Abstract
We hypothesized that in congestive heart failure (CHF) slow-twitch but not fast-twitch muscles exhibit decreased fatigue resistance in the sense of accelerated reduction of muscle force during activity. Experiments were carried out on anaesthetized rats 6 weeks after induction of myocardial infarction or a sham operation (Sham). Animals with left ventricular end-diastolic pressure (LVEDP) > 15 mmHg under anaesthesia were selected for the CHF group. There was no muscle atrophy in CHF. Force generation by in situ perfused soleus (Sol) or extensor digitorum longus (EDL) muscles was recorded during stimulation (trains at 5 Hz for 6 s (Sol) or 10 Hz for 1.5 s (EDL) at 10 or 2.5 s intervals, respectively) for 1 h in Sol and 10 min in EDL at 37 °C. Initial force was almost the same in Sol from CHF and Sham rats, but relaxation was slower in CHF. Relaxation times (95–5 % of peak force) were 177 ± 55 and 131 ± 44 ms in CHF and Sham, respectively, following the first stimulation train. After 2 min of stimulation the muscles transiently became slower and maximum relaxation times were 264 ± 71 and 220 ± 45 ms in CHF and Sham, respectively (P < 0.05). After 60 min they recovered to 204 ± 60 and 122 ± 55 ms in CHF and Sham, respectively (P < 0.05). In CHF but not in Sham rats the force of contraction of Sol declined from the second to the sixtieth minute to 70 % of peak force. The EDL of both CHF and Sham fatigued to 24–28 % of initial force, but no differences in contractility pattern were detected. Thus, slow-twitch muscle is severely affected in CHF by slower than normal relaxation and significantly reduced fatigue resistance, which may explain the sensation of both muscle stiffness and fatigue in CHF patients.
Fatigue is a prominent symptom in patients with congestive heart failure (CHF). Low anaerobic threshold and exertional dyspnoea due to lung congestion, low cardiac output and restricted perfusion of skeletal muscles can to some extent explain the fatigue. However, it is also clear that the contractile properties and fatigue resistance inherent to the muscle itself are altered in this condition. In CHF patients there is a weak association between clinical symptoms, for instance fatigue, and left ventricular function (Harrington & Coats, 1997). Furthermore, even when using small hand muscles that do not put any demand on the heart, these patients experience increased fatigue (Kemp et al. 1996). Since it is difficult to sort out the relative contribution of detraining, adaptation to chronic hypoxaemia and muscle effects of medication in patient studies, several investigators have also examined muscle changes in experimental models of heart failure (reviewed by Lunde et al. 2001b). Alterations have been reported for several enzymes and metabolites as well as a modest fibre type switch. However, the reason for the reduced fatigue resistance seems to reside in the SR. We recently reported that in single fibres from fast-twitch muscle of rats with experimentally induced CHF there is slowed Ca2+ reuptake by the SR compared to muscles from sham-operated (Sham) rats after fatiguing contractions (Lunde et al. 2001a). We also showed contractile data from the isolated slow-twitch soleus muscle (Sol) during frequent tetanic contractions at room temperature. Relaxation was markedly slower in Sol from CHF compared to Sham rats, causing partial fusion of contractions although force was maintained.
It is striking that the most prominent deterioration of muscle function in CHF seems to occur in slow-twitch muscles, which share a lot of properties with the heart. For instance, the Ca2+-ATPase isoform of the SR (SERCA2a) is the same in Sol and myocardium of the rat (Froemming et al. 2000). The present study was carried out on both Sol and extensor digitorum longus (EDL) to identify functional alterations of these muscles in CHF rats compared to Sham rats during stimulation leading to fatigue. We performed experiments on the in situ perfused muscles of anaesthetized rats. We chose this model for several important reasons. First, the experiments could be carried out at physiological temperatures. The various steps of the excitation-contraction-relaxation cycle have different temperature sensitivies. Hence, the steps causing fatigue could shift with temperature (Fryer & Neering, 1986). Second, we wanted to avoid the ischaemia that can occur in isolated working muscles and the associated major alterations of metabolites that have been linked to fatigue. This was important since muscles from CHF rats could be more prone to ischaemia and lactate accumulation (Thompson et al. 1995). Finally we chose stimulation protocols that, especially in the Sol, could be maintained for an hour with only minor contractile changes in the Sham rats, since this is close to the way the muscle normally works. We found that the slightly slower relaxation of Sol in CHF compared to Sham rats in resting muscle was greatly amplified after a few minutes of stimulation, but only transiently, and after 1 h developed force was very low in spite of unchanged tissue content of high-energy phosphates and lactate.
METHODS
Animal preparation
Under halothane anaesthesia (Fluothane, Zeneca Pharmaceuticals, UK; 1–2 % in 30 % O2, 70 % N2O) male Wistar rats (300 g) were subjected to ligation of the left coronary artery (CHF group, n = 32) as described by Tønnessen et al. (1997). A sham operation, in which the coronary artery was exposed but not ligated, was performed in 25 other rats (Sham group). Postoperatively the rats were given 0.2 mg kg−1 buprenorphine and kept under daily surveillance. The animals were housed in separate cages with a light-dark cycle of 12 h-12 h and had free access to food and water. The overall survival rate was 80 % over 6 weeks.
Six weeks after surgery rats were anaesthetized as above and the adequacy of the anaesthesia was then monitored throughout the experiment. The blood pressure and left ventricular end-diastolic pressure (LVEDP) were determined by a 0.67 mm (2 F) microtip pressure catheter (model SPR-407, Millar Instruments Inc., Houston, TX, USA) inserted through the right carotid artery. The skin was then carefully removed from the right leg and either the Sol or the EDL muscle was dissected free with their blood supply intact. The foot and the tibia of the rat were fixed by two clamps and the distal tendon of either the Sol or the EDL muscle was attached to a force transducer (FT03, Grass Instrument Company, Quincy, MA, USA) and positioned in parallel to the tibia. In both cases the sciatic nerve was cut. The temperature of the muscles was continuously monitored and kept at 37 °C by superfusing with preheated Krebs-Ringer solution (mm: 101 NaCl, 30 Na-acetate, 4 KCl, 2 CaCl2 and 1 MgCl2). Stimulation electrodes were attached to each end of the muscle and connected to a pulse generator (Pulsar 6bp, FHC, Bowdoinham, ME, USA). The muscle was stretched and stimulation voltage was adjusted to give maximal force of twitch contractions. At the end of the experiment the exercising muscle and the contralateral control were rapidly dissected free and frozen in liquid nitrogen. The muscles were stored at −70 °C until analysis for ATP, creatine phosphate (CrP) and lactate. The heart was dissected free and the infarct scar was evaluated macroscopically. The rats were kept on a heating pad to maintain normal body temperature during the whole experiment.
The experiments were conducted in accordance with ‘Regulations on Animal Experimentation’ under The Norwegian Animal Welfare Act and were approved by the Norwegian Animal Research Authority (NARA).
Stimulation protocols
Force-frequency relationship
Each muscle was stimulated at 11 different frequencies between 1 and 50 Hz for 2 s to produce a force-frequency relationship.
Fatigue protocol
The muscles were stimulated with pulses of 15 ms duration, which produces single contractions (single-pulse contractions) with peak force about 1.76 times that of a twitch, but still well below tetanic force (Close & Hoh, 1968a). About 10 min after the muscles were dissected free they were stimulated at 1 and then at 50 Hz (Sol) or 100 Hz (EDL) for 1–3 s (test contractions). The muscles were then allowed to rest for 10–15 min before the fatigue protocol was started. Sol was stimulated at 5 Hz for 6 s at 10 s intervals for 1 h and EDL was stimulated at 10 Hz for 1.5 s at 2.5 s intervals for 10 min.
Recovery experiments
The animals undergoing the fatigue protocol were divided into two groups. In one group the muscles were rapidly dissected free and frozen for metabolite analysis and in the other group the muscles were allowed to recover for 1 h (Sol) or 30 min (EDL). During the recovery period the muscles were again stimulated at 1 and 50 Hz (Sol) or 100 Hz (EDL) at intervals.
Data registration and sampling
Force and blood pressure data were continuously displayed on an oscilloscope and chart recorder. At various time points both blood pressure and force were sampled on a PC at 250 Hz using a Metrabyte DAS1800 AD-board and ASYST4.0 software (Asyst/Keithley Software Technologies Inc., Rochester, NY, USA) for 30 s periods. From the digitized data, maximum force during the train, contraction rates (maximum derivative, time from 5 to 95 % of maximum force) of the first contraction and relaxation rates (maximum negative derivative, time from 95 to 50 % and from 50 to 5 % of maximum force) of the last contraction of the train were calculated using ASYST4.0 software (Verburg et al. 2001).
Metabolite analysis
Immediately at the end of the stimulation protocol the exercising muscle was cut free, excess buffer removed and the muscle frozen in liquid nitrogen within 5 s. The contralateral control muscle was then dissected free and frozen in the same manner. The muscles were analyzed for ATP, CrP and lactate within 1 week by the methods of Lowry & Passonneau (1972).
Histochemical fibre classification
The fibre classification was done according to a modification (Bye et al. 1989) of the method of Brooke & Kaiser (1970), which differentiates between type I and type II fibres.
mRNA analysis
Poly A+ RNA was extracted from homogenized tissue from the left ventricle of the heart from Sham and CHF rats using oligo(dT)-conjugated paramagnetic beads according to the manufacturer's instructions (Dynal A/S, Oslo, Norway). Northern blotting was performed as previously described (Tønnessen et al. 1997) using a cDNA probe (650 bp) for atrial natriuretic factor (ANF).
Statistics
Data are presented as means ±s.e.m. Comparisons were done by MANOVA for repeated measures, followed by ad hoc tests (Statistica, StatSoft Inc., Tulsa, OK, USA). Student's t test was used when appropriate for group comparison. Significance level was set at P < 0.05.
RESULTS
Animal and muscle characteristics
Six weeks after the operation the myocardial infarction (MI) comprised most of the free wall of the left ventricle. LVEDP was > 15 mmHg (Table 1), which correlates with significant slowing of contraction of the remaining viable myocardium and dilation of the left atrium as a sign of pulmonary congestion in CHF rats (Sjaastad et al. 2000). Also the mRNA signal for ANF was significantly upregulated in the viable myocardium (Fig. 1), heart weight was increased and arterial systolic blood pressure was reduced in CHF compared with Sham rats (Table 1). Thus, several features of heart failure in humans are present in this rat model (Lunde et al. 2001b).
Table 1.
Haemodynamic parameters of CHF and Sham rats
| Sham (n = 28) | CHF (n = 36) | |
|---|---|---|
| Arterial systolic pressure (mmHg) | 130.6 ± 3.7 | 109.4 ± 2.7* |
| LVEDP (mmHg) | 3.2 ± 0.5 | 25.7 ± 2.0* |
| Body weight (g) | 403 ± 4.3 | 396 ± 4.9 |
| ANF (relative mRNA amount) | 100 ± 22 | 698 ± 157* |
| (n = 12) | (n = 6) |
Values are means ±s.e.m.
P < 0.05.
Figure 1. Western blot of ANF mRNA in left ventricle from CHF and Sham (SH) rats.
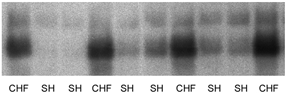
The ANF mRNA expression was significantly higher in CHF (n = 6) compared to Sham rats (n = 12; P < 0.05).
Body weight was not different in CHF and Sham rats (Table 1), and there was no sign of muscle atrophy, since weights of Sol and EDL muscles were not significantly different between the CHF and Sham groups (Table 2). Sol was dominated by type I fibres and EDL by type II; the relative abundance and size of the dominant fibre types was not changed in CHF compared with Sham groups (Table 2).
Table 2.
Muscle data and contractile parameters of test contractions of Sol and EDL from CHF and Sham rats
| Sol, Sham | Sol, CHF | EDL, Sham | EDL, CHF | |
|---|---|---|---|---|
| Morphology | ||||
| Muscle weight (mg) | 118 ± 2.7 (20) | 115 ± 3.2 (14) | 125 ± 3.6 (19) | 121 ± 3.4 (14) |
| Fibre type (type I/type II) (%) | 90.4/9.6 (8) | 100/0 * (3) | 0.5/99.5 (10) | 0.7/99.3 (11) |
| Fibre size | ||||
| Type I (μm2) | 4503 ± 260 (8) | 4710 ± 214 (3) | — | — |
| Type II (μm2) | 3126 ± 135 (8) | — | 5482 ± 236 (10) | 5313 ± 172 (11) |
| Contractile properties (test contractions) | (n = 8) | (n = 15) | (n = 8) | (n = 15) |
| Single-pulse contraction | ||||
| Maximal force (mN) | 824 ± 49 | 765 ± 20 | 1569 ± 98 | 1422 ± 196 |
| Contraction rate (max dF/dt) (s−1) | 27.5 ± 0.7 | 25.1 ± 0.4 * | 47.0 ± 1.5 | 48.5 ± 1.9 |
| Relaxation rate (max −dF/dt) (s−1) | 13.7 ± 0.5 | 10.2 ± 0.3 * | 38.4 ± 1.5 | 37.9 ± 1.6 |
| Tetanic contraction | ||||
| Maximal force (mN) | 2078 ± 10 | 1922 ± 39 * | 2108 ± 127 | 2677 ± 78 * |
| Contraction rate (max dF/dt) (s−1) | 12.1 ± 0.3 | 10.7 ± 0.1 * | 40.6 ± 1.7 | 34.6 ± 0.7 |
| Relaxation rate (max −dF/dt) (s−1) | 13.5 ± 0.4 | 11.2 ± 0.2 * | 37.3 ± 1.5 | 34.7 ± 0.6 * |
| Recovery of force at given time after last stimulation train | ||||
| 2 min | ||||
| Single-pulse contraction (% of control) | 101 ± 37 (5) | 110 ± 15 (5) | 40 ± 7 (5) | 35 ± 4 (5) |
| Tetanic contraction (% of control) | 95 ± 9 (5) | 106 ± 7 (5) | 43 ± 10 (5) | 32 ± 1 (5) |
| 10 min | ||||
| Single-pulse contraction (% of control) | 92 ± 33 (5) | 112 ± 11 (5) | 50 ± 22 (5) | 72 ± 5 (5) |
| Tetanic contraction (% of ctontrol) | 91 ± 10 (5) | 103 ± 5 (5) | 49 ± 1 (5) | 72 ± 21 (5) |
Values are means ±s.e.m. (n). Contraction rate and relaxation rate are normalized by peak force.
P < 0.05.
Contractile properties
Test contractions
In Sol tetanic (50 Hz), but not single-pulse force (1 Hz) was slightly lower in CHF compared with Sham rats (8 %; Table 2; P < 0.05), whereas both contraction and relaxation rates were significantly slower (16–18 and 30–20 %, respectively; Table 2; P < 0.05). In contrast, in the EDL tetanic force (100 Hz) was higher in CHF compared with Sham rats (27 %, P < 0.05). At lower frequencies (10 and 1 Hz) no differences were detected in force (Table 2) and contraction and relaxation rates were not significantly different at any stimulation frequency in this muscle.
As a result of the slowing of relaxation, the force- frequency curve for Sol from CHF rats was shifted to the left (not shown), so that 50 % of maximal force was obtained at 9.9 Hz (n = 9) compared with 12.3 Hz in Sham rats (n = 5; P < 0.05). The force-frequency relationship was not significantly changed by the fatigue protocol (data not shown). In the EDL the force-frequency relationship was not significantly different in CHF and Sham rats.
Fatigue development of Sol
Sol from CHF rats showed obvious signs of altered contractile properties soon after the start of stimulation, most notably a further slowing of relaxation and a subsequent gradual decline of maximum force during the rest of the stimulation period. These alterations were very much smaller or not seen at all in Sham rats. Figure 2 shows selected trains of contractions at various time points during the 1 h stimulation period and Fig. 3 shows selected single-pulse contractions at the beginning and end of trains. Figure 4 summarizes the mean values of contractile performance of Sol.
Figure 2.
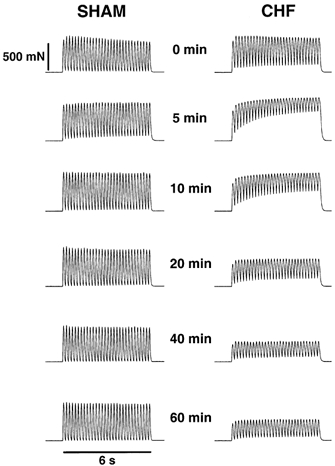
Force recordings (5 Hz, 6 s) from in situ stimulated Sol from representative CHF and Sham rats at different time points during the 1 h fatigue protocol. Each stimulation train is separated by 4 s of rest.
Figure 3.
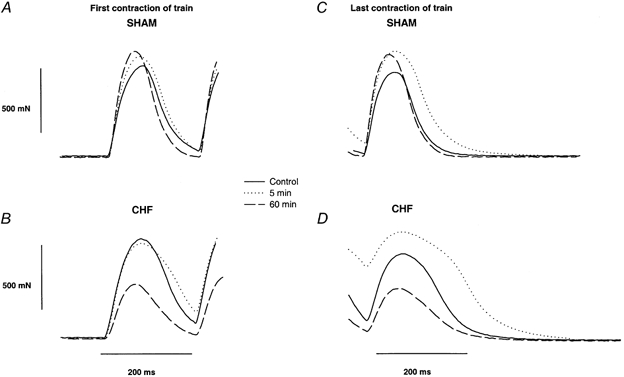
Selected force curves from in situ stimulated Sol from a Sham (A and C) and a CHF rat (B and D) on a expanded scale (same rats as in Fig. 2). The curves are the first (A and B) and last contractions (C and D) in a 6 s, 5 Hz stimulation train at different time points during the stimulation protocol. Continuous lines are control recordings, dotted lines are recordings at 5 min and dashed lines are recordings at the end of the protocol (60 min).
Figure 4. Fatigue development in Sol from CHF (○) and Sham rats (•).
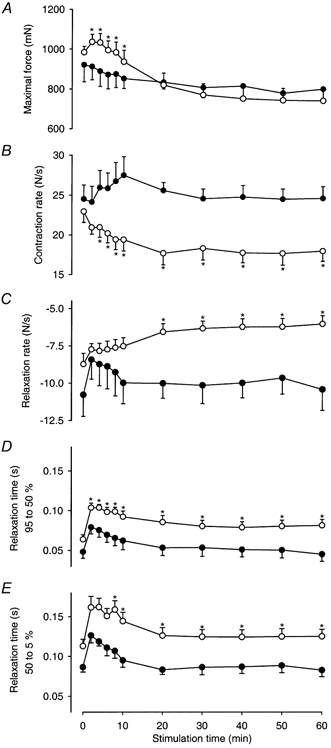
A, maximum developed force; B, maximum contraction rate (maximum dF/dt of first contraction in stimulation train; C, maximum relaxation rate (minimum -dF/dt of last contraction in stimulation train; D, relaxation time from 95 to 50 % of maximum force; and E, relaxation time from 50 to 5 % of maximum force at various time points during the 1 h stimulation period. Data are mean values ±s.e.m. Asterisk indicates value significantly different from Sham at a given time point. Time-dependent significance not shown (see text).
Interestingly, maximum force (Fig. 4A) increased in CHF rats initially, but from the second minute it fell throughout the 1 h stimulation period to 70 % of the force observed at 2 min (P < 0.05). In contrast, maximum force only fell nominally, to 87 % of the control value, during the same period in Sham rats. Maximum force was significantly higher in CHF compared to Sham rats between 2 and 10 min from the start of the protocol.
The contraction rate (maximum +dF/dt) for Sol was initially not different in CHF and Sham rats, but soon after the start of stimulation contraction rate became slower in CHF rats (overall P < 0.05; Figs 3A and B and 4B). In Sol of CHF rats the maximum contraction rate for each 5 Hz train fell during the first 20 min by 23 % (P < 0.05) and was then stable throughout the rest of the protocol. In contrast, the contraction rate in Sol from Sham rats remained stable throughout the protocol. Contraction rates closely followed maximum force in both groups, except at the very beginning of stimulation in CHF rats (CHF, r = 0.98, P < 0.05; Sham, r = 0.77, P < 0.05).
Maximum relaxation rate from the last contraction of the trains (maximum -dF/dt) was at the outset nominally slower in Sol from CHF than from Sham rats, but showed a significant slowing with time in CHF rats (P < 0.05) that was not detectable in Sham rats. Overall maximum relaxation rate was slower in the CHF group (P < 0.05) and at the end of the 60 min protocol the relaxation rate of Sol from CHF rats was merely 58 % of that of Sham rats (P < 0.05). However, the maximum relaxation rate does not adequately describe the changing shape of the relaxation curves. Figure 3D illustrates that after 5 min, relaxation of Sol from CHF rats was clearly biphasic with a slow initial phase and a faster second phase that tailed off very slowly. Relaxation times for both the first 45 % and the subsequent 45 % decline of force are summarized in Fig. 4D and E. Both phases of the relaxation were significantly longer in CHF compared to Sham rats (overall P < 0.05) and this amounted to 180 and 162 %, respectively, of that in the Sham group at the end of the protocol (P < 0.05). However, the overall patterns were similar in the two groups. Both phases of relaxation initially became longer and then returned to their initial values during the course of 15–20 min (time effects, P < 0.05). At 2 min 95–5 % relaxation time was 264 ± 71 ms in CHF and 220 ± 45 ms in Sham rats (P < 0.05), and at 60 min the corresponding values were 204 ± 60 and 122 ± 55 ms, respectively (P < 0.05).
After 5 min of stimulation the slowing of Sol in CHF rats led to partially fused contractions, and the muscle only relaxed to 80 % of peak single-pulse force before the last stimulus of the train (Fig. 2). This is in striking contrast to Sham rats, where almost no fusion of contractions occurred. The partial fusion of contractions led to a pronounced decline of developed force (difference between single-pulse force and the preceding baseline force between contractions) during the first 5 min in Sol from CHF but not from Sham rats (Fig. 2). Even though relaxation times recovered in CHF rats, the developed force remained low.
Interestingly, developed force fell throughout the stimulation train, but only in trains from about the second to the fifteenth minute of the protocol (see the 5 and 10 min trains in Fig. 2). This is because baseline force increased due to a gradual decline of relaxation rate. Thus, especially in CHF rats, there is a rapid and dynamic regulation of relaxation rate. The muscle becomes slower during the initial 3 s of the train and recovers partly during the 4 s rest period between the trains. The effect is evident as a reduction of developed force.
Fatigue development of EDL
There were no significant differences between EDL of CHF and Sham rats regarding contractile parameters at any time point during the 10 min fatigue protocol. In both groups twitch force fell to between 30 and 40 % of the initial value over 2 min and then remained stable (Fig. 5A). Contraction rates closely followed peak twitch force (Fig. 5B), whereas relaxation rates fell substantially more in both groups (by about 94 %; Fig. 5C).
Figure 5. Fatigue development in EDL from CHF and Sham rats.
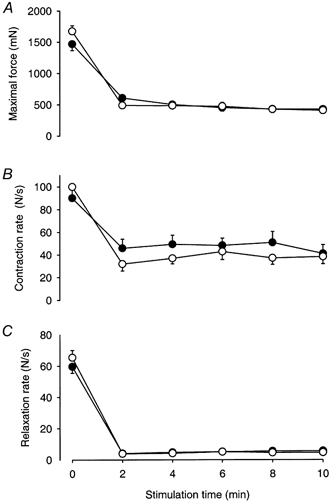
Maximal developed force from 10 Hz trains at selected time points during the stimulation of Sham (•, n = 9) and CHF rats (○, n = 12). Data are mean values ±s.e.m.
The initial contraction rate was about four times higher in EDL compared to Sol for both CHF and Sham rats. At the start of the stimulation protocol, the relaxation rate for EDL was 5.5 and 7.5 times faster than for Sol in CHF and Sham rats, respectively, but after 2 min of stimulation the relaxation rate for EDL was in fact slower than in Sol in both groups.
Force recovery of Sol and EDL
Single-pulse and tetanic force of the Sol were recorded for 1 h after the last train of the 60 min fatigue protocol, and recovered to more than 91 % during the first 30 s (Table 2).
In contrast, single-pulse and tetanic force of EDL recovered much more slowly and poorly after the 10 min stimulation protocol (Table 2). Approximately 1 min after the end of the stimulation protocol the force of single-pulse contractions was 34–40 % of the control value. During the next 30 min, EDL from CHF rats showed little recovery of single-pulse force, but tetanic force increased to 57 % of the control value. Recovery in Sham rats was significantly better. Both single-pulse force and tetanic force were about 70 % of the control value after 30 min.
Muscle metabolites
Concentrations of ATP, CrP and lactate in Sol and EDL after the 60 min fatigue protocol are presented in Table 3. In Sol, ATP was slightly lower in the stimulated compared with the unstimulated leg. However, there were no significant differences in the metabolite concentrations between the CHF and Sham rats in either leg.
Table 3.
Metabolite concentrations in stimulated and unstimulated contralateral Sol and EDL after fatigue protocol from CHF and Sham rats
| Sham | CHF | |||
|---|---|---|---|---|
| Unstimulated | Stimulated | Unstimulated | Stimulated | |
| SOL | ||||
| ATP | 5.3 ± 0.6 (4) | 3.2 ± 0.6 (6) * | 4.0 ± 0.5 (10) | 3.1 ± 0.4 (9) |
| CrP | 9.2 ± 1.0 (4) | 6.8 ± 0.8 (6) | 6.4 ± 0.9 (10) | 4.7 ± 0.7 (9) |
| Lactate | 2.6 ± 0.8 (4) | 3.1 ± 0.5 (6) | 2.5 ± 0.4 (10) | 3.2 ± 0.5 (9) |
| EDL | ||||
| ATP | 8.1 ± 0.5 (6) | 4.1 ± 0.3 (6) * | 7.5 ± 0.6 (6) | 2.9 ± 0.3 (6) *† |
| CrP | 11.9 ± 1.2 (6) | 6.1 ± 0.5 (6) * | 13.1 ± 1.7 (6) | 7.0 ± 1.1 (6) * |
| Lactate | 2.8 ± 0.9 (6) | 5.5 ± 0.7 (6) * | 3.7 ± 0.9 (6) | 4.5 ± 0.9 (6) * |
All values are in mmol (kg wet weight)−1. Values are means ±s.e.m. (n).
P < 0.05 exercise vs. control.
P < 0.05 CHF vs. Sham.
In EDL, both in the Sham and the CHF group, all the measured metabolites were significantly different in the stimulated compared to the contralateral unstimulated muscles. Metabolite concentrations were not significantly different in unstimulated muscles from CHF and Sham rats, whereas in stimulated muscles ATP concentration was lower in CHF than in Sham rats. CrP and lactate concentrations were not significantly different.
DISCUSSION
In this study we show that, under physiological conditions, intermittent stimulation of the perfused in situ Sol of CHF rats caused a pronounced but transient slowing of relaxation over 5 min and a remarkable decline of force during the following 1 h stimulation period. Relaxation of the Sol of Sham rats was faster than that of CHF rats, but also showed a transient slowing of relaxation, although force was maintained throughout the 1 h stimulation period. These changes occurred in the absence of significant alterations of high-energy substrates or lactate, although fluctuations of local concentrations of these metabolites may have occurred during the stimulation protocol. The EDL of both CHF and Sham rats fatigued rapidly, and we could not detect significant differences between the two groups. We therefore conclude that slow-twitch muscle especially is affected in CHF. The slowing of relaxation upon muscle usage may explain an increasing feeling of stiffness in CHF patients and, if exercise can be sustained, fatigue will be prominent.
Gradual development of fatigue
The important new finding in the present study is that in the non-ischaemic working Sol from CHF rats perfused at normal body temperature, pronounced fatigue develops during prolonged intermittent stimulation. This is not seen in Sol from Sham rats. The observation of reduced fatigue resistance in our CHF soleus muscles seems to contrast with previous results (Lunde et al. 2001a) from isolated Sol. However, in that study the isolated muscle was stimulated intensively (70 Hz) for only a few minutes and, although force fell, it did not fall more in CHF than in Sham rats and the experimental protocol did not allow for recording of muscle endurance during aerobic, submaximal muscle activity. In both the present study and that of Lunde et al. (2001a), a pronounced slowing of relaxation was observed, causing baseline force to increase, as can be seen in Fig. 2. Thus, in both studies, developed force declined more in CHF than in Sham rats. The gradual recovery of relaxation (early and late phase) taking place during continued prolonged stimulation was only observed in the present study and might be associated with the development of fatigue.
The fatigue observed in CHF rats resembles the fatigue process that was recently described by Verburg et al. (2001). In that study, we hypothesized that the development of fatigue can be causally related to the transient slowing of relaxation. Briefly, in this scenario the reuptake of Ca2+ by the SR is gradually reduced during the first minute of stimulation. Hence, at the time of the next stimulus the concentration of Ca2+ in the cytosol is still high and consequently there is insufficient time for recycling through the SR of more than a small fraction of the available Ca2+ during the stimulation trains. Thus, Ca2+ release with each stimulus is small and developed force is reduced. The fraction of non-recycled Ca2+ will first remain in the cytosol and would therefore be responsible for the increasing baseline force and increasing fusion of contractions. Interestingly, it seems that this pool of Ca2+ gradually becomes unavailable for the contractile elements, since baseline force subsequently returns to basal level (Verburg et al. 2001). This picture fits nicely with several studies showing that fatigue can be due to reduced release of Ca2+ from the SR (Westerblad & Allen, 1991; Westerblad et al. 1991). Reduced release can be attributed to the release process itself or to trapping of calcium so that it becomes unavailable for release. Although force recovered rapidly, our data do not allow us to distinguish between these possibilities. It is less likely that the non-recycled Ca2+ was trapped by phosphate in the SR as has recently been suggested (Fryer et al. 1995; Dahlstedt et al. 2001), since high-energy phosphates did not change in our study. Mitochondria have a large capacity for calcium uptake and storage, but this has been regarded as a slow process that is mostly responsible for protecting cells against calcium overload (Carafoli, 1987). However, Gillis (1997) recently reported that in muscle cells rich in mitochondria (such as the slow-twitch soleus muscle) calcium may be rapidly stored and released from the mitochondrial compartment and contribute to faster relaxation of these muscles, which do not contain parvalbumin. In fast-twitch mammalian muscle there is no evidence for calcium accumulation during tetanic stimulation (Lännergren et al. 2001). It is therefore, in our opinion, still an open question why it seems that some Ca2+ is not released or becomes unavailable for release from the SR.
Transient slowing of relaxation due to reduced Ca2+ reuptake by SR
We recently reported (Verburg et al. 2001) that with this stimulation protocol normal Sol also shows a transient slowing of relaxation, as we observed in the present study for the Sham muscle. In that study, muscle temperature was kept slightly lower, and maximal force fell throughout the stimulation period. This is in accordance with the study of Close & Hoh (1968b), who found a reduction of developed tension and slowing of relaxation with a decrease in temperature. We intentionally raised the temperature in the present study to be closer to the normal working temperature of the muscle, and consequently did not detect significant fatigue in the Sham group. The slowing of relaxation can probably be ascribed to reduced Ca2+ reuptake by the SR (Fryer & Neering, 1986; Wahr et al. 1998).
Unfortunately, it has hitherto not been possible to measure Ca2+ transients in slow-twitch skeletal muscle of the mouse or the rat for technical reasons (Lunde et al. 2001a). We therefore have no direct evidence for a reduced rate of Ca2+ reuptake, but there are arguments in favour of this explanation. First, at normal body temperature the rate of relaxation of skeletal muscle has been shown to be closely associated with the rate of Ca2+ reuptake (Fryer & Neering, 1986). Second, in frog muscle it has been shown that the first part of the force relaxation curve is related to the rate of decline of intracellular Ca2+ (Wahr et al. 1998). Third, we have recently shown that in isolated single fibres of the flexor digitorum brevis muscle of rats with CHF, fatiguing contractions lead to a slowing of the decline of the Ca2+ transient (Lunde et al. 2001a). Therefore, we conclude that Ca2+ reuptake by the SR is slower in Sol from CHF compared with Sham rats and that activating the muscle will significantly reduce the rate of Ca2+ reuptake and relaxation rate further, especially the early and late parts of the relaxation. The molecular mechanism for this is at present unclear, especially since the rat soleus muscle lacks phospholamban, which is the direct regulator of the Ca2+-ATPase in the heart (Damiani et al. 2000). Slowing of relaxation will probably be experienced as muscle stiffness, since antagonist muscles will still develop force during activation of an agonist unless movements are slowed down considerably.
Little effect of CHF on fast-twitch muscle
The present stimulation protocol did not reveal any differences between EDL of CHF and Sham rats. EDL contains more than 99 % type II fibres in both CHF and Sham rats. Also, in the fast-twitch flexor digitorum brevis we did not see any significant differences between CHF and Sham rats regarding contractile performance of single muscle fibres, although there was a tendency for the fibres to relax more slowly after the fatigue protocol (Lunde et al. 2001a). However, the rate of decline of the Ca2+ transient became significantly slower in CHF rats. Thus, the changes in fast-twitch muscle in CHF are minimal, but the effect on the Ca2+ transient is compatible with a similar but much more pronounced effect in Sol.
Unaltered muscle metabolism
In this study, muscles were examined in situ and were well perfused during the stimulation protocol, as evidenced by the analysis of high-energy phosphates. The concentration of high-energy phosphates is of the same magnitude as previously reported in a similar model (Verburg et al. 2001). Thus, by stimulating only one muscle of one leg, the demand for increased cardiac output did not seem to exceed the capacity of the failing heart. We did not detect lower muscle content of creatine phosphate in CHF, which is in agreement with others (Brunotte et al. 1995; Thompson et al. 1995), but with our moderate stimulation protocol we avoided the more pronounced breakdown of creatine phosphate in muscles from CHF animals that others have observed (Chati et al. 1994; Brunotte et al. 1995). We also avoided the lactacidosis that can develop in isolated muscles or with higher intensity exercise and stimulation protocols. Also, muscle temperature was kept at 37 °C. It is well known that at lower temperatures acidosis will reduce the developed force at a given intracellular [Ca2+] (Westerblad et al. 1997), which could have been the case in our previous study (Lunde et al. 2001a). Therefore, we have now shown that in Sol from CHF and Sham rats there were no detectable differences in the aerobic or anaerobic metabolism or high-energy phosphates that can explain the differences in contractile performance.
Severe heart failure, but no muscle atrophy
Interestingly, in our rat model of CHF we did not observe muscle atrophy or any fibre type switch. Muscle disuse would be expected to cause atrophy of single fibres but, as previously reported, these animals seemed to be as active in their cages as the Sham rats (Lunde et al. 2001a). Furthermore, muscle unloading leads to atrophy (Bigard et al. 1998), but unloaded muscles become faster, not slower as we observed. Several investigators have indeed reported a fibre type switch towards faster muscles (Simonini et al. 1996; Vescovo et al. 1998). These changes might not be specific to the heart failure condition, but could be due to different disease models and experimental conditions. Our rats showed typical signs of severe heart failure: tachypnoea, pulmonary oedema and sometimes ascites (data not shown). We have previously demonstrated that by excluding rats that have LVEDP below 15 mmHg we avoid including animals that have MI, but not CHF (Sjaastad et al. 2000; Lunde et al. 2001a). We also show in the present paper that there is a significant expression of ANF mRNA in the left ventricle of CHF rats that cannot be seen in Sham rats. We therefore conclude that our rat model of severe CHF is not confounded by the effects of muscle disuse and atrophy.
Conclusions
We show that low-frequency stimulation gives rise to slowing of relaxation and pronounced fatigue in the slow soleus muscle from CHF rats, whereas little fatigue was seen in soleus muscles from Sham rats. These changes appeared without any detectable difference in the tissue content of the high-energy phosphates ATP and CrP, or of lactate, which suggests that these metabolic factors are not likely to play a role in the fatigue. The reduced muscle performance was not due to inactivity or atrophy, but seemed to be an effect of CHF on muscle that is very similar to the effect of ageing (Narayanan et al. 1996). We therefore postulate that the observed differences in contractile properties of soleus from CHF and Sham rats are due to altered SR Ca2+ handling in CHF.
Acknowledgments
The study was supported by the Anders Jahres Fund for the Promotion of Science, The Research Council of Norway and Rachel and Otto Chr. Bruun's Fund. We thank Unni Lie Henriksen, Severin Leraand, Thea Sandsbråcten Solum, Line Solberg and Gerd Torgersen for expert technical assistance.
REFERENCES
- Bigard A-X, Boehm E, Veksler V, Mateo P, Anflous K, Ventura-Clapier R. Muscle unloading induces slow to fast transitions in myofibrillar but not mitochondrial properties. Relevance to skeletal muscle abnormalities in heart failure. Journal of Molecular and Cellular Cardiology. 1998;30:2391–2401. doi: 10.1006/jmcc.1998.0798. [DOI] [PubMed] [Google Scholar]
- Brooke MH, Kaiser KK. Three “myosin adenosine triphosphatase” systems: the nature of their pH lability and sulphydryl dependence. Journal of Histochemistry and Cytochemistry. 1970;18:670–672. doi: 10.1177/18.9.670. [DOI] [PubMed] [Google Scholar]
- Brunotte F, Thompson CH, Adamopoulos S, Coats A, Unitt J, Lindsay D, Kaklamanis L, Radda GK, Rajagopalan B. Rat skeletal muscle metabolism in experimental heart failure: effects of physical training. Acta Physiologica Scandinavica. 1995;154:439–447. doi: 10.1111/j.1748-1716.1995.tb09929.x. [DOI] [PubMed] [Google Scholar]
- Bye E, Grrønnerød O, Vogt NB. Multivariate classification of histochemically stained human skeletal muscle fibers by the SIMCA method. Histochemical Journal. 1989;21:15–22. doi: 10.1007/BF01002467. [DOI] [PubMed] [Google Scholar]
- Carafoli E. Intracellular calcium homeostasis. Annual Reviews of Biochemistry. 1987;56:395–433. doi: 10.1146/annurev.bi.56.070187.002143. [DOI] [PubMed] [Google Scholar]
- Chati Z, Zannad F, Michel C, Lherbier B, Mertes PM, Escanye JM, Ribout C, Robert J, Villemot JP, Aliot E. Skeletal muscle phosphate metabolism abnormalities in volume-overload experimental heart failure. American Journal of Physiology. 1994;267:H2186–H2192. doi: 10.1152/ajpheart.1994.267.6.H2186. [DOI] [PubMed] [Google Scholar]
- Close R, Hoh JF. The after-effects of repetitive stimulation on the isometric twitch contraction of rat fast skeletal muscle. Journal of Physiology. 1968a;197:461–477. doi: 10.1113/jphysiol.1968.sp008570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Close R, Hoh JF. Influence of temperature on isometric contractions of rat skeletal muscles. Nature. 1968b;217:1179–1180. doi: 10.1038/2171179a0. [DOI] [PubMed] [Google Scholar]
- Dahlstedt A, Katz A, Westerblad H. Role of myoplasmic phosphate in contractile function of skeletal muscle: studies on creatine kinase-deficient mice. Journal of Physiology. 2001;533:379–388. doi: 10.1111/j.1469-7793.2001.0379a.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damiani E, Sacchetto R, Margreth A. Variation of phospholamban in slow-twitch muscle sarcoplasmic reticulum between mammalian species and a link to the substrate specificity of endogenous Ca2+-calmodulin-dependent protein kinase. Biochimica et Biophysica Acta. 2000;1464:231–241. doi: 10.1016/s0005-2736(00)00153-x. [DOI] [PubMed] [Google Scholar]
- Froemming GR, Murray BE, Harmon S, Pette D, Ohlendieck K. Comparative analysis of the isoform expression pattern of Ca2+-regulatory membrane proteins in fast-twitch, slow-twitch, cardiac, neonatal and chronic low-frequency stimulated muscle fibers. Biochimica et Biophysica Acta. 2000;1466:151–168. doi: 10.1016/s0005-2736(00)00195-4. [DOI] [PubMed] [Google Scholar]
- Fryer MW, Neering IR. Relationship between intracellular calcium concentration and relaxation of rat fast and slow muscles. Neuroscience Letters. 1986;64:231–235. doi: 10.1016/0304-3940(86)90106-0. [DOI] [PubMed] [Google Scholar]
- Fryer MW, Owen VJ, Lamb GD, Stephenson DG. Effects of creatine phosphate and Pi on Ca2+ movements and tension development in rat skinned skeletal muscle fibres. Journal of Physiology. 1995;482:123–140. doi: 10.1113/jphysiol.1995.sp020504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillis JM. Inhibition of mitochondrial calcium uptake slows down relaxation in mitochondria-rich skeletal muscles. Journal of Muscle Research and Cell Motility. 1997;18:473–483. doi: 10.1023/a:1018603032590. [DOI] [PubMed] [Google Scholar]
- Harrington D, Coats AJ. Skeletal muscle abnormalities and evidence for their role in symptom generation in chronic heart failure. European Heart Journal. 1997;18:1865–1872. doi: 10.1093/oxfordjournals.eurheartj.a015194. [DOI] [PubMed] [Google Scholar]
- Kemp GJ, Thompson CH, Stratton JR, Brunotte F, Conway M, Adamopoulos S, Arnolda L, Radda GK, Rajagopalan B. Abnormalities in exercising skeletal muscle in congestive heart failure can be explained in terms of decreased mitochondrial ATP synthesis, reduced metabolic efficiency, and increased glycogenolysis. British Heart Journal. 1996;76:35–41. doi: 10.1136/hrt.76.1.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lännergren J, Westerblad H, Bruton JD. Changes in mitochondrial Ca2+ detected with Rhod-2 in single frog and mouse skeletal muscle fibres during and after repeated tetanic contractions. Journal of Muscle Research and Cell Motility. 2001;22:265–275. doi: 10.1023/a:1012227009544. [DOI] [PubMed] [Google Scholar]
- Lowry OH, Passonneau JV. A Flexible System of Enzymatic Analysis. New York, NY, USA: Academic Press; 1972. A collection of metabolite assays; pp. 146–218. [Google Scholar]
- Lunde PK, Dahlstedt AJ, Bruton JD, Lännergren J, Thorén P, Sejersted OM, Westerblad H. Contraction and intracellular Ca2+ handling in isolated skeletal muscle of rats with congestive heart failure. Circulation Research. 2001a;88:1299–1305. doi: 10.1161/hh1201.092041. [DOI] [PubMed] [Google Scholar]
- Lunde PK, Sjaastad I, Thorud H-M S, Sejersted OM. Skeletal muscle disorders in heart failure. Acta Physiologica Scandinavica. 2001b;171:277–294. doi: 10.1046/j.1365-201x.2001.00830.x. [DOI] [PubMed] [Google Scholar]
- Narayanan N, Jones DL, Xu A, Yu JC. Effects of aging on sarcoplasmic reticulum function and contraction duration in skeletal muscles of the rat. American Journal of Physiology. 1996;271:C1032–C1040. doi: 10.1152/ajpcell.1996.271.4.C1032. [DOI] [PubMed] [Google Scholar]
- Simonini A, Long CS, Dudley GA, Yue P, McElhinny J, Massie BM. Heart failure in rats causes changes in skeletal muscle morphology and gene expression that are not explained by reduced activity. Circulation Research. 1996;79:128–136. doi: 10.1161/01.res.79.1.128. [DOI] [PubMed] [Google Scholar]
- Sjaastad I, Sejersted OM, Ilebekk A, Bjørnerheim R. Echocardiographic criteria for detection of post-infarction congestive heart failure in rats. Journal of Applied Physiology. 2000;89:1445–1454. doi: 10.1152/jappl.2000.89.4.1445. [DOI] [PubMed] [Google Scholar]
- Thompson CH, Kemp GJ, Rajagopalan B, Radda GK. Abnormal ATP turnover in rat leg muscle during exercise and recovery following myocardial infarction. Cardiovascular Research. 1995;29:344–349. [PubMed] [Google Scholar]
- Tønnessen T, Christensen G, Oie E, Holt E, Kjekshus H, Smiseth OA, Sejersted OM, Attramadal H. Increased cardiac expression of endothelin-1 mRNA in ischemic heart failure in rats. Cardiovascular Research. 1997;33:601–610. doi: 10.1016/s0008-6363(96)00266-0. [DOI] [PubMed] [Google Scholar]
- Verburg E, Thorud H-M S, Eriksen M, Vøllestad NK, Sejersted OM. Muscle contractile properties during intermittent non-tetanic stimulation in rat skeletal muscle. American Journal of Physiology. 2001;281:R1952–R1965. doi: 10.1152/ajpregu.2001.281.6.R1952. [DOI] [PubMed] [Google Scholar]
- Vescovo G, Ceconi C, Bernocchi P, Ferrari R, Carraro U, Ambrosio GB, Libera LD. Skeletal muscle myosin heavy chain expression in rats with monocrotaline-induced cardiac hypertrophy and failure. Relation to blood flow and degree of muscle atrophy. Cardiovascular Research. 1998;39:233–241. doi: 10.1016/s0008-6363(98)00041-8. [DOI] [PubMed] [Google Scholar]
- Wahr PA, Johnson JD, Rall JA. Determinants of relaxation rate in skinned frog skeletal muscle fibers. American Journal of Physiology. 1998;274:C1608–C1615. doi: 10.1152/ajpcell.1998.274.6.C1608. [DOI] [PubMed] [Google Scholar]
- Westerblad H, Allen DG. Changes of myoplasmic calcium concentration during fatigue in single mouse muscle fibers. Journal of General Physiology. 1991;98:615–635. doi: 10.1085/jgp.98.3.615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westerblad H, Bruton JD, Lännergren J. The effect of intracellular pH on contractile function of intact, single fibres of mouse muscle declines with increasing temperature. Journal of Physiology. 1997;500:193–204. doi: 10.1113/jphysiol.1997.sp022009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westerblad H, Lee JA, Lännergren J, Allen DG. Cellular mechanisms of fatigue in skeletal muscle. American Journal of Physiology. 1991;261:C195–C209. doi: 10.1152/ajpcell.1991.261.2.C195. [DOI] [PubMed] [Google Scholar]


