Abstract
Previous evidence suggests that the heart rate (HR) increase observed with isometric exercise is dependent on different afferent mechanisms to those eliciting the increase in blood pressure (BP). Central command and muscle metaboreceptors have been shown to contribute to this differential effect. However, in experimental animals passive stretch of the hindlimb increases HR suggesting that small fibre mechanoreceptors could also have a role. This has not been previously shown in humans and was investigated in this study. Healthy human volunteers were instrumented to record BP, ECG, respiration, EMG of rectus femoris and gastrocnemius and contraction force of triceps surae. Voluntary isometric contraction of triceps surae elicited a significant HR change in the first three respiratory cycles at 40 % of maximum voluntary contraction whereas BP did not change significantly until after 30 s. This suggests that different mechanisms are involved in the initiation of the cardiovascular changes. Sustained passive stretch of triceps surae for 1 min, by dorsiflexion of the foot, caused a significant (P < 0.05) increase in HR (5 ± 2.6 beats min−1) with no significant change in BP. A time domain measure of cardiac vagal activity was reduced significantly during passive stretch from 69.7 ± 12.9 to 49.6 ± 8.9 ms. Rapid rhythmic passive stretch (0.5 Hz for 1 min) was without significant effect suggesting that large muscle proprioreceptors are not involved. We conclude that in man small fibre muscle mechanoreceptors responding to stretch, inhibit cardiac vagal activity and thus increase HR. These afferents could contribute to the initial cardiac acceleration in response to muscle contraction.
During an isometric muscle contraction heart rate (HR) and blood pressure (BP) increase. However, the increase in HR is more immediate than the more slowly developing increase in BP. Furthermore at the end of contraction if blood flow is occluded to the exercising muscle, BP remains elevated above baseline levels whereas HR rapidly returns to baseline levels. These features suggest there are different mechanisms eliciting the two cardiovascular responses.
Central motor command, whereby the signal to contract muscles also stimulates cardiovascular centres, has been proposed as a mechanism to increase HR and there is good evidence to support this (Thornton et al. 2001). However, sensors in the muscle must also contribute since HR still rises even when central command is eliminated, for example during direct activation of the muscle using electrical stimulation (Hollander & Bouman, 1975; Hultman & Sjoholm, 1982; Bull et al. 1989; Al Ani et al. 1997; Fisher & White, 1999). Studies using experimental animals like the cat have shown that during muscle contractions evoked by ventral root stimulation HR and BP rise, an effect which is abolished by dorsal root section, indicating that feedback via the muscle afferents is important (Coote et al. 1971; McCloskey & Mitchell, 1972; McMahon & McWilliam, 1992). The muscle afferents that cause the cardiovascular responses are the smaller myelinated (group III) and unmyelinated (group IV) afferents (Coote et al. 1971; McCloskey & Mitchell, 1972; Coote, 1995; Secher, 1999). The group III afferents are mainly stimulated by mechanical stimuli like stretch, contraction or pressure (therefore termed mechanoreceptors) and respond abruptly when a muscle contracts (Kaufman et al. 1983; Mense & Stahnke, 1983; Kaufman & Rybicki, 1987). The majority of the group IV afferents are stimulated by metabolic or chemical products of contraction such as potassium, bradykinin and inorganic phosphate, and can therefore be termed metaboreceptors. However, both groups of afferents include receptors that are polymodal, responding to both mechanical and chemical stimuli (Kniffki et al. 1981; Mense & Stahnke, 1983; Kaufman et al. 1983; Mense & Meyer, 1985).
In anaesthetised cats, activating the mechanoreceptors alone by passively stretching hind limb muscles increases HR and BP, providing evidence that mechanoreceptors could play a role in causing the cardiovascular responses during a contraction (Stebbins et al. 1988; Wilson et al. 1994b; Murata & Matsukawa, 2001; Leshnower et al. 2001).
In humans, although muscle metaboreceptors have been shown to be important in causing cardiovascular responses during exercise (Alam & Smirk, 1937; Lind et al. 1964; Bull et al. 1989), there is no clear evidence that mechanoreceptors play a role. In attempts to study the receptors in man, direct pressure on the muscle (cuff-induced compression, Williamson et al. 1994; McClain et al. 1994), distension of the vascular bed (venous congestion, Baum et al. 1990, 1993; McClain et al. 1993), stretch (Baum et al. 1995) or rhythmic passive movement (Nobrega et al. 1994) have been used. None of these studies reported significant changes in HR in response to the various procedures. This is curious since the rapidity of the HR increase during electrically evoked muscle contractions strongly suggests mechanoreceptor involvement (Al Ani et al. 1997).
Therefore the present study was designed to adequately and selectively activate muscle mechanosensitive receptors and to measure the effect on HR and BP. Passive stretch was chosen as it provides distortion of the muscle receptors in a similar plane to that occurring normally in isometric muscle contractions.
METHODS
Two studies, approved by the South Birmingham Hospital research ethics committee and in accordance with the Declaration of Helsinki, were conducted. The first examined the time course of the HR and BP changes to different intensities of voluntary isometric contraction of the triceps surae. The second study investigated the cardiovascular changes in response to passive muscle stretch of the same muscles. All subjects gave informed consent and attended the laboratory for a preliminary familiarisation visit.
In both studies HR, BP and respiratory data were collected into a computer during the experiment. HR was determined from an electrocardiogram (ECG) with a standard lead II configuration using silver chloride electrodes (Leonhard Lang GmbH, Austria) placed on the chest. BP was recorded from a cuff on the middle finger, with the hand placed at heart level using a Finapres blood pressure monitor (Ohmeda 2300, Louisville, CO, USA). The servo-self adjust mechanism was turned off during recordings to permit continuous BP data collection. Respiration was measured using a strain gauge attached to a strap fitted around the abdomen, the voltage output of which increased and decreased with varying stretch caused by breathing movements. The ECG and respiratory signals were amplified (MS2000 cardiac-respiratory monitor, Telesound Ltd, UK) and together with the BP signal were digitised via an analog to digital converter (National Instruments, Austin, TX, USA) at 500 Hz, and then displayed and collected by an Apple Power Macintosh computer (8100/100) into a custom-written suite of programs in the LabView application (Version 4, National Instruments; H. Ross, University of Birmingham).
The rate of ventilation was controlled by asking the subject to breathe in time to a metronome, which was set at a frequency which they found most comfortable. This frequency was kept the same throughout all the experiments. An oscillographic display of respiratory movement gave an approximate indication of inspiratory volume and the subject was asked to keep this the same throughout the tests.
In the passive muscle stretch experiments EMG activity of rectus femoris and gastrocnemius of the same limb was measured using two silver-coated electrodes 10 mm in diameter placed 15 mm apart on the belly of these muscles with the reference plate placed on the same limb. The signal was amplified (Grayden Electronics, UK) filtered (LF,30 Hz, HF3000 Hz) and displayed on an oscilloscope (Scopestation 140 digital oscilloscope, LeCroy, UK). In some cases data were sampled at 1000 Hz and stored on an Apple Power Macintosh computer via the MacLab/e series (ADInstruments, UK) using PowerLab version 3.6.
Isometric muscle contraction
The methods used to assess the cardiovascular responses to voluntary isometric contraction of triceps surae of the right limb were as described by Bull and co-workers (Bull et al. 1989). Subjects were seated in a dynamometer with the thigh clamped horizontally just above the knee and the ankle set at 85 deg. Upward force generated by triceps surae was detected by a transducer and displayed on an oscilloscope and computer. Maximum voluntary contraction (MVC) was taken as the highest force produced from three attempts. Graded contractions expressed as a percentage of MVC were then displayed on the oscilloscope for the subject to view.
Each subject performed a voluntary isometric contraction of the plantar flexors on a command signal at the beginning of expiration. The desired force was maintained for 1 min and the mean values of HR, systolic and diastolic BP were calculated for each of the first three respiratory cycles and then at 30 s and 60 s. The mean value of the changes, compared to values determined for similar periods in 1 min of pre-test baseline, was calculated together with the standard error of the mean.
Passive muscle stretch
Subjects lay semi-supine on a couch with the right knee and ankle supported in a purpose-designed ergometer (Fig. 1). The left leg remained relaxed on the couch.
Figure 1. Diagram of experimental set up for sustained muscle stretch.

Top, schematic diagram showing the position of the subject in the ergometer indicating the passive dorsiflexion of the foot (arrow). Middle, measurements registered and processed. Bottom, protocol of experiment: base, 1 min baseline; stretch, 1 min sustained passive stretch of triceps surae; recovery, 1 min period immediately after release of foot dorsiflexion.
The ergometer was constructed to enable the right foot to be passively and rapidly rotated around the subject's normal dorsal-plantar axis (just below the maleolus) and locked into position to obtain a sustained stretch in the triceps surae (Fig. 1). The equipment comprised a metal plate for the foot, with a heel support and velcro straps to secure the foot in place. The position of the heel and the height of the foot could be adjusted to ensure the same point of rotation for each subject. The angle of flexion of the foot was measured for both plantar- and dorsiflexion. The knee was also supported at an angle of 150 deg as in preliminary studies this was found to be the optimum angle to achieve a significant stretch in the triceps surae.
Maximum triceps surae extension was accomplished by brief passive dorsiflexion of the foot to an angle just below where the subject reported discomfort. With the subject fully relaxed, a 1 min baseline period was followed by 1 min sustained passive stretch, following which the foot was allowed to return to its resting position. The test was repeated three times with a 10 min rest period between each. In a further series of tests four subjects underwent a 1 min passive rapid back and forth rotation of the foot at a frequency of 0.5 Hz to an angle similar to that held during sustained dorsiflexion.
The data from these muscle stretch experiments were calculated as the mean value of the first three respiratory cycles and each 30 s together with the standard error of the mean. Baseline was determined similarly from the average of two 30 s periods immediately preceding stretch.
For all subjects the total angle of rotation was 58 ± 14 deg and the calculated length of extension (stretch) of the plantar flexors from the resting position of the foot was 59 ± 13 mm.
Measurement of cardiac vagal tone
In the muscle stretch experiments vagal activity was assessed using the standard deviation of successive differences (SDSD) in R-R intervals and pNN50. pNN50 is derived by assessing the proportion of R–R intervals that differ from the previous one by more than 50 ms (an arbitrary level set to represent a significant change in HR). Both of these measurements are considered to be sensitive indices of vagal activity (Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology, 1996)
Data and statistical analysis
ANOVA for repeated measures was used to identify significant differences. If there was a significance (P < 0.05) a Bonferroni-Dunn post hoc test was used to identify where the significant differences lay. Results are expressed as means ± standard error of the mean (s.e.m.).
RESULTS
Calf contraction at different intensities
Six subjects (mean age, 29 ± 5 years) attended the laboratory. When seated in the dynamometer they had resting mean systolic BP of 113 ± 7 mmHg, diastolic BP 66 ± 6 mmHg and HR of 77 ± 11 beats min−1. Four intensities of contraction, 20 %, 30 %, 40 % and 50 % MVC were studied. Peak changes at the end of the 1 min period of contraction were graded according to the intensity of MVC, ranging from 3 ± 2 to 9 ± 1.2 beats min−1 for HR, 8 ± 2 to 17.5 ± 2 mmHg for systolic BP and 4 ± 1 to 10.5 ± 1.5 mmHg for diastolic BP. Regression analysis showed that all these responses were significantly correlated with intensity (HR and systolic BP, P < 0.05; diastolic BP P < 0.001).
Examination of the effects of the different intensities of MVC on the occurrence and time course of the changes revealed that during the first three respiratory cycles no significant BP changes occurred at any force whereas significant changes in HR (P < 0.05) were first observed at 40 % MVC (Fig. 2). However, by 30 s and 60 s at each strength of the isometric contractions both BP and HR showed significant changes (P < 0.03).
Figure 2. Cardiovascular changes at beginning of a muscle contraction.

Bar charts showing the mean changes (± s.e.m.) from baseline in heart rate (HR, beats min−1, A), in systolic blood pressure (SBP, B) and in diastolic blood pressure (DBP, C) in each of the three respiratory cycles c1, c2, c3 immediately following an isometric contraction of triceps surae at two intensities: 20 % and 40 % of maximum voluntary contraction (MVC). Data are for 6 subjects (positive values indicate increases, negative values indicate decreases). By the third respiratory cycle there is a significant increase (P < 0.003) in heart rate whereas there is no significant change in blood pressure.
During these trials fixed respiratory frequency was used as described in Methods and there was no evidence of a Valsalva manoeuvre.
At the end of the isometric contraction both systolic and diastolic BP decreased quickly to control values over three respiratory cycles, whereas HR remained significantly above baseline at 7 ± 1 beats min−1 (P < 0.05).
Sustained stretch
Seven subjects with a mean age of 27 ± 2.3 years participated. When resting supine in the muscle stretch apparatus they had a mean systolic BP of 128 ± 13 mmHg, a diastolic BP of 64.4 ± 7.3 mmHg and HR of 59 ± 5.5 beats min−1.
Sustained passive stretch of triceps surae for 1 min caused a significant (P < 0.05) increase in HR without a significant change in BP. The effect of passive stretch is shown for a typical subject in Fig. 3 in this subject. During the control period, HR displays an oscillation in phase with the respiratory cycle (respiratory sinus arrhythmia, RSA) with a minimum value of 55 beats min−1 and a maximum of 65 beats min−1 (mean RSA, 9 ± 2 beats min−1). On stretching the triceps surae there is an immediate increase in HR which after three beats falls and then rises again to peak above 70 beats min−1, and RSA is decreased to 6 ± 1 beats min−1. On cessation of stretch HR is maintained for a further 10 cardiac cycles before quickly recovering to resting value (Fig. 3A). Mean arterial BP shows an increase at onset of stretch and then falls to similar values to rest for the remainder of the stretch period. The respiratory trace shows that the subject continued to breath at the fixed rate and depth throughout the test, although there is a DC shift in the recording due to a small passive movement of the upper body when the foot was passively dorsiflexed. The group mean data shown in Fig. 4 reveals that HR increased significantly above baseline in the first three respiratory cycles of the stretch period (6 ± 1.5, 5 ± 2.6 and 5 ± 2.8 beats min−1 for each respiratory cycle). It declined slightly at 30 s but was maintained significantly above baseline until the end of the stretch at 60 s (P < 0.05). In contrast the fluctuations in BP are not significantly different from resting values.
Figure 3. Cardiovascular response to sustained passive stretch of triceps surae.

Computer plots of heart rate (A), mean blood pressure (B) and respiratory movements (C). Between the vertical lines the foot was passively dorsiflexed with an angle of rotation of 60 deg and this stretch was maintained for 1 min. The DC shift in the trace in C was due to a small passive movement of the thorax when the foot was rotated. Trace is from one subject.
Figure 4. The effects of sustained passive stretch on cardiovascular variables.
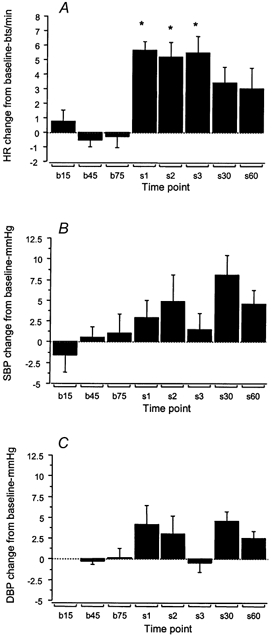
Histograms showing the group mean data (n = 7) of changes in heart rate (A), systolic blood pressure (B) and diastolic blood pressure (C) at 0, 30 and 60 s (b15, b45, b75) prior to passive stretch of triceps surae, and in each of the first three respiratory cycles (s1, s2, s3) and at 30 and 60 s during passive stretch. Mean (± s.e.m.) changes from baseline are shown. * P < 0.01.
The indices of cardiac vagal activity decreased during the stretch period: pNN50, from 0.41 ± 0.08 during baseline to 0.31 ± 0.09 during stretch (not significant, n.s.) and SDSD, from 71 ± 12 to 61.5 ± 11.5 ms (n.s.). Although the SDSD decrease for the whole period of stretch did not reach statistical significance, a calculation of SDSD for 20 cardiac cycles following the onset of stretch and comparing this to a 20 heart beat period pre-stretch revealed a highly significant (P < 0.01) reduction with values of 69.7 ± 12.9 ms pre-stretch and 49.6 ± 8.9 ms during stretch (Fig. 5). Recovery values of SDSD (77.0 ± 47.3 ms) were also significantly different from stretch values (P < 0.03). A similar estimation of pNN50 was not conducted because there were too few data points in 20 cardiac cycles to enable accurate assessment. However, the minute recovery period values for pNN50, 0.45 ± 0.08, were significantly greater than the values during the full minute of the stretch period, 0.31 ± 0.09 (P < 0.05).
Figure 5. Cardiac vagal activity before, during and after passive stretch.

Bar graphs showing the group mean data (± s.e.m.; n = 7) of the standard deviation of successive differences (SDSD), * P < 0.05 comparing stretch to base and recovery values.
Rhythmic passive stretch
To test whether or not the rapidly adapting large proprioceptive muscle afferent fibres were contributing to the HR change a further experiment was performed on four of the subjects in which a robust response to sustained passive stretch was obtained. The triceps surae muscle was subjected to brief rapid passive dorsiflexion of the foot to an angle similar to that used for sustained stretch at a frequency of 0.5 Hz for 1 min. This resulted in no significant change in HR (increase in HR of 1.1 ± 1 beats min−1)
Similarly there were non-significant changes in BP, systolic BP rising 5.0 ± 4 mmHg and diastolic BP remaining the same as control values.
Throughout the passive stretch tests, sustained and rhythmic, there was no EMG activity detected in rectus femoris or gastrocnemius muscles of the same limb.
DISCUSSION
The present study has confirmed that the HR increases soon after the onset of a muscle contraction (Hollander & Bouman, 1975; Hultman & Sjoholm, 1982; Bull et al. 1989; Al-Ani et al. 1997; Fisher & White, 1999). Furthermore, it has shown that the pressor response has a higher threshold of activation than does the HR change. This suggests that different afferent mechanisms might initiate each of the two cardiovascular responses to exercise. Several earlier studies have drawn attention to this possibility. Lind and co-workers (Lind et al. 1968) observed that the loss of small sensory fibres in a patient with syringomyelia led to the absence of a pressor response to handgrip on the affected side whereas the HR increase remained. A similar dissociation was reported in a study in normal subjects undergoing dynamic leg exercise before and during anaesthesia of small afferent fibres (Fernandes et al. 1990). Conversely during a period of post-exercise circulatory occlusion, the pressor response is retained, whereas the HR returns rapidly to control levels (Bull et al. 1989; Fisher & White, 1999).
These differences in the characteristics of the HR change and the pressor response to exercise have strengthened the idea that central motor command to contract muscles also strongly influences the autonomic control of the heart (Secher, 1999). A recent ingenious and elegant study using hypnosis provides strong evidence to support this (Thornton et al. 2001). However, there are circumstances when HR is increased in response to electrically induced contraction of muscle when central command is absent (Bull et al. 1989; Al Ani et al. 1997) suggesting a muscle feedback mechanism can contribute. The present study reveals that afferent feedback from mechanoreceptors could fulfil this role. Passive stretch of the triceps surae to stimulate mechanoreceptors without voluntary command elicited significant increases in HR without a change in BP. As we have shown this is similar to events at the beginning of a voluntary contraction of the same muscles.
The study also addressed the question as to which type of muscle receptor was involved in these effects of stretch. We showed that rapid, transient, rhythmic stretch, which would have stimulated muscle spindle afferents, and possibly also some Golgi tendon organs, did not elicit any cardiovascular response. This is supported by data from cats showing that the large muscle afferents (groups I and II) do not elicit HR or BP increases (Coote & Perez-Gonzalez, 1970; McCloskey et al. 1972; Goodwin et al. 1972; Tibes, 1977).
Interestingly, studies of cat muscle afferent fibres show that group III receptors are sensitive to both stretch and contraction of muscles (Mense & Stahnke, 1983; Kaufman et al. 1983, 1984) unlike group IV fibres. Therefore we speculate that, during isometric contraction of muscles in humans, these group III afferents support the central command signal to increase HR. Clearly in studies in which central command is bypassed by ventral root stimulation in the cat or by electrical stimulation of muscles in humans, activation of muscle group III mechanoreceptors will be the major cause of HR change at the onset of exercise (Gelsema et al. 1983; Bull et al. 1989; McMahon & McWilliam, 1992; Al Ani et al. 1997; Fisher & White, 1999). The build-up of metabolites as contraction proceeds will of course activate the metaboreceptors so that the magnitude of the reflex cardiovascular response will be determined by the amount of afferent input from both types of receptors as has been suggested by a recent study in the anaesthetised cat (Leshnower et al. 2001).
The heart rate changes in response to passive muscle stretch, although quite small, should only be considered as indicative of the influence that muscle mechanoreceptors can have on heart rate. Muscle stretch as applied in the present experiments was used as a surrogate for the natural stimulus to these receptors and the ability to activate them adequately was limited by the degree to which the triceps surae could be lengthened. Studies on the anaesthetised cat in which the triceps surae tendon was cut and muscles were stretched to exert similar forces to those of an isometric contraction showed that both procedures produced similar heart rate changes of up to 30 beats min−1 (Leshnower et al. 2001). The importance of this mechanoreceptor reflex lies in supporting central command to elicit a relatively rapid increase in heart rate at exercise onset, although clearly its effect is sustained throughout the period of stretch. This interpretation is supported by the demonstration in the decerebrate cat that muscle stretch leads to a sustained increase in cardiac vagal nerve activity (Murata & Matsukawa, 2001).
The data obtained from an animal model (Murata & Matsukawa, 2001) make it tempting to speculate that the increase in HR produced by passive stretch of the triceps surae in the present human study was probably mainly a consequence of reflex inhibition of cardiac vagal activity. This is also strongly suggested by the rapid onset of the HR increase, by the decrease in HR variability, and by significant decreases in SDSD values immediately following the onset of muscle stretch. Although this is the first indication in humans, a direct demonstration of inhibition of cardiac vagal nerve activity induced by muscle stretch was provided by an electrophysiological study of vagal nerve activity in decerebrate cats by Murata and co-workers (Murata & Matsukawa, 2001). Our evidence suggested that there was a selective effect of muscle mechanoreceptors on cardiac parasympathetic activity. However, the cat studies indicate that sympathetic nerve activity may also be increased, but this is a transient response in the decerebrate cat (Murata & Matsukawa, 2001). Unlike the latter studies we were unable to obtain evidence in humans that muscle stretch increased sympathetic activity significantly, since there was no significant change in diastolic BP. In contrast, in anaesthetised cats, stretching triceps surae results in a more sustained increase in sympathetic activity to the heart (Murata & Matsukawa, 2001) and to the kidney (Victor et al. 1989; Matsukawa et al. 1990; Matsukawa et al. 1994; Wilson et al. 1994a). This could be an effect of anaesthesia or due to the magnitude of stretch in the cat studies being proportionally greater than those used in the present study in man.
A reduction in vagal tone caused by activation of muscle mechanoreceptors would suggest that baroreceptor excitation of vagal neurones will be compromised. This accords with previous work in man showing that the baroreflex control of heart rate is attenuated (decrease in sensitivity and gain) during dynamic exercise (Bristow et al. 1971; Potts et al. 1993). This was conclusively demonstrated in animal studies where a baroreceptor induced decrease in heart rate was prevented by involuntary contraction of hind limb muscles produced by ventral root stimulation (McMahon & McWilliam, 1992). This contrasts with baroreceptor control of vasomotor activity which, according to a recent report, is enhanced during isometric muscle contraction. The authors showed that during static handgrip the operating point of the baroreflex moved to the right to a higher diastolic pressure and there was a greater decrease in muscle sympathetic nerve activity for a given change in diastolic pressure compared to that at rest (Kamiya et al. 2001). Thus the set point and sensitivity of the baroreflex influence on vasomotor tone was increased. This is a further indication of the differential regulation of the heart (Murata & Matsukawa, 2001) and blood vessels during exercise.
Our finding of a positive influence of stretch on heart rate is in contrast to a previous study in man (Baum et al. 1995). In the latter study subjects sat upright in a frame which enabled downward pressure to be applied to the quadriceps muscle just above the knee. This forced the heel to drop below the level of the ball of the foot which was fixed, thus producing a sudden rapid dorsiflexion. During this procedure, HR either showed a small decrease or remained virtually unchanged. We can suggest three possible reasons for this difference. Firstly, our subjects were tested in the semi-supine position to ensure higher cardiac vagal tone. This should have made our tests more sensitive since the response we are observing is a removal of vagal tone. Secondly, it is likely that mechanoreceptor input was low in the study by Baum et al. (1995) since the degree of muscle stretch would have been less than in the present study. Thirdly, downward pressure applied to the quadriceps and skin of the thigh could have set up opposing reflexes which modified the effect of stretching the triceps surae.
In conclusion our results confirm that the increase in HR at exercise onset is mainly determined by different afferent mechanisms to those determining the pressor response. The results strongly suggest that part of the effect on HR is mediated by muscle mechanoreceptors since we showed for the first time that stimulation of these receptors by sustained passive muscle stretch selectively decreases parasympathetic activity and increases HR. We speculate that this effect is due to the small group III mechanoreceptors in the muscle reflexly inhibiting cardiac vagal neurones. We suggest that this reflex, together with central command, contributes to the prompt cardiac acceleration that occurs during isometric contractions and probably also during dynamic exercise.
Acknowledgments
The authors would like to thank Dr S. J. Gladwell for his technical advice and assistance in this study, and the Mechanical Workshop of the Medical School, University of Birmingham for building the muscle stretch apparatus.
REFERENCES
- Alam M, Smirk FH. Observations in man upon a blood pressure raising reflex arising from the voluntary muscles. Journal of Physiology. 1937;89:372–384. doi: 10.1113/jphysiol.1937.sp003485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al Ani M, Robins K, Al Khalidi AH, Vaile J, Townend J, Coote JH. Isometric contraction of arm flexor muscles as a method of evaluating cardiac vagal tone in man. Clinical Science. 1997;92:175–180. doi: 10.1042/cs0920175. [DOI] [PubMed] [Google Scholar]
- Baum K, Essfeld D, Sondermann C, Leyk D, Stegemann J. Effect of graded changes in extracellular muscle volume on cardiovascular drives during static exercise. European Journal of Applied Physiology. 1993;67:245–249. doi: 10.1007/BF00864223. [DOI] [PubMed] [Google Scholar]
- Baum K, Essfeld D, Stegemann J. Reduction in extracellular muscle volume increases heart rate and blood pressure to isometric exercise. European Journal of Applied Physiology and Occupational Physiology. 1990;60:217–221. doi: 10.1007/BF00839162. [DOI] [PubMed] [Google Scholar]
- Baum K, Selle K, Leyk D, Essfeld D. Comparison of blood pressure and heart rate responses to isometric exercise and passive muscle stretch in humans. European Journal of Applied Physiology. 1995;70:240–245. doi: 10.1007/BF00238570. [DOI] [PubMed] [Google Scholar]
- Bristow JD, Brown EB, Cunningham DJC, Howson MG, Peterson ES, Pickering TG, Sleight P. Effect of bicycling on the baroreflex regulation of the pulse interval. Circulation Research. 1971;28:582–592. [Google Scholar]
- Bull RK, Davies CT, Lind AR, White MJ. The human pressor response during and following voluntary and evoked isometric contraction with occluded local blood supply. Journal of Physiology. 1989;411:63–70. doi: 10.1113/jphysiol.1989.sp017560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coote JH. Cardiovascular responses to exercise: central and reflex contribution. In: Jordan D, Marshall JM, editors. Cardiovascular Regulation. London: Portland Press; 1995. pp. 93–112. [Google Scholar]
- Coote JH, Hilton SM, Perez-Gonzalez JF. The reflex nature of the pressor response to muscular exercise. Journal of Physiology. 1971;215:789–804. doi: 10.1113/jphysiol.1971.sp009498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coote JH, Perez-Gonzalez JF. The response of some sympathetic neurones to volleys in various afferent nerves. Journal of Physiology. 1970;208:261–278. doi: 10.1113/jphysiol.1970.sp009118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandes A, Galbo H, Kjaer M, Mitchell JH, Secher NH, Thomas SN. Cardiovascular and ventilatory responses to dynamic exercise during epidural anaesthesia in man. Journal of Physiology. 1990;420:281–293. doi: 10.1113/jphysiol.1990.sp017912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher WJ, White MJ. Training-induced adaptations in the central command and peripheral reflex components of the pressor response to isometric exercise of the human triceps surae. Journal of Physiology. 1999;520:621–628. doi: 10.1111/j.1469-7793.1999.00621.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gelsema AJ, De Groot G, Bouman LN. Instantaneous cardiac acceleration in the cat elicited by peripheral nerve stimulation. Journal of Applied Physiology. 1983;55:703–710. doi: 10.1152/jappl.1983.55.3.703. [DOI] [PubMed] [Google Scholar]
- Goodwin GM, McCloskey DI, Mitchell JH. Cardiovascular and respiratory responses to changes in central command during isometric exercise at constant muscle tension. Journal of Physiology. 1972;226:173–190. doi: 10.1113/jphysiol.1972.sp009979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollander AP, Bouman LN. Cardiac acceleration in man elicited by a muscle-heart reflex. Journal of Applied Physiology. 1975;38:272–278. doi: 10.1152/jappl.1975.38.2.272. [DOI] [PubMed] [Google Scholar]
- Hultman E, Sjoholm H. Blood pressure and heart rate response to voluntary and nonvoluntary static exercise in man. Acta Physiologica Scandinavica. 1982;115:499–501. doi: 10.1111/j.1748-1716.1982.tb07110.x. [DOI] [PubMed] [Google Scholar]
- Kamiya A, Michikami D, Fu Q, Niimi Y, Iwase S, Mano T, Suzumura A. Static handgrip exercise modifies arterial baroreflex control of vascular sympathetic outflow in humans. American Journal of Physiology - Regulatory, Integrative and Comparative Physiology. 2001;281:R1134–1139. doi: 10.1152/ajpregu.2001.281.4.R1134. [DOI] [PubMed] [Google Scholar]
- Kaufman MP, Longhurst JC, Rybicki KJ, Wallach JH, Mitchell JH. Effects of static muscular contraction on impulse activity of groups III and IV afferents in cats. Journal of Applied Physiology. 1983;55:105–112. doi: 10.1152/jappl.1983.55.1.105. [DOI] [PubMed] [Google Scholar]
- Kaufman MP, Rybicki KJ. Discharge properties of group III and IV muscle afferents: their responses to mechanical and metabolic stimuli. Circulation Research. 1987;61(4 Pt 2):I60–I65. [PubMed] [Google Scholar]
- Kaufman MP, Waldrop TG, Rybicki KJ, Ordway GA, Mitchell JH. Effects of static and rhythmic twitch contractions on the discharge of group III and IV muscle afferents. Cardiovascular Research. 1984;18:663–668. doi: 10.1093/cvr/18.11.663. [DOI] [PubMed] [Google Scholar]
- Kniffki KD, Mense S, Schmidt RF. Muscle receptors with fine afferent fibres which may evoke circulatory reflexes. Circulation Research. 1981;48((6 Pt 2)):I25–I31. [PubMed] [Google Scholar]
- Leshnower BG, Potts JT, Garry MG, Mitchell JH. Reflex cardiovascular responses evoked by selective activation of skeletal muscle ergoreceptors. Journal of Applied Physiology. 2001;90:308–316. doi: 10.1152/jappl.2001.90.1.308. [DOI] [PubMed] [Google Scholar]
- Lind AR, McNicol GW, Bruce RA, Macdonald HR, Donald KW. The cardiovascular responses to sustained contractions of a patient with unilateral syringomyelia. Clinical Science. 1968;35:45–53. [PubMed] [Google Scholar]
- Lind AR, Taylor SH, Humphreys PW, Kennelly BM, Donald KW. The circulatory effects of sustained voluntary muscle contraction. Clinical Science. 1964;27:229–244. [PubMed] [Google Scholar]
- McClain J, Hardy C, Enders B, Smith M, Sinoway L. Limb congestion and sympathoexcitation during exercise. Journal of Clinical Investigation. 1993;92:2353–2359. doi: 10.1172/JCI116840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClain J, Hardy JC, Sinoway LI. Forearm compression during exercise increases sympathetic nerve traffic. Journal of Applied Physiology. 1994;77:2612–2617. doi: 10.1152/jappl.1994.77.6.2612. [DOI] [PubMed] [Google Scholar]
- McCloskey DI, Matthews PB, Mitchell JH. Absence of appreciable cardiovascular and respiratory responses to muscle vibration. Journal of Applied Physiology. 1972;33:623–626. doi: 10.1152/jappl.1972.33.5.623. [DOI] [PubMed] [Google Scholar]
- McCloskey DI, Mitchell JH. Reflex cardiovascular and respiratory responses originating in exercising muscle. Journal of Physiology. 1972;224:173–186. doi: 10.1113/jphysiol.1972.sp009887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMahon SE, McWilliam PN. Changes in R-R interval at the start of muscle contraction in the decerebrate cat. Journal of Physiology. 1992;447:549–562. doi: 10.1113/jphysiol.1992.sp019017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsukawa K, Wall PT, Wilson LB, Mitchell JH. Reflex responses of renal nerve activity during isometric muscle contraction in cats. American Journal of Physiology. 1990;259:H1380–1388. doi: 10.1152/ajpheart.1990.259.5.H1380. [DOI] [PubMed] [Google Scholar]
- Matsukawa K, Wall PT, Wilson LB, Mitchell JH. Reflex stimulation of cardiac sympathetic nerve activity during static muscle contraction in cats. American Journal of Physiology. 1994;267:H821–827. doi: 10.1152/ajpheart.1994.267.2.H821. [DOI] [PubMed] [Google Scholar]
- Mense S, Meyer H. Different types of slowly conducting afferent units in cat skeletal muscle and tendon. Journal of Physiology. 1985;363:403–417. doi: 10.1113/jphysiol.1985.sp015718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mense S, Stahnke M. Responses in muscle afferent fibres of slow conduction velocity to contractions and ischaemia in the cat. Journal of Physiology. 1983;342:383–397. doi: 10.1113/jphysiol.1983.sp014857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murata J, Matsukawa K. Cardiac vagal and sympathetic efferent discharges are differentially modified by stretch of skeletal muscle. American Journal of Physiology - Heart and Circulatory Physiology. 2001;280:H237–245. doi: 10.1152/ajpheart.2001.280.1.H237. [DOI] [PubMed] [Google Scholar]
- Nobrega AC, Williamson JW, Araujo CG, Friedman DB. Heart rate and blood pressure at the onset of dynamic exercise: effect of Valsalva manoeuvre. European Journal of Applied Physiology. 1994;68:336–340. doi: 10.1007/BF00571453. [DOI] [PubMed] [Google Scholar]
- Potts JT, Shi XR, Raven PB. Carotid baroreceptor responsiveness during dynamic exercise in humans. American Journal of Physiology. 1993;265:H1928–1938. doi: 10.1152/ajpheart.1993.265.6.H1928. [DOI] [PubMed] [Google Scholar]
- Secher NH. Cardiovascular function and oxygen delivery during exercise. In: Whipp BJ, Sargeant AJ, editors. Physiological Determinants of Exercise Tolerance in Humans. London: Portland Press; 1999. pp. 93–114. [Google Scholar]
- Stebbins CL, Brown B, Levin D, Longhurst JC. Reflex effect of skeletal muscle mechanoreceptor stimulation on the cardiovascular system. Journal of Applied Physiology. 1988;65:1539–1547. doi: 10.1152/jappl.1988.65.4.1539. [DOI] [PubMed] [Google Scholar]
- Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. Heart rate variability. Standards of measurement, physiological interpretation, and clinical use. Circulation. 1996;93:1043–1065. [PubMed] [Google Scholar]
- Thornton JM, Guz A, Murphy K, Griffith AR, Pedersen DL, Kardos A, Leff A, Adams L, Casadei B, Paterson DJ. Identification of higher brain centres that may encode the cardiorespiratory response to exercise in humans. Journal of Physiology. 2001;533:823–836. doi: 10.1111/j.1469-7793.2001.00823.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tibes U. Reflex inputs to the cardiovascular and respiratory centers from dynamically working canine muscles. Some evidence for involvement of group III or IV fibres. Circulation Research. 1977;41:332–341. doi: 10.1161/01.res.41.3.332. [DOI] [PubMed] [Google Scholar]
- Victor RG, Rotto DM, Pryor SL, Kaufman MP. Stimulation of renal sympathetic activity by static contraction: Evidence for mechanoreceptor-induced reflexes from skeletal muscle. Circulation Research. 1989;64:592–599. doi: 10.1161/01.res.64.3.592. [DOI] [PubMed] [Google Scholar]
- Williamson JW, Mitchell JH, Olesen HL, Raven PB, Secher NH. Reflex increase in blood pressure induced by leg compression in man. Journal of Physiology. 1994;475:351–357. doi: 10.1113/jphysiol.1994.sp020076. erratum appears in Journal of Physiology 1994 476, 554–555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson LB, Dyke CK, Pawelczyk JA, Wall PT, Mitchell JH. Cardiovascular and renal nerve responses to static muscle contraction of decerebrate rabbits. Journal of Applied Physiology. 1994a;77:2449–2455. doi: 10.1152/jappl.1994.77.5.2449. [DOI] [PubMed] [Google Scholar]
- Wilson LB, Wall PT, Pawelczyk JA, Matsukawa K. Cardiorespiratory and phrenic nerve responses to graded muscle stretch in anaethetised cats. Respiration Physiology. 1994b;98:251–266. doi: 10.1016/0034-5687(94)90075-2. [DOI] [PubMed] [Google Scholar]


