Abstract
The excitatory action of muscarine on spinal motoneurones was investigated with intracellular recordings in a slice preparation from adult turtles. In these cells muscarine is known to facilitate a persistent inward current mediated by L-type Ca2+ channels. When this effect was blocked by nifedipine, muscarine still increased the excitability. In voltage clamp, a slowly activating outward current, generated during depolarizing voltage commands and deactivating as a tail current on return to the holding voltage, was reduced by muscarine. This outward current was activated when the voltage was stepped to potentials positive to −60 mV, was voltage sensitive and had a deactivation time constant of ≈80 ms. These findings are compatible with an M-current. This possibility was also supported by the finding that the current was reduced by XE-991 - a selective blocker of the KCNQ potassium channels underlying M-currents in other cell types. Our findings suggest that an M-like current, mediated by a KCNQ channel, contributes to the intrinsic response properties of motoneurones in the adult spinal cord by increasing adaptation of repetitive firing and decreasing the slope of the frequency-current relation.
Motoneurones transform synaptic input to the discharge patterns that underlie movement. Each spike in a motor axon induces a contraction in the muscle fibres innervated. For this reason spike frequency regulation in motoneurones is a functionally important element in this transformation.
It has been known for many years, that motoneurones respond to a sustained depolarizing current by a train of action potentials that gradually adapts from a high initial firing frequency to a lower frequency in steady state (Kernell, 1965). This activity-dependent change in excitability is important for the mechanical output of the muscle fibres innervated by a motoneurone (Binder et al. 1996). The adaptation can be separated in three phases: initial, early and late (Sawczuk et al. 1997). It is currently thought that different mechanisms might contribute to the three phases of adaptation (Kernell, 1999). However, the underlying cellular properties and their regulation by metabotropic transmitter receptors have not been elucidated.
Among the possible mechanisms that could contribute to regulation of excitability in motoneurones, activation of an M-current is a good candidate (Aiken et al. 1995), especially for the early phase of adaptation. The M-current, so called because it is reduced by acetylcholine acting at muscarinic receptors, is a slowly activating voltage-regulated K+ current (for review see Brown & Yu, 2000). It activates near the resting membrane potential and does not inactivate during a sustained depolarization. It is now accepted that most M-currents are mediated by members of the KCNQ K+ channel family because these channels generate a current that shares many characteristics with the M-current, including voltage dependence, kinetics, and pharmacology (Wang et al. 1998; Schroeder et al. 2000). An M-like current has been recorded previously in primary cultures of dissociated cells from the spinal cord of the mouse (Nowak & Macdonald, 1983). Moreover, in situ hybridization experiments show that mRNAs for subunits are present in the anterior horn of the spinal cord of the adult mouse (Dedek et al. 2001). However, the existence of an M-current in spinal motoneurones has not yet been demonstrated.
This study was undertaken to investigate whether an M-current, carried through KCNQ channels, contributes to the regulation of the excitability in mature spinal motoneurones. In the absence of slice preparations with viable motoneurones from the spinal cord of adult mammals, experiments were performed using turtles. The intrinsic response properties of motoneurones in in vitro preparations from the spinal cord of the adult turtle are remarkably similar to the properties of spinal motoneurones in the cat and seem to be generated by the same set of ion channels (Perrier & Hounsgaard, 2000). Using intracellular recordings in voltage- and current-clamp mode, we now show that an M-like current, probably mediated by KCNQ channels, contributes to the adaptation of discharge frequency in motoneurones. Part of this work has been published previously in abstract form (Alaburda et al. 2001).
METHODS
Slice preparation
Transverse slices (1.5–2 mm thick) were obtained from the lumbar enlargement of adult turtles (Chrysemys scripta elegans) anaesthetized by intraperitoneal injection of 100 mg sodium pentobarbitone and killed by decapitation. The surgical procedures complied with Danish legislation and were approved by the controlling body under the Ministry of Justice. Experiments were performed at room temperature (20–2 °C) in normal Ringer solution containing (mm): 120 NaCl; 5 KCl; 15 NaHCO3; 2 MgCl2; 3 CaCl2; and 20 glucose, saturated with 98 % O2 −2 % CO2 to obtain pH 7.6. Calcium-free cobalt solution was prepared by replacing CaCl2 with CoCl2.
Recordings
Intracellular recordings in current-clamp and voltage-clamp mode were performed with an Axoclamp-2A amplifier (Axon Instruments, Inc., Union City, CA, USA). The sharp electrode technique used allows stable intracellular recordings lasting up to 4 h. Glass pipettes were filled with 1 m potassium acetate. To reduce electrode capacitance in voltage-clamp recordings, pipette tips were coated with Sylgard. Voltage-clamp recordings were performed in discontinuous service mode at a sample rate of 5–8 kHz, with a gain of 0.7–1.5 nA mV−1 and a low-pass filter of 0.1 kHz. Motoneurones were selected for study if they had a stable membrane potential more negative than −50 mV. Data were sampled at 20 kHz with a 12-bit analog-to-digital converter (Digidata 1200, Axon Instruments) and displayed by means of Axoscope and Clampex software (Axon Instruments) and stored on a hard disk for later analysis.
Drugs
In all experiments, synaptic potentials were eliminated with 6-cyano-7-nitroquinoxaline-2, 3-dione (CNQX, 25 μm; Tocris Cookson Ltd, Bristol, UK), either dl-2-amino-5-phosphonopentanoic acid (dl-AP5, 50 μm; Tocris) or d(−)-2-amino-7-phosphonoheptanoic acid (AP7, 25 μm; Tocris), and strychnine (10 μm). In voltage-clamp experiments, action potentials were blocked with tetrodotoxin (TTX, 1–2 μm; Alomone Labs, Jerusalem, Israel). The hyperpolarization-activated inward mixed cation current was blocked with ZD 7288 (50–100 μm, Tocris). L-type Ca2+ channels were blocked with nifedipine (20–40 μm; Sigma). Other drugs used: (±)-muscarine chloride (muscarine, 5–12.5 μm; Sigma), 10, 10-bis(4-pyridinylmethyl)-9(10 H)-anthracenone (XE991, 1–10 μm; provided by NeuroSearch A/S, Denmark) and atropine (3.5 μm).
Data quantification and representation
Instantaneous firing frequency was calculated as the inverse value of interspike intervals. The steady state frequency was measured during the last 500 ms of current pulses lasting 3s. Frequency- current plots (f-I) were obtained using the steady state frequency as a function of current. The slope of the f-I relation was measured as the slope of the straight line fitting the initial linear part of f-I (least squares method; Microcal Origin software, OriginLab Corp., Northampton, MA, USA).
Input resistance at the resting membrane potential was obtained from the relation between the amplitude of a small current pulse and the steady state voltage change induced by this stimulus.
The voltage-clamp protocol consisted of 2 s depolarizing commands of stepwise increasing amplitude from a level of −60 mV. A presumed M-current was studied as the slow outward deactivation tail current observed when stepping back to −60 mV from different levels of depolarization. The peak of the tail current was quantified as the average amplitude of the current measured from 30 to 50 ms after stepping to −60 mV (Shapiro et al. 2000). The time constant of the tail current was evaluated by fitting the current during the 200 ms following the peak of the tail with a single exponential (Microcal Origin software). For some of the recordings, the signal was filtered by means of the substitute average technique (Clampfit software).
Data were analysed statistically by using a two populations (paired and independent) t test (Microcal Origin software). Significance was accepted when P < 0.05. Data are presented as means ± standard error of means (s.e.m.).
RESULTS
Multiple excitatory actions of muscarine
In spinal motoneurones from adult turtles, muscarine increases excitability by facilitating a persistent inward current mediated by L-type Ca2+ channels (Delgado-Lezama et al. 1997; Svirskis & Hounsgaard, 1998). In agreement, we found that extracellularly applied muscarine altered the firing pattern evoked by a depolarizing current pulse. Rather than adapting from an early high firing frequency to a lower frequency in steady state (Fig. 1A and B), the rate of discharge, in the presence of muscarine, accelerated after the initial adaptation to a higher level in steady state and an afterdepolarization was observed after the pulse (n = 4; Fig. 1A and B). In addition to the delayed increase in firing frequency, however, we noted that the initial rate of firing during the pulse was also facilitated by muscarine (Fig. 1A and B). While nifedipine, a selective blocker of L-type Ca2+ channels, readily blocked the delayed increase in discharge rate and the afterdepolarization (n = 2), the general increase in excitability in the presence of muscarine was unaffected (n = 8, Fig. 1A and B). Atropine reduced the nifedipine-insensitive increase in excitability induced by muscarine (Fig. 1A and B; n = 3).
Figure 1. Multiple excitatory effects of muscarine.
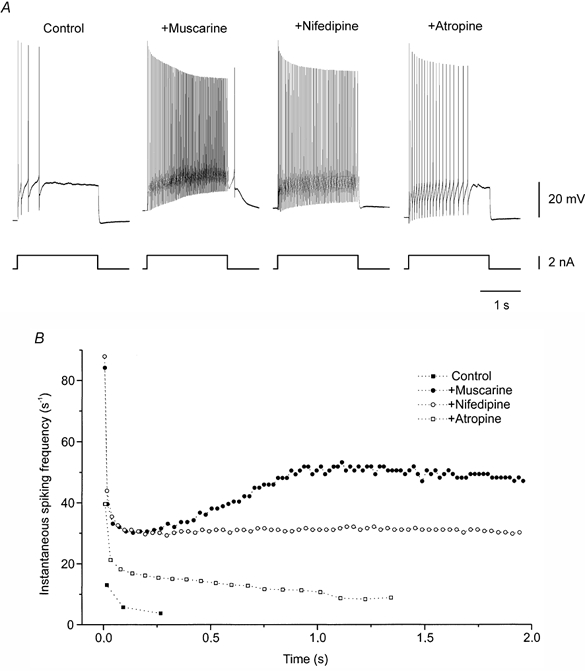
A, response of a motoneurone to a depolarizing current pulse. Muscarine (12.5 μm) increased early spiking, induced discharge acceleration and afterdepolarization. Addition of nifedipine (20 μm) blocked discharge acceleration and afterdepolarization, but did not affect the increase in excitability. Atropine (3.5 μm) partially blocked excitatory effect of muscarine. B, instantaneous spike frequency as a function of time (same data as in A). All recordings from the same cell.
The muscarine-induced increase in excitability that remains in the presence of nifedipine is compatible with the reduction of an M-like K+ current (Marrion, 1997). The experiments presented in the remaining part of this paper aimed at testing this hypothesis. These experiments were performed in the presence of nifedipine.
Muscarine sensitivity of the tail current
To test whether the muscarine-induced increase in excitability was due to the reduction of an M-like current, we performed voltage-clamp experiments. In the presence of TTX, the tail current following voltage commands from depolarized levels to −60 mV was used as an index of the M-current (see Methods). Over the accessible voltage range, the amplitude of the tail current increased with increasing amplitude of the depolarizing voltage step (Fig. 2A, arrow on left traces; n = 32). The deactivation time constant for the tail current was 78.2 ± 6.4 ms. When the membrane potential was stepped from −60 mV to −30 mV the tail current accounted for 6.2 ± 0.6 % of the total current evoked during voltage commands.
Figure 2. Muscarine-sensitive tail current.
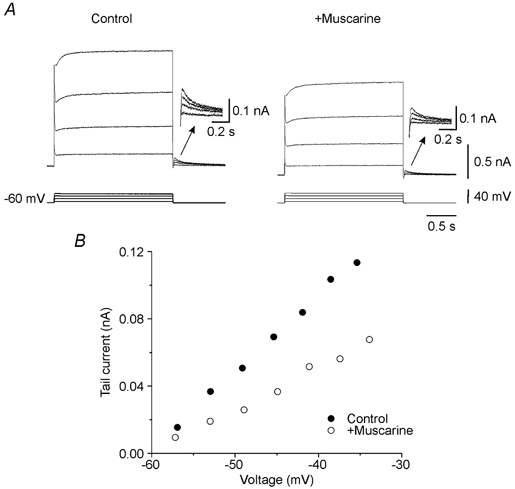
In the presence of nifedipine, depolarizing voltage steps activated a voltage-sensitive slowly activating non-inactivating outward current relaxing as a tail (A, left). Addition of muscarine (12.5 μm) reduced this current (A, right). Insets: tail currents at a higher resolution. Voltage sensitivity of the tail current was not affected by muscarine (B). Action potentials were blocked by TTX.
Muscarine significantly reduced the amplitude of the tail current (−47.4 ± 2.7 %; n = 4; P < 0.05) (Fig. 2A and B), but not its voltage sensitivity (Fig. 2B).
Firing pattern and tail current are sensitive to block of KCNQ channel
XE991 is known to be a selective blocker of the KCNQ family potassium channels underlying M-currents in other cell types (Wang et al. 1998). In all 10 cells tested, XE991 added to the extracellular medium increased the firing rate during depolarizing current pulses (Fig. 3A and B). The slope of f-I was increased by 17.6 ± 2.3 % (P < 0.00005) and the f-I was shifted to the left (Fig. 3C). The effect of XE991 on the tail current was tested in voltage clamp in the presence of TTX. Application of XE991 reduced the amplitude of the tail current by 71.4 ± 4.7 % (n = 6; P < 0.005) (Fig. 4A). A similar voltage-sensitive tail current was present after replacement of the extracellular calcium by cobalt, a non-selective Ca2+ channel blocker (Fig. 4B; n = 9). The tail current amplitudes evoked by stepping voltage from −30 mV back to −60 mV in normal and Ca2+-free media were not significantly different (P > 0.05). Therefore Ca2+-dependent potassium currents cannot account for the tail current. XE991 significantly reduced the amplitude of the tail current in Ca2+-free medium (51 ± 3.9 %; n = 5; P < 0.00005) (Fig. 4C).
Figure 3. Block of KCNQ channels increases excitability of motoneurones.
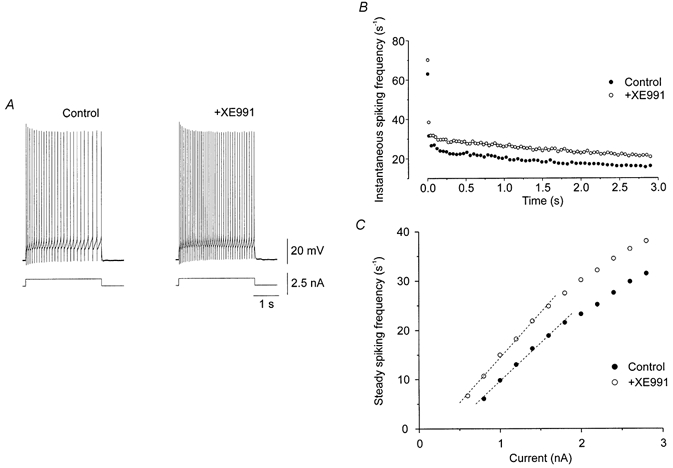
In current clamp, addition of XE991 (1 μm) enhanced the response to a suprathreshold depolarizing current pulse (A). XE991 reduced the early adaptation of firing frequency (B), shifting the steady state f-I relation to the left and increasing its steepness (C). The data in B were obtained in response to a 1.4 nA depolarizing current pulse lasting 3s. Recordings from the same cell, in the presence of nifedipine.
Figure 4. Block of KCNQ channels reduces the tail current.
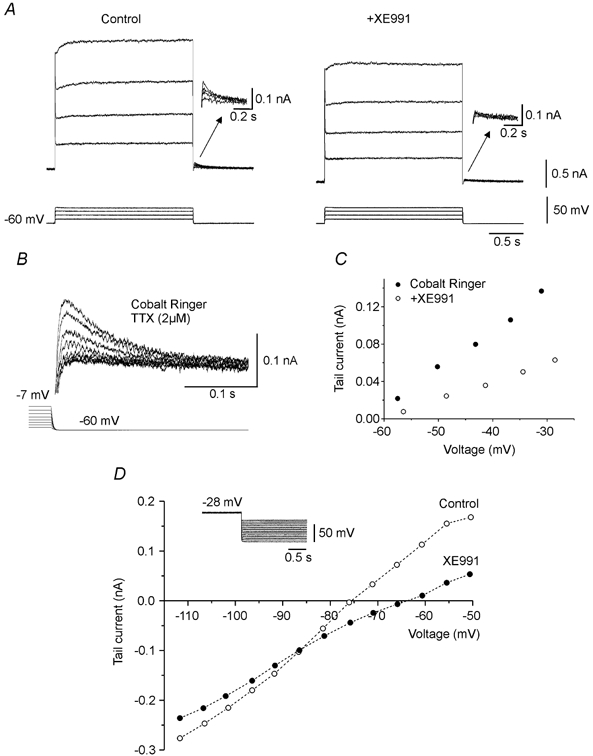
In voltage clamp, addition of XE991 (1 μm) reduced the voltage-sensitive tail current (arrows in A). A similar voltage-sensitive tail current present in cobalt Ringer (B and C) was reduced by XE991 (5 μm) (C). B and C are from different motoneurones. The ionic basis of the XE991 sensitive current was investigated by applying hyperpolarized voltage steps of increasing amplitude (protocol illustrated in D) in the presence of ZD 7288 (100 μm). The amplitude of the tail current was measured as a function of the potential before and after addition of XE991 (5 μm) (D). The 2 curves were crossing at −86 mV, value which indicates the reversal potential for ions carrying the tail current. Action potentials were blocked by TTX. A and D in the presence of nifedipine.
Ionic basis of the M-like current in motoneurones
The ionic basis of the XE991-sensitive tail current was investigated in voltage clamp in the presence of TTX. The tail current was evoked by steps from a depolarized potential (from −28 mV to −36 mV) to a test level that was decremented by 5 mV in successive trials from −50 mV to −110 mV (inset in Fig. 4D). The slow, hyperpolarization-activated mixed cation current, which is known to be present in motoneurones (Hounsgaard et al. 1988), was blocked by extracellular application of ZD 7288 (BoSmith et al. 1993; Harris & Constanti, 1995). The tail current recorded under these conditions was quantified and plotted as a function of voltage (Fig. 4D). In all experiments the I–V relations for the tail current before and after application of XE991 crossed between −80 mV and −90 mV (−86.4 ± 1.4 mV; n = 5), a value compatible with the reversal potential for K+ ions.
DISCUSSION
In the present study we have demonstrated that an M-like current contributes to the intrinsic response properties in spinal motoneurones in the adult turtle. Several arguments favour the hypothesis that this current is an M-current. It activates slowly during depolarizing voltage commands from −60 mV and deactivates as a tail current when voltage is stepped back. It does not inactivate during a sustained depolarization of several seconds. It is voltage sensitive. It persists in the presence of cobalt. The deactivation time constant of the tail current was ∼80 ms, compatible with previously reported values (Brown & Adams, 1980). It is inhibited by XE991, a specific blocker of KCNQ family potassium channels. The XE991-sensitive tail current was inverted between −80 and −90 mV, i.e. at a value compatible with a potassium current. Finally, muscarine inhibits the tail current without affecting its voltage sensitivity, as shown before for M-currents in other cell types (Shapiro et al. 2000).
The amplitude of the M-like current recorded in motoneurones is small compared to the global current. However, the fact that the current activates just below the threshold for spike generation makes this current important for regulating spike initiation and excitability in spinal motoneurones.
M-currents in other neurones are mediated by K+ channels expressing KCNQ2 and KCNQ3 subunits (Wang et al. 1998) and KCNQ5 subunits (Schroeder et al. 2000). Although expression of K+ channels of the KCNQ family in motoneurones has not yet been shown, the presence of mRNAs for the KCNQ2 and KCNQ3 subunits in the ventral horn provides good candidates (Dedek et al. 2001). Benign familial neonatal convulsions is a form of epilepsy associated with frequent seizures in the first weeks of life. It is believed that the disease is due to a mutation of KCNQ2 and KCNQ3 subunits (Singh et al. 1998; Charlier et al. 1998). For some of the patients, the disease is followed later in life by myokymia and involuntary contractions of skeletal muscles (Dedek et al. 2001). Under physiological conditions, the presence of the M-current in motoneurones could play a role in preventing involuntary movements by reducing their excitability.
The M1 muscarinic acetylcholine receptor is a candidate for muscarinic M-current inhibition (Marrion et al. 1989; Selyanko et al. 2000). However, recent data obtained from M1 receptor knock-out mice (Shapiro et al. 2001) suggest that activation of M1 receptors inhibits L-type Ca2+ channels. The subtypes of muscarinic receptors facilitating L-type Ca2+ channels and suppressing M-like current in turtle motoneurones remain to be identified.
In addition to up-regulation of L-channels and down-regulation of the channel responsible for the M-like current, as reported in the present paper, muscarine clearly has additional, undescribed effects on motoneurones. These include increased input resistance (Svirskis & Hounsgaard, 1998) and reduced spike afterhyperpolarization (Lape & Nistri, 2000). It is not yet known if each cholinergic synapse controls all these channels in parallel or only a subset of channels.
Acknowledgments
This work was kindly funded by the European Union, the Danish MRC, The Lundbeck Foundation, The Novo-Nordisk Foundation and The Foundation Agnes and Poul Friis. J.-F. P. is supported by a grant from the Danish MRC.
REFERENCES
- Aiken SP, Lampe BJ, Murphy PA, Brown BS. Reduction of spike frequency adaptation and blockade of M-current in rat CA1 pyramidal neurones by linopirdine (DuP 996), a neurotransmitter release enhancer. British Journal of Pharmacology. 1995;115:1163–1168. doi: 10.1111/j.1476-5381.1995.tb15019.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alaburda A, Perrier JF, Hounsgaard J. M-like outward current in spinal motoneurons. Society for Neuroscience Abstracts. 2001;31:714.4. [Google Scholar]
- Binder MD, Heckman CJ, Powers RK. The physiological control of motoneuron activity. In: Rowell LB, Shepherd JT, editors. Handbook of Physiology, section 12, Exercise: Regulation and Integration of Multiple Systems. New York: Oxford University Press; 1996. pp. 3–53. [Google Scholar]
- BoSmith RE, Briggs I, Sturgess NC. Inhibitory actions of ZENECA ZD7288 on whole-cell hyperpolarization activated inward current (If) in guinea-pig dissociated sinoatrial node cells. British Journal of Pharmacology. 1993;110:343–349. doi: 10.1111/j.1476-5381.1993.tb13815.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown BS, Yu SP. Modulation and genetic identification of the M channel. Progress in Biophysics and Molecular Biology. 2000;73:135–166. doi: 10.1016/s0079-6107(00)00004-3. [DOI] [PubMed] [Google Scholar]
- Brown DA, Adams PR. Muscarinic suppression of a novel voltage-sensitive K+ current in a vertebrate neurone. Nature. 1980;283:673–676. doi: 10.1038/283673a0. [DOI] [PubMed] [Google Scholar]
- Charlier C, Singh NA, Ryan SG, Lewis TB, Reus BE, Leach RJ, Leppert M. A pore mutation in a novel KQT-like potassium channel gene in an idiopathic epilepsy family. Nature Genetics. 1998;18:53–55. doi: 10.1038/ng0198-53. [DOI] [PubMed] [Google Scholar]
- Dedek K, Kunath B, Kananura C, Reuner U, Jentsch TJ, Steinlein OK. Myokymia and neonatal epilepsy caused by a mutation in the voltage sensor of the KCNQ2 K+ channel. Proceedings of the National Academy of Sciences of the USA. 2001;98:12272–12277. doi: 10.1073/pnas.211431298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delgado-Lezama R, Perrier J-F, Nedergaard S, Svirskis G, Hounsgaard J. Metabotropic synaptic regulation of intrinsic response properties of turtle spinal motoneurones. Journal of Physiology. 1997;504:97–102. doi: 10.1111/j.1469-7793.1997.097bf.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris NC, Constanti A. Mechanism of block by ZD 7288 of the hyperpolarization-activated inward rectifying current in guinea-pig substantia nigra neurons in vitro. Journal of Neurophysiology. 1995;74:2366–2378. doi: 10.1152/jn.1995.74.6.2366. [DOI] [PubMed] [Google Scholar]
- Hounsgaard J, Kiehn O, Mintz I. Response properties of motoneurones in a slice preparation of the turtle spinal cord. Journal of Physiology. 1988;398:575–589. doi: 10.1113/jphysiol.1988.sp017058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kernell D. The adaptation and the relation between discharge frequency and current strength of cat lumbosacral motoneurons stimulated by long-lasting injected currents. Acta Physiologica Scandinavica. 1965;65:65–73. doi: 10.1111/j.1748-1716.1965.tb04081.x. [DOI] [PubMed] [Google Scholar]
- Kernell D. Repetitive impulse firing in motoneurons: facts and perspectives. Progress in Brain Research. 1999;123:31–37. doi: 10.1016/s0079-6123(08)62841-1. [DOI] [PubMed] [Google Scholar]
- Lape R, Nistri A. Current and voltage clamp studies of the spike medium afterhyperpolarization of hypoglossal motoneurons in a rat brain stem slice preparation. Journal of Neurophysiology. 2000;83:2987–2995. doi: 10.1152/jn.2000.83.5.2987. [DOI] [PubMed] [Google Scholar]
- Marrion NV. Control of M-current. Annual Reviews Physiology. 1997;59:483–504. doi: 10.1146/annurev.physiol.59.1.483. [DOI] [PubMed] [Google Scholar]
- Marrion NV, Smart TG, Marsh SJ, Brown DA. Muscarinic suppression of the M-current in the rat sympathetic ganglion is mediated by receptors of the M1-subtype. British Journal of Pharmacology. 1989;98:557–573. doi: 10.1111/j.1476-5381.1989.tb12630.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nowak LM, Macdonald RL. Ionic mechanism of muscarinic cholinergic depolarization of mouse spinal cord neurons in cell culture. Journal of Neurophysiology. 1983;49:792–803. doi: 10.1152/jn.1983.49.3.792. [DOI] [PubMed] [Google Scholar]
- Perrier JF, Hounsgaard J. Development and regulation of response properties in spinal cord motoneurons. Brain Research Bulletin. 2000;53:529–535. doi: 10.1016/s0361-9230(00)00386-5. [DOI] [PubMed] [Google Scholar]
- Sawczuk A, Powers RK, Binder MD. Contribution of outward currents to spike-frequency adaptation in hypoglossal motoneurons of the rat. Journal of Neurophysiology. 1997;78:2246–2253. doi: 10.1152/jn.1997.78.5.2246. [DOI] [PubMed] [Google Scholar]
- Schroeder BC, Hechenberger M, Weinreich F, Kubisch C, Jentsch TJ. KCNQ5, a novel potassium channel broadly expressed in brain, mediates M- type currents. Journal of Biological Chemistry. 2000;275:24089–24095. doi: 10.1074/jbc.M003245200. [DOI] [PubMed] [Google Scholar]
- Selyanko AA, Hadley JK, Wood IC, Abogadie FC, Jentsch TJ, Brown DA. Inhibition of KCNQ1–4 potassium channels expressed in mammalian cells via M1 muscarinic acetylcholine receptors. Journal of Physiology. 2000;522:349–355. doi: 10.1111/j.1469-7793.2000.t01-2-00349.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapiro MS, Gomeza J, Hamilton SE, Hille B, Loose MD, Nathanson NM, Roche JP, Wess J. Identification of subtypes of muscarinic receptors that regulate Ca2+ and K+ channel activity in sympathetic neurons. Life Sciences. 2001;68:2481–2487. doi: 10.1016/s0024-3205(01)01042-6. [DOI] [PubMed] [Google Scholar]
- Shapiro MS, Roche JP, Kaftan EJ, Cruzblanca H, Mackie K, Hille B. Reconstitution of muscarinic modulation of the KCNQ2/KCNQ3 K+ channels that underlie the neuronal M current. Journal of Neuroscience. 2000;20:1710–1721. doi: 10.1523/JNEUROSCI.20-05-01710.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh NA, Charlier C, Stauffer D, Dupont BR, Leach RJ, Melis R, Ronen GM, Bjerre I, Quattlebaum T, Murphy JV, Mcharg ML, Gagnon D, Rosales TO, Peiffer A, Anderson VE, Leppert M. A novel potassium channel gene, KCNQ2, is mutated in an inherited epilepsy of newborns. Nature Genetics. 1998;18:25–29. doi: 10.1038/ng0198-25. [DOI] [PubMed] [Google Scholar]
- Svirskis G, Hounsgaard J. Transmitter regulation of plateau properties in turtle motoneurons. Journal of Neurophysiology. 1998;79:45–50. doi: 10.1152/jn.1998.79.1.45. [DOI] [PubMed] [Google Scholar]
- Wang HS, Pan Z, Shi W, Brown BS, Wymore RS, Cohen IS, Dixon JE, Mckinnon D. KCNQ2 and KCNQ3 potassium channel subunits: molecular correlates of the M-channel. Science. 1998;282:1890–1893. doi: 10.1126/science.282.5395.1890. [DOI] [PubMed] [Google Scholar]


