Abstract
There are only a few reports of the influence of imposed flow on an active lymph pump under conditions of controlled intraluminal pressure. Thus, the mechanisms are not clearly defined. Rat mesenteric lymphatics and thoracic ducts were isolated, cannulated and pressurized. Input and output pressures were adjusted to impose various flows. Lymphatic systolic and diastolic diameters were measured and used to determine contraction frequency and pump flow indices. Imposed flow inhibited the active lymph pump in both mesenteric lymphatics and in the thoracic duct. The active pump of the thoracic duct appeared more sensitive to flow than did the active pump of the mesenteric lymphatics. Imposed flow reduced the frequency and amplitude of the contractions and accordingly the active pump flow. Flow-induced inhibition of the active lymph pump followed two temporal patterns. The first pattern was a rapidly developing inhibition of contraction frequency. Upon imposition of flow, the contraction frequency immediately fell and then partially recovered over time during continued flow. This effect was dependent on the magnitude of imposed flow, but did not depend on the direction of flow. The effect also depended upon the rate of change in the direction of flow. The second pattern was a slowly developing reduction of the amplitude of the lymphatic contractions, which increased over time during continued flow. The inhibition of contraction amplitude was dependent on the direction of the imposed flow, but independent of the magnitude of flow. Nitric oxide was partly but not completely responsible for the influence of flow on the mesenteric lymph pump. Exposure to NO mimicked the effects of flow, and inhibition of the NO synthase by NG-monomethyl-l-arginine attenuated but did not completely abolish the effects of flow.
Lymph flow is the result of complicated combinations of different driving forces. The rate of lymph formation is an important factor that generates the pressure gradient along the lymphatic vessels. But there are many factors that alter this gradient, one of the most important being the intrinsic contractile activity of lymphatics, or active lymph pump. The sequence of contractile events in lymphatics is often described using terms of the cardiac cycle (McHale & Roddie, 1976; Benoit et al. 1989; Gashev, 1991). During lymphatic systole, contraction of muscle cells in the lymphatic wall increases the intraluminal pressure. Due to the pressure increase and to the fact that adjacent lymphangions do not all contract at the exact same time, some lymph flows in the retrograde direction, closing the input valve of the lymphangion during the beginning of systole (McHale & Roddie, 1976; Hargens & Zweifach, 1977; Gashev, 1991). However, most lymph is ejected from the lymphangion centripetally during the lymphatic systole. Moreover, there are several extrinsic forces that influence lymph flow, i.e. the pulsation of the nearest blood vessels, motion of skeletal muscles or tissues surrounding the lymphatics and the suction effects of inspiration. Most of the time these forces have effects that help to move lymph centripetally. For example, when contraction of skeletal muscle begins, it squeezes lymphatics and aids lymph flow. However, sustained skeletal muscle activity decreases lymph flow (Olszewski & Engeset, 1980) by increasing outflow resistance of peripheral lymphatics. Another important feature of the lymphatic beds, which makes the flow profile in lymphatics even more complicated, is the branching pattern of the lymphatic vessels. The junctures of lymphatic branch points often have no lymphatic valves and the asymmetric contractile activity of the nearest branches of lymphatic vessels could cause significant retrograde flow. As a result of all of these factors, lymph flow exhibits a complicated pattern during normal lymphatic function that not only shows great changes in magnitude but also transient changes in its direction. Thus, if lymphatic contractile activity is sensitive to the shear stresses exerted by lymph, lymph flow is not only generated by the lymph pump but the flow can also provide feedback to influence the pump activity.
In spite of the potential importance of the effects of flow on lymphatic contractility, there are only a few reports of the influence of imposed flow on an active lymph pump under conditions of controlled intraluminal pressures. Experimental conditions in studies that increase the flow rate by raising the rate of lymph formation (Benoit et al. 1989) do not allow separation of the effects of changes in flow and intraluminal pressure, which will both increase under these conditions. An inhibition of the amplitude and frequency of contractions induced by a controlled increase in imposed flow was shown in experiments in isolated bovine mesenteric vessels (Gashev, 1989). Recently (Koller et al. 1999), reductions in the amplitude of the contractions of isolated rat iliac microlymphatics were reported as a result of increases in controlled perfusate flow. In this study, the authors also described an increase in the contraction frequency as a result of the increase in flow rate.
Therefore, it is not clearly understood if and/or how changes in the magnitude of flow can modulate the contractility of different lymphatic vessels. Additionally, the answers to several more questions concerning the influence of changes in flow conditions in lymphatics are still unclear. Specifically, there are few or no data to help the understanding of how lymphatics react to changes in the direction of flow and whether the flow-induced changes in lymphatic contractility are constant or change over time after a change in flow rate.
METHODS
The animal facilities used for these studies are accredited by the Association for the Assessment and Accreditation of Laboratory Animal Care (AAALAC International) and adhere to the regulations, policies and principles detailed in the Public Health Service Policy for the Humane Care and Use of Laboratory Animals (PHS Policy, 1996) and the United States Department of Agriculture's (USDA) Animal Welfare Regulations (Animal Welfare Act, AWA, 9CFR, 1985, 1992). All animal procedures performed for this study were reviewed and approved by our institutional animal care and use committee, the Texas A&M University Laboratory Animal Care Committee, before the activity was initiated.
Male Sprague-Dawley rats weighing between 125 and 300 g were used. All rats were fasted for 1 day before surgery. During this period, water was available ad libitum. The rats were anaesthetized with a combination of a droperidol-fentanyl solution (droperidol 20 mg ml−1, fentanyl 0.4 mg ml−1; 0.3 ml kg−1i.m.) and diazepam (2.5 mg kg−1i.m.).
To isolate mesenteric lymphatics, a 3 cm long midline abdominal incision was made through the skin, underlying fascia and abdominal muscle layers. A small loop of intestine, 6–7 cm long, was exteriorized through the incision. A section of the mesentery containing lymphatic vessels was centred over a viewing window in the preparation board. The exteriorized tissues were continuously suffused with a modified physiological salt solution. A suitable lymphatic (80–120 μm in diameter) was found using a dissecting microscope and the isolation procedure begun. To isolate the lymphatic, a segment was identified and cleared of all connective and fat tissue. Extreme caution was used not to grab or pinch the vessel at any time so as to reduce the likelihood of damage. Sections of the mesenteric lymphatics 0.8–1.0 cm were used. After isolating the mesenteric lymphatic the animal was killed by injection of pentobarbitone (120 mg (kg body weight)−1i.p.).
To isolate the thoracic duct, the animal was killed by injection of pentobarbitone (120 mg (kg body weight)−1i.p.) and a midline incision was made through the ventral chest wall. The lungs and heart were carefully removed so as to expose the thoracic duct between the aorta and vertebral column. The thoracic duct was then carefully excised between the thoracic aorta and vertebral arteries all along the thoracic cavity. Sections of the thoracic duct 1–2 cm long were used.
Once thoroughly cleaned of all surrounding tissues, the lymphatic segment was cut and transferred to a chilled well (0‐4 °C), where the lymphatic was prepared using modifications of techniques originally developed in arterioles (Duling et al. 1981; Gore & Davis, 1984; Osol & Halpern, 1985; Kuo et al. 1988, 1990). The microscope stage plate used for cannulating the vessel was filled with an albumin-enriched physiological salt solution (APSS) (mm: 145.00 NaCl, 4.7 KCl, 2.0 CaCl2, 1.17 MgSO4, 1.2 NaH2PO4, 5.0 dextrose, 2.0 sodium pyruvate, 0.02 EDTA, 3.0 Mops and 10 g l−1 bovine serum albumin) and chilled to 0–4 °C. The isolated lymphatic was cannulated and tied onto two carefully matched glass pipettes (80–100 μm tip diameter for mesenteric lymphatics and 250–300 μm for thoracic duct). For these experiments it was critical to use pipettes with carefully matched resistances that were within 10 % of one another (Kuo et al. 1990). These glass pipettes were connected to independently adjustable pressure reservoirs. Once cannulated, the vessel was slowly warmed over 60 min (from 4 to 25 °C) and allowed to regain tone. Slight positive pressure (2–3 cmH2O) was applied to the vessel to detect leaks and to ensure that the vessel was undamaged and untwisted. The vessel was set to its approximate in situ length and positioned just above the glass coverslip chamber bottom. This isolated vessel preparation was then transferred to the stage of a microscope. The vessel was set to an equilibration pressure of 1 cmH2O and warmed further to 37–38 °C. Once tone and spontaneous contractions were observed, the vessel was allowed to equilibrate at 1 cmH2O for 15 min. A video camera and high-resolution monitor were used to observe the lymphatic and a video recorder used to record all experiments. The lymphatic diameters were later measured from the videotape using techniques described previously (Zawieja et al. 1991, 1999; Zawieja & Davis, 1993). Briefly, a rectangular window (typically including a length of the lymphatic 1 to 3 times its resting diameter) of the video frame was selected and digitized using a frame grabber (Imaging Technologies, Woburn, MA, USA) and the digital signal analysed for the vessel diameter using vessel wall tracking software (Diamtrak, Vollum Institute, Portland, OR, USA) (Neild, 1989). This method allowed temporal measurement of lymphatic diameters at a rate of approximately 10 to 15 times per second. This diameter record was digitized and stored on computer for further analysis. The data were collected in 3–5 min intervals at each set of inflow-outflow pressures. From the lymphatic diameter tracing we used cardiac pump analogies to define systolic and diastolic lymphatic diameters in reference to the lymphatic contractile cycle (Benoit et al. 1989; Zawieja et al. 1991). At the end of each experiment, the passive (relaxed) diameters were measured at each pressure after the vessels were exposed to a nominally calcium-free (EDTA, 3.0 mm) APSS for 15 min. The anatomy and basal contraction frequencies of lymphatic vessels in many species (rats, dogs, guinea-pigs, humans etc.) are quite variable from vessel to vessel, even when similar vessels from age-, weight- and sex-matched animals are observed (Yoffey & Courtice, 1970; Gnepp, 1984; Borisov, 1997). Thus, to study lymphatic function effectively, the lymphatic diameters were normalized to the passive diameters and frequencies to zero flow conditions. The normalization of lymphatic parameters minimizes the animal-to-animal variability in basal lymphatic parameters and allows for a more sensitive investigation of the controlled parameters. Additionally, since one of the goals of this project was to evaluate the effectiveness and sensitivity of two anatomically divergent lymph pumps, mesenteric lymphatics (≈100 μm) and thoracic duct (≈500 μm), it is necessary to normalize the diameters for the gross anatomical differences. From the lymphatic diameter tracings the following lymph pump parameters were determined: contraction frequency, ejection fraction (the part of the end-diastolic volume which was ejected during lymphatic contraction) and fractional pump flow (an index of active lymph flow calculated as the ejection fraction multiplied by the contraction frequency, producing the fractional change in lymphatic volume per minute). In the experiments to study the time dependencies of the effects of flow on the lymph pump, ejection fraction and fractional pump flow were normalized to the values seen at the start of these protocols (0 cmH2O pressure gradient conditions). Normalizations of a similar nature are commonly used in lymphatic (Zawieja et al. 1991; von der Weid & Van Helden, 1996; von der Weid et al. 1996, 2001; Hollywood et al. 1997; Mizuno et al. 1998, 1999; von der Weid, 1998; Koller et al. 1999; Shirasawa et al. 2000) and other microcirculatory studies (Kuo et al. 1988, 1990; Meininger et al. 1991; Hill & Gould, 1997; Hiramatsu et al. 1998; Guibert & Beech, 1999).
Experimental protocol
The inflow and outflow pipettes were attached to independently adjustable pressure reservoirs by three-way stopcocks. Care was taken to ensure that there were no air bubbles in the tubing or the pipettes. After the equilibration period, the vessel was again inspected for leaks. Leaky vessels were discarded. The vessels were exposed to a range of transmural pressures (at zero imposed flow) from 0 to 7 cmH2O to determine whether they had a normal response to transmural pressure for mesenteric lymphatics (Zawieja et al. 1999) or for thoracic ducts (Gashev & Zawieja, 2001). If a normal pressure response was noted, the vessels were then exposed to a range of imposed transaxial pressure gradients to control flow through the isolated lymphatic while carefully maintaining the average transmural pressure at some given level (Kuo et al. 1990). Great care was used to prepare and select sets of resistance-matched pipettes. For each experiment we chose pairs of pipettes with matching diameters and tip lengths, and tested them to ensure that the difference between their measured electrical resistances did not exceed 10 % (Kuo et al. 1990). A relatively constant transmural pressure at the different flow-pressure profiles was verified at the end of the experiments by evaluating the change in diameter of the vessels in calcium-free solution over the range of pressures and flows used. Since the vessels were very sensitive to changes in transmural pressure, it was important to maintain a constant transmural pressure at any given set of imposed flows. For studies on mesenteric lymphatics, we chose 5 cmH2O as the mean transmural pressure and a range of transaxial pressure gradients from 0 to 7 cmH2O. To produce each pressure gradient, we increased input pressure and decreased output pressure by the same amount (for example, for a transaxial pressure gradient of 1 cmH2O we set input pressure to 5.5 cmH2O and output pressure to 4.5 cmH2O). For thoracic ducts we chose 3 cmH2O as transmural pressure and a range of transaxial pressure gradients from 0 to 5 cmH2O.
In a subset of studies, the same transaxial pressure gradients, i.e. flows, were applied in both the orthograde and retrograde directions in segments of mesenteric lymphatics without valves. These experiments were designed to compare the flow-induced changes in lymphatic pumping produced by orthograde flow (+1 to +3 cmH2O transaxial pressure gradient) and retrograde flow (−1 to - 3 cmH2O transaxial pressure gradient). In another subset of studies, we applied different sequences of flows to mesenteric lymphatic segments without valves to compare the influence of rapid and slow changes in the direction of flow. For example, we compared conditions where flow was reversed immediately from +1 to −1 cmH2O gradients with conditions where there was a 5 min time interval with no flow interposed between the orthograde and retrograde flow periods.
In a separate group of experiments, we determined whether NO could produce an inhibition of the lymph pump similar to what we observed as a result of imposed flow. In these studies, we applied the NO donor (nitroprusside, 1–10 μm) abluminally to isolated mesenteric lymphatics. The vessels were set at a transmural pressure of 5 cmH2O with no imposed flow and then nitroprusside was applied at increasing concentrations. To determine whether nitric oxide was involved in the observed flow response, we treated some of the rat mesenteric lymphatics with the nitric oxide synthase inhibitor NG-monomethyl-l-arginine (l-NMMA, 10 μm) and re-evaluated the response of the lymphatics to imposed flow.
Data analysis
In the Results section, the numbers of animals used in the reported data are shown separately for each group of experiments, where n depicts the number of vessels used for each experimental protocol. The data in this paper reflect studies from 37 different animals. Since we could use only a single vessel from each animal, the number of vessels reported on and the number of animals from which these vessels were derived is the same. Each subgroup of vessels described came from separate experiments, except for the studies of the time dependencies of the flow-induced inhibitory responses of rat mesenteric lymphatics and the effects of rapid and slow changes in the directions of imposed flow on the active lymph pump. These two analyses were performed using data from one set of vessels (numbers of animals and vessels are also shown). Statistical differences were determined by ANOVA and Student's t test and considered significant at P < 0.05.
RESULTS
Effects of imposed flow on lymph pump parameters in rat mesenteric lymphatics
We evaluated the influence of imposed flow on the pump function of rat mesenteric vessels at an transmural pressure of 5 cmH2O. This is the pressure at which these lymphatics show maximal active pumping (Zawieja et al. 1999). After a period of zero imposed flow, we gradually increased the imposed transaxial pressure gradient to 1, 3, 5 and 7 cmH2O at 5 min intervals (n = 7). Data from seven animals are reported.
Figure 1 illustrates the influence of imposed flow on the contractile activity in rat mesenteric lymphatics. In this lymphatic the imposed flow decreased the frequency of contractions and substantially increased the systolic diameter. In spite of an accompanying small increase in the diastolic diameter, the amplitude of lymphatic contractions fell. Table 1 shows the changes in the absolute values of the parameters of the active lymph pump in mesenteric lymphatics during the period of highest imposed flow (7 cmH2O).
Figure 1. The effect of imposed flow on the contractions of rat mesenteric lymphatics.

A, the input and output pressures are 5 cmH2O, imposed flow gradient 0 cmH2O. B, input pressure 8.5 cmH2O, output pressure 1.5 cmH2O, imposed flow gradient 7 cmH2O.
Table 1.
The influence of imposed flow on active lymph pump parameters in rat mesenteric lymphatics and thoracic duct
| SD (μm) | DD (μm) | CF (min−1) | EF | FPF (min−1) | |
|---|---|---|---|---|---|
| Mesenteric lymphatics (n = 7) | |||||
| Control (no flow) | 63 ± 7 | 99 ± 6 | 9.0 ± 1.6 | 0.59 ± 0.05 | 5.1 ± 1.0 |
| Imposed flow (7 cmH2O) | 89 ± 8 * | 102 ± 6 | 3.1 ± 1.4 * | 0.22 ± 0.05 * | 1.0 ± 0.5 * |
| Thoracic duct (n = 9) | |||||
| Control (no flow) | 456 ± 27 | 548 ± 24 | 4.6 ± 0.6 | 0.31 ± 0.03 | 1.4 ± 0.2 |
| Imposed flow (5 cmH2O) | 562 ± 24 * | 567 ± 23 | 0.1 ± 0.1 * | 0.02 ± 0.02 * | 0.01 ± 0.01 * |
Values are means ±s.e.m. SD, systolic diameter; DD, diastolic diameter; CF, contraction frequency; EF, ejection fraction; FPF, fractional pump flow.
Significantly different from control (P < 0.05).
Figure 2 shows, in detail, the influence of imposed flow on the parameters (5 min average) of the active lymph pump in rat mesenteric lymphatics. The major changes in the lymph pump occurred when the imposed pressure gradient was increased from 0 to 3 cmH2O. During such changes in imposed flow, the diastolic or resting diameter did not significantly increase, but the systolic diameter increased dramatically (Fig. 2A). Therefore, the amplitude of the lymphatic contractions fell more than twofold between no-flow conditions and an imposed flow of 3 cmH2O. (The normalized diastolic and systolic diameters during zero imposed flow were 88 ± 1 %/ 56 ± 4 %, respectively, and at an imposed flow of 3 cmH2O they were 90 ± 1 %/76 ± 6 %, respectively.) Due to this negative inotropic effect of the imposed flow, we observed a significant drop in the ejection fraction, from 0.60 to 0.26 (Fig. 2C).
Figure 2. Effects of imposed flow on parameters of active pump in rat mesenteric lymphatics.

A, increasing flow from the imposed transaxial pressure gradient increases the lymphatic systolic diameter dramatically and results in subtle increases in diastolic diameter (diameters normalized to the passive diameter at 0 cmH2O pressure gradient condition). B, the increasing flow produces a strong inhibition of the lymphatic contraction frequency (normalized to the zero pressure gradient condition). C, the strength of the lymphatic contractions as measured by the ejection fraction is strongly attenuated by increasing flows. D, these effects combine to produce a strong inhibition of the lymph pump by increasing imposed flow. * Significant difference of parameters between no-flow conditions and the level of imposed pressure gradient (P < 0.05, n = 7).
The imposed pressure gradient of 3 cmH2O also caused a decrease in the frequency of lymphatic contractions, to 44 ± 16 % of the frequency in zero-flow conditions (Fig. 2B). This negative chronotropic effect, combined with the negative inotropic response to imposed flow, led to a strong inhibition of the active lymph pump. The fractional pump flow was 5.1 ± 0.7 min−1 at an imposed flow gradient of 0 cmH2O and fell to 1.8 ± 0.9 min−1 at 3 cmH2O (Fig. 2D).
During further increases of the imposed flow (from 3 to 7 cmH2O) flow-induced inhibition of the active lymph pump in rat mesenteric lymphatics continued. However, the differences between the parameters of the lymph pump at the imposed flows of 3 and 7 cmH2O were not as large as they were between 0 and 3 cmH2O. At an imposed flow gradient of 7 cmH2O, the frequency of contractions was 30 ± 11 % of control. At this imposed flow, the fractional pump flow was only 20 % of control (1.0 ± 0.5 min−1).
Effects of imposed flow on lymph pump parameters in the rat thoracic duct
To investigate the influence of imposed flow on the pump function of the rat thoracic duct, we performed experiments at an transmural pressure of 3 cmH2O, at which this vessel shows maximal active pumping (Gashev & Zawieja, 2001). After the period of zero imposed flow, we gradually increased the imposed transaxial pressure gradient to 1, 2, 3, 4 and 5 cmH2O at 5 min intervals (n = 9). Data from nine animals are reported in this experimental protocol.
The rat thoracic duct was more sensitive to the imposed flow than were the mesenteric lymphatics. Table 1 shows the changes in the absolute values of the parameters of the active lymph pump in thoracic duct after the period of highest imposed flow that was tested (5 cmH2O). Figure 3 shows in detail the influence of the imposed flow on the parameters (5 min average) of the active lymph pump in the rat thoracic duct. When the imposed pressure gradient was increased from 0 to 3 cmH2O, the diastolic diameter showed a small increase, while the systolic diameter increased even more, expressed as a percentage, than in mesenteric lymphatics. The amplitude of the contractions of thoracic duct fell approximately tenfold between no-flow conditions and an imposed pressure gradient of 3 cmH2O. The normalized diastolic and systolic diameters during no-flow conditions were 93 ± 1 %/77 ± 2 %, respectively, and at an imposed pressure gradient of 3 cmH2O they were 96 ± 1 %/94 ± 1 %, respectively (Fig. 3A). Due to this negative inotropic effect of the imposed flow, we observed a ninefold decrease of the ejection fraction (Fig. 3C).
Figure 3. Effects of imposed flow on parameters of active pump in rat thoracic duct.

A, a potent inhibition of the lymphatic contraction by increasing imposed flow results in increases of the lymphatic systolic diameter. Again, increasing imposed flow results in small increases in the thoracic duct diastolic diameter (diameters normalized to the passive diameter at 0 cmH2O pressure gradient condition). B, the increasing flow produces a nearly complete inhibition of the lymphatic contraction frequency (normalized to the zero pressure gradient condition). C, the thoracic duct ejection fraction is about half that of the mesenteric lymphatic pump and is very strongly attenuated by increasing flows. D, these effects combine to produce an extremely strong inhibition of the lymph pump by increasing imposed flow. * Significant difference of parameters between no-flow conditions and the level of imposed pressure gradient (P < 0.05, n = 9).
The flow resulting from an imposed pressure gradient of 3 cmH2O also caused a great decrease in the contraction frequency in thoracic duct down to a value only 6 ± 4 % of the frequency in zero-flow conditions (Fig. 3B). The combined negative inotropic and chronotropic effects produced a profound inhibition (98 %) of the thoracic duct active lymph pump. The fractional pump flow was 1.4 ± 0.2 and 0.04 ± 0.03 min−1 at imposed pressure gradients of 0 and 3 cmH2O, respectively (Fig. 3D).
Further increases in the imposed pressure gradient in the thoracic duct (from 3 to 5 cmH2O) essentially caused a complete inhibition of the active lymph pump. Specifically, at 5 cmH2O imposed flow gradient, the frequency of contractions fell by 99 % and the fractional pump flow fell by 100 % from the values at no flow.
Time dependence of the flow-induced inhibitory responses in rat mesenteric lymphatics: the influence of different directions of imposed flow on active pump parameters
For this and the next group of experiments we used vessels from seven animals. As we noted earlier, for the experiments with changes in the directions of imposed flow, we used mesenteric lymphatic segments without valves. We found that the flow-mediated responses in the active lymphatic pump were not constant but changed over time even when the imposed pressure gradient remained constant. Figure 4 shows the differences between the parameters of the active pump at no-flow conditions and at the first, third and fifth minute after the initiation of imposed pressure gradients of either +1 (n = 9) or −1 cmH2O (n = 11). The imposed flow caused an increase of the systolic diameter, i.e. a reduction in the strength of the contraction. The systolic diameter rose over time and was larger in the fifth minute of imposed flow when compared to the first minute (Fig. 4A). The diastolic diameter did not change significantly during flow (data not shown). As a result of the changes in systolic diameter, the amplitude of the lymphatic contractions decreased over time during the presence of an imposed flow gradient. This accordingly led to a decrease in the ejection fraction (Fig. 4C). Continuing to use analyses of the cardiac cycle to describe the contractile events in lymphatics, we can describe this flow-dependent inhibition of amplitude of the lymphatic contractions as a slowly developing negative inotropic response to imposed flow.
Figure 4. The temporal pattern of the parameters of active pump in rat mesenteric lymphatics during orthograde and retrograde imposed flows (+1 and −1 cmH2O imposed transaxial pressure gradients).
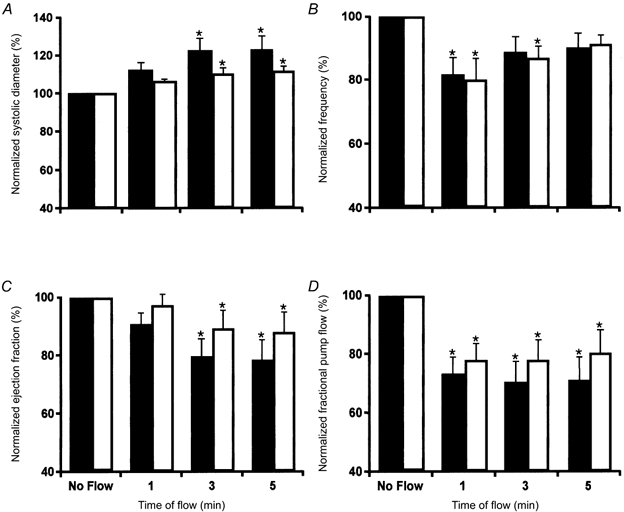
A, the normalized lymphatic systolic diameter increases slowly over 5 min after the imposition of flows of +1 cmH2O (▪, n = 9) and −1 cmH2O (□, n = 11). B, the imposition of flows produces a rapid inhibition of the lymphatic contraction frequency that slowly returns towards the values seen before the imposition of flow. C, the strength of the lymphatic contractions as measured by the ejection fraction slowly falls after the imposition of flows. D, these effects combine to produce a sustained inhibition of the lymph pump by imposed flow. * Significant difference between parameters in no-flow conditions and specified minute of imposed flow (P < 0.05). All parameters normalized to the 0 cmH2O pressure gradient condition.
The frequency of the lymphatic contractions dropped immediately after the imposed flow was applied to the vessel (Fig. 4B), but this effect subsided over time during the continued presence of the imposed pressure gradient. During the first minute of imposed flow, the frequency of lymphatic contractions was lower than at the fifth minute of imposed flow. This behaviour suggests that flow-dependent inhibition of the lymphatic contraction frequency is a rapidly developing negative chronotropic response to imposed flow. The fractional pump flow reflected the complicated combination of these two inhibitory effects of the imposed flow and was significantly decreased throughout the presence of flow (Fig. 4D).
It is important to note that the flow-induced inhibition of frequency did not depend on the direction of imposed flow (+1 versus −1 cmH2O), but the inhibition of the amplitude of contractions did appear to be dependent upon the direction of flow. Systolic diameter at the fifth minute of retrograde flow (−1 cmH2O) was smaller (though not quite significant) than that seen during the fifth minute at a flow of +1 cmH2O. Due to this, the ejection fraction and fractional pump flow were larger during retrograde flow than during orthograde flow.
A similar time dependence of active pumping parameters was seen at the higher imposed flows of +3 (n = 12) or −3 cmH2O (n = 7). Figure 5A shows that systolic diameter increased significantly during the continued presence of flow, becoming greater at the fifth minute than at the first minute of flow. The diastolic diameter did not change during the imposed flow gradients (data not shown). As a result of the decrease in amplitude of the lymphatic contractions, the ejection fraction dropped over time during the presence of imposed flow (Fig. 5C). However, there were no significant differences between the flow-dependent inhibition of amplitude of the lymphatic contractions by +1 and by +3 cmH2O flow. Thus, the slowly developing negative inotropic response during imposed flow does not appear to be dependent on the magnitude of the imposed flow gradient over the range tested.
Figure 5. The temporal pattern of the parameters of active pump in rat mesenteric lymphatics during orthograde and retrograde imposed flows (+3 and −3 cmH2O imposed transaxial pressure gradients).
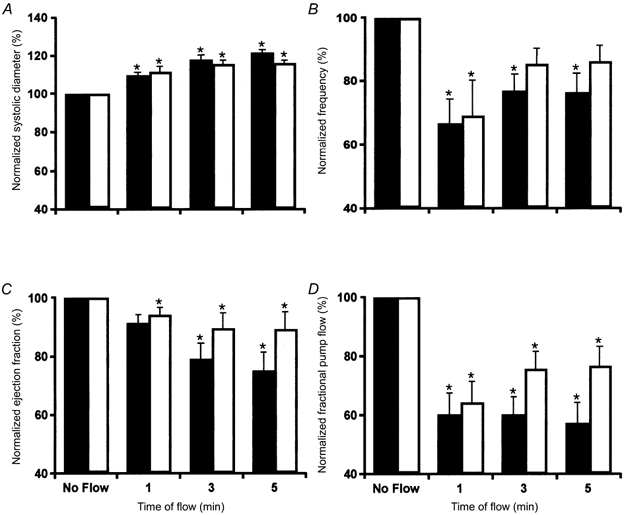
A, the normalized lymphatic systolic diameter increases over 5 min after the imposition of flows of +3 cmH2O (▪, n = 12) and −3 cmH2O (□, n = 7). B, the imposition of flows produces a rapid potent inhibition of the lymphatic contraction frequency that returns towards control values. C, the ejection fraction slowly falls after the imposition of flows. D, these effects also combine to produce a sustained inhibition of the lymph pump by imposed flow. * Significant difference between parameters in no-flow conditions and specified minute of imposed flow (P < 0.05). All parameters normalized to the 0 cmH2O pressure gradient condition.
The frequency of the lymphatic contractions dropped immediately after the imposed flow gradient of +3 cmH2O was applied (Fig. 5B). The contraction frequency then recovered over the next 5 min. The inhibition of frequency fell over time during the continued presence of the flow gradient. This is similar to what we observed during the presence of +1 cmH2O flow. However, contrary to what was observed in the flow-induced decrease in the contraction amplitude, the flow-induced inhibition of contraction frequency was about twice as great at +3 than at +1 cmH2O flow. Therefore, the rapidly developing negative chronotropic response of imposed flow appears to be dependent upon the magnitude of the imposed flow. Due to the greater decline of frequency by the imposed flow gradient of +3 cmH2O, the fractional pump flow under these conditions (Fig. 5D) was also less than that seen at +1 cmH2O flow gradient.
The flow-induced inhibition of contraction frequency was not significantly different between orthograde and retrograde flows (+3 versus −3 cmH2O). Systolic diameter at the fifth minute of flow gradient of −3 cmH2O was smaller than that at an imposed flow gradient of +3 cmH2O (although not quite significant). As a result, the ejection fraction and fractional pump flow were also higher during retrograde than during orthograde flow.
Effects of rapid and slow changes in the directions of imposed flow on the active lymph pump
As described above, we found flow-induced inhibition of the active lymph pump. In vivo, situations where the flow gradient changes direction occur often. Due to the complicated interaction of factors in vivo, fast or slow changes in the direction of flow gradient will occur (discussed in Introduction).
In these experiments we compared the reactions of rat mesenteric lymphatics to rapid or slow changes in the direction of imposed flow. To initiate a rapid change in the direction of flow, we immediately changed the imposed pressure gradient from +1 to −1 cmH2O (n = 7). To make a slow change in the direction of flow, we returned imposed flow to zero for 5 min after the initial period of +1 cmH2O flow gradient before we applied the −1 cmH2O imposed flow gradient (n = 6).
Figure 6 illustrates our findings. During fast changes in the direction of the flow gradient, we observed that the previous flow-induced inhibition of frequency was transiently eliminated. As shown in Fig. 6, on reversing the direction of flow from +1 to −1 cmH2O pressure gradient (dashed lines), the contraction frequency and, accordingly, the fractional pump flow were significantly increased immediately after this change. Later on, during the third and fifth minute after the change in the gradient direction, the retrograde flow inhibited the contraction frequency and fractional pump flow.
Figure 6. The flow-induced inhibition of contraction frequency in lymphatics depends on the speed at which the changes in the direction of flow occur.

A rapid change in the imposed flow direction temporarily ameliorates the preceding flow-mediated inhibition of the contraction frequency and fractional pump flow in rat mesenteric lymphatics. During slow changes in the flow direction, retrograde flow immediately causes a flow-mediated inhibition of parameters of the active lymph pump. (The dashed line indicates changing the imposed flow directly from the orthograde to retrograde directions (n = 7), while the continuous line indicates a period of no-flow conditions interposed between the orthograde and retrograde flows (n = 6).) All parameters are normalized to the 0 cmH2O pressure gradient condition.
During the slow change in the direction of the flow gradient (continuous lines), we interposed a 5 min period with no flow between the periods of orthograde and retrograde flow. Zero-flow conditions returned the parameters of the lymph pump back to control values. An immediate flow-induced inhibition of frequency occurred in the first minute of retrograde flow, when the vessel was exposed to a minus;1 cmH2O pressure gradient following zero-flow conditions. Fractional pump flow decreased immediately following the imposition of retrograde flow (Fig. 6B).
We also evaluated the temporal pattern of these effects at different imposed flow gradients: +3 to −3 cmH2O (n = 7); and +3 to 0 and then to −3 cmH2O (n = 7) and saw similar patterns to those described above. Additionally, we evaluated these effects with a change from retrograde to orthograde flow gradients from −1 to +1 cmH2O (n = 6) and from −1 to 0 and then to +1 cmH2O (n = 6), and with a change from −3 to +3 cmH2O (n = 7) and from −3 to 0 and then to +3 cmH2O (n = 6). We did not find any significant differences when starting with retrograde flow and compared with results of the experiments starting with the corresponding orthograde flow.
Effects of NO and the NO synthase inhibitor l-NMMA on the rat mesenteric lymph pump
Our present data indicate that the lymphatic endothelium is sensitive to shear caused by the imposed flow. It is well known that shear stimulates the blood vessel endothelium to produce NO, which can then act upon the vascular smooth muscle. Previous studies have shown that pharmacological agonists can induce the release of NO in lymphatics (Ohhashi & Takahashi, 1991; von der Weid et al. 1996) and that NO induces hyperpolarization of the lymphatic muscle cells by activation of ATP-sensitive K+ channels via cyclic GMP (von der Weid, 1998). Recently it was shown that applied NO decreases pacemaking activity in lymphatic vessels by activation of protein kinases via the cyclic GMP pathway (von der Weid et al. 2001). To determine whether NO could produce an inhibition of the lymph pump similar to what we observed as a result of imposed flow, we applied the NO donor nitroprusside (1–10 μm) abluminally to isolated mesenteric lymphatics (n = 7). Data from seven animals are reported in this experimental protocol. The vessels were set at a transmural pressure of 5 cmH2O with no imposed flow and then exposed to nitroprusside. The results of these studies are seen in Table 2. Nitroprusside (10 μm) produced significant inhibition of the contraction strength, contraction frequency, ejection fraction and fractional pump flow, although the magnitude of inhibition produced by 10 μm nitroprusside was not as great as that observed during the imposed flow. Thus, the general pattern of lymph pump inhibition produced by the exogenous NO donor, nitroprusside, was similar to that seen in response to flow. However, it was interesting to note that 10 μm nitroprusside produced an inhibition of the contraction strength (i.e. changes in diastolic diameter, systolic diameter and ejection fraction) similar to that observed at an imposed transaxial pressure gradient of 1 cmH2O, while the inhibition of contraction frequency produced by this same dose of nitroprusside (10 μm) was only about 50 % of the contraction frequency inhibition produced by an imposed transaxial pressure gradient of 1 cmH2O. Thus, it appears that applied NO can partly, but not completely, mimic the inhibition of the rat mesenteric lymph pump mediated by flow. The intriguing differences in the chronotropic influences of flow and applied NO require further investigation.
Table 2.
Effects of NO donor nitroprusside (NP) on active lymph pump parameters in rat mesenteric lymphatics
| DD (%) | SD (%) | CF (%) | EF | FPF (min−1) | |
|---|---|---|---|---|---|
| Control | 91 ± 5 | 62 ± 5 | 100 ± 0.0 | 0.54 ± 0.03 | 5.3 ± 0.8 |
| NP (10−6m) | 95 ± 6 | 67 ± 5 | 85 ± 13 * | 0.50 ± 0.02 | 4.2 ± 0.8 |
| NP (10−5m) | 97 ± 6 | 72 ± 5 * | 79 ± 13 * | 0.45 ± 0.03 * | 3.6 ± 0.8 * |
Transmural pressure of 5 cmH2O, no imposed flow. Values are means ±s.e.m. SD, normalized systolic diameter; DD, normalized diastolic diameter; CF, normalized contraction frequency; EF, ejection fraction; FPF, fractional pump flow. (Diameters are normalized to the passive diameter at 0 cmH2O pressure gradient condition; contraction frequencies are normalized to the 0 cmH2O pressure gradient condition.)
Significantly different from control (P < 0.05, n = 7).
While the nitroprusside experiments demonstrate the potential for NO to inhibit the lymph pump, they do not establish whether NO is involved in the response seen during the imposition of flow. To determine whether nitric oxide is involved in the observed flow response, we treated rat mesenteric lymphatics (n = 7) with the nitric oxide synthase inhibitor l-NMMA (10 μm) and re-evaluated the response of these lymphatics to imposed flows. This dose of l-NMMA completely inhibits flow-induced dilatation of arterioles, in which NO is the primary mechanism of dilatation (Kuo et al. 1990). Table 3 depicts the results of these studies. Seven animals were used for this experimental protocol. l-NMMA (10 μm) attenuated the inhibition by imposed flow of the contraction strength by about 35–40 % (as judged by the change in diameter during contraction and the ejection fraction), the contraction frequency by 20–30 %, and the fractional pump flow by about 15 %. While 10 μm l-NMMA did not produce complete attenuation of the flow-induced inhibition of the lymph pump, it clearly produced a significant attenuation of the effect.
Table 3.
Effects of imposed flow and the nitric oxide synthase inhibitor L-NMMA (10 μm) on active lymph pump parameters in rat mesenteric lymphatics
| Imposed flow (cmH2O) | Treatment | DD % | SD % | CF % | EF | FPF (min−1) |
|---|---|---|---|---|---|---|
| 0 | Control | 88 ± 1 | 56 ± 4 | 100 ± 0 | 0.59 ± 0.05 | 5.1 ± 0.7 |
| 0 | l-NMMA | 87 ± 2 | 54 ± 2 | 107 ± 14 | 0.61 ± 0.03 | 5.7 ± 0.4 |
| 3 | Control | 90 ± 2 | 76 ± 6 | 44 ± 16 | 0.26 ± 0.11 | 1.9 ± 0.9 |
| 3 | l-NMMA | 88 ± 2 | 67 ± 5 * | 66 ± 14 * | 0.40 ± 0.08 * | 2.4 ± 0.6 |
| 7 | Control | 91 ± 1 | 79 ± 5 | 30 ± 11 | 0.22 ± 0.09 | 1.0 ± 0.5 |
| 7 | l-NMMA | 91 ± 2 | 72 ± 4 * | 44 ± 9 * | 0.35 ± 0.08 * | 1.6 ± 0.5 |
Values are means ±s.e.m. SD, normalized systolic diameter; DD, normalized diastolic diameter; CF, normalized contraction frequency; EF, ejection fraction; FPF, fractional pump flow. (Diameters are normalized to the passive diameter at 0 cmH2O pressure gradient condition; contraction frequencies are normalized to the 0 cmH2O pressure gradient condition.)
Significantly different from control (P < 0.05, n = 7).
DISCUSSION
In this study we found significant flow-dependent inhibition of the active lymph pump in mesenteric lymphatics as well as in the thoracic duct. Imposed flow caused reductions in the frequency and amplitude of lymphatic contractions. As a result of these negative chronotropic and inotropic effects, the active pumping of lymphatics was greatly diminished. However, it is difficult to conclude that such flow-dependent inhibition of the active lymph pump decreases the total lymph flow in vivo. Because total lymph flow is the sum of passive and active flows, it is likely that the increase in imposed (or passive) flow could overwhelm any decreases in active lymph flow. A potentially important overriding factor would be an enhanced rate of lymph formation. At high levels of lymph formation, passive lymph flow could become a greater driving force to move lymph than the active lymph pump. Flow-dependent inhibition of the active lymph pump in such situations could be a reasonable physiological mechanism to save metabolic energy by temporarily decreasing or stopping contractions during the time when the lymphatic does not need it. An additional outcome of the inhibition of the lymph pump under these conditions would be a reduction in lymph outflow resistance. This reduction in outflow resistance is a result of the net increase in average lymphatic diameter that occurs when contractions are inhibited. For example, complete cessation of the mesenteric active lymph pump (at zero imposed flow and 5 cmH2O transmural pressure gradient) would result in a net increase in the time-averaged diameter by about 23 %, thus theoretically reducing resistance by approximately 56 %. This reduction in the outflow resistance could ease the removal of fluid from the affected compartment that is producing the high lymph flows and facilitate the resolution of oedema.
There are many forces and conditions that influence lymph flow. Different lymphatic beds have their own specific combinations of these forces. For example, because of their anatomic size and position, the outflow resistance of the mesenteric lymphatics is significantly higher than that in the thoracic duct, where the influence of inspiration also helps to drive lymph centripetally. Due to such regional differences we could also expect regional differences in the role of the active lymph pumps. Previous studies in our laboratory to compare the active lymph pump and the muscle cells responsible for this pump between mesenteric lymphatics and the thoracic duct in rats indicate that the thoracic duct lymph pump is weaker than that in the mesenteric lymphatics (Gashev & Zawieja, 2001). Our present findings strongly support this general idea. We found that responses to imposed flow were not the same for mesenteric lymphatics as for the thoracic duct. Even at higher imposed flows, the active pump in mesenteric lymphatics was comparatively more effective, whereas lower rates of imposed flow in the thoracic duct caused almost complete inhibition of the active pump. In other words, even at a high rate of flow generated by increased lymph formation, the mesenteric lymphatics may still need a strong active pump to overcome the high outflow resistance. In the thoracic duct under the same conditions, there is no need to apply a greater motive force to drive lymph, because the thoracic duct is in essence the final outflow path of the lymphatic circulation. In the case of a high rate of passive flow in the thoracic duct, passive driving forces such as the suction effect of inspiration and the influence of low or negative pressures in the central veins could be enough to drive lymph centripetally and there may be no need to develop additional force by active contractions of the duct. Indeed, active pumping would only increase the outflow resistance of the lymphatic circulation as described above.
The time-dependent analysis of experiments with increased flow shows that the flow-induced inhibition of the lymph pump is a complex process including different temporal patterns of inhibition for different contractile characteristics. One pattern is a rapidly developing inhibition of lymphatic contraction frequency, which diminishes over time even during the continued presence of flow. The inhibition of contraction frequency is dependent on the magnitude of the imposed flow, but does not depend upon the direction of flow. A second pattern is a more slowly developing inhibition of the amplitude of lymphatic contractions. This inhibition increases over time during the continued presence of flow and is dependent upon the direction of flow, but is independent of the magnitude of the imposed flows studied.
Therefore, the chronotropic and inotropic flow-induced inhibitory responses appear somewhat different. In the first minute of the initiation of flow, the lymphatic response to flow occurs primarily through a rapid inhibition of the contraction frequency. In vivo, short periods of increased flow occur very often due to the contractions of upstream lymphangions. It is possible that a fast chronotropic response of lymphatics is an important short-term regulatory reaction to rapid but short-lasting periods of increased flow. At high rates of lymph formation, which can be present in the mesenteric lymphatic bed in vivo, long periods of increased flow may occur. The slow inotropic effect, which develops in lymphatics in minutes, could be an important long-term regulatory reaction to slow but long-lasting periods of increased flow. This slowly developing flow-induced inhibition of lymphatic contractility could conserve energy in lymphatics when there are sufficient passive forces to move lymph without the active lymph pump and decrease local outflow resistance. In this context, we should emphasize one important detail. In experiments with imposed flow gradients, the slowly developing negative inotropic effect caused an increase in the average diameter of lymphatics over the time period of the imposed pressure gradient. This diameter increase was not large, but it was enough to cause a slow reduction in the resistance of the lymphatics. The decrease in the vessel resistance will lead to a slow rise in flow during the continuous imposed pressure gradient. So theoretically, in any experiments with fixed imposed pressure gradients, the slow increase in average diameter we observed will produce a slow rise in flow and shear rate through the vessel. Despite this slow, subtle rise in flow and shear rate during our imposed pressure gradients, the lymphatic contraction frequency fell quickly and then slowly returned back towards normal. Additionally, flow-induced inhibition of contraction frequency did not depend on the direction of flow, whereas flow-induced inhibition of the amplitude of lymphatic contractions did. Long-term inhibition of lymphatic contractility was stronger during orthograde flow, which occurs in lymphatics more often in comparison with long periods of retrograde flow, which rarely occur because of the competency of the lymphatic valves. This evidence leads us to speculate that the mechanisms of the rapidly developing chronotropic and the slowly developing inotropic lymphatic responses to flow could be different. Currently, there are insufficient data to allow understanding whether different mechanisms are indeed responsible for flow-induced rapidly developing inhibition of lymphatic contraction frequency and the slowly developing inhibition of the amplitude of lymphatic contractions. Future studies are needed in this area to evaluate the mechanisms responsible.
Our finding that retrograde flow caused a fast chronotropic inhibition could be important for a better understanding of the lymphatic contractile cycle. Normally, contraction of the upstream lymphangion would produce a relatively high local flow, leading to the filling of the downstream lymphangion. However, at the beginning of systole of the downstream lymphangion, lymph moves in the retrograde direction due to the local pressure gradients and closes the input valve in the lymphangion (McHale & Roddie, 1976; Gashev, 1991). The inhibitory effect of retrograde flow described above only occurs during slow changes in the direction of flow. It would not happen during the rapid changes in flow direction that occur during the normal lymphatic contractile cycle. The rapid changes in the direction of flow from orthograde to retrograde would cause a short, partial block of flow-induced inhibition. These situations frequently occur in lymphatics. We propose that the fast temporal block is a physiological mechanism to prevent unneeded inhibition of lymphatic contractions during regular pumping activity.
In numerous studies on isolated lymphatics performed by us and others (Mawhinney & Roddie, 1973; McHale & Roddie, 1976; Kirkpatrick & McHale, 1977; Orlov & Lobacheva, 1977; Ohhashi et al. 1980; Johnston & Gordon, 1981; Gashev et al. 1990; Orlov et al. 1991; Zawieja & Davis, 1993; Zawieja et al. 1993), experiments without imposed flow were very useful to investigate the separate effects of transmural pressure, pharmacological agents etc. on lymphatic pumping. But of course these conditions are somewhat artificial because it is very difficult to imagine lymphatics in vivo without any lymph flow for an extended period of time. The actions of the different external driving forces and intrinsic contractile activity of lymphatics constantly change and do not always support unidirectional lymph flow. Our findings that lymphatics react differently to slow or rapid changes in the direction of flow also help us to describe lymph flow as the complicated net sum of local orthograde and retrograde flows that are the result of different forces, which finally drive lymph centripetally.
At this time, there is not enough information to completely define the mechanisms of flow-induced inhibition of the lymph pump. However, the results of our nitroprusside and l-NMMA studies, as well as previous work by others (von der Weid, 1998; von der Weid et al. 2001), indicate that NO is clearly involved in this mechanism. The NO donor, nitroprusside, simulated the general effects of flow on the lymph pump, although the maximum inhibition produced by nitroprusside (10 μm) did not reach the maximum inhibition produced by imposed flow. However, the inhibition of contraction frequency by nitroprusside was not as strong as the inhibition of the strength of contraction when compared to levels of inhibition produced by imposed flows. Thus it appears that applied NO can only partly mimic the inhibition of the rat mesenteric lymph pump mediated by flow. Blocking the production of NO during flow using l-NMMA (10 μm) attenuated the inhibition of the lymph pump produced by imposed flow. The attenuation of the flow-induced inhibition by l-NMMA of the contraction strength (35–40 %) was greater than attenuation of the flow-induced inhibition by l-NMMA of the contraction frequency (20–30 %). Thus while it appears that NO is involved in the flow-mediated inhibition of the lymph pump, there are differences in the apparent effects of NO versus imposed flow on chronotropic and inotropic aspects of the lymph pump that require further investigation. Such studies would provide valuable information regarding the detailed mechanisms involved in the flow-induced inhibition of the lymph pump.
Our results agree in part with those seen in isolated rat iliac microlymphatics (Koller et al. 1999). These authors reported decreases in both systolic and diastolic diameter as well as reductions in the amplitude of the contractions as a result of increases in imposed flow. However, in that study the authors described an increase in the contraction frequency as a result of the increase in flow rate, opposite to what we have observed in both the rat mesenteric lymphatics and the thoracic duct. The reasons for the differences between their observations and ours are unclear. Since we have shown strong quantitative differences between characteristics of the lymph pump in rat mesenteric lymphatics and rat thoracic duct, it is possible that there are more profound differences between the lymphatics of the iliac tissue and other regions.
In summary, imposed flow induces inhibition of the active lymph pump in rat mesenteric lymphatics and thoracic duct. The active pump of the thoracic duct appears much more sensitive to flow than does the active pump of the mesenteric lymphatics. Imposed flow causes a decrease in both frequency and amplitude of contractions and, accordingly, in active pump flow. The flow-induced inhibition of the active lymph pump includes two patterns. One pattern is a rapidly developing inhibition of contraction frequency, which falls over time during the continued presence of flow and is dependent on the magnitude but not the direction of imposed flow. The other pattern is a slowly developing inhibition of the amplitude of the lymphatic contractions, which increases over time during the continued presence of flow and depends on the direction but not the magnitude of the imposed flow. Additionally, the flow-induced inhibition of contraction frequency in lymphatics is dependent upon how fast the change in the direction of flow occurs. The precise mechanisms by which this flow-mediated inhibition of the lymph pumps occurs are not clearly understood, although it appears that NO is involved.
Acknowledgments
This study was supported by National Institutes of Health grants HL-21498 and HL-07688, and by National Space Biomedical Research Institute grant NCC9-58-42 CA00209. The authors thank Eduard Kossmann and John Pullin for their assistance in these studies.
REFERENCES
- Benoit JN, Zawieja DC, Goodman AH, Granger HJ. Characterization of intact mesenteric lymphatic pump and its responsiveness to acute edemagenic stress. American Journal of Physiology. 1989;257:H2059–2069. doi: 10.1152/ajpheart.1989.257.6.H2059. [DOI] [PubMed] [Google Scholar]
- Borisov AV. The theory of the design of the lymphangion. Morfologiia. 1997;112:7–17. (in Russian) [PubMed] [Google Scholar]
- Duling BR, Gore RW, Dacey RG, Jr, Damon DN. Methods for isolation, cannulation, and in vitro study of single microvessels. American Journal of Physiology. 1981;241:H108–116. doi: 10.1152/ajpheart.1981.241.1.H108. [DOI] [PubMed] [Google Scholar]
- Gashev AA. The pump function of the lymphangion and the effect on it of different hydrostatic conditions. Fiziologicheskii Zhurnal SSSR Imeni I. M. Sechenova. 1989;75:1737–1743. (in Russian) [PubMed] [Google Scholar]
- Gashev AA. The mechanism of the formation of a reverse fluid filling in the lymphangions. Fiziologicheskii Zhurnal SSSR Imeni I. M. Sechenova. 1991;77:63–69. (in Russian) [PubMed] [Google Scholar]
- Gashev AA, Orlov RS, Borisov AV, Kliuchin'ski T, Andreevskaya MV, Bubnova NA, Borisova RP, Andreev YA, Erofeev NP, Priklonskaya EG. The mechanisms of lymphangion interaction in the process of the lymph movement. Fiziologicheskii Zhurnal SSSR Imeni I. M. Sechenova. 1990;76:1489–508. (in Russian) [PubMed] [Google Scholar]
- Gashev AA, Zawieja DC. 7th World Congress for Microcirculation. Sydney, Australia: Australian and New Zealand Microcirculation Society; 2001. Comparison of the active lymph pumps of the rat thoracic duct and mesenteric lymphatics; pp. 1–19. [Google Scholar]
- Gnepp DR. Lymphatics. In: Staub NC, Taylor AE, editors. Edema. New York, NY, USA: Raven Press; 1984. pp. 263–298. [Google Scholar]
- Gore RW, Davis MJ. Mechanics of smooth muscle in isolated single microvessels. Annals of Biomedical Engineering. 1984;12:511–520. doi: 10.1007/BF02363920. [DOI] [PubMed] [Google Scholar]
- Guibert C, Beech DJ. Positive and negative coupling of the endothelin ETA receptor to Ca2+-permeable channels in rabbit cerebral cortex arterioles. Journal of Physiology. 1999;514:843–856. doi: 10.1111/j.1469-7793.1999.843ad.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hargens AR, Zweifach BW. Contractile stimuli in collecting lymph vessels. American Journal of Physiology. 1977;233:H57–65. doi: 10.1152/ajpheart.1977.233.1.H57. [DOI] [PubMed] [Google Scholar]
- Hill CE, Gould DJ. Pathway-specific effects of calcitonin gene-related peptide on irideal arterioles of the rat. Journal of Physiology. 1997;505:797–809. doi: 10.1111/j.1469-7793.1997.797ba.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiramatsu O, Goto M, Yada T, Kimura A, Chiba Y, Tachibana H, Ogasawara Y, Tsujioka K, Kajiya F. In vivo observations of the intramural arterioles and venules in beating canine hearts. Journal of Physiology. 1998;509:619–628. doi: 10.1111/j.1469-7793.1998.619bn.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollywood MA, Cotton KD, Thornbury KD, McHale NG. Tetrodotoxin-sensitive sodium current in sheep lymphatic smooth muscle. Journal of Physiology. 1997;503:13–20. doi: 10.1111/j.1469-7793.1997.013bi.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston MG, Gordon JL. Regulation of lymphatic contractility by arachidonate metabolites. Nature. 1981;293:294–297. doi: 10.1038/293294a0. [DOI] [PubMed] [Google Scholar]
- Kirkpatrick CT, McHale NG. Electrical and mechanical activity of isolated lymphatic vessels. Journal of Physiology. 1977;272:33P–34P. [PubMed] [Google Scholar]
- Koller A, Mizuno R, Kaley G. Flow reduces the amplitude and increases the frequency of lymphatic vasomotion: role of endothelial prostanoids. American Journal of Physiology. 1999;277:R1683–1689. doi: 10.1152/ajpregu.1999.277.6.R1683. [DOI] [PubMed] [Google Scholar]
- Kuo L, Chilian WM, Davis MJ. Coronary arteriolar myogenic response is independent of endothelium. Circulation Research. 1990;66:860–866. doi: 10.1161/01.res.66.3.860. [DOI] [PubMed] [Google Scholar]
- Kuo L, Davis MJ, Chilian WM. Myogenic activity in isolated subepicardial and subendocardial coronary arterioles. American Journal of Physiology. 1988;255:H1558–1562. doi: 10.1152/ajpheart.1988.255.6.H1558. [DOI] [PubMed] [Google Scholar]
- Kuo L, Davis MJ, Chilian WM. Endothelium-dependent, flow-induced dilation of isolated coronary arterioles. American Journal of Physiology. 1990;259:H1063–1070. doi: 10.1152/ajpheart.1990.259.4.H1063. [DOI] [PubMed] [Google Scholar]
- Mawhinney HJ, Roddie IC. Spontaneous activity in isolated bovine mesenteric lymphatics. Journal of Physiology. 1973;229:339–348. doi: 10.1113/jphysiol.1973.sp010141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McHale NG, Roddie IC. The effect of transmural pressure on pumping activity in isolated bovine lymphatic vessels. Journal of Physiology. 1976;261:255–269. doi: 10.1113/jphysiol.1976.sp011557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meininger GA, Zawieja DC, Falcone JC, Hill MA, Davey JP. Calcium measurement in isolated arterioles during myogenic and agonist stimulation. American Journal of Physiology. 1991;261:H950–959. doi: 10.1152/ajpheart.1991.261.3.H950. [DOI] [PubMed] [Google Scholar]
- Mizuno R, Koller A, Kaley G. Regulation of the vasomotor activity of lymph microvessels by nitric oxide and prostaglandins. American Journal of Physiology. 1998;274:R790–796. doi: 10.1152/ajpregu.1998.274.3.R790. [DOI] [PubMed] [Google Scholar]
- Mizuno R, Ono N, Ohhashi T. Involvement of ATP-sensitive K(+) channels in spontaneous activity of isolated lymph microvessels in rats. American Journal of Physiology. 1999;277:H1453–1456. doi: 10.1152/ajpheart.1999.277.4.H1453. [DOI] [PubMed] [Google Scholar]
- Neild TO. Measurement of arteriole diameter changes by analysis of television images. Blood Vessels. 1989;26:48–52. [PubMed] [Google Scholar]
- Ohhashi T, Azuma T, Sakaguchi M. Active and passive mechanical characteristics of bovine mesenteric lymphatics. American Journal of Physiology. 1980;239:H88–95. doi: 10.1152/ajpheart.1980.239.1.H88. [DOI] [PubMed] [Google Scholar]
- Ohhashi T, Takahashi N. Acetylcholine-induced release of endothelium-derived relaxing factor from lymphatic endothelial cells. American Journal of Physiology. 1991;260:H1172–1178. doi: 10.1152/ajpheart.1991.260.4.H1172. [DOI] [PubMed] [Google Scholar]
- Olszewski WL, Engeset A. Intrinsic contractility of prenodal lymph vessels and lymph flow in human leg. American Journal of Physiology. 1980;239:H775–783. doi: 10.1152/ajpheart.1980.239.6.H775. [DOI] [PubMed] [Google Scholar]
- Orlov RS, Borisova RP, Bubnova NA, Gashev AA, Erofeev NP, Lobov GI, Pan'kova MN, Petunov SG. The lymphatic vessels: their tonus, motility and regulation. Fiziologicheskii Zhurnal SSSR Imeni I. M. Sechenova. 1991;77:140–149. (in Russian) [PubMed] [Google Scholar]
- Orlov RS, Lobacheva TA. Intravascular pressure and spontaneous lymph vessels contractions. Bulletin of Experimental Biology and Medicine. 1977;83:392–394. (in Russian) [PubMed] [Google Scholar]
- Osol G, Halpern W. Myogenic properties of cerebral blood vessels from normotensive and hypertensive rats. American Journal of Physiology. 1985;249:H914–921. doi: 10.1152/ajpheart.1985.249.5.H914. [DOI] [PubMed] [Google Scholar]
- Shirasawa Y, Ikomi F, Ohhashi T. Physiological roles of endogenous nitric oxide in lymphatic pump activity of rat mesentery in vivo. American Journal of Physiology. 2000;278:G551–556. doi: 10.1152/ajpgi.2000.278.4.G551. [DOI] [PubMed] [Google Scholar]
- von der Weid PY. ATP-sensitive K+ channels in smooth muscle cells of guinea-pig mesenteric lymphatics: role in nitric oxide and beta-adrenoceptor agonist-induced hyperpolarizations. British Journal of Pharmacology. 1998;125:17–22. doi: 10.1038/sj.bjp.0702026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von der Weid PY, Crowe MJ, Van Helden DF. Endothelium-dependent modulation of pacemaking in lymphatic vessels of the guinea-pig mesentery. Journal of Physiology. 1996;493:563–575. doi: 10.1113/jphysiol.1996.sp021404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von der Weid PY, Van Helden DF. Beta-adrenoceptor-mediated hyperpolarization in lymphatic smooth muscle of guinea pig mesentery. American Journal of Physiology. 1996;270:H1687–1695. doi: 10.1152/ajpheart.1996.270.5.H1687. [DOI] [PubMed] [Google Scholar]
- von der Weid PY, Zhao J, Van Helden DF. Nitric oxide decreases pacemaker activity in lymphatic vessels of guinea pig mesentery. American Journal of Physiology. 2001;280:H2707–2716. doi: 10.1152/ajpheart.2001.280.6.H2707. [DOI] [PubMed] [Google Scholar]
- Yoffey JM, Courtice FC. Lymphatics, Lymph and the Lymphomyeloid Complex. London, New York: Academic Press; 1970. p. 924. [Google Scholar]
- Zawieja DC, Davis KL. Inhibition of the active lymph pump in rat mesenteric lymphatics by hydrogen peroxide. Lymphology. 1993;26:135–142. [PubMed] [Google Scholar]
- Zawieja DC, Davis KL, Schuster R, Hinds WM, Granger HJ. Distribution, propagation, and coordination of contractile activity in lymphatics. American Journal of Physiology. 1993;264:H1283–1291. doi: 10.1152/ajpheart.1993.264.4.H1283. [DOI] [PubMed] [Google Scholar]
- Zawieja DC, Greiner ST, Davis KL, Hinds WM, Granger HJ. Reactive oxygen metabolites inhibit spontaneous lymphatic contractions. American Journal of Physiology. 1991;260:H1935–1943. doi: 10.1152/ajpheart.1991.260.6.H1935. [DOI] [PubMed] [Google Scholar]
- Zawieja DC, Kossman E, Pullin J. Dynamics of the microlymphatic system. Journal of Progress in Applied Microcirculation. 1999;23:100–109. [Google Scholar]


