Abstract
We have measured monocular and binocular contrast sensitivities in response to medium to high spatial frequencies of vertical sinusoidal grating patterns in normal subjects, anisometropic amblyopes, strabismic amblyopes and non-amblyopic esotropes. On binocular viewing, contrast sensitivities were slightly but significantly increased in normal subjects, markedly increased in anisometropes and esotropes with anomalous binocular single vision (BSV) and significantly reduced in esotropes and exotropes without BSV. Application of a prismatic correction to the strabismic eye in order to achieve bifoveal stimulation resulted in a significant reduction in contrast sensitivity in esotropes with and without anomalous BSV, in exotropes and in non-amblyopic esotropes. Control experiments in normal subjects with monocular viewing showed that degradative effects of the prism occurred only with high prism powers and at high spatial frequencies, thus establishing that the reduced contrast sensitivities were the consequence of bifoveal stimulation rather than optical degradation. Displacement of the image of the grating pattern by 2 deg in normal subjects and anisometropes by a dichoptic method to simulate a small angle esotropia had no effect on the contrast sensitivities recorded through the companion eye. By contrast, esotropes showed similar reductions in contrast sensitivity to those obtained with the prism experiments, confirming a fundamental difference between subjects with normal and abnormal ocular alignments. The results have thus established a suppressive action of the fovea of the amblyopic eye acting on the companion, non-amblyopic eye and indicate that correction of ocular misalignments in adult esotropes may be disadvantageous to binocular visual performance.
Amblyopia is a relatively common condition in man, with an incidence of 2.0–2.5 %, in which the visual acuity through one eye is subnormal despite the absence of any overt pathologies (von Noorden, 1990). It arises as a result of deprivation of adequate visual stimulation during early childhood and once established is, in many cases, irreversible. The major causes are occlusion, squint (strabismus) or unequal refraction between the two eyes (anisometropia). Strabismus falls into two main categories: an inward deviation of one eye (esotropia) or, less frequently, an outward deviation (exotropia). Deviations in other directions arise but are not considered further. An important but unanswered question concerns the quality of vision that amblyopes have on binocular viewing. When visual performance is measured as the contrast sensitivity for the just supra-threshold detection of vertically orientated, sinusoidal grating patterns of different spatial frequencies, the contrast sensitivities are enhanced on binocular viewing compared with monocular viewing by some 40 % (Campbell & Green, 1965a; Ross et al. 1985; Pardhan & Gilchrist, 1990). The situation in amblyopes who are combining visual information from a normal eye and an amblyopic eye is far from clear. With respect to reaction times for detection of a grating pattern, those for binocular viewing have been shown to be no different from those for monocular viewing with the non-amblyopic eye, thus implying that the contribution from the amblyopic eye has been disregarded (Levi et al. 1979).
In testing this assumption, the type of binocular vision must be taken into account. Simple anisometropic amblyopes have normal binocular single vision (BSV), which is not the case in strabismics. On binocular viewing, esotropes and exotropes are characterized by suppression of the visual input from the misaligned eye so that BSV is absent. However, in esotropes with a small to moderate angle deviation and in microtropes who are defined as having esotropia of 5 deg or less, abnormal retinal correspondence may develop between the fovea of the normal eye and the extrafoveal region aligned with the visual axis of the deviating eye; in these cases, anomalous BSV may develop (Lyle & Wybar, 1967).
We have, therefore, undertaken contrast sensitivity measurements in different categories of amblyopes with particular regard being paid to the status of their BSV i.e. whether present, absent or anomalous. First, binocular contrast sensitivities were compared with those for monocular viewing. Since all types of amblyopes show a loss of contrast sensitivity at high spatial frequencies (Hess & Howell, 1977; Leguire et al. 1989), we carried out our determinations over the high spatial frequency limb of the contrast sensitivity function. Second, bifoveal viewing in strabismics was effected by a prism that compensated for the angle of squint and the effects on contrast sensitivity were determined. It was then ascertained with dichoptic viewing whether a shift of the retinal image in one eye affected the contrast sensitivities through the other eye in normal subjects. Our results have shown that binocular contrast sensitivities depend on the presence or absence of BSV rather than the type of amblyopia.
METHODS
Subjects
A total of 42 subjects participated in the experiments. Each subject gave his/her informed consent to participation in the study, which conformed to the requirements of the Declaration of Helsinki and the Western District Ethics Committee of the Greater Glasgow Health Board, UK.
Visual assessments
For each subject, the following assessments were carried out.
Visual acuity
This was determined as the best Snellen acuity for each eye, with additional spherical or cylindrical refraction if necessary. The subjective refraction was then confirmed objectively by retinoscopy using a streak retinoscope at a working distance of 1 m.
Fixation pattern
The uniocular fixation pattern was determined by ophthalmoscopy in which the subject was requested to fixate the central graticule of the ophthalmoscope. By observation of the small glint of light reflected by the foveola, fixation was assessed as being foveal if the glint was located centrally or extra-foveal if located non-centrally. For the latter, the deviation in degrees was determined from the graticule circles. It was also noted whether the fixation was steady, unsteady or, in the presence of eccentric fixation, wandering (Duke Elder & Wybar, 1973).
Extra-ocular muscle balance
Non-strabismic subjects may exhibit heterophoria, which is an imbalance in eye position in the absence of a visual image and is compensated for on normal viewing. By contrast, strabismic subjects exhibit heterotropia, which is an error of alignment during normal viewing. The direction and magnitude of heterophoria and heterotropia were determined with the prism and cover test carried out with the subject fixating at the viewing distance employed in the experiments. The principle of this test is that when one eye is covered, since there is no visual input to guide fixation, that eye will assume its resting position. Hence, on rapid removal of the cover, the eye will be seen to move from its resting position in order to fixate the visual scene. Temporal movement on removal of the cover is indicative of esophoria since the eye must have assumed a nasally directed resting position. The converse is true for exophoria. In practice, this test is carried out alternating the cover over each eye in turn. By placing the appropriate power of prism in front of the dominant eye in normal subjects or the non-amblyopic eye in amblyopes and an alternate cover test carried out, no movement of the eyes is seen. In normal subjects, the prism power equals the magnitude of heterophoria and in strabismics it equals the magnitude of heterotropia and any associated heterophoria. In the cases of microtropia without identity, the magnitude of the manifest deviation was determined by the simultaneous prism and cover test (Dale, 1982). The reason for this is that microtropes who possess anomalous BSV fall into two groups: microtropes with identity who use the parafoveal point which serves as a pseudofovea in both monocular and binocular viewing and microtropes without identity who use a different parafoveal point in monocular and binocular viewing. In the simultaneous prism and cover test, a prism positioned base-out is placed in front of the squinting eye while the non-amblyopic eye is occluded. When the angle of microtropia has been neutralized by the prism, no movement of this eye occurs behind the prism on occlusion of the non-amblyopic eye. This test gives the magnitude of microtropia.
BSV status
The presence or absence of BSV was determined by application of three tests. The Bagolini Striated Lornette generates a line of light at 45 deg in one eye and 135 deg in the other eye. In cases of normal or anomalous BSV, a saltire is perceived, the central part of which may or may not be absent in anomalous BSV, whereas in the absence of BSV one diagonal of the saltire is completely absent (Lyle & Wybar, 1967). The macular Worth four-dot test consists of two laterally placed green spots, a superior red spot and an inferior white spot. The subject views through a red filter in one eye and a green filter in the other. If normal or anomalous BSV is present, the subject reports the presence of the four spots of light. If central suppression is present in one eye, then the number of spots reported is that seen by the other eye viz. three spots with the green filter or two spots with the red filter. The Four Dioptre Prism Reflex Test involves placing a prism of power 4 (resulting in ∼2 deg deviation) in front of the subject's eye in order to deflect the incoming light rays from a point light source: this leads to a conjugate movement in both eyes as the eye behind the prism re-establishes foveal fixation. In subjects with normal BSV, this is then followed by a compensatory movement of the non-prism eye in order to regain fusion of the image while, when central suppression is present, no subsequent movement is observed (Irvine, 1944; Romano & von Noorden, 1969).
Accommodation
The amplitude of accommodation was determined monocularly by adding concave lenses in order to stimulate an increase in accommodation that is equal and opposite to the concave lens. The amplitude of accommodation was the largest power of concave lens with which the subject still possessed best Snellen acuity. Horizontal pupil diameter was estimated for distance viewing with the millimetre rule on the handle of the Romanes occluder.
Stimulus display
The stimulus display consisted of a vertical sinusoidal grating pattern generated on a Tektronix 606B monitor (Tektronix UK Ltd, Bracknell, UK) with a P31 phosphor of peak emission at 520 nm. Fixation was guided by a centrally placed small black spot. The display had a surround of similar hue and luminance to the display. The timebase of the monitor was provided by the ramp output of a Tektronix 5103 oscilloscope running at 0.5 ms div−1, which was fed into the X input of the monitor. A uniform green raster was generated by feeding a 770 kHz triangular wave into the Y input of the monitor. Sine wave modulation of the raster horizontally across the monitor was obtained by feeding a variable sine wave from a Farnell LFP1 oscillator (Farnell Instruments Ltd, Wetherby, UK) into the Z input of the monitor. A stationary sinusoidal grating pattern was obtained by using the trigger output of the oscillator to trigger the Tektronix 5103 oscilloscope which was also used to monitor the frequency and amplitude of the Z modulation sine wave. A calibration graph between the contrast of the sinusoidal grating pattern and the Z modulation voltage was determined psychophysically by the method of Campbell & Green (1965b). The luminance of the display, measured with a UDT Optometer (Optilas Ltd, Milton Keynes, UK), was 8.4 cd m−2 and the room illumination was 1 mcd.
Contrast sensitivity measurements
These were made by the ascending method which provides values very similar to those derived psychometrically based on 50 % detection (Morrison & Reilly, 1986). Starting with a uniform display, the subject increased the display contrast via a 10-turn potentiometric control unit until the grating pattern was just detectable, at which point the peak-to-peak Z modulation voltage was measured. This was repeated six times and a mean contrast threshold was calculated. The reciprocal of this value is the contrast sensitivity. Initially, the subject carried out practice runs at 10, 20 and 30 cycles deg−1 before undertaking the main experiments which were as follows.
Binocular and monocular contrast sensitivities
In order to compare the binocular contrast sensitivities with those for monocular viewing, determinations were undertaken in normal subjects over the range 10–40 cycles deg−1 in 5 cycles deg−1 steps in pseudo-random order for, first, viewing monocularly with the right eye, second, with the left eye and, third, for binocular viewing. For monocular viewing, the companion eye was occluded. In amblyopic subjects, monocular viewing was first with the normal eye and then with the amblyopic eye. This was followed by binocular viewing which, in anisometropes, was bifoveal and, in strabismics, was foveal with the normal eye and extra-foveal with the strabismic eye. In these subjects, an additional spatial frequency of 8 cycles deg−1 was tested since vision at higher spatial frequencies with the amblyopic eye was curtailed.
Control monocular experiments
The possibility that the presence of illumination per se in the companion eye may have affected the contrast sensitivities of the test eye was investigated by comparing monocular viewing with the companion eye occluded against the companion eye viewing through a diffuser. The diffuser, which consisted of medium duty polythene stretched over a trial lens blank, was effective in degrading the highest contrast with minimal loss of luminance. Six contrast threshold readings taken in two groups of three readings were obtained for each viewing condition at 10, 20 and 30 cycles deg−1.
Prismatic correction in strabismics
The effect of bifoveal viewing was determined in strabismics by application of a full diameter prism of appropriate power and orientation, which neutralized the angle of heterotropia as determined with the prism and cover test or simultaneous prism and cover test. The power of the prism was divided between both eyes and generally did not exceed 8 Δ. In microtropes without identity, the angle of deviation neutralized was equal to the difference between the eccentric point in the microtropic eye used on binocular viewing and the pseudofovea used by that eye on monocular viewing. For ease of terminology, this is still referred to as bifoveal viewing. In these experiments binocular contrast sensitivities were determined over the range 8–40 cycles deg−1.
Dichoptic viewing in normal subjects
For comparison with the results from bifoveal viewing in strabismic amblyopes, normal subjects undertook binocular viewing in which the image in one eye was displaced eccentrically from the fovea. This could not be achieved prismatically since an external prism would induce a compensatory translation of the eye in order to maintain bifoveal viewing and hence binocular fusion. Thus, in order to effect stimulation of non-corresponding retinal points, dichoptic viewing of the grating pattern was undertaken. The grating pattern which had a subtense of 2 deg was viewed through an optical system consisting of a 2 inch (50.8 mm) beamsplitter and front silvered mirrors as shown in Fig. 1. Positioned at 2 deg to the left of the centre of the grating pattern was a green light-emitting diode (LED) of subtense 5 min arc. When the right eye fixated on the LED, the grating pattern was located 2 deg temporally, thus falling on the nasal retina and simulating an esotropia of that magnitude. The left eye and right eye grating pattern beams were equalized by inclusion of the appropriate neutral density filter in the path of one beam. Contrast sensitivities were then determined over the range 5–40 cycles deg−1 for: (i) left eye foveal viewing of the grating pattern, (ii) left eye foveal viewing of the grating pattern together with right eye foveal viewing of the green LED in order to determine whether the superimposed image of the LED affected the left eye contrast sensitivities, and (iii) left eye foveal viewing of the grating pattern together with right eye foveal viewing of the green LED and right eye eccentric viewing of the grating pattern, in order to determine whether the eccentric grating pattern affected the left eye contrast sensitivities. These experiments were also performed on simple anisometropic amblyopes in whom foveal viewing of the grating pattern was made with the non-amblyopic eye and eccentric viewing of the grating pattern was made with the amblyopic eye.
Figure 1. Schematic representation of the dichoptic viewing apparatus.
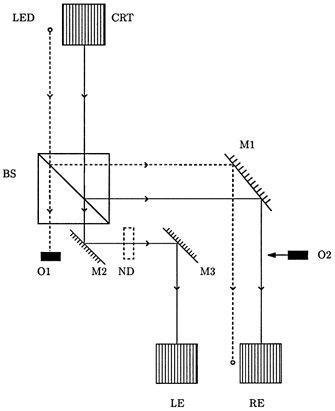
This shows the path from the cathode ray tube (CRT) display and light emitting diode (LED) to the left eye (LE) and right eye (RE) of the subject who is positioned at the lower margin of the figure, looking upwards. The beams are divided by the beamsplitter BS. The reflected beams are further reflected through 90 deg by the front silvered mirror M1. The right eye may thus view either both the LED and CRT or, after occlusion of the CRT beam by the movable occluder O2, the LED beam alone. Of the beams transmitted by the beamsplitter, the LED beam is occluded by the fixed occluder O1. The CRT beam is reflected twice through 90 deg by the front silvered mirrors M2 and M3. The two CRT beams are equalized by inclusion of the neutral density filter ND. Mirror M1 is mounted on a rotatable stage to allow adjustment of its orientation.
Dichoptic viewing in strabismics
In order to test the repeatability of the results for bifoveal viewing in strabismics, the dichoptic apparatus was also employed in a number of these subjects. Monocular contrast sensitivities were first determined for foveal viewing of the grating pattern with the non-amblyopic eye. The use of the green LED to guide fixation of the strabismic eye was not practicable in these subjects since they exerted a constant magnitude of heterotropia. Therefore, the apparatus was first arranged for bifoveal viewing for a normal subject without the LED. After the angle of heterotropia in the strabismic subject had been determined, the apparatus was adjusted in order to effect bifoveal stimulation in that subject. This procedure was followed for each strabismic subject.
Control prism experiments
In order to determine whether the prisms used for prismatic correction in strabismics had any degradative effect on contrast sensitivity, control experiments were undertaken by placing a prism in front of one eye which then executed a compensatory translation to maintain foveal fixation. Contrast sensitivities were determined over the range 8–40 cycles deg−1 for normal viewing and with prisms of power 2, 4, 6, 8, 10 and 12 Δ. The prism was placed base-out in cases of esophoria and base-in in cases of exophoria.
Binocular and monocular contrast sensitivities, prismatic correction in strabismics and control prism experiments were carried out at a viewing distance of 2.86 m. Control monocular experiments, dichoptic viewing in normal subjects and dichoptic viewing in strabismics were carried at 2.43 m. The display subtense was arranged to be 2 deg for both viewing distances. The total combined experimental time for binocular and monocular contrast sensitivities and prismatic correction in strabismics was 3–4 h, for control monocular experiments the total time was 0.5–0.75 h, and for dichoptic viewing in normal subjects, dichoptic viewing in strabismics and control prism experiments the total combined time was 2–3 h. The experiments were carried out at a measured pace with frequent breaks.
Statistical analysis
Analyses of the data were performed post hoc at the end of each group of experiments with the Minitab 11 (Minitab Ltd, Coventry, UK) statistical package (Ryan & Joiner, 1994). Depending on the subject group, the difference between better eye and poorer eye viewing, binocular and better eye viewing, viewing with and without a prism and, in the dichoptic viewing experiments, viewing in the absence and presence of the eccentrically located grating pattern was calculated at each spatial frequency. The dependence of the calculated difference on spatial frequency was tested by linear regression analysis. In all cases, the differences in contrast sensitivity were not related to spatial frequency (R2 < 56 %, P > 0.1). This allowed the calculation for each subject of a mean overall difference which was the difference in contrast sensitivity between the two viewing states averaged over the spatial frequency range. These values were expressed as a percentage of the reference contrast sensitivities, e.g. better eye viewing in the comparison between binocular and better eye viewing. Within each group of subjects, these values were compared against zero, i.e. application of Student's paired t test. Between different groups of subjects, the values were compared with a two sample t test. Values are given as the mean ±s.e.m. The relationships between logarithm contrast sensitivity and spatial frequency and between contrast sensitivity differences and decimal Snellen acuity were analysed by linear regression analysis. Statistical significance was taken when P < 0.05.
RESULTS
Initially, the subject groups were based on the criteria that normal vision required a Snellen acuity of 6/6 or better, corrected if necessary, while amblyopia was defined as 6/9 or poorer. All amblyopic subjects had uniocular amblyopia of varying severity. Normal subjects had foveolar fixation in both eyes and normal BSV. Simple anisometropic amblyopes also had foveolar fixation in both eyes and normal BSV together with a refractive error difference between eyes of at least 1.00 D in any meridian without the presence of microtropia. Microtropes exhibited an esotropia of no more than 10 Δ, the presence of superimposed heterophoria, extra-foveolar fixation and central suppression in the microtropic eye, and well established anomalous BSV. Esotropic amblyopes with anomalous BSV consisted of small to moderate angle esotropes who exhibited an absence of heterophoria, foveolar fixation in each eye for monocular viewing, and anomalous BSV. Esotropic and exotropic amblyopes without BSV exhibited heterotropia, showed an absence of BSV and the state of fixation of the amblyopic eye, i.e. whether foveolar or eccentric, was not critical. Non-amblyopic esotropes and exotropes exhibited Snellen acuity of 6/6 or better in both eyes, heterotropia of constant magnitude and no clinical evidence of BSV as indicated by the presence of constant central and peripheral suppression.
However, after completion of the contrast sensitivity measurements, several anomalies became apparent. Two of the original 11 normal subjects showed a mean overall difference in contrast sensitivity between eyes of 40 and 43 % despite having Snellen acuities of 6/5: these were significantly different from the distribution for the remaining nine subjects (P < 0.001). On the basis that these subjects could not be considered normal, they were excluded from the normal group. In two non-amblyopic esotropes, mean overall differences between eyes of 44 and 51 % suggested that the poorer eye was amblyopic and these subjects were incorporated into the group of esotropic amblyopes without BSV. Similarly, two non-amblyopic exotropes whose mean overall differences were 41 and 44 % were incorporated into the group of exotropic amblyopes without BSV. Finally, on the basis of the similarity of the magnitude of esotropia and the contrast sensitivity results, the microtropes and esotropic amblyopes with anomalous BSV were combined into one group. The revised groups are shown in Table 1. In addition, all subjects had adequate accommodation to focus the displays and there were no abnormalities in pupil diameters, which were broadly equal within the precision of the measurements.
Table 1.
Clinical data for revised groups of subjects
| Subject category | n | Age (years) | LE or NE | RE or AE | Diff (Sph Eq) | Tropia |
|---|---|---|---|---|---|---|
| Normal + BSV | 9 | 20–45 | 6/4–6/5 | 6/4–6/5 | 0–0.75 DS | 0 |
| Anisometropes + BSV | 9 | 27–48 | 6/4–6/6 | 6/9–6/36 | 1.25–5.00 DS | 0 |
| Esotropes + aBSV | 9 | 19–47 | 6/4–6/6 | 6/9–6/12 | 0–2.50 DS | 4–6 Δ |
| Esotropes – BSV | 7 | 19–46 | 6/5–6/6 | 6/9–6/60 | 0–1.75 DS | 6–30 Δ |
| Exotropes – BSV | 4 | 34–52 | 6/4–6/9 | 6/4–6/36 | 0–2.00 DS | 12–24 Δ |
| Non-amblyp Eso – BSV | 2 | 18–26 | 6/4–6/6 | 6/4–6/6 | 0–0.50 DS | 18–40 Δ |
BSV, binocular single vision (+ present, − absent); aBSV, anomalous binocular single vision; LE, left eye; NE, normal eye; RE, right eye; AE, amblyopic eye; Diff (Sph Eq), difference in refraction in spherical equivalents; Tropia, magnitude of esotropia or exotropia in prism dioptres.
Monocular and binocular contrast sensitivities
Over the range of spatial frequencies studied, logarithm contrast sensitivity declined monotonically until the high spatial frequency cut-off was reached (Fig. 2A). For each of the nine normal subjects, the relationship was well described by a straight line of negative slope which was statistically significant (R2 > 87 %, P < 0.02). In terms of the mean overall contrast sensitivity, one eye performed better than the companion eye with a range of mean differences over the spatial frequency range studied of 6–29 %. For the group as a whole, the mean overall difference between the better eye and the poorer eye was 18 ± 3 %, which was statistically significant (P = 0.002).
Figure 2. Comparison of binocular and monocular contrast sensitivities.
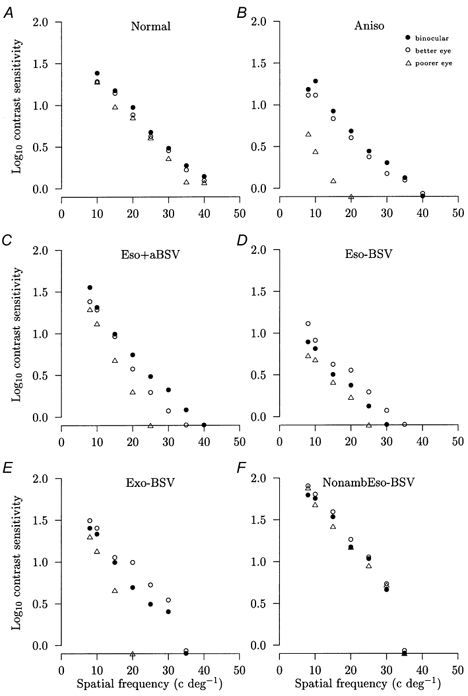
Comparisons of the mean logarithm contrast sensitivities against the spatial frequency of the sinusoidal grating pattern display for binocular viewing (•), viewing through the better or non-amblyopic eye (○) and viewing through the poorer or amblyopic eye (▵) for representative subjects. For each subject, the change in contrast sensitivity averaged over the range of spatial frequencies is shown for the poorer eye (PE) and binocular viewing (BIN) compared with the better eye taken as 100 %. A, normal subject with BSV: PE = −17 %, BIN = +11 %. B, anisometropic amblyope with BSV: PE = −65 %, BIN = +28 %. C, esotropic amblyope with anomalous BSV: PE = −40 %, BIN = +37 %. D, esotropic amblyope without BSV: PE = −44 %, BIN = −26 %. E, exotropic amblyope without BSV: PE = −56 %, BIN = −29 %. F, non-amblyopic esotrope without BSV: PE = −18 %, BIN = −14 %. All changes for PE and BIN are significant (P < 0.05). The s.e.m. is smaller than the symbol size and symbols falling on the x axis denote that that spatial frequency was not detected.
The amblyopic groups, as might be expected, showed a much more pronounced reduction between the better eye and the amblyopic eye, reflecting the downwards shift of the contrast sensitivity function (Fig. 2B-E). The mean overall reduction in anisometropes was 45 ± 9 % (range −8 to −88 %); in esotropes with anomalous BSV, it was 53 ± 7 % (range −26 to −87 %); in esotropes without BSV, it was 60 ± 7 % (range −31 to −51 %); and in exotropes without BSV, it was 57 ± 1 % (range −41 to −87 %). In each case the difference was statistically significant (P = 0.001). In the two non-amblyopic esotropes, the mean overall reductions were 18 and 22 % (Fig. 2F), which were similar to the values for the normal subjects.
A further outcome was that the non-amblyopic eye of our amblyopic subjects did not perform as well as the better eye of our normal subjects, which was inferred from the following analysis. For each subject the contrast sensitivity values were summed over the range 10–40 cycles deg−1 and the values for the normal group were normalized so that they gave a mean value of 100 % for the group. This normalization factor was then applied to the results of the amblyopic subjects, which are shown in Table 2. The mean value for the 30 amblyopes was 76.1 %, which was significantly different from that of the normal group (P = 0.008). Hence, these results indicate that the non-amblyopic eye of amblyopes was, in fact, not normal.
Table 2.
Percentage change in total contrast sensitivity
| Subject category | n | Better eye (%) | P | Binocular (%) | P |
|---|---|---|---|---|---|
| Normal + BSV | 9 | 100 ± 6.3 | — | 100 ± 5.9 | — |
| Anisometropes + BSV | 9 | 68.7 ± 9.9 | 0.02 | 76.0 ± 9.4 | 0.05 |
| Esotropes + aBSV | 9 | 74.1 ± 11.0 | 0.061 | 83.0 ± 8.2 | 0.11 |
| Esotropes – BSV | 7 | 84.4 ± 8.8 | 0.18 | 72.7 ± 8.5 | 0.023 |
| Exotropes – BSV | 4 | 83.0 ± 7.1 | 0.12 | 67.8 ± 5.7 | 0.004 |
| All amblyopes | 30 | 76.1 ± 5.0 | 0.008 | — | — |
| Amblyopes with BSV | 18 | — | — | 79.5 ± 6.1 | 0.025 |
Comparison of contrast sensitivities of amblyopic groups against the normal group whose values were normalized to give a mean of 100 %. Values are expressed as means ±s.e.m. and P values were obtained with Student's two-sample t test. BSV, binocular single vision (+ present, − absent); aBSV, anomalous binocular single vision.
With respect to the comparison between binocular and better eye viewing, the normal group showed an overall mean increase of 13 ± 5 % (range 3–49 %) which, while small, was nonetheless statistically significant (P = 0.02) (Fig. 2A). Both the anisometropes and esotropes with anomalous BSV showed much larger increases in the mean overall contrast sensitivity of 35 ± 7 % (range +6 to +77 %) and 38 ± 11 % (range +15 to +67 % with one subject at −26 %), respectively (P < 0.01) (Fig. 2B and C). The enhancement present in the anisometropic group was significantly higher than that of the normal group (P = 0.03), as was the enhancement shown by the esotropes with anomalous BSV after the omission of one subject who showed a reduction (P = 0.01). By contrast, the groups without BSV showed different results. Binocular contrast sensitivities showed a mean overall reduction of 14 ± 3 % (range −5 to −26 %) in esotropes without BSV and 26 ± 6 % (range −11 to −41 %) in exotropes without BSV (Fig. 2D and E). These reductions, which were significant (P = 0.003), were not related to the depth of amblyopia expressed as the decimal Snellen acuity (R2 = 0 %, P = 0.71). The two non-amblyopic esotropes showed a mean overall increase of 2 % and a decrease of 14 % (Fig. 2F). When the binocular contrast sensitivities over the range 10–40 cycles deg−1 were normalized and summed, the amblyopes with BSV collectively showed a mean value which was 20.5 % less than that for the normal group (Table 2, P = 0.025). Hence, while the binocular contrast sensitivities of amblyopes with BSV showed a marked increased over those of their non-amblyopic eye alone, they still did not attain the values of normal subjects.
Control monocular experiments
These experiments were undertaken to determine whether an isoluminant uniform display presented to the companion eye affected the contrast thresholds of the test eye. Comparisons were made at 10, 20 and 30 cycles deg−1 between the contrast sensitivities when the companion eye viewed through the diffuser against when the companion eye was occluded. In two normal subjects, two esotropes without BSV and an exotrope without BSV, no significant differences occurred at any of the three spatial frequencies tested (P > 0.05). The mean overall differences between the diffuser and occluder contrast sensitivities were +2.0 and +5.5 % for the two normal subjects, +7.8 and +4.3 % for the two esotropes and −1.5 % for the exotrope.
Prismatic correction in strabismics
The application of the appropriate prismatic correction to effect bifoveal stimulation in strabismics on binocular viewing caused a significant reduction in contrast sensitivity compared with natural binocular viewing in these groups (Fig. 3A-D). In the esotropes with anomalous BSV, the mean overall reduction was 25 ± 4 % (range −7 to −39 %) (P = 0.0002) and, in the esotropes without BSV, it was 27 ± 5 % (range −4 to −44 %) (P = 0.003) (Fig. 3A and B). Three exotropes without BSV showed a reduction (range −6 to −44 %) while one exotrope showed an increase of 8 % giving a mean reduction of 15 ± 11 % which was not significant (P = 0.26) (Fig. 3C). The two non-amblyopic esotropes without BSV showed reductions of 33 and 53 % (Fig. 3D). Hence, out of the 22 strabismics in whom bifoveal stimulation was effected, 21 showed showed a reduction in contrast sensitivity. This reduction was not related to the depth of amblyopia expressed as the decimal Snellen acuity (R2 = 0 %, P = 0.48).
Figure 3. Bifoveal viewing experiments.
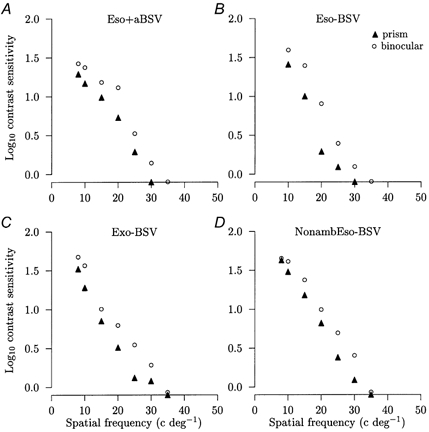
Comparisons of the mean logarithm contrast sensitivities against the spatial frequency of the sinusoidal grating pattern display between normal binocular viewing (○) and binocular viewing with bifoveal stimulation effected by prismatic correction (▴) for representative subjects. The percentage difference between binocular viewing with the prism (BIN +Δ) and binocular viewing is given for each subject. A, esotropic amblyope with anomalous BSV: (BIN +Δ) = −37 %. B, esotropic amblyope without BSV: (BIN +Δ) = −44 %. C, exotropic amblyope without BSV: (BIN +Δ) = −44 %. D, non-amblyopic esotrope without BSV: (BIN +Δ) = −33 %. The reductions are all significant (P < 0.05). The s.e.m. is smaller than the symbol size and symbols falling on the x axis denote that that spatial frequency was not detected.
Control prism experiments
Monocular contrast sensitivities were determined for the better eye of four normal subjects for viewing through prisms of increasing power. Comparison of the contrast sensitivities at each spatial frequency with and without the prism showed that there were no significant changes over the range 8–35 cycles deg−1 with prisms of power 2, 4, 6 and 8 Δ (P > 0.05). For the subject shown in Fig. 4A-D, no significant changes occurred at 8–25 cycles deg−1 with 10 and 12 Δ (P > 0.05) while significant reductions did occur at 30–40 cycles deg−1 (P < 0.05). In the group data, with prisms of 10 and 12 Δ, no significant changes occurred at 8–15 cycles deg−1 (P > 0.05) while significant reductions did occur at 20–35 cycles deg−1 (P < 0.05). This indicated that degradation did occur, but only with the largest prism powers at the higher spatial frequencies.
Figure 4. Control prism experiments.
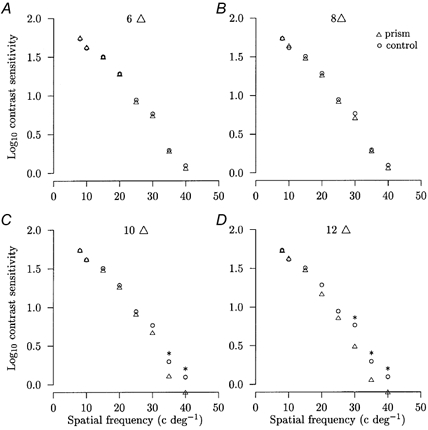
Mean logarithm contrast sensitivity against the spatial frequency of the sinusoidal grating pattern display between normal viewing with the better eye (○) and viewing through a prism of stated power (▵) for a representative subject. *Statistical significance (P < 0.05). The s.e.m. is smaller than the symbol size and symbols falling on the x axis denote that that spatial frequency was not detected.
Dichoptic viewing in normal and strabismic subjects
Since prisms could not be used to create an eccentrically positioned image in normal subjects, recourse was made to dichoptic stimulation in which one eye fixated on the green LED so that the grating pattern was positioned 2 deg temporally. Contrast sensitivities were determined monocularly over the range 5–35 cycles deg−1 in six normal subjects. The range of spatial frequencies was slightly truncated compared with previous experiments due to the reduced luminance of the CRT display resulting from division of the beam. With the left eye viewing the grating pattern, the superimposition of the green LED seen by the right eye had no effect on the monocular contrast sensitivities of the left eye alone (P = 0.43). Comparison of the former set of results with contrast sensitivities for left eye foveal viewing of the grating pattern and right eye foveal viewing of the green LED with the eccentrically located grating pattern also showed no significant change. The mean overall difference between the results with and without the eccentrically located grating pattern in the right eye was −1 ± 1 % (P = 0.17). In two anisometropes, the mean overall contrast sensitivity change was +2 and −1 %. These results, which are illustrated in Fig. 5A and B, showed that the eccentrically positioned grating pattern was without effect on contrast sensitivities recorded through the companion eye.
Figure 5. Dichoptic viewing experiments.
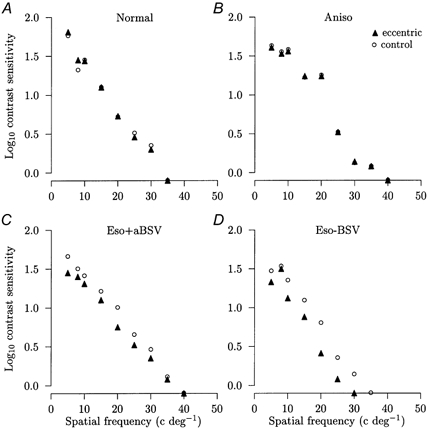
Comparisons of the logarithm contrast sensitivities against the spatial frequency of the sinusoidal grating pattern display between normal monocular viewing of the display with the better or non-amblyopic eye and foveal viewing of the green LED in the other eye (○ in A-D) and monocular viewing of the display with the better or non-amblyopic eye and foveal viewing of the green LED together with eccentric viewing of the grating display by the other eye (▴ in A-D). The percentage difference as a result of additionally viewing the eccentric display (ECC) is given for each subject. A, normal subject: ECC = +2 % (not significant). B, anisometropic amblyope: ECC = −1 % (not significant). C, esotropic amblyope with anomalous BSV: ECC = −25 % (P < 0.01). D, esotropic amblyope without BSV: ECC = −33 % (P < 0.01). The s.e.m. is smaller than the symbol size and symbols falling on the x axis denote that that spatial frequency was not detected.
By contrast, under conditions of bifoveal stimulation, two esotropic amblyopes with anomalous BSV showed mean overall reductions in contrast sensitivity of 23 and 25 % (Fig. 5C), while three esotropic amblyopes without BSV showed reductions of 33, 33 and 50 % (Fig. 5D). Clearly, the presence of the grating pattern image on the fovea of the strabismic eye caused a marked reduction in contrast sensitivity, thus confirming the results of the prism experiments.
DISCUSSION
Our measurements of contrast sensitivities over the high spatial frequency limb of the contrast sensitivity function have produced two clear cut results. The first, which is summarized in Fig. 6A, shows that the presence of BSV was associated with binocular facilitation in nine normal subjects and in 17 out of 18 amblyopes with normal or anomalous BSV. Furthermore, the amount of binocular facilitation was significantly greater in both anisometropes and esotropes with anomalous BSV than in normal subjects. The second result, which is summarized in Fig. 6B, is that in esotropic amblyopes with and without BSV, non-amblyopic esotropes without BSV and exotropes without BSV, bifoveal stimulation effected by prismatic correction caused a marked reduction in contrast sensitivities compared with normal binocular viewing.
Figure 6. Summary of results.
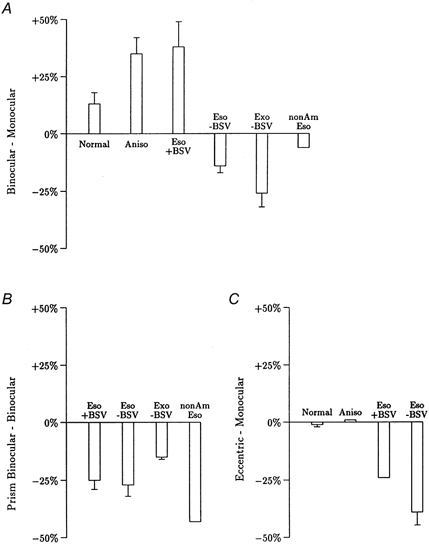
Overall percentage change in contrast sensitivity averaged over the spatial frequencies studied. Columns show mean ±s.e.m. except where n = 2 where mean only is shown. A, differences between binocular and better/non-amblyopic eye monocular viewing in normal subjects (Normal, n = 9), anisometropes with BSV (Aniso, n = 9), esotropes with anomalous BSV (Eso + BSV, n = 9), esotropes without BSV (Eso - BSV, n = 7), exotropes without BSV (Exo - BSV, n = 4) and non-amblyopic esotropes without BSV (nonAmEso, n = 2). B, difference between bifoveal viewing effected by prismatic correction and normal binocular viewing (Prism Binocular - Binocular) for the same heterotropes as in A. C, differences between normal monocular viewing with the better/non-amblyopic eye plus foveal viewing of the green LED together with eccentric viewing of the grating display by the other eye and normal monocular viewing with the better/non-amblyopic eye plus foveal viewing of the green LED in the other eye (Eccentric - Monocular) for six normal subjects, two anisometropes, two esotropic amblyopes with anomalous BSV and three esotropic amblyopes without BSV.
Subjects with BSV or anomalous BSV
On binocular viewing by normal subjects, the mean increase in contrast sensitivity of 13 % compared with the better eye was less than previous reports of 40 % (Campbell & Green, 1965a; Ross et al. 1985; Pardhan & Gilchrist, 1990). The value reported by Campbell & Green (1965a) is for comparison against the mean value, while Ross et al. (1985) and Pardhan & Gilchrist (1990) did not state the basis of their comparisons. Against the better eye, our result is comparable with the 17 % increase in contrast sensitivity recorded by Marshall et al. (2001) and the 10 % reduction in reaction time recorded by Blake et al. (1980). A possible confounding factor in the interpretation of our results was that monocular contrast sensitivities were obtained with the companion eye occluded so that the presence of a bright field in this eye on binocular viewing may have had a depressive action on the binocular contrast sensitivities. However, since monocular contrast sensitivities were not affected when the companion eye viewed through a diffuser compared with when the eye was occluded, this can be excluded. This is in agreement with previous results for contrast sensitivities (Campbell & Green, 1965a; Pardhan & Gilchrist, 1990) and reaction times for detection of a grating pattern (Harwerth et al. 1980).
The increases in binocular contrast sensitivities of 35 and 38 % in anisometropes and esotropes with anomalous BSV have no precedents in the literature. A small number of microtropes has been shown to have a small improvement in visual acuity on binocular viewing while a single anisometrope showed none (Sireteanu et al. 1981). In another study, a small number of anisometropes was shown to have an absence of binocular summation at low spatial frequencies (Holopigian et al. 1986). By contrast, in patients with Duane syndrome who had reduced abduction but attained and maintained BSV by adopting an abnormal head posture, binocular summation was 32 % when compared with the better eye (Marshall et al. 2001); this complements the present results very well.
Amblyopes without BSV
The absence of BSV was associated with binocular inhibition which occurred in all 11 heterotropes without BSV. Whether a reduction also occurs in non-amblyopic esotropes without BSV cannot be resolved from our sample of two subjects. Since monocular contrast sensitivities were the same irrespective of whether the companion eye was occluded or illuminated, which is consistent with the reaction time data of Levi et al. (1979), this confirms that the binocular inhibition required the presence of spatial contrast and not just illumination. Pardhan & Gilchrist (1990) have demonstrated an inhibitory action on monocular contrast sensitivities of a positive defocusing lens of 2–3 DS worn in front of the companion eye in normal subjects. This, however, is unlikely to be related to the inhibition present in amblyopes since it can be accounted for by the 180 deg phase shift (i.e. contrast reversal) of the defocused image and the subsequent combination of out-of-phase images (Hopkins, 1955). Nor is the binocular inhibition a straightforward manifestation of suppression as conventionally understood. Normally, suppression is exerted by the normal eye on the input from the strabismic eye, though not in the reverse direction (Travers, 1938; Sireteanu, 1982). The suppression affects the fovea and the region of nasal retina aligned with the visual axis of esotropes and may encompass the intervening retina (Lyle & Wybar, 1967) while, in exotropes, the suppression area may consist of the entire temporal retina of the deviating eye (Jampolsky & Schlor, 1955). While the operation of suppression, as conventionally understood, accounts for the absence of binocular enhancement in heterotropes without BSV, it does not explain the reduction of contrast sensitivities to below those of the non-amblyopic eye. This may be regarded as suppression of the non-amblyopic eye by the amblyopic eye, which is a new finding. An additional result of importance is that, in all types of amblyope, the non-amblyopic eye had subnormal contrast sensitivities (Table 2) which confirms the previous reports of Leguire et al. (1990) and Holopigian et al. (1991) though not of Hess & Howell (1977) who concluded that the non-amblyopic eye was normal.
Bifoveal viewing
All heterotropes, regardless of whether BSV was present or absent, showed marked reductions in binocular contrast sensitivities on binocular viewing. An effect of prismatic degradation in our experiments may be excluded since this occurred only for prism powers of at least 10 and 12 Δ and, then, only at higher spatial frequencies (Fig. 4). The only group which might have been affected were the two non-amblyopic esotropes who required 18 and 40 Δ and who detected spatial frequencies of up to 30 cycles deg−1. Further evidence, which excludes prismatic degradation as an important factor, is that the reduction in contrast sensitivity was also reproduced in the dichoptic viewing experiments in five esotropes who had previously undertaken the prism experiments (Fig. 6C). Hence, it may be asserted with confidence that bifoveal stimulation results in diminished contrast sensitivities that may be inferred to arise from an inhibitory action of the neural input of the fovea of the misaligned eye. In these cases, the fovea of the misaligned eye may be deemed to be non-corresponding to the fovea of the normal eye, irrespective of whether or not the individual had anomalous BSV. This is in marked contrast to normal subjects and anisometropes, both of whom possess normal BSV. In these groups, there was no reduction in contrast sensitivity on binocular viewing when one grating pattern was located eccentrically, which indicates that the eccentric pattern was essentially disregarded. This is consistent with the lack of effect of an illuminated field in one eye on the contrast sensitivities through the other eye. Hence, the binocular inhibition present on bifoveal viewing is specific to the presence of ocular misalignment. While this again represents the operation of suppression of the non-amblyopic eye by the amblyopic eye, it would be premature to view it as conventional suppression working in the reverse direction. Conventional suppression is inversely related to the depth of amblyopia (Holopigian et al. 1988), which is not the case with suppression phenomenon we have described (see Results).
The model
In order to explain our results, a model is presented in Fig. 7, which shows the proposed neural projections in normal and amblyopic subjects. The question as to whether the differences in inputs arise from reduced numbers of neurones or from reduced firing rates is deferred until later. The basis of the neural projections to both left and right visual cortices is that the grating pattern which subtended 2 deg is viewed by the central fovea from which 50 % nasal retinal ganglion cells project temporally and 50 % temporal retinal ganglion cells project nasally within in a vertical strip of visual field of width 1 deg (Bunt et al. 1977). Additionally, callosal fibres projecting between the V1-V2 boundary extend into V1 by 0.5–1 mm (Van Essen & Zeki, 1978), which corresponds to about 0.1 deg (Daniel & Whitteridge, 1961), leading to further bilateral representation of the central foveal projections. For normal viewing (Fig. 7A), convergence of the left and right eye inputs onto the binocular integrating centre occurs at visuotopically corresponding locations in the left and right cortices which, while shown as primary visual cortex, may reside in prestriate cortex. The strong excitatory inputs (shown in blue) are proposed to be balanced by mutual inhibition (shown in red) so that the overall binocular facilitation is rather modest. A difference arises in anisometropes for whom the excitatory inputs are shown to be slightly reduced for the normal eye and markedly reduced for the amblyopic eye (Fig. 7B). The mutual inhibition is proposed to be of high threshold so that it may be reduced or, as shown in Fig. 7B, absent. The net effect is enhancement of binocular facilitation compared with normal. This is not the state of affairs in esotropes without BSV (Fig. 7C) in whom the stimulated part of the right eye (nasal retina) is shown as projecting to a different binocular integrating centre (dotted circle) in the left hemisphere, since this is a non-corresponding retinal area. This input is proposed to weakly stimulate its own binocular integrating centre while exerting appreciable inhibition on the integrating centre innervated by the left eye. This would account for the inhibition recorded on binocular viewing. A comparable arrangement may be proposed for exotropes without BSV with the difference that, since the image falls on the temporal retina which projects ipsilaterally, the inhibitory interactions occur in the right hemisphere. In esotropes with anomalous BSV, a new retinal correspondence has been established so that the nasal retina of the right (esotropic) eye has an excitatory projection to the same binocular integrating centre as the left eye (Fig. 7D). As in the case of anisometropes, the mutual antagonism mechanism is proposed to be subthreshold so that binocular facilitation occurs.
Figure 7. Schematic model.
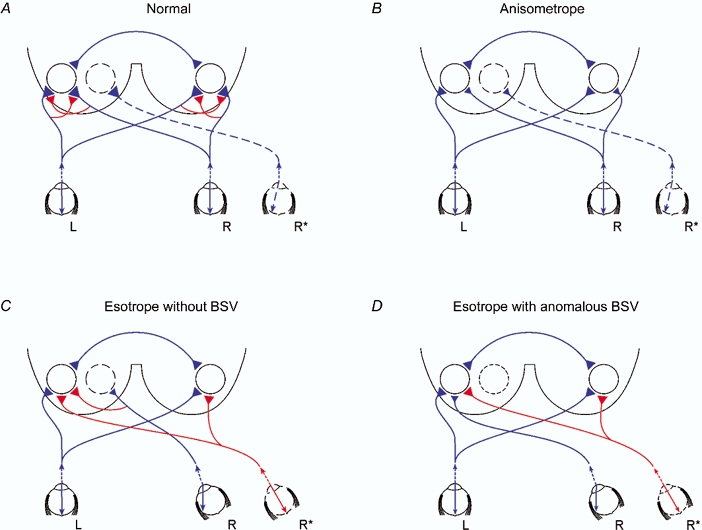
Proposed neural projections to account for present results, showing image projection into each eye and the neural projection, omitting the lateral geniculate nucleus, to the binocular integrating unit represented by the circles in the left and right visual cortices. Excitatory projections are shown in blue and inhibitory projections in red, without any implication as to how the inhibition is mediated. Magnitude of input to visual cortex is represented qualitatively by the size of the projection terminal. Binocular integrating centres in the two hemispheres are shown to be connected through the corpus callosum. L, left eye; R, right eye; R* is right eye with deviation of image onto nasal retina (A and B) or fovea (C and D). A, normal subject showing balanced left and right eye excitatory inputs which are attenuated by the action of high threshold mutual inhibition. The displaced image in the nasal retina of the right eye gives rise to a projection to the left visual cortex. This is shown not to be connected to the binocular integrating centre driven by the foveae of the left and right eyes. B, anisometrope amblyope showing reduced excitatory inputs both from the non-amblyopic eye and amblyopic eye and an absence of mutual inhibition to account for the enhanced binocular facilitation. As in A, the nasally directed image in the right eye has no adverse effect on contrast sensitivities. C, esotropic amblyope without BSV showing reduced excitatory inputs both from the non-amblyopic eye and amblyopic eye. In this case, there is a powerful inhibitory action of the esotropic eye input on the binocular integrating centre. Both binocular integrating centres are subjected to inhibition from foveal stimulation of the right eye. A similar scheme would apply to exotropes without BSV except that there is stimulation of the temporal retina and the binocular interactions are reflected onto reverse cortices. D, esotropic amblyope with anomalous BSV showing reduced excitatory inputs both from the non-amblyopic eye and amblyopic eye but this time leading to binocular facilitation due to establishment of new retinal correspondence; however, both binocular integrating centres are subjected to inhibition from foveal stimulation of the right eye as in C.
The projections activated during the deviation of the image in the right eye either prismatically or dichoptically are represented by R*. In normal subjects and anisometropes, the image that falls on the nasal retina projects to a binocular integrating centre in the contralateral hemisphere, which is proposed to be essentially disconnected from the binocular integrating centre for foveal viewing (Fig. 7A and B). By contrast, in both esotropes without BSV and esotropes with anomalous BSV, stimulation of the fovea of the right eye is proposed to lead to inhibition of the binocular integrating centre for foveal viewing (Fig. 7C and D). This is a marked difference from normal subjects and anisometropes.
Comparisons with psychophysical data
Steady viewing of a high contrast display is a well established method of investigating the characteristics of excitatory and inhibitory visual processes. The presence of a stationary grating pattern mask in one eye leads to increased contrast thresholds for detection of the grating pattern through the other eye so that the test contrast threshold is approximately linearly related to the mask contrast (Legge, 1979). This process of dichoptic masking which involves the inter-ocular transfer of adaptation is appreciably more powerful than masking in the same eye. Since binocular summation is said to depend on balanced signals from both eyes, as a possible mechanism for amblyopia an imbalance would give rise to dichoptic masking resulting in impaired vision. However, this can be excluded since there was no relationship between binocular summation and the depth of amblyopia expressed as decimal acuity, which is taken as a measure of binocular imbalance, in both anisometropes and esotropes with anomalous BSV (R2 = 12 %, P = 0.4; R2 = 21 %, P = 0.2, respectively). Dichoptic masking experiments carried out in anisometropes for whom the grating contrast was normalized against the contrast threshold showed the amblyopic eye to have a reduced or normal masking effect on the non-amblyopic eye while the normal eye had a reduced masking effect on the amblyopic eye (Harrad & Hess, 1992). Reduced masking effects may reasonably be reconciled with the proposed model on the basis of the reduced excitatory drive from both non-amblyopic and amblyopic eyes. With respect to the cases of a normal masking action from the amblyopic eye, this may be a consequence of the normalization of contrast against contrast threshold thus lifting the absolute contrast to above that required to recruit the high threshold inhibition proposed in the model. With respect to esotropes, the model predicts that those with anomalous BSV should show a reduced or normal masking effect of the amblyopic eye on the non-amblyopic eye while those without BSV should have an absence of a masking effect. Both types have been described by Harrad & Hess (1992), though correlation with BSV status is not possible from the clinical data presented. All esotropes showed an enhanced masking effect of the normal eye on the amblyopic eye that is not readily explained by the present model unless it is postulated that esotropes have a loss of mutual inhibition in excess of that shown by anisometropes, resulting in greater dichoptic masking. So, while the proposed model accommodates many of the results of Harrad & Hess (1992), some problems remain. However, the effects of adaptation may not necessarily reflect the dynamics of neural interactions investigated in the present study, not least because the spatial frequency ranges employed were markedly different.
Comparisons with neuronal recording data
These have been undertaken with data mainly from primate experiments though, more recently, data have been provided by brain imaging experiments in man. Generally, recourse to cat data has been made only when none are available for primate, since cat cortical neuronal responses show a much greater incidence of binocularity and poorer spatial resolution (Hubel & Wiesel, 1962, 1968).
Normal binocular viewing
On binocular viewing, we showed that contrast sensitivities were enhanced by a small though significant amount. This is likely to have as its neuronal basis the binocular summation which occurs as a result of the convergence of left and right eye inputs onto cortical neurones located in layers 2, 3, 4B and 4Cα of area V1 of primate (Hubel & Wiesel, 1968; Poggio, 1995). However, binocular summation is by no means a general property of cortical neurones since the majority show the same response magnitude on binocular as on monocular stimulation of the better eye alone (Hubel & Livingstone, 1987; Crawford et al. 1996). So it is not unreasonable to surmise that the modest summation present in our experiments is attributable to a modest average increase in firing across a large population of cortical neurones. The operation of concurrent inhibitory processes also has a basis in that inhibitory projections are present in primate cortex (Callaway, 1998). Additionally cortical neurones of the cat are characterized by diminishing response gain with increasing stimulus contrast for both monocular and binocular stimulation (Truchard et al. 2000).
Binocular viewing in anisometropia and esotropia
One problem in relating the present results to neuronal data from amblyopic primates is that the status of an animal's BSV is generally not known. However, it may reasonably be inferred that anisometropic animals have BSV, while animals with large angle esotropia and exotropia must have an absence of BSV. Monocular deprivation studies are generally not relevant to the present discussion since the resultant amblyopia is the most severe due to the presence of all three amblyopiogeneic factors viz. light deprivation, form deprivation and abnormal binocular interactions (von Noorden, 1976). Data for anisometropic primates are scarce but there is a loss of binocularly activated neurones from area V1 (von Noorden & Crawford, 1977; Movshon et al. 1987; Kiorpes et al. 1998). Similarly, surgically induced esotropia and prism rearing which simulates esotropia lead to a dramatic loss of binocularly activated neurones in area V1 (Crawford & von Noorden, 1979; Wiesel, 1982; Crawford et al. 1996), though more recent results indicate that the loss may be far from as devastating as first reported. Kiorpes et al. (1998) described the spatial resolution and contrast sensitivity of neurones driven by the amblyopic eye as being similar to those driven by the normal eye and an abnormality was apparent only on a population basis. A neural deficit in area V1 in esotropic amblyopes has also been confirmed by magnetoencephalography (MEG; Anderson et al. 1999) and functional magnetic resonance imaging (fMRI; Goodyear et al. 2000; Barnes et al. 2001). The fMRI studies are especially noteworthy since the reduced amplitude of response was associated with a reduced number of active voxels, which indicates that a reduced number of neurones were activated. Hence, on balance, amblyopia appears to have as its basis a reduced number of neurones driven by the amblyopic eye, which clarifies the nature of the projections in Fig. 7.
There also exists evidence for the neuronal basis of the binocular inhibition that occurred in our esotropes without BSV. Crawford et al. (1996) showed that, as well as a reduction in the incidence of binocular summation in prism reared primates, there was an increase of ∼2-fold in the incidence of cortical neurones showing binocular inhibition. Similar results have been reported for a single primate with surgically induced esotropia (Sengpiel & Blakemore, 1996). However, there are no data against which the adverse effect of bifoveal viewing in heterotropes can be compared. Binocular inhibition may arise in several ways. In esotropic cats, binocularity may be compromised by extended response latencies and lack of synchronization of inputs from the esotropic eye (Chino et al. 1983; Roelfsema et al. 1994). While these results have been questioned on the basis that the animals were bilateral amblyopes (Kiorpes & McKee, 1999), extended response latencies have been reported from human amblyopes (Anderson et al. 1999). Some cortical neurones display binocular inhibition as a result of stimulation of inappropriate positional disparities, though the spatial extent of these interactions is too limited to account for the present results (Poggio & Fischer, 1977; Hubel & Livingstone, 1987; Poggio, 1995). A further mechanism is through cross orientation suppression which occurs when one eye is stimulated with an orientation different from that stimulating the companion eye. This suppression is normally of modest effect in cortical neurones of normal cats (Walker et al. 1998) though in strabismic animals, the suppression occurred when iso- as well as non-iso-orientated stimuli were presented to the esotropic eye (Sengpiel & Blakemore, 1996).
Sengpiel & Blakemore (1996) have proposed a model based on the interactions between neighbouring ocular dominance columns to account for suppression. Normally, binocular facilitation occurs when the two eyes are stimulated with the same orientation but are inhibited when the stimulus orientations are different. However, in strabismic suppression, it is proposed that the excitatory interactions between ocular dominance columns are lost whilst the inhibitory interactions remain extant. The model, however, fails to take into account the effects of BSV status and does not address the enhanced binocular facilitation which occurs in anisometropes and esotropes with anomalous BSV. While this model is consistent with the binocular inhibition that we have shown to occur in heterotropes without BSV, there remains the problem of the considerable spatial extent of the inhibitory interactions which arise in response to bifoveal stimulation.
Binocular integrating centre
Since area V1 is the first level of the visual pathway to show widespread binocular interactions, this must be the obvious candidate for the binocular integration centres described in Fig. 7. The bilateral representation of binocular integration in V1 is supported by fMRI studies in man, which showed bilateral activation in response to stimulation of both the non-amblyopic and amblyopic eye, though the activation was much reduced in the latter (Barnes et al. 2001). However, in amblyopic monkeys, the deficits recorded from area V1 have been shown to be insufficient to explain the behavioural deficits, thus requiring the postulation of further stages of amplification of the deficit (Kiorpes & McKee, 1999). This may involve area V2, which has an important binocularity function (Zeki, 1978; Hubel & Livingstone, 1987) and is bilaterally activated on non-amblyopic and amblyopic eye viewing (Barnes et al. 2001). However, strong support for area V1 comes from human studies of binocular rivalry in which presentation of dissimilar images to each eye leads to alternate appreciation of the two images in sequence. During binocular rivalry, activity in area V1 of man as determined by fMRI was shown to be correlated with the monocular image perceived at that time (Polonsky et al. 2000).
Summary
Considerable concordance exits between the proposed model and existing experimental data, though several major issues remain unresolved. The application of MEG and fMRI studies might provide further information concerning the unusually high level of binocular summation in amblyopes with some form of BSV, the binocular inhibition in strabismics without BSV and the inhibitory effects of bifoveal viewing in all types of strabismics. A more intractable problem is to account for the relatively long range neural processes which underlie these inhibitory interactions since their extent is equivalent to the angle of squint.
Clinical relevance
The important outcomes from this study are as follows. In both anisometropes and heterotropes, the non-amblyopic eye is not normal but performs appreciably less well than normal eyes of normal individuals. Amblyopes with some form of BSV show enhancement of contrast sensitivities on binocular viewing whereas heterotropes without BSV show binocular inhibition. While the absolute magnitude of this deficit is relatively small, it is nonetheless a matter of concern given that vision through the non-amblyopic eye is subnormal. In the context of orthoptic treatment, it is certainly a priority to improve vision in the amblyopic eye, though it is also important to try to establish some form of BSV in order to shift the individual from a position of binocular inhibition to one of binocular summation. In all heterotropes, bifoveal viewing resulted in binocular inhibition, which has to be viewed in the context that normally binocular enhancement should occur. This may be considered to be one of the more important results from this study since it implies that correction of strabismus in adults would be predicted to impair visual performance on binocular viewing. While a systematic study of preoperative and postoperative contrast sensitivities after correction of strabismus has yet to be undertaken, we are aware of anecdotal evidence that vision with both eyes open is perceived by some individuals to be poorer following strabismic surgery.
Acknowledgments
Our appreciation is expressed to our subjects for their participation in the experiments. We thank Mr J. J. Morrison for the preparation of Fig. 1, Miss C. Maciver for Fig. 7 and Drs B. Torsney and C. Weir for statistical advice.
REFERENCES
- Anderson SJ, Holliday IE, Harding GFA. Assessment of cortical dysfunction in human strabismic amblyopia using magnetoencephalography (MEG) Vision Research. 1999;39:1723–1738. doi: 10.1016/s0042-6989(98)00259-4. [DOI] [PubMed] [Google Scholar]
- Barnes GR, Hess RF, Dumoulin SO, Achtman RL, Pike GB. The cortical deficit in humans with strabismic amblyopia. Journal of Physiology. 2001;533:281–297. doi: 10.1111/j.1469-7793.2001.0281b.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blake R, Martens WL, DiGianfilippo A. Reaction time as a measure of binocular interaction in human vision. Investigative Ophthalmology and Visual Science. 1980;19:930–941. [PubMed] [Google Scholar]
- Bunt AH, Minckler DS, Johanson GW. Demonstration of bilateral projection of the central retina of the monkey with horseradish peroxidase neuronography. Journal of Comparative Neurology. 1977;171:619–630. doi: 10.1002/cne.901710412. [DOI] [PubMed] [Google Scholar]
- Callaway EM. Local circuits in primary visual cortex of the macaque monkey. Annual Review of Neuroscience. 1998;21:47–74. doi: 10.1146/annurev.neuro.21.1.47. [DOI] [PubMed] [Google Scholar]
- Campbell FW, Green DG. Monocular vs. binocular visual acuity. Nature. 1965a;208:191–192. doi: 10.1038/208191a0. [DOI] [PubMed] [Google Scholar]
- Campbell FW, Green DG. Optical and retinal factors affecting visual resolution. Journal of Physiology. 1965b;181:576–593. doi: 10.1113/jphysiol.1965.sp007784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chino YM, Shansky MS, Jankowski WL, Banser FA. Effect of rearing kittens with convergent strabismus on development of receptive field properties in striate cortex neurones. Journal of Neurophysiology. 1983;50:265–286. doi: 10.1152/jn.1983.50.1.265. [DOI] [PubMed] [Google Scholar]
- Crawford MLJ, Harwerth RS, Chino YM, Smith EL. Binocularity in prism-reared monkeys. Eye. 1996;10:161–166. doi: 10.1038/eye.1996.41. [DOI] [PubMed] [Google Scholar]
- Crawford MLJ, von Noorden GK. The effects of experimental strabismus on the visual system of Macaca mulatta. Investigative Ophthalmology and Visual Science. 1979;18:496–504. [PubMed] [Google Scholar]
- Dale RT. Fundamentals of Ocular Motility and Strabismus. New York: Grune Stratton Inc.; 1982. [Google Scholar]
- Daniel PM, Whitteridge D. The representation of the visual field on the cerebral cortex in monkeys. Journal of Physiology. 1961;159:203–221. doi: 10.1113/jphysiol.1961.sp006803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duke Elder S, Wybar KC. System of Ophthalmology, Ocular Motility and Strabismus. VI. London: Henry Kimpton; 1973. [Google Scholar]
- Goodyear BG, Nicolle DA, Humphrey GK, Menon RS. BOLD fMRI responses of early visual areas to perceived contrast in human amblyopia. Journal of Neurophysiology. 2000;84:1907–1913. doi: 10.1152/jn.2000.84.4.1907. [DOI] [PubMed] [Google Scholar]
- Harrad R, Hess RF. Binocular integration of contrast information in amblyopia. Vision Research. 1992;32:2135–2150. doi: 10.1016/0042-6989(92)90075-t. [DOI] [PubMed] [Google Scholar]
- Harwerth RS, Smith E, Levi DM. Suprathreshold binocular interactions for grating patterns. Perception and Psychophysics. 1980;27:43–50. [Google Scholar]
- Hess RF, Howell ER. Threshold contrast sensitivity function in strabismic amblyopia: evidence for a two type classification. Vision Research. 1977;17:1049–1055. doi: 10.1016/0042-6989(77)90009-8. [DOI] [PubMed] [Google Scholar]
- Holopigian K, Blake R, Greenwald MJ. Selective losses in binocular vision in anisometropic amblyopes. Vision Research. 1986;26:621–630. doi: 10.1016/0042-6989(86)90010-6. [DOI] [PubMed] [Google Scholar]
- Holopigian K, Blake R, Greenwald MJ. Clinical suppression and amblyopia. Investigative Ophthalmology and Visual Science. 1988;29:444–451. [PubMed] [Google Scholar]
- Holopigian K, Seiple W, Kupersmith M. VEP threshold and suprathreshold deficits in amblyopia. Clinical Vision Science. 1991;6:109–117. [Google Scholar]
- Hopkins HH. The frequency response of the defocused optical system. Proceedings of the Royal Society A. 1955;231:91–103. [Google Scholar]
- Hubel DH, Livingstone MS. Segregation of form, colour, and stereopsis in primate area 18. Journal of Neuroscience. 1987;7:3378–3415. doi: 10.1523/JNEUROSCI.07-11-03378.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubel DH, Wiesel TN. Receptive fields, binocular interaction and functional architecture in cat's visual cortex. Journal of Physiology. 1962;160:106–154. doi: 10.1113/jphysiol.1962.sp006837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubel DH, Wiesel TN. Receptive fields and functional architecture of monkey striate cortex. Journal of Physiology. 1968;195:215–243. doi: 10.1113/jphysiol.1968.sp008455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irvine SR. A simple test for binocular fixation: clinical application useful in the appraisal of ocular dominance, amblyopia, exanopsia, minimal strabismus and malingerers. American Journal of Ophthalmology. 1944;27:740–744. [Google Scholar]
- Jampolsky A, Schlor CM. Characteristics of suppression in strabismus. Archives of Ophthalmology. 1955;54:683–696. doi: 10.1001/archopht.1955.00930020689010. [DOI] [PubMed] [Google Scholar]
- Kiorpes L, Kiper DC, O'Keefe LP, Cavanaugh JR, Movshon JA. Neuronal correlates of amblyopia in the visual cortex of macaque monkeys with experimental strabismus and anisometropia. Journal of Neuroscience. 1998;18:6411–6424. doi: 10.1523/JNEUROSCI.18-16-06411.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiorpes L, McKee SP. Neural mechanisms underlying amblyopia. Current Opinion in Neurobiology. 1999;9:480–486. doi: 10.1016/s0959-4388(99)80072-5. [DOI] [PubMed] [Google Scholar]
- Legge GE. Spatial frequency masking in human vision: binocular interactions. Journal of the Optical Society of America. 1979;69:838–874. doi: 10.1364/josa.69.000838. [DOI] [PubMed] [Google Scholar]
- Leguire LE, Rogers GL, Bremer DL. Amblyopia: the normal eye is not normal. Journal of Pediatric Ophthalmology and Strabismus. 1990;27:32–38. doi: 10.3928/0191-3913-19900101-10. [DOI] [PubMed] [Google Scholar]
- Leguire LE, Rogers GL, Bremer DL, Wali N. A comparison of contrast sensitivity functions (CSF) between strabismic and anisometropic amblyopia in children. Binocular Vision Quarterly. 1989;4:179–186. [Google Scholar]
- Levi DM, Harwerth RS, Manny RE. Suprathreshold spatial frequency detection and binocular interaction in strabismic and anisometropic amblyopes. Investigative Ophthalmology and Visual Science. 1979;18:714–730. [PubMed] [Google Scholar]
- Lyle TK, Wybar KC. Practical Orthoptics in the Treatment of Squint. 5. London: H. K. Lewis & Co.; 1967. [Google Scholar]
- Marshall WE, Dawson E, Neveu MM, Morgan MJ, Sloper JJ. Increased binocular enhancement of contrast sensitivity and reduced stereoacuity in Duane syndrome. Investigative Ophthalmology and Visual Science. 2001;42:2821–2825. [PubMed] [Google Scholar]
- Morrison JD, Reilly J. An assessment of decision-making as a possible factor in the age-related loss of contrast sensitivity. Perception. 1986;15:541–552. doi: 10.1068/p150541. [DOI] [PubMed] [Google Scholar]
- Movshon JA, Eggers HM, Gizzi MS, Hendrickson AE, Kiorpes L, Boothe RG. Effects of early unilateral blur on the macaque's visual system. III. Physiological observations. Journal of Neuroscience. 1987;7:1340–1351. doi: 10.1523/JNEUROSCI.07-05-01340.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pardhan S, Gilchrist J. The effect of monocular defocus on binocular contrast sensitivity. Ophthalmic and Physiological Optics. 1990;10:33–36. [PubMed] [Google Scholar]
- Poggio GF. Mechanisms of stereopsis in monkey visual cortex. Cerebral Cortex. 1995;3:193–204. doi: 10.1093/cercor/5.3.193. [DOI] [PubMed] [Google Scholar]
- Poggio GF, Fischer B. Binocular interaction and depth sensitivity in striate and prestriate cortex of behaving rhesus monkey. Journal of Neurophysiology. 1977;40:1392–1405. doi: 10.1152/jn.1977.40.6.1392. [DOI] [PubMed] [Google Scholar]
- Polonsky A, Blake R, Braun J, Heeger DJ. Neuronal activity in human primary visual cortex correlates with perception during binocular rivalry. Nature Neuroscience. 2000;3:1153–1159. doi: 10.1038/80676. [DOI] [PubMed] [Google Scholar]
- Roelfsema PR, Konig P, Engel AK, Sireteanu R, Singer W. Reduced synchronization in the visual cortex of cats with strabismic amblyopia. European Journal of Neuroscience. 1994;6:1645–1655. doi: 10.1111/j.1460-9568.1994.tb00556.x. [DOI] [PubMed] [Google Scholar]
- Romano PE, von Noorden GK. Atypical responses to the 4 prism test. American Journal of Ophthalmology. 1969;67:935–939. doi: 10.1016/0002-9394(69)90089-0. [DOI] [PubMed] [Google Scholar]
- Ross JE, Clarke DD, Bron AJ. Effect of age on contrast sensitivity function: uniocular and binocular findings. British Journal of Ophthalmology. 1985;69:51–56. doi: 10.1136/bjo.69.1.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan BE, Joiner BL. Minitab Handbook. 3. Belmont, CA, USA: Duxbury Press; 1994. [Google Scholar]
- Sengpiel F, Blakemore C. The neural basis of suppression and amblyopia in strabismus. Eye. 1996;10:250–258. doi: 10.1038/eye.1996.54. [DOI] [PubMed] [Google Scholar]
- Sireteanu R. Binocular vision strabismic humans with alternating fixation. Vision Research. 1982;22:889–896. doi: 10.1016/0042-6989(82)90025-6. [DOI] [PubMed] [Google Scholar]
- Sireteanu R, Fronius M, Singer W. Binocular interactions in the peripheral visual field of humans with strabismic and anisometropic amblyopia. Vision Research. 1981;21:1065–1074. doi: 10.1016/0042-6989(81)90011-0. [DOI] [PubMed] [Google Scholar]
- Travers TaB. Suppression of vision in squint and its association with retinal correspondence and amblyopia. British Journal of Ophthalmology. 1938;22:577–604. doi: 10.1136/bjo.22.10.577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Truchard AM, Ohzawa I, Freeman RD. Contrast gain control in the visual cortex: monocular vs. binocular mechanisms. Journal of Neuroscience. 2000;20:3017–3032. doi: 10.1523/JNEUROSCI.20-08-03017.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VanEssen DC, Zeki SM. The topographical organization of rhesus monkey prestriate cortex. Journal of Physiology. 1978;277:193–226. doi: 10.1113/jphysiol.1978.sp012269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Noorden GK. Current concepts of amblyopia. In: Moore S, Mein J, Stockbridge S, editors. Orthoptics Past, Present and Future. Boston, MA, USA: Symposia Specialist Medical Books; 1976. [Google Scholar]
- von Noorden GK. Binocular Vision and Ocular Motility: Theory and Management of Strabismus. 4. St Louis: The C. V. Mosby Co.; 1990. [Google Scholar]
- von Noorden GK, Crawford MLJ. Form deprivation without light deprivation produces the visual deprivation syndrome in Macaca mulatta. Brain Research. 1977;129:37–44. doi: 10.1016/0006-8993(77)90968-4. [DOI] [PubMed] [Google Scholar]
- Walker GA, Ohzawa I, Freeman RD. Binocular cross-orientation suppression in the cat's striate cortex. Journal of Neurophysiology. 1998;79:227–239. doi: 10.1152/jn.1998.79.1.227. [DOI] [PubMed] [Google Scholar]
- Wiesel TN. Postnatal development of the visual cortex and the influence of environment. Nature. 1982;299:583–591. doi: 10.1038/299583a0. [DOI] [PubMed] [Google Scholar]
- Zeki SM. Uniformity and diversity of structure and function in rhesus monkey prestriate visual cortex. Journal of Physiology. 1978;277:273–290. doi: 10.1113/jphysiol.1978.sp012272. [DOI] [PMC free article] [PubMed] [Google Scholar]


