Abstract
Previous studies have demonstrated that post-junctional α1- and α2-adrenoceptors mediate vasoconstriction in the human forearm. However, the relative contributions of the α-adrenoceptor subtypes to basal limb vascular tone are unknown. In healthy young men, forearm blood flow (FBF; venous occlusion plethysmography) responses to brachial artery administration of prazosin (an α1-adrenoceptor antagonist), yohimbine (an α2-adrenoceptor antagonist) and phentolamine (a non-selective α-adrenoceptor antagonist) were determined after local β-adrenoceptor blockade with propranolol. In 10 subjects, prazosin increased FBF from 2.4 ± 0.3 to 5.8 ± 1.0 ml (100 ml)−1 min−1 (∼140 %; P < 0.001vs. baseline). Subsequently, phentolamine further increased FBF to 11.7 ± 1.6 ml (100 ml)−1 min−1 (∼ 385 %; P < 0.001vs. baseline). Thus, the average calculated increase in FBF due to removal of α2-vasoconstrictor tone was greater than that due to removal of α1-tone (5.9 ± 0.8 vs. 3.4 ± 0.8 ml (100 ml)−1 min−1; P < 0.01) and represented ∼ 63 % of basal sympathetic tone. Complete α1-adrenoceptor blockade was confirmed by a minimal reduction in FBF in response to phenylephrine after prazosin (46 ± 3 vs. 6 ± 4 %; before vs. after blockade) and in a separate group of four subjects, increasing the dose of prazosin threefold did not evoke further forearm vasodilatation. Additionally, the reduction in FBF in response to tryamine (evokes endogenous noradrenaline release) was abolished after phentolamine (40 ± 3 vs. 2 ± 1 %; before vs. after blockade), documenting complete pharmacological sympathectomy. In another group of seven subjects, administering yohimbine prior to phentolamine resulted in similar findings. These observations indicate that vasoconstricting post-junctional α2-adrenoceptors contribute more to basal vascular tone than α1-adrenoceptors in the forearms of young healthy men. The potential physiological and pathophysiological implications of these findings are discussed.
Acute sympathectomy of the human forearm results in a significant increase in limb blood flow (Duff, 1951), consistent with the presence of a ‘tonic’ vasoconstriction mediated by sympathetic nerves. Traditionally, it was thought that this was due to noradrenaline stimulating post-junctional α1-adrenoceptors on vascular smooth muscle cells resulting in vasoconstriction (see Ruffolo et al. 1991; Piascik et al. 1996). In addition to post-junctional α1-adrenoceptors, pre-junctional α2-adrenoceptors were demonstrated to be located in the sympathetic nerve endings, where they modulate noradrenaline release via a negative feedback mechanism (see Ruffolo et al. 1991). However, several pharmacological studies in experimental animals and humans have consistently demonstrated the presence of vasoconstricting post-junctional α2-adrenoceptors (Kiowski et al. 1983; Jie et al. 1984). Thus, it is now recognized that post-junctional α2-adrenoceptors contribute to sympathetic vasoconstriction.
Our current understanding of the relative contribution of α1- and α2-adrenoceptors to basal vascular tone in humans is not clear. Previous studies have suggested that basal α1-adrenoceptor vasoconstriction in the human forearm is greater than that mediated through α2-adrenoceptors (Kiowski et al. 1983; Jie et al. 1987b). In contrast, other studies have suggested approximately equal contribution of the adrenoceptor subtypes to basal and stimulated vascular tone (Bolli et al. 1983; Jie et al. 1984; Taddei et al. 1988). These equivocal results are probably due to different experimental approaches and the interpretation difficulties associated with them. For example, in some studies, α-adrenoceptor antagonists (selective or non-selective) were administered to different groups of subjects (Kiowski et al. 1983; Taddei et al. 1988), limiting the ability to determine the relative contribution of the receptor subtypes to basal vascular tone within individuals. In other studies, selective α-adrenoceptor antagonists were given to the same subject, but α2-adrenoceptor blockade was performed before α1-adrenoceptor blockade (Jie et al. 1984, 1987b). This may inhibit pre-junctional α2-adrenoceptors, increase noradrenaline release, and (in the presence of α2-adrenoceptor blockade) result in α1-adrenoceptor stimulation and a subsequent overstimation of α1-adrenoceptor tone (Jie et al. 1987a; Grossman et al. 1991). In this context, it is possible that the relative contribution of post-junctional α2-adrenoceptors to basal vascular tone may have been underestimated. Finally, the degree of selective α-adrenoceptor blockade often was not demonstrated (Bolli et al. 1983; Jie et al. 1987a, b). Therefore, the relative contribution of α1- and α2-adrenoceptors to basal limb vascular tone in humans is currently unknown.
With this information as background, the purpose of this study was to test the hypothesis that vasoconstricting post-junctional α2-adrenoceptors are responsible for most of the basal sympathetic vascular tone observed in the forearms of healthy men. To test this hypothesis, forearm blood flow responses to brachial artery administration of prazosin (a selective α1-adrenoceptor antagonist), yohimbine (a selective α2-adrenoceptor antagonist) and phentolamine (a non-selective α-adrenoceptor antagonist) were determined after local β-adrenoceptor blockade to eliminate any β-mediated effects of the study drugs.
METHODS
Subjects
With Institutional Review Board approval and after written informed consent, a total of 21 young healthy men (age, 26 ± 1 years; weight, 84.3 ± 2.8 kg; height, 182 ± 2 cm; body mass index, 25.3 ± 0.6 kg m−2; means ± s.e.m.) participated in the present study. All were non-smokers, non-obese, normotensive, and not taking any medications. Studies were performed after an overnight fast with the subjects in the supine position. All studies were performed according to the Declaration of Helsinki.
Arterial catheterization
A 20 gauge, 5 cm catheter was placed in the brachial artery of the non-dominant arm under aseptic conditions after 1 % lidocaine (lignocaine) local anaesthesia. The catheter was connected to a pressure transducer for mean arterial blood pressure (MAP) measurement and continuously flushed at 3 ml h−1 with heparanized saline (Dietz et al. 1994). All study drugs were administered via the brachial artery catheter at rates of 2–3 ml min−1.
Forearm blood flow and vascular conductance
Forearm blood flow (FBF) was measured using venous occlusion plethysmography with mercury-in-silastic strain gauges (Greenfield et al. 1963). Briefly, a paediatric blood pressure cuff was placed around the wrist and inflated to suprasystolic levels (220 mmHg) to arrest the circulation of the hand, and a venous occlusion cuff was placed on the upper arm and rapidly inflated to 50 mmHg every 7.5 s, yielding one blood flow every 15 s. FBF was expressed as millilitres per 100 millilitres of tissue per minute (ml (100 ml)−1 min−1). To account for any changes in blood pressure, forearm vascular conductance (FVC) was calculated as (FBF/MAP) × 100, and expressed as‘arbitrary units’ (a.u.).
Protocol 1. Relative α-adrenoceptor control of basal limb vascular tone assessed by selective α1-adrenoceptor blockade followed by non-selective blockade
The main experimental protocol (n = 10) is shown in Fig. 1A. Propranolol was given at 10 μg (100 ml forearm volume)−1 min−1 (μg (100 ml)−1 min−1) for 5 min to control for the potential of noradrenaline binding to β-adrenoceptors during complete α-adrenoceptor blockade and evoking vasodilatation that is not mediated by the removal of α-adrenergic vasoconstrictor tone (Saeed et al. 1982), as well as to control for any β-stimulating effects of phenylephrine (Torp et al. 2001). This dose has been documented to block forearm vasodilatation to isoproteronol (Johnsson, 1967). A‘maintenance’ dose of propranolol (5 μg min−1) was then infused throughout the protocol. Prazosin was given at 0.5 μg (100 ml)−1 min−1 for 10 min to block α1-adrenoceptors (Kiowski et al. 1983). Phenylephrine was administered at 0.125 μg (100 ml)−1 min−1 for 2 min to stimulate α1-adrenoceptors before and after prazosin to document the degree of α1-adrenoceptor blockade. In subjects that demonstrated significant vasoconstriction to phenylephrine during α1-adrenoceptor blockade (n = 6), an additional loading dose (50 % of the original) of prazosin was given to ensure complete blockade. Phenylephrine infusion was then repeated.
Figure 1. Experimental protocols.
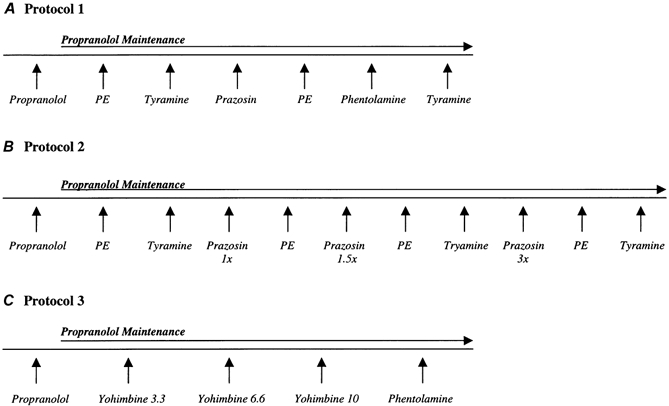
The order of phenylephrine and tyramine was randomized prior to α-adrenoceptor antagonist adminstration. FBF, MAP and HR were recorded before and during infusions of study drugs. Appropriate rest periods were allowed for FBF to return to levels prior to α-adrenoceptor agonist administration; PE indicates phenylephrine. See text for further details.
Susequently, phentolamine was administered at 12 μg (100 ml)−1 min−1 for 10 min to block both α1- and α2-adrenoceptors (Egan et al. 1987). To document complete α-adrenoceptor blockade, tyramine was administered at 6 μg (100 ml)−1 min−1 for 3 min to evoke endogenous noradrenaline release and stimulate both α1- and α2-adrenoceptors before and after phentolamine (Frewin & Whelan, 1968; Jie et al. 1987a, b). In subjects that demonstrated significant vasoconstriction to tyramine during non-selective α-adrenoceptor blockade (n = 3) an additional dose (50 % of the original) of phentolamine was given to provide greater non-selective α-adrenoceptor blockade and the tyramine infusion was repeated.
The relative contribution of α1-adrenoceptors to limb basal vascular tone was calculated as: (prazosin FBF–rest FBF)/(phentolamine FBF–rest FBF) × 100, expressed as a percentage of the total increase in limb blood flow during pharmacological sympathectomy. Similarly, the relative contribution of α2-adrenoceptors to basal limb vascular tone was calculated as (phentolamine FBF–prazosin FBF)/(phentolamine FBF–rest FBF) × 100. These calculations were also performed for FVC.
Protocol 2. Effects of increasing doses of prazosin on α1-adrenoceptor vasodilatation and vasoconstrictor responses to tyramine
In an additional group of four subjects, we determined whether the dose of prazosin administered in Protocol 1 elicited maximal vasodilatation and whether this influenced the α2-adrenoceptor vasoconstrictor response to tyramine during selective α1-adrenoceptor blockade. This experimental protocol is shown in Fig. 1B. The doses of propranolol, phenylephrine, tyramine, and the first two doses of prazosin (1 × and 1.5 ×) were identical to those used in Protocol 1. The third dose of prazosin (3 ×) was 1.5 μg (100 ml)−1 min−1, and was administered for 10 min.
Protocol 3. Relative α-adrenoceptor control of basal limb vascular tone assessed by selective α2-adrenoceptor blockade followed by non-selective blockade
In another group of seven subjects, the relative contribution of α1- and α2-adrenoceptors to basal limb vascular tone was determined by blocking α2-adrenoceptors prior to non-selective blockade with phentolamine (Fig. 1C). Yohimbine was administered at 3.3, 6.6 and 10 μg (100 ml)−1 min−1 for 2 min to selectively block α2-adrenoceptors. The highest dose of yohimbine has been shown to elicit maximal forearm vasodilatation when given for 4 min, but significantly increases forearm venous noradrenaline concentrations (Kubo et al. 1989). Thus, our rationale to administer this dose for 2 min was an attempt to maximize forearm blood flow responses while minimizing pre-junctional effects of yohimbine facilitating noradrenaline release. The doses for propranolol and phentolamine were identical to those in Protocol 1, except the duration of phentolamine adminstration was 15 min for all subjects (total dose equivalent to the highest given in Protocol 1).
In this protocol, an 18 gauge, 3 cm catheter was also inserted in an antecubital vein of the experimental forearm (n = 4) and directed toward the hand so the tip was located in a deep vein that drained the forearm muscles (Joyner et al. 1992). Arterial and venous blood samples were obtained prior to yohimbine administration, and venous samples taken at the end of the infusion of each dose of yohimbine for determination of plasma noradrenaline concentrations via high-performance liquid chromatography (Minson et al. 2000). Noradrenaline spillover was calculated as the product of the difference in arterial and venous noradrenaline and forearm blood flow (Jie et al. 1987a). Similar to Protocol 1, α2-adrenoceptor contribution to basal vascular tone (highest dose of yohimbine) was calculated as (yohimbine FBF–rest FBF)/(phentolamine FBF–rest FBF) × 100, and α1-adrenoceptor contribution as (phentolamine FBF–yohimbine FBF)/ (phentolamine FBF–rest FBF) × 100. These calculations were also performed for FVC.
Data analysis and statistics
Data were digitised and stored on a computer at 250 Hz and analysed off-line with signal-processing software (WinDaq, DATAQ Instruments, Akron, OH, USA). FBF was determined from the derivative of the forearm plethysmogram. Heart rate (HR) was derived from the electrocardiogram signal (5-lead ECG) and MAP was derived from the arterial pressure waveform. For phenylephrine and tyramine, the data reported represent an average of the last minute of each dose of drug infusion. Similarly, the data reported for yohimbine represent an average of the last minute of each dose of drug infusion. For prazosin and phentolamine, we observed an initial vasodilatation that was sustained throughout the infusion; thus the data reported represent an average of the last 3 min of drug infusion.
All values reported are means ± s.e.m. Student's paired t tests were used to compare each subject's baseline and blood flow responses across drug infusion conditions. Statistical significance was set a priori at P < 0.05.
RESULTS
Protocol 1
The group and individual FBF values at baseline and after the α-antagonists are presented in Table 1. Selective blockade of α1-adrenoceptors with prazosin resulted in an ∼140 % increase in FBF from 2.4 ± 0.3 to 5.8 ± 1.0 ml (100 ml)−1 min−1 (P < 0.001vs. baseline). Non-selective α-adrenoceptor blockade with phentolamine resulted in a further increase in FBF to 11.7 ± 1.6 ml (100 ml)−1 min−1 (∼ 385 %; P < 0.001vs. baseline). Similar results were obtained when vasodilator responses to the α-adrenoceptor antagonists are expressed as FVC (baseline: 2.6 ± 0.3 a.u.; prazosin: 6.6 ± 1.3 a.u.; phentolamine: 13.4 ± 2.2 a.u.). On average, the calculated increase in FBF and FVC due to removal of α2-vasoconstrictor tone was greater than that due to removal of α1-tone (5.9 ± 0.8 vs. 3.4 ± 0.8 ml (100 ml)−1 min−1; 6.8 ± 1.1 vs. 4.0 ± 1.0 a.u.; P < 0.01). Thus, of the total increase in limb vasodilatation to pharmacological sympathectomy, 63 % was due to removal of α2-vasoconstrictor tone.
Table 1.
The effects of a-adrenoceptor antagonists on forearm blood flow in individual subjects from Protocol 1
| Subject | Baseline | After prazosin | Calculated α1-tone | After phentolamine | Calculated α2-tone |
|---|---|---|---|---|---|
| 1 | 3.3 | 8.7 | 5.4 | 18.3 | 9.6 |
| 2 | 2.9 | 5.4 | 2.5 | 8.3 | 2.9 |
| 3 | 1.4 | 3.5 | 2.1 | 8.8 | 5.3 |
| 4 | 2.3 | 3.5 | 1.2 | 11.3 | 7.7 |
| 5 | 1.7 | 5.7 | 4.0 | 10.6 | 4.9 |
| 6 | 2.1 | 4.2 | 2.1 | 7.8 | 3.6 |
| 7 | 3.0 | 12.5 | 9.5 | 21.3 | 8.8 |
| 8 | 3.8 | 7.5 | 3.7 | 15.9 | 8.4 |
| 9 | 2.4 | 4.3 | 1.9 | 10.5 | 6.3 |
| 10 | 1.3 | 2.3 | 1.0 | 4.7 | 2.5 |
| Means ± s.e.m. | 2.4 ± 0.3 | 5.8 ± 1.0* | 3.4 ± 0.8 | 11.7 ± 1.6*† | 5.9 ± 0.8‡ |
All values reported are ml (100 ml)−1 min−1; α1-vasoconstrictor tone is expressed as the difference in FBF after selective α1-adrenoceptor blockade and that at baseline; α2-vasoconstrictor tone is expressed as the difference in FBF after combined α-adrenoceptor blockade (phentolamine) and that after selective α1-adrenoceptor blockade (prazosin).
P < 0.01 vs. baseline
P < 0.01 vs. prazosin
P < 0.01 vsα1-tone.
The reduction in FBF to phenylephrine was significantly reduced by prazosin administration, documenting effective α1-adrenoceptor blockade (46 ± 3 vs. 6 ± 4 %; before vs. after blockade; Fig. 2A). Similarly, forearm vasoconstrictor responses to tyramine were abolished after non-selective α-blockade with phentolamine (40 ± 3 vs. 2 ± 1 %; before vs. after blockade), documenting complete α-adrenoceptor blockade (Fig. 2B).
Figure 2. Effectiveness of selective α1- (A) and non-selective1 (B) α-adrenoceptor blockade.
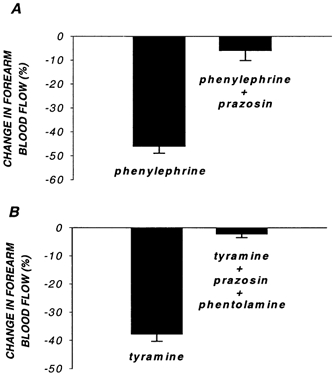
Reductions in forearm blood flow to phenylephrine (A) and tyramine (B) are nearly abolished by α1-adrenoceptor blockade (prazosin) and non-selective α-adrenoceptor blockade (prazosin + phentolamine), respectively.
MAP was not significantly different after prazosin (95 ± 2 vs. 92 ± 3 mmHg; baseline vs. prazosin) or phentolamine (91 ± 3 mmHg). HR was similar under all conditions (baseline: 56 ± 2 beats min−1; prazosin: 58 ± 2 beats min−1; phentolamine: 58 ± 2 beats min−1). The relative α-adrenoceptor contribution to forearm vascular tone was not different in the subjects whose MAP tended to be lower (n = 5) compared with those that did not demonstrate any change during α-adrenoceptor antagonist infusion. Importantly, in the two subjects that demonstrated reductions in MAP (∼ 4–;6 mmHg) and possibly baroreflex-mediated increases in HR (∼ 6 beats min−1), the relative α-adrenoceptor contribution to forearm vascular tone was similar to the pooled group (α2: 67 %; α1: 33 %).
Protocol 2
The degree of α1-adrenoceptor blockade was similar for the two higher doses of prazosin (Table 2). This is demonstrated by the comparable blunting of the vasoconstrictor responses to phenylephrine, as well as the lack of further increase in FBF and FVC. Increasing the dose threefold (3 ×) compared with the dose administered in Protocol 1 did not evoke further vasodilatation. MAP and HR were similar under all conditions of this protocol. Vasoconstrictor responses to tyramine during selective α1-adrenoceptor blockade are also presented in Table 2. This vasoconstrictor response mediated via α2-adrenoceptors was ∼ 10 % lower during selective α1-adrenoceptor blockade, but was similar during the two higher doses of prazosin.
Table 2.
Forearm vascular responses and systemic haemodynamics from Protocol 2
| Baseline | 1 × Prazosin | 1.5 × Prazosin | 3 × Prazosin | |
|---|---|---|---|---|
| FBF (ml (100 ml)−1 min−1) | 1.7 ± 0.2 | 2.6 ± 0.2 | 2.4 ± 0.3 | 2.6 ± 0.2 |
| FVC (U) | 2.0 ± 0.2 | 3.0 ± 0.1 | 2.8 ± 0.2 | 3.0 ± 0.2 |
| Phenylephrine (%) | −57 ± 6 | −15 ± 3 | −8 ± 3 | −7 ± 2 |
| Tyramine (%) | −41 ± 5 | — | −31 ± 4 | −29 ± 3 |
| MAP (mmHg) | 85 ± 3 | 87 ± 4 | 84 ± 4 | 84 ± 3 |
| Heart rate (beats min−1) | 56 ± 2 | 57 ± 4 | 57 ± 4 | 57 ± 4 |
FBF, forearm blood flow; FVC, forearm vascular conductance; MAP, mean arterial blood pressure.
Protocol 3
Yohimbine elicited a dose-response increase in FBF and FVC (Table 3), and phentolamine caused further vasodilatation (P < 0.001). In these subjects, the calculated relative contribution of α2-adrenoceptors to basal vascular tone during pharmacological sympathectomy was 59 %. Baseline noradrenaline spillover was 6 ± 20 pg (100 ml)−1 min−1 and did not consistently change during yohimbine administration (yohimbine 3.3: −13 ± 26 pg (100 ml)−1 min−1; yohimbine 6.6: 17 ± 36 pg (100 ml)−1 min−1; yohimbine 10: −29 ± 32 pg (100 ml)−1 min−1). MAP and HR were not affected by either yohimbine or phentolamine administration in this protocol.
Table 3.
Forearm vascular responses and systemic haemodynamics from Protocol 3
| Baseline | Yohimbine 3.3 | Yohimbine 6.6 | Yohimbine 10 | Phentolamine | |
|---|---|---|---|---|---|
| FBF (ml (100 ml)−1 min−1) | 2.5 ± 0.4 | 3.2 ± 0.4* | 3.4 ± 0.4* | 3.5 ± 0.5* | 4.4 ± 0.5*† |
| FVC (U) | 2.7 ± 0.4 | 3.5 ± 0.4* | 3.7 ± 0.4* | 3.8 ± 0.4* | 4.8 ± 0.5*† |
| MAP (mmHg) | 91 ± 2 | 91 ± 1 | 90 ± 2 | 91 ± 2 | 91 ± 2 |
| Heart rate (beats min−1) | 55 ± 3 | 54 ± 3 | 54 ± 3 | 54 ± 3 | 56 ± 3 |
FBF, FVC, MAP, see definitions above. Numbers after yohimbine indicate dose administered in μg (100 ml)−1 min−1.
P < 0.01 vs. baseline
P < 0.01 vs. Yohimbine (all doses).
DISCUSSION
The primary finding of the present study is that the relative post-junctional α2-adrenoceptor contribution to basal vascular tone in young healthy men is greater than that mediated through α1-adrenoceptors. The main experimental evidence supporting this is that the proportion of the total forearm vasodilatation during pharmacological sympathectomy that could be accounted for by selective α1-adrenoceptor blockade is significantly less than that accounted for by removal of α2-adrenoceptor vasoconstrictor tone.
Previous studies attempting to address α1- and α2-adrenoceptor subtype contribution to basal vascular tone have yielded equivocal results. This is probably due to differences in experimental approaches and the interpretation difficulties associated with them. Potential problems of these earlier studies (alone or in combination) include the lack of administration of selective α-adrenoceptor antagonists to the same groups of subjects (Kiowski et al. 1983; Taddei et al. 1988), or the administration of α2-adrenoceptor antagonists prior to α1-adrenoceptor blockade (Jie et al. 1984, 1987b). Another potential limitation with some of these previous studies is the lack of evidence for selective α-adrenoceptor blockade (Bolli et al. 1983; Jie et al. 1987a, b). The experimental protocols utilized in the present study were designed to minimize, if not eliminate, these previous limitations.
Evidence for greater relative post-junctional α2-adrenoceptor contribution to basal forearm sympathetic vasoconstrictor tone
In the present study, we employed several experimental approaches to demonstrate greater basal vasoconstriction mediated via post-junctional α2-adrenoceptors in humans. In Protocol 1, we determined the forearm vasodilator responses to selective post-junctional α1-adrenoceptor blockade (prazosin), followed by non-selective α-adrenoceptor blockade (phentolamine). Expressed as a percentage of the total vasodilatation observed during complete post-junctional α-adrenoceptor blockade, the amount of vasoconstriction mediated via α1-adrenoceptors was 37 %, whereas that mediated via α2-adrenoceptors was 67 %. For this approach to be valid, we first needed to demonstrate complete α1-adrenoceptor blockade. The experimental evidence supporting complete α1-adrenoceptor blockade includes: (1) the significant blunting of forearm vasoconstrictor responses to the selective α1-adrenoceptor agonist phenylephrine (Fig. 1A; Table 2); (2) the lack of further vasodilatation when the dose of prazosin administered was increased threefold above the highest dose used in Protocol 1 (Table 2).
In addition to providing evidence for complete selective α1-adrenoceptor blockade, it was equally as necessary to document complete non-selective α-adrenoceptor blockade (i.e. α1 and α2). In this context, we demonstrated that the vasoconstrictor responses to endogenous norepinephrine release (evoked via tyramine) was abolished after administration of both prazosin and phentolamine (Fig. 1B), consistent with complete pharmacological sympathectomy. Further, in Protocol 2, we demonstrated that selective α1-adrenoceptor blockade only modestly blunted the vasoconstriction evoked via tyramine (by ∼ 10 %). This suggests that non-selective α-adrenoceptor blockade with phentolamine in Protocol 1 was responsible for abolishing most of the tyramine-induced vasoconstriction. Collectively, the data from Protocols 1 and 2 strongly suggest complete selective α1-and non-selective α-adrenoceptor blockade with the doses of prazosin and phentolamine administered.
In Protocol 3, we determined the relative contributions of post-junctional α1-and α2-adrenoceptors to basal sympathetic vasconstrictor tone by selective blockade of α2-adrenoceptors (yohimbine) prior to non-selective α-adrenoceptor (i.e. reversed the order of α-adrenoceptor blockade). Given that α2-adrenoceptor blockade has been documented to facilitate noradrenaline release (Jie et al. 1987a; Grossman et al. 1991), we estimated noradrenaline spillover in four of seven subjects in this protocol. Although the doses of yohimbine administered in the present study resulted in negligible changes in noradrenaline spillover, we cannot exclude this possibility as we did not determine this using radiolabelled isotopes. Nevertheless, using this approach, we found that the relative contribution of α1-adrenoceptors to basal sympathetic vasoconstrictor tone was 41 %, and that mediated via α2-adrenoceptors was 59 %. Taken together, the findings from Protocols 1 and 3 indicate that post-junctional α2-adrenoceptors contribute ∼ 60 % to basal forearm sympathetic vasoconstrictor tone in humans.
Critique of methodology
Determination of the relative contribution of post-junctional α-adrenoceptors to basal sympathetic vasoconstrictor tone in humans is challenging. However, we feel that the experimental approach employed in Protocol 1 may be used to determine the relative contribution of α1- and α2-adrenoceptors to basal sympathetic tone for the following reasons. First, previous studies have not only demonstrated the selective nature of prazosin for post-junctional α1-adrenoceptors, but have also shown that α1-adrenoceptor blockade with prazosin does not affect noradrenaline release (Cambridge et al. 1977). Therefore, assessment of the vasodilator responses to local α1-adrenoceptor blockade should represent only the removal of vasoconstriction mediated through these receptors. Second, although phentolamine has similar affinity for both α1- and α2-adrenoceptors (Doxey et al. 1977) and can evoke noradrenaline release by inhibiting pre-junctional α2-adrenoceptors (Saeed et al. 1982), blockade of β-adrenoceptors prior to phentolamine will eliminate any confounding vasodilatation that does not represent removal of sympathetic α-adrenergic vasoconstrictor tone (Saeed et al. 1982). Thus, the magnitude of vasodilatation in response to non-selective α-adrenoceptor blockade with phentolamine after selective α1-adrenoceptor blockade with prazosin should represent only the removal of α2-vasoconstrictor tone.
Experimental limitations
One assumption of the present study is that the pharmacological agents administered to selectively (yohimbine) or non-selectively (phentolamine) block post-junctional α2-adrenoceptors act primarily at the level of the vascular smooth muscle cell. Data from experimental animals suggest that stimulation of endothelial α2-adrenoceptors evokes a nitric oxide-mediated vasodilatation (Angus et al. 1986). Whether these adrenoceptors are involved in tonic (basal) nitric oxide synthesis and release in humans is unknown. We cannot exclude this possibility and, if this were the case, blockade of these adrenoceptors would reduce the vasodilator response (by inhibition of nitric oxide) and understimate our calculation of the relative α2-adrenoceptor contribution to basal sympathetic vascular tone. Another issue not addressed with the present study design relates to the subdivision of post-junctional α1- (α1A, α1B and α1D) and α2- (α2A, α2B and α2C) adrenoceptors, which have recently been identified and cloned in humans (see Bylund et al. 1995). Currently there are no highly selective α-adrenoceptor antagonists that can be administered to humans. Therefore, whether these selectively participate in the control of basal or stimulated sympathetic vasoconstriction in humans awaits further study.
General observations
Of interest, the range of increases in FBF from Protocol 1 during complete pharmacological sympathectomy within this homogenous group of young men was 3.4 to 18.3 ml (100 ml)−1 min−1. This biological variability may reflect individual differences in basal muscle sympathetic nerve activity (MSNA), noradrenaline release, α-adrenoceptor responsiveness, or interactions with other factors that can modulate α-adrenergic vasoconstrictor tone. Another interesting observation from the present study was that the magnitude of forearm vasodilatation in the subjects from Protocol 1 was greater compared to the vasodilatation observed in the other two protocols. It is possible that this again reflects normal interindividual biological variability. Another possiblity is that seasonal variations in basal MSNA and, hence, vasoconstrictor tone exist in humans. In this context, preliminary evidence suggests that basal MSNA and plasma noradrenaline are ∼ 40 % greater in winter months compared with summer (Niimi et al. 1999). This is consistent with our observation of greater forearm vasodilatation to pharmacological sympathectomy in the subjects from Protocol 1, who were studied in winter (January-March), compared with subjects in Protocols 2 and 3 who were studied in summer/early autumn (June-October). The possibility of seasonal differences in sympathetic vasoconstrictor tone deserves further study.
Potential significance
Skeletal muscle blood flow responses during a variety of hyperaemic conditions depend critically on the interaction between local vasodilator and neural vasoconstrictor influences. Recent studies in experimental animals (Anderson & Faber, 1991; Thomas et al. 1994; Buckwalter et al. 2001) and humans (Hansen et al. 2000) have demonstrated that the vasoconstrictor responses to sympathetic nerve stimulation, α-adrenoceptor agonist administration, and/or noradrenaline release are blunted in contracting skeletal muscle (functional sympatholysis). The data from the animal models have consistently demonstrated that this effect is mediated primarily through α2-adrenoceptors (Anderson & Faber, 1991; Thomas et al. 1994; Buckwalter et al. 2001) and might be specific to fast-twitch skeletal muscle fibres (Anderson & Faber, 1991; Thomas et al. 1994). One interpretation of this data is that the proportion of α2-adrenoceptors is greater in the arterioles that subserve fast-twitch compared with slow-twitch skeletal muscle. Whether this is true for humans is unknown. However, if this were the case, we might expect that any conditions that influence skeletal muscle fibre type distribution (e.g. ageing, heart failure, muscular dystrophy) may be associated with similar changes in post-junctional α-adrenoceptor subtypes and their respective contribution to vascular tone.
Another factor that may influence relative post-junctional α-adrenoceptor control of basal limb vasoconstrictor tone is chronic levels of MSNA. For example, Ohyanagi et al. have demonstrated that low frequency stimulation of sympathetic nerves preferentially stimulates post-junctional α2-adrenoceptors, whereas post-junctional α1-adrenoceptors are preferentially recruited during high frequency nerve stimulation (Ohyanagi et al. 1991). Thus, it appears plausible to speculate that this may explain the greater contribution of α2-adrenoceptors observed in the young healthy men (low basal MSNA) in the current study. Whether this relative contribution is shifted to greater α1-adrenoceptor vasoconstriction in humans with chronically elevated MSNA (e.g. older adults, heart failure patients) has yet to be determined. However, the experimental design employed in the present study (Protocol 1) will allow in vivo determination of whether changes in relative post-junctional α-adrenoceptor contribution to vascular tone occur in these populations. It also remains to be determined whether differences in α1- and α2-adrenoceptor control of vascular tone in conditions such as ageing and heart failure influence skeletal muscle blood flow responses and systemic blood pressure regulation during exercise.
Conclusions
In contrast to the classical view that sympathetic vasoconstriction in humans is mediated primarily by post-junctional α1-adrenoceptors, our data suggest that the relative α2-adrenoceptor contribution to basal limb vascular tone is greater than that mediated via α1-adrenoceptors. The potential physiological and pathophysiological implications of these findings remain to be determined.
Acknowledgments
We thank Shelly Roberts, Karen Krucker, Landon Clark, and Annette Nelson for their technical assistance, and the subjects who volunteered for this study. This research was supported by NIH grants HL-46493 and NS-32352 (MJJ), NIH General Research Center Grant RR-00585 (to the Mayo Clinic, Rochester, MN, USA) and an Individual National Research Service Award AG-05912 (FAD).
REFERENCES
- Anderson KM, Faber JE. Differential sensitivity of arteriolar α 1- and α2-adrenoceptor constriction to metabolic inhibition during rat skeletal muscle contraction. Circulation Research. 1991;69:178–184. doi: 10.1161/01.res.69.1.174. [DOI] [PubMed] [Google Scholar]
- Angus JA, Cocks TM, Satoh K. The α adrenoceptors on endothelial cells. Federation Proceedings. 1986;45:2355–2359. [PubMed] [Google Scholar]
- Bolli PE, Kiowski W, Ji BH, Amann FW, Buhler FR. Important contribution of post-junctional α2 adrenoceptor-mediated vasoconstriction to arteriolar tone in man. Journal of Hypertension. 1983;1:257–259. [Google Scholar]
- Buckwalter JB, Naik JS, Valic Z, Clifford PS. Exercise attenuates α-adrenergic-receptor responsiveness in skeletal muscle vasculature. Journal of Applied Physiology. 2001;90:172–178. doi: 10.1152/jappl.2001.90.1.172. [DOI] [PubMed] [Google Scholar]
- Bylund DB, Regan JA, Faber JE, Hieble JP, Triggle CR, Ruffolo RR. Vascular α-adrenoceptors: from the gene to the human. Canadian Journal of Physiology and Pharmacology. 1995;73:533–543. doi: 10.1139/y95-068. [DOI] [PubMed] [Google Scholar]
- Cambridge D, Davey MJ, Massingham R. Prazosin, a selective antagonist of post-synaptic α-adrenoceptors. British Journal of Pharmacology. 1977;59:514–515. [PMC free article] [PubMed] [Google Scholar]
- Dietz NM, Rivera JM, Eggener ES, Fix RJ, Warner DO, Joyner MJ. Nitric oxide contributes to the rise in forearm blood flow during mental stress in humans. Journal of Physiology. 1994;480:361–368. doi: 10.1113/jphysiol.1994.sp020366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doxey JC, Smith GFC, Walker JM. Selectivity for blocking agents for pre- and postsynaptic α-adrenoceptors. British Journal of Pharmacology. 1977;60:91–96. doi: 10.1111/j.1476-5381.1977.tb16752.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duff RS. Circulatory changes in the forearm following sympathectomy. Clinical Science. 1951;10:529–540. [PubMed] [Google Scholar]
- Egan B, Panis R, Hinderliter A, Schork N, Julius S. Mechanism of increased α adrenergic vasoconstriction in human essential hypertension. Journal of Clinical Investigation. 1987;80:812–817. doi: 10.1172/JCI113138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frewin DB, Whelan RF. The mechanism of action of tyramine on the blood vessels of the forearm in man. British Journal of Pharmacology. 1968;22:105–116. doi: 10.1111/j.1476-5381.1968.tb00478.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenfield ADM, Whitney RJ, Mowbray JF. Methods for the investigation of peripheral blood flow. British Medical Bulletin. 1963;19:101–109. doi: 10.1093/oxfordjournals.bmb.a070026. [DOI] [PubMed] [Google Scholar]
- Grossman E, Chang PC, Hoffman A, Tamrat M, Goldstein DS. Evidence for functional α2-adrenoceptors on vascular sympathetic nerve endings in the human forearm. Circulation Research. 1991;69:887–897. doi: 10.1161/01.res.69.4.887. [DOI] [PubMed] [Google Scholar]
- Hansen J, Sander M, Hald CF, Victor RG, Thomas GD. Metabolic modulation of sympathetic vasoconstriction in human skeletal muscle: role of tissue hypoxia. Journal of Physiology. 2000;527:387–396. doi: 10.1111/j.1469-7793.2000.00387.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jie K, van Brummelen P, Vermey P, Timmermans P, van Zwieten PA. Identification of vascular postsynaptic α-1 and α-2 adrenoceptors in man. Circulation Research. 1984;54:447–452. doi: 10.1161/01.res.54.4.447. [DOI] [PubMed] [Google Scholar]
- Jie K, van Brummelen P, Vermey P, Timmermans P, van Zwieten PA. Modulation of noradrenaline release by peripheral presynaptic α2-adrenoceptors in humans. Journal of Cardiovascular Pharmacology. 1987a;9:407–413. doi: 10.1097/00005344-198704000-00005. [DOI] [PubMed] [Google Scholar]
- Jie K, van Brummelen P, Vermey P, Timmermans P, van Zwieten PA. Postsynaptic α1 and α2-adrenoceptors in human blood vessels: interactions with exogenous and endogenous catecholamines. European Journal of Clinical Investigation. 1987b;17:174–181. doi: 10.1111/j.1365-2362.1987.tb02397.x. [DOI] [PubMed] [Google Scholar]
- Johnsson G. The effects of intra-arterially administered propranolol and H 56-28 on blood flow in the forearm - a comparative study of two β-adrenergic receptor antagonists. Acta Pharmacologica Toxicologica. 1967;25:63–74. doi: 10.1111/j.1600-0773.1967.tb02997.x. [DOI] [PubMed] [Google Scholar]
- Joyner MJ, Nauss LA, Warner MA, Warner DO. Sympathetic modulation of blood flow and O2 uptake in rhythmically contracting human forearm muscles. American Journal of Physiology. 1992;263:H1078–1083. doi: 10.1152/ajpheart.1992.263.4.H1078. [DOI] [PubMed] [Google Scholar]
- Kiowski W, Hulthen UL, Ritz R, Buhler FR. α2-Adrenoceptor-mediated vasoconstriction of arteries. Clinical Pharmacology and Therapeutics. 1983;34:565–569. doi: 10.1038/clpt.1983.216. [DOI] [PubMed] [Google Scholar]
- Kubo SH, Rector TS, Heifetz SM, Cohn JM. α2-Receptor-mediated vasoconstriction in patients with congestive heart failure. Circulation. 1989;80:1660–1667. doi: 10.1161/01.cir.80.6.1660. [DOI] [PubMed] [Google Scholar]
- Minson CT, Halliwill JR, Young TM, Joyner MJ. Influence of the menstrual cycle on sympathetic activity, baroreflex sensitivity, and vascular transduction in young women. Circulation. 2000;101:862–868. doi: 10.1161/01.cir.101.8.862. [DOI] [PubMed] [Google Scholar]
- Niimi Y, Matsukawa T, Sugiyama S, Shamsuzzaman AS, Mano T. Comparison of sympathetic nerve response to head-up tilt in summer and winter. Journal of Gravitational Physiology. 1999;6:43–44. [PubMed] [Google Scholar]
- Ohyanagi M, Faber JE, Nishigaki K. Differential activation of α1- and α2-adrenoceptors on microvascular smooth muscle during sympathetic nerve stimulation. Circulation Research. 1991;68:232–244. doi: 10.1161/01.res.68.1.232. [DOI] [PubMed] [Google Scholar]
- Piascik MT, Soltis EE, Piascik MM, Macmillan LB. α-Adrenoceptors and vascular regulation: molecular, pharmacologic and clinical correlates. Pharmacology and Therapeutics. 1996;72:215–241. doi: 10.1016/s0163-7258(96)00117-9. [DOI] [PubMed] [Google Scholar]
- Ruffolo RR, Nichols AJ, Stadel JM, Hieble JP. Structure and function of α-adrenoceptors. Pharmacological Reviews. 1991;43:475–505. [PubMed] [Google Scholar]
- Saeed M, Sommer O, Holtz J, Bassenge E. α-Adrenoceptor blockade by phentolamine causes β-adrenergic vasodilation by increased catecholamine release due to presynaptic α-blockade. Journal of Cardiovascular Pharmacology. 1982;4:44–52. doi: 10.1097/00005344-198201000-00008. [DOI] [PubMed] [Google Scholar]
- Taddei S, Salvetti A, Pedrinelli R. Further evidence for the existence of α2-mediated adrenergic vasoconstriction in human vessels. European Journal of Clinical Pharmacology. 1988;34:407–410. doi: 10.1007/BF00542444. [DOI] [PubMed] [Google Scholar]
- Thomas GD, Hansen J, Victor RG. Inhibition of α2- adrenergic vasoconstriction during contraction of glycolytic, not oxidative, rat hindlimb muscle. American Journal of Physiology. 1994;266:H920–929. doi: 10.1152/ajpheart.1994.266.3.H920. [DOI] [PubMed] [Google Scholar]
- Torp KD, Tschakovsky ME, Halliwill JR, Minson CT, Joyner MJ. β-receptor agonist activity of phenylephrine in the human forearm. Journal of Applied Physiology. 2001;90:1855–1859. doi: 10.1152/jappl.2001.90.5.1855. [DOI] [PubMed] [Google Scholar]


