Maruyama M, Nagashima K. The central organization of the thermoregulatory system. In: Kosaka M, Sugahara T, Schmidt KL, Simon E, editors. Thermotherapy for Neoplasia, Inflammation, and Pain. Tokyo: Springer; 2001. pp. 2–11. [Google Scholar]
- Kanosue K, Zhang YH, Yanase-Fujiwara M, Hosono T. Hypothalamic network for thermoregulatory shivering. American Journal of Physiology. 1994b;267R:275–282. doi: 10.1152/ajpregu.1994.267.1.R275. [DOI] [PubMed] [Google Scholar]
- Key BJ, Wigfield CC. Changes in the tail surface temperature of the rat following injection of 5-hydroxytryptamine into the ventrolateral medulla. Neuropharmacology. 1992;31:717–723. doi: 10.1016/0028-3908(92)90032-k. [DOI] [PubMed] [Google Scholar]
- Lovick TA. Cardiovascular responses to 5-HT in the ventrolateral medulla of the rat. Journal of the Autonomic Nervous System. 1989;28:35–41. doi: 10.1016/0165-1838(89)90005-2. [DOI] [PubMed] [Google Scholar]
- Lumb WV, Jones EW. Veterinary Anaesthesia. Philadelphia: Lea & Febiger; 1984. [Google Scholar]
- McAllen RM, Dampney RAL. The selectivity of descending vasomotor control by subretrofacial neurons. Progress in Brain Research. 1989;81:233–242. doi: 10.1016/s0079-6123(08)62013-0. [DOI] [PubMed] [Google Scholar]
- Morrison SF. RVLM and raphe differentially regulate sympathetic outflows to splanchnic and brown adipose tissue. American Journal of Physiology. 1999;276R:962–973. doi: 10.1152/ajpregu.1999.276.4.R962. [DOI] [PubMed] [Google Scholar]
- Morrison SF, Sved AF, Passerin AM. GABA-mediated inhibition of raphe pallidus neurons regulates sympathetic outflow to brown adipose tissue. American Journal of Physiology. 1999;276R:290–297. doi: 10.1152/ajpregu.1999.276.2.R290. [DOI] [PubMed] [Google Scholar]
- Murphy AZ, Rizvi TA, Ennis M, Shipley MT. The organization of preoptic-medullary circuits in the male rat: evidence for interconnectivity of neural structures involved in reproductive behavior, antinociception and cardiovascular regulation. Neuroscience. 1999;91:1103–1116. doi: 10.1016/s0306-4522(98)00677-0. [DOI] [PubMed] [Google Scholar]
- Nagashima K, Nakai S, Tanaka M, Kanosue K. Neuronal circuitries involved in thermoregulation. Autonomic Neuroscience. Basic and Clinical. 2000;85:18–25. doi: 10.1016/S1566-0702(00)00216-2. [DOI] [PubMed] [Google Scholar]
- Nakayama T. Thermosensitive neurons in the brain. Japanese Journal of Physiology. 1985;35:375–389. doi: 10.2170/jjphysiol.35.375. [DOI] [PubMed] [Google Scholar]
- O'Leary DS, Johnson JM, Taylor WF. Mode of neural control mediating rat tail vasodilation during heating. Journal of Applied Physiology. 1985;59:1533–1538. doi: 10.1152/jappl.1985.59.5.1533. [DOI] [PubMed] [Google Scholar]
- Ootsuka Y, Terui N. Functionally different neurons are organized topographically in the rostral ventrolateral medulla of rabbits. Journal of the Autonomic Nervous System. 1997;67:67–78. doi: 10.1016/s0165-1838(97)00094-5. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. 2. San Diego: Academic Press; 1986. [DOI] [PubMed] [Google Scholar]
- Rathner JA, McAllen RM. The lumbar preganglionic sympathetic supply to rat tail and hindpaw. Journal of the Autonomic Nervous System. 1997;69:127–131. doi: 10.1016/s0165-1838(98)00014-9. [DOI] [PubMed] [Google Scholar]
- Rathner JA, McAllen RM. Differential control of sympathetic drive to the rat tail artery and kidney by medullary premotor cell groups. Brain Research. 1999;834:196–199. doi: 10.1016/s0006-8993(99)01568-1. [DOI] [PubMed] [Google Scholar]
- Rathner JA, Owens NC, McAllen RM. Cold-activated raphé-spinal neurons in rats. Journal of Physiology. 2001;535:841–854. doi: 10.1111/j.1469-7793.2001.t01-1-00841.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizvi TA, Murphy AZ, Ennis M, Behbehani MM, Shipley MT. Medial preoptic area afferents to periaqueductal grey medullo-output neurons: a combined Fos and tract tracing study. Journal of Neuroscience. 1996;16:333–344. doi: 10.1523/JNEUROSCI.16-01-00333.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothlin CV, Katz E, Verbitsky M, Elgoyhen AB. The alpha9 nicotinic acetylcholine receptor shares pharmacological properties with type A gamma-aminobutyric acid, glycine, and type 3 serotonin receptors. Molecular Pharmacology. 1999;55:248–254. doi: 10.1124/mol.55.2.248. [DOI] [PubMed] [Google Scholar]
- Simerly RB, Swanson LW. Projections of the medial preoptic nucleus: a phaseolus vulgaris leucoagglutinin anterograde tract-tracing study in the rat. Journal of Comparative Neurology. 1988;270:209–242. doi: 10.1002/cne.902700205. [DOI] [PubMed] [Google Scholar]
- Smith JE, Gilbey MP. Segmental origin of sympathetic preganglionic neurones regulating the tail circulation in the rat. Journal of the Autonomic Nervous system. 1998;68:109–114. doi: 10.1016/s0165-1838(97)00124-0. [DOI] [PubMed] [Google Scholar]
- Smith JE, Jansen ASP, Gilbey MP, Loewy AD. CNS cell groups projecting to sympathetic outflow of tail artery: neural circuits involved in heat loss in the rat. Brain Research. 1998;786:153–164. doi: 10.1016/s0006-8993(97)01437-6. [DOI] [PubMed] [Google Scholar]
- Zhang YH, Hosono T, Yanase-Fujiwara M, Chen XM, Kanosue K. Effect of midbrain stimulations on thermoregulatory vasomotor responses in rats. Journal of Physiology. 1997;503:177–86. doi: 10.1111/j.1469-7793.1997.177bi.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhange YH, Yanase-Fujiwara M, Hosono T, Kanosue K. Warm and cold signals from the preoptic area: which contribute more to the control of shivering in rats? Journal of Physiology. 1995;485:195–202. doi: 10.1113/jphysiol.1995.sp020723. [DOI] [PMC free article] [PubMed] [Google Scholar]


