Abstract
In this study we have investigated mechanisms underlying enhancement by group II metabotropic glutamate (mGlu) receptors of group I mGlu receptor-induced calcium mobilization. Inhibition of protein kinase A (PKA) caused an enhancement of mGlu5 receptor-mediated calcium mobilization and occluded the enhancement by group II mGlu receptors. A peptide (Ht31) that prevents interaction between A-kinase anchoring protein (AKAP) and PKA also enhanced mGlu5-mediated calcium mobilization. Enhancement of mGlu5 function, by inhibition of PKA or by activation of group II mGlu receptors, was prevented by the protein phosphatase 2B (PP2B) inhibitor cyclosporin A. Furthermore, the enhancement by activation of group II mGlu receptors was prevented by raising intracellular cAMP. These results suggest that the regulation by PKA and PP2B of phosphorylation of a substrate on mGlu5 and/or on group II mGlu receptors is intimately involved in the mechanisms underlying interaction between group II mGlu and mGlu5 receptors. Long-term depression (LTD) in perirhinal cortex requires group I, group II and NMDA receptor activation at resting membrane potentials but does not require group II mGlu receptor activation at depolarized potentials. We previously suggested that interaction between group I and group II mGlu receptors is required for induction of LTD at resting potentials. In support of this, we demonstrate in perirhinal cortex slices that blocking mechanisms underlying mGlu receptor synergy (by raising intracellular cAMP or by inhibition of PP2B) selectively prevented LTD at resting membrane potentials. This study thus provides a potential explanation for the co-requirement in LTD of group I and group II mGlu receptor activation. Similar mechanisms of synergistic interaction may also be important in other physiological processes dependent on mGlu receptors.
Glutamate is the primary excitatory transmitter in the CNS and acts via ionotropic and metabotropic glutamate receptors (mGlu receptors). Ionotropic receptors couple directly to ion channels and mediate fast synaptic transmission. Metabotropic receptors couple to G-proteins and modulate intracellular biochemical cascades. Within each of the ionotropic and metabotropic receptor groups there is a variety of different receptor types and various different functions have been attributed to each receptor subtype (for reviews see Conn & Pin, 1997; Anwyl, 1999). Briefly, metabotropic glutamate receptors are divided into groups I, II and III. Group I receptors (mGlu 1,5) couple to phosphoinositide signalling whilst groups II (mGlu 2,3) and III (mGlu 4,6,7,8) negatively couple to forskolin-stimulated adenylyl cyclase.
In addition to the direct signalling mechanisms attributed to each ionotropic glutamate and mGlu receptor subtype there is an added level of complexity in that ionotropic and metabotropic glutamate receptor subtypes can interact with and modulate the function of one another (Anwyl, 1999). For example, NMDA receptor activation can enhance mGlu receptor function (Challiss et al. 1994; Luthi et al. 1994; Algarsamy et al. 1999) and vice versa (Aniksztejn et al. 1992; Harvey et al. 1993). Interestingly, there is also evidence that different metabotropic glutamate receptors can interact with and modulate one another. For example, Group II mGlu receptors can enhance phosphoinositide turnover that results from group I mGlu receptor function and group I mGlu receptor activation can enhance the function of group II mGlu receptors which can result in an increase in cAMP (Nicoletti et al. 1993; Schoepp et al. 1996; Mistry et al. 1998; Cho et al. 2000). However, there is very little known about the possible mechanisms underlying, or the functions of, interactions between these different mGlu receptors.
Group I and group II mGlu receptors are present in many brain regions and are involved in the regulation of synaptic transmission, plasticity and neurodegeneration (Anwyl, 1999). Therefore an understanding of the mechanisms of interaction between mGlu receptors has implications for furthering understanding of synaptic physiology. In a recent study we demonstrated that the induction of activity-dependent LTD in perirhinal cortex relies on the activation of NMDA, group I and group II mGlu receptors at resting membrane potentials but not depolarized potentials (Cho et al. 2000). Furthermore, we showed that group II mGlu receptor activation enhanced group I receptor-mediated calcium mobilization (Cho et al. 2000).
In the present study we have examined potential mechanisms by which group II mGlu receptors enhance group I mGlu receptor function. We present evidence that the interaction between these receptors occurs through protein phosphatase 2B (PP2B)-dependent dephosphorylation in an A kinase anchoring protein (AKAP)-dependent manner. However, the precise substrates that undergo (de)phosphorylation remain to be determined. Interestingly, disruption of the mechanisms of mGlu receptor synergy prevents the induction of LTD under conditions where this normally relies on co-activation of group I and group II mGlu receptors. Thus this study provides an understanding of mechanisms underlying cross-talk between mGlu receptors and of the potential physiological role of synergy between mGlu receptors.
METHODS
Cell culture
Perirhinal cultures were prepared according to methods previously described (Cho et al. 2000). All efforts were made to minimize animal suffering and the number of animals used. Briefly, 3–5-day-old rats were killed by cervical dislocation and decapitated. The perirhinal cortex was dissected out and neurones recovered by enzymatic digestion (trypsin and DNase) and mechanical dissociation. Cells were plated onto coverslips in 35 mm Petri dishes. Cultures were maintained at 37 °C in a 95 % O2-5 % CO2-humidified incubator. The culture media comprised Minimal Essential Medium (Gibco): glucose, 30 mm; glutamine, 2 mm; Hepes, 15 mm; bovine transferrin, 100 μg ml−1 and insulin, 30 μg ml−1, complemented with fetal calf serum (5–10 %). From the second day in culture, the media were supplemented with cytosine-β-d-arabinofuranoside (2.5 μm; Sigma). Neurones were used for calcium imaging studies 12–25 days after plating.
Calcium imaging
Imaging techniques were used as described previously (Doherty et al. 1997; Cho et al. 2000). Briefly, cells were washed three times in Hepes-buffered saline (HBS) (NaCl, 119 mm; KCl, 5 mm; Hepes, 25 mm; glucose, 33 mm; CaCl2, 2 mm; MgCl2, 2 mm; TTX 500 nm; glycine, 1 μm; picrotoxin, 100 μm; pH 7.4; osmolarity 300–310 mosmol l−1) and loaded with 5 μm of the membrane-permeable Ca 2+ indicator fluo-3-AM made up in 1 mg ml−1 bovine serum albumin-HBS at 37 °C for 30 min. Cells were then washed three times in HBS and incubated for 20 min in a 5 % CO2 atmosphere at 22 °C in order to allow for the de-esterification of the flurophore. Cells were viewed on a BioRad MRC600 confocal microscope equipped with an argon ion laser using standard green filter sets and perfused continuously at ∼2 ml min−1 with HBS buffer to which AP5 (50 μm) was added. Integrations of five individual images were obtained every 10 s before, during and after agonist application. The fluorescence of individual cells in each preparation was measured using the public domain NIH program (http://rsb.info.nih.gov/nihimage/) and expressed relative to baseline. The mean peak fluorescence was then calculated. In some experiments glial cells were also visualized. These cells did not respond reliably or consistently to bath application of mGlu receptor agonists. All neurones that initially responded to (RS)-3,5-dihydroxyphenylglycine (DHPG), 2-chloro-5-hydroxyphenylglycine (CHPG) or carbachol (as appropriate) with an increase in fluorescence were then used in analysis. Group I mGlu receptor agonists were applied for 1 min (20–30 min between each application). Several such applications did not produce any noticeable run-down in mGlu receptor calcium mobilization (data not shown). Drugs used were the N-methyl-d-aspartate (NMDA) receptor antagonist d-2-amino-5-phosphonopentanoate (AP5), the cholinergic agonist carbachol, the metabotropic glutamate (mGlu) receptor antagonist MPEP, the mGlu receptor agonists DHPG, CHPG and (2S,2′R,3′R)-2-(2′,3′-dicarboxycyclopropyl)glycine (DCG-IV), the PKA activators forskolin and 8-bromo-cAMP, the PKA inhibitor KT5720, the protein phosphatase inhibitors okadaic acid and cyclosporin A (all from Tocris, Bristol, UK), and the human thyroid A-kinase anchoring proteins Ht31 and Ht31p (in 50 mm Tris-HCl; from Promega, Madison, USA). Ht31 had the following sequence: N-Stearate-DLIEEAASRIVDAVIEQVKAAGAY; Ht31p was identical except for substitution of I(18) by P.
Electrophysiology
Slices of perirhinal cortex were prepared from adult male DA rats (150–270 g, 7-–2 weeks, Bantin and Kingman, UK). All efforts were made to minimize animal suffering and the number of animals used. Animals were anaesthetized with halothane, decapitated in accordance with the UK Animals (Scientific Procedures) Act 1986, the brain rapidly removed and placed in ice-cold artificial cerebrospinal fluid (aCSF; bubbled with 95 % O2-5 % CO2) which comprised (mm): NaCl, 124; KCl, 3; NaHCO3, 26; NaH2PO4, 1.25; CaCl2, 2; MgSO4, 1; d-glucose, 10. A mid-sagittal section was made and the rostral and caudal parts were removed by single scalpel cuts made at approximately 45 deg to the dorso-ventral axis. Each half was then glued by its caudal end to a vibroslice stage (Campden Instruments, Sileby, UK). Slices (400 μm) which included perirhinal, entorhinal and temporal cortices were stored submerged in aCSF (20–25 °C). A single slice was placed in a submerged recording chamber (28–30 °C, flow rate ∼2 ml min−1) when required. In experiments testing effects of cyclosporin A, the slices were stored in cyclosporin A (10 μm)-containing medium. Picrotoxin (5 μm) (and cyclosporin A, if appropriate) was then perfused for the duration of the experiment. Blind whole cell recordings were obtained from neurones in layer II/III. Pipette (4–7 MΩ) solutions (280 mosmol l−1, pH 7.2) comprised (mm): CsMeSO4, 130; NaCl, 8; Mg-ATP, 4; Na-GTP, 0.3; EGTA, 0.5; Hepes 10; QX-314, 6. In some experiments 3′,5′-monophosphothioate (sp-cAMP) a nonhydrolysable analogue of cAMP was added to the pipette solution (20 μm; Calbiochem). One stimulating electrode was placed dorso-rostrally on the temporal cortex side (area 35/36) and one ventro-caudally on the entorhinal cortex side (area 35/entorhinal cortex) of the rhinal sulcus. Stimuli (constant voltage) were delivered alternately to the two electrodes (each electrode 0.033 Hz). Neurones were voltage clamped at −70 mV unless otherwise indicated. Low frequency stimulation (LFS): 200 stimuli, 1 Hz) was delivered at −70 mV or paired with depolarization to −40 mV, as appropriate. Where the membrane potential was changed this was done for the duration of LFS only. The amplitude of the evoked EPSCs was measured and expressed relative to the normalized pre-conditioning baseline. Effects of LFS were measured at appropriate time points (averaged over a 5 min period) after delivering LFS. Data were only analysed from one slice per rat (number = n), unless otherwise indicated. Data pooled across slices are expressed as the mean ± s.e.m. and significance (P < 0.05) tested using either paired or unpaired t tests as appropriate. Data were recorded using an Axopatch 200 amplifier (Axon Instruments, Foster City, CA, USA), monitored and analysed on-line and re-analysed off-line (Anderson & Collingridge, 1997).
RESULTS
We have previously reported (Cho et al. 2000) that application of the group I mGlu receptor agonist DHPG results in an increase in intracellular calcium that is significantly enhanced by co-application of the selective group II mGlu receptor agonist DCG-IV (Ishida et al. 1993). To determine whether mGlu5 and mGlu1 receptors are both similarly enhanced by group II mGlu receptor activation, we now demonstrate that application of the mGlu5 selective agonist CHPG (1 mm; Doherty et al. 1997) also significantly increases fluorescence of fluo-3 filled neurones cultured from perirhinal cortex (Fig. 1). Furthermore, the mGlu5 receptor-mediated mobilization of intracellular calcium was significantly enhanced (P < 0.05) by the co-activation of group II mGlu receptors with 1 μm DCG-IV (CHPG: 140 ± 4 %; DCG-IV + CHPG: 164 ± 5 %, n = 40 cells from 5 dishes; Fig. 1A–C).
Figure 1. Enhancement of mGlu5 receptor function by co-activation of group II mGlu receptors.
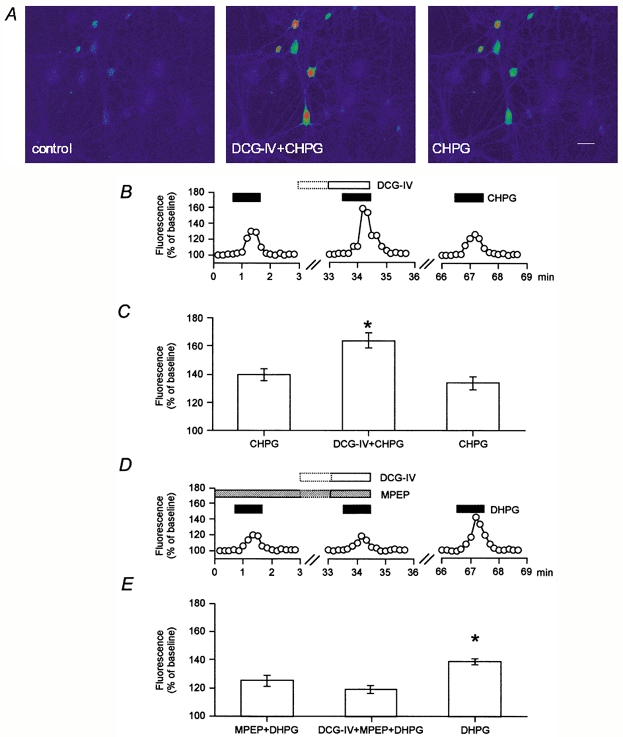
A-C, illustration of a field of cultured perirhinal cortex neurones (A), a single analysed cell (B) and pooled data from 40 cells (C) showing that application of the mGlu5 receptor selective agonist CHPG (1 mm) produced an increase in intracellular calcium, as assessed by the increase in fluorescence compared to baseline. The mGlu5 receptor-induced increase in intracellular calcium was further enhanced (P < 0.05) by co-activation of group II mGlu receptors by DCG-IV (1 μm), in a reversible manner. Scale bar in A, 30 μm. *Significant difference from the first CHPG response. D and E, single example (D) and pooled data (35 cells; E) illustrating that the group I mGlu receptor agonist DHPG (20 μm), in the presence of the mGlu5 receptor selective antagonist MPEP (0.5 μm), produced an mGlu1 receptor-dependent increase in intracellular calcium that was not enhanced further by the co-application of DCG-IV (1 μm). mGlu5 and mGlu1 receptor activation by DHPG produced a bigger increase in intracellular calcium than activation of mGlu1 alone. *Significantly different from DCG-IV + MPEP + DHPG.
Selective activation of mGlu1 receptors (by 20 μm DHPG in the presence of 0.5 μm of the selective mGlu5 receptor antagonist MPEP; Gasparini et al. 1999; Salt et al. 1999) also produced an increase in intracellular calcium (125 ± 4 %, n = 35 cells from 4 dishes; Fig 1D, E). However, calcium mobilization following activation of mGlu1 receptors was not enhanced by co-activation of group II mGlu receptors (120 ± 3 %; Fig. 1D, E). To test whether the activation of group II mGlu receptors interacts with other PLC-coupled receptors, carbachol (50 μm) was bath-applied and this also produced an increase in intracellular calcium (142 ± 3 % n = 15 cells from 3 dishes). However, calcium mobilization resulting from muscarinic receptor activation was not significantly enhanced by co-activation of group II mGlu receptors (DCG-IV + carbachol: 145 ± 3 %; data not shown). Therefore under the conditions of our experiments, group II mGlu receptor activation selectively enhances mGlu5 but not mGlu1 or muscarinic receptor-induced calcium mobilization. Because of the selectivity of interaction between mGlu5 and group II mGlu receptors, most subsequent experiments were carried out using the group I mGlu receptor agonist DHPG.
Since group II mGlu receptors negatively couple to forskolin-stimulated adenylyl cyclase, we tested whether the phosphorylation state of protein kinase A (PKA) may regulate the enhancement of mGlu5 receptor function. Therefore, we tested effects of cAMP activators on mGlu5 receptor signalling and on the synergy with group II mGlu receptors. Increasing cAMP levels, by application of either forskolin (50 μm) or 8-bromo-cAMP (5 μm), prevented the DCG-IV-induced enhancement of mGlu5 receptor-mediated calcium signalling (DCG-IV + DHPG + forskolin: 127 ± 5 %; DCG-IV + DHPG: 153 ± 5 %, n = 13 cells from 3 dishes, data not shown. DCG-IV + DHPG + 8-Br-cAMP: 123 ± 5 %; DCG-IV + DHPG: 161 ± 6 %; n = 20 cells from 5 dishes; Fig. 2A, B). Furthermore, the DHPG-induced calcium signal was unaffected by application of 8-bromo-cAMP (DHPG: 139 ± 4 %; DHPG + 8-Br-cAMP: 134 ± 4 %, n = 15 cells from 3 dishes, Fig. 2C). These results suggest that PKA-dependent phosphorylation prevents DCG-IV-induced enhancement of mGlu5 receptor function.
Figure 2. Group II mGlu receptor enhancement of mGlu5 receptor-mediated calcium mobilization relies on a decrease in cAMP/protein kinase A activity.
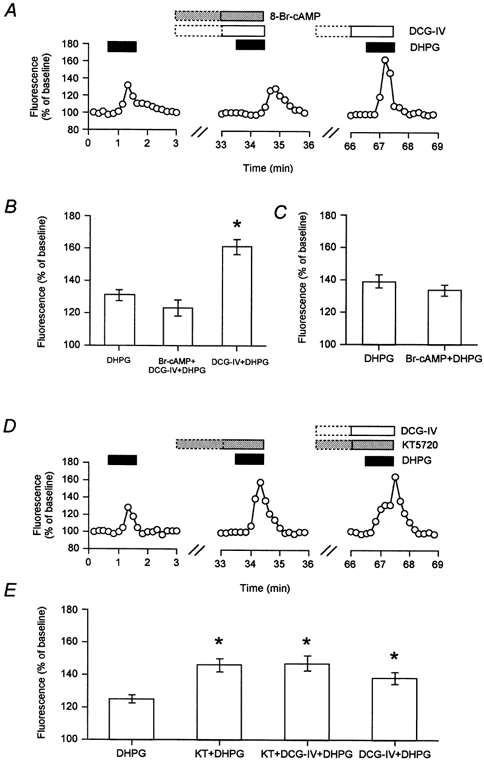
A and B, single example (A) and pooled data (25 cells; B) showing that increasing cAMP (8-bromo-cAMP; 5 μm) prevented the normal enhancement of mGlu5 receptor function (20 μm DHPG) by co-activation of group II mGlu receptors (1 μm DCG-IV). *Significantly different from 8-Br-cAMP + DCG-IV + DHPG response. C, pooled data (15 cells) to illustrate that increasing cAMP had no effect on DHPG-induced calcium mobilization. D and E, single example (D) and pooled data (44 cells; E) showing that the protein kinase A inhibitor KT5720 (0.5 or 1 μm) significantly enhanced mGlu5 receptor-induced calcium mobilization (P < 0.05). This enhancement occluded the normal DCG-IV enhancement of mGlu5 receptor function, in a reversible manner. *Significantly different from DHPG response.
The effects of blocking protein kinase A (PKA) on mGlu5 receptor-mediated calcium mobilization was tested next. Interestingly, application of the PKA inhibitor KT5720 (1 μm; Cabell & Audesirk, 1993) by itself significantly (P < 0.05) enhanced the mGlu5 receptor-dependent calcium signal (DHPG: 125 ± 5 %; DHPG + KT5720: 146 ± 4 %; n = 25 cells from 4 dishes; Fig. 2D, E). This shows that mGlu5 dephosphorylation can increase mGlu5 function. Furthermore, the enhancement of mGlu5 receptor function by KT5720 occluded any further enhancement by co-activation of group II mGlu receptors (DCG-IV + DHPG + KT5720: 147 ± 5 %; n = 25 cells; Fig. 2D, E). In addition, the enhancement of mGlu5 receptor signalling by DCG-IV prevented further enhancement by KT5720 (data not shown). Taken together, the results so far suggest that a decrease in phosphorylation at a PKA phosphorylation site may be responsible for the enhancement of mGlu5 receptor function by group II mGlu receptor activation. However, these results do not indicate whether phosphorylation of mGlu5 or mGlu2/3 regulates the interaction between these receptors.
It is known that PKA is anchored to its cellular targets through interaction with A-kinase anchoring protein (AKAP) via an amphipathic helix binding motif (Carr et al. 1992; Colledge et al. 1999; Edwards & Scott, 2000). We therefore used a cell-permeant anchoring inhibitor peptide (human thyroid A-kinase anchoring protein; Ht31: see Methods for details) that selectively prevents AKAP's interaction with the RII regulatory subunit of PKA (Carr et al. 1992; Rosenmund et al. 1994; Vijayaraghavan et al. 1997; Herberg et al. 2000). The consequences of this manipulation on the interaction between group I and group II mGlu receptors were then examined. Interestingly, the application of Ht31 by itself produced a significant enhancement (P < 0.05) of the group I mGlu receptor-induced calcium signal (DHPG: 132 ± 6 %; Ht31 + DHPG: 160 ± 9 %, n = 35 cells from 4 dishes; Fig. 3A). Subsequent application of DCG-IV, in the presence of Ht31, did not produce any further enhancement of the DHPG-induced response (156 ± 8 %; Fig. 3A). Furthermore, calcium mobilization resulting from selective activation of mGlu5 receptors by application of CHPG was also enhanced by Ht31 (CHPG: 174 ± 7 %; Ht31 + CHPG: 261 ± 11 %, n = 27 cells from 4 dishes; Fig. 3B). A control peptide (Ht31p; identical to Ht31 except for a single proline to isoleucine substitution to prevent helix formation) had no significant effect on mGlu5 receptor signalling (DHPG: 135 ± 4 %; DHPG + Ht31p: 128 ± 4 %, n = 25 cells from 4 dishes; Fig. 3C). The similarity of the results with Ht31 to the effects with the PKA inhibitor KT5720 further suggests that modulation of PKA-dependent phosphorylation can directly regulate mGlu5 receptor function. Whether a decrease in PKA-dependent phosphorylation of mGlu5 per se provides the underlying mechanism for group II mGlu receptor enhancement of mGlu5 remains to be determined.
Figure 3. The function of A-kinase anchoring protein (AKAP) is required for the regulation of mGlu5 receptor function.
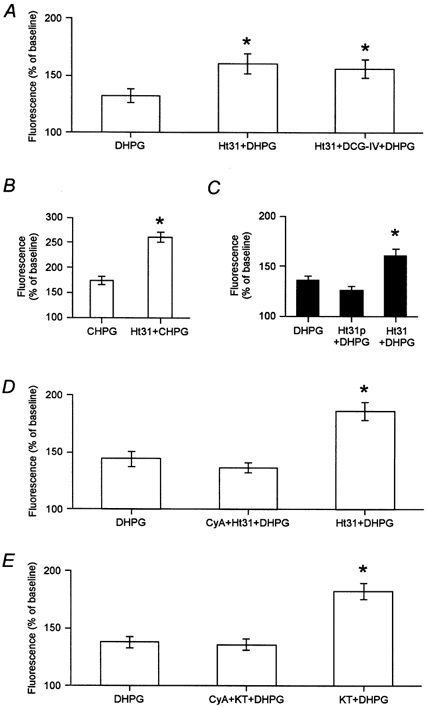
A, the peptide Ht31 that prevents PKA-AKAP interaction significantly enhanced mGlu5 receptor-induced calcium mobilization (P < 0.05). The enhancement by Ht31 occluded the normal DCG-IV-induced enhancement of mGlu5 receptor function (35 cells). *Significantly different from DHPG response. B, Ht31 also enhanced CHPG-induced calcium mobilization. C, a control version of Ht31 (Ht31p) did not enhance mGlu5 receptor function (25 cells). *Significantly different from Ht31p + DHPG response. D, the enhancement of mGlu5 receptor function by Ht31 relies on protein phosphatase 2B activity since the enhancement was blocked by cyclosporin A (CyA), in a reversible manner (21 cells). *Significantly different from CyA + Ht31 + DHPG. E, the enhancement of mGlu5 receptor function by KT5720 also relies on protein phosphatase 2B activity since this enhancement was also blocked by cyclosporin A (23 cells). *Significantly different from CyA + KT5720 + DHPG.
We next examined whether the decrease in PKA activity might allow protein phosphatase-dependent dephosphorylation and a subsequent increase in mGlu5 receptor function. Therefore, the above experiment with Ht31 was repeated but in the presence of the protein phosphatase 2B inhibitor cyclosporin A (Fruman et al. 1992; 10 μm). Under these conditions there was no significant enhancement (P > 0.05) of mGlu5 receptor signalling by Ht31 (DHPG: 144 ± 6 %; DHPG + Ht31 + cyclosporin A: 137 ± 4 %; DHPG + Ht31: 186 ± 8 % Fig. 3D, n = 21 cells from 4 dishes). Furthermore, the enhancement of mGlu5 receptor function by KT5720 was also prevented (P > 0.05) by cyclosporin A (DHPG: 138 ± 5 %; DHPG + KT5720 + cyclosporin A: 136 ± 5 %; DHPG + KT5720: 183 ± 7 %, n = 23 cells from 4 dishes; Fig. 3E). These results suggest that decreasing PKA activity allows enhancement of mGlu5 receptor function via PP2B-dependent dephosphorylation.
We next determined whether the synergistic enhancement of mGlu5 receptor function by group II mGlu receptor activation also occurred via a PP2B-dependent mechanism. In the presence of cyclosporin A there was no significant enhancement (P > 0.05) of group I mGlu function by co-activation of group II mGlu receptors (DHPG: 140 ± 4 %; DHPG + DCG-IV + cyclosporin A: 148 ± 6 %; DHPG + DCG-IV: 179 ± 5 %; n = 24 cells from 4 dishes; Fig. 4A, B). To confirm that this effect did not rely indirectly on activation of mGlu1, the above experiments were repeated with the mGlu5 receptor selective agonist CHPG. We found that the enhancement by DCG-IV of CHPG-dependent calcium mobilization was also prevented by cyclosporin A (CHPG: 194 ± 11 %; cyclosporin A + DCG-IV + CHPG: 181 ± 12 %; DCG-IV + CHPG: 278 ± 18 %, n = 22 cells from 4 dishes. Fig. 4C). In contrast, okadaic acid (inhibitor of protein phosphatase 1/2A) did not prevent the DCG-IV-induced enhancement of mGlu5 receptor function (DHPG: 139 ± 5 %; DHPG + DCG-IV + okadaic acid: 173 ± 5 %; n = 26 cells from 3 dishes; Fig. 4A, D). Therefore activation of PP2B, but not PP1/2A, appears to be required for the interaction between mGlu5 and group II mGlu receptors. Whilst PKA and PP2B can directly affect mGlu5 function these results do not provide evidence as to whether phosporylation of mGlu5 or phosphorylation of mGlu2/3 regulates the interaction between these receptor types.
Figure 4. The group II mGlu receptor enhancement of mGlu5 receptor function is dependent on PP2B but not PP1/2A activity.
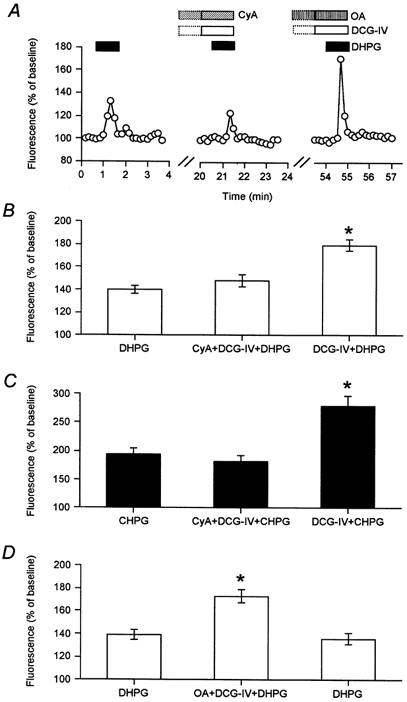
A and B, single example (A) and pooled data (24 cells; B) showing that the PP2B inhibitor cyclosporin A (10 μm) prevented the normal enhancement of mGlu5 function by group II mGlu receptor activation. *Significantly different from CyA + DCG-IV + DHPG. C, the enhancement by DCG-IV of calcium mobilization by CHPG was also prevented by an inhibitor of PP2B. A and D, single example and pooled data (26 cells) showing that the PP1/2A inhibitor okadaic acid (OA) did not prevent the enhancement of mGlu5 function by group II receptor activation. *Significantly different from DHPG.
In the final series of experiments we tested whether there is a physiological role for the interaction between group I and group II mGlu receptors. We have previously shown that the induction of LTD in perirhinal cortex relies on activation of group I and group II mGlu receptors and NMDA receptors at resting membrane potentials. However, LTD does not require group II mGlu receptor activation under depolarized conditions (Cho et al. 2000). To test whether mGlu receptor synergy may be required for the induction of LTD at resting membrane potentials we performed experiments in slices of perirhinal cortex in the presence of agents that, in the experiments described above, prevented the synergistic enhancement of mGlu5 receptor function by group II mGlu receptor activation. Thus postsynaptic cAMP was raised by including sp-cAMP (20 μm) within the whole-cell filling solution; this would be expected to prevent mGlu5/group II interaction. Under these conditions the induction of LTD was prevented when LFS was delivered at −70 mV (97 ± 4 %, n = 3, Fig. 5A) but LTD was induced with LFS delivered at −40 mV (45 ± 7 %, n = 3, Fig. 5B). Furthermore, LFS delivered at −70 mV did not induce LTD in the presence of cyclosporin A (92 ± 7 % relative to baseline; n = 5; Fig. 6A, B), which prevents mGlu5- group II interaction. In contrast however, LTD induced at −40 mV was not blocked by cyclosporin A (63 ± 8 % relative to baseline; n = 4, Fig. 6C).
Figure 5. LTD that requires group I and group II mGlu receptor activation relies on mechanisms of interaction between mGlu receptors.
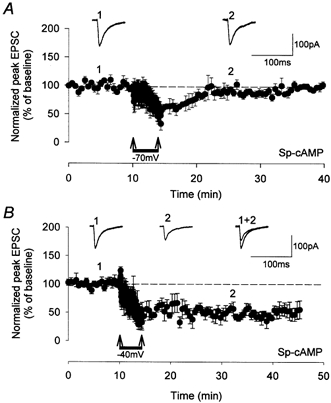
A, LTD induced at −70 mV was prevented when sp-cAMP was present within the whole-cell filling solution. Under these conditions, LTD required activation of both group I and group II mGlu receptors (Cho et al. 2000). B, LTD induced at −40 mV was not prevented by raising postsynaptic cAMP. Therefore, blocking mechanisms of mGlu receptor synergy prevents induction of LTD at resting potentials. Synaptic responses (1, 2) are taken from time points indicated on the graphs.
Figure 6. LTD that requires group I and group II mGlu receptor activation is blocked by inhibition of PP2B.
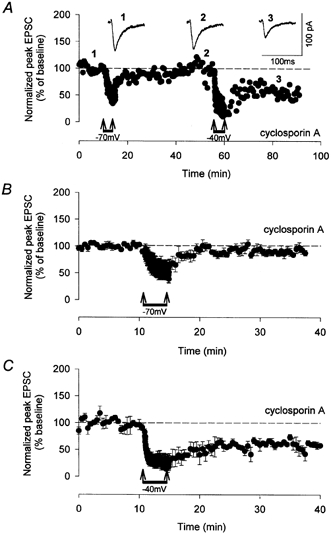
A and B, single example (A) and pooled data (n = 5; B) illustrating that LTD was blocked by cyclosporin A (10 μm) when LFS was delivered at −70 mV. Under these conditions, LTD required activation of both group I and group II mGlu receptors (Cho et al. 2000). However, LTD that does not require group II mGlu receptor activation (LFS delivered at −40 mV) was not blocked by the presence of cyclosporin A; single example (A) and pooled data (C; n = 4). Therefore, blocking mechanisms of mGlu receptor synergy prevents induction of LTD at resting potentials. Synaptic responses (1, 2, 3) are taken from time points indicated on the graphs.
These results strongly support the hypothesis that when LTD requires group I and group II mGlu receptor activation (at −70 mV) this depends on a synergy between group I and group II mGlu receptors that relies on a decrease in cAMP and on PP2B-dependent dephosphorylation (Fig. 6). However, these mechanisms of synergy are not necessary at −40 mV, conditions under which LTD does not require group II mGlu receptor activation (Cho et al. 2000).
DISCUSSION
The results of the present study demonstrate that the synergy between group II and mGlu5 receptors that we (Cho et al. 2000) and others (Nicoletti et al. 1993; Schoepp et al. 1996; Mistry et al. 1998) have previously reported occurs through a decrease in PKA function and PP2B-mediated dephosphorylation. Crucially, we demonstrate that the synergy between mGlu receptors may explain the requirement of group II mGlu receptors in the induction of LTD at resting but not depolarized membrane potentials.
We were particularly interested in investigating the mechanisms responsible for the synergy between group I and group II mGlu receptors (Nicoletti et al. 1993; Schoepp et al. 1996; Mistry et al. 1998) because of the role of group I and group II mGlu receptors in synaptic plasticity in the perirhinal cortex (McCaffery et al. 1999; Cho et al. 2000) and other brain regions (Anwyl, 1999). Since group II mGlu receptors negatively couple to forskolin-stimulated cAMP (for review, see Conn & Pin, 1997) a decrease in phosphorylation at a PKA phosphorylation site (on mGlu5 or group II mGlu receptors) might underlie the synergy with group II mGlu receptors. In keeping with this hypothesis, increasing cAMP levels prevented the enhancement of mGlu5 receptor function by group II mGlu receptors, as has also been suggested for other receptor systems linked to these same pathways (Fowler & Tiger, 1991; Undie & Frieman, 1994). In addition, raising cAMP levels with forskolin or 8-Br-cAMP had no effect on the mGlu5 receptor-mediated calcium signal. Furthermore, the PKA inhibitor KT5720 by itself enhanced mGlu5 receptor function. Together these results suggest a constitutive PKA-dependent phosphorylation of mGlu5; modulation of the phosphorylation state can alter mGlu5 receptor function. Modulation by PKA has not always been found to affect mGlu5 receptor function however (Gereau & Heinemann, 1998). The reasons for this reported lack of effect of PKA on mGlu5 are not clear, especially since mGlu5 receptors contain at least one potential PKA consensus site (Schaffhauser et al. 2000).
Further evidence for the importance of PKA signalling came from the results showing that a peptide (human thyroid A-kinase anchoring protein; Ht31; Carr et al. 1992; Rosenmund et al. 1994; Vijayaraghavan et al. 1997) known to bind to the RII subunit of PKA, and thus prevent binding of the RII subunit to the kinase binding region of AKAP, also enhanced the mGlu5 receptor signal. Importantly, the synergy between group II mGlu receptors and mGlu5 receptors was also occluded by inhibition of PKA or by Ht31. This study provides the first demonstration of the involvement of AKAP in regulation of mGlu5 receptor function. Whilst we do not know which AKAP is involved in regulation of mGlu5 receptors, it is interesting that AKAP79 binds both PKA and PP2B, as well as PKC (Colledge & Scott, 1999; Edwards & Scott, 2000) indicating that AKAP79 may be one of the critical AKAPs involved. Furthermore, this also raises the possibility that PKC regulation of mGlu5 (Alagarsamy et al. 1999) may rely on an interaction with AKAP79.
We present evidence using the inhibitor cyclosporin A that the enhancement of mGlu5 receptor function by inhibition of PKA or by group II mGlu receptor activation is dependent on PP2B-mediated dephosphorylation. Whilst cyclosporin A and other PP2B inhibitors may affect other phosphatases such inhibitors have nevertheless been used previously to demonstrate a role for PP2B in forms of LTD (Mulkey et al. 1993; Kirkwood & Bear, 1994; O'Dell & Kandel, 1994). A role for PP2B-dependent dephosphorylation at a PKC-dependent site has previously been shown to underlie NMDA receptor-dependent enhancement of mGlu5 function (Alagarsamy et al. 1999). The results of the present study suggest that PP2B-dependent dephosphorylation at a PKA site may also increase mGlu5 receptor function in a group II mGlu receptor-dependent manner.
Whilst our results show that modulation of the phosphorylation state of mGlu5 can regulate its function it remains to be determined whether this is the mechanism underlying the enhancement of mGlu5 function by group II mGlu receptors. Thus one possible mechanism is that group II mGlu receptor-mediated decreases in cAMP/PKA activity and an increase in PP2B activity cause dephosphorylation of and thus enhancement of mGlu5 function. However, there are other possible scenarios: A previous study has suggested that interaction between group II and group I mGlu receptors may depend on a direct interaction between the G-proteins that couple to group I and group II mGlu receptors (Mistry et al. 1998). In addition, it has been demonstrated that PKA phosphorylation prevents G-protein coupling to group II mGlu receptors (Schaffhauser et al. 2000) but has no direct effect on mGlu5 (Gereau & Heinemann, 1998). Given this evidence it is possible therefore, that the regulation of mGlu receptor interactions that we have described may rely on the phosphorylation state of mGlu2/3. Thus PKA and PP2B regulation of mGlu2/3 phosphorylation may control G-protein coupling with mGlu2/3 and may therefore regulate the direct interactions with mGlu5-associated G-proteins. This is an alternative mechanism by which PKA may control the enhancement of mGlu5 function. Thus, we have identified certain molecular candidates that may underlie enhancement of mGlu5 function by group II mGlu receptor activation, but the precise substrates that are (de)phosphorylated remain to be determined.
Previous studies have demonstrated that group II mGlu receptor activation decreases forkolin-stimulated cAMP, with little effect on basal cAMP, whilst co-activation of group I and group II mGlu receptors can enhance cAMP (see Schoepp et al. 1996; Conn & Pin, 1997). However, the present data suggest that under the conditions of our experiments, basal cAMP negatively regulates mGlu5 receptors. Thus, inhibition of PKA or activation of group II mGlu receptors enhanced mGlu5 calcium mobilization. Additionally, the evidence from the present study does not indicate that an increase in cAMP is responsible for enhancing mGlu5-induced calcium mobilization since the application of cAMP activators (with forskolin or 8-Br-cAMP) did not affect mGlu5 receptor-mediated calcium mobilization but completely prevented the enhancement by group II mGlu receptor activation.
The synergy between group I receptors and group II mGlu receptors occurred between mGlu5 and group II mGlu receptors but not between mGlu1 and group II receptors; it is possible that this lack of synergy between mGlu1 and group II mGlu receptors is due to the lack of a PKA consensus site on the C-terminal domain of mGlu1 (Schaffhauser et al. 2000). The action of group II mGlu receptors in enhancing the function of PLC-coupled receptors was specific for mGlu receptors since we found no enhancement of muscarinic receptor-mediated calcium signals (Mistry et al. 1998). Whether such a synergy may occur between group II mGlu receptors and other PLC-coupled receptors that we have not yet investigated remains to be determined (Fowler & Tiger, 1991; Undie & Friedman, 1994).
We have previously described that LTD in perirhinal cortex relies on activation of group I, group II mGlu and NMDA receptors at resting membrane potentials, but only on group I and NMDA receptors at depolarized membrane potentials (Cho et al. 2000). It was suggested that group II mGlu receptor activation may enhance group I mGlu receptor function and thereby increase the release of calcium from intracellular stores; this may be important for the induction of LTD at resting membrane potentials when the function of NMDA receptors is reduced. In the present study we have demonstrated that LTD at resting membrane potentials (−70 mV), but not depolarized potentials (−40 mV), relies on the same mechanisms (decrease in cAMP, activation of PP2B) as the synergy between mGlu5 and group II mGlu receptors. These results provide strong support for the hypothesis that the requirement in LTD for activation of group II mGlu receptors may be to provide enhancement of mGlu5 function.
A role for protein phosphatases in LTD has been described previously (Mulkey et al. 1993; Kirkwood & Bear, 1994; O'Dell & Kandel, 1994) and thus it is possible that the role of PP2B in LTD in the present study does not reflect a requirement for mGlu receptor synergy but reflects some other requirement for phosphatase activation, such as direct dephosphorylation of GluR1. However, this is unlikely since inhibition of PP2B only blocked LTD under conditions where mGlu5 and group II mGlu receptors were required for LTD. Inhibition of PP2B or raised cAMP did not prevent the induction of LTD at −40 mV; conditions in which group II mGlu receptor activation plays no role in LTD (Cho et al. 2000). Furthermore, it has been reported previously that the phosphorylation state of Ser-845 and Ser-831 on GluR1 is unaffected by inhibition of either PP1/2A or PP2B (Kameyama et al. 1998), indicating that these phosphatases might play a role in LTD by some mechanism other than direct modulation of GluR1. Therefore, the role of PP2B in LTD under the conditions of the present study may be explained by a PP2B-dependent enhancement of mGlu5 receptor function, either directly or via dephosphorylation of group II mGlu receptors.
The mechanisms that we have described are likely to be widespread within the CNS and therefore may be important in regulating synaptic plasticity and other functions not only in the perirhinal cortex but also in many other brain regions.
Acknowledgments
This work was supported by the BBSRC and MRC.
REFERENCES
- Alagarsamy S, Marino MJ, Rouse ST, Gereau RW, Heinemann SF, Conn PJ. Activation of NMDA receptors reverses desensitisation of mGluR5 in native and recombinant systems. Nature Neuroscience. 1999;2:234–240. doi: 10.1038/6338. [DOI] [PubMed] [Google Scholar]
- Anderson WW, Collingridge GL. A data acquisition programme for on-line analysis of long-term potentiation and long-term depression. Society for Neuroscience Abstracts. 1997;23:665. [Google Scholar]
- Aniksztejn L, Otani S, Ben-ari Y. Quisqualate metabotropic receptors modulate NMDA currents and facilitate induction of long-term potentiation through protein kinase C. European Journal of Neuroscience. 1992;4:500–505. doi: 10.1111/j.1460-9568.1992.tb00900.x. [DOI] [PubMed] [Google Scholar]
- Anwyl R. Metabotropic glutamate receptors: elctrophysiological properties and role in plasticity. Brain Research Reviews. 1999;29:83–120. doi: 10.1016/s0165-0173(98)00050-2. [DOI] [PubMed] [Google Scholar]
- Cabell L, Audesirk G. Effects of selective inhibition of protein kinase C, cyclicAMP-dependent protein kinase, and Ca-calmodulin-dependent protein kinase on neurite development in cultured rat hippocampal neurones. International Journal of Developmental Neuroscience. 1993;11:357–368. doi: 10.1016/0736-5748(93)90007-z. [DOI] [PubMed] [Google Scholar]
- Carr DW, Hausken ZF, Fraser ID, Stofko-Hahn RE, Scott JD. Association of the type II cAMP-dependent protein kinase with a human thyroid RII-anchoring protein. Cloning and characterization of the RII-binding domain. Journal of Biological Chemistry. 1992;267:13376–13382. [PubMed] [Google Scholar]
- Challiss RAJ, Mistry R, Gray DW, Nahorski RR. Modulatory effects of NMDA on phosphoinositide responses evoked by the metabotropic glutamate receptor agonist 1S, 3R-ACPD in neonatal rat cerebral cortex. British Journal of Pharmacology. 1994;112:231–239. doi: 10.1111/j.1476-5381.1994.tb13057.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho K, Kemp N, Noel J, Aggleton JP, Brown MW, Bashir ZI. A novel form of long-term depression in the perirhinal cortex. Nature Neuroscience. 2000;3:150–156. doi: 10.1038/72093. [DOI] [PubMed] [Google Scholar]
- Colledge M, Scott JD. AKAPs: from structure to function. Trends in Cell Biology. 1999;9:216–221. doi: 10.1016/s0962-8924(99)01558-5. [DOI] [PubMed] [Google Scholar]
- Conn PJ, Pin J-P. Pharmacology and functions of metabotropic glutamate receptors. Annual Review Pharmacology and Toxicology. 1997;37:167–203. doi: 10.1146/annurev.pharmtox.37.1.205. [DOI] [PubMed] [Google Scholar]
- Doherty AJ, Palmer MJ, Henley JM, Collingridge GL. (RS)-2-chloro-5-hydroxyphenylglycine (CHPG) activates mGlu5 but not mGlu1 receptors expressed in CHO cells and potentiates NMDA responses in the hippocampus. Neuropharmacology. 1997;36:265–267. doi: 10.1016/s0028-3908(97)00001-4. [DOI] [PubMed] [Google Scholar]
- Edwards AS, Scott JD. A-kinase anchoring proteins: protein kinase A and beyond. Current Opinion in Cell Biology. 2000;12:217–221. doi: 10.1016/s0955-0674(99)00085-x. [DOI] [PubMed] [Google Scholar]
- Fowler CJ, Tiger G. Modulation of receptor mediated inositol phospholipid breakdown in the brain. Neurochemistry International. 1991;19:171–206. [Google Scholar]
- Fruman DA, Klee CB, Bierer BE, Burakoff SJ. Calcineurin phosphatase activity in T lymphocytes is inhibited by FK 506 and cyclosporin A. Proceedings of the National Academy of Sciences of the USA. 1992;89:3685–3686. doi: 10.1073/pnas.89.9.3686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasparini F, Lingenhöahl K, Stoehr N, Flor PJ, Heinrich M, Vranesic I, Biollaz M, Allgeier H, Heckendorn R, Urwyler S, Varney MA, Johnson EC, Hess SD, Rao SP, Sacaan AI, Santori EM, Veliçerebi G, Kuhn R. 6-Methyl-2-(phenylethynyl)-pyridine (MPEP), a potent, selective and systemically active mGlu5 receptor antagonist. Neuropharmacology. 1999;38:1493–1503. doi: 10.1016/s0028-3908(99)00082-9. [DOI] [PubMed] [Google Scholar]
- Gereau RW, Heinemann SF. Role of protein kinase C phosphorylation in rapid desensitisation of metabotropic glutamate receptor 5. Neuron. 1998;20:143–151. doi: 10.1016/s0896-6273(00)80442-0. [DOI] [PubMed] [Google Scholar]
- Harvey J, Collingridge GL. Signal transduction pathways involved in the acute potentiation of NMDA responses by 1S, 3R-ACPD in rat hippocampal slices. British Journal of Pharmacology. 1993;109:1085–1090. doi: 10.1111/j.1476-5381.1993.tb13733.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herberg FW, Maleszka A, Eide T, Vossebein L, Tasken K. Analysis of A-kinase anchoring protein (AKAP) interaction with protein kinase A (PKA) regulatory subunits: PKA isoform specificity in AKAP binding. Journal of Molecular Biology. 2000;298:329–339. doi: 10.1006/jmbi.2000.3662. [DOI] [PubMed] [Google Scholar]
- Ishida M, Saitoh T, Shimamoto K, Ohfune Y, Shinozaki H. A novel metabotropic glutamate receptor agonist: marked depression of monosynaptic excitation in the isolated rat spinal cord. British Journal of Pharmacology. 1993;109:1169–1177. doi: 10.1111/j.1476-5381.1993.tb13745.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kameyama K, Lee H-Y, Bear MF, Huganir RL. Involvement of a postsynaptic protein kinase A substrate in the expression of homosynaptic long-term depression. Neuron. 1998;21:1163–1175. doi: 10.1016/s0896-6273(00)80633-9. [DOI] [PubMed] [Google Scholar]
- Kirkwood A, Bear MF. Homosynaptic long-term depression in the visual cortex. Journal of Neuroscience. 1994;14:3404–3412. doi: 10.1523/JNEUROSCI.14-05-03404.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luthi A, Gahwiler BH, Gerber U. Potentiation of a metabotropic glutamatergic response following NMDA receptor activation. Pflügers Archiv. 1994;427:197–202. doi: 10.1007/BF00585965. [DOI] [PubMed] [Google Scholar]
- McCaffery B, Cho K, Bortolotto ZA, Aggleton J, Brown MW, Conquet F, Collingridge GL, Bashir ZI. Synaptic depression induced by pharmacological activation of metabotropic glutamate receptors in the perirhinal cortex in vitro. Neuroscience. 1999;93:977–984. doi: 10.1016/s0306-4522(99)00205-5. [DOI] [PubMed] [Google Scholar]
- Mistry R, Golding N, Challiss RAJ. Regulation of phosphoinositide turnover in neonatal rat cerebral cortex by group I and II selective metabotropic glutamate receptor agonists. British Journal of Pharmacology. 1998;123:581–589. doi: 10.1038/sj.bjp.0701626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulkey RM, Herron CE, Malenka RC. An essential role for protein phosphatases in hippocampal long-term depression. Science. 1993;261:1051–1055. doi: 10.1126/science.8394601. [DOI] [PubMed] [Google Scholar]
- Nicoletti F, Casabona G, Genazzani AA, L'Esiscopo MR, Shinozaki H. (2S, 1′R, 2″R, 3″R)-2-(2, 3-dicarboxycyclopropyl) glycine enhances quisqualate-stimulated inositol phospholipid hydrolysis in hippocampal slices. European Journal of Pharmacology. 1993;245:297–298. doi: 10.1016/0922-4106(93)90111-l. [DOI] [PubMed] [Google Scholar]
- O' Dell TJ, Kandel ER. Low frequency stimulation erases LTP through an NMDA receptor-mediated activation of protein phosphatases. Learning and Memory. 1994;1:129–139. [PubMed] [Google Scholar]
- Rosenmund C, Carr DW, Bergeson SE, Nilaver G, Scott JD, Westbrook GL. Anchoring of protein kinase A is required for modulation of AMPA/kainate receptors on hippocampal neurones. Nature. 1994;368:853–856. doi: 10.1038/368853a0. [DOI] [PubMed] [Google Scholar]
- Salt TE, Binns KE, Turner JP, Gasparini F, Kuhn R. Antagonism of the mGlu5 agonist 2-chloro-5-hydroxypheylglycine by the novel selective mGlu5 antagonist 6-methyl-2-(phenylethynyl)-pyridine (MPEP) in the thalamus. British Journal of Pharmacology. 1999;127:1057–1059. doi: 10.1038/sj.bjp.0702677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaffhauser H, Cai Z, Hubalek F, Macek TA, Pohl J, Murphy TJ, Conn PJ. cAMP-dependent protein kinase A inhibits mGluR2 coupling to G-proteins by direct receptor phosphorylation. Journal of Neuroscience. 2000;20:5663–5670. doi: 10.1523/JNEUROSCI.20-15-05663.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoepp DD, Salhoff CR, Wright RA, Johnson BG, Burnett JP, Mayne NG, Belagage R, Wu S, Monn JA. The novel metabotropic glutamate receptor agonist 2R,4R-APDC potentiates stimulation of phosphoinositide hydrolysis in the rat hippocampus by 3,5-dihydroxyphenylglycine: evidence for a synergistic interaction between group I and group II receptors. Neuropharmacology. 1996;35:1661–1672. doi: 10.1016/s0028-3908(96)00121-9. [DOI] [PubMed] [Google Scholar]
- Undie AS, Friedman E. Inhibition of dopamine agonist-induced phosphoinositide hydrolysis by concomitant stimulation of cyclic AMP formation in brain slices. Journal of Neurochemistry. 1994;63:222–230. doi: 10.1046/j.1471-4159.1994.63010222.x. [DOI] [PubMed] [Google Scholar]
- Vijayaraghavan S, Goueli SA, Davey MP, Carr DW. Protein kinase A-anchoring inhibitory peptides arrest mammalian sperm motility. Journal of Biological Chemistry. 1997;272:4747–4752. doi: 10.1074/jbc.272.8.4747. [DOI] [PubMed] [Google Scholar]


