Abstract
Porcine pulmonary arterial endothelial cells accumulated myo-inositol and taurine, as well as betaine, during adaptation to hypertonic stress. The cells grew and maintained their normal morphology during culture in hypertonic (0.5 osmol (kg H2O)−1) medium that contained osmolytes such as betaine, myo-inositol or taurine at concentrations close to reported physiological values. The cells did not grow well in hypertonic medium depleted of potential compatible osmolytes. After a few days, cell density decreased by about 50 % and many cells rounded up and detached from the plates, their nuclei showing clear apoptotic morphology. The caspase-3 activity of the cells also increased dramatically under these conditions, but remained negligibly low when betaine and myo-inositol were added to the medium. Addition of betaine and myo-inositol to hypertonic medium depleted of compatible osmolytes increased the number of colonies remaining after 12 days of culture; with each solute at 30–100 μmol l−1 the number increased about sixfold. In the absence of compatible osmolytes, increased mRNA levels and corresponding activities of betaine/γ-aminobutyric acid transporter (BGT1) and sodium/myo-inositol transporter (SMIT) induced by hypertonicity remained high after 72 h incubation, whereas they were down regulated in the presence of betaine and myo-inositol. Similarly, the down regulation of the amino acid System A transporter (ATA2) was markedly slowed in the absence of compatible osmolytes. We conclude that these compatible osmolytes at concentrations close to physiological values enable the endothelial cells to adapt to hypertonic stress, protecting them from apoptosis, and also modulate the adaptation process.
Extreme hypertonic stress (greater than about 0.6 osmol (kg H2O)−1) has been shown to induce apoptosis in a number of mammalian cells in culture, including human SH SY5Y neuroblastoma cells (Matthews & Feldman, 1996), thymocytes (Bortner & Cidlowski, 1996), DT40 chicken B cells (Qin et al. 1997), Madin-Darley canine kidney (MDCK) cells (Hizoh et al. 1998; Horio et al. 2001) and murine renal inner medullary collecting duct (mIMCD3) cells (Santos et al. 1998; Michea et al. 2000). Usually, however, many types of mammalian cells can survive culture in a more moderate hypertonic environment (up to about 0.5 osmol (kg H2O)−1) because of a specific adaptation process that eventually results in the cells accumulating compatible osmolytes (Burg, 1995; Burg et al. 1997). This adaptation involves changes of gene expression that result in an increased synthesis either of a compatible osmolyte itself (e.g. sorbitol) or of transporters for the osmolytes, such as ATA2 for neutral amino acids (Alfieri et al. 2001), BGT1 for betaine, SMIT for myo-inositol and TAUT for taurine (see review by Burg et al. 1997). The usual explanation of this phenomenon is the need to replace the early cellular accumulation of inorganic ions, the initial rapid response to hypertonicity, with small organic molecules (compatible osmolytes) that do not perturb macromolecular structures, as the increased concentrations of inorganic ions do.
The adaptive response to hyperosmolarity has been studied mainly in kidney-derived epithelial cells, since these are physiologically exposed to marked variations in osmolarity during their normal function. In the last few years, however, similar increased transport activities and subsequent accumulation of compatible osmolytes have been detected in chondrocytes (De Angelis et al. 1999), macrophages (Denkert et al. 1998), endothelial (Petronini et al. 2000) and mesothelial (Matsuoka et al. 1999) cells exposed to hypertonic media. Although the responses of renal cells and chondrocytes to a hypertonic environment are readily understandable in terms of their physiology, the reason for the similar adaptive behaviour of other cells is less evident. Yet the existence of these responses to hypertonicity, which are quite expensive in terms of gene information, gene expression and metabolic input, suggests a physiological role. Such a role remains obscure, but it is worth noting that endothelial cells are constantly exposed to the shear and stress of blood flow, and eventually to moderate changes in blood osmolarity. It is also likely that certain pathological conditions, such as diabetic hyperglycaemia, hyperosmolar coma or severe dehydration, might require fast osmotic responses. Such osmotic adaptation might be particularly important to endothelial cells that physiologically mediate the exchange of water, small solutes, such as ions and nutrients, and even macromolecules, between plasma and interstitial fluid.
As we reported recently (Petronini et al. 2000), the immediate shrinkage of endothelial cells in hypertonic (0.5 osmol (kg H2O)−1) medium is followed by a slow regulatory volume increase, during which an increase in cell K+ concentration occurs. Then the activity of amino acid transport System A increases, accompanied by an accumulation of ninhydrin-positive solutes, and this is followed by an induction of the BGT1 transporter, while cell K+ concentration gradually decreases. Estimation of the cellular concentrations of ninhydrin-positive solutes and betaine, however, failed to account for osmotic equilibrium, suggesting that some other solute, or solutes, had also been accumulated. We have, therefore, extended the previous study by examining the hypertonic induction of other transporters and determining whether cell survival under prolonged hypertonic conditions does indeed depend on the presence in the culture medium of specific compatible osmolytes. Our results confirm this latter contention, show that the cells do accumulate several compatible osmolytes and reveal a modulating effect of some osmolytes on the hypertonic induction of membrane transport activities.
METHODS
Materials
α-[32P]dCTP, γ-amino[2,3-3H]butyric acid, [1,2-3H]taurine and myo-[3H]inositol were obtained from Amersham International, Amersham, Bucks., UK. 2-[1-14C]methylaminoisobutyric acid (MeAIB) was obtained from Dupont/New England Nuclear, Boston, MA, USA. Choline oxidase, betaine, amino acids and myo-inositol were bought from Sigma Chemical Co., Poole, Dorset, UK. [14C]Betaine was prepared from [14C]choline by enzymic oxidation (Petronini et al. 1994). A human cDNA probe for ATA2 (Sugawara et al. 2000) was kindly supplied by Dr V. Ganapathy, Department of Biochemistry and Molecular Biology, Medical College of Georgia, Augusta, GA, USA. Canine BGT1 and SMIT cDNA probes (Yamauchi et al. 1992) were kindly provided by Dr Moo Kwon, Division of Nephrology, John Hopkins University School of Medicine, Baltimore, MD, USA. The probe for 28S rRNA was obtained from Dr Lorenza Tacchini, University of Milan, Italy. Media, fetal calf serum and antibiotics for culturing the cells were purchased from Gibco, Grand Island, NY, USA. Disposable plastics for laboratory use were obtained from Costar, Broadway, Cambridge, MA, USA. Other reagents of analytical grade were purchased from Sigma Chemical Co., St. Louis, MO, USA.
Cell culture
Pig pulmonary arteries were obtained from a local abattoir and the endothelial cells cultured as described previously (Petronini et al. 2000). The growth medium was minimal essential medium (MEM) containing 100 U ml−1 penicillin, 100 μg ml−1 streptomycin and supplemented with 2 mmol l−1 glutamine (final concentration 4 mmol l−1), 2 mmol l−1 sodium pyruvate, 10 mmol l−1 Hepes (pH 7.4), 10 % fetal calf serum, 50 μg ml−1 endothelial cell growth factor. All cultures were kept in an incubator at 37 °C in a water-saturated atmosphere of 5 % CO2 in air. Cells used in this study were from early (up to the tenth) passages and were routinely checked for the expression of von Willebrand Factor by immuno-cytochemistry with the use of rabbit anti-human von Willebrand Factor (DAKO Ltd, UK).
The osmolalities of the culture media were checked with a vapour-pressure osmometer (Wescor). Normal growth medium was about 0.3 osmol (kg H2O)−1. Hypertonic growth medium was made by addition of 200 mmol l−1 sucrose to normal growth medium, giving a final osmolality of about 0.5 osmol (kg H2O)−1.
Culture medium depleted of compatible osmolytes
A mixture of MEM ingredients without myo-inositol was obtained from Gibco (Grand Island, NY, USA) and reconstituted with the use of either dialysed serum or bovine serum albumin (0.1 %, w/v) in the place of normal serum.
Determination of cell survival and growth
To test their viability under hypertonic conditions, endothelial cells were first seeded at a density of about 104 cells cm−2 and cultured for 2 days in the usual isotonic medium. Then the latter was replaced with hypertonic medium, either depleted of compatible osmolytes or with 0.1 mmol l−1 betaine and 0.1 mmol l−1myo-inositol added. After incubation under these conditions for 24, 48 and 72 h, cell density was assayed in terms of the protein content per dish (Petronini et al. 1993).
For measurement of colony formation, the cells were seeded at a density of 20 cells cm−2 and cultured for 1 day in isotonic MEM medium supplemented with fetal calf serum (10 %). Then the medium was replaced with hypertonic medium, either depleted of compatible osmolytes or with 0.1 mmol l−1 betaine and 0.1 mmol l−1myo-inositol added. Colonies were allowed to grow for 12 days and then the cells were fixed with 95 % ethanol, stained with 0.1 % crystal violet and counted (Petronini et al. 1993).
Detection of apoptosis
Cell apoptosis was assessed both morphologically and in terms of increased caspase-3 activity. For the former, detached cells were stained with the dye Hoechst 33342 and their nuclear morphology examined by fluorescence microscopy. For determination of caspase activity, adherent endothelial cells were scraped from dishes and added to any cells that had detached spontaneously. The mixture of cells was washed by sedimentation and resuspension before being incubated in lysis buffer on ice for 10 min. Cell debris was then sedimented by centrifugation at 12 000 g for 2 min at 4 °C and the caspase-3 activity of the supernatant solution was assayed colourimetrically with the use of a kit from MBL International Corporation, Watertown, MA, USA.
Ninhydrin-positive substances (NPS)
The intracellular content of NPS was measured by the method of Law and Turner (1987) and expressed as nmol mg−1 of protein.
Protein
Cell protein, precipitated by TCA, was dissolved in 0.2 n NaOH and its concentration determined by a dye-fixation method (Bio-Rad) using bovine serum albumin as standard (Bradford, 1976).
Transport measurements
The rates of uptake of amino acids, betaine, and myo-inositol by endothelial cells were measured after the latter had been incubated in control (0.3 osmol (kg H2O)−1) or test (0.5 osmol (kg H2O)−1) medium for the desired time. The cell monolayers were quickly washed with Earle's balanced salt solution containing 0.1 % glucose and then incubated in this solution for 15 min at 37 °C to diminish the cellular pool of amino acids. The cells were washed again and immediately incubated at 37 °C for the desired time in the presence of [3H]- or [14C]- labelled solute. MeAIB was used as the characterising substrate of amino acid transport System A (Christensen, 1975) and GABA for BGT1 (De Angelis et al. 1999). The incubations were stopped by removal of the medium and the cells were quickly washed three times with fresh cold medium. Trichloroacetic acid (5 %, w/v) was added to denature the cells and the radioactivity in samples of the acid extracts was measured by scintillation counting.
Northern blotting
Total RNA was extracted from cultured cells using the Ultraspec RNA Isolation System from Biotecx (Chomczynski & Sacchi, 1987). RNA samples (20 μg) were fractionated by electrophoresis through 1 % agarose gels and the separated bands transferred to nylon filters. The quality and quantity of RNA blotted on the membranes were checked by u.v. absorption. Full length ATA2, BGT1 and SMIT cDNAs and 28S rRNA were nick-translated (Amersham kit N. 5000) with [α-32P]dCTP (3000 Ci mmol−1). Hybridisation, washing and autoradiography were carried out exactly as described previously (Petronini et al. 2000).
RESULTS
Effect of hypertonicity on cellular accumulation of compatible osmolytes
The compatible solutes, in addition to betaine, most likely to be involved in the endothelial cells responses to hypertonicity were taurine, sorbitol and myo-inositol (Petronini et al. 2000). Sorbitol, however, seems not to be an important osmolyte in these cells in the presence of normal concentrations of glucose. A hypertonic induction of mRNA for aldose reductase was observed, but the cellular concentration of sorbitol reached only about 5 mmol l−1 after 48 h incubation in hypertonic (0.5 osmol (kg H2O)−1) medium containing 25 mmol l−1 glucose: with the normal 5 mmol l−1 glucose it was not reliably detectable (result not shown). In contrast, endothelial cells accumulated taurine, myo-inositol and betaine against their concentration gradients under both isotonic and hypertonic conditions when incubated in media containing the solutes at 0.1 mmol l−1, a concentration close to the physiological ranges (Allen et al. 1993; Rim et al. 1998; Weik et al. 1998). The uptakes were, however, much greater under hypertonic conditions, the intracellular concentration reaching 45–55 mmol l−1 after 48 h incubation in 0.5 osmol (kg H2O)−1 medium (Fig. 1). Since the cells are usually cultured in MEM (that contains myo-inositol) supplemented with fetal calf serum (containing betaine, taurine and myo-inositol), this finding suggests that the combined accumulation of these solutes could account for the cells' adaptation to prolonged hypertonicity.
Figure 1. Accumulation of betaine, taurine and myo-inositol by endothelial cells incubated in hypertonic medium.
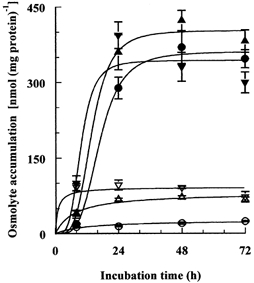
Endothelial cells were incubated in isotonic (0.3 osmol (kg H2O)−1) or hypertonic (0.5 osmol (kg H2O)−1) medium containing, separately, 0.1 mmol l−1 radio-labelled betaine, taurine or myo-inositol and the cellular concentration of each solute was measured at the indicated times. Sucrose was used as the extra osmolyte. Mean values (±s.d.) of four measurements are shown. The final concentration values noted in the text were calculated with the use of measured cell volumes, which had returned to the control value of 7–8 μl mg−1 of protein after a few hours of hypertonic incubation (Petronini et al. 2000). Open symbols, isotonic medium; closed symbols, hypertonic medium; •, betaine; ▴, myo-inositol; ▾, taurine.
Effect of compatible osmolytes on the growth and survival of cells exposed to hypertonicity
To test the conclusion noted above, we cultured cells in media depleted of potential compatible osmolytes (see Methods) or with selected ones added at 0.1 mmol l−1. Cell growth in isotonic media was the same with or without betaine, myo-inositol and taurine, but was over 60 % less in hypertonic medium depleted of these osmolytes (Table 1). Addition of one, two or all three (each at 0.1 mmol l−1) to the hypertonic medium for 56 h restored growth to 52–67 % of the value under isotonic conditions. In view of these results, we decided to use just betaine (a zwitterion) and myo-inositol (an uncharged poly-alcohol) for further experiments, keeping each at 0.1 mmol l−1.
Table 1.
Effect of compatible osmolytes on cell growth under hypertonic conditions
| Cell density (μg protein cm−2) | ||
|---|---|---|
| Addition | Isotonic | Hypertonic |
| None | 51.0 ± 3.4 (6) | 18.8 ± 1.5 (6) |
| Betaine +myo-inositol + taurine | 49.7 ± 1.5 (3) | 32.8 ± 2.3 (3) |
| Betaine +myo-inositol | — | 34.5 ± 1.9 (4) |
| Betaine + taurine | — | 32.7 ± 2.2 (4) |
| myo-Inositol + taurine | — | 32.8 ± 1.4 (4) |
| Betaine | — | 30.2 ± 2.6 (4) |
| myo-Inositol | — | 27.3 ± 1.4 (4) |
| Taurine | — | 26.7 ± 0.2 (4) |
Endothelial cells were cultured for 24 h in isotonic (0.3 osmol (kg H2O)−1) medium depleted of potential compatible osmolytes and then for a further 56 h under the conditions noted above. The hypertonic medium was about 0.5 osmol (kg H20)−1 and, when added, each osmolyte (betaine, myo-inositol or taurine) was present at 0.1 mmol l−1. Cell growth was then estimated by measurement of cell protein. The values given are means (±s.d.) of the number of independent measurements indicated in brackets.
First, we simply monitored cell morphology during incubation under hypertonic conditions with and without the compatible osmolytes. Although use of the depleted medium had no obvious effect on cell growth and survival under isotonic (0.3 osmol (kg H2O)−1) conditions, it was clearly detrimental under hypertonic (0.5 osmol (kg H2O)−1) conditions (Fig. 2). The cells not only failed to grow but also some began to round up, lose adhesion and detach from the plates (Fig. 2B). Staining with the DNA dye Hoechst 33342 revealed that most of these detached cells had clear apoptotic morphology; their chromatin either had the classic semi-lunar appearance or had collapsed into patches and dense spheres (Fig. 2C). In contrast, the endothelial cells grew well and maintained their normal morphology during culture for 72 h in hypertonic (0.5 osmol (kg H2O)−1) medium that contained only 0.1 mmol l−1 of both betaine and myo-inositol (Fig. 2D). In keeping with these observations, the caspase-3 activity of the cells increased dramatically during their prolonged incubation in the hypertonic medium lacking the compatible osmolytes, but remained negligibly low when both betaine and myo-inositol were present (Fig. 3).
Figure 2. Effect of deprivation of compatible osmolytes on the morphology of cells exposed to hypertonicity.
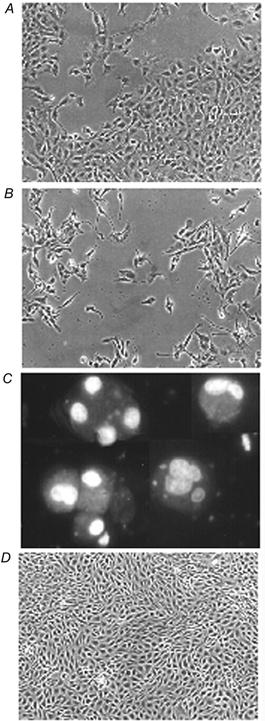
A, cells were seeded and cultured for 24 h in isotonic (0.3 osmol (kg H2O)−1) medium and then incubated for a further 72 h in either (B) depleted hypertonic (0.5 osmol (kg H2O)−1) medium or (D) depleted hypertonic medium with added betaine and myo-inositol (each at 0.1 mmol l−1). The cells were then photographed under phase contrast. Cells that had detached after 72 h of hypertonic incubation in the absence of compatible osmolytes (C) were treated with the fluorescent DNA stain Hoechst 33342. Magnification: A, B and D, × 100; C, × 400. All the cells in field C exhibit the typical chromatin condensation of apoptosis.
Figure 3. Effect of deprivation of compatible osmolytes on cell caspase-3 activity during hypertonic incubation.
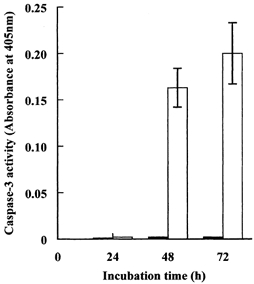
Cells were incubated for the times indicated in depleted hypertonic (0.5 osmol (kg H2O)−1) medium with or without added betaine and myo-inositol, each at 0.1 mmol l−1. Caspase-3 activity was measured in cell extracts as described in the Methods section. Mean values (±s.d.) of three independent determinations are given. □, depleted of compatible osmolytes; ▪, with betaine and myo-inositol.
More quantitative information was obtained by repeating the measurements of cell density as a function of time of incubation. After allowing 24 h for cell attachment in isotonic medium, cell monolayers were again incubated in the depleted hypertonic medium with or without the addition of 0.1 mmol l−1 betaine and myo-inositol. Although there was no significant difference in cell growth rate during the first 24 h under these two conditions, during more prolonged incubation the cells in hypertonic medium depleted of compatible osmolytes stopped growing, and some of them started to detach from the substratum. The net result was that after 72 h of this treatment the density of the cells was 50 % lower than that of cells cultured in the presence of the compatible osmolytes (Fig. 4). This effect on cell growth was corroborated by examination of the effect of the compatible osmolytes on the cells' ability to form colonies. Cells were plated for 24 h in isotonic medium, then switched to the depleted hypertonic medium supplemented with various concentrations of betaine and myo-inositol and cultured for 12 days. The number of colonies (of more than 50 cells) was then measured. As shown in Fig. 5, the number of colonies remaining after 12 days of hypertonic culture increased markedly in the presence of relatively low concentrations of both osmolytes, doubling with concentrations of only 1–3 μmol l−1, and increasing about sixfold when each was at 30–100 μmol l−1.
Figure 4. Effect of removal of compatible osmolytes on cell growth and survival under hypertonic conditions.
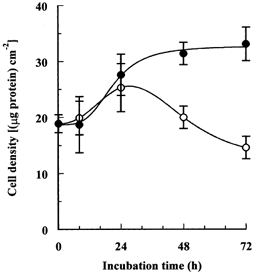
Cells were seeded and cultured for 24 h in isotonic (0.3 osmol (kg H2O)−1) medium and then transferred to depleted hypertonic (0.5 osmol (kg H2O)−1) medium with or without added betaine and myo-inositol, each at 0.1 mmol l−1. Culture in both media was continued for the indicated times and cell growth was estimated by measurement of cell protein. Mean values (±s.d.) of three independent measurements are given. ○, depleted of compatible osmolytes; •, with betaine and myo-inositol.
Figure 5. Effect of betaine and myo-inositol on the colony-forming efficiency of cells incubated in depleted hypertonic medium.
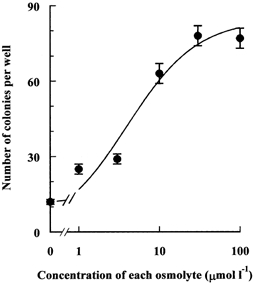
Endothelial cells were seeded at a density of about 200 cells per well (area 9 cm2) in isotonic (0.3 osmol (kg H2O)−1) medium and cultured for 24 h. Then the medium was replaced with the depleted hypertonic (0.5 osmol (kg H2O)−1) one containing added betaine and myo-inositol at the indicated concentrations. Colonies were allowed to grow for 12 days and then the number of colonies larger than 50 cells was counted. Values are the means (±s.d.) of three independent determinations. (The maximum number of colonies possible was less than 200.)
Taken together, these results show these endothelial cells do require compatible osmolytes to survive prolonged exposure to hypertonicity, and that the presence of just 0.1 mmol l−1 betaine and myo-inositol protects them against apoptosis induced by hypertonicity.
Modulation of induced transport activities
We showed previously that the addition of a relatively high concentration (5 mmol l−1) of betaine to the culture medium drastically reduced the hypertonic induction of BGT1 and significantly decreased that of System A (Petronini et al. 2000). Since the results described above showed that even the low concentrations of compatible osmolytes normally present in the incubation media have dramatic effects, we also checked the effect of their removal on the hypertonic induction of the various transport activities. Cells were cultured in the depleted hypertonic medium with or without the addition of betaine and myo-inositol (each at 0.1 mmol l−1) and the transport activities of ATA2, BGT1 and SMIT were monitored over 3 days.
The response of ATA2 transport activity (measured as the initial rate of MeAIB influx) in cells incubated under hypertonic conditions in the presence of betaine and myo-inositol was similar to that noted previously (Petronini et al. 2000; Alfieri et al. 2001). The transport activity increased quickly, reached a maximum after about 8 h of hypertonic treatment, and then declined to the isotonic control value. In the absence of the added osmolytes, however, the increased uptake induced by hypertonicity declined much more slowly and at 72 h was still 50 % of the peak value (Fig. 6A). We also monitored the cellular content of ninhydrin-positive solutes (NPS), mostly amino acids, some of which may behave as compatible osmolytes, under the conditions described above. The absence of compatible osmolytes from the hypertonic medium again resulted in a markedly slower decline from the induced maximum cell content of NPS (Fig. 6B).
Figure 6. Modulation of hypertonic induction of amino acid transport activity and accumulation of NPS.
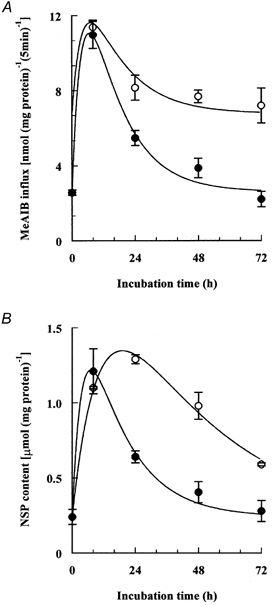
Initial rates of cell uptake of 2-[1-14C]methylaminoisobutyric acid (MeAIB) (A) and cell contents of ninhydrin-positive solutes (NPS) (B) were measured after the cells had been incubated for the indicated times in depleted hypertonic (0.5 osmol (kg H2O)−1) medium with or without added betaine and myo-inositol, each at 0.1 mmol l−1. The extracellular concentration of MeAIB was 0.1 mmol l−1. Mean values (±s.d.) of four measurements are given. ○, depleted of compatible osmolytes; •, with betaine and myo-inositol.
The removal of the osmolytes produced similar, but much more dramatic, changes to the hypertonic induction of BGT1 and SMIT activities, assayed as the initial rates of uptake of GABA (De Angelis et al. 1999) and myo-inositol, respectively (Fig. 7). In the presence of added betaine and myo-inositol, the induced activities of both transporters reached maximum values after 24 h of treatment and then slowly declined towards the values of the isotonic controls. In contrast, in the absence of betaine and myo-inositol, the induced transport activities were quite different, being just detectable at 8 h and then increasing throughout the 72 h period to values six to seven times higher than those of the hypertonic controls, and 18–20 times higher than the activities of isotonic control cells.
Figure 7. Modulation of hypertonic induction of BGT1 and SMIT transport activities induced by hypertonicity.
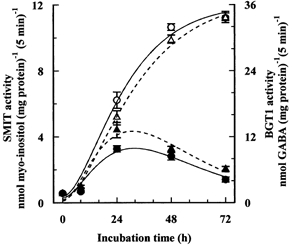
The initial rates of uptake of GABA and myo-inositol (extracellular concentrations 0.1 mmol l−1) by endothelial cells were measured after the cells had been incubated for the indicated times in depleted hypertonic (0.5 osmol (kg H2O)−1) medium with or without added betaine and myo-inositol, each at 0.1 mmol l−1. Sucrose was used as the extra osmolyte. Mean values (±s.d.) of four measurements are given. Open symbols, depleted of compatible osmolytes; filled symbols, with betaine and myo-inositol; ▴, GABA; •, myo-inositol.
Modulation of induction of transporter mRNAs
The findings noted above obviously raise the question of where in the induction pathway the compatible osmolytes act. It has been known for some time that changes in the gene expression of BGT1, TAUT and SMIT transporters parallel the hypertonic induction of their activities (see Burg et al. 1997). More recently, we reported a similar correlation for amino acid transport System A and the ATA2 gene, showing that changes in the expression of ATA2 mRNA preceded both the induction and subsequent down regulation of transport activity in these endothelial cells (Alfieri et al. 2001). Hence, we next examined the effect of the compatible osmolytes on the mRNA levels of all three transporters during the exposure of the cells to hypertonicity. As expected, Northern blotting revealed the presence of mRNA for each of the transporters (ATA2, BGT1 and SMIT) in endothelial cells exposed to hypertonicity, but only small or insignificant amounts in control cells incubated in isotonic conditions (Fig. 8). Removal of the compatible osmolytes from the hypertonic medium both intensified and prolonged the induction of mRNAs, at least up to 48 h, in agreement with the higher transport activities observed under the same culturing conditions. The only apparently discrepant finding was the lack of correspondence between the influx of myo-inositol (Fig. 7) and the amount of SMIT mRNA detected in cells incubated in the presence of betaine and inositol (Fig. 8C). Instead of paralleling the small decrease of inositol influx, the amount of SMIT mRNA detected after 48 h incubation always increased markedly. A possible explanation is lack of specificity of the Northern blot, some other induced mRNA being abundant and detected after 48 h. This explanation is supported by the results for the cells incubated in the depleted medium, where the increase in apparent mRNA level between 24 h and 48 h was somewhat larger than expected from the increase in transport activity.
Figure 8. Modulation of expression of mRNA for ATA2, BGT1 and SMIT induced by hypertonicity.
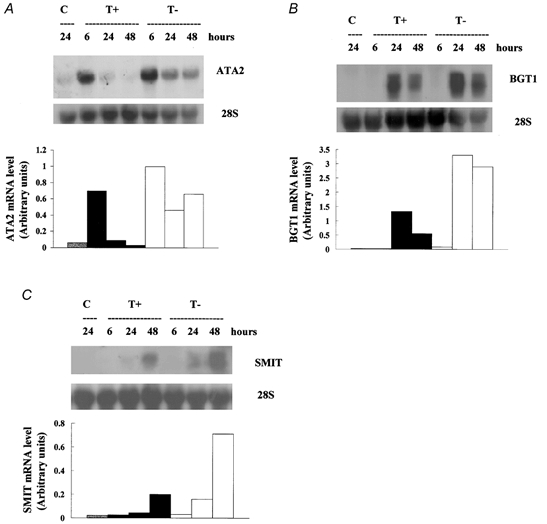
Endothelial cells were incubated in isotonic (0.3 osmol (kg H2O)−1) medium (C) and depleted hypertonic (0.5 osmol (kg H2O)−1) medium with (T+) or without (T-) added betaine and myo-inositol, each at 0.1 mmol l−1. After the indicated times, total cellular RNA was extracted and analysed by Northern blotting for (A) ATA2, (B) BGT1 and (C) SMIT mRNA, as described under Methods. 28S rRNA was used for standardisation, the densitometric quantification of the blots being normalised to the levels of 28S rRNA.
In general, these observations confirm that the increased transport activities in endothelial cells cultured in hypertonic medium follow increased expression of the corresponding genes, and show that the absence of compatible osmolytes inhibits subsequent down-regulation of gene expression to normal levels.
DISCUSSION
Amino acids accumulation, sustained by increased activity of transport System A, was sufficient for the complete volume recovery that occurs during the first few hours of hypertonic treatment in vascular endothelial cells (Dall'Asta et al. 1999). When the osmotic stress persists for a longer period of time, however, osmolytes that are more compatible might be mandatory for cell survival. Our findings show that pulmonary artery endothelial cells undergo apoptosis during prolonged culture in a hypertonic medium depleted of potential compatible osmolytes, such as betaine, taurine and myo-inositol. The dramatic induction of the transporters (BGT1, TAUT and SMIT) for these solutes during hypertonic incubation of the cells, and their impressive accumulation from a concentration (0.1 mmol l−1) near the physiological range, clearly support the notion that they are important. This is confirmed by the growth and survival of the cells when betaine and myo-inositol were added back to the depleted medium.
We did not attempt to test all the possible concentrations and combinations of these osmolytes; besides being impracticable, the details of their physiological concentrations are not certain. Since we found little difference among the possibilities we did examine, we used mainly the combination of one of the zwitterions (betaine) and the uncharged myo-inositol, to cover any possible specific requirements of which we are unaware. Removal of these osmolytes from the hypertonic culture medium had no detectable effect on the induced increase of the transport activities, but did markedly alter subsequent events. In addition to the lack of cell proliferation and eventual activation of the apoptotic pathway that occurred in their absence, the BGT1 and SMIT mRNA levels and transport activities were no longer down-regulated. Moreover, the induced ATA2 mRNA level and System A activity, and the increased NPS content, were only partly down-regulated. The usual presence of compatible osmolytes thus appears to be involved in the down regulation of these transport activities. This modulation of the down-regulation clearly acts at, or before, the gene expression steps in the induction pathway. It is not simple feed-back inhibition because betaine and myo-inositol stimulated the down-regulation of ATA2, as well BGT1 and SMIT. The lack of specificity was confirmed by comparison of the induced transport activities remaining after 48 h incubation in depleted hypertonic (0.48 osmol (kg H2O)−1) medium with or without the addition of either betaine or myo-inositol. With betaine alone at 0.1 mmol l−1, the induced activities of ATA2, BGT1 and SMIT were down-regulated by 33, 34 and 16 %, respectively. With 0.1 mmol l−1myo-inositol alone the corresponding reductions were 54, 48 and 72 %. When the concentration of the added osmolyte was increased to 1 mmol l−1, the down-regulation of transport activities ranged from 57 % to 87 %. Amino acids in general, and most of the other ninhydrin-positive solutes, clearly did not cause such down-regulations. Hence these modulating effects of betaine and myo-inositol seem to be of a general nature and presumably stem from intrinsic properties that distinguish them as compatible osmolytes. The ability to stabilise proteins under various extreme conditions (Yancey et al. 1982; Santoro et al. 1992) may be most important, but a similar effect on nucleic acids also seems possible. Taken together, these results show that betaine and myo-inositol at concentrations (each 0.1 mmol l−1) close to the physiological range (Allen et al. 1993; Weik et al. 1998; Rim et al. 1998) act both as components and modulators of the sequential steps of the adaptation process to hypertonicity, enabling the endothelial cells to avoid apoptosis.
Our results are consistent with and extend previous observations that the survival and growth of Madin-Darby canine kidney (MDCK) and mesothelial cells under hypertonic conditions were markedly suppressed in the presence of a specific inhibitor of myo-inositol transport (Kitamura et al. 1997; Matsuoka et al. 1999). Similarly, our demonstration that betaine and myo-inositol counteract hypertonicity-induced apoptosis in endothelial cells supports the finding that 1 mmol l−1 betaine (a concentration ten times the physiological level) protected hypertonicity-induced apoptosis of MDCK cells (Horio et al. 2001).
Although the physiological necessity for the existence of this adaptation process in these endothelial cells remains speculative (see Introduction), the protection of endothelial cells from hypertonicity-induced apoptosis by low concentrations of compatible osmolytes might have a clinical application. Malek et al. (1998) reported that intravenous administration of mannitol at concentrations of 0.4–0.6 osmol l−1, as used in the treatment of a number of clinical conditions, could induce apoptosis in vascular endothelium. If mannitol mimics sucrose osmotically, the use of appropriate concentrations of osmolytes such as betaine and myo-inositol in these hypertonic infusion therapies might help the survival of endothelial cells.
Acknowledgments
We thank the Ministero della Istruzione, della Universitaé della Ricerca Scientifica, Rome, Italy for financial support.
REFERENCES
- Alfieri RR, Petronini PG, Bonelli MA, Caccamo AE, Cavazzoni A, Borghetti AF, Wheeler KP. Osmotic regulation of ATA2 mRNA expression and amino acid transport System A activity. Biochemical and Biophysical Research Communications. 2001;283:174–178. doi: 10.1006/bbrc.2001.4729. [DOI] [PubMed] [Google Scholar]
- Allen RH, Stabler SP, Lindenbaum J. Serum betaine, N,N-dimethylglycine and N-methylglycine levels in patients with cobalamin and folate deficiency and related inborn errors of metabolism. Metabolism. 1993;42:1448–1460. doi: 10.1016/0026-0495(93)90198-w. [DOI] [PubMed] [Google Scholar]
- Bortner CD, Cidlowski JA. Absence of volume regulatory mechanisms contributes to the rapid activation of apoptosis in thymocytes. American Journal of Physiology. 1996;271C:950–961. doi: 10.1152/ajpcell.1996.271.3.C950. [DOI] [PubMed] [Google Scholar]
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Analytical Biochemistry. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Burg MB. Molecular basis of osmotic regulation. American Journal of Physiology. 1995;268F:983–996. doi: 10.1152/ajprenal.1995.268.6.F983. [DOI] [PubMed] [Google Scholar]
- Burg MB, Kwon HM, Kultz D. Regulation of gene expression by hypertonicity. Annual Review of Physiology. 1997;59:437–455. doi: 10.1146/annurev.physiol.59.1.437. [DOI] [PubMed] [Google Scholar]
- Chomczynski P, Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Analytical Biochemistry. 1987;162:156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Christensen HN. Recognition sites for material transport and information transfer. Current Topics in Membranes and Transport. 1975;6:227–258. [Google Scholar]
- Dall'Asta V, Bussolati O, Sala R, Parolari A, Alamanni F, Biglioli P, Gazzola GC. Amino acids are compatible osmolytes for volume recovery after hypertonic shrinkage in vascular endothelial cells. American Journal Physiology. 1999;276C:865–872. doi: 10.1152/ajpcell.1999.276.4.C865. [DOI] [PubMed] [Google Scholar]
- De Angelis E, Petronini PG, Borghetti P, Borghetti AF, Wheeler KP. Induction of betaine-γ-aminobutyric acid transport activity in porcine chondrocytes exposed to hypertonicity. Journal of Physiology. 1999;518:187–194. doi: 10.1111/j.1469-7793.1999.0187r.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denkert C, Warskulat U, Hensel F, Haussinger D. Osmolyte strategy in human monocytes and macrophages: involvement of p38MAPK in hyperosmotic induction of betaine and myo-inositol transporters. Archives of Biochemistry and Biophysics. 1998;354:172–180. doi: 10.1006/abbi.1998.0661. [DOI] [PubMed] [Google Scholar]
- Hizoh I, Strater J, Schick CS, Kubler W, Haller C. Radiocontrast-induced DNA fragmentation of renal tubular cells in vitro: role of hypertonicity. Nephrology, Dialysis and Transplantation. 1998;13:911–918. doi: 10.1093/ndt/13.4.911. [DOI] [PubMed] [Google Scholar]
- Horio M, Ito A, Matsuoka Y, Moriyama T, Orita Y, Takenaka M, Imai E. Apoptosis induced by hypertonicity in Madin-Darley canine kidney cells: protective effect of betaine. Nephrology, Dialysis and Transplantation. 2001;16:483–490. doi: 10.1093/ndt/16.3.483. [DOI] [PubMed] [Google Scholar]
- Kitamura H, Yamauchi A, Nakanishi T, Takamitsu Y, Sugiura T, Akagi A, Moriyama T, Horio M, Imai E. Effects of inhibition of myo-inositol transport on MDCK cells under hypertonic environment. American Journal of Physiology. 1997;272:F267–272. doi: 10.1152/ajprenal.1997.272.2.F267. [DOI] [PubMed] [Google Scholar]
- Law RO, Turner DPJ. Are ninhydrin-positive substances volume-regulatory osmolytes in rat renal papillary cells? Journal of Physiology. 1987;386:45–61. doi: 10.1113/jphysiol.1987.sp016521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malek AM, Goss GG, Jiang L, Izumo S, Alper SL. Mannitol at clinical concentrations activates multiple signaling pathways and induces apoptosis in endothelial cells. Stroke. 1998;29:2631–2640. doi: 10.1161/01.str.29.12.2631. [DOI] [PubMed] [Google Scholar]
- Matsuoka Y, Yamauchi A, Nakanishi T, Sugiura T, Kitamura H, Horio M, Takamitsu Y, Ando A, Imai E, Hori M. Response to hypertonicity in mesothelial cells: role of Na+/myo-inositol co-transporter. Nephrology, Dialysis and Transplantation. 1999;14:1217–1223. doi: 10.1093/ndt/14.5.1217. [DOI] [PubMed] [Google Scholar]
- Matthews CC, Feldman EL. Insulin-like growth factor 1 rescues SH-SY5Y human neuroblastoma cells from hyperosmotic induced programmed cell death. Journal of Cellular Physiology. 1996;166:323–331. doi: 10.1002/(SICI)1097-4652(199602)166:2<323::AID-JCP10>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- Michea L, Ferguson DR, Peters EM, Andrews PM, Kirby MR, Burg MB. Cell cycle delay and apoptosis are induced by high salt and urea in renal medullary cells. American Journal of Physiology. 2000;278F:209–218. doi: 10.1152/ajprenal.2000.278.2.F209. [DOI] [PubMed] [Google Scholar]
- Petronini PG, Alfieri R, De Angelis E, Campanini C, Borghetti AF, Wheeler KP. Different HSP70 expression and cell survival during adaptive responses of 3T3 and transformed 3T3 cells to osmotic stress. British Journal of Cancer. 1993;67:493–499. doi: 10.1038/bjc.1993.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petronini PG, Alfieri RR, Losio MN, Caccamo AE, Cavazzoni A, Bonelli MA, Borghetti AF, Wheeler KP. Induction of BGT1 and amino acid System A transport activities in endothelial cells exposed to hyperosmolarity. American Journal of Physiology. 2000;279R:1580–1589. doi: 10.1152/ajpregu.2000.279.5.R1580. [DOI] [PubMed] [Google Scholar]
- Petronini PG, De Angelis E, Borghetti AF, Wheeler KP. Osmotically inducible uptake of betaine via amino acid transport system A in SV-3T3 cells. Biochemical Journal. 1994;300:45–50. doi: 10.1042/bj3000045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin S, Minami Y, Kurosaki T, Yamamura H. Distinctive functions of Syk and Lyn in mediating osmotic stress- and ultraviolet C irradiation- induced apoptosis in chicken B cells. Journal of Biological Chemistry. 1997;272:17994–17999. doi: 10.1074/jbc.272.29.17994. [DOI] [PubMed] [Google Scholar]
- Rim JS, Atta MG, Dahl SC, Berry GT, Handler JS, Kwon HM. Transcription of the sodium/myo-inositol cotransporter gene is regulated by multiple tonicity-responsive enhancers spread over 50 kilobase pairs in the 5′-flanking region. Journal of Biological Chemistry. 1998;273:20615–20621. doi: 10.1074/jbc.273.32.20615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santoro MM, Liu Y, Khan SM, Hou LX, Bolen DW. Increased thermal stability of proteins in the presence of naturally occurring osmolytes. Biochemistry. 1992;31:5278–5283. doi: 10.1021/bi00138a006. [DOI] [PubMed] [Google Scholar]
- Santos BC, Chevaile A, Hebert MJ, Zagajeski J, Gullans SR. A combination of NaCl and urea enhances survival of IMCD cells to hyperosmolality. American Journal of Physiology. 1998;274F:1167–1173. doi: 10.1152/ajprenal.1998.274.6.F1167. [DOI] [PubMed] [Google Scholar]
- Sugawara M, Nakanishi T, Fei Y -J, Huang W, Ganapathy ME, Leibach FH, Ganapathy V. Cloning of an amino acid transporter with functional characteristics and tissue expression pattern identical to that of System A. Journal of Biological Chemistry. 2000;275:16473–16477. doi: 10.1074/jbc.C000205200. [DOI] [PubMed] [Google Scholar]
- Weik C, Warskulat U, Bode J, Peters-Regehr T, Haussinger D. Compatible osmolytes in rat liver sinusoidal endothelial cells. Hepatology. 1998;27:569–575. doi: 10.1002/hep.510270235. [DOI] [PubMed] [Google Scholar]
- Yamauchi A, Uchida S, Kwon HM, Preston AS, Robey BR, Garcia-Perez A, Burg MB, Handler JS. Cloning of a Na+-dependent and Cl−-dependent betaine transporter that is regulated by hypertonicity. Journal of Biological Chemistry. 1992;267:649–652. [PubMed] [Google Scholar]
- Yancey PH, Clark ME, Hand SC, Bowlus RD, Somero GN. Living with water stress: evolution of osmolyte systems. Science. 1982;217:1214–1222. doi: 10.1126/science.7112124. [DOI] [PubMed] [Google Scholar]


