Abstract
Protein composition and mechanical function of intermediate filaments were examined in arteries of different sizes using desmin deficient mice (Des−/−) and their wild-type controls (Des+/+). Using SDS-PAGE gels and Western blots we found a gradient in desmin expression in the arterial tree; the desmin content increased from the elastic artery aorta, via the muscular mesenteric artery to the resistance-sized mesenteric microarteries ∼150 μm in diameter in Des+/+ mice. Mechanical experiments were performed on the aorta, the mesenteric artery and resistance-sized arteries using wire myographs. For aorta and mesenteric artery, no differences in passive or active circumference- stress relations were found between Des−/− and Des+/+ mice. In microarteries, both passive and active stress were lower in the Des−/− group. In conclusion, large elastic and muscular arteries contain a relatively low amount of desmin, and the desmin intermediate filaments do not seem to play a major role in the mechanical properties of these larger arterial vessels. In the microarteries, where expression of desmin is high, desmin plays a role in supporting both passive and active tension.
The intermediate filaments are a large family of cytoskeletal structures composed of several classes of proteins (Lazarides, 1982). In smooth muscle, the intermediate filaments are mainly composed of two major proteins, desmin and vimentin (Berner et al. 1981; Osborn et al. 1981), although other intermediate filament proteins have been described, e.g. cytokeratins (Jahn & Franke, 1989; Johansson et al. 1997). Desmin is the predominant intermediate filament protein in visceral smooth muscle (Lazarides, 1982). Vimentin, which is typically expressed in mesenchymally derived non-muscle cells, has been found to be the major intermediate filament in smooth muscle in the aorta (Frank & Warren, 1981; Gabbiani et al. 1981; Schmid et al. 1982). Although vimentin is the predominant intermediate filament protein in large arteries, a gradient in desmin and vimentin expression is found (Osborn et al. 1981); larger arteries contain a small number of desmin-positive cells whereas muscular arteries and veins have a larger number of desmin-positive cells. This gradient in the vimentin/ desmin ratio in the vascular tree has also been reported for human vascular tissues (Johansson et al. 1997). Vimentin and desmin can coexist in the same vascular smooth muscle cell during cell culture (Travo et al. 1982) and in vessels (Berner et al. 1981; Osborn et al. 1987).
The studies of the gradient in vimentin/desmin expression within the vascular tree, discussed above, have mainly been focused on larger elastic and muscular arteries, whereas information on intermediate filament protein expression in smaller resistance-sized arterial vessels is, to our knowledge, not available. Arteries with a diameter of 100–300 μm play a key role in determining resistance within the circulation (Christensen & Mulvany, 2001) and their physiological function is most probably influenced by both their passive and active mechanical properties. If the gradient in desmin/ vimentin expression, observed in larger arterial vessels, also extends to resistance arteries it is likely that desmin intermediate filaments are present in higher amounts and possibly have a more prominent function in the smaller arteries.
The detailed function of the intermediate filaments in smooth muscle is unresolved. In striated muscle, desmin intermediate filaments appear to have a structural role in maintaining the cell integrity and to anchor Z-disks and other cellular structures (Lazarides, 1980; Thornell & Price, 1991; Vigoreaux, 1994). In smooth muscle the desmin intermediate filaments bind to the dense bodies (Small & Sobieszek, 1977; Small, 1995), and might provide an anchoring of the contractile units. Recently, a desmin deficient mouse strain has been developed (Li et al. 1996). In these animals active force generation of visceral smooth muscle is significantly lower, suggesting that the desmin intermediate filaments function in transmission of active force (Sjuve et al. 1998).
The purpose of the present investigation was to examine whether the previously reported gradient in desmin expression in the vasculature extends to the small arterial resistance vessels, i.e. to explore whether these vessels have a higher relative content of desmin compared to larger muscular and elastic arteries. Comparisons were made between a large elastic artery (aorta), a muscular artery (superior mesenteric artery) and resistance arteries (mesenteric microarteries, diameter approximately 150–200 μm) from the mouse. A second objective was to examine the role of desmin intermediate filaments in passive and active mechanical properties of these vessels, which were obtained from different parts of the arterial tree and exhibited a large difference in diameter. To address this question we have used a wire myograph technique (Mulvany et al. 1978) and compared vessels from desmin deficient mice (Li et al. 1996) with their wild-type controls.
Some of these data have been presented in preliminary form at the European Muscle Conference, Pavia, Italy, September 2001.
METHODS
Animals and preparation
The experiments were performed according to the Swedish national ethical guidelines for animal research and approved by the local ethics committee.
Desmin deficient mice (Des−/−), obtained by introducing a null mutation in the desmin gene of C57BL/6J mice as previously described (Li et al. 1996), were compared with age-matched wild-type (Des+/+) mice. The animals were adults of either sex (Des+/+: 7 female, 7 male; Des−/−: 14 female, 5 male). The animals were killed by cervical dislocation and the whole mesentery, the thoracic aorta and the urinary bladder were quickly taken out and transferred to a Hepes-buffered solution (see Solutions). The thoracic aorta, the mesenteric artery and microarteries from the second arch of the mesentery were carefully dissected free from fat and connective tissue under a microscope and mounted for mechanical experiments as described below. Samples from these tissues and from the urinary bladder were also frozen in liquid nitrogen for biochemical analysis.
Solutions
For the mechanical experiments, a Krebs solution of the following composition was used (mm): NaCl 118, KCl 4.7, MgCl2 1.2, KH2PO4 1.2, NaHCO3 24 and glucose 11.5. The solution was gassed with 5 % CO2-95 % O2 giving a pH of 7.4 at 37 °C. When calcium was included in the solution, a concentration of 2.5 mm CaCl2 was used. During dissection and preparation at room temperature, a physiological saline solution buffered with N-(2-hydroxyethyl)piperazine-N′-(2-ethanesulfonic acid) (Hepes) of the following composition was used (mm): NaCl 118, KCl 5, Na2HPO4 1.2, MgCl2 1.2, Hepes 24 (pH 7.4), glucose 10 and CaCl2 1.5. This solution was gassed with oxygen immediately before use.
Experimental procedure
Thin steel wires (diameter 30 μm) were inserted through the lumen of the vessels and the preparations were mounted in either a myograph described previously (Boels et al. 1994) (aorta and mesenteric artery) or in a Model 410A (Danish Myo Technology A/S, Aarhus, Denmark) myograph (microarteries). The vessels were kept at 37 °C in Ca2+-containing Krebs solution and stretched to a low passive tension. The measurements were started after approximately 45 min of equilibration under these conditions. At the start of each experiment, 2–3 high-K+ (80 mm)-induced contractions were performed at the initial length, to obtain reproducible contractions. Thereafter the optimal circumference (L0) for high-K+-induced contractions was determined by contracting the vessel preparations at different circumferences, creating circumference-tension curves. After the 5–7 min-long contractions, the preparations were relaxed in Ca2+-free solution for 5 min before being stretched to a new circumference. At the new circumference the muscle preparations were relaxed for another 5 min before the next contraction was performed by adding 2.5 mm CaCl2 followed by 80 mm KCl. Passive tension was recorded immediately before the addition of CaCl2 and active tension was recorded at the maximum force within 5 min of activation.
After determination of L0, the preparations were equilibrated for 10 min in Ca2+-containing Krebs solution at L0 before activating with 10−5m noradrenaline for 7 min. At the end of each experiment the internal circumference (at L0) and the segmental length of the vessels were measured using a microscope with an ocular scale. The preparations were fixed for microscopy in Ca2+-free Krebs solution with 1 % glutaraldehyde for 1–2 h at room temperature, removed from the apparatus, and thereafter kept at 4 °C in Cacodylate buffer (0.125 m; Merck, Darmstadt, Germany) with 0.125 % glutaraldehyde. The samples were post-fixed in OsO4, embedded in Epon, sectioned (2 μm thickness), stained with Azur III and analysed using light microscopy. The wall thickness was determined using a CCD camera and a digital imaging system (Hamamatsu, Argus-10 Image Processor, Hamamatsu City, Japan). Six measurements of the media thickness were obtained along the vessel circumference. Active and passive stress (i.e. force per unit vessel media area) values were calculated by dividing the tension values (i.e. force per unit vessel wall length) by the vessel wall thickness at L0.
Gel electrophoretic analysis
The frozen samples of the aorta, mesenteric artery, mesenteric microarteries and urinary bladder were homogenised (∼50 μl mg−1) on ice in a sample buffer solution containing: 25 mm Tris-HCl (pH 6.8), 10 % glycerol, 5 % mercaptoethanol, 1 mg ml−1 bromophenol blue and 2 % SDS. The samples were boiled for 2 min, briefly centrifuged and separated on 8 % polyacrylamide gels using a Bio-Rad MiniGel system (Bio-Rad, Richmond, CA, USA). Two gels were run in parallel and on each gel samples of urinary bladder, aorta, mesenteric artery and microarteries were loaded in different concentrations. One of the gels was stained with Coomassie brilliant blue, destained and scanned using a GS-710 calibrated imaging densitometer (Bio-Rad) and evaluated using Quantity One software (Bio-Rad). The areas of the peaks corresponding to myosin heavy chain, actin and intermediate filament proteins were evaluated. The other gel, loaded with the same samples and run in parallel, was used for Western blots. The desmin band was detected using a primary antibody (Sigma cat. No. D1033) and an enhanced bioluminescence kit (ECL, Amersham Biosciences, UK). The reaction was recorded and analysed using a Fluor-S MultiImager system (Bio-Rad).
The intermediate filament protein band on the Coomassie-stained gels (comprising vimentin and desmin) was related to the actin band. Using Western blot we confirmed that the tissues of the Des−/− mice lacked desmin. The relative desmin content of the different tissues was evaluated in the Des+/+ group. As described above, the same volumes of sample were separated in parallel on the Coomassie-stained gels and on the gels used for the Western blots. The area of the desmin band on the Western blots (ECL reaction) was expressed relative to the area of the actin band on the Coomassie-stained gels. This ratio, in arbitrary units, can be used to compare samples within one gel. To compare different sets of gels, the ECL/Coomassie ratio of the bladder tissue (containing a high amount of desmin) was used to normalise the values.
Statistics
All values are given as means ± s.e.m. with the number of animals in parentheses unless stated otherwise. Student's t test was performed to find significant differences; * corresponds to P < 0.05 and ** corresponds to P < 0.01.
RESULTS
Characteristics of the animals
The characteristics of the animals are shown in Table 1. The average body weight of the Des−/− mice was significantly lower than that of the Des+/+ group. The Des−/− had an increased heart/body weight ratio compared to the Des+/+. During dissection we observed calcifications on the heart surface in the Des−/− mice consistent with previous observations on this mouse strain (Li et al. 1996).
Table 1.
Characteristics of the Des+/+ and Des−/− animals
| Des+/+ | Des−/− | P | |
|---|---|---|---|
| Body weight (g) | 29.4 ± 1.7 | 21.1 ± 0.56 | < 0.001 |
| Heart weight (mg) | 177 ± 21 | 185 ± 23 | n.s. |
| Heart weight/body weight (mg g−1) | 5.7 ± 0.3 | 8.1 ± 0.8 | < 0.05 |
Tissue content of contractile and intermediate filament proteins
The biochemical analysis of cytoskeletal and contractile protein expression in the tissue was performed on the mesenteric microarteries, the mesenteric artery and the aorta. For comparison we also examined the urinary bladder tissue. We performed two sets of experiments to determine the relative expression of desmin in the arterial tissues. In the first set we used the Coomassie-stained polyacrylamide gels and evaluated the intermediate filament band, which is mainly composed of desmin and vimentin in smooth muscle. Figure 1A shows photographs of Coomassie-stained gels and corresponding Western blots to show the position of desmin. In the urinary bladder a strong desmin band was observed in the Des+/+ tissue. In the vascular tissues we could resolve the desmin band and on some gels a vimentin band at a position corresponding to the higher molecular weight was also visible. Since the intensity and resolution of these two bands did not enable evaluation of vimentin and desmin separately we chose to quantify an intermediate filament (IF) band (desmin + vimentin). The amounts of samples loaded on the gels used for the analysis (Fig. 2) were all between 3 and 11 μl of the tissue homogenate (corresponding to 0.2–0.73 units in Fig. 1B). The relations between the IF and actin bands and the loaded amount of tissue were linear (Fig. 1B), although they extrapolated to an intensity slightly different from zero at zero loading. This might introduce a small error into the estimates, but since all sample volumes were within the same range, the comparisons between tissues would still be valid. It should also be noted that the staining intensities of actin and IF proteins might differ and the IF/actin ratio might therefore not reflect the absolute ratios of proteins. The IF band relative to actin was significantly weaker in the urinary bladder and microarterial vessels from Des−/− mice (Fig. 2A) whereas no significant difference could be observed in the mesenteric artery and aorta. We could not detect any desmin in the samples from Des−/− mice on Western blots. The data in Fig. 2A thus suggest that the desmin content is higher in the urinary bladder and microarterial vessels. In a second series of experiments the tissue expression of desmin in Des+/+ tissues was examined using quantitative Western blot analysis. The desmin ECL intensity on the Western blots was related to the actin band on the Coomassie-stained gels (cf. Fig. 1C). Urinary bladder tissue, which contains high amount of desmin, was used as a standard. As seen in Fig. 2B the relative ratio of desmin to actin gradually decreased from smaller to larger vessels. The myosin/actin ratio, determined on the Coomassie-stained gels, was not different between the Des+/+ and Des−/− animals (Microarterial vessels Des+/+: 0.31 ± 0.03, Des−/−: 0.32 ± 0.03; Mesenteric artery Des+/+: 0.36 ± 0.02, Des−/−: 0.36 ± 0.04; Aorta Des+/+: 0.50 ± 0.03, Des−/−: 0.51 ± 0.04, n = 5–8).
Figure 1. Gel electrophoretic analysis.
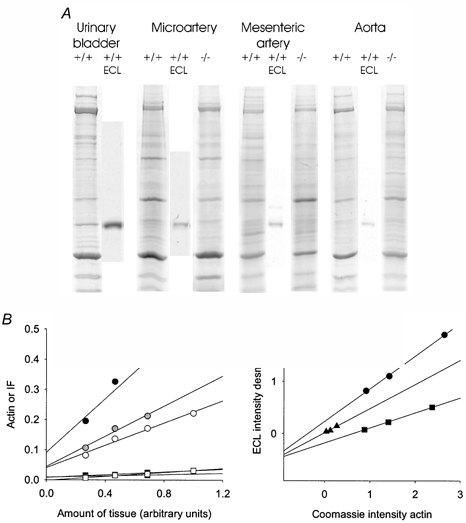
A, original picture of Coomassie-stained SDS gels and Western blots for desmin (ECL reactions) on tissue from the urinary bladder, microartery, mesenteric artery and aorta from Des+/+ and Des−/− mice. B, plots of actin (circles) and intermediate filament bands (IF, squares) vs. the amount of tissue loaded on gels (1 unit corresponds to 15 μl tissue extract). Black symbols show results from a microartery, grey symbols from a mesenteric artery and open symbols from the aorta. Data were fitted by linear regression (r2 > 0.9). C, plots of the ECL intensity of the desmin band in Western blots against the corresponding actin band in the Coomassie blue-stained gel. The values were normalised to the intensities of the bands in a urinary bladder extract (i.e. 1 unit corresponds to the amount of actin or desmin in 1 μl urinary bladder extract). Circles show results from a microartery, triangles from a mesenteric artery and squares from an aorta. Data were fitted by linear regression (r2 > 0.95). All tissues in B and C were from wild-type animals.
Figure 2. Intermediate filament protein content.
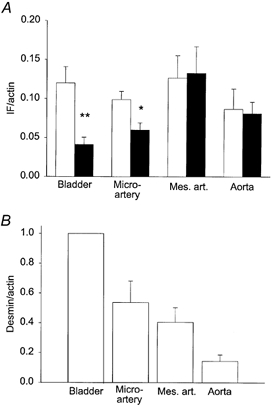
A, intermediate filament protein content in tissues from Des+/+ (open bars) and Des−/− (filled bars). Intermediate filament protein content (IF) is given relative to actin, evaluated using scans of SDS gels stained with Coomassie blue. Statistical differences are indicated, * P < 0.05, ** P < 0.01, n = 6–8. B, desmin content of tissues from Des+/+ expressed as relative units of ECL intensity on Western blots compared to Coomassie blue intensity of the actin band from corresponding SDS gels. To enable comparisons between gels, data were normalised to the ratio in urinary bladder tissue. n = 5–8.
Mechanical properties of arterial vessels
Circumference-tension relations were determined for the three different vessels. Muscles were activated at different circumferences using Ca2+-containing, high-K+ solution and relaxed in Ca2+-free solution. Figure 3 shows the relations for the aorta and the mesenteric artery. Both vessel types had high passive stress and at the optimal circumference for active stress, passive stress was approximately three times the active. No difference in passive or active properties could however be noted for these two vessels between the Des−/− and Des+/+ groups. In Fig. 4, the corresponding mechanical data for the microarteries are shown. The passive stress was generally lower compared to the larger vessels (cf. Fig. 3). Both passive and active stress were significantly lower in the microarteries from the Des−/− compared to the Des+/+ animals.
Figure 3. Circumference-stress relations of the aorta and mesenteric artery.
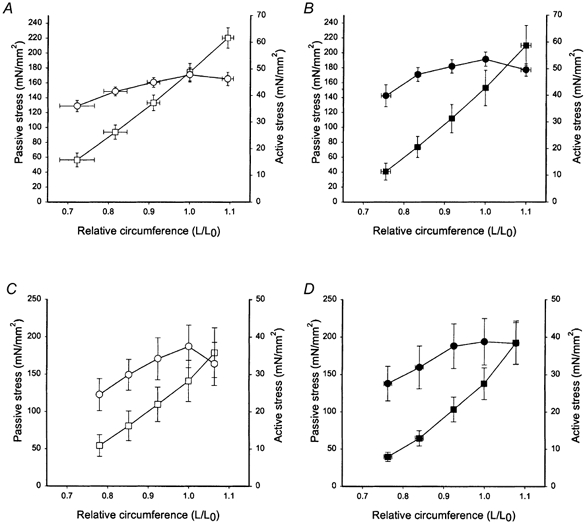
A and B, aorta; C and D, the mesenteric artery of Des+/+ (open symbols, A and C) and Des−/− (filled symbols, B and D) animals. Passive (squares) and active (circles, high-K+ activation) stress values were calculated using wall thickness at L0. Circumference (L) is given relative to the optimal circumference for active force (L0). Data are shown as means ± s.e.m., n = 6–7.
Figure 4. Circumference-stress relations of microarteries.
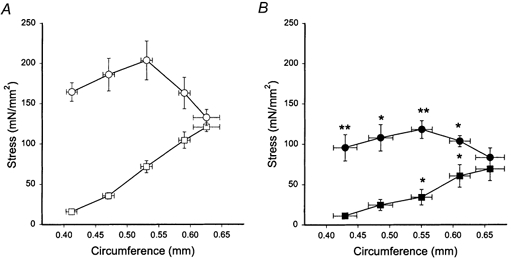
A, Des+/+ (open symbols), and B, Des−/− (filled symbols). Passive (squares) and active (circles, high-K+ activation) stress values were calculated using wall thickness at L0. Data are shown as means ± s.e.m. Statistical differences between Des+/+ and Des−/− data are indicated, * P < 0.05, ** P < 0.01, n = 6.
Table 2 summarises the mechanical and morphological data. The ratios of the circumferences were approximately 1:3:7 for the microarteries, mesenteric artery and aorta. The corresponding ratios in wall thickness were approximately 1:2.5:4.5. No difference in circumference or wall thickness could be detected between the Des+/+ and Des−/− groups for any of the vessels. For the aorta the number of elastic lamellae was similar (4–5) in the Des+/+ and Des−/− groups. No significant difference was observed between the Des+/+ and Des−/− groups with regard to the active and passive stress (force/vessel media cross-sectional area, calculated from wall tension and wall media thickness at L0) for the mesenteric artery or the aorta. For the microarteries both active and passive stress at optimal circumference were significantly lower by approximately 50 %.
Table 2.
Mechanical characteristics of vessels from Des+/+ and Des−/− animals
| Des+/+ | Des−/− | |||||
|---|---|---|---|---|---|---|
| Microartery | Mesenteric artery | Aorta | Microartery | Mesenteric artery | Aorta | |
| Circumference at L0 (mm) | 0.53 ± 0.009 | 1.96 ± 0.080 | 3.77 ± 0.097 | 0.55 ± 0.016 | 1.81 ± 0.059 | 3.50 ± 0.163 |
| Active tension at L0 (mN mm−1) | 1.58 ± 0.07 | 0.81 ± 0.17 | 1.83 ± 0.13 | 1.03 ± 0.12 ** | 0.75 ± 0.07 | 2.04 ± 0.11 |
| Passive tension at L0 (mN mm−1) | 0.56 ± 0.03 | 2.79 ± 0.47 | 6.64 ± 0.40 | 0.30 ± 0.08 * | 2.68 ± 0.35 | 5.94 ± 0.79 |
| Active stress (mN mm−2) | 203.46 ± 24.13 | 37.48 ± 5.71 | 47.81 ± 2.63 | 118.05 ± 10.95 ** | 38.79 ± 6.20 | 53.55 ± 2.69 |
| Passive stress (mN mm−2) | 71.62 ± 7.46 | 141.12 ± 27.63 | 174.25 ± 11.68 | 34.23 ± 9.69 * | 137.89 ± 21.29 | 152.75 ± 23.74 |
| Medial thickness (μm) | 8.1 ± 0.6 | 21.2 ± 2.2 | 38.3 ± 1.2 | 9.0 ± 0.4 | 21.3 ± 2.2 | 39.1 ± 2.8 |
| NA-tension, peak (%) | 117 ± 6 | 94 ± 13 | 53 ± 3 | 112 ± 1 | 104 ± 12 | 75 ± 11 |
| NA-tension, plateau (%) | 119 ± 8 | 165 ± 19 | 24 ± 4 | 108 ± 6 | 191 ± 9 | 92 ± 19** |
Active tension and optimal circumference (L0) for active tension were determined in circumference–tension experiments using K+ activation; n = 6–7. Using medial thickness at L0, active and passive stress were calculated. Tension at peak (within 30 s) and plateau (after 7 min) after noradrenaline (NA, 10−5 m) activation at L0 are given relative to the K+-activated tension; n = 4–6. Statistical comparisons were made for corresponding vessels between the Des+/+ and Des−/− groups.
P < 0.05
P < 0.01.
We also evaluated the sustained phase (5 min after activation onset) of contraction, compared to the peak force developed within 1 min of onset of high-K+ activation. No significant differences in the tension during the later phase relative to the initial were observed between the Des+/+ and the Des−/− groups for either of the vessels. Peak/sustained force values at L0 for the respective groups were: for microarteries, 1.18 ± 0.09 (Des+/+, n = 6) and 1.07 ± 0.11 (Des−/−, n = 5); for the mesenteric artery, 0.77 ± 0.02 (Des+/+, n = 6) and 0.77 ± 0.04 (Des−/−, n = 7) and for aorta; 0.80 ± 0.04 (Des+/+, n = 6) and 0.75 ± 0.02 (Des−/−, n = 7). These results show that Des−/− arteries did not lose more force during sustained contractions than the Des+/+ arteries, although absolute stress was lower in Des−/− microarteries.
The circumference-tension relations above were determined using high-K+ activation. For comparison, we also activated the vessels at the optimal circumference with noradrenaline (10−5m). We evaluated the initial tension reached within 30 s and the sustained component (measured after 7 min). The data from these experiments are shown in Table 2. In the microarteries and mesenteric artery the tension after the noradrenaline activation compared to the high-K+-induced tension was similar in both the Des+/+ and Des−/− groups. In the aorta preparations, peak tension of noradrenaline-induced contractions, expressed relative to K+ contractions was slightly lower than in the other vessel groups. In the Des+/+ aortas the sustained phase of the noradrenaline contractions was lower than the initial peak. In contrast, the tension of the sustained phase of the noradrenaline contractions of the Des−/− aortas was higher than the initial peak and was significantly higher than the corresponding phase of noradrenaline contractions in the Des+/+ group.
DISCUSSION
We report here that resistance-sized microarteries have a high content of desmin intermediate filament protein and that this cellular component has a mechanical function in these vessels. Our data from the mouse, showing a comparatively higher content of desmin in a muscular artery (mesenteric artery), compared to an elastic artery (aorta), are consistent with previous reports from other animals (Berner et al. 1981; Frank & Warren, 1981) and man (Kocher & Gabbiani, 1986; Johansson et al. 1997). The finding that microarteries have high desmin content shows that the gradient in expression of desmin in the arterial tree extends to the vessels considered to determine systemic resistance. Due to the small size of the vessel samples we could not determine the absolute desmin content. However, a complete ablation of the desmin expression resulted in a decrease of the total intermediate filament band by about 60 % (Fig. 2A), in the microarterial vessels. Corresponding data for the mesenteric artery and the aorta suggest low amounts of desmin in these vessels. To obtain specific data for desmin we performed Western blots on Des+/+ vessels, which gave relative contents of desmin in microartery/mesenteric artery/aorta of 1/0.75/ 0.27 (Fig. 2B). These data show a gradient in desmin expression among the vessels towards more desmin in the microarteries compared to the larger arteries.
Our studies of the circumference-tension relations show that passive and active properties are not altered in the larger arteries of the Des−/− mice. Thus the desmin filaments do not have a major mechanical role in these vessels. This is in contrast to the results from the microarterial resistance vessels where both active and passive tensions were reduced in the Des−/− group. Intermediate filaments of the vimentin type or other vascular structures probably have mechanical functions in the larger vessels.
No major differences in vessel structure, as determined by light microscopy, were observed between the Des+/+ and Des−/− groups. In a previous study we have shown that the cellular ultrastructure is essentially unaltered in visceral smooth muscle from Des−/− mice, except that intermediate filaments are absent (Sjuve et al. 1998). Previous studies on the mesenteric microvessels from rats have shown that altered flow can lead to alterations in microarterial structure. Lower flow was associated with reduced wall and media cross-sectional area and circumference at 100 mmHg and unaltered wall stress (Pourageaud & De Mey, 1997). Although the Des−/− mice have a cardiomyopathy with impaired cardiac function (Li et al. 1996; Milner et al. 1999) mean blood pressures have been reported to be unaltered (Lacolley et al. 2001). Our results show that the media thickness at optimal circumference was unaltered in vessels of the Des−/− mice which is not consistent with a major pressure or flow-induced remodelling of vascular structure as an explanation for the lower active wall stress.
In an early study of muscle tissue from Des−/− mice (Li et al. 1996), it was reported that the smooth muscle layer between the elastic lamellae in the aorta was reduced, suggesting a degeneration of the smooth muscle tissue. In an in vivo study of Des−/− mice, it was found that the viscoelasticity of the carotid artery was altered and that the rupture pressure was decreased (Lacolley et al. 2001). In the present study of isolated vessels in vitro we did not find major alterations in the aortic media thickness or in the elastic or contractile properties in the circumference range near optimal for active stress. We have thus not been able to confirm the structural alterations in the aorta described by Li et al. (1996). In addition, other studies using extensive electron microscopy have found essentially normal smooth muscle structure in Des−/− mice (Sjuve et al. 1998). These data and the mechanical measurements in our study suggest that if structural alterations are present in the elastic arteries, they do not seem to affect the static mechanical properties to a major extent. It is possible that the altered elasticity of the carotid artery observed in vivo might reflect that the Des−/− and Des+/+ vessels in the living animal are operating at different parts of the circumference-tension curves or that their level of active tone is different.
An interesting alteration was noted in the contractile behaviour of the aorta following noradrenaline activation; the sustained phase was stronger in the Des−/− group. We have not pursued the nature of this phenomenon, but it might reflect an effect of desmin filaments on cellular functions or secondary alterations in cellular signalling in the large arteries in response to, for example, a cardiac failure in the Des−/− mice. This mechanism might lead to an altered level of adrenergic tone and possibly contribute to altered vessel elasticity in vivo.
The data from microarterial resistance-sized vessels show that the desmin intermediate filament system contributes to both passive and active tension in these vessels. The microarteries from the wild-type Des+/+ mouse, used in the present investigation, had an approximate passive transmural pressure at optimal circumference of 50 mmHg. The total transmural pressure (high-K+ activation) was 191 mmHg. Corresponding data from the Des−/− mice were 26 (passive) and 115 mmHg (total). These pressure values show that the investigated vessels can operate over a pressure range relevant for resistance arteries. Mean arterial blood pressure of anaesthetised Des+/+ and Des−/− mice has been reported to be similar (Lacolley et al. 2001). The exact pressure in this microvascular segment of the awake, living mouse is not known, but if it is in the range 100 mmHg, the Des−/− mice would have to operate at a higher active tone to support the pressure. Interestingly, the Des−/− vessels have not altered their circumference- tension relations towards lower circumference values or adapted by increased wall thickness in response to the lower active and passive wall stress.
A marked alteration in the Des−/− group is a lower tension at optimal circumference in the microarteries compared to the corresponding vessels from the Des+/+ animals. We did not observe any difference in the relation between the initial and the sustained phase of the contraction, suggesting that the microarteries of the Des−/− animals are equally capable of sustaining the developed force after activation. Desmin is located in the vicinity of the dense bodies and plaques in smooth muscle, and it has been suggested that it has a role in anchoring to dense bodies and alignment of the contractile units in smooth muscle cells (Somlyo et al. 1973; Cooke, 1976; Small, 1995). Desmin deficiency could thus result in an inhomogeneity in the length of contractile units in the contractile apparatus and a less effective passive and active force transduction both within and between the cells in the blood vessel. A mis-alignment in the contractile units could also explain the flatter active circumference-tension curve of the microarteries in the Des−/− group, since the length distribution of the contractile units would be broader. To further investigate the difference in active tension development between Des−/− and Des+/+ microarteries we also activated the vessels with noradrenaline. With this agonist we still found a significant reduction in active tension in the microarteries of Des−/− animals, suggesting that the decreased active tension is due to mechanical alterations, rather than differences in signalling events. This interpretation is also consistent with previous findings on skinned visceral smooth muscle from Des−/− mice (Sjuve et al. 1998), where less active force was found even under conditions where cellular signalling events were bypassed. Although our data thus are consistent with a primary defect in force transmission, we cannot at present exclude the possibility that the lack of desmin also influences processes in the excitation-contraction coupling in the microarteries.
The decreased tension in the microarteries could also be due to fewer smooth muscle cells or to a lower myosin content of the existing smooth muscle cells. We have previously shown (Sjuve et al. 1998) that there is no major difference in myosin content between visceral smooth muscle tissue from Des−/− and Des+/+ animals. Also, our data show similar medial wall thicknesses and myosin/ actin ratios of the resistance arteries of Des−/− and Des+/+ animals suggesting that there is no difference in either the amount or the myosin content of smooth muscle cells from the respective groups. These results are consistent with a model, discussed above, where desmin intermediate filaments have a mechanical function in the generation and transmission of active force. With regard to the passive tension in the microvascular wall, our data show that a large portion is supported by the desmin intermediate filaments. Other structures are, however, able to maintain the integrity of the vessel and support about half of the passive tension at optimal circumference.
In conclusion, desmin content is low in larger arteries of the mouse. The desmin intermediate filaments in larger arteries do not have a major mechanical role but they might contribute to other cell functions, possibly cellular signalling following receptor activation. Desmin is the predominant intermediate filament component in resistance-sized microarterial vessels and these filaments contribute to maintenance of passive tension and to the transmission of active force in these vessels.
Acknowledgments
This study was supported by the Swedish Medical Research Council 04X-8268, the Swedish Heart Lung Foundation, the Medical Faculty Lund University and AFM (French Association against Myopathies).
REFERENCES
- Berner PF, Frank E, Holtzer H, Somlyo AP. The intermediate filament proteins of rabbit vascular smooth muscle: immunofluorescent studies of desmin and vimentin. Journal of Muscle Research and Cell Motility. 1981;2:439–452. [Google Scholar]
- Boels PJ, Arner A, Malmqvist U, Uvelius B. Structure and mechanics of growing arterial microvessels from hypertrophied urinary bladder in the rat. Pflügers Archiv. 1994;426:506–515. doi: 10.1007/BF00378528. [DOI] [PubMed] [Google Scholar]
- Christensen KL, Mulvany MJ. Location of resistance arteries. Journal of Vascular Research. 2001;38:1–12. doi: 10.1159/000051024. [DOI] [PubMed] [Google Scholar]
- Cooke P. A filamentous cytoskeleton in vertebrate smooth muscle fibers. Journal of Cell Biology. 1976;68:539–556. doi: 10.1083/jcb.68.3.539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank ED, Warren L. Aortic smooth muscle cells contain vimentin instead of desmin. Proceedings of the National Academy of Sciences of the USA. 1981;78:3020–3024. doi: 10.1073/pnas.78.5.3020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabbiani G, Schmid E, Winter S, Chaponnier C, De Ckhastonay C, Vandekerckhove J, Weber K, Franke WW. Vascular smooth muscle cells differ from other smooth muscle cells: predominance of vimentin filaments and a specific alpha-type actin. Proceedings of the National Academy of Sciences of the USA. 1981;78:298–302. doi: 10.1073/pnas.78.1.298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jahn L, Franke WW. High frequency of cytokeratin-producing smooth muscle cells in human atherosclerotic plaques. Differentiation. 1989;40:55–62. doi: 10.1111/j.1432-0436.1989.tb00813.x. [DOI] [PubMed] [Google Scholar]
- Johansson B, Eriksson A, Virtanen I, Thornell LE. Intermediate filament proteins in adult human arteries. The Anatomical Record. 1997;247:439–448. doi: 10.1002/(SICI)1097-0185(199704)247:4<439::AID-AR1>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- Kocher O, Gabbiani G. Cytoskeletal features of normal and atheromatous human arterial smooth muscle cells. Human Pathology. 1986;17:875–880. doi: 10.1016/s0046-8177(86)80637-2. [DOI] [PubMed] [Google Scholar]
- Lacolley P, Challande P, Boumaza S, Cohuet G, Laurent S, Boutouyrie P, Grimaud J, Paulin D, Lamaziere JD, Li Z. Mechanical properties and structure of carotid arteries in mice lacking desmin. Cardiovascular Research. 2001;51:178–187. doi: 10.1016/s0008-6363(01)00278-4. [DOI] [PubMed] [Google Scholar]
- Lazarides E. Intermediate filaments as mechanical integrators of cellular space. Nature. 1980;283:249–256. doi: 10.1038/283249a0. [DOI] [PubMed] [Google Scholar]
- Lazarides E. Intermediate filaments: a chemically heterogeneous, developmentally regulated class of proteins. Annual Review of Biochemistry. 1982;51:219–250. doi: 10.1146/annurev.bi.51.070182.001251. [DOI] [PubMed] [Google Scholar]
- Li Z, Colucci-Guyon E, Pincon-Raymond M, Mericskay M, Pournin S, Paulin D, Babinet C. Cardiovascular lesions and skeletal myopathy in mice lacking desmin. Developmental Biology. 1996;175:362–366. doi: 10.1006/dbio.1996.0122. [DOI] [PubMed] [Google Scholar]
- Milner DJ, Taffet GE, Wang X, Pham T, Tamura T, Hartley C, Gerdes AM, Capetanaki Y. The absence of desmin leads to cardiomyocyte hypertrophy and cardiac dilation with compromised systolic function. Journal of Molecular and Cellular Cardiology. 1999;31:2063–2076. doi: 10.1006/jmcc.1999.1037. [DOI] [PubMed] [Google Scholar]
- Mulvany MJ, Hansen OK, Aalkjaer C. Direct evidence that the greater contractility of resistance vessels in spontaneously hypertensive rats is associated with a narrowed lumen, a thickened media, and an increased number of smooth muscle cell layers. Circulation Research. 1978;43:854–864. doi: 10.1161/01.res.43.6.854. [DOI] [PubMed] [Google Scholar]
- Osborn M, Caselitz J, Puschel K, Weber K. Intermediate filament expression in human vascular smooth muscle and in arteriosclerotic plaques. Virchows Archiv. A, Pathological Anatomy and Histopathology. 1987;411:449–458. doi: 10.1007/BF00735226. [DOI] [PubMed] [Google Scholar]
- Osborn M, Caselitz J, Weber K. Heterogeneity of intermediate filament expression in vascular smooth muscle: a gradient in desmin positive cells from the rat aortic arch to the level of the arteria iliaca communis. Differentiation. 1981;20:196–202. doi: 10.1111/j.1432-0436.1981.tb01176.x. [DOI] [PubMed] [Google Scholar]
- Pourageaud F, De Mey JG. Structural properties of rat mesenteric small arteries after 4-wk exposure to elevated or reduced blood flow. American Journal of Physiology. 1997;273:H1699–1706. doi: 10.1152/ajpheart.1997.273.4.H1699. [DOI] [PubMed] [Google Scholar]
- Schmid E, Osborn M, Rungger-Brandle E, Gabbiani G, Weber K, Franke WW. Distribution of vimentin and desmin filaments in smooth muscle tissue of mammalian and avian aorta. Experimental Cell Research. 1982;137:329–340. doi: 10.1016/0014-4827(82)90034-9. [DOI] [PubMed] [Google Scholar]
- Sjuve R, Arner A, Li Z, Mies B, Paulin D, Schmittner M, Small JV. Mechanical alterations in smooth muscle from mice lacking desmin. Journal of Muscle Research and Cell Motility. 1998;19:415–429. doi: 10.1023/a:1005353805699. [DOI] [PubMed] [Google Scholar]
- Small JV. Structure-function relationships in smooth muscle: the missing links. Bioessays. 1995;17:785–792. doi: 10.1002/bies.950170908. [DOI] [PubMed] [Google Scholar]
- Small JV, Sobieszek A. Studies on the function and composition of the 10-NM100-A filaments of vertebrate smooth muscle. Journal of Cell Science. 1977;23:243–268. doi: 10.1242/jcs.23.1.243. [DOI] [PubMed] [Google Scholar]
- Somlyo AP, Devine CE, Somlyo AV, Rice RV. Filament organization in vertebrate smooth muscle. Philosophical Transactions of the Royal Society B. 1973;265:223–229. doi: 10.1098/rstb.1973.0027. [DOI] [PubMed] [Google Scholar]
- Thornell LE, Price MG. The cytoskeleton in muscle cells in relation to function. Biochemical Society Transactions. 1991;19:1116–1120. doi: 10.1042/bst0191116. [DOI] [PubMed] [Google Scholar]
- Travo P, Weber K, Osborn M. Co-existence of vimentin and desmin type intermediate filaments in a subpopulation of adult rat vascular smooth muscle cells growing in primary culture. Experimental Cell Research. 1982;139:87–94. doi: 10.1016/0014-4827(82)90321-4. [DOI] [PubMed] [Google Scholar]
- Vigoreaux JO. The muscle Z band: lessons in stress management. Journal of Muscle Research and Cell Motility. 1994;15:237–255. doi: 10.1007/BF00123477. [DOI] [PubMed] [Google Scholar]


