Abstract
The components and properties of the delayed rectifier K+ current (IK) in isolated guinea-pig sino-atrial (SA) node pacemaker cells were investigated using the whole-cell configuration of the patch-clamp technique. An envelope of tails test was conducted by applying depolarizing pulses from a holding potential of −50 mV to +30 mV for various durations ranging from 40 to 2000 ms. The ratio of the tail current amplitude elicited upon return to the holding potential to the magnitude of the time-dependent outward current activated during depolarizing steps was dependent on the pulse duration, while after exposure to the selective IKr inhibitor E-4031 (5 μm) this current ratio became practically constant irrespective of the pulse duration. These observations are consistent with the presence of the E-4031-sensitive, rapidly activating and E-4031-resistant, slowly activating components of IK (IKr and IKs, respectively) in guinea-pig SA node cells. The activation range for IKr, defined as the E-4031-sensitive current (half-maximal activation voltage (V1/2) of −26.2 mV) was much more negative than that for IKs, defined as the E-4031-resistant current (V1/2 of +17.2 mV). IKr exhibited a marked inward rectification at potentials positive to −50 mV, whereas IKs showed only a slight rectification. In the current-clamp experiments, bath application of E-4031 (0.5 and 5 μm) initially slowed the repolarization at potentials negative to approximately −30 mV and produced a significant depolarization of the maximum diastolic potential, followed by the arrest of electrical activity, thus indicating that the late phase of the repolarization leading to the maximum diastolic potential at around −60 mV in spontaneous action potentials is primarily produced by IKr in guinea-pig SA node cells. External application of the selective IKs inhibitor 293B (30 μm) also delayed the repolarization process at potentials negative to about −20 mV and induced moderate depolarization of the maximum diastolic potential leading to the arrest of the spontaneous activity. These results provide evidence to suggest that both IKr and IKs are present and play crucial roles in the spontaneous electrical activity of guinea-pig SA node pacemaker cells.
It has been demonstrated that the spontaneous electrical activity of the sino-atrial (SA) node pacemaker cells in the mammalian heart is generated by the interaction of multiple ionic currents (for a review see Irisawa et al. 1993). An inward current is generated by the hyperpolarization-activated cation current (If; DiFrancesco et al. 1986), the background non-selective cation current (Hagiwara et al. 1992), the sustained inward current (Guo et al. 1995) and the T- and L-type Ca2+ currents (ICa,T and ICa,L, respectively; Hagiwara et al. 1988; Doerr et al. 1989), while the delayed rectifier K+ current (IK) mainly contributes outward current for the generation of pacemaking activity in SA node cells under physiological conditions. Spontaneous openings of the muscarinic K+ channels are also known to produce a substantial outward current which may affect the electrical activity of the pacemaker cells (Ito et al. 1994). The activation of IK during the plateau phase of spontaneous action potentials plays an important role in the repolarization of the cell membrane toward the K+ equilibrium potential, while a gradual deactivation of IK at negative potentials during membrane repolarization, together with the activation of the inward currents, is responsible for generating the slow diastolic depolarization (pacemaker potential). IK thus plays a crucial role not only in the repolarizing phase but also in the pacemaker depolarization in the spontaneous action potentials of SA node cells.
Electrophysiological and pharmacological studies have revealed two kinetically and pharmacologically distinct components of IK in atrial and ventricular myocytes of a variety of mammalian species, including guinea-pig (Sanguinetti & Jurkiewicz, 1990a, b, 1991), dog (Liu & Antzelevitch, 1995; Gintant, 1996) and humans (Wang et al. 1994; Li et al. 1996). IKr activates rapidly in response to depolarization to levels positive to −40 mV, displays a marked inward rectification and is specifically blocked by lanthanum ions and by methanesulfonanilide class III antiarrhythmic drugs such as E-4031, sotalol and dofetilide. In contrast, IKs activates more slowly and at more depolarized potentials, shows a weak inward rectification and is resistant to these drugs. In recent years, evidence has been presented regarding the molecular structures of these two distinct IK channels; human ether-à-go-go related gene (HERG) protein coassembles with the minK-related peptide 1 (MiRP1) to form IKr channels (Curran et al. 1995; Sanguinetti et al. 1995; Trudeau et al. 1995; Abbott et al. 1999), whereas, in coassembly with the minK, the potassium channel protein KVLQT1 encodes the IKs channels (Barhanin et al. 1996; Sanguinetti et al. 1996).
It is unclear at present whether these two components of IK are distributed in the SA node pacemaker cells of mammalian species. Previous studies on IK in this cell type have been mostly confined to the rabbit heart, and in this preparation it has been shown that IKr is readily distinguished and is essential for the development of spontaneous action potentials (Shibasaki, 1987; Ono & Ito, 1995; Verheijck et al. 1995; Lei & Brown, 1996). However, such a functional role for IKr in the SA node pacemaking activity has not yet been studied in detail in other mammalian species. On the other hand, in guinea-pig SA node cells IK has been reported to be largely composed of one component which resembles IKs (Anumonwo et al. 1992; Freeman & Kass, 1993).
The present study was undertaken to characterize the components and properties of IK in isolated SA node pacemaker cells of guinea-pig using the whole-cell patch-clamp technique together with a potent methanesulfonanilide IKr blocker E-4031. Our results provide the first detailed evidence to show that IKr is indeed present and plays an essential role in the late repolarization in spontaneously contracting SA node cells of the guinea-pig heart.
METHODS
SA node cell isolation
Single SA node cells were isolated from the hearts of guinea-pigs (250–400 g body weight) using an enzymatic dispersion procedure similar to that described previously (Guo et al. 1997). Briefly, guinea-pigs were deeply anaesthetized with an overdose of sodium pentobarbitone (80 mg kg−1, i.p.) and then the chest cavity was opened under artificial respiration. The ascending aorta was cannulated in situ and the heart was then excised and retrogradely perfused via the aortic cannula on a Langendorff perfusion apparatus at 37 °C, initially for 4 min with normal Tyrode solution and then for 4 min with nominally Ca2+-free Tyrode solution. This was followed by 8–12 min of perfusion with nominally Ca2+-free Tyrode solution containing 0.4 mg ml−1 collagenase (Wako Pure Chemical Industries, Osaka, Japan). All these solutions were oxygenated with 100 % O2. The digested heart was then removed from the Langendorff perfusion apparatus, and the SA node region was dissected out and cut perpendicular to the crista terminalis into small strips measuring about 0.5 mm in width. These SA node tissue strips were further digested at 37 °C for 20 min with nominally Ca2+-free Tyrode solution containing 1.0 mg ml−1 collagenase and 0.1 mg ml−1 elastase (Boehringer Mannheim, Mannheim, Germany). Finally, the enzyme-digested SA node strips were mechanically agitated in a high-K+, low-Cl− Kraftbrühe (KB) solution (Isenberg & Klöckner, 1982) to disperse the cells. The isolated cells thus obtained were then stored at 4 °C in the KB solution until required for experimental use.
All these experimental procedures were reviewed and approved by the Shiga University of Medical Science Animal Care Committee, Japan.
Whole-cell patch-clamp technique and data analysis
The membrane potentials and whole-cell currents were recorded using the whole-cell patch-clamp technique (Hamill et al. 1981) in the current- and voltage-clamp modes, respectively. An EPC-7 patch-clamp amplifier (List-electronic, Darmstadt, Germany) was used for these recordings. The patch electrodes were fabricated from glass capillaries (o.d., 1.5 mm; i.d., 0.9 mm; Narishige Scientific Instrument Laboratory, Tokyo, Japan) using a three-stage horizontal microelectrode puller (P-80; Sutter Instrument Co., Novato, CA, USA), and the tips were then fire-polished with a microforge. Electrode resistance was 2.0–4.0 MΩ when filled with the pipette solution. An aliquot of dissociated cells was transferred to a recording chamber (0.5 ml in volume) mounted on the stage of an inverted microscope (TMD300, Nikon, Tokyo) and superfused at a constant flow rate of 2 ml min−1 with normal Tyrode solution at 35–37 °C. After a tight seal (resistance, 5–50 GΩ) was established between the electrode tip and the cell membrane by gentle suction (−20 to −30 cm H2O), the membrane patch was ruptured by a brief period of more vigorous suction, controlled manually with a 2.5 ml syringe.
The SA node cells were identified by the presence of both spontaneous action potentials recorded in the current-clamp mode and the hyperpolarization-activated cation current (If) recorded in the voltage-clamp mode during superfusion with normal Tyrode solution. IK was activated by the depolarizing test pulses, under conditions where the Na+ channels (INa) were inactivated by setting the holding potential to −40 or −50 mV and the L-type Ca2+ channels (ICa,L) were blocked by adding 0.4 μm nisoldipine to the normal Tyrode solution. Nisoldipine was shown to have no effect on the cardiac IK at this concentration (Sanguinetti & Jurkiewicz, 1991). Under these experimental conditions changes in amplitude of IK after rupture of the patch membrane were monitored by measuring the outward tail currents elicited on repolarization to a holding potential of −50 mV following a 500 ms depolarizing step to +30 mV every 8 s. IK was found to reach a steady level (about 60–80 % of the initial value) within 3–5 min of membrane rupture and thereafter remained practically stable for at least 10 min. Accordingly, IK was measured during the time period approximately between 4 and 12 min after rupture of the patch membrane. However, there was a gradual decline (run-down) in the amplitude of If over this time period; the amplitude of If activated during hyperpolarization to −100 mV at 4 and 12 min after membrane rupture was 86.8 ± 3.5 % and 79.3 ± 2.9 % of the initial amplitude (498 ± 18 pA, n = 4), respectively, thus showing that If at −100 mV was reduced by 37 ± 8 pA on average during this time period (between 4 and 12 min after rupture of the patch membrane).
Current-clamp experiments showed that spontaneous activity was maintained in a regular rhythm at a firing rate of ≥ 150 min−1 for at least 8 min in approximately 70 % of cells examined (12 out of 17 cells), although the spontaneous rhythm became irregular, decelerated or even stopped after rupture of the patch membrane in the remaining 30 % of cells (5 out of 17). The effect of E-4031 or 293B was therefore examined in cells which were found to exhibit stable spontaneous action potentials for at least 1 min after establishment of whole-cell recording. These current-clamp experiments were usually completed within about 7 min (see Fig. 8 and Fig. 9). The voltage- and current-clamp experiments were thus conducted in an approximately similar time period after the whole-cell clamp mode was established.
Figure 8. Effect of block of IKr by E-4031 on the spontaneous electrical activity.
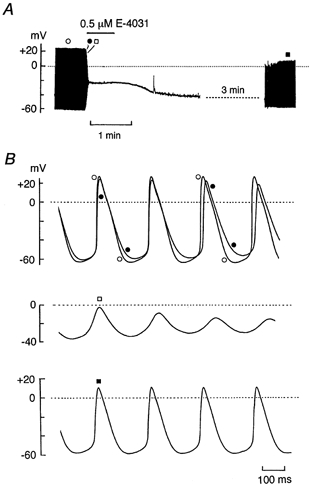
A, chart record of membrane potential before, during the exposure to and after washing off 0.5 μm E-4031. E-4031 was added to normal Tyrode solution during the period indicated by the horizontal bar. B, action potentials on a faster time scale recorded before (top trace, •), 3 s (top trace, ○) and 6 s (middle trace, □) after exposure to E-4031, and about 5.5 min after washout of the drug (bottom trace, ▪). Firing rates of spontaneous activity before exposure to (○) and after washing off (▪) the drug were 262 and 255 min−1, respectively.
Figure 9. Effect of chromanol 293B on spontaneous action potentials.
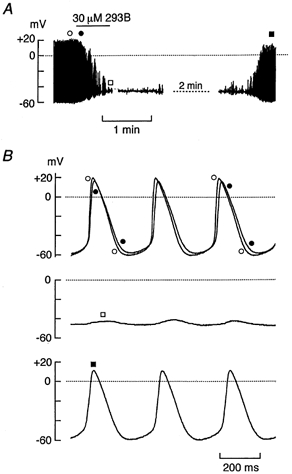
A, a spontaneously active SA node cell at a firing rate of 192 min−1 was exposed to 30 μm 293B for 40 s, as indicated by the horizontal bar over the chart record of membrane potential. Membrane potential recordings at points indicated by symbols are demonstrated in B on a faster time scale. B, top trace shows spontaneous action potentials recorded before application of 293B (○) superimposed with those recorded 8 s after drug application (•). Middle and bottom traces show the membrane potentials recordings 40 s after drug application (□) and those about 4 min after washout of the drug (▪), respectively. The firing rate after washing off the drug was 181 min−1.
The current and voltage signals were stored on a digital audiotape (DM120, Hitachi Maxell, Tokyo, Japan) using a PCM data recorder (RD-101T, TEAC, Tokyo, Japan) and were later fed to the computer (PC98RL, NEC, Tokyo, Japan) every 1 ms through a low-pass filter (48 dB per octave, E-3201A, NF, Yokohama, Japan) at a cut-off frequency of 3 kHz for data analysis. Cell membrane capacitance (Cm) was calculated from the capacitive transients elicited by 20 ms voltage-clamp steps (± 5 mV) according to the relationship (Bénitah et al. 1993): Cm = τCI0/ΔVm(1 − I∞/I0), where τC is the time constant of the capacitive transient, I0 is the initial peak current amplitude, I∞ is the steady state current value, and ΔVm is the amplitude of the voltage step. The sampling rate for these measurements of cell capacitance was 50 kHz with a low-pass 10 kHz filter. The average values for τC and Cm in SA node cells used in the present study were 0.257 ± 0.023 ms and 38.9 ± 1.8 pF (mean ± s.e.m.; n = 15 cells), respectively. The access resistance (Ra), estimated by dividing τC by Cm, averaged 6.5 ± 0.7 MΩ (n = 15). The tail current amplitudes of IKr and IKs were divided by the cell capacitance to provide an estimate of current density (pA pF−1).
Results are expressed as the mean ± s.e.m.; n indicates the number of cells studied. Statistical comparisons were made using the Student's t test, and differences were regarded as significant at the 95 % confidence level.
Solutions and chemicals
Normal Tyrode solution contained (mm): NaCl 140, KCl 5.4, CaCl2 1.8, MgCl2 0.5, NaH2PO4 0.33, glucose 5.5 and Hepes 5.0 (pH adjusted to 7.4 with NaOH). The nominally Ca2+-free Tyrode solution used for isolating the cells was prepared by simply omitting CaCl2 from the normal Tyrode solution. The extracellular solution used for measuring whole-cell IK was normal Tyrode solution supplemented with 0.4 μm nisoldipine (a generous gift from Bayer, Germany). Nisoldipine was prepared as a 1 mm stock solution in ethanol. E-4031 (N-(4-((1-(2-(6-methyl-2-pyridinyl) ethyl)-4-piperidinyl) carbonyl)phenyl)methanesulfonamide dihydrochloride dihydrate; a generous gift from Eisai Pharmaceutical Co., Tokyo, Japan) was dissolved in distilled water as a 1 mm stock solution and then added to the extracellular solution to give a final concentration of either 0.5 or 5 μm in some experiments as indicated. The chromanol derivative 293B (trans-6-cyano-4-(N-ethylsulphonyl-N-methylamino)-3-hydroxy-2,2-dimethyl-chromane; a generous gift from Hoechst Marion Roussel, Frankfurt, Germany) was dissolved in dimethylsulfoxide (DMSO, Sigma Chemical Co., MO, USA) to make a 100 mm stock solution and then diluted in the external solution to achieve a concentration of 30 μm. The pipette solution contained (mm): potassium aspartate 70, KCl 50, KH2PO4 10, MgSO4 1, Na2ATP 3, Li2GTP 0.1, EGTA 5, CaCl2 2 and Hepes 5 (pH adjusted to 7.2 with KOH). The concentration of free Ca2+ in the pipette solution was calculated to be approximately 0.1 μm (Fabiato & Fabiato, 1979; Tsien & Rink, 1980). The KB solution for cell preservation contained (mm): potassium glutamate 70, KCl 30, KH2PO4 10, MgCl2 1, taurine 20, EGTA 0.3, glucose 10 and Hepes 10 (pH adjusted to 7.2 with KOH).
RESULTS
Characteristics of guinea-pig SA node pacemaker cells
When single cells enzymatically dissociated from the SA node region of the guinea-pig heart were superfused with normal Tyrode solution, less than 5 % of the cells showed spontaneous and regular contraction. These pacemaker SA node cells were typically spindle-shaped with faint striations and had a small bulge around their centre. Such a scarce presence of pacemaker SA node cells in guinea-pig heart appears to be consistent with previous morphological studies demonstrating that the amount of cells in the primary pacemaker area of the guinea-pig (less than 1000 cells) was much less than that of the rabbit (about 5000 cells; Bleeker et al. 1980; Opthof et al. 1985). In the present study, all electrophysiological measurements were made from these spontaneously and regularly contracting SA node cells.
Figure 1 demonstrates a representative recording of membrane potential and whole-cell currents of an isolated guinea-pig SA node cell superfused with normal Tyrode solution. As illustrated in Fig. 1A, an SA node cell exhibited spontaneous action potentials that arose smoothly from the preceding slow diastolic depolarization (pacemaker depolarization). The firing rate of spontaneous action potentials and the maximum diastolic potential recorded from isolated guinea-pig SA node cells were 205 ± 11 min−1 and −59.3 ± 1.8 mV (n = 20), respectively. These values are comparable to those of rabbit SA node cells (Ono & Ito, 1995; Verheijck et al. 1995). Figure 1B shows membrane currents in response to 500 ms depolarizing (up to +40 mV) and hyperpolarizing (up to −120 mV) test pulses applied in 10 mV steps from a holding potential of −40 mV. The depolarizing clamp steps first activated an inward current which peaked at potentials around 0 mV (Fig. 1C) and was sensitive to inhibition by 0.4 μm nisoldipine (see Fig. 3A), thus confirming that the inward current is due to the L-type Ca2+ current (ICa,L). During depolarization the inactivation of the inward ICa,L was followed by the time-dependent increase in outward current representing an activation of IK, while a slowly decaying outward tail current associated with the deactivation of IK was observed after a return to a holding potential of −40 mV (Fig. 1B, top traces).
Figure 1. Membrane potential and whole-cell currents recorded from an isolated guinea-pig SA node pacemaker cell.
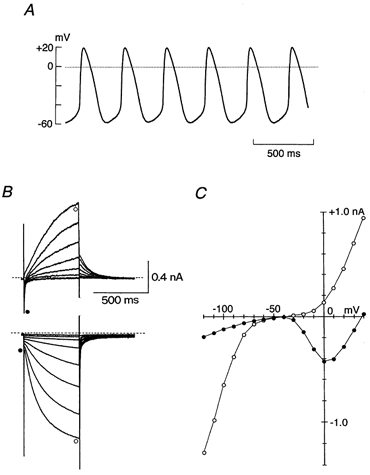
A, spontaneous action potentials recorded from a regularly contracting SA node cell in the normal Tyrode solution under current-clamp mode. Maximum diastolic potential, −58 mV; firing rate, 178 min−1. B, superimposed current traces elicited by 500 ms command steps to membrane potentials of −to +40 mV (top traces) and −50 to −120 mV (bottom traces) in 10 mV steps applied from a holding potential of −40 mV. The dashed lines indicate the zero current level. These voltage-clamp records were obtained from the same cell as in A. C, current-voltage (I–V) relationships measured at the initial (•) and end (○) of 500 ms voltage steps. Initial current was measured either at the peak of the ICa,L during the depolarizing test pulse or immediately after the decay of the capacitive transient during the hyperpolarizing test pulse. The late current was measured near the end of the 500 ms voltage step. Continuous lines through data points were fitted by eye.
Figure 3. Voltage-dependence of IKr activation.
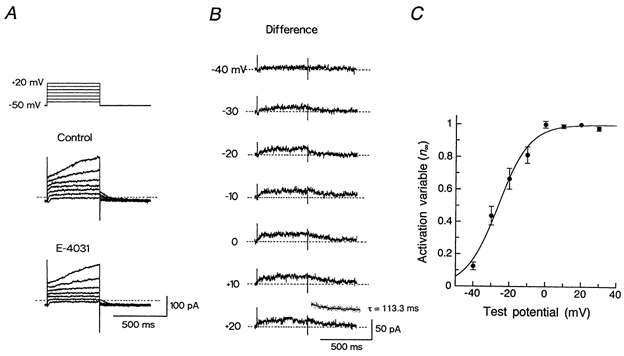
A, voltage-clamp protocol (top) and superimposed current traces in response to 500 ms voltage steps to membrane potentials of −40 to +20 mV applied in 10 mV steps from a holding potential of −50 mV, under control conditions (top traces) and during the exposure to 5 μm E-4031 (bottom traces). B, IKr, obtained by digitally subtracting the current trace in the presence of E-4031 from the control trace at each test potential (shown in A). Inset, single exponential fit (continuous line) to the tail current (dotted points) elicited following depolarization to +20 mV with a time constant (τ) of 113.3 ms. C, peak amplitude of IKr tail currents elicited upon return to the holding potential was normalized with reference to its amplitude at +20 mV and plotted against the test potential. The continuous curve was drawn by a least-squares fit of a Boltzmann equation, yielding V½ of −26.2 mV and k of 8.7 mV (see text). Data represent the means ± s.e.m. of three cells.
Changes in the membrane currents in response to hyperpolarizing pulses were characterized by an instantaneous current jump of small amplitude in the inward direction followed by the time- and voltage-dependent activation of the inward current (Fig. 1B, bottom traces), thus showing that the hyperpolarization-activated cation current (If) is present while the inwardly rectifying K+ current (IK1) is either absent or very small in guinea-pig SA node pacemaker cells. The activation of If, detected as the difference between the initial (Fig. 1C, •) and late current levels (Fig. 1C, ○) during hyperpolarizations, became evident at levels negative to −60 mV.
Two components of IK revealed by the envelope of tails test
We next examined the components and properties of IK in guinea-pig SA node pacemaker cells, under conditions in which ICa,L was blocked by adding 0.4 μm nisoldipine to the normal Tyrode solution. An envelope of tails test (Noble & Tsien, 1969) was conducted to clarify whether IK arises from a single or multiple component of IK. An SA node pacemaker cell was depolarized from a holding potential of −50 mV to +30 mV for various durations ranging from 40 to 2000 ms (Fig. 2A, top) and the amplitude of the tail current (IK,tail) elicited upon repolarization to the holding potential after the depolarizing test pulses was compared with the magnitude of the outward current (IK,pulse) activated during depolarization for each test pulse duration (n = 3). Under control conditions, the IK,tail elicited following brief depolarizations (40 and 60 ms) was greater than or nearly equal to the IK,pulse, while IK,pulse predominated over IK,tail at longer pulse durations (≥100 ms; Fig. 2A, top traces and Fig. 2B, top traces). The ratio of IK,tail to IK,pulse progressively declined with the lengthening of the pulse duration and reached a practically steady value of about 0.38 at a pulse duration of 500 ms (Fig. 2C, ○). The current ratio was thus dependent upon the duration of the pulse, suggesting the presence of more than one component of IK in SA node cells. After the same cell was exposed to 5 μm E-4031 to selectively inhibit IKr, there was a marked decrease in IK,tail elicited following brief depolarizations (Fig. 2A, bottom traces and Fig. 2B, bottom traces), and the ratio of IK,tail to IK,pulse became fairly constant (approximately 0.35) over an entire range of pulse durations (Fig. 2C, •), suggesting that only one component remains in the presence of the drug. These observations are consistent with the presence of E-4031-sensitive and E-4031-resistant components of IK (IKr and IKs, respectively) in guinea-pig SA node pacemaker cells.
Figure 2. Envelope of tails test for IK under control conditions and during exposure to E-4031.
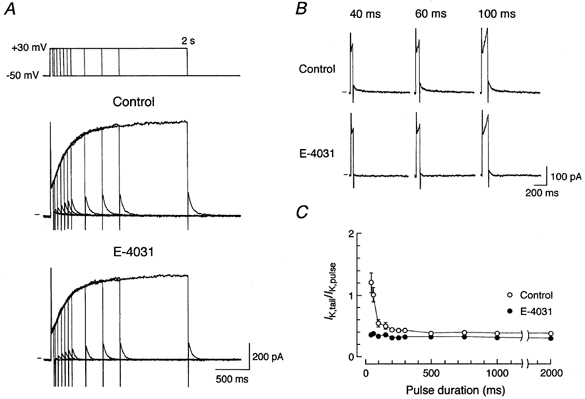
A, voltage protocol (top) and superimposed current traces elicited by depolarizing pulses from a holding potential of −50 mV to +30 mV for various durations (40, 60, 100, 150, 200, 250, 300, 500, 750, 1000 and 2000 ms) under control conditions (top traces) and after 3 min exposure to 5 μm E-4031 (bottom traces). The zero current level is indicated to the left of the current records by the horizontal line. B, expanded current traces in response to depolarizing steps of 40 ms (left-hand traces), 60 ms (middle traces) and 100 ms (right-hand traces) in duration under control conditions (top traces) and during exposure to 5 μm E-4031 (bottom traces), from data in A. C, ratio of peak tail current amplitude elicited upon return to a holding potential of −50 mV (IK,tail) to the amplitude of time-dependent currents activated during the depolarizing test pulses (IK,pulse) plotted as a function of pulse duration, under control conditions (○) and during the exposure to E-4031 (•). IK,tail was determined by digitally subtracting the steady state current level from the peak current level measured after the step to a holding potential of −mV. IK,pulse was determined by digital subtraction of the current level measured after a decay of the capacitive transient from that at the end of the depolarizing pulse. Data represent the means ± s.e.m. of three cells.
Electrophysiological properties of IKr in guinea-pig SA node cells
We investigated the electrophysiological properties of IKr, determined as an E-4031-sensitive current, in an SA node cell. Figure 3 shows a representative analysis of the voltage-dependence of IKr activation. IK was activated by 500 ms depolarizing steps to membrane potentials between −40 and +20 mV in the absence (Fig. 3A, top traces) and the presence (bottom traces) of 5 μm E-4031, and IKr at each test potential was obtained by digitally subtracting IK in the presence of E-4031 from that in its absence (Fig. 3B). The activation of IKr in response to depolarizing steps was relatively rapid, reaching a steady level within a few hundred milliseconds. The voltage dependence of IKr activation was evaluated by measuring the amplitude of IKr tail current elicited on return to −50 mV (n = 3), which reflects the degree of activation at the preceding depolarizing test potential. The activation variable (n∞) was then obtained by normalizing the amplitude of the tail current at each test potential with reference to its peak amplitude at +20 mV, and was plotted as a function of membrane potential to provide the steady state activation curve (Fig. 3C). The relationship between n∞ and the test potential was reasonably well fitted by a Boltzmann equation:
| (1) |
where Vm is the test potential, V½ is the voltage at which the activation is half-maximal (−26.2 mV), and k is the slope factor (8.7 mV). The values of V½ and k are comparable to those reported for IKr in other cardiac preparations including guinea-pig atrial cells (V½, −19.3 mV; k, 5.2 mV; Sanguinetti & Jurkiewicz, 1991), guinea-pig ventricular cells (V½, −21.5 mV; k, 7.5 mV; Sanguinetti & Jurkiewicz, 1990a), rabbit SA node cells (V½, −25.1 mV; k, 7.4 mV; Shibasaki, 1987; V½, −23.2 mV; k, 10.6 mV; Ono & Ito, 1995), human atrial cells (V½, −14.0 mV; k, 6.5 mV; Wang et al. 1994) and human ventricular cells (V½, −14.0 mV; k, 7.7 mV; Li et al. 1996). Deactivation kinetics of IKr at a membrane potential of −50 mV were assessed by fitting a single exponential function to the outward tail current and a time constant of 113.3 ms was obtained, as shown in the inset of Fig. 3B. The current density of IKr, obtained by normalizing the peak amplitude of IKr tail current measured upon return to the −50 mV holding potential following depolarization to +20 mV with reference to the cell capacitance, was 0.45 ± 0.03 pA pF−1 (n = 6).
A relatively small outward current of IKr activated during depolarizing voltage steps was unchanged or somewhat reduced in amplitude with the increasing magnitude of depolarization to positive potentials (Fig. 3B). This observation is consistent with the presence of the inwardly rectifying property of IKr, which has been proposed to arise from a fast transition of the channels to an inactivated state either from an open state or directly from a closed state during depolarizing steps (Shibasaki, 1987; Sanguinetti & Jurkiewicz, 1990a; Ono & Ito, 1995; Sanguinetti et al. 1995; Trudeau et al. 1995; Smith et al. 1996; Spector et al. 1996). We assessed the conductance properties of IKr in guinea-pig SA node pacemaker cells by measuring the amplitude of outward tail currents during a double-pulse protocol; the cell was first depolarized to +20 mV for 500 ms to fully activate IKr (see Fig. 3C), and then repolarized to potentials ranging from +10 to −70 mV before (Fig. 4A, •) and during (Fig. 4A, ○) the exposure to 5 μm E-4031. It should be noted that membrane currents during hyperpolarization to −70 mV both in the absence and presence of E-4031 exhibited a time-dependent increase in the inward direction, which appears to reflect the activation of If.
Figure 4. Inwardly rectifying properties of IKr.
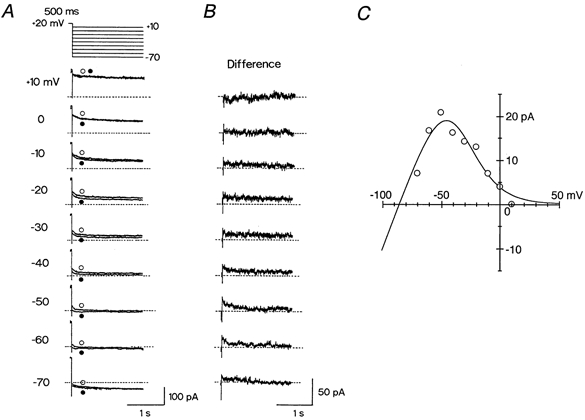
A, voltage-clamp protocol (top panel) and the membrane currents elicited by 2 s voltage steps to potentials of +10 to −70 mV in 10 mV steps after 500 ms depolarization to +20 mV. Membrane currents were recorded at all test potentials initially in the absence of 5 μm E-4031 (○) and then during its presence (•). Test potential is indicated to the left of the current traces. Dashed line indicates the zero current level (also in B). B, difference current at each test potential obtained by digital subtraction of current trace in the presence of E-4031 (•) from the corresponding trace in its absence (○) (shown in A). C, peak amplitudes of IKr tail currents plotted as a function of test potential. A continuous line through the data point represents the least-squares fit of eqns (2) and (3).
An outward tail current of IKr at each test potential was measured as the E-4031-sensitive current, obtained by the digital subtraction of the current trace in the presence of E-4031 from that in its absence. The IKr tail current was not detected at +10 mV but did become evident at negative potentials (≤ −10 mV, Fig. 4B), indicating that channel inactivation was alleviated during the step back to these negative potentials. Previous studies have proposed that repolarization to negative potentials is accompanied by rapid recovery of channels from inactivation to the open state followed by the gradual transitions to the closed state (deactivation), thereby giving rise to slowly decaying outward tail currents (Shibasaki, 1987; Sanguinetti & Jurkiewicz, 1990a; Ono & Ito, 1995; Sanguinetti et al. 1995; Trudeau et al. 1995; Smith et al. 1996; Spector et al. 1996). Figure 4C illustrates the peak amplitude of IKr tail current plotted against the test potential, which clearly shows a prominent inward rectification at potentials positive to −50 mV. The amplitude of IKr tail current at around and negative to EK was too small to be accurately isolated by use of this subtraction method, since concurrently activated If frequently exhibited a run-down in the course of the experiments (DiFrancesco et al. 1986; Ono & Ito, 1995).
Given that this inward rectification is due to the voltage-dependent inactivation of the channels, the amplitude of IKr can be described as:
| (2) |
and
| (3) |
where GK,r is the maximum conductance of IKr, Pi,∞ is the steady state inactivation given by a Boltzmann equation (eqn (3)), V½ is the voltage at which steady state inactivation is half maximal, k is the slope factor, and ER is the reversal potential. Assuming ER to be −85 mV, a calculated equilibrium potential for K+ under the present experimental conditions (Sanguinetti & Jurkiewicz, 1990a, 1991; Ono & Ito, 1995), the data points could be reasonably well fitted by eqn (2) and eqn (3) with V½ of −35.4 mV and k of 13.4 mV, supporting the view that inward rectification of IKr in guinea-pig SA node cells is caused by a voltage-dependent inactivation process (Shibasaki, 1987; Sanguinetti & Jurkiewicz, 1990a; Ono & Ito, 1995; Sanguinetti et al. 1995; Trudeau et al. 1995; Smith et al. 1996; Spector et al. 1996). These parameters relating to the voltage dependence of IKr inactivation are comparable either to those reported for IKr in other cardiac preparations (rabbit SA node cells: V½, −28.6 mV; k, 17.1 mV; Ono & Ito, 1995; V½, −42.1 mV; k, 9.35 mV, Ho et al. 1996; guinea-pig ventricular cells: V½, −9.0 mV; k, 22.4 mV; Sanguinetti & Jurkiewicz, 1990a) or to those for the HERG K+ current (V½, −49.0 mV; k, 28.0 mV; Sanguinetti et al. 1995).
Properties of IKs in guinea-pig SA node cells
The voltage-dependence of IKs activation was examined by measuring the amplitude of the outward tail currents elicited upon return to the holding potential of −50 mV after 4 s depolarizing voltage steps to various potentials in the presence of 5 μm E-4031 (Fig. 5A). The activation variable (n∞) for IKs was calculated by normalizing the amplitude of IKs tail current at each test potential with respect to its peak amplitude obtained at +70 mV and then plotted as a function of test potential (Fig. 5B). A continuous curve through the data points shows the least squares fit of a Boltzmann equation (eqn (1)), yielding values for V½ of +17.2 mV and k of 13.5 mV. These values were similar to those reported for IKs in atrial (V½, 24.0 mV; k, 15.7 mV; Sanguinetti & Jurkiewicz, 1991) and ventricular myocytes (V½, 15.7 mV; k, 12.7 mV; Sanguinetti & Jurkiewicz, 1990a) of guinea-pig. The current density of IKs (obtained by normalizing the amplitude of the peak tail current elicited upon return to the holding potential of −50 mV following depolarization to +70 mV with reference to the cell capacitance) was 8.71 ± 0.40 pA pF−1 (n = 6).
Figure 5. Voltage dependence of IKs activation.
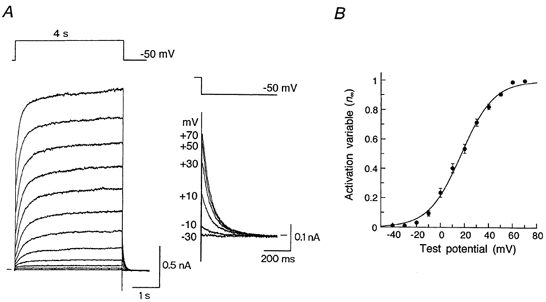
A, current traces during 4 s voltage-clamp steps to membrane potentials of −40 to +70 mV in 10 mV steps applied from a holding potential of −50 mV. These recordings were conducted in the presence of 5 μm E-4031 to inhibit IKr. Tail currents elicited after depolarizing steps to −30, −10, +10, +30, +50 and +70 mV were illustrated on an expanded scale (inset). Zero current level is indicated to either the left or the right (inset) of current traces by a horizontal bar. B, amplitude of IKs tail current at each test potential was normalized to the maximum amplitude at +70 mV and then plotted as a function of the test potential. Data points were fitted to a Boltzmann equation (eqn 1) using the least-squares method with V½ of +17.2 mV and k of 13.5 mV (see text). Data represent the means ± s.e.m. of three cells.
We examined the fully activated I–V relationship of IKs in guinea-pig SA node pacemaker cells. For this purpose, the cell was first depolarized to +40 mV for 4 s, and then repolarized to potentials in the range of +30 to −110 mV in the presence of 5 μm E-4031 to abolish IKr (Fig. 6A, left-hand panel). The decaying tail current elicited at potentials > −50 mV can be regarded as solely representing the deactivation of IKs, whereas the time-dependent change in the membrane currents observed at potentials <−60 mV appears to arise not only from the deactivation of IKs but also from the activation of If (see Fig. 1B and C). The deactivation of IKs elicited at potentials <−60 mV after depolarization to +40 mV (Fig. 6A, right-hand panel) was therefore obtained by subtracting If activated by hyperpolarization from a holding potential of −50 mV (middle panel) from the membrane current elicited upon hyperpolarization from +40 mV (left-hand panel). The amplitude of IKs tail current was then divided by the expected changes in the activation variable (n∞) for IKs (Fig. 5B) and plotted against the membrane potential (Fig. 6B). The fully activated I–V relationship for IKs was nearly linear in the potential range between −110 and −30 mV and rectified slightly in the inward direction at more positive potentials, which was similar to the conductance of IKs previously recorded in other cardiac cell types (Matsuura et al. 1987; Sanguinetti & Jurkiewicz, 1991; Zhou & Lipsius, 1994). In addition, IKs tail current reversed its polarity at −81.2 ± 2.5 mV (n = 3), which is close to the predicted equilibrium potential for K+ (−85 mV), indicating that IKs was predominantly carried by K+ ions, as has previously been demonstrated in guinea-pig atrial cells (Sanguinetti & Jurkiewicz, 1991).
Figure 6. Conductance properties of IKs in guinea-pig SA node cells.
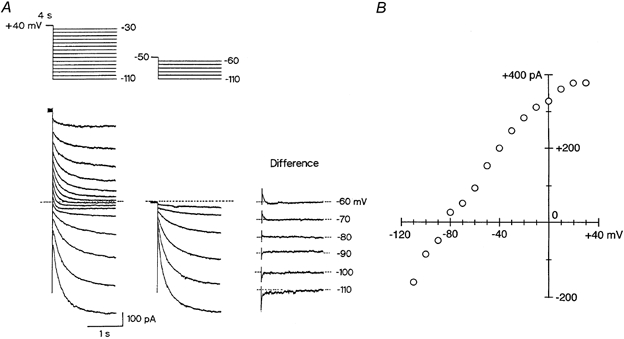
A, cell was depolarized from a holding potential of −50 mV to +40 mV for 4 s to activate IKs, and then repolarized to various test potentials between +30 and −110 mV in 10 mV steps (left-hand panel). The same cell was repolarized from the −50 mV holding potential to membrane potentials between −60 and −110 mV without the depolarizing pulse (middle panel). IKs tail current elicited on return to the test potentials between −60 and −110 mV after a 4 s depolarizing pulse to +40 mV was determined by subtracting the membrane current without the depolarizing pulse to +40 mV from that with the depolarizing pulse (right-hand panel). A schematic diagram of the voltage protocol is given above the current traces. These recordings were conducted in the presence of 5 μm E-4031 to inhibit IKr. The dashed line indicates the zero current level. B, amplitude of the IKs tail current at each test potential was divided by the expected decrease in the activation variable and then plotted as a function of membrane potential.
Deactivation kinetics of IKr and IKs in guinea-pig SA node cells
The deactivation time course of IKr and IKs was then analyzed by fitting either a single or double exponential function to the deactivating tail currents. As shown in Fig. 3 and Fig. 4, the tail currents of IKr were mostly very small in amplitude and somewhat noisy (due to the digital subtraction procedure), which may hamper the accurate analysis of the deactivation of IKr. We therefore decided to assess the deactivation kinetics of IKr at potentials of −40, −50 and −60 mV, where relatively large amplitudes (≥ 15 pA) of IKr tail currents were elicited, by fitting to a single exponential function, as described in the inset of Fig. 3B. Time constants of IKr tail currents at −40, −50 and −60 mV, determined as E-4031-sensitive current during the voltage clamp protocol shown either in Fig. 3 or Fig. 4, were 156.9 ± 14.2 ms (n = 4), 108.3 ± 10.9 ms (n = 6) and 88.0 ± 9.8 ms (n = 4), respectively.
On the other hand, amplitudes of IKs tail currents, elicited during a double-pulse protocol demonstrated in Fig. 6A, were large at all the test potentials except near EK (−80 and −90 mV). Figure 7A illustrates an example of the kinetic analysis of IKs tail currents elicited at −50 mV following a 4 s pulse to +40 mV in the presence of 5 μm E-4031. The time course of the tail current was well fitted by two exponential components having fast (τf = 44.4 ms) and slow (τs = 151.5 ms) decay time constants. The amplitudes of the fast and slow decay phases (Af and As, respectively), obtained by extrapolating the fitted line to 0 time in a semilogarithmic analysis, were 0.060 and 0.059 nA, respectively. The inset to Fig. 7A shows the original trace of tail current at −50 mV superimposed with the theoretical curve representing the sum of two exponential functions:
| (4) |
where Af, As, τf and τs were 0.060 nA, 0.059 nA, 44.4 ms and 151.5 ms, respectively.
Figure 7. Deactivation kinetics of IKs.
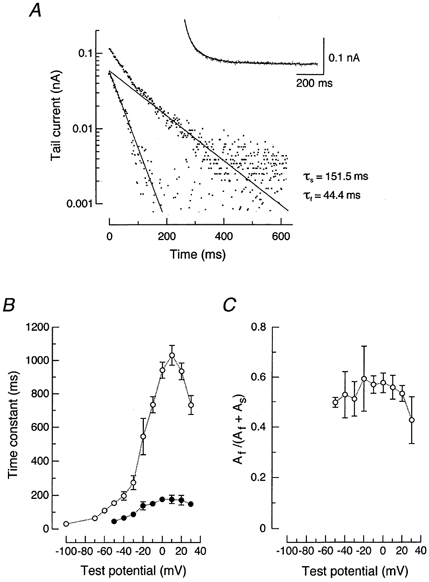
A, semilogarithmic plots of the IKs tail current elicited on return to −50 mV after a 4 s voltage step to +40 mV in the presence of 5 μm E-4031 (shown in Fig. 6A). Time constants used for exponential fitting were indicated in the figure. τs and τf represent the slow and fast components, respectively. The inset shows the original current trace (dotted points) fitted with the theoretical curve (continuous line) representing the sum of two exponential functions (see text). B, deactivation time constants for the fast (•) and slow (○) components of IKs plotted against membrane potential. The time constants were obtained from IKs tail currents elicited during a double-pulse protocol as demonstrated in Fig. 6A. C, relative amplitude of the fast component to the total amplitude (Af /(Af + As)) plotted against membrane potential where the fast and slow components are present. Data in panels B and C represent the means ± s.e.m. of four cells.
The IKs tail currents elicited at test potentials > −50 mV can be generally fitted with the sum of two exponential functions, while a single exponential function adequately describes the IKs tail current elicited at potentials <−60 mV. Figure 7B shows the time constants for the fast (•) and slow components (○) of IKs tail currents (τf and τs, respectively) plotted as a function of membrane potential between −100 and +30 mV. Both τf and τs values were largest at around +10 mV, and decreased at more positive and more negative potentials. The relative amplitude of the fast component to the total amplitude (Af/(Af + As)) was between 0.4 and 0.6 at potentials where the fast and slow components were present (Fig. 7C). These kinetic properties of IKs deactivation are comparable to those recently reported for IKs in porcine SA node pacemaker cells (Ono et al. 2000).
Role of IKr and IKs in the spontaneous activity of guinea-pig SA node cells
Voltage-clamp experiments so far described in the present study provide evidence for the presence of IKr as well as IKs in guinea-pig SA node pacemaker cells. In order to elucidate whether IKr contributes to the spontaneous pacemaker activity of SA node cells, we examined the effect of the block of IKr on spontaneous action potentials. Figure 8 illustrates a representative example of the effect of E-4031 at 0.5 μm, a concentration known to produce more than 50 % inhibition of IKr without affecting other ionic currents (Sanguinetti & Jurkiewicz, 1990a; Ito & Ono, 1995; Verheijck et al. 1995). Under control conditions, an SA node cell exhibited regular and stable pacemaker activity with a firing rate of 262 min−1 and a maximum diastolic potential of −61 mV (Fig. 8B, top trace, •). An application of 0.5 μm E-4031 initially slowed the repolarizing phase of the action potentials at levels negative to approximately −30 mV, accompanied by a progressive and marked depolarization of the maximum diastolic potential (Fig. 8B, top trace, ○). These effects of E-4031 were followed by either a cessation of the spontaneous activity which was observed in most of the cells examined (5 out of 7 cells; Fig. 8A), or an irregular and decelerated firing of the action potentials in the remaining cells (2 out of 7 cells). The membrane potential of the cells in which spontaneous electrical activity was abolished during exposure to E-4031 stabilized at −30.1 ± 2.8 mV (n = 5; Fig. 8A).
The gradual hyperpolarization of the membrane potential observed after washout of the drug (Fig. 8A) appears to reflect the recovery of IKr from the block by E-4031. The cell eventually restored its regular and stable spontaneous action potentials with a firing rate of 255 min−1 and maximum diastolic potential of −59 mV, about 5.5 min after washout of the drug (Fig. 8B, bottom trace, ▪). Higher concentrations (5 μm) of E-4031 also preferentially affected the late repolarizing phase at first and then caused a rapid and complete arrest of the electrical activity, in all cells examined, which was not reversed after washing out the drug (n = 3; data not shown). These results strongly suggest that IKr contributes primarily to the late phase of repolarization and that blocking IKr leads to either an arrest or deceleration of the spontaneous activity in guinea-pig SA node cells.
The role of IKs in the SA node pacemaker activity in guinea-pig heart was also evaluated using a selective IKs blocker 293B. It has been demonstrated in human and guinea-pig ventricular myocytes that the chromanol derivative 293B at a concentration of 30 μm largely (by more than 80 %) inhibits IKs without appreciably affecting other ionic currents including IKr and ICa,L (Busch et al. 1996; Bosch et al. 1998). Figure 9 demonstrates the effect of 30 μm 293B on spontaneous action potentials in a guinea-pig SA node cell superfused with normal Tyrode solution. Bath application of 30 μm 293B consistently slowed the repolarizing phase at potentials negative to approximately −20 mV and produced a gradual depolarization of the maximum diastolic potential (n = 6; Fig. 9B, top trace, •). A complete arrest in electrical activity was evoked during exposure to 30 μm 293B in 3 out of 6 cells (Fig. 9B, middle trace, □) while in the remaining 3 cells, sporadic firings with irregular intervals remained. The membrane potential of SA node cells that exhibited a complete arrest was found to be −45.6 ± 2.1 mV (n = 3; Figs 9A and B, middle trace). After washout of 293B the spontaneous activity resumed in a regular rhythm in most of cells examined (4 out of 6 cells; Fig. 9C, bottom trace, ▪). These results suggest that IKs also plays a crucial role in the repolarization process of spontaneous action potentials in guinea-pig SA node pacemaker cells.
Amplitudes of IKr and IKs during voltage-clamp protocol simulating action potentials
The tail current amplitude of fully activated IKr (0.45 ± 0.03 pA pF−1; n = 6; see Fig. 3) is about 5 % of the fully activated IKs (8.71 ± 0.40 pA pF−1; n = 6; see Fig. 5) in guinea-pig SA node pacemaker cells. However, IKr activates more rapidly and at more negative potentials than IKs, which may be favourable conditions for the contribution of IKr to the repolarizing outward current during the action potential. We therefore compared the amplitudes of IKr and IKs during a voltage-clamp protocol simulating spontaneous action potentials at a firing rate of 200 min−1 (50 ms depolarizing pulses to +20 mV followed by repolarization to −50 mV for 250 ms at a rate of 200 pulses min−1) in the experiments represented in Fig. 10. The peak levels of IK (IKr + IKs) tail currents (denoted by arrow) elicited upon repolarization to −50 mV progressively increased during the initial repetitive depolarizing pulses (Fig. 10B), representing accumulation of IK activation (Viswanathan et al. 1999). Considering the time constants of deactivation of IKr (τ = 108.3 ± 10.9 ms, n = 6) and IKs (τf = 45.1 ± 4.3 ms; τs = 153.6 ± 17.0 ms; Af/(Af + As) = 0.498, n = 4; Fig. 7) at −50 mV, deactivation of both IKr and IKs was expected to be incomplete during the brief interval (250 ms) at the −50 mV holding potential. The IK response to repetitive stimulation reached a practically steady level approximately 5–10 s after the start of a series of depolarizing pulses, indicating that the amount of activation during depolarization became equivalent to that of deactivation at the holding potential of −50 mV. E-4031 (5 μm) was added to the bath 1 min after starting repetitive depolarizations, and it rapidly reduced the amplitude of IK to a steady level within 30 s. IKr during these repetitive stimulations was identified as an E-4031-sensitive current (Fig. 10C), which was obtained by digital subtraction of IK after 1 min exposure to 5 μm E-4031 (IK at P400, Fig. 10B) from that before its exposure (IK at P200, Fig. 10B), while IK after 1 min exposure to E-4031 (IK at P400) was defined as IKs. During successive depolarizations simulating action potential firings, the amplitude of IKs tail current measured at −50 mV (0.74 ± 0.09 pA pF−1, n = 4; Fig. 10D) was found to be significantly larger than the IKr tail current at the same potential (0.43 ± 0.05 pA pF−1).
Figure 10. Comparison of tail current amplitude of IKr and IKs.
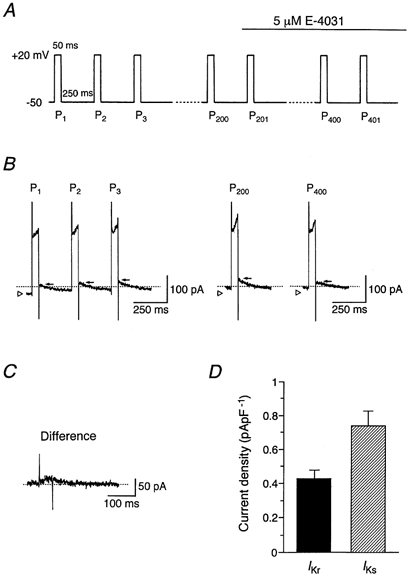
A, pulse protocol. Depolarizing pulses of 50 ms were applied to +20 mV from a holding potential of −50 mV at a rate of 200 pulses min−1. After an SA node cell was subjected to a train of successive depolarizing pulses for 1 min (200 pulses), 5 μm E-4031 was added to the bath, as marked by the horizontal bar. B, IK at P1 (first pulse), P2 (second pulse), P3 (third pulse), P200 (two hundredth pulse) and P400 (four hundredth pulse). The open triangle indicates the current level before applying the pulse train and the arrow denotes the peak level of IK tail current. The zero current level is indicated by the dashed line. C, IKr obtained by digital subtraction of IK after 1 min exposure to E-4031 (IK at P400) from that before its exposure (IK at P200) (shown in B). D, amplitudes of tail currents of IKr and IKs (n = 4), determined during successive depolarizations (shown in A). The amplitude of IKr tail current was measured from IKr obtained as shown in C. The magnitude of IKs tail current was determined from IK at P400 by subtracting the current level before applying the pulse train (denoted by open triangle) from the peak of tail current (indicated by arrow). The current amplitudes were normalized with reference to cell capacitance. Columns and bars represent the means and s.e.m., respectively, and the amplitude of IKs was significantly larger than the amplitude of IKr (Student's paired t test).
DISCUSSION
The present investigation characterized IK in spontaneously active SA node cells in the guinea-pig heart using a methanesulfonanilide class III antiarrhythmic agent E-4031, which has been demonstrated to specifically inhibit IKr in cardiac myocytes (Sanguinetti & Jurkiewicz, 1990a, 1991). Under control conditions, IK does not satisfy the envelope of tails test, consistent with the activation of more than one current system exhibiting distinct properties of kinetics and conductance (Noble & Tsien, 1969), whereas after the blockade of the IK that is sensitive to E-4031, the remaining IK completely satisfies the test, supporting the view that only a single current system exists during the exposure to E-4031 (Fig. 2). This test clearly indicates that IK in guinea-pig SA node pacemaker cells is generated by activation of both the E-4031-sensitive and E-4031-resistant IK (IKr and IKs, respectively). In addition, the ratio (approximately 0.35) of the tail current to the time-dependent current for IKs, recorded in the presence of E-4031 (Fig. 2C, •), is slightly larger than the predicted ratio of the driving force at +30 and −50 mV for a non-rectifying membrane current having the reversal potential of −81 mV ((−50 − (−81))/(+30 − (−81)) = 0.28), which can be accounted for by the presence of a weak inward rectification in IKs (Fig. 6).
Sanguinetti & Jurkiewics (1990a, b, 1991) have shown that IK in guinea-pig atrial and ventricular myocytes can be divided into two distinct components based on the sensitivity to inhibition by lanthanum ions and by the methanesulfonanilide E-4031: IKr, defined as the E-4031-sensitive current, exhibits a rapid activation and a marked inward rectification; whereas IKs, defined as the E-4031-resistant current, activates more slowly and exhibits a slight rectification. These two distinct components of IK have since been identified in atrial and ventricular myocytes of various mammalian species including humans (Wang et al. 1994; Liu & Antzelevitch, 1995; Gintant, 1996; Li et al. 1996). Previous studies investigating the composition and properties of IK in mammalian pacemaker cells were mostly undertaken using rabbit SA node cells. In this preparation, exposure to E-4031 nearly completely abolishes IK (Ono & Ito, 1995; Verheijck et al. 1995; Ho et al. 1996), thus suggesting that IKr is a predominant component of IK in rabbit SA node cells. These studies have further demonstrated that a bath application of E-4031 prolonged the action potential duration, depolarized the maximum diastolic potential and eventually abolished the spontaneous electrical activity, indicating that IKr is essential for maintaining the SA node automaticity. Lei & Brown (1996) showed that a part of IK is resistant to dofetilide at 1 μm, a concentration known to completely abolish cardiac IKr (Carmeliet 1992), and that the dofetilide-resistant IK shows electrophysiological properties which are comparable to IKs previously recorded in other cardiac cells, such as a half-maximal activation voltage (V½) of 15.6 mV and a slope factor (k) of 14.7 mV, thus suggesting that IKs is also present in rabbit SA node cells.
On the other hand, it has been demonstrated in guinea-pig SA node pacemaker cells that IK activates at potentials positive to −mV and is little affected by exposure to either E-4031 (5 μm) or lanthanum ions (100 μm). In addition, IK has been shown to satisfy the envelope of tails test with the ratio of tail current to time-dependent current expected for a slightly rectifying current system (Anumonwo et al. 1992; Freeman & Kass, 1993). These observations indicate that IKs is the major component of IK in this preparation. In the present study, we consistently detected an E-4031-sensitive current which activates rapidly, reaching a steady state within 200–300 ms after depolarization (Fig. 3), and displays a marked inward rectification at depolarized potentials (Fig. 4) in spontaneously active SA node cells of the guinea-pig heart. We found that the tail current density of fully activated IKr measured upon repolarization to −50 mV following 500 ms depolarization to +20 mV (0.45 ± 0.03 pA pF−1; n = 6; see Fig. 3) is approximately one-twentieth of that of fully activated IKs recorded at −50 mV after 4 s depolarization to +70 mV (8.71 ± 0.40 pA pF−1; n = 6; see Fig. 5) in guinea-pig SA node cells, and this small amplitude of IKr relative to the large amplitude of IKs might have precluded the detailed analysis of IKr in this cell type.
Exposure to a specific IKr blocker E-4031 was found to initially slow the repolarizing phase at potentials negative to approximately −30 mV without producing any appreciable effect on repolarization at more depolarized potentials in spontaneous action potentials (Fig. 8). These results strongly indicate that the late phase of repolarization (at potentials negative to −30 mV) leading to the maximal diastolic potential at around −60 mV is primarily produced by IKr in guinea-pig SA node cells. In addition, the observation that the block of IKr has little effect on the early phase of repolarization appears to be consistent with the prominent inwardly rectifying property of IKr which minimizes the contribution of repolarizing outward current through the IKr channel at depolarized potentials. The inward rectification of IKr in guinea-pig SA node cells can be accounted for by the voltage-dependent inactivation process (Fig. 4), as has been demonstrated in other cardiac cells (Shibasaki, 1987; Sanguinetti & Jurkiewicz, 1990a; Ono & Ito, 1995; Smith et al. 1996; Spector et al. 1996).
The chromanol derivative 293B has been demonstrated to inhibit IKs in guinea-pig and human ventricular myocytes in a highly selective manner (Busch et al. 1996; Bosch et al. 1998). The present experiments revealed that application of 293B first slowed the rate of repolarization at levels negative to about −20 mV with relatively small depolarization of the maximum diastolic potential (Fig. 9) compared with the effect of E-4031 (Fig. 8), which suggests that IKs contributes to the repolarization process at more depolarized levels than IKr in guinea-pig SA node pacemaker cells. In addition, spontaneous electrical activity of SA node cells was found to be greatly diminished by exposure to 293B (Fig. 9), indicating an essential role of IKs in the pacemaker activity in guinea-pig heart. The observation that the membrane potential following arrest of spontaneous action potentials induced by inhibition of IKs by 293B (−45.6 ± 2.1 mV, n = 3; Fig. 9) was much more negative than that evoked by blockade of IKr induced by E-4031 (−30.1 ± 2.8 mV, n = 5; Fig. 8) may reflect a major contribution of IKr to the late phase of repolarization in spontaneous action potentials of guinea-pig SA node cells. The present pharmacological studies using E-4031 and 293B thus strongly suggest that both components of IK are indispensable for the spontaneous electrical activity of guinea-pig SA node cells. The decrease in the action potential amplitude and overshoot in the presence of either E-4031 (Fig. 8) or 293B (Fig. 9), which eventually leads to the arrest of spontaneous activity, is likely to be secondarily induced by the voltage-dependent inactivation of ICa.L associated with the depolarization of the maximum diastolic potential.
It has been demonstrated that both IKr and IKs represent a potentially relevant target for the actions of neurotransmitters and drugs. In addition to the methanesulfonanilides, a relatively broad spectrum of medications, including some antiarrhythmic (Follmer & Colatsky, 1990), antihistaminic (Rampe et al. 1993; Woosley, 1996), antibiotic (Daleau et al. 1995) and psychoactive agents (Drolet et al. 1999), have been demonstrated to preferentially block IKr and thereby slow the cardiac repolarization. In contrast, the stimulation of both β- and α-adrenoceptors was shown to selectively enhance IKs in cardiac cells (Sanguinetti et al. 1991; Tohse et al. 1992; Wang et al. 1994; Mamas et al. 1996), thus showing that IKs primarily represents a target for the actions of sympathetic neurotransmitters. The prolongation of the refractory period induced by the class III antiarrhythmic agents through the inhibition of IKr was demonstrated to be reversed by the enhancement of IKs by β-adrenergic stimulation (Sanguinetti et al. 1991), thus showing that the electrical activity of cardiac cells can be modulated by a differential regulation of two IK components by neurotransmitters and drugs. Since both IKr and IKs are present and also play a crucial role in the development of pacemaker activity in guinea-pig SA node cells, these cell preparations should provide a suitable experimental model for future studies investigating the interactions of neurotransmitters and drugs affecting the IK components in the SA node pacemaking activity.
Acknowledgments
We thank Professor A. Noma (Kyoto University, Japan) for teaching us the method for isolating guinea-pig sino-atrial node cells, Dr T. Shioya (Saga Medical School, Japan) for providing us with the computer programs used for the data analysis, Dr B. Quinn for critically reading the manuscript and Ms Y. Tanigaki for secretarial assistance. This study was supported by Grants-in-Aid for Scientific Research (Nos 09670048 and 11670040) from the Ministry of Education, Science, Sports and Culture of Japan.
REFERENCES
- Abbott GW, Sesti F, Splawski I, Buck ME, Lehmann MH, Timothy KW, Keating MT, Goldstein SAN. MiRP1 forms IKr potassium channels with HERG and is associated with cardiac arrhythmia. Cell. 1999;97:175–187. doi: 10.1016/s0092-8674(00)80728-x. [DOI] [PubMed] [Google Scholar]
- Anumonwo JMB, Freeman LC, Kwok WM, Kass RS. Delayed rectification in single cells isolated from guinea pig sinoatrial node. American Journal of Physiology. 1992;262:H921–925. doi: 10.1152/ajpheart.1992.262.3.H921. [DOI] [PubMed] [Google Scholar]
- Barhanin J, Lesage F, Guillemare E, Fink M, Lazdunski M, Romey G. KVLQT1 and IsK (minK) proteins associate to form the IKs cardiac potassium current. Nature. 1996;384:78–80. doi: 10.1038/384078a0. [DOI] [PubMed] [Google Scholar]
- Bénitah J-P, Gomez AM, Bailly P, Da Ponte J-P, Berson G, Delgado C, Lorente P. Heterogeneity of the early outward current in ventricular cells isolated from normal and hypertrophied rat hearts. Journal of Physiology. 1993;469:111–138. doi: 10.1113/jphysiol.1993.sp019807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bleeker WK, Mackaay AJC, Masson-Pevet M, Bouman LN, Becker AE. Functional and morphological organization of the rabbit sinus node. Circulation Research. 1980;46:11–22. doi: 10.1161/01.res.46.1.11. [DOI] [PubMed] [Google Scholar]
- Bosch RF, Gaspo R, Busch AE, Lang HJ, Li G-R, Nattel S. Effects of the chromanol 293B, a selective blocker of the slow component of the delayed rectifier K+ current, on repolarization in human and guinea pig ventricular myocytes. Cardiovascular Research. 1998;38:441–450. doi: 10.1016/s0008-6363(98)00021-2. [DOI] [PubMed] [Google Scholar]
- Busch AE, Suessbrich H, Waldegger S, Sailer E, Greger R, Lang H-J, Lang F, Gibson KJ, Maylie JG. Inhibition of IKs in guinea pig cardiac myocytes and guinea pig IsK channels by the chromanol 293B. Pflügers Archiv. 1996;432:1094–1096. doi: 10.1007/s004240050240. [DOI] [PubMed] [Google Scholar]
- Carmeliet E. Voltage- and time-dependent block of the delayed K+ current in cardiac myocytes by dofetilide. Journal of Pharmacology and Experimental Therapeutics. 1992;262:809–817. [PubMed] [Google Scholar]
- Curran ME, Splawski I, Timothy KW, Vincent GM, Green ED, Keating MT. A molecular basis for cardiac arrhythmia: HERG mutations cause long QT syndrome. Cell. 1995;80:795–803. doi: 10.1016/0092-8674(95)90358-5. [DOI] [PubMed] [Google Scholar]
- Daleau P, Lessard E, Groleau M-F, Turgeon J. Erythromycin blocks the rapid component of the delayed rectifier potassium current and lengthens repolarization of guinea pig ventricular myocytes. Circulation Research. 1995;91:3010–3016. doi: 10.1161/01.cir.91.12.3010. [DOI] [PubMed] [Google Scholar]
- DiFrancesco D, Ferroni A, Mazzanti M, Tromba C. Properties of the hyperpolarizing-activated current (if) in cells isolated from the rabbit sino-atrial node. Journal of Physiology. 1986;377:61–88. doi: 10.1113/jphysiol.1986.sp016177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doerr T, Denger R, Trautwein W. Calcium currents in single SA nodal cells of the rabbit heart studied with action potential clamp. Pflügers Archiv. 1989;413:599–603. doi: 10.1007/BF00581808. [DOI] [PubMed] [Google Scholar]
- Drolet B, Vincent F, Rail J, Chahine M, Deschenes D, Nadeau S, Khalifa M, Hamelin BA, Turgeon J. Thioridazine lengthens repolarization of cardiac ventricular myocytes by blocking the delayed rectifier potassium current. Journal of Pharmacology and Experimental Therapeutics. 1999;288:1261–1268. [PubMed] [Google Scholar]
- Fabiato A, Fabiato F. Calculator programs for computing the composition of the solutions containing multiple metals and ligands used for experiments in skinned muscle cells. Journal de Physiologie. 1979;75:463–505. [PubMed] [Google Scholar]
- Follmer CH, Colatsky TJ. Block of delayed rectifier potassium current, IK, by flecainide and E-4031 in cat ventricular myocytes. Circulation. 1990;82:289–293. doi: 10.1161/01.cir.82.1.289. [DOI] [PubMed] [Google Scholar]
- Freeman LC, Kass RS. Delayed rectifier potassium channels in ventricle and sinoatrial node of the guinea pig: molecular and regulatory properties. Cardiovascular Drugs and Therapy. 1993;7:627–635. doi: 10.1007/BF00877630. [DOI] [PubMed] [Google Scholar]
- Gintant GA. Two components of delayed rectifier current in canine atrium and ventricle: Does IKs play a role in the reverse rate dependence of class III agents? Circulation Research. 1996;78:26–37. doi: 10.1161/01.res.78.1.26. [DOI] [PubMed] [Google Scholar]
- Guo J, Mitsuiye T, Noma A. The sustained inward current in sino-atrial node cells of guinea-pig heart. Pfügers Archiv. 1997;433:390–396. doi: 10.1007/s004240050293. [DOI] [PubMed] [Google Scholar]
- Guo J, Ono K, Noma A. A sustained inward current activated at the diastolic potential range in rabbit sino-atrial node cells. Journal of Physiology. 1995;483:1–13. doi: 10.1113/jphysiol.1995.sp020563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagiwara N, Irisawa H, Kameyama M. Contribution of two types of calcium currents to the pacemaker potentials of rabbit sino-atrial node cells. Journal of Physiology. 1988;395:233–253. doi: 10.1113/jphysiol.1988.sp016916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagiwara N, Irisawa H, Kasanuki H, Hosoda S. Background current in sino-atrial node cells of the rabbit heart. Journal of Physiology. 1992;448:53–72. doi: 10.1113/jphysiol.1992.sp019029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamill OP, Marty A, Neher E, Sakmann B, Sigworth FJ. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflügers Archiv. 1981;391:85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Ho W-K, Earm YE, Lee SH, Brown HF, Noble D. Voltage- and time-dependent block of delayed rectifier K+ current in rabbit sino-atrial node cells by external Ca2+ and Mg2+ Journal of Physiology. 1996;494:727–742. doi: 10.1113/jphysiol.1996.sp021528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irisawa H, Brown HF, Giles W. Cardiac pacemaking in the sinoatrial node. Physiological Reviews. 1993;73:197–227. doi: 10.1152/physrev.1993.73.1.197. [DOI] [PubMed] [Google Scholar]
- Isenberg G, Klöckner U. Calcium tolerant ventricular myocytes prepared by preincubation in a ‘KB medium’. Pflügers Archiv. 1982;395:6–18. doi: 10.1007/BF00584963. [DOI] [PubMed] [Google Scholar]
- Ito H, Ono K. A rapidly activating delayed rectifier K+ channel in rabbit sinoatrial node cells. American Journal of Physiology. 1995;269:H443–452. doi: 10.1152/ajpheart.1995.269.2.H443. [DOI] [PubMed] [Google Scholar]
- Ito H, Ono K, Noma A. Background conductance attributable to spontaneous opening of muscarinic K+ channels in rabbit sino-atrial node cells. Journal of Physiology. 1994;476:55–68. [PMC free article] [PubMed] [Google Scholar]
- Lei M, Brown HF. Two components of the delayed rectifier potassium current, IK, in rabbit sino-atrial node cells. Experimental Physiology. 1996;81:725–741. doi: 10.1113/expphysiol.1996.sp003972. [DOI] [PubMed] [Google Scholar]
- Li G-R, Feng J, Yue L, Carrier M, Nattel S. Evidence for two components of delayed rectifier K+ current in human ventricular myocytes. Circulation Research. 1996;78:689–696. doi: 10.1161/01.res.78.4.689. [DOI] [PubMed] [Google Scholar]
- Liu D-W, Antzelevitch C. Characteristics of the delayed rectifier current (IKr and IKs) in canine ventricular epicardial, midmyocardial, and endocardial myocytes: A weaker IKs contributes to the longer action potential of the M cell. Circulation Research. 1995;76:351–365. doi: 10.1161/01.res.76.3.351. [DOI] [PubMed] [Google Scholar]
- Mamas MA, Connors SC, Terrar DA. Effects of protein kinase C on the components of the delayed rectifier potassium current in guinea-pig isolated ventricular myocytes (abstract) Journal of Physiology. 1996;493.P:26–27P. [Google Scholar]
- Matsuura H, Ehara T, Imoto Y. An analysis of the delayed outward current in single ventricular cells of the guinea-pig. Pflügers Archiv. 1987;410:596–603. doi: 10.1007/BF00581319. [DOI] [PubMed] [Google Scholar]
- Noble D, Tsien RW. Outward membrane currents activated in the plateau range of potentials in cardiac Purkinje fibres. Journal of Physiology. 1969;200:205–231. doi: 10.1113/jphysiol.1969.sp008689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ono K, Ito H. Role of rapidly activating delayed rectifier K+ current in sinoatrial node pacemaker activity. American Journal of Physiology. 1995;269:H453–462. doi: 10.1152/ajpheart.1995.269.2.H453. [DOI] [PubMed] [Google Scholar]
- Ono K, Shibata S, Iijima T. Properties of the delayed rectifier potassium current in porcine sino-atrial node cells. Journal of Physiology. 2000;524:51–62. doi: 10.1111/j.1469-7793.2000.00051.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Opthof T, De Jonge B, Mackaay AJC, Bleeker WK, Masson-Pevet M, Jongsma HJ, Bouman LN. Functional and morphological organization of the guinea-pig sinoatrial node compared with the rabbit sinoatrial node. Journal of Molecular and Cellular Cardiology. 1985;17:549–564. doi: 10.1016/s0022-2828(85)80024-9. [DOI] [PubMed] [Google Scholar]
- Rampe D, Wible B, Brown AM, Dage RC. Effects of terfenadine and its metabolites on a delayed rectifier K+ channel cloned from human heart. Molecular Pharmacology. 1993;44:1240–1245. [PubMed] [Google Scholar]
- Sanguinetti MC, Curran ME, Zou A, Shen J, Spector PS, Atkinson DL, Keating MT. Coassembly of KVLQT1 and minK (IsK) proteins to form cardiac IKs potassium channel. Nature. 1996;384:80–83. doi: 10.1038/384080a0. [DOI] [PubMed] [Google Scholar]
- Sanguinetti MC, Jiang C, Curran ME, Keating MT. A mechanistic link between an inherited and an acquired cardiac arrhythmia: HERG encodes the IKr potassium channel. Cell. 1995;81:299–307. doi: 10.1016/0092-8674(95)90340-2. [DOI] [PubMed] [Google Scholar]
- Sanguinetti MC, Jurkiewicz NK. Two components of cardiac delayed rectifier K+ current: differential sensitivity to block by class III antiarrhythmic agents. Journal of General Physiology. 1990a;96:195–215. doi: 10.1085/jgp.96.1.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanguinetti MC, Jurkiewicz NK. Lanthanum blocks a specific component of IK and screens membrane surface charge in cardiac cells. American Journal of Physiology. 1990b;259:H1881–1889. doi: 10.1152/ajpheart.1990.259.6.H1881. [DOI] [PubMed] [Google Scholar]
- Sanguinetti MC, Jurkiewicz NK. Delayed rectifier outward K+ current is composed of two currents in guinea pig atrial cells. American Journal of Physiology. 1991;260:H393–399. doi: 10.1152/ajpheart.1991.260.2.H393. [DOI] [PubMed] [Google Scholar]
- Sanguinetti MC, Jurkiewicz NK, Scott A, Siegl PKS. Isoproterenol antagonizes prolongation of refractory period by the class III antiarrhythmic agent E-4031 in guinea pig myocytes. Mechanism of action. Circulation Research. 1991;68:77–84. doi: 10.1161/01.res.68.1.77. [DOI] [PubMed] [Google Scholar]
- Shibasaki T. Conductance and kinetics of delayed rectifier potassium channels in nodal cells of the rabbit heart. Journal of Physiology. 1987;387:227–250. doi: 10.1113/jphysiol.1987.sp016571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith PL, Baukrowitz T, Yellen G. The inward rectification mechanism of the HERG cardiac potassium channel. Nature. 1996;379:833–836. doi: 10.1038/379833a0. [DOI] [PubMed] [Google Scholar]
- Spector PS, Curran ME, Zou A, Keating MT, Sanguinetti MC. Fast inactivation causes rectification of the IKr channel. Journal of General Physiology. 1996;107:611–619. doi: 10.1085/jgp.107.5.611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tohse N, Nakaya H, Kanno M. α1-adrenoceptor stimulation enhances the delayed rectifier K+ current of guinea pig ventricular cells through the activation of protein kinase C. Circulation Research. 1992;71:1441–1446. doi: 10.1161/01.res.71.6.1441. [DOI] [PubMed] [Google Scholar]
- Trudeau MC, Warmke JW, Ganetzky B, Robertson GA. HERG, a human inward rectifier in the voltage-gated potassium channel family. Science. 1995;269:92–95. doi: 10.1126/science.7604285. [DOI] [PubMed] [Google Scholar]
- Tsien RY, Rink TJ. Neutral carrier ion-selective microelectrodes for measurement of intracellular free calcium. Biochimica et Biophysica Acta. 1980;599:623–638. doi: 10.1016/0005-2736(80)90205-9. [DOI] [PubMed] [Google Scholar]
- Verheijck EE, van Ginneken ACG, Bourier J, Bouman LN. Effects of delayed rectifier current blockade by E-4031 on impulse generation in single sinoatrial nodal myocytes of the rabbit. Circulation Research. 1995;76:607–615. doi: 10.1161/01.res.76.4.607. [DOI] [PubMed] [Google Scholar]
- Viswanathan PC, Shaw RM, Rudy Y. Effects of IKr and IKs heterogeneity on action potential duration and its rate dependence. A simulation study. Circulation Research. 1999;99:2466–2474. doi: 10.1161/01.cir.99.18.2466. [DOI] [PubMed] [Google Scholar]
- Wang Z, Fermini B, Nattel S. Rapid and slow components of delayed rectifier current in human atrial myocytes. Cardiovascular Research. 1994;28:1540–1546. doi: 10.1093/cvr/28.10.1540. [DOI] [PubMed] [Google Scholar]
- Woosley RL. Cardiac actions of antihistamines. Annual Review of Pharmacology and Toxicology. 1996;36:233–252. doi: 10.1146/annurev.pa.36.040196.001313. [DOI] [PubMed] [Google Scholar]
- Zhou Z, Lipsius SL. Delayed rectifier potassium current (IK) in latent atrial pacemaker cells isolated from cat right atrium. Pflügers Archiv. 1994;426:341–347. doi: 10.1007/BF00374791. [DOI] [PubMed] [Google Scholar]


