Abstract
The current study has investigated the electrophysiological responses evoked by histamine in bovine adrenal chromaffin cells using perforated-patch techniques. Histamine caused a transient hyperpolarization followed by a sustained depolarization of 7.2 ± 1.4 mV associated with an increase in spontaneous action potential frequency. The hyperpolarization was abolished after depleting intracellular Ca2+ stores with thapsigargin (100 nm), and was reduced by 40 % with apamin (100 nm). Membrane resistance increased by about 60 % during the histamine-induced depolarization suggesting inhibition of a K+ channel. An inward current relaxation, typical of an M-current, was observed in response to negative voltage steps from a holding potential of −30 mV. This current reversed at −81.6 ± 1.8 mV and was abolished by the M-channel inhibitor linopirdine (100 μm). During application of histamine, the amplitude of M-currents recorded at a time corresponding with the sustained depolarization was reduced by 40 %. No inward current rectification was observed in the range −150 to −70 mV, and glibenclamide (10 μm) had no effect on either resting membrane potential or the response to histamine. The results show that an M-current is present in bovine chromaffin cells and that this current is inhibited during sustained application of histamine, resulting in membrane depolarization and increased discharge of action potentials. These results demonstrate for the first time a possible mechanism coupling histamine receptors to activation of voltage-operated Ca2+ channels in these cells.
Histamine is a potent secretagogue of the chromaffin cells of the adrenal medulla, causing substantial and sustained release of both adrenaline and noradrenaline (Livett & Marley, 1986; Noble et al. 1988). This action of circulating histamine may be lifesaving during anaphylactic shock, and in addition, local release of histamine within the adrenal medulla may also have a paracrine function (Häppöalä et al. 1985; Tuominen et al. 1993).
The secretory effect of histamine is mediated exclusively by H1 receptors, which are expressed in high abundance in the adrenal medulla (Chang et al. 1979). In chromaffin cells, as in many other tissues, stimulation of H1 receptors activates phospholipase C via a pertussis toxin-insensitive G-protein and causes the production of inositol 1,4,5-trisphosphate (IP3; Noble et al. 1986; Plevin & Boarder, 1988), and the subsequent release of Ca2+ from intracellular stores (Stauderman & Pruss, 1990; Stauderman & Murawsky, 1991). The Ca2+ response caused by activation of histamine receptors on chromaffin cells has two components. The first component is transient and is mediated by release of Ca2+ from intracellular Ca2+ stores. The second component is sustained, and generally thought to depend on the presence of Ca2+ in the extracellular solution (O'Sullivan et al. 1989; Stauderman et al. 1990; Goh & Kurosawa, 1991; Cheek et al. 1993; Zerbes et al. 1998; Bödding, 2000). While the Ca2+ released from intracellular stores can cause secretion of catecholamines, this release is transient, lasting less than 1 min, and is quantitatively small (Bunn & Boyd, 1992). In contrast, the substantial release of catecholamines evoked by sustained application of histamine is abolished in Ca2+-free solution and reduced 70–80 % by inhibition of voltage-operated Ca2+ channels (VOCCs; O'Farrell & Marley, 1999; Livett & Marley, 1986; Noble et al. 1988; Bunn & Boyd, 1992). These results indicate that H1 receptors on chromaffin cells are coupled to the activation of VOCCs and influx of extracellular Ca2+, and that the latter is responsible for mediating the majority of the secretory effects of histamine. Other G-protein-coupled receptor agonists also evoke catecholamine secretion by activating Ca2+ entry through VOCCs (O'Farrell & Marley, 1997). However, the nature of the coupling between such receptors and VOCCs is currently unclear.
Studies on the electrophysiological responses to histamine in chromaffin cells have shown that, paradoxically, histamine causes membrane hyperpolarization (Artalejo et al., 1993; Bödding, 2000) and an inhibition of Ca2+ currents (Currie & Fox, 2000). Artalejo et al. (1993) and Bödding (2000) showed that short applications of histamine activated a rapid outward current and membrane hyperpolarization that was largely blocked with apamin, suggesting it resulted from activation of small conductance Ca2+-activated K+ (SK) channels. However, these authors did not report on the responses to prolonged application of histamine. Currie & Fox (2000) reported that histamine caused a 28 % reduction in Ca2+ currents via a pertusis toxin-insensitive mechanism. To date no study has shown an electrophysiological effect of histamine that can explain the secretory responses to this agonist.
Action potentials have been demonstrated in human, bovine, guinea-pig, mouse, gerbil and rat chromaffin cells (Biales et al. 1976; Fenwick et al. 1982a; Nassar-Gentina et al. 1988; Zhou & Misler, 1995; Barbara et al. 1998; Holman et al. 1998). At least in rat and mouse chromaffin cells these action potentials have contributions from both Na+ and Ca2+ (Nassar-Gentina et al. 1988; Holman et al. 1994). Further, Zhou & Misler (1995) showed that bursts of action potentials are accompanied by secretion (as detected by amperometry). Activation of muscarinic receptors in rat, guinea-pig, gerbil and mouse chromaffin cells has been shown to promote excitability by causing a depolarization associated with a discharge of action potentials (Douglas et al. 1967; Nassar-Gentina et al. 1988; Barbara et al. 1998; Holman et al. 1998). In the rat, this depolarization is associated with a small inward current resulting from activation of cation channels (Barbara et al. 1998), while in the guinea-pig the depolarization has either been attributed to activation of a similar inward cation current (Inoue et al. 1998) or associated with a reduction in membrane conductance, presumably reflecting the closure of a K+ channel (Holman et al. 1998). Comparable studies have not been reported on bovine chromaffin cells. Furthermore, tetrodotoxin only reduces histamine-induced catecholamine secretion by around 20 % (O'Farrell & Marley, 1999), indicating tetrodotoxin-sensitive action potentials cannot be the only mechanism coupling H1 receptors to the activation of VOCCs.
The present study therefore aimed to characterize the electrophysiological effects of prolonged histamine stimulation on cultured bovine adrenal chromaffin cells, to help elucidate how stimulation of G-protein-coupled receptors can lead to activation of VOCCs in these cells. In addition, relatively few studies have described the spontaneous electrical activity occurring in chromaffin cells of any species and none on bovine chromaffin cells. Consequently, this study also presents some detail on the spontaneous changes in membrane potential recorded from bovine chromaffin cells. A preliminary report of this study has been published (Wallace & Marley, 2001).
METHODS
Preparation of bovine adrenal chromaffin cell cultures
Cultures of bovine chromaffin cells were prepared from adrenal glands, obtained from an abattoir, by collagenase digestion followed by Percoll density gradient centrifugation (Livett, 1984). Cells were plated at densities between 0.2 and 0.4 × 106 cells in 2 ml of culture medium in 35 mm Falcon tissue culture dishes (Becton Dickinson Labware, Franklin Lakes, NJ, USA) coated with rat tail collagen. Cultures were maintained in a humidified incubator at 37 °C in an atmosphere of 95 % air and 5 % CO2 (see Livett et al. (1984) for full details). Cells were cultured for 2–5 days prior to use to allow them to recover from the collagenase digestion and to attach to the culture dishes. After 2 days in culture, the chromaffin cells displayed a variety of morphological appearances, as previously noted (Marley et al. 1989). In the current study, electrophysiological recordings were made from single, round cells. These cells ranged in diameter from ∼10 to 30 μm, and had an average membrane capacitance of 10.7 ± 0.2 pF (n = 87), and an average membrane resistance of 7.2 ± 0.4 GΩ (n = 87).
Electrophysiology
Cells were viewed on an inverted microscope (Olympus IX50, Tokyo, Japan) equipped with Hoffman modulation contrast optics. Recordings of membrane potential or membrane currents were made from single cells using an Axopatch 200B amplifier (Axon Instruments, Foster City, CA, USA). In most experiments, recordings were made in the perforated whole-cell configuration using nystatin as the perforating agent, though open-tip whole-cell configuration was employed in a few experiments. Patch pipettes were pulled from borosilicate glass (Harvard Apparatus, Kent, UK) using a P97 Flaming/Brown micropipette puller (Sutter Instrument Co., Novato, CA, USA) and had resistances after fire-polishing of ∼1.5–2.5 MΩ (using the perforated patch pipette solution). The average stable access resistance achieved using perforated whole-cell technique in a sample of 30 cells was 18.9 ± 0.8 MΩ, and in a sample of eight cells using open-tip whole-cell technique was 13.5 ± 3.0 MΩ. For voltage-clamp recordings, access resistance and cell capacitance were compensated using the circuits built into the Axopatch amplifier. Signals were low-pass filtered at 2 kHz using an 8-pole Bessel filter, digitized using a DigiData 1200 series interface (Axon Instruments) at a sampling frequency of 4 kHz and stored on a PC computer. Voltage- and current-clamp protocols were executed using Clampex 8 software (Axon Instruments) and analysis conducted using either Clampfit 8 (Axon Instruments) or Origin 5.0 software (Microcal Software, Northampton, MA, USA). Results are presented as mean ± s.e.m. from n cells, and statistical comparisons made using Student's unpaired or paired t test or ANOVA, as indicated. All experiments were conducted at room temperature (∼24 °C). No attempt has been made to adjust any of the data for junction potentials or leak currents.
Repeated responses to sustained applications of histamine were found to desensitize in many cells, as has been previously reported for histamine receptors in chromaffin cells (Stauderman et al. 1990; Warashina, 1997). Consequently, the reported responses to histamine alone and to histamine in the presence of drug solutions are from separate populations of cells. Furthermore, not all cells were found to respond to histamine (in agreement with previous studies: see Stauderman et al. 1990; Pender & Burgoyne, 1992). As desensitization following a single brief pulse (< 5 s) of histamine (1 μm) was negligible, responding cells were initially identified using 2–3 s pulses of histamine (1 μm).
In experiments recording membrane potential, control responses to histamine were assessed after recording 6–10 min of baseline spontaneous activity. Where the effects of drugs on the response to histamine have been assessed, baseline spontaneous activity was recorded for 3–6 min prior to the application of the appropriate drug solution. Cells were exposed to the drug solution for 10–15 min before the response to histamine was recorded. The one exception to this protocol was when thapsigargin was used to deplete intracellular stores of Ca2+. For this, cells were exposed to thapsigargin for 20 min prior to assessing the response to histamine. Separate time control experiments were also performed to ensure that observed effects were not due to additional rundown of histamine responsiveness. The duration of the application of histamine was 6 min unless stated otherwise.
To assess the effects of histamine applications on resting membrane potential, records were first low-pass filtered off-line at 0.1 Hz. An average control resting membrane potential was then calculated for the 3 min immediately prior to the application of histamine, and this was compared with the average membrane potential calculated for the last 3 min of histamine application.
Action potential amplitude was defined as the difference between the peak membrane potential recorded during the action potential and the membrane potential recorded ∼50 ms prior to the peak, and action potential afterhyperpolarization defined as the difference between the peak negative potential reached during the action potential and the membrane potential recorded ∼50 ms prior to the peak. The amplitude of the histamine-induced membrane hyperpolarization was measured as the difference between the peak negative potential reached during the hyperpolarization and the membrane potential recorded ∼50 ms prior to the application of histamine.
Where responses to prolonged application of histamine were assessed using voltage-clamp mode, the holding potential was −50 mV, and a 1 s hyperpolarizing voltage step to −80 mV was applied every 10 s to assess effects of histamine on membrane resistance. A record of the spontaneous changes in the holding current was made for 3 min prior to application of histamine, which was then applied for 5 min. Data were low-pass filtered at 100 Hz off-line. Voltage-clamp recordings were made using the perforated whole-cell technique with the K+ pipette solution.
For voltage-clamp recordings of K+ M-currents, holding potential was set at −30 mV and 900 ms hyperpolarizing voltage steps applied over the range −40 to −100 mV in 10 mV increments. Currents for analysis were the average of 10 repeat sweeps. The amplitude of the resulting M-current relaxation was measured as the difference between the average current recorded in a 10 ms segment beginning 25 ms after the start of the hyperpolarizing step and the average current recorded in the final 50 ms of the hyperpolarizing step (after Cruzblanca et al. 1998). Where the effects of histamine on this current were assessed, control records were made prior to application of histamine, and the current in the presence of histamine recorded 3 min after the onset of the application of histamine. Where the effects of linopirdine were assessed, control currents were again recorded prior to drug application and the effect of linopirdine assessed after exposure of the cell to drug-containing solution for 10 min.
Solutions
For perforated whole-cell configuration, the pipette solution contained (mm): 55 KCl, 75 K2SO4, 8 MgSO4 and 10 Hepes-free acid (pH 7.3, adjusted with KOH). For experiments using Cs+-filled pipettes, perforated whole-cell configuration was used, and the pipette solution contained (mm): 55 CsCl, 75 Cs2SO4, 8 MgSO4 and 10 Hepes-free acid (pH 7.3, adjusted with CsOH). Nystatin was dissolved in dimethylsulphoxide (DMSO) and included in the pipette solution at a final concentration of ∼250 μg ml−1. For open-tip whole-cell configuration, the pipette solution was that used by Artalejo et al. (1993) and contained (mm): 145 potassium glutamate, 1 MgCl2, 0.5 MgATP, 0.3 LiGTP and 10 Hepes-free acid (pH 7.3, adjusted with KOH). The bath solution contained (mm): 140 NaCl, 2.8 KCl, 2.5 CaCl2, 2 MgCl2, 10 Hepes-free acid and 10 glucose (pH 7.3, adjusted with NaOH). Nominally Ca2+-free bath solution was made by simple omission of CaCl2 from the above solution. When N-methyl-d-glucamine (NMDG) was used to replace Na+, the bath solution contained 140 mm NMDG in place of NaCl and the pH was adjusted to 7.3 using 1 m HCl. The osmolarity of the recording solutions used was in the range 285–310 mosmol l−1.
During all experiments, the bath volume was maintained at ∼600 μl, and the bath was perfused at a rate of ∼ 2.5–3.0 ml min−1 using a Watson-Marlow peristaltic pump (Model 502S). Histamine was applied from a delivery pipette located ∼500 μm from the cell at a rate of ∼0.5–1.0 ml min−1. Application of drug-free bath solution from the delivery pipette had no effect on either resting membrane potential or resting membrane current. Drugs other than histamine were applied by including them in the bath solution as well as in the histamine-containing delivery pipette solution.
Drugs used
Tetrodotoxin and thapsigargin were from Calbiochem, San Diego, CA, USA). N-Methyl-d-glucamine, histamine dihydrochloride, apamin, tetraethylammonium chloride and linopirdine were from Sigma, St Louis, MO, USA). Glibenclamide was from Tocris Cookson Ltd, Bristol, UK. Thapsigargin, linopirdine and glibenclamide were all dissolved in DMSO, and appropriate stock solutions made so that the final concentration of DMSO in working solutions did not exceed 0.1 %. Stock solutions of all other drugs were prepared in distilled water.
RESULTS
Resting electrical properties of bovine adrenal chromaffin cells
Using perforated whole-cell technique, the average resting membrane potential of the chromaffin cells was −52.0 ± 0.9 mV (n = 59), and was characterized by the presence of continuous but erratic small fluctuations of ± 5 mV amplitude (Fig. 1A). Spontaneous action potentials were observed in 80 % of cells. The discharge frequency of the spontaneous action potentials was not constant over time for any given cell and was highly variable between cells. The highest average frequency of spontaneous action potentials measured over a 5 min control period in 20 cells was 0.94 Hz, and the average frequency over all 20 cells was 0.21 ± 0.06 Hz (n = 20). The amplitude of the spontaneous action potentials in any given cell often varied markedly, not infrequently by over 20 mV. As the sampling frequency for all recordings was 4 kHz it is unlikely this variation was due to sampling error, and may be caused by variation in the extent of Na+ channel inactivation associated with the wandering resting membrane potential. Using open-tip whole-cell technique the average resting membrane potential was −47.6 ± 1.9 mV (n = 11), with small fluctuations as described above. Spontaneous action potentials were also observed during open-tip whole-cell recordings, though the average action potential amplitude was larger for open-tip than for perforated whole-cell recordings (see Table 1).
Figure 1. Membrane potential recordings from an isolated bovine chromaffin cell in culture using the perforated whole-cell patch technique.
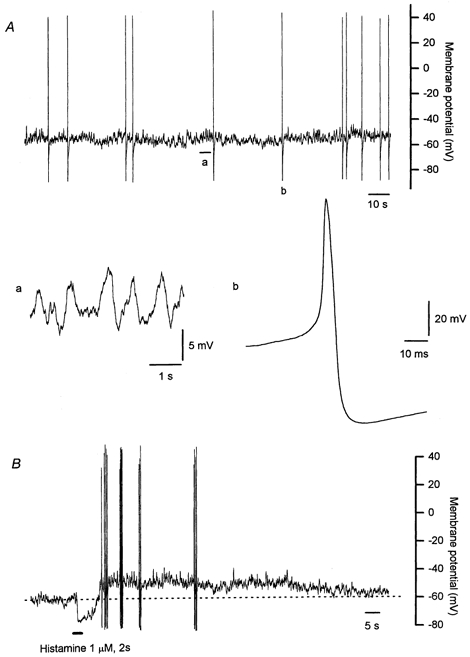
A, an example of the spontaneous electrical activity, with expanded segments showing the continuous small fluctuations in resting membrane potential (a), and detail of an action potential (b). The resting membrane potential was characterized by the presence of small fluctuations of around 5 mV in amplitude. Most cells also had an irregular discharge of action potentials. The action potentials recorded in any one cell varied in amplitude, and invariably had a pronounced afterhyperpolarization. B, the electrical response to a brief application of histamine. The response to a 2 s pulse of histamine (1 μm) consisted of a rapid initial hyperpolarization that was followed by a membrane depolarization which was generally associated with an increase in frequency of action potentials. Records in panel A and B are from different cells.
Table 1.
Amplitude and frequency of action potentials recorded in bovine chromaffin cells before and during application of histamine (5 μm)
| Action potential amplitude (mV) | Action potential frequency (Hz) | |||||
|---|---|---|---|---|---|---|
| Before histamine | n | During histamine | n | Before histamine | During histamine | |
| Perforated whole-cell | ||||||
| Control | 64.5 ± 7.7 | 13 (132) | 47.3 ± 4.2a | 13 (242) | 0.17 ± 0.07 | 0.31 ± 0.11a |
| Cs+ pipette solution | 73.6 ± 4.9 | 5 (60) | 66.5 ± 3.5a | 5 (95) | 0.04 ± 0.02 | 0.06 ± 0.03 |
| Apamin (100 nm) | 76.1 ± 6.7 | 6 (72) | 51.1 ± 5.3a | 6 (153) | 0.20 ± 0.09 | 0.43 ± 0.14a |
| Glibenclamide (10 μm) | 78.2 ± 7.1 | 3 (20) | 52.5 ± 3.9 | 3 (50) | 0.11 ± 0.11 | 0.28 ± 0.13a |
| Tetrodotoxin (10 μm) | 33.1 | 1 (5)b | 41.1 ± 5.4 | 6 (101) | 0.08b | 0.28 ± 0.08 |
| Thapsigargin (100 nm) | 61.9 ± 13.7 | 7 (53) | 46.3 ± 13.6a | 7 (112) | 0.22 ± 0.05 | 0.47 ± 0.27 |
| Ca2+-free solution | 76.9 ± 26.0 | 2 (6)c | 73.0 ± 2.9 | 3 (60) | 0.01 ± 0.01 | 0.33 ± 0.22 |
| Open-tip whole-cell | ||||||
| Control | 85.95 ± 8.7 | 5 (27) | 79.5 ± 7.9 | 5 (51) | 0.14 ± 0.04d | 0.14 ± 0.03d |
| 0.02 ± 0.02e | 0.21 ± 0.17e | |||||
n values stated are the number of cells, with the total number of action potentials measured in parentheses.
Significant difference before compared with during histamine, P < 0.05, Student's paired t test.
Spontaneous action potentials were only recorded in one of six cells in the presence of tetrodotoxin prior to application of histamine, but in all cells during histamine application.
Spontaneous action potentials were only recorded in two of four cells in the presence of Ca2+-free solution prior to application of histamine, and three of four cells during histamine application.
Average action potential frequencies for cells in which histamine failed to cause a sustained depolarization in open-tip whole-cell configuration (three of seven cells).
Average action potential frequencies for the cells in which histamine caused a sustained depolarization in open-tip whole-cell configuration and spontaneous action potentials were observed (two of four cells).
Responses to histamine
Membrane potential responses to histamine
The response to a brief 2–3 s pulse of histamine consisted of an initial membrane hyperpolarization, as previously reported by others (Artalejo et al. 1993; Bödding, 2000). This was followed by a prolonged membrane depolarization, generally lasting for several minutes, which was usually associated with a discharge of action potentials (Fig 1B). When histamine was applied during a discharge of spontaneous action potentials, the discharge was interrupted by the histamine-induced hyperpolarization, and the subsequent depolarization was associated with an increase in the frequency of the spontaneous action potentials. Around 80 % of cells (68 of a sample group of 84 cells) responded to histamine, with the remaining cells showing neither the hyperpolarization nor the depolarization. Both the histamine-induced membrane hyperpolarization and depolarization were blocked by mepyramine (10 μm), confirming that the responses were mediated by H1 histamine receptors (data not shown). Application of bath solution alone from the delivery pipette had no effect on the membrane potential.
In response to prolonged (6 min) applications of histamine, the initial hyperpolarization was followed by a sustained depolarization that lasted for the duration of the histamine application (Fig. 2A and B). This depolarization was also usually associated with an increase in the frequency of action potentials. The average amplitude of the spontaneous action potentials recorded during the sustained depolarization was smaller than that recorded prior to histamine application (see Table 1). The average amplitude of the initial hyperpolarization was −23.7 ± 1.8 mV (n = 13), reached an average peak potential of −76.5 ± 2.4 mV (n = 13) and had an average duration of 11.2 ± 0.8 s (n = 13). In 62 % of cells (8 of 13), the initial hyperpolarization was followed by several similar hyperpolerizations of reducing amplitude, which were then followed by the sustained depolarization (Fig. 2A). The average amplitude of the histamine-induced sustained depolarization was 7.2 ± 1.4 mV (range from 1.8 to 17.5 mV, n = 13).
Figure 2. Membrane potential responses to sustained (6 min) application of histamine.
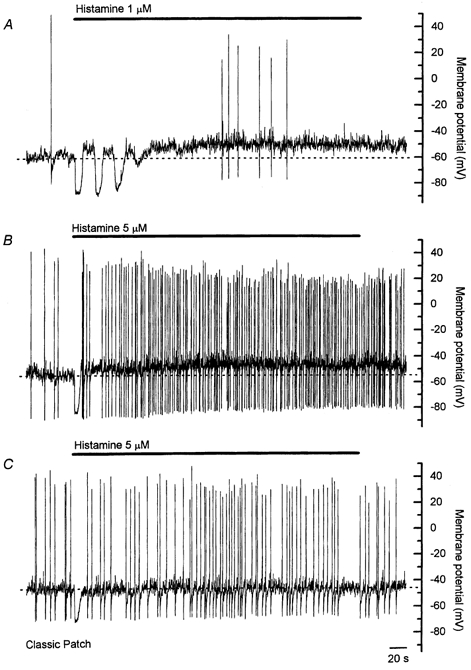
Panels A and B were made using the perforated whole-cell patch configuration, and panel C was made using open-tip whole-cell configuration. Using perforated whole-cell configuration, responses to prolonged application of histamine consisted of an initial hyperpolarization followed by a sustained depolarization that was associated with an increase in the frequency of action potentials. The initial hyperpolarization was followed by several similar hyperpolarizations in some cells, such as that shown in A. The depolarization and increase in action potential frequency persisted for the duration of the application of histamine. Action potential amplitude progressively decreased over the course of the histamine application, as shown in B. When records were made using open-tip whole-cell configuration, a sustained depolarization in response to histamine was observed in less than 50 % of cells, and when present it was smaller in amplitude than that observed using the perforated whole-cell configuration. Time scale in C applies to all panels.
The general characteristics of the responses to histamine recorded using open-tip whole-cell technique were similar to those described above (Fig 2C). However, both the initial hyperpolarization (average amplitude −18.7 ± 1.6 mV, n = 7) and the sustained depolarization (average amplitude 3.9 ± 0.9 mV, n = 4) were smaller in amplitude than equivalent responses in cells using perforated whole-cell technique, and the sustained depolarization was only observed in four of seven cells. Spontaneous action potentials were observed in two of the cells in which histamine induced a sustained depolarization, and in both cases action potential frequency was increased in the presence of histamine (see Table 1). These differences between open-tip and perforated whole-cell technique may be due to diffusion into the patch pipette of a soluble second messenger involved in the histamine-evoked signalling pathway, or to the disturbance of the intracellular milieu inherent to the open-tip whole-cell recording technique. As a consequence of these possibilities, the remainder of the experiments described below employed the perforated whole-cell technique.
Membrane current responses to histamine
When voltage-clamp recordings were made with a holding potential of −50 mV, a small positive resting holding current was observed in the majority of cells (average 3.8 ± 0.8 pA, range −3 to 14.8 pA, n = 25). Prolonged application of histamine (5 μm) evoked a substantial initial outward current, followed by a small inward shift in holding current, paralleling the changes in membrane potential described above (Fig. 3). The average membrane resistance was increased by about 60 % during the inward shift in holding current (13.2 ± 4.1 GΩ compared with 8.2 ± 2.2 GΩ prior to histamine application, n = 8, P < 0.05, Student's paired t test). These results suggest that the inward shift in holding current and corresponding depolarization produced by histamine may result from inhibition of a K+ channel.
Figure 3. Membrane current response to prolonged application of histamine recorded in voltage-clamp using perforated whole-cell configuration.
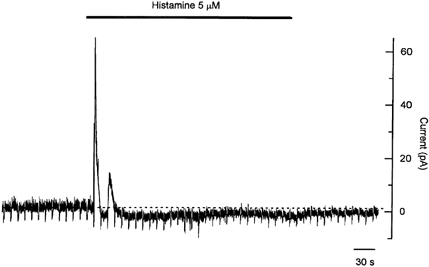
The holding potential was −50 mV, with a 1 s hyperpolarizing voltage step to −80 mV applied once every 10 s. Application of histamine caused a pronounced initial outward current that corresponded with the initial membrane hyperpolarization observed in membrane potential records. Note the second outward current in this cell, correlating with the multiple hyperpolarizations noted in other cells. The initial outward current was followed by a maintained inward shift in holding current that correlated with the sustained membrane depolarization. This inward current was associated with an increase in membrane resistance of about 60 %.
Role of K+ channels in histamine-induced responses
M-currents
Membrane depolarization is mediated in a variety of cells by closure of a class of K+ channel known as the M-channel, which was first described in neurons in lumbar sympathetic ganglia (Brown & Adams, 1980). M-currents are characterized by a gradual closure of the M-channel in response to membrane hyperpolarization, which gives rise to a slow inward current relaxation in response to hyperpolarizing voltage command-steps. Figure 4A shows whole-cell currents recorded in response to a series of hyperpolarizing voltage command-steps from a holding potential of −30 mV. At the holding potential of −30 mV, the holding current was considerably more positive (average 33.7 ± 3.3 pA, n = 7) than when the holding potential was −50 mV, as may be expected given that the M-current is characteristically an outwardly rectifying, non-inactivating K+ current. At the onset of the hyperpolarizing command-step there was a rapid ohmic change in the membrane current due to the prevailing conductance of the membrane (labelled a in the inset in Fig. 4A), followed by an obvious inward current relaxation that reversed in direction at around −80 mV (average −81.6 ± 1.8 mV, n = 7, Fig. 4B). Fast Na+ currents were activated on repolarization from steps more negative than −60 mV. However, in the absence of the fast Na+ current, the ohmic step in membrane current on repolarization at the end of the voltage command-step was smaller than at the beginning (see b compared with a in the inset in Fig. 4A), consistent with a reduction in membrane conductance associated with the closure of M-channels during the inward current relaxation.
Figure 4. Characteristics of the M-current in bovine chromaffin cells and its inhibition by histamine.
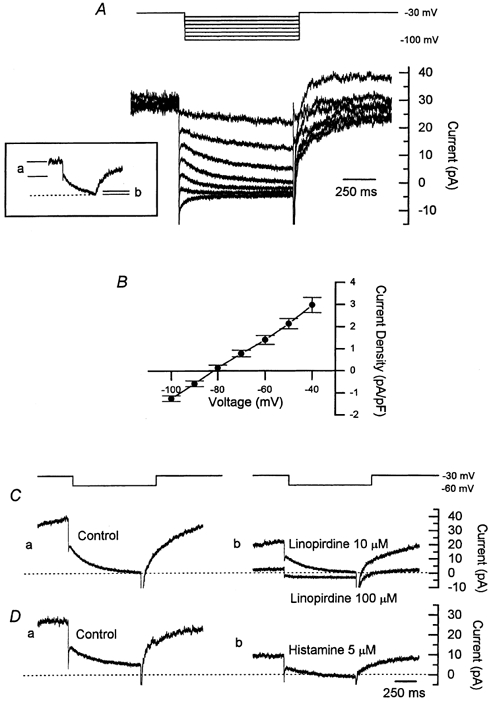
A, hyperpolarizing voltage steps from a holding potential of −30 mV gave rise to a slow inward current relaxation. This inward current relaxation is characteristic of an M-current and is due to the closure of the M-channels. The inset shows the inward current relaxation evoked by a step to −50 mV, highlighting the increase in membrane resistance occurring during the voltage step, consistent with channel closure, as indicated by the reduction in the ohmic current step represented by a and b. B, average current-voltage relation for seven cells showing a reversal potential for the current of about −80 mV. Current was measured over a 10 ms segment beginning 25 ms after the start of the voltage step. C, M-currents recorded in response to a voltage step to −60 mV from a holding potential of −30 mV in standard bath solution (a), and in the presence of linopirdine (10 and 100 μm, b). Linopirdine blocked the M-current in a concentration-dependent fashion. D, M-currents recorded in response to a voltage step to −60 mV in standard bath solution (a), and after exposure to histamine (5 μm) for 3 min. Histamine inhibited M-currents recorded in response to hyperpolarizing voltage steps by around 40 %. Records in A and D are from the same cell; records in C are from a different cell.
The properties of these M-currents were further characterized using linopirdine, a neurotransmitter release-enhancing drug that has been shown to inhibit M-currents in rat sympathetic neurons and NG108–15 cells (Lamas et al. 1997; Meves et al. 1999). Linopirdine caused a dramatic drop in resting holding current and a concentration-dependent decrease in the magnitude of the inward current relaxation (Fig. 4C). Thus, the inward current relaxation observed during a command-step to −70 mV was reduced by around 45 % at 10 μm linopirdine (average amplitudes in control and linopirdine were −10.3 ± 2.2 pA and −5.9 ± 1.7 pA respectively, n = 6), and by around 95 % at 100 μm linopirdine (average amplitudes in control and linopirdine were −11.4 ± 1.7 pA and −0.8 ± 0.3 pA respectively, n = 5). As described above, in voltage-clamped cells, application of histamine caused an initial outward current followed by a inward shift in holding current (see Fig. 3). Both the resting holding current and the amplitude of the inward M-current relaxation were reduced after exposure to histamine for 3 min (Fig. 4D). For a hyperpolarizing step from −30 to −70 mV, the average amplitude of the inward current relaxation was reduced by around 40 % (from −9.4 ± 1.5 to −5.4 ± 1.0 pA, n = 8). M-currents were not observed if Cs+-rich pipette solutions were used (data not shown). These results demonstrate for the first time the presence of an M-current in chromaffin cells, and indicate that histamine can cause a partial inhibition of this current. This M-current inhibition is consistent with the histamine-induced inward shift in holding current observed in voltage-clamp experiments.
To examine the role of M-channel inhibition in the sustained depolarization evoked by histamine, the effects of linopirdine on histamine responses were examined in current-clamp experiments. Linopirdine (100 μm) caused a rapid membrane depolarization of around 10 mV (from −54.4 ± 2.6 mV to −43.7 ± 1.0 mV, n = 5) that was associated with a marked increase in action potential frequency in all cells (Fig. 5). Prolonged exposure to linopirdine at this concentration resulted in a decline in spontaneous action potentials, such that after 10 min the remaining spontaneous action potentials were reduced in frequency and amplitude. These effects of linopirdine on action potential characteristics are consistent with our observation that linopirdine at this concentration also reduced currents through voltage-gated Ca2+ channels (data not shown). In the presence of linopirdine (100 μm), histamine continued to cause an initial hyperpolarization (average amplitude −35.8 ± 1.8 mV, average duration 13.4 ± 0.9 s, n = 5), but the average amplitude of the sustained depolarization was reduced to 3.8 ± 1.2 mV (n = 5). In the presence of linopirdine, there was no change in the frequency of action potential discharge during the application of histamine, but a clear interpretation of this result is complicated by the effects of linopirdine on voltage-gated Ca2+ channels mentioned above. This result suggests that histamine-induced M-channel inhibition contributes to the sustained depolarization, but the residual depolarization persisting in the presence of linopirdine suggests that histamine also causes depolarization via another mechanism.
Figure 5. Effect of linopirdine on the resting membrane potential and on the membrane potential response to prolonged application of histamine.
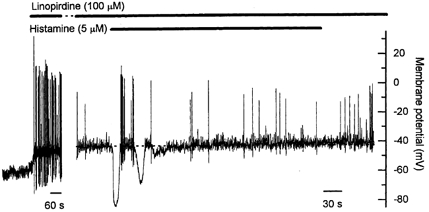
Linopirdine (100 μm), an inhibitor of M-channels, caused a rapid depolarization of the resting membrane potential that was associated with an increase in the frequency of action potentials. The initial histamine-induced hyperpolarization persisted in the presence of linopirdine, but the sustained depolarization was markedly reduced in amplitude. The increase in action potential frequency was not observed, though this may be in part due to non-specific effects of linopirdine (see Results for details). The gap represents 11 min. Note the different time scales in the two parts of the figure.
The role of other K+ channels in responses to histamine
To further assess the involvement of K+ channels in shaping responses to histamine, experiments were conducted using the perforated whole-cell technique with Cs+-rich pipette solutions. All cells were first patched with the normal perforated K+ pipette solution to confirm a positive response to brief histamine application, and then subsequently re-patched with a different pipette containing the Cs+-rich solution. Neither resting properties of the cells nor responses to histamine were significantly affected by sequential patching of a cell with two pipettes that both contained the perforated K+ pipette solution. Average resting membrane potential using the Cs+-rich pipette solution was −42.9 ± 1.5 mV (n = 5). Action potential frequency was considerably lower than that observed when using K+ pipette solutions, with the spontaneous discharge frequency never exceeding 0.1 Hz. Spontaneous action potentials were dramatically prolonged in all cells, consistent with the known blocking effect of internal Cs+ on voltage-gated K+ channels. Neither the initial hyperpolarization nor the sustained depolarization was observed in response to application of histamine when using the Cs+-rich pipette solution (Fig. 6A). Action potential frequency was also not altered by histamine (see Table 1). The finding that histamine failed to evoke a sustained depolarization when Cs+-rich pipette solutions were used, suggests that any mechanism activated by histamine to cause depolarization in addition to inhibition of the M-current probably also involves inhibition of a Cs+-sensitive K+ channel.
Figure 6. Effect of various K+ channel blockers on responses to prolonged application of histamine.
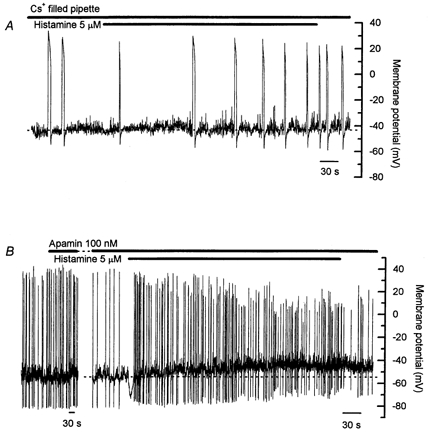
A, using Cs+ pipette solution the resting membrane potential was slightly less negative, at around −40 mV, than that recorded with the K+ pipette solution. Action potential duration was dramatically lengthened, and action potential frequency was low. Neither the histamine-induced hyperpolarization nor the sustained depolarization was observed. B, apamin (100 nm) caused a slight hyperpolarization of the resting membrane potential but had no effect on the characteristics of spontaneous action potentials. The initial histamine-induced hyperpolarization was reduced by around 40 % in apamin, though the sustained depolarization and increase in action potential frequency induced by histamine persisted. Note the different time scales in the two parts of panel B. The gap in B represents 5 min. Records in A and B are from different cells.
Previous studies have found that the Ca2+-activated K+ current activated by histamine in bovine chromaffin cells could be blocked by 75–80 % with apamin (Artalejo et al. 1993; Bödding, 2000). In the current study, apamin (100 nm) caused a slight hyperpolarization of the resting membrane potential (average prior to and after apamin −50.5 ± 2.0 mV and −53.8 ± 1.4 mV respectively, n = 6, P < 0.05, Student's paired t test). The amplitude of the histamine-induced hyperpolarization was reduced in apamin by around 40 % (average amplitude −15.0 ± 2.0 mV, average duration 9.1 ± 1.2 s in apamin, n = 6), and the average amplitude of the sustained depolarization was 8.7 ± 1.7 mV (n = 6, Fig. 6B). The frequency of spontaneous action potentials was increased and the average action potential amplitude reduced during the histamine-induced sustained depolarization in the presence of apamin, as seen in control responses (see Table 1). The amplitude of the afterhyperpolarization associated with the spontaneous action potentials was unaffected by apamin (average amplitude before apamin −22.6 ± 4.1 mV, n = 56 action potentials from six cells, average amplitude after apamin −21.2 ± 3.5 mV, n = 60 action potentials from six cells, P > 0.05, Student's paired t test), indicating that SK channels do not contribute to action potential afterhyperpolarization in these cells. These results suggest that inhibition of SK channels does not contribute to the histamine-induced sustained depolarization, and further that Ca2+ entering through the activated Ca2+ channels during the action potentials does not access the SK channels, though the SK channels are activated by released store Ca2+ and contribute to the histamine-induced hyperpolarization.
KATP and KIR channels
ATP-sensitive K+ (KATP) channels regulate resting membrane potential in a number of cells including neurons (Ashcroft & Rorsman, 1989; Ohno-Shosaku & Yamamoto, 1992; Hogg & Adams, 2001). In these cells, block of KATP channels by increasing intracellular ATP concentrations causes membrane depolarization. Further, recent studies have shown that this inhibition of KATP channels by ATP is reduced in the presence of the membrane phospholipids phosphatidylinositol 4,5-bisphosphate (PIP2) or phosphatidylinositol 4-phosphate (PIP) (Baukrowitz et al. 1998; Shyng & Nichols, 1998). Activation of H1 receptors by histamine in bovine chromaffin cells is known to activate phospholipase C, leading to hydrolysis of PIP2 (Bunn & Dunkley, 1997; Roberts-Thomson et al. 2000). Therefore, another possible mechanism through which histamine may cause depolarization is via a reduction in KATP current due to hydrolysis of membrane phospholipids. This possibility was tested using glibenclamide (10 μm), a known blocker of KATP channels. Glibenclamide was found to have no effect on either resting membrane potential (average prior to glibenclamide −52.7 ± 3.9 mV, and after −52.4 ± 5.0 mV, n = 3, P > 0.05, Student's paired t test), or on the membrane potential responses evoked by application of histamine (Fig. 7A, Table 1), so it is unlikely that KATP channels contribute to histamine-evoked responses in these cells.
Figure 7. Lack of involvement of KATP channels or inward rectifier K+ channels in the response to prolonged histamine application.
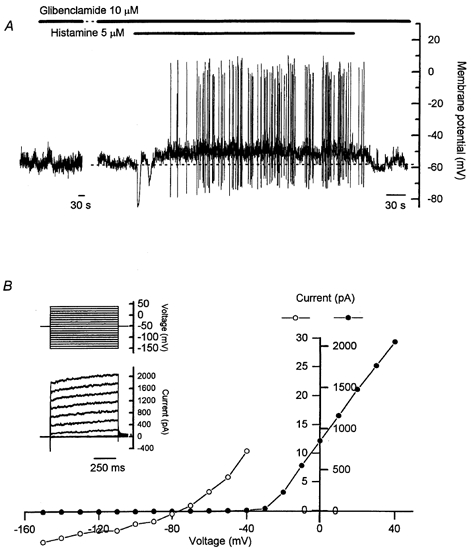
A, glibenclamide (10 μm) had no effect on either the resting membrane potential or the characteristics of spontaneous action potentials. Both the initial histamine-induced hyperpolarization and the sustained depolarization and increase in action potential frequency were unaffected by glibenclamide. B, no inward rectification of K+ currents was observed under voltage-clamp over the range −150 to −70 mV. The current-voltage relation in B was taken from the data shown in the inset, with current measurements made over the last 50 ms of the voltage step. Closed circles correlate with the scale shown on the right of the current axis and represent the current-voltage relation measured over all voltage steps from −150 to +40 mV. Open circles correlate with the scale shown on the left of the current axis and show the current-voltage relation for voltage steps over the range −150 to −40 mV on an expanded scale. Note the different time scales in the two parts of A. The gap in A represents 14 min. Records in A and B are from different cells.
Inwardly rectifying K+ (KIR) channels also contribute to setting the resting membrane potential in numerous cell types (Reiner & Kamondi, 1994). Histamine has been shown to inhibit KIR channels (Nilius et al. 1993), and the activity of cloned KIR channels can be regulated directly by PIP2 (Huang et al. 1998). However, in the current study, no evidence was found for an inwardly rectifying K+ current, with a linear current-voltage relation observed in response to command-steps over the range −150 to −70 mV from a holding potential of −50 mV (Fig. 7B). A contribution to the sustained depolarization evoked by histamine from inhibition of KIR channels is therefore unlikely.
Role of external Na+ in responses to histamine
As no other studies have documented the electro physiological response to sustained application of histamine in chromaffin cells, a number of other experiments have also been done to characterize the role of external Na+ and Ca2+ and the role of intracellular Ca2+ stores in this response.
The importance of fast Na+ channels in generating action potentials both at rest and in response to sustained application of histamine was examined using tetrodotoxin. Tetrodotoxin was used at 10 μm because a small, tetrodotoxin-insensitive, voltage-sensitive Na+ current was detected in these cells that was only blocked by high concentrations of tetrodotoxin (data not shown). In five of six cells, tetrodotoxin rapidly abolished all spontaneous action potentials (Fig. 8A). In the remaining cell, a discharge of spontaneous action potentials persisted, but both the frequency and amplitude of the action potentials was reduced. The initial membrane hyperpolarization in response to histamine persisted in the presence of tetrodotoxin (average amplitude −27.1 ± 1.9 mV, n = 6), though the average duration was increased compared with control cells (average duration 40.4 ± 13.0 s, n = 6, P < 0.01, ANOVA). The average amplitude of the sustained depolarization evoked by histamine in the presence of tetrodotoxin was 9.1 ± 1.6 mV (n = 6), and in all cells the depolarization was associated with a discharge of action potentials (Fig. 8A, Table 1). These observations clearly show that tetrodotoxin-resistant action potentials can occur during histamine stimulation, and raise the possibility that spontaneous action potentials, and action potentials evoked during histamine stimulation, may be initiated by separate mechanisms.
Figure 8. Effect of tetrodotoxin and replacement of external Na+ on membrane potential responses to prolonged application of histamine.
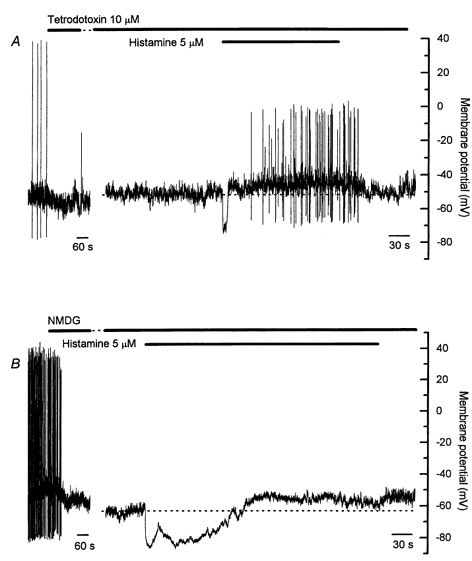
A, tetrodotoxin (10 μm) rapidly abolished spontaneous action potentials in most cells. Tetrodotoxin increased the duration of the initial hyperpolarization in response to histamine in 50 % of cells, but did not influence the sustained depolarization, which in all cells was associated with a discharge of tetrodotoxin-resistant action potentials. B, replacement of external Na+ with NMDG had variable effects on resting membrane potential. The initial histamine-induced hyperpolarization was markedly prolonged in the presence of NMDG. Action potentials were rarely observed in the presence of NMDG. Note the different time scales in the two parts of both A and B. The gap in A represents 4 min, and that in B represents 6 min. Records in A and B are from different cells.
The role played by external Na+ was further examined in experiments where external Na+ was replaced with an equimolar concentration of NMDG (Fig. 8B). NMDG itself had variable effects on resting membrane potential, causing a depolarization of 5–10 mV in three of six cells, and a hyperpolarization of 5–10 mV in the remaining cells. This observation suggests that while a resting Na+ conductance may contribute to setting the resting membrane potential in these cells, other resting conductances, such as a resting Cl− conductance or the activity of an electrogenic ion pump, must also be present. Spontaneous action potentials were only recorded in one cell in the presence of NMDG. The average amplitude of the histamine-induced hyperpolarization in the presence of NMDG was −26.9 ± 4.5 mV (n = 6) and, similar to responses in the presence of tetrodotoxin, the average hyperpolarization duration was increased compared with control cells (average duration 106.2 ± 26.1 s, n = 6, P < 0.001, ANOVA). The average amplitude of the histamine-induced sustained depolarization in the presence of NMDG was 5.8 ± 1.0 mV (n = 6), though sustained action potential discharge was only seen in one cell. These results confirm the importance of external Na+ in the generation of spontaneous action potentials, and may suggest that the action potentials evoked during histamine stimulation in the presence of tetrodotoxin are initiated by a Na+-dependent channel such as a non-selective cation channel or tetrodotoxin-insensitive Na+ channel.
Role of external Ca2+ and intracellular Ca2+ stores in responses to histamine
Involvement of internally released Ca2+ in the observed electrophysiological responses to histamine was assessed by depleting intracellular Ca2+ stores using a 20 min pretreatment with thapsigargin (100 nm). Resting membrane potential was unchanged by thapsigargin over the 20 min pretreatment (n = 7). After thapsigargin pretreatment, histamine failed to evoke the initial membrane hyperpolarization, but the sustained depolarization was still observed in all cells (Fig. 9A). The average amplitude of the sustained depolarization in these experiments was 6.8 ± 1.1 mV (n = 7). Spontaneous action potentials were observed in four of the seven cells in this series of experiments. Spontaneous action potential frequency was again increased during the histamine-induced sustained depolarization after thapsigargin pretreatment, and the reduction in action potential amplitude again noted (see Table 1). These results clearly demonstrate that histamine activates at least two intracellular pathways in bovine chromaffin cells. One pathway is dependent on intact intracellular stores of Ca2+ and, when activated, causes an initial transient membrane hyperpolarization, while the other pathway causes membrane depolarization in a manner independent of Ca2+ stores and in part by inhibiting M-channels. Further, these results suggest that release of Ca2+ from intracellular stores is not required for initiation of spontaneous action potentials.
Figure 9. Effect of thapsigargin and Ca2+-free bath solutions on membrane potential responses to prolonged application of histamine.
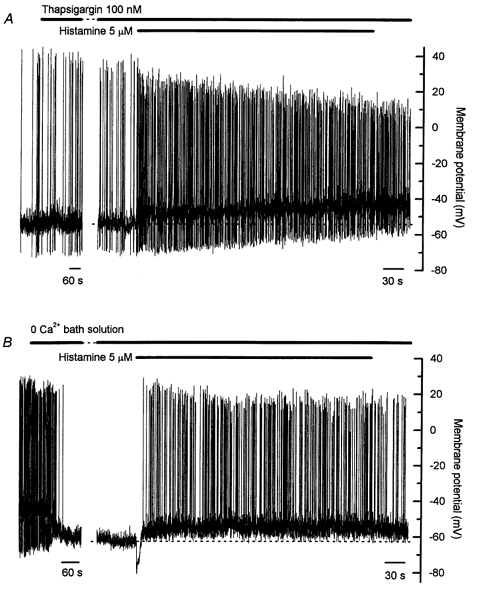
A, 20 min pretreatment with thapsigargin (100 nm) had no effect on either the resting membrane potential or the spontaneous action potentials, but the initial histamine-induced hyperpolarization was abolished. Both the sustained histamine-induced depolarization and the increase in action potential frequency persisted after thapsigargin pretreatment. B, Ca2+-free bath solution invariably caused a membrane hyperpolarization of around 15 mV and stopped the discharge of spontaneous action potentials in most cells. Both the initial histamine-induced hyperpolarization and the sustained depolarization persisted in Ca2+-free bath solution. The sustained depolarization was still associated with an increased action potential frequency, though the action potentials evoked in Ca2+-free solution were notably lacking afterhyperpolarizations. Note the different time scales in the two parts of both A and B. The gap in A represents 16 min, and that in B represents 5 min. Records in A and B are from different cells.
Experiments were also conducted in nominally Ca2+-free bath solution to assess the contribution made by Ca2+ conductances to the observed responses. Perfusion of Ca2+-free solution caused a membrane hyperpolarization of around −15 mV in all cells (n = 4, Fig. 9B). In two of the four cells, this hyperpolarization stopped the discharge of spontaneous action potentials. Changes in [Ca2+]o are known to affect gating of K+ channels through surface-potential effects (see Hille, 1992), and a recent study has demonstrated that reduction of [Ca2+]o can cause a −9 mV shift in the voltage-activation curve for shaker K+ channels (Hong et al. 2001). It is therefore possible that the hyperpolarization observed in the current study on perfusion with Ca2+-free solution results from a shift in K+ channel gating properties. In the two cells in which action potentials persisted, the action potentials observed in Ca2+-free solution were larger in amplitude than those observed in control bath solution, and lacked afterhyperpolarizations. The initial histamine-induced membrane hyperpolarization persisted in Ca2+-free solution (average amplitude −19.5 ± 0.8 mV, average duration 11.6 ± 1.2 s, n = 4), and a sustained depolarization of reduced amplitude was also observed (average amplitude 4.2 ± 2.0 mV, n = 4, Fig. 9B). In three of the four cells, the depolarization was associated with a maintained discharge of action potentials (Fig. 9B, see Table 1), and these action potentials also lacked afterhyperpolarizations. It is clear from these experiments that the afterhyperpolarization associated with action potentials in these cells is dependent on activation of Ca2+-activated K+ channels with an absolute requirement for Ca2+ entry during the action potential.
DISCUSSION
This study has demonstrated the presence of an M-current in bovine chromaffin cells and shown that histamine inhibits this current and causes a membrane depolarization associated with an increase in membrane resistance. Consistent with this, linopirdine, a known blocker of M-channels, inhibited the M-current observed in these cells and also caused a membrane depolarization similar to that observed in response to application of histamine. While histamine has previously been shown to inhibit a variety of K+ conductances in other preparations, including Ca2+-activated K+ (Haas & Konnerth, 1983), inward rectifier K+ (Nilius et al. 1993) and leak K+ conductances (Reiner & Kamondi, 1994; Li & Hatton, 1996), as far as we are aware this is the first demonstration of inhibition of an M-current by histamine. This study has also examined for the first time the electrical responses in bovine chromaffin cells induced by sustained exposure to histamine, and demonstrates a possible mechanism coupling histamine receptors to activation of voltage-operated Ca2+ channels in these cells.
M-currents in chromaffin cells
M-currents were initially described in neurons of amphibian lumbar sympathetic ganglia (Brown & Adams, 1980), and have subsequently been described in a number of other central and peripheral neurons (Constanti & Brown, 1981; Constanti & Galvan, 1983; Halliwell, 1986; Griffith et al. 1988; Moore et al. 1988; Womble & Moises, 1992). M-currents or M-like currents have also been shown in other cell types such as smooth muscle cells (Sims et al. 1985), lactotrophs (Sankaranarayanan & Simasko, 1996), rod photoreceptors (Wollmuth, 1994) and retinal pigment epithelial cells (Takahira & Hughes, 1997), as well as in PC12 cells (Villarroel et al. 1989; Villarroel, 1996). Characteristically, the M-current is a non-inactivating, outwardly rectifying K+ current with a half-activation voltage of around −40 mV. Another defining characteristic of the current is that the M-channels close gradually in response to hyperpolarizing voltage steps from depolarized potentials, giving rise to a slow inward current relaxation due to the closure of the open M-channels (Brown & Adams, 1980). The K+ current recorded in the present study exhibits this characteristic response to hyperpolarizing voltage steps, and is also blocked by linopirdine, a known inhibitor of M-channels (Lamas et al. 1997).
Holman et al. (1998) discussed the possibility that muscarinic agonists may inhibit an M-current in guinea-pig chromaffin cells on the basis of an observed increase in membrane resistance in response to application of acetylcholine or bethanecol. In addition, inhibition of an M-current by muscarine in rat PC12 cells, a tumour cell line initially derived from rat adrenal chromaffin cells, has also been reported (Villarroel et al. 1989; Villarroel, 1996). In the current study, we have shown that histamine inhibits the M-current observed in bovine chromaffin cells by around 40 % at a time that corresponds with the histamine-induced membrane depolarization. Consistent with this effect of histamine, direct inhibition of M-channels with linopirdine also results in membrane depolarization. This effect of linopirdine also indicates that M-channels in bovine chromaffin cells are active at rest and contribute to setting the resting membrane potential. The observation that the sustained depolarization was either absent or smaller in amplitude in open-tip whole-cell configuration may reflect the presence of a soluble intracellular messenger in the pathway causing inhibition of the M-channels (see Marrion, 1997). However, other studies have shown that the M-current is sensitive to the availability of hydrolysable ATP (Simmons & Schneider, 1998), or that these currents are not observed with open-tip whole-cell configuration (Xu & Adams, 1992), so it is also possible that the disturbance of the intracellular milieu inherent to the open-tip patch recording technique may prevent the observation of the effects of histamine on the M-current.
Recent molecular studies have shown that M-channels are coded for by members of the KCNQ K+ channel gene family, with expression of KCNQ2, KCNQ3, KCNQ4 or KCNQ5 giving rise to currents with characteristics similar to those of native M-currents (see Robbins, 2001). A possible molecular correlate for M-currents of small amplitude was described by Wang et al. (1998), who showed that that expression in oocytes of KCNQ2 or KCNQ3 alone gave rise to a current around eleven-fold smaller than that observed when both genes were expressed together, but no study has yet reported on the expression of these genes in chromaffin cells.
Effects of histamine
As discussed above, the sustained depolarization evoked by prolonged application of histamine results at least in part from inhibition by histamine of an M-current. However, as there is a significant residual depolarization evoked by histamine in the presence of linopirdine, it is likely that histamine can also cause depolarization in these cells through another mechanism. Histamine causes depolarization in gastrointestinal smooth muscle that is associated with a substantial increase in conductance, presumably through activation of non-selective cation channels (Bolton et al. 1981). While the results of the current study cannot exclude the possibility that the residual depolarization is due to activation of a similar channel, the finding that the depolarization is abolished by internal Cs+ implies that it is more likely to be due to inhibition of another type of K+ channel. As the results from the current study suggest that inhibition of KATP or KIR channels is unlikely to be involved in the histamine-induced depolarization, inhibition of a K+ leak conductance, as reported for cortical and supraoptic neurons (Reiner & Kamondi, 1994; Li & Hatton, 1996), emerges as the most likely explanation for the residual depolarization. This possibility remains to be tested.
The initial response to histamine was a rapid but transient membrane hyperpolarization, as observed previously by Artalejo et al. (1993) and Bödding (2000). These authors found that histamine caused an outward current associated with a concurrent increase in [Ca2+]i. They also showed that the outward current was due to activation of channels with a unitary conductance of about 4 pS, and that the hyperpolarization was reduced by about 75 % with high concentrations of apamin (1 μm), suggesting that histamine activated a population of SK Ca2+-activated K+ channels. We also found that the hyperpolarization was partially sensitive to apamin, and also showed that it was abolished by pretreatment of cells with thapsigargin. We suggest then that histamine causes release of Ca2+ from intracellular stores, and that this released Ca2+ activates a population of Ca2+-activated K+ channels to cause membrane hyperpolarization. Stauderman et al. (1990) and Bödding (2000) both showed that histamine could evoke several distinct releases of store Ca2+, leading to an oscillating level of [Ca2+]i, and it is likely that the oscillating pattern of hyperpolarizations seen in some cells in the current study (e.g. Fig 2A) corresponds with multiple store release events evoked by the sustained application of histamine. As the hyperpolarization was only reduced by 40 % with 100 nm apamin in the current study and that even with 1 μm apamin 20–30 % of the response remained (Artalejo et al. 1993), it seems likely that histamine activates both an SK channel and an apamin-insensitive Ca2+-activated K+ channel. Further, several studies have shown that histamine induces release of Ca2+ from caffeine-sensitive stores, presumably as a consequence of Ca2+-induced Ca2+ release (CICR), in addition to release from IP3-sensitive stores (Stauderman & Murawsky, 1991; Stauderman et al. 1991), raising the possibility that Ca2+ released from the different intracellular stores may differentially activate distinct populations of Ca2+-activated K+ channels.
The observation in the current study that the action potential afterhyperpolarization is lost in Ca2+-free bath solution indicates that these cells also possess a population of Ca2+-activated K+ channels that can be activated by Ca2+ entering through voltage-operated Ca2+ channels, most probably BK channels (Marty, 1981; Marty & Neher, 1985). The afterhyperpolarization was not affected by thapsigargin pretreatment indicating that CICR is not important for activation of Ca2+-activated K+ channels during the afterhyperpolarization, in contrast to several central and peripheral neurons (Sah & McLachlan, 1991; Jobling et al. 1993; Hillsley et al. 2000; Shah & Haylett, 2000; Vogalis et al. 2001). Further, as the afterhyperpolarization was unaffected by apamin, it is unlikely that SK channels play a significant role in the afterhyperpolarization. This suggests a refined distribution of the K+ channels in the cell membrane, such that the SK channels are functionally linked with the Ca2+ stores while being largely inaccessible to Ca2+ entering the cell through voltage-operated Ca2+ channels, while the BK channels are activated by Ca2+ entry.
Following the initial hyperpolarization, histamine caused a maintained depolarization that was associated with an increase in action potential frequency. This depolarization was not seen in two previous studies (Artalejo et al. 1993; Bödding, 2000), presumably because they both used open-tip patch techniques and this phase of the histamine response is smaller or absent in open-tip patch recordings (see Fig. 2C). As discussed above, our results indicate that this depolarization results in part from inhibition of an M-current. Histamine has been reported to depolarize a wide variety of central neurons by a variety of different mechanisms (Brown et al. 2001). However, to our knowledge histamine-induced depolarization by inhibition of an M-current has not been previously reported in any tissue.
Linopirdine had a similar effect to histamine on the frequency of spontaneous action potentials, and this suggests that the main cause of the increase in action potential frequency during sustained histamine application is the combination of the depolarization and increased membrane resistance associated with the closure of M-channels. The observed reduction in action potential amplitude in the presence of histamine is likely to be largely due to increased inactivation of Na+ channels at the more depolarized membrane potential (Fenwick et al. 1982b). However, histamine has been shown in bovine chromaffin cells to cause a reduction in Ca2+ currents through a pertussis toxin-insensitive pathway (Currie & Fox, 2000). As it has also been suggested that feedback of released transmitters may also inhibit Ca2+ currents in bovine chromaffin cells (Doupnik & Pun, 1994), a phenomenon that could be due to activation of either α2-adrenergic (Kleppisch et al. 1992), opioid (Albillos et al. 1996) or P2Y purinergic receptors (Gandia et al. 1993; Powell et al. 2000), it is unclear whether this inhibitory effect of histamine on Ca2+ currents is direct or indirect. Regardless, it is possible that a mechanism causing a reduction in Ca2+ currents may also contribute to the observed reduction in action potential amplitude.
Resting electrical properties of bovine chromaffin cells
The spontaneous action potentials observed in chromaffin cells in the current study appear to have both Na+ and Ca2+ components, the former being indicated by the sensitivity of action potentials to tetrodotoxin, the latter by the lack of afterhyperpolarization observed in Ca2+-free bath solutions. Similar observations have previously been made regarding the action potentials recorded in chromaffin cells from other species (Nassar-Gentina et al. 1988; Holman et al. 1994). The mechanism initiating the spontaneous action potentials has yet to be established, but given the observation that spontaneous action potentials were abolished by tetrodotoxin it is likely that they are initiated by a membrane potential event that is supra-threshold for activation of fast tetrodotoxin-sensitive Na+ channels. However, it is clear that there is also another tetrodotoxin-insensitive mechanism capable of initiating action potentials during the histamine-induced depolarization. Considering that action potentials were rarely recorded after substitution of Na+ with NMDG, it seems likely that these action potentials result from activation of tetrodotoxin-insensitive Na+ channels similar to those found in cardiac muscle (Gellens et al. 1992) and in some sensory neurons (Caffrey et al. 1992; Akopian et al. 1996). Even though the tetrodotoxin-insensitive Na+ currents observed in these cells are small, the high input resistance of the cells, compounded by the additional membrane resistance associated with the closure of the M-channels during the histamine-induced depolarization, may result in a deflection in membrane potential large enough to recruit the voltage-operated Ca2+ channels and generate a full action potential. These observations are also consistent with the finding that histamine-induced secretion of catecholamines from these cells is largely insensitive to tetrodotoxin (O'Farrell & Marley, 1999).
We conclude that sustained application of histamine to bovine chromaffin cells causes a membrane depolarization that results in part from inhibition of an M-current and that this depolarization, and the increase in membrane resistance associated with M-channel closure, promotes cell excitability resulting in an increase in action potential frequency. These action potentials cause extracellular Ca2+ entry through VOCCs, which in turn evokes catecholamine secretion. This mechanism may be shared by other G-protein-coupled receptor agonists that promote secretion from chromaffin cells.
Acknowledgments
This project was supported by grant 980675 from the Australian NH & MRC, and by equipment grants from the Clive and Vera Ranaciotti Foundation, the ANZ Charitable Trust and the University of Melbourne. P. D. M. is an NH & MRC Principal Research Fellow. We would like to thank Drs Fivos Vogalis and Lea Delbridge and Professor David Adams for their help and comments.
REFERENCES
- Akopian AN, Sivilotti L, Wood JN. A tetrodotoxin-resistant voltage-gated sodium channel expressed by sensory neurons. Nature. 1996;379:257–262. doi: 10.1038/379257a0. [DOI] [PubMed] [Google Scholar]
- Albillos A, Carbone E, Gandia L, Garcia AG, Pollo A. Opioid inhibition of Ca2+ channel subtypes in bovine chromaffin cells: selectivity of action and voltage-dependence. European Journal of Neuroscience. 1996;8:1561–1570. doi: 10.1111/j.1460-9568.1996.tb01301.x. [DOI] [PubMed] [Google Scholar]
- Artalejo AR, Garcia AG, Neher E. Small-conductance Ca2+-activated K+ channels in bovine chromaffin cells. Pflügers Archiv. 1993;423:97–103. doi: 10.1007/BF00374966. [DOI] [PubMed] [Google Scholar]
- Ashcroft FM, Rorsman P. Electrophysiology of the pancreatic beta-cell. Progress in Biophysics and Molecular Biology. 1989;54:87–143. doi: 10.1016/0079-6107(89)90013-8. [DOI] [PubMed] [Google Scholar]
- Barbara JG, Lemos VS, Takeda K. Pre- and post-synaptic muscarinic receptors in thin slices of rat adrenal gland. European Journal of Neuroscience. 1998;10:3535–3545. doi: 10.1046/j.1460-9568.1998.00349.x. [DOI] [PubMed] [Google Scholar]
- Baukrowitz T, Schulte U, Oliver D, Herlitze S, Krauter T, Tucker SJ, Ruppersberg JP, Fakler B. PIP2 and PIP as determinants for ATP inhibition of KATP channels. Science. 1998;282:1141–1144. doi: 10.1126/science.282.5391.1141. [DOI] [PubMed] [Google Scholar]
- Biales B, Dichter M, Tischler A. Electrical excitability of cultured adrenal chromaffin cells. Journal of Physiology. 1976;262:743–753. doi: 10.1113/jphysiol.1976.sp011618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolton TB, Clark JP, Kitamura K, Lang RJ. Evidence that histamine and carbachol may open the same ion channels in longitudinal smooth muscle of guinea-pig ileum. Journal of Physiology. 1981;320:363–379. doi: 10.1113/jphysiol.1981.sp013955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bödding M. Histamine evoked sustained elevations of cytosolic Ca2+ in bovine adrenal chromaffin cells independently of Ca2+ entry. Cell Calcium. 2000;27:139–151. doi: 10.1054/ceca.1999.0100. [DOI] [PubMed] [Google Scholar]
- Brown DA, Adams PR. Muscarinic suppression of a novel voltage-sensitive K+ current in a vertebrate neurone. Nature. 1980;283:673–676. doi: 10.1038/283673a0. [DOI] [PubMed] [Google Scholar]
- Brown RE, Stevens DR, Haas HL. The physiology of brain histamine. Progress in Neurobiology. 2001;63:637–672. doi: 10.1016/s0301-0082(00)00039-3. [DOI] [PubMed] [Google Scholar]
- Bunn SJ, Boyd TL. Characterization of histamine-induced catecholamine secretion from bovine adrenal medullary chromaffin cells. Journal of Neurochemistry. 1992;58:1602–1610. doi: 10.1111/j.1471-4159.1992.tb10031.x. [DOI] [PubMed] [Google Scholar]
- Bunn SJ, Dunkley PR. Histamine-stimulated phospholipase C signalling in the adrenal chromaffin cell: effects on inositol phospholipid metabolism and tyrosine hydroxylase phosphorylation. Clinical and Experimental Pharmacology and Physiology. 1997;24:624–631. doi: 10.1111/j.1440-1681.1997.tb02103.x. [DOI] [PubMed] [Google Scholar]
- Caffrey JM, Eng DL, Black JA, Waxman SG, Kocsis JD. Three types of sodium channels in adult rat dorsal root ganglion neurons. Brain Research. 1992;592:283–297. doi: 10.1016/0006-8993(92)91687-a. [DOI] [PubMed] [Google Scholar]
- Chang R S L, Tran VT, Snyder SH. Characteristics of histamine H1-receptors in peripheral tissues labelled with [3H]mepyramine. Journal of Pharmacology and Experimental Therapeutics. 1979;209:437–442. [PubMed] [Google Scholar]
- Cheek TR, Morgan A, O'Sullivan AJ, Moreton RB, Berridge MJ, Burgoyne RD. Spatial localisation of agonist-induced Ca2+ entry in bovine adrenal chromaffin cells: different patterns induced by histamine and angiotensin II, and relationship to catecholamine release. Journal of Cell Science. 1993;105:913–921. doi: 10.1242/jcs.105.4.913. [DOI] [PubMed] [Google Scholar]
- Constanti A, Brown DA. M-Currents in voltage-clamped mammalian sympathetic neurones. Neuroscience Letters. 1981;24:289–294. doi: 10.1016/0304-3940(81)90173-7. [DOI] [PubMed] [Google Scholar]
- Constanti A, Galvan M. M-current in voltage-clamped olfactory cortex neurones. Neuroscience Letters. 1983;39:65–70. doi: 10.1016/0304-3940(83)90166-0. [DOI] [PubMed] [Google Scholar]
- Cruzblanca H, Koh DS, Hille B. Bradykinin inhibits M current via phospholipase C and Ca2+ release from IP3-sensitive Ca2+ stores in rat sympathetic neurons. Proceedings of the National Academy of Sciences of the USA. 1998;95:7151–7156. doi: 10.1073/pnas.95.12.7151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Currie KP, Fox AP. Voltage-dependent, pertussis toxin insensitive inhibition of calcium currents by histamine in bovine adrenal chromaffin cells. Journal of Neurophysiology. 2000;83:1435–1442. doi: 10.1152/jn.2000.83.3.1435. [DOI] [PubMed] [Google Scholar]
- Douglas WW, Kanno T, Sampson SR. Influence of the ionic environment on the membrane potential of adrenal chromaffin cells and on the depolarizing effect of acetylcholine. Journal of Physiology. 1967;191:107–121. doi: 10.1113/jphysiol.1967.sp008239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doupnik CA, Pun RY. G-protein activation mediates prepulse facilitation of Ca2+ channel currents in bovine chromaffin cells. Journal of Membrane Biology. 1994;140:47–56. doi: 10.1007/BF00234485. [DOI] [PubMed] [Google Scholar]
- Fenwick EM, Marty A, Neher E. A patch-clamp study of bovine chromaffin cells and of their sensitivity to acetylcholine. Journal of Physiology. 1982a;331:577–597. doi: 10.1113/jphysiol.1982.sp014393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenwick EM, Marty A, Neher E. Sodium and calcium channels in bovine chromaffin cells. Journal of Physiology. 1982b;331:599–635. doi: 10.1113/jphysiol.1982.sp014394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gandia L, Garcia AG, Morad M. ATP modulation of calcium channels in chromaffin cells. Journal of Physiology. 1993;470:55–72. doi: 10.1113/jphysiol.1993.sp019847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gellens ME, George AL, Jr, Chen LQ, Chahine M, Horn R, Barchi RL, Kallen RG. Primary structure and functional expression of the human cardiac tetrodotoxin-insensitive voltage-dependent sodium channel. Proceedings of the National Academy of Sciences of the USA. 1992;89:554–558. doi: 10.1073/pnas.89.2.554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goh Y, Kurosawa A. Characterization and Ca2+ requirement of histamine-induced catecholamine secretion in cultured bovine chromaffin cells. Journal of Neurochemistry. 1991;57:1249–1257. doi: 10.1111/j.1471-4159.1991.tb08286.x. [DOI] [PubMed] [Google Scholar]
- Griffith WH, Hills JM, Brown DA. Substance P-mediated membrane currents in voltage-clamped guinea pig inferior mesenteric ganglion cells. Synapse. 1988;2:432–441. doi: 10.1002/syn.890020411. [DOI] [PubMed] [Google Scholar]
- Haas HL, Konnerth A. Histamine and noradrenaline decrease calcium-activated potassium conductance in hippocampal pyramidal cells. Nature. 1983;302:432–434. doi: 10.1038/302432a0. [DOI] [PubMed] [Google Scholar]
- Halliwell JV. M-current in human neocortical neurones. Neuroscience Letters. 1986;67:1–6. doi: 10.1016/0304-3940(86)90198-9. [DOI] [PubMed] [Google Scholar]
- Häppöalä O, Soinila S, Päivärinta H, Joh TH, Panula P. Histamine-immunoreactive endocrine cells in the adrenal medulla of the rat. Brain Research. 1985;339:393–396. doi: 10.1016/0006-8993(85)90113-1. [DOI] [PubMed] [Google Scholar]
- Hille B. Ionic Channels of Excitable Membranes. 2. Sunderland, MA, USA: Sinauer Associates Inc.; 1992. [Google Scholar]
- Hillsley K, Kenyon JL, Smith TK. Ryanodine-sensitive stores regulate the excitability of AH neurons in the myenteric plexus of guinea-pig ileum. Journal of Neurophysiology. 2000;84:2777–2785. doi: 10.1152/jn.2000.84.6.2777. [DOI] [PubMed] [Google Scholar]
- Hogg RC, Adams DJ. An ATP-sensitive K+ conductance in dissociated neurones from adult rat intracardiac ganglia. Journal of Physiology. 2001;534:713–720. doi: 10.1111/j.1469-7793.2001.00713.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holman ME, Coleman HA, Tonta MA, Parkington HC. Synaptic transmission from splanchnic nerves to the adrenal medulla of guinea-pigs. Journal of Physiology. 1994;478:115–124. doi: 10.1113/jphysiol.1994.sp020235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holman ME, Tonta MA, Coleman HA, Parkington HC. Muscarinic receptor activation in guinea-pig chromaffin cells causes decreased membrane conductance and depolarization. Journal of the Autonomic Nervous System. 1998;68:140–144. doi: 10.1016/s0165-1838(97)00122-7. [DOI] [PubMed] [Google Scholar]
- Hong KH, Armstrong CM, Miller C. Revisiting the role of Ca2+ in Shaker K+ channel gating. Biophysical Journal. 2001;80:2216–2220. doi: 10.1016/S0006-3495(01)76194-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang CL, Feng S, Hilgemann DW. Direct activation of inward rectifier potassium channels by PIP2 and its stabilization by Gβγ. Nature. 1998;391:803–806. doi: 10.1038/35882. [DOI] [PubMed] [Google Scholar]
- Inoue M, Fujishiro N, Imanaga I. Hypoxia and cyanide induce depolarization and catecholamine release in dispersed guinea-pig chromaffin cells. Journal of Physiology. 1998;507:807–818. doi: 10.1111/j.1469-7793.1998.807bs.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jobling P, McLachlan EM, Sah P. Calcium induced calcium release is involved in the afterhyperpolarization in one class of guinea pig sympathetic neurone. Journal of the Autonomic Nervous System. 1993;42:251–257. doi: 10.1016/0165-1838(93)90370-a. [DOI] [PubMed] [Google Scholar]
- Kleppisch T, Ahnert-Hilger G, Gollasch M, Spicher K, Hescheler J, Schultz G, Rosenthal W. Inhibition of voltage-dependent Ca2+ channels via alpha 2-adrenergic and opioid receptors in cultured bovine adrenal chromaffin cells. Pflügers Archiv. 1992;421:131–137. doi: 10.1007/BF00374819. [DOI] [PubMed] [Google Scholar]
- Lamas JA, Selyanko AA, Brown DA. Effects of a cognition-enhancer, linopirdine (DuP 996), on M-type potassium currents (IKM) and some other voltage- and ligand-gated membrane currents in rat sympathetic neurons. European Journal of Neuroscience. 1997;9:605–616. doi: 10.1111/j.1460-9568.1997.tb01637.x. [DOI] [PubMed] [Google Scholar]
- Li Z, Hatton GI. Histamine-induced prolonged depolarization in rat supraoptic neurons: G-protein-mediated, Ca2+-independent suppression of K+ leakage conductance. Neuroscience. 1996;70:145–158. doi: 10.1016/0306-4522(95)00373-q. [DOI] [PubMed] [Google Scholar]
- Livett BG. Adrenal medullary chromaffin cells in vitro. Physiological Reviews. 1984;64:1103–1161. doi: 10.1152/physrev.1984.64.4.1103. [DOI] [PubMed] [Google Scholar]
- Livett BG, Marley PD. Effects of opioid peptides and morphine on histamine-induced catecholamine secretion from cultured, bovine adrenal chromaffin cells. British Journal of Pharmacology. 1986;89:327–334. doi: 10.1111/j.1476-5381.1986.tb10264.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marley PD, Bunn SJ, Wan DCC, Allen AM, Mendelsohn FAO. Localization of angiotensin II binding sites in the bovine adrenal medulla using a labelled specific antagonist. Neuroscience. 1989;28:777–787. doi: 10.1016/0306-4522(89)90022-5. [DOI] [PubMed] [Google Scholar]
- Marrion NV. Control of M-current. Annual Review of Physiology. 1997;59:483–504. doi: 10.1146/annurev.physiol.59.1.483. [DOI] [PubMed] [Google Scholar]
- Marty A. Ca2+-dependent K+ channels with large unitary conductance in chromaffin cell membranes. Nature. 1981;291:497–500. doi: 10.1038/291497a0. [DOI] [PubMed] [Google Scholar]
- Marty A, Neher E. Potassium channels in cultured bovine adrenal chromaffin cells. Journal of Physiology. 1985;367:117–141. doi: 10.1113/jphysiol.1985.sp015817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meves H, Schwarz JR, Wulfsen I. Separation of M-like current and ERG current in NG108-15 cells. British Journal of Pharmacology. 1999;127:1213–1223. doi: 10.1038/sj.bjp.0702642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore SD, Madamba SG, Joels M, Siggins GR. Somatostatin augments the M-current in hippocampal neurons. Science. 1988;239:278–280. doi: 10.1126/science.2892268. [DOI] [PubMed] [Google Scholar]
- Nassar-gentina V, Pollard HB, Rojas E. Electrical activity in chromaffin cells of intact mouse adrenal gland. American Journal of Physiology. 1988;254:C675–683. doi: 10.1152/ajpcell.1988.254.5.C675. [DOI] [PubMed] [Google Scholar]
- Nilius B, Schwarz G, Droogmans G. Modulation by histamine of an inwardly rectifying potassium channel in human endothelial cells. Journal of Physiology. 1993;472:359–371. doi: 10.1113/jphysiol.1993.sp019951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noble EP, Bommer M, Liebisch D, Herz A. H1-histaminergic activation of catecholamine release by chromaffin cells. Biochemical Pharmacology. 1988;37:221–228. doi: 10.1016/0006-2952(88)90721-6. [DOI] [PubMed] [Google Scholar]
- Noble EP, Bommer M, Sincini E, Costa T, Herz A. H1-Histaminergic activation stimulates inositol-1-phosphate accumulation in chromaffin cells. Biochemical and Biophysical Research Communications. 1986;135:566–573. doi: 10.1016/0006-291x(86)90031-8. [DOI] [PubMed] [Google Scholar]
- O'Farrell M, Marley PD. Multiple calcium channels are required for pituitary adenylate cyclase-activating polypeptide-induced catecholamine secretion from bovine cultured adrenal chromaffin cells. Naunyn-Schmiedeberg's Archives of Pharmacology. 1997;356:536–542. doi: 10.1007/pl00005088. [DOI] [PubMed] [Google Scholar]
- O'Farrell M, Marley PD. Different contributions of voltage-sensitive Ca2+ channels to histamine-induced catecholamine release and tyrosine hydroxylase activation in bovine adrenal chromaffin cells. Cell Calcium. 1999;25:209–217. doi: 10.1054/ceca.1999.0025. [DOI] [PubMed] [Google Scholar]
- O'Sullivan AJ, Cheek TR, Moreton RB, Berridge MJ, Burgoyne RD. Localization and heterogeneity of agonist-induced changes in cytosolic calcium concentration in single bovine adrenal chromaffin cells from video imaging of fura-2. The EMBO Journal. 1989;8:401–411. doi: 10.1002/j.1460-2075.1989.tb03391.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohno-Shosaku T, Yamamoto C. Identification of an ATP-sensitive K+ channel in rat cultured cortical neurons. Pflügers Archiv. 1992;422:260–266. doi: 10.1007/BF00376211. [DOI] [PubMed] [Google Scholar]
- Pender N, Burgoyne RD. Histamine stimulates exocytosis in a sub-population of bovine adrenal chromaffin cells. Neuroscience Letters. 1992;144:207–210. doi: 10.1016/0304-3940(92)90751-r. [DOI] [PubMed] [Google Scholar]
- Plevin R, Boarder MR. Stimulation of formation of inositol phosphates in primary cultures of bovine adrenal chromaffin cells by angiotensin II, histamine, bradykinin, and carbachol. Journal of Neurochemistry. 1988;51:634–641. doi: 10.1111/j.1471-4159.1988.tb01085.x. [DOI] [PubMed] [Google Scholar]
- Powell AD, Teschemacher AG, Seward EP. P2Y purinoceptors inhibit exocytosis in adrenal chromaffin cells via modulation of voltage-operated calcium channels. Journal of Neuroscience. 2000;20:606–616. doi: 10.1523/JNEUROSCI.20-02-00606.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiner PB, Kamondi A. Mechanisms of antihistamine-induced sedation in the human brain: H1 receptor activation reduces a background leakage potassium current. Neuroscience. 1994;59:579–588. doi: 10.1016/0306-4522(94)90178-3. [DOI] [PubMed] [Google Scholar]
- Robbins J. KCNQ potassium channels: physiology, pathophysiology, and pharmacology. Pharmacology and Therapeutics. 2001;90:1–19. doi: 10.1016/s0163-7258(01)00116-4. Roberts-Thomson, EL, Saunders, HI, Palmer, SM, Powis, DA, Dunkley, PR, Bunn, SJ, (2000). Ca2+ influx stimulated phospholipase C activity in bovine adrenal chromaffin cells: responses to K+ depolarization and histamine, European Journal of Pharmacology, 398, 199-207. [DOI] [PubMed] [Google Scholar]
- Sah P, McLachlan EM. Ca2+-activated K+ currents underlying the afterhyperpolarization in guinea pig vagal neurons: a role for Ca2+-activated Ca2+ release. Neuron. 1991;7:257–264. doi: 10.1016/0896-6273(91)90264-z. [DOI] [PubMed] [Google Scholar]
- Sankaranarayanan S, Simasko SM. Characterization of an M-like current modulated by thyrotropin-releasing hormone in normal rat lactotrophs. Journal of Neuroscience. 1996;16:1668–1678. doi: 10.1523/JNEUROSCI.16-05-01668.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah M, Haylett DG. Ca2+ channels involved in the generation of the slow afterhyperpolarization in cultured rat hippocampal pyramidal neurons. Journal of Neurophysiology. 2000;83:2554–2561. doi: 10.1152/jn.2000.83.5.2554. [DOI] [PubMed] [Google Scholar]
- Shyng SL, Nichols CG. Membrane phospholipid control of nucleotide sensitivity of KATP channels. Science. 1998;282:1138–1141. doi: 10.1126/science.282.5391.1138. [DOI] [PubMed] [Google Scholar]
- Simmons MA, Schneider CR. Regulation of M-type potassium current by intracellular nucleotide phosphates. Journal of Neuroscience. 1998;18:6254–6260. doi: 10.1523/JNEUROSCI.18-16-06254.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sims SM, Singer JJ, Walsh JV., Jr Cholinergic agonists suppress a potassium current in freshly dissociated smooth muscle cells of the toad. Journal of Physiology. 1985;367:503–529. doi: 10.1113/jphysiol.1985.sp015837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stauderman KA, McKinney RA, Murawsky MM. The role of caffeine-sensitive Ca2+ stores in agonist- and inositol 1,4,5-trisphosphate-induced Ca2+ release from bovine adrenal chromaffin cells. Biochemical Journal. 1991;278:643–650. doi: 10.1042/bj2780643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stauderman KA, Murawsky MM. The inositol 1,4,5-trisphosphate-forming agonist histamine activates a ryanodine-sensitive Ca2+ release mechanism in bovine adrenal chromaffin cells. Journal of Biological Chemistry. 1991;266:19 150–19 153. [PubMed] [Google Scholar]
- Stauderman KA, Murawsky MM, Pruss RM. Agonist-dependent patterns of cytosolic Ca2+ changes in single bovine adrenal chromaffin cells: relationship to catecholamine release. Cell Regulation. 1990;1:683–691. doi: 10.1091/mbc.1.9.683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stauderman KA, Pruss RM. Different patterns of agonist-stimulated increases of 3H-inositol phosphate isomers and cytosolic Ca2+ in bovine adrenal chromaffin cells: comparison of the effects of histamine and angiotensin II. Journal of Neurochemistry. 1990;54:946–953. doi: 10.1111/j.1471-4159.1990.tb02342.x. [DOI] [PubMed] [Google Scholar]
- Takahira M, Hughes BA. Isolated bovine retinal pigment epithelial cells express delayed rectifier type and M-type K+ currents. American Journal of Physiology. 1997;273:C790–803. doi: 10.1152/ajpcell.1997.273.3.C790. [DOI] [PubMed] [Google Scholar]
- Tuominen RK, Karhunen T, Panula P, Yamatodani A. Endogenous histamine in cultured bovine adrenal chromaffin cells. European Journal of Neuroscience. 1993;5:1436–1441. doi: 10.1111/j.1460-9568.1993.tb00211.x. [DOI] [PubMed] [Google Scholar]
- Villarroel A. M-current suppression in PC12 cells by bradykinin is mediated by a pertussis toxin-insensitive G-protein and modulated by intracellular calcium. Brain Research. 1996;740:227–233. doi: 10.1016/s0006-8993(96)00870-0. [DOI] [PubMed] [Google Scholar]
- Villarroel A, Marrion NV, Lopez H, Adams PR. Bradykinin inhibits a potassium M-like current in rat pheochromocytoma PC12 cells. FEBS Letters. 1989;255:42–46. doi: 10.1016/0014-5793(89)81057-9. [DOI] [PubMed] [Google Scholar]
- Vogalis F, Furness JB, Kunze WA. Afterhyperpolarization current in myenteric neurons of the guinea pig duodenum. Journal of Neurophysiology. 2001;85:1941–1951. doi: 10.1152/jn.2001.85.5.1941. [DOI] [PubMed] [Google Scholar]
- Wallace DJ, Marley PD. Activation of voltage-operated Ca2+ channels by histamine in chromaffin cells does not require Ca2+ release from intracellular stores. Proceedings of the Australian Neuroscience Society. 2001;12:35. [Google Scholar]
- Wang HS, Pan Z, Shi W, Brown BS, Wymore RS, Cohen IS, Dixon JE, McKinnon D. KCNQ2 and KCNQ3 potassium channel subunits: molecular correlates of the M-channel. Science. 1998;282:1890–1893. doi: 10.1126/science.282.5395.1890. [DOI] [PubMed] [Google Scholar]
- Warashina A. Involvement of protein kinase C in homologous desensitization of histamine-evoked secretory responses in rat chromaffin cells. Brain Research. 1997;762:40–46. doi: 10.1016/s0006-8993(97)00346-6. [DOI] [PubMed] [Google Scholar]
- Wollmuth LP. Mechanism of Ba2+ block of M-like K channels of rod photoreceptors of tiger salamanders. Journal of General Physiology. 1994;103:45–66. doi: 10.1085/jgp.103.1.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Womble MD, Moises HC. Muscarinic inhibition of M-current and a potassium leak conductance in neurones of the rat basolateral amygdala. Journal of Physiology. 1992;457:93–114. doi: 10.1113/jphysiol.1992.sp019366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu ZJ, Adams DJ. Resting membrane potential and potassium currents in cultured parasympathetic neurones from rat intracardiac ganglia. Journal of Physiology. 1992;456:405–424. doi: 10.1113/jphysiol.1992.sp019343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zerbes M, Bunn SJ, Powis DA. Histamine causes Ca2+ entry via both a store-operated and a store-independent pathway in bovine adrenal chromaffin cells. Cell Calcium. 1998;23:379–386. doi: 10.1016/s0143-4160(98)90094-x. [DOI] [PubMed] [Google Scholar]
- Zhou Z, Misler S. Action potential-induced quantal secretion of catecholamines from rat adrenal chromaffin cells. Journal of Biological Chemistry. 1995;270:3498–3505. [PubMed] [Google Scholar]


