Abstract
Rat hippocampal interneurons express diverse subtypes of functional nicotinic acetylcholine receptors (nAChRs), including α7-containing receptors that have properties unlike those expected for homomeric α7 nAChRs. We previously reported a strong correlation between expression of the α7 and of the β2 subunits in individual neurons. To explore whether co-assembly of the α7 and β2 subunits might occur, these subunits were co-expressed in Xenopus oocytes and the functional properties of heterologously expressed nAChRs were characterized by two-electrode voltage clamp. Co-expression of the β2 subunit, both wild-type and mutant forms, with the α7 subunit significantly slowed the rate of nAChR desensitization and altered the pharmacological properties. Whereas ACh, carbachol and choline were full or near-full agonists for homomeric α7 receptor channels, both carbachol and choline were only partial agonists in oocytes expressing both α7 and β2 subunits. In addition the EC50 values for all three agonists significantly increased when the β2 subunit was co-expressed with the α7 subunit. Co-expression with the β2 subunit did not result in any significant change in the current-voltage curve. Biochemical evidence for the co-assembly of the α7 and β2 subunits was obtained by co-immunoprecipitation of these subunits from transiently transfected human embryonic kidney (TSA201) cells. These data provide direct biophysical and molecular evidence that the nAChR α7 and β2 subunits co-assemble to form a functional heteromeric nAChR with functional and pharmacological properties different from those of homomeric α7 channels. This co-assembly may help to explain nAChR channel diversity in rat hippocampal interneurons, and perhaps in other areas of the nervous system.
Neuronal nicotinic acetylcholine receptors (nAChRs) are ligand-gated ion channels that are widely expressed in the central and peripheral nervous system where they are involved in a variety of physiological processes, including cognition and development (Jones et al. 1999). Furthermore, dysfunctions in nAChRs may be a factor in a variety of neurodegenerative diseases including Alzheimer's disease (Paterson & Nordberg, 2000; Court et al. 2001; Dani, 2001), and in ageing (Jones et al. 1999). For example in Alzheimer's disease, the extensive accumulation of the β-amyloid peptide (Aβ1–42) has been proposed to lead to the progressive loss of cognitive function, and Aβ1–42 has recently been shown to inhibit nAChR function in rat hippocampal neurons (Liu et al. 2001; Pettit et al. 2001). In the brain, nAChRs are known to function both at presynaptic sites (i.e. to regulate the release of neurotransmitter) and postsynaptic sites (i.e. to mediate fast excitatory synaptic transmission) (Wonnacott, 1997; Jones et al. 1999). Currently there are at least 11 different nAChR subunits known to be widely expressed in the rat nervous system; nine of these (α2-α7 and β2-β4) are expressed in the adult rat CNS (McGehee & Role, 1995; Boyd, 1997; Elgoyhen et al. 2001). To enhance our understanding of the biophysical and pharmacological properties of functional nAChRs, we must understand how these subunits assemble to form functional channels.
Rat hippocampal interneurons express diverse subtypes of functional nAChRs (Alkondon et al. 1997; Jones & Yakel, 1997; Frazier et al. 1998; McQuiston & Madison, 1999; Ji & Dani, 2000). While the majority of these nAChRs contain the α7 subunit, the properties of these α7-containing receptors are not identical to the observed properties of recombinant homomeric α7 receptors; in particular these native α7-containing receptors desensitize more slowly and have a smaller single channel conductance (Shao & Yakel, 2000; Sudweeks & Yakel, 2000). This suggests the possibility that α7-containing nAChRs expressed in rat hippocampal interneurons are not homomeric assemblies of α7 subunits. It had been previously thought that mammalian α7 subunits mostly form homomeric receptors as no direct evidence for co-assembly had been reported (McGehee & Role, 1995; Chen & Patrick, 1997; Drisdel & Green, 2000). In addition the properties of native chick α7 nAChRs often do not match those of heterologously expressed homomeric α7 nAChRs (Anand et al. 1993; Yu & Role, 1998a; Girod et al. 1999), and α7-containing heteromeric chick nAChRs can be formed in heterologous expression systems (Girod et al. 1999; Palma et al. 1999). Multiple functional subtypes of α7-containing nAChRs have been reported in rat intracardiac ganglion and superior cervical ganglion neurons (Cuevas & Berg, 1998; Cuevas et al. 2000) and chick sympathetic neurons (Yu & Role, 1998a), suggesting further the possibility for heteromeric α7-containing nAChRs. Combining patch-clamp electrophysiological and single-cell RT-PCR analysis, we previously reported (Sudweeks & Yakel, 2000) a strong correlation between expression of the α7 and β2 subunits in individual rat hippocampal interneurons.
In this study, we tested whether the rat nAChR α7 and β2 subunits can co-assemble to form a functional heteromeric nAChR channel in Xenopus oocytes. Previously no evidence had been reported demonstrating the heteromeric assembly of mammalian α7-containing nAChRs. The co-expression of the β2 subunit with the α7 subunit significantly slowed the rate of desensitization as compared to homomeric α7 channels, and altered the pharmacological properties. The co-expression of the α7 subunit with a mutant form of the β2 subunit, containing a cysteine residue in place of a leucine residue in the presumed pore region, also dramatically slowed the rate of desensitization. Biochemical evidence for the co-assembly of the α7 and β2 subunits was obtained by co-immunoprecipitation of these subunits from transiently transfected human embryonic kidney (TSA201) cells. These data provide direct biophysical and molecular evidence that the rat nAChR α7 and β2 subunits co-assemble to form a functional heteromeric nAChR.
METHODS
RNA preparation
mRNA was transcribed in vitro from plasmids using mMessage Machine kit (Ambion Inc., Austin, TX, USA) according to conditions suggested by the manufacturer. The rat nAChR α7 and β2 plasmids were kindly provided by J. Patrick, and the β2 subunit was subcloned into pcDNA 3.1 (Invitrogen, Carlsbad, CA. USA) prior to mutagenesis. The leucine to cysteine point mutation at amino acid position 277 of the β2 subunit (numbering from protein accession no. JH0174) was made with the Stratagene (La Jolla, CA, USA) QuickChange mutation kit. The following oligonucleotide primers (synthesized by Life Technologies, Rockville, MD, USA) were used: TATTTCTGTGCTGTGCGCACTCA-CGGTGT (sense strand), and ACACCGTGAGTGCGCACAGCACAGAAATA (antisense strand). All conditions for the mutagenesis were as suggested by the manufacturer. The entire sequence of the open reading frame from the resulting plasmid was sequenced in order to confirm the presence of the desired mutation, and that no other mutations were present.
Expression in Xenopus oocytes
All experiments were carried out in accordance with guidelines approved by the National Instsitute of Environmental Health Sciences (NIEHS) Animal Care and Use Committee. Female Xenopus laevis frogs were anaesthetized by immersion in cold water containing 0.2 % ethylmetaaminobenzoate (MS-222; Sigma) for 60 min, and decapitated. Oocytes were dissected and defolliculated by treatment with collagenase B (Boehringer Mannheim, 3–4 mg ml−1) for 2–4 h (Kriegler et al. 1999). The total amount of RNA injected for each nAChR subunit was 25 ng for the α7 subunit and either 25 or 75 ng for the β2 subunit. Experiments were performed 1–12 days after injection.
Electrophysiological recording
Current responses were obtained by two-electrode voltage-clamp recording at a holding potential of −60 mV using a GeneClamp 500 amplifier and pCLAMP 8 software (Axon Instruments, Union City, CA, USA). The bath was grounded via a silver chloride pellet; the maximum expected voltage error was estimated to be 1–2 mV. Typically traces were filtered at 0.2–1 kHz and sampled at 0.5–5 kHz. Electrodes contained 3 m KCl with 0.4 m BAPTA and had resistances of < 1 MΩ. The slow leak of BAPTA into oocytes from the electrodes for several minutes (e.g. > 5 min) was enough to significantly reduce the IP3 receptor-mediated Ca2+ signalling in Xenopus oocytes (authors' unpublished observations). Traces obtained for the quantification of the desensitization rate were usually taken 5–15 min after impaling oocytes, a time that should have allowed BAPTA to enter the oocytes to chelate the expected Ca2+ influx through the α7-containing nAChRs. These precautions were taken to minimize any activation of the endogenous Ca2+-dependent chloride conductance in Xenopus oocytes that might significantly alter the amplitude or kinetics of the nAChR-mediated responses; that this was the case was suggested by the fact that the kinetics of desensitization were stable for up to 1 h and not significantly affected by the chloride channel blocker niflumic acid (300 μm), and the reversal potential values of responses (see Results section). Carbachol, ACh, and choline (Sigma) were freshly prepared in bath solution from a frozen stock and applied either by suction of respective solutions from dishes using the OTC-20 rapid solution exchange system (ALA Scientific Instruments, Westbury, NY, USA), or via a synthetic quartz perfusion tube (0.7 mm i.d.) operated by a computer-controlled valve. With the OTC-20 system, solution exchange can be achieved in ∼10 ms (Madeja et al. 1991). Only oocytes injected with α7 nAChR subunit RNA expressed functional channels; the injection of either wild-type or mutant β2 subunit RNA alone did not result in the expression of functional channels when tested using carbachol (1 mm).
Solutions
Oocytes were defolliculated in a solution containing (mm): NaCl (85.2), KCl (2), MgCl2 (1) and Hepes (5). Recordings were performed in a solution containing m: NaCl (96), KCl (2), CaCl2 (1.8), MgCl2 (1) and Hepes (10) (pH 7.4). Oocytes were maintained in culture in the same solution, with the addition of 2.5 mm sodium pyruvate, 0.5 mm theophylline and 50 μg ml−1 gentamicin.
cDNAs, antibodies and cell lines
Mammalian plasmid expression vectors containing rat nAChR β2 subunit cDNA (pRK5-β2) and an epitope-tagged rat α7 subunit cDNA (pcDNA1neo-α7FLAG) have been described previously (Cooper & Millar, 1997; Cooper et al. 1999). Monoclonal antibody mAb270, which recognizes an extracellular epitope on the nAChR β2 subunit (Whiting & Lindstrom, 1987), was purified from the hybridoma cell line HB189 (obtained from the American Type Culture Collection, Rockville, MD, US). Monoclonal antibody mAbFLAG-M2 which recognizes an eight amino acid epitope tag (Hopp et al. 1988) introduced into the intracellular loop region of the α7 subunit (α7FLAG) (Cooper & Millar, 1997) was obtained from Scientific Imaging Systems. Mammalian cell line TSA201, a derivative of the human embryonic kidney HEK293 cell line which expresses the simian virus 40 large T-antigen, was obtained from W. Green.
Transfection, metabolic labelling and immunoprecipitation
Human TSA201 cells were cultured in Dulbecco's modified Eagle's medium (Life Technologies) containing 2 mm L-Glutamax™ (Life Technologies) plus 10 % heat-inactivated fetal calf serum (FCS) (Sigma), with penicillin (100 U ml−1) and streptomycin (100 μg ml−1) and were maintained in a humidified incubator containing 5 % CO2 at 37 °C. Cells were transfected using Effectene transfection reagent (Qiagen Inc., Valencia, CA, USA) according to the manufacturer's instructions with a total of 0.6 μg plasmid DNA. In all cases where different subunit cDNA combinations were compared, the amount of each plasmid construct and the total amount of plasmid DNA used during transfection experiments was kept constant. This was achieved by including appropriate amounts of ‘empty’ expression plasmid (pRK5 without a cDNA insert). Cells were transiently transfected overnight in 10 cm tissue culture dishes pre-coated with poly-l-lysine (Sigma). To starve cells of methionine, cells were washed twice with, and bathed for 10 min in l-methionine- (Met) and l-cysteine- (Cys) free DMEM (Life Technologies) containing 10 mm Hepes and 3.7 g l−1 NaHCO3. Cells were labelled with 125 μCi ‘Redivue Pro-mix’ (Amersham Pharmacia Biotech, Little Chalfont, UK; a mixture of [35S]methionine and [35S]cysteine) in 3.5 ml of Met/Cys-free DMEM for 4 h, followed by a 2 h incubation with an additional 5 ml of complete growth medium containing normal concentrations of Cys and Met. Metabolically labelled cells were rinsed with phosphate-buffered saline, harvested and solubilized in ice-cold lysis buffer (LB) containing protease inhibitors (150 mm NaCl, 50 m Tris-Cl pH 8.0, 5 mm EDTA, 1 % Triton X-100, 0.25 mm phenyl-methylsulfonyl fluoride, and 10 μg ml−1 each of leupeptin, aprotinin and pepstatin). Samples were immunoprecipitated with mAb270 or mAbFLAG-M2, followed by Protein G-sepharose (Amersham Pharmacia Biotech) and analysed by SDS-polyacrylamide gel electrophoresis, as has been described (Cooper & Millar, 1997).
Data analysis
Peak currents, decay kinetics and curve fitting were measured and analysed using Clampfit (Axon Instruments,) and Origin (OriginLab Corp., Northampton, MA, USA) software. Tests of significance were determined using either Student's t test (P values less than 0.05 were considered significant), or the Fisher's least significant difference (LSD) test to make pairwise comparisons among multiple treatment groups. The variance-stabilizing logarithmic transformation was used in some of these statistical analyses. Fits were made to the Hill equation: I = Imax(xn/(Kn+xn)), where Imax is the maximum response amplitude to agonist, x is the agonist concentration, n is the Hill coefficient, and K is the EC50 value, using either Origin or the CVFIT program provided by Dr David Colquhoun (http://www.ucl.ac.uk/Pharmacology/dcpr95.html#notes). Fits to a two-component Hill equation were made using either the CVFIT program or by statisticians at NIEHS (Drs Joe Haseman and Shyamal Peddada). The performance of the two-component fit was compared with the single-component fit using the standard F statistic which compares the excess mean error sum of squares. Fits to full concentration-response curves for individual oocytes were made independently and then averaged in order to compare significant differences between groups. All agonist data was normalized to the amplitude produced from the same oocyte by a 1 mm dose of ACh; this amplitude was assigned a value of 1.0.
RESULTS
The rapid application of ACh, carbachol, or choline to oocytes expressing the rat α7 nAChR subunit induced inward current responses at a holding potential of −60 mV that activated rapidly, and then desensitized (i.e. inactivated) in the continued presence of agonist. An example of a carbachol- (1 mm) activated response is shown in Fig. 1A. The onset of desensitization was a biphasic process; the fast time constant of decay (τfast) averaged 0.106 ± 0.012 s (12 cells) and comprised 85 ± 2 % of the fit, and the slow time constant of decay (τslow) averaged 1.11 ± 0.15 s and comprised 13 ± 2 % of the fit (Fig. 1C and D). Co-expression of the β2 with α7 subunits also resulted in rapidly activating responses (Fig. 1B), but the rate of desensitization was significantly slower (Fig. 1C) than for the α7 subunit alone. When equal amounts of the α7 and β2 subunits (25 ng each) were injected (these will be referred to as α7β2 channels), the fast and slow decay phases averaged (13 cells), respectively, 0.210 ± 0.033 s (68 ± 5 % of the fit) and 1.27 ± 0.22 s (29 ± 5 % of the fit; Fig. 1B and D). The rate of the fast decay phase and the relative fractions of both phases were significantly different from those for α7 alone (Fig. 1C and D). Injecting a three times greater amount (75 ng) of β2 subunit RNA (i.e. α7β2(×3) channels) with the α7 subunit RNA (25 ng) did not significantly alter the kinetics of desensitization versus the oocytes injected with equal amounts (i.e. 25 ng) of the α7 and β2 subunit RNA; the fast and slow decay phases averaged (10 cells; Fig. 1C and D), respectively, 0.231 ± 0.026 s (54 ± 7 % of the fit) and 1.15 ± 0.24 s (44 ± 6 % of the fit).
Figure 1. Co-expression of the β2 along with the α7 subunit alters the kinetics of desensitization.
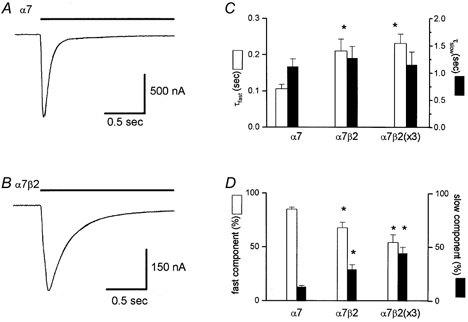
In oocytes expressing the α7 subunit alone (A) or with the β2 subunit (B), the rapid application of carbachol (1 mm; indicated by the horizontal bar) induced a rapid inward current response at a holding potential of −60 mV that rapidly desensitized. In A, the fast and slow time constants of decay were 0.088 s (89 % of the fit) and 1.37 s (11 % of the fit), respectively. In B, these values were 0.230 s (84 % of the fit) and 1.25 s (13 % of the fit). These curve fits overlay the data trace in both A and B. The relative time constant values and proportions of fit for all three groups (i.e. α7, α7β2, α7β2(×3) are shown in C and D, respectively. In C and D, the asterisks denote a significant difference (P < 0.05) compared to homomeric α7 channels.
Dose-response curves for ACh, carbachol and choline for α7, α7β2 and α7β2(×3) channels are shown in Fig. 2, and average EC50 and Hill coefficient values are shown in Table 1. The average amplitude for ACh-activated responses (1 mm; 3–12 days after injection) for α7, α7β2 and α7β2(×3) channels, respectively, was 3099 ± 105 nA (14 cells), 2602 ± 161 nA (11 cells), and 1965 ± 211 nA (5 cells). The doseresponse curve for ACh (Fig. 2A) showed no significant difference when the β2 subunit was co-expressed with the α7 subunit in equal amounts. However for the α7β2(×3) channels, there was a slight but significant increase in the EC50 value without any significant change in the Hill coefficient (Table 1). Both carbachol (Fig. 2B) and choline (Fig. 2C) were nearly full agonists for α7 channels, and both had higher EC50 values than for ACh. However for both α7β2 and α7β2(×3) channels, carbachol and choline were only partial agonists. Carbachol (10 mm) activated responses that were only 57 % of the amplitude of ACh (10 mm) for alpha;7β2 channels, and 45 % for α7β2(×3). Choline (40 mm) activated responses that were only 58 % (α7β2 channels) and 41 % (α7β2(×3)) of the amplitude of ACh (10 mm). The EC50 values for carbachol and choline for both α7β2 and α7β2(×3) channels were significantly larger than that of α7 channels; the values for α7β2 and α7β2(×3) channels were not significantly different from each other (Table 1).
Figure 2. Concentration-response curves shows altered pharmacological properties when co-expressing α7 and β2 subunits.
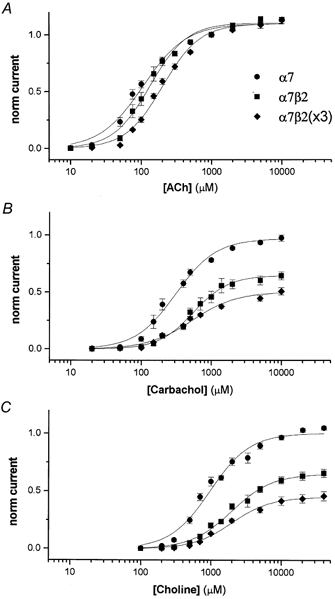
The concentration-response curves for activation by ACh, carbachol and choline are shown in A, B and C, respectively. Each point represents the average (3–6 cells) from data normalized (see Methods) to the amplitude of 1 mm ACh. The traces are one-component Hill fits (see Methods) to the averaged data.
Table 1.
Effect of co-expression of β2 with α7 subunit on concentration–Vresponse curves
| N | EC50 (μm) | n | Maximum amplitude (norm.) | ||
|---|---|---|---|---|---|
| ACh | α7 | 4 | 105 ± 10 | 1.52 ± 0.06 | 1.13 ± 0.01 |
| α7β2 | 3 | 131 ± 18 | 1.67 ± 0.03 | 1.13 ± 0.01 | |
| α7β2(×3) | 4 | 213 ± 19* | 1.66 ± 0.12 | 1.13 ± 0.06 | |
| Carbachol | α7 | 6 | 285 ± 42 | 1.88 ± 0.12 | 0.97 ± 0.05 |
| α7β2 | 5 | 572 ± 96* | 1.62 ± 0.12 | 0.64 ± 0.06 | |
| α7β2(×3) | 4 | 773 ± 40* | 1.29 ± 0.07* | 0.51 ± 0.06 | |
| Choline | α7 | 5 | 907 ± 78 | 1.82 ± 0.15 | 1.05 ± 0.02 |
| α7β2 | 4 | 1797 ± 150* | 1.58 ± 0.04 | 0.66 ± 0.05 | |
| α7β2(×3) | 5 | 2028 ± 99* | 1.54 ± 0.11 | 0.46 ± 0.09 |
Values were obtained from individual fits to concentration–Vresponse curves. N, number of oocytes in each group. Maximum dose of agonist was 10 mm for ACh and carbachol, and 40 mm for choline. The maximum amplitude values were normalized to a value of 1.0 for the response obtained from 1 mm ACh. The asterisks denote a significant difference (P < 0.05) compared to homomeric α7 channels.
Co-expression of the β2 with the α7 subunit did not significantly alter the shape of the current-voltage (I–V) curve (data not shown). The reversal potential values for responses for the α7, α7β2, and α7β2(×3) channels were, respectively, −5 ± 1 mV (3 cells), −5 ± 2 mV (3 cells), and −7 ± 1 mV (4 cells). These values suggest that there was no significant change in ionic permeabilities when co-expressing the β2 along with the α7 subunit, and furthermore that there was probably no significant contribution of the endogenous Ca2+-dependent chloride conductance under our experimental recording conditions (see Methods).
Co-expressing the α7 and β2 subunits could lead to the expression of a heterogeneous population of channels, containing homomeric α7 channels and β2-containing α7 channels. Perhaps variable numbers of the β2 subunit might also exist per channel. If so, attempting to fit the concentration-response data to a single-component Hill fit might be inadequate. When the data was fitted to a two-component Hill fit (see Methods), the fits were significantly better in most cases, even for the homomeric α7 channels. This was not expected since there should be a homogeneous population of channels in these oocytes. Therefore, the significant improvement with a two-component Hill fit was not necessarily indicative of a heterogeneous population of nAChRs and thus this type of analysis was not conclusive.
The leucine at amino acid position 277 of the β2 subunit was mutated to cysteine (β2L277C) in order to use the substituted cysteine accessibility method (SCAM; Akabas et al. 1994; Kriegler et al. 1999) to test for the co-assembly of the nAChR α7 and β2 subunits. This position corresponds to the leucine at position 251 of the nicotinic α1 subunit (Akabas et al. 1994) that is thought to line the pore of the channel. However for technical reasons, this method was apparently unsuitable for rat α7-containing nAChRs. However in expressed α7 nAChRs, mutating this position alters the kinetics of desensitization (Revah et al. 1991). We co-expressed equal amounts (i.e. 25 ng each) of this mutant nicotinic β2 subunit along with the α7 subunit and tested whether carbachol-activated responses were altered. The mutant β2L277C subunit also slowed the rate of nAChR desensitization as compared to the α7 subunit alone (Fig. 3). The fast and slow decay phases averaged (15 cells), respectively, 0.088 ± 0.015 s (41 ± 3 % of the fit) and 0.90 ± 0.1 s (52 ± 3 % of the fit). Although the time constant values were not significantly different from homomeric α7 channels, the relative fraction of the fast decay phase significantly decreased by 52 % (i.e. from 85 % to 41 %), and the fraction of the slow decay phase significantly increased by 300 % (i.e. from 13 % to 52 %). These changes in the proportions of the fast and slow decay phases resulted in an overall decrease in the rate of desensitization for α7β2L277Cversus homomeric α7 channels.
Figure 3. Co-expression of mutant β2 subunit with the α7 subunit alters the kinetics of desensitization.
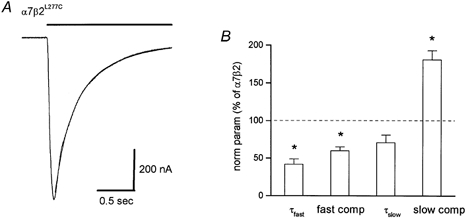
In oocytes expressing the mutant β2L277C subunit along with the α7 subunit, the rate of desensitization is slowed, compared to homomeric α7 channels. The fast and slow time constants of decay for α7β2L277C trace (A) were 0.136 s (40 % of the fit) and 0.542 s (57 % of the fit). The curve fit overlays the data trace. The relative change in the fast decay phase, the relative fraction of the fast decay phase, the slow decay phase, and the fraction of the slow decay phase versus expression with the wild-type β2 subunit are shown in B. The asterisks denote a significant difference (P < 0.05) compared to α7β2 channels.
The kinetics of desensitization for the α7β2L277C channels were significantly different from those for the α7β2 channels. The time constant value and relative fraction of the fast decay phase significantly decreased (by 58 % and 40 %, respectively), and the relative fraction of the slow decay phase significantly increased by 79 % (i.e. from 29 % to 52 %; Fig. 3B). Comparing the kinetics of decay for the α7β2L277C and α7β2 channels (utilizing the average values from above), the initial rate of decay (up to ∼250 ms) for the α7β2L277C channels was faster, but the sustained component of decay (> 250 msec) for these channels was slower than for the wild-type α7β2 channels. These data are also consistent with the notion that the nAChR α7 and β2 subunits co-assemble to form a functional heteromeric nAChR in Xenopus oocytes.
Further evidence for the co-assembly of the nAChR α7 and β2 subunits was obtained by co-immunoprecipitation of these subunits with subunit-specific antibodies. Due to difficulties in obtaining sufficient material from heterologous expression in oocytes, these experiments were performed by transfection of subunit cDNAs into human embryonic kidney (TSA201) cells (Fig. 4). Cells were transiently transfected with plasmid expression constructs encoding either β2 (pRK5-β2) (Cooper et al. 1999) or an epitope tagged α7 subunit (pcDNA1neo-α7FLAG) (Cooper & Millar, 1997). Transfected cells were metabolically labelled (with a mixture of [35S]methionine and [35S]cysteine) and the subunit co-assembly examined by immunoprecipitation of subunit proteins from detergent-solubilized cell extracts. Cell lysates were immunoprecipitated with either mAb270, which recognizes an epitope on the extracellular domain of β2 (Whiting & Lindstrom, 1987), or mAbFLAG-M2, which recognizes an eight amino acid epitope tag (Hopp et al. 1988) introduced into the intracellular loop region of the α7 subunit (α7FLAG) (Cooper & Millar, 1997). Anti-β2 antibody mAb270 immunoprecipitated the β2 subunit but showed no cross-reactivity with α7FLAG. Co-precipitation of α7FLAG by mAb270 was seen when the two subunits were co-expressed. Similarly, anti-FLAG antibody mAbFLAG-M2 immunoprecipitated α7FLAG but showed no cross-reactivity with β2. Co-precipitation of β2 by mAbFLAG-M2 was seen when the two subunits were co-expressed. This provides direct evidence for co-assembly of the nAChR α7 and β2 subunits when expressed heterologously in a mammalian cell line. It should be noted that preliminary experiments have not detected functional nAChRs in TSA201 cells transfected with α7 and/or β2 subunits. This is not unexpected considering the considerable difficulties that have previously been encountered in detecting functional α7 nAChRs in transfected mammalian cell lines (Cooper & Millar, 1997; Kassner & Berg, 1997; Rangwala et al. 1997; Chen et al. 1998).
Figure 4. Co-immunoprecipitation of α7 and β2 subunits.
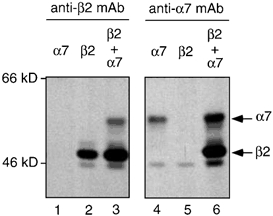
Human TSA201 cells were transiently transfected with plasmid expression constructs encoding either β2 (pRK5-β2) (Cooper et al. 1999) or an epitope-tagged α7 subunit (pcDNA1neo- α7FLAG) (Cooper & Millar, 1997). Co-assembly of β2 and α7FLAG was examined by immunoprecipitation of metabolically labelled subunit proteins from detergent-solubilized cells. Cell lysates were immunoprecipitated with either mAb270, which recognizes an epitope on the extracellular domain of β2 (Whiting & Lindstrom, 1987), or mAbFLAG-M2, which recognizes an eight-amino-acid epitope tag (Hopp et al. 1988) introduced into the intracellular loop region of the α7 subunit (α7FLAG) (Cooper & Millar, 1997). Anti-β2 antibody mAb270 immunoprecipitated the β2 subunit (lane 2) but showed no cross-reactivity with α7FLAG (lane 1). Co-precipitation of α7FLAG by mAb270 was seen when the two subunits were co-expressed (lane 3). Similarly, anti-FLAG antibody mAbFLAG-M2 immunoprecipitated α7FLAG (lane 4) but showed no cross-reactivity with β2 (lane 5). Co-precipitation of β2 by mAbFLAG-M2 was seen when the two subunits were co-expressed (lane-6). The positions of molecular weight markers are shown. Minor immunoprecipitated bands are visible above and below the main β2 band. After immunoprecipitation with the anti-β2 mAb (lanes 1–3), these bands are only visible in cells transfected with β2 (lanes 2 and 3). This would suggest that they correspond to differently migrating forms of the β2 subunit. In addition, after immunoprecipitation with the anti-α7 mAb (lanes 4–6), a band is visible which co-migrates with the lower molecular weight band seen in lanes 2 and 3. Since this band is visible in cells transfected with both α7 alone (lane 4) and β2 alone (lane 5), it would appear that it corresponds to a non-specific protein (which is recognized by the anti-α7, but not by the anti-β2 mAb). These two co-migrating bands would both be expected to be present in lane 6, which may explain the greater intensity of this low molecular weight band in this lane. In all cases cells were transfected under identical conditions and similar amounts of immunoprecipitated material was loaded. All lanes are from a single experiment and x-ray films that were exposed for the same length of time. Co-precipitation of α7 and β2 has been confirmed in two independent experiments. It should be noted that these results correspond to total (surface and intracellular) proteins.
DISCUSSION
We have shown that the rat nAChR α7 and β2 subunits can co-assemble to form a functional heteromeric nAChR. The co-expression of β2 with the α7 subunit in Xenopus oocytes resulted in nAChR channels with a slower rate of desensitization, and altered sensitivity to agonists. Furthermore, the α7 and β2 subunits were co-immunoprecipitated from transiently transfected human embryonic kidney (TSA201) cells. These data suggest the possibility that the α7 and β2 subunits may co-assemble in vivo, which might help to explain nAChR channel diversity in rat hippocampal interneurons, and perhaps in other areas of the nervous system as well.
Although at least nine different genes encode nAChR subunits expressed in the rat brain (α2–α7 and β2–β4), with the potential of forming thousands of different combinations of functional channels, a few subunits are known to predominate in specific brain regions (Jones et al. 1999). For example in the hippocampus, functional data have led to the notion that the major types of channels are composed of α7 and α4β2 subunits (Alkondon & Albuquerque, 1993; Alkondon et al. 1997; Jones & Yakel, 1997; Frazier et al. 1998,McQuiston & Madison, 1999; Ji & Dani, 2000). However when comparing the properties of heterologously expressed channels formed from these subunits with those of native nAChRs from hippocampal recordings, it is clear that the composition of the native receptors is more complex (i.e. nAChRs are not simply composed of α4β2 and α7 subunits) (McQuiston & Madison, 1999; Sudweeks & Yakel, 2000). One major task, therefore, is to understand the molecular makeup of nAChRs in various brain regions. Previously we provided evidence for a link between the expression of the α7 and β2 nAChR subunits (Shao & Yakel, 2000; Sudweeks & Yakel, 2000), and hypothesized that co-assembly of these subunits might help to explain nAChR diversity in these hippocampal interneurons. Here we have shown, for the first time, that co-assembly between these two subunits from rat can occur.
Preliminary evidence for the co-assembly of the α7 and β2 subunits in a heterologous expression system utilizing chick nAChR subunits has been reported (Girod et al. 1999; Crabtree et al. 2000). When co-expressing the α7 and β2 subunits in Xenopus oocytes and measuring membrane current, the EC50 value for ACh decreased 100-fold compared to homomeric α7 channels (Crabtree et al. 2000); this is clearly different from the rat α7 and β2 subunits investigated here. The chick β3 subunit was also found to co-assemble with the α7 subunit in Xenopus oocytes (Palma et al. 1999). Co-expression of the β3 subunit with the wild-type α7 subunit resulted in the cell-surface expression of heteromeric nAChRs, but these channels could not be activated by ACh. However co-expressing the β3 subunit with a mutant α7 subunit (α7L247T) resulted in functional heteromeric channels with a lower affinity for ACh, a faster rate of desensitization, a more non-linear I–V relation with a shift in Erev, and a smaller single-channel conductance than for homomeric α7L247T channels (Palma et al. 1999). Co-expressing a much higher ratio of β3 subunit RNA also dramatically reduced the cell-surface expression of functional receptors and the amplitude of the ACh-induced currents. It was proposed that this effect might be due to the sequestering of α7β3 dimers by the β3 subunit, thus hindering functional pentamer formation (Palma et al. 1999). In the current study, the amplitude of responses to ACh was not dramatically different for the rat α7, α7β2, and α7β2(×3) channels. If the maximum open probability and single channel conductance values were not different for these three groups of channels, this would then suggest that for rat nAChR subunits, the β2 subunit does not appear to dramatically alter the formation of functional α7-containing channels in Xenopus oocytes. However we cannot rule out the possibility that co-expression with β2 subunits alters the efficacy of the channel response to ACh or the single channel conductance, and that there are in fact significant changes in the number of functional channels. Nevertheless in the chick, it appears likely that while β subunits (i.e. β2 and β3) can co-assemble with the α7 subunit, the change in functional properties is different from that of co-assembled rat α7 and β2 subunits.
Native chick α7-containing nAChRs also exhibit diversity consistent with the notion of heteromeric α7 nAChRs. The chick α7 subunit has previously been suggested to co-assemble and form functional channels with the α5 subunit (Yu & Role, 1998b) and α8 subunit (Keyser et al. 1993) in native tissues, and perhaps with other subunits as well (Pugh et al. 1995; Yu & Role, 1998a; Girod et al. 1999). The α7 subunit is thought to contribute to the function of at least three subtypes of nAChR in embryonic chick sympathetic neurons, all distinct from heterologously expressed homomeric α7 nAChRs (Yu & Role, 1998a). We have previously shown that the α7-containing nAChRs in rat hippocampal interneurons desensitize more slowly and have a unitary single channel conductance different from that expected for homomeric α7 nAChRs (Shao & Yakel, 2000; Sudweeks & Yakel, 2000). Our observation that co-expression of β2 with the α7 subunit significantly slowed the rate of desensitization is consistent with the notion that co-assembly of the α7 and β2 subunits might occur in rat hippocampal interneurons.
When co-expressing the α7 and β2 subunits, it is possible that a heterogeneous population of channels (e.g. α7 and α7β2 channels) is expressed in individual oocytes. However, strong evidence to suggest that this occurred was not apparent. First, the kinetics of desensitization were biphasic both in oocytes expressing only the α7 subunit (i.e. presumed homomeric α7 channels), and in oocytes expressing both α7 and β2 subunits. A heterogeneous population of channels might be expected to result in a more complex decay phase, but this appeared not to be the case (Fig. 1). However, it should be noted that extra exponential components might not be distinguishable; this depends in part on the area of each component and on the separation of the time constant values. Second, fitting the concentration-response curves to single- and double-component Hill fits, which might be used to test for a heterogeneous population of channels, proved inconclusive. Studies other than concentration-response curves (e.g. single channel studies) might help to solve this point more rigorously.
Interestingly, expressing a higher proportion of the β2 subunit produced further shifts in pharmacological properties (Fig. 2; Table 1), without any significant change in the kinetics of desensitization. These data could suggest that certain subunit combinations are favoured. From the concentration-response curves and kinetic analysis, it seems likely that in the different batches of oocytes (i.e. α7, α7β2 and α7β2(×3)), one type of channel predominated. The pharmacological differences between α7 and α7β2 oocytes could indicate that most of the channels in the latter case contain a β2 subunit (perhaps one), and the pharmacological differences between α7β2 and α7β2(×3) oocytes could indicate that the number of β2 subunits per channel in the latter case might be higher than for α7β2 channels. For the nicotinic α4 and β2 subunits, four pharmacologically distinct subtypes were expressed in Xenopus oocytes by altering the ratio of the cDNAs encoding these two subunits (Zwart & Vijverberg, 1998). Perhaps a similar situation is occurring with the α7 and β2 subunits. Unlike chick α7β3 channels expressed in Xenopus oocytes (Palma et al. 1999), the number of β2 subunits in a rat nAChR complex appears not to affect the cooperativity of receptor activation.
In summary, we have shown that the rat nAChR α7 and β2 subunits can co-assemble to form a functional heteromeric nAChR, with altered biophysical and pharmacological properties. Thus it is becoming clear that in heterologous expression systems as well as in native tissues, the α7 nAChR subunit can and does co-assemble with other nAChR subunits. This might help to explain nAChR channel diversity in rat hippocampal interneurons, and perhaps in other areas of the nervous system as well. For example the kinetics of desensitization of native α7-containing receptors in rat hippocampal interneurons is much slower than that expected for recombinant homomeric α7 receptors (Sudweeks & Yakel, 2000). The fact that co-expression of the β2 with the α7 subunit in Xenopus oocytes slows the rate of desensitization, combined with the fact that these two subunits are significantly co-expressed in the same neurons (Sudweeks & Yakel, 2000), is consistent with the idea that the α7 andβ2 subunits may co-assemble in the rat hippocampal interneurons in vivo. Taken together these data lead to the conclusion that nAChR diversity, at least in the brain, is much more complex than previously imagined.
Acknowledgments
We would like to thank D. Armstrong and C. Erxleban for advice in preparing the manuscript, J. Patrick for providing us with plasmid DNA, W. Green for providing us with TSA201 cells, Joe Haseman and Shyamal Peddada (NIEHS) for statistical advice, and Meyer Jackson, David Colquhoun, Joe Haseman and Shyamal Peddada for advice on curve fitting of concentration-response relations.
REFERENCES
- Akabas MH, Kaufmann C, Archdecon P, Karlin A. Identification of acetylcholine receptor channel-lining residues in the entire M2 segment of the alpha subunit. Neuron. 1994;13:919–927. doi: 10.1016/0896-6273(94)90257-7. [DOI] [PubMed] [Google Scholar]
- Alkondon M, Albuquerque EX. Diversity of nicotinic acetylcholine receptors in rat hippocampal neurons. I. Pharmacological and functional evidence for distinct structural subtypes. Journal of Pharmacology and Experimental Therapeutics. 1993;265:1455–1473. [PubMed] [Google Scholar]
- Alkondon M, Pereira EF, Barbosa CT, Albuquerque EX. Neuronal nicotinic acetylcholine receptor activation modulates gamma-aminobutyric acid release from CA1 neurons of rat hippocampal slices. Journal of Pharmacology and Experimental Therapeutics. 1997;283:1396–1411. [PubMed] [Google Scholar]
- Anand R, Peng X, Lindstrom J. Homomeric and native α7 acetylcholine receptors exhibit remarkably similar but non-identical pharmacological properties, suggesting that the native receptor is a heteromeric protein complex. FEBS Letters. 1993;327:241–246. doi: 10.1016/0014-5793(93)80177-v. [DOI] [PubMed] [Google Scholar]
- Boyd RT. The molecular biology of neuronal nicotinic acetylcholine receptors. Critical Reviews in Toxicology. 1997;27:299–318. doi: 10.3109/10408449709089897. [DOI] [PubMed] [Google Scholar]
- Chen D, Dang H, Patrick JW. Contributions of N-linked glycosylation to the expression of a functional α7-nicotinic receptor in Xenopus oocytes. Journal of Neurochemistry. 1998;70:349–357. doi: 10.1046/j.1471-4159.1998.70010349.x. [DOI] [PubMed] [Google Scholar]
- Chen D, Patrick JW. The α-bungarotoxin-binding nicotinic acetylcholine receptor from rat brain contains only the α7 subunit. Journal of Biological Chemistry. 1997;272:24024–24029. doi: 10.1074/jbc.272.38.24024. [DOI] [PubMed] [Google Scholar]
- Cooper ST, Harkness PC, Baker ER, Millar NS. Up-regulation of cell-surface α4β2 neuronal nicotinic receptors by lower temperature and expression of chimeric subunits. Journal of Biological Chemistry. 1999;274:27145–27152. doi: 10.1074/jbc.274.38.27145. [DOI] [PubMed] [Google Scholar]
- Cooper ST, Millar NS. Host cell-specific folding and assembly of the neuronal nicotinic acetylcholine receptor α7 subunit. Journal of Neurochemistry. 1997;68:2140–2151. doi: 10.1046/j.1471-4159.1997.68052140.x. [DOI] [PubMed] [Google Scholar]
- Court J, Martin-Ruiz C, Piggott M, Spurden D, Griffiths M, Perry E. Nicotinic receptor abnormalities in Alzheimer's disease. Biological Psychiatry. 2001;49:175–184. doi: 10.1016/s0006-3223(00)01116-1. [DOI] [PubMed] [Google Scholar]
- Crabtree GW, Chen J, Ramirez-Latorre JA, Davis TI, Role LW. α7-containing heteromeric neuronal nicotinic acetylcholine receptors expressed in Xenopus oocytes. Society for Neuroscience Abstracts. 2000;26:1633. [Google Scholar]
- Cuevas J, Berg DK. Mammalian nicotinic receptors with α7 subunits that slowly desensitize and rapidly recover from α-bungarotoxin blockade. Journal of Neuroscience. 1998;18:10335–10344. doi: 10.1523/JNEUROSCI.18-24-10335.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuevas J, Roth AL, Berg DK. Two distinct classes of functional α7-containing nicotinic receptor on rat superior cervical ganglion neurons. Journal of Physiology. 2000;525:735–746. doi: 10.1111/j.1469-7793.2000.t01-1-00735.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dani JA. Overview of nicotinic receptors and their roles in the central nervous system. Biological Psychiatry. 2001;49:166–174. doi: 10.1016/s0006-3223(00)01011-8. [DOI] [PubMed] [Google Scholar]
- Drisdel RC, Green WN. Neuronal α-bungarotoxin receptors are α7 subunit homomers. Journal of Neuroscience. 2000;20:133–139. doi: 10.1523/JNEUROSCI.20-01-00133.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elgoyhen AB, Vetter DE, Katz E, Rothlin CV, Heinemann SF, Boulter J. α10: A determinant of nicotinic cholinergic receptor function in mammalian vestibular and cochlear mechanosensory hair cells. Proceedings of the National Academy of Sciences of the USA. 2001;98:3501–3506. doi: 10.1073/pnas.051622798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frazier CJ, Rollins YD, Breese CR, Leonard S, Freedman R, Dunwiddie TV. Acetylcholine activates an alpha-bungarotoxin-sensitive nicotinic current in rat hippocampal interneurons, but not pyramidal cells. Journal of Neuroscience. 1998;18:1187–1195. doi: 10.1523/JNEUROSCI.18-04-01187.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girod R, Crabtree G, Ernstrom G, Ramirez-Latorre J, McGehee D, Turner J, Role L. Heteromeric complexes of α5 and/or α7 subunits. Effects of calcium and potential role in nicotine-induced presynaptic facilitation. Annals of the New York Academy of Sciences. 1999;868:578–590. doi: 10.1111/j.1749-6632.1999.tb11331.x. [DOI] [PubMed] [Google Scholar]
- Hopp TP, Prickett KS, Price VL, Libby RT, March CJ, Cerretti DP, Urdal DL, Conlon PJ. A short polypeptide marker sequence useful for recombinant protein identification and purification. Biotechnology. 1988;6:1204–1210. [Google Scholar]
- Ji D, Dani JA. Inhibition and disinhibition of pyramidal neurons by activation of nicotinic receptors on hippocampal interneurons. Journal of Neurophysiology. 2000;83:2682–2690. doi: 10.1152/jn.2000.83.5.2682. [DOI] [PubMed] [Google Scholar]
- Jones S, Sudweeks S, Yakel JL. Nicotinic receptors in the brain: correlating physiology with function. Trends in Neurosciences. 1999;22:555–561. doi: 10.1016/s0166-2236(99)01471-x. [DOI] [PubMed] [Google Scholar]
- Jones S, Yakel JL. Functional nicotinic ACh receptors on interneurones in the rat hippocampus. Journal of Physiology. 1997;504:603–610. doi: 10.1111/j.1469-7793.1997.603bd.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kassner PD, Berg DK. Differences in the fate of neuronal acetylcholine receptor protein expressed in neurons and stably transfected cells. Journal of Neurobiology. 1997;33:968–982. doi: 10.1002/(sici)1097-4695(199712)33:7<968::aid-neu8>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- Keyser KT, Britto LR, Schoepfer R, Whiting P, Cooper J, Conroy W, Brozozowska-Prechtl A, Karten HJ, Lindstrom J. Three subtypes of α-bungarotoxin-sensitive nicotinic acetylcholine receptors are expressed in chick retina. Journal of Neuroscience. 1993;13:442–454. doi: 10.1523/JNEUROSCI.13-02-00442.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kriegler S, Sudweeks S, Yakel JL. Communication: The nicotinic α4 receptor subunit contributes to the lining of the ion channel pore when expressed with the 5-HT3 receptor subunit. Journal of Biological Chemistry. 1999;274:3934–3936. doi: 10.1074/jbc.274.7.3934. [DOI] [PubMed] [Google Scholar]
- Liu Q, Kawai H, Berg DK. β-Amyloid peptide blocks the response of α7-containing nicotinic receptors on hippocampal neurons. Proceedings of the National Academy of Sciences of the USA. 2001;98:4734–4739. doi: 10.1073/pnas.081553598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGehee DS, Role LW. Physiological diversity of nicotinic acetylcholine receptors expressed by vertebrate neurons. Annual Review of Physiology. 1995;57:521–546. doi: 10.1146/annurev.ph.57.030195.002513. [DOI] [PubMed] [Google Scholar]
- McQuiston AR, Madison DV. Nicotinic receptor activation excites distinct subtypes of interneurons in the rat hippocampus. Journal of Neuroscience. 1999;19:2887–2896. doi: 10.1523/JNEUROSCI.19-08-02887.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madeja M, Mußhoff U, Speckmann E-J. A concentration-clamp system allowing two-electrode voltage-clamp investigations in oocytes of Xenopus laevis. Journal of Neuroscience Methods. 1991;38:267–269. doi: 10.1016/0165-0270(91)90178-3. [DOI] [PubMed] [Google Scholar]
- Palma E, Maggi L, Barabino B, Eusebi F, Ballivet M. Nicotinic acetylcholine receptors assembled from the α7 and β3 subunits. Journal of Biological Chemistry. 1999;274:18335–18340. doi: 10.1074/jbc.274.26.18335. [DOI] [PubMed] [Google Scholar]
- Paterson D, Nordberg A. Neuronal nicotinic receptors in the human brain. Progress in Neurobiology. 2000;61:75–111. doi: 10.1016/s0301-0082(99)00045-3. [DOI] [PubMed] [Google Scholar]
- Pettit DL, Shao Z, Yakel JL. β-Amyloid 1–42 peptide directly modulates nicotinic receptors in the rat hippocampal slice. Journal of Neuroscience. 2001;21:RC120. doi: 10.1523/JNEUROSCI.21-01-j0003.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pugh PC, Corriveay RA, Conroy WG, Berg DK. Novel subpopulation of neuronal acetylcholine receptors among those binding α-bungarotoxin. Molecular Pharmacology. 1995;47:717–725. [PubMed] [Google Scholar]
- Rangwala F, Drisdel RC, Rakhilin S, Ko E, Atluri P, Harkins AB, Fox AP, Salman SS, Green WN. Neuronal alpha-bungarotoxin receptors differ structurally from other nicotinic acetylcholine receptors. Journal of Neuroscience. 1997;17:8201–8212. doi: 10.1523/JNEUROSCI.17-21-08201.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Revah F, Bertrand D, Galzi J-L, Devillers-Thiéry A, Mulle C, Hussy N, Bertrand S, Ballivet M, Changeux J-P. Mutations in the channel domain alter desensitization of a neuronal nicotinic receptor. Nature. 1991;353:846–849. doi: 10.1038/353846a0. [DOI] [PubMed] [Google Scholar]
- Shao Z, Yakel JL. Single channel properties of neuronal nicotinic ACh receptors in stratum radiatum interneurons of rat hippocampal slices. Journal of Physiology. 2000;527:507–513. doi: 10.1111/j.1469-7793.2000.00507.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sudweeks SN, Yakel JL. Functional and molecular characterization of neuronal nicotinic ACh receptors in rat CA1 hippocampal neurones. Journal of Physiology. 2000;527:515–528. doi: 10.1111/j.1469-7793.2000.00515.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whiting P, Lindstrom J. Purification and characterization of a nicotinic acetylcholine receptor from rat brain. Proceedings of the National Academy of Sciences of the USA. 1987;84:595–599. doi: 10.1073/pnas.84.2.595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wonnacott S. Presynaptic nicotinic ACh receptors. Trends in Neurosciences. 1997;20:92–98. doi: 10.1016/s0166-2236(96)10073-4. [DOI] [PubMed] [Google Scholar]
- Yu CR, Role LW. Functional contribution of the α7 subunit to multiple subtypes of nicotinic receptors in embryonic chick sympathetic neurones. Journal of Physiology. 1998a;509:651–665. doi: 10.1111/j.1469-7793.1998.651bm.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu CR, Role LW. Functional contribution of the α5 subunit to neuronal nicotinic channels expressed by chick sympathetic ganglion neurones. Journal of Physiology. 1998b;509:667–681. doi: 10.1111/j.1469-7793.1998.667bm.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zwart R, Vijverberg HP. Four pharmacologically distinct subtypes of α4β2 nicotinic acetylcholine receptor expressed in Xenopus laevis oocytes. Molecular Pharmacology. 1998;54:1124–1131. [PubMed] [Google Scholar]


