Abstract
A forelimb-withdrawal classical conditioning paradigm was used in awake cats (n = 4) to investigate changes in transmission in climbing fibre (CF) pathways during motor learning. The conditioned stimulus was an auditory tone, while the unconditioned stimulus was a low-intensity, single or double (0.1 ms) electrical pulse applied to the ipsilateral superficial radial nerve. Microwires chronically implanted into the paravermal cerebellar cortex (lobule V) were used to record the CF field potentials evoked by nerve stimulation, and fields at 22 recording sites (9 C1, 7 C2 and 6 C3 zone sites) were monitored during the complete sequence of at least one training run (i.e. over a period of about 2-3 weeks of conditioning). At 19 sites (86 %) conditioning led to a significant reduction in mean size of field. Similar reductions occurred at four sites studied over two successive training runs. At 10 sites (45 %) there was a statistically significant increase prior to the reduction. The three sites that failed to exhibit a significant reduction were all located in the C1 zone. Controls showed that the changes in CF transmission were dependent on the animal being conditioned. The mean size of CF field for trials in which a conditioned EMG response was present (in either the cleidobrachialis or biceps muscle) was not significantly different from the mean size for trials in which a conditioned response was absent. Similarly, on a trial-by-trial basis, CF pathway excitability was not correlated with the conditioned EMG activity in the flexor muscles under study. Overall, the results demonstrate that (1) the capacity of spino-olivocerebellar pathways (SOCPs) to forward information to the cerebellar cortex can be altered by recent experience, (2) establishment of a conditioned forelimb flexor reflex to a tone reduces SOCP excitability at most but not all sites within a forelimb-related region of the cerebellar cortex, (3) the extent of reductions differ at different sites and some are preceded by transient increases, and (4) the changes in transmission may not be related to the conditioned movement. The implications of these findings for some key theories of cerebellar cortical function are discussed.
Although the climbing fibres of the inferior olive form a major input to the cerebellar cortex and are generally regarded as a vital component of the cerebellar contribution to motor learning, their precise role in the acquisition of new motor skills remains enigmatic (for reviews see Thach et al. 1992; Bloedel & Bracha, 1998; Yeo & Hesslow, 1998). Nevertheless, detailed studies of eye-blink circuits have provided valuable insights into the neural mechanisms underlying classical conditioning. This has led to the cerebellar cortical conditioning hypothesis in which climbing fibres are thought to relay the unconditioned stimulus to the cerebellar cortex, thereby signalling unpredictable sensory events and, in so doing, serving as ‘teachers’ during the acquisition process (for a review and references see Yeo & Hesslow, 1998). In particular, the dorsal accessory olive (DAO) and its climbing fibre projections to the paravermal cerebellar cortical C1 and C3 zones are implicated in this process (e.g. McCormick et al. 1985; Yeo et al. 1985, 1986; Steinmetz et al. 1989; Hesslow, 1994). On the other hand, it is now clear that climbing fibre pathways targeting the same paravermal zones are also subject to movement-related changes in transmission (for a review see Apps, 1999). During well-rehearsed movements such as locomotion, powerful modulatory influences regulate pathway excitability so that climbing fibre inputs to the C1 and C3 zones in the cerebellar anterior lobe are suppressed during the stance phase of the step cycle in the ipsilateral forelimb, but facilitated during the swing phase (e.g. Apps et al. 1995). Similar gating mechanisms may also operate during motor learning, but to date, only reductions have been observed during conditioning of eye-blink reflexes. Interpretation of such studies is also complicated by the fact that the efficacy of the unconditioned stimulus was not monitored (Sears & Steinmetz, 1991; Hesslow & Ivarsson, 1996), and it remains uncertain whether such effects are movement rather than learning related.
To obtain further knowledge of the way in which transmission is modified during the complete sequence of training required to establish a conditioned reflex should therefore provide useful insights into the signalling capabilities of the climbing fibre pathways targeting the three different zones (C1, C2 and C3) of the paravermis. If, for example, information forwarded to the cerebellum via the climbing fibres is gated out (i.e. pathway transmission is closed) early on during learning of a conditioned response, then this would limit the capacity of the climbing fibres to act as teachers. Furthermore, if modulation of transmission in climbing fibre pathways is to be considered a phenomenon associated with motor learning in general, it is necessary that conditioning experiments are carried out on cerebellar pathways other than eye-blink circuits. In this regard, Kolb et al. (1997) have shown that inactivation of nucleus interpositus (the output nucleus of the paravermal cortex) disrupts severely the flexor component of classically conditioned and unconditioned forelimb withdrawal responses in awake cats, indicating that forelimb (like eye-blink) conditioning is cerebellar-dependent (see also Chambers & Sprague, 1955). Thus, if gating of climbing fibre pathways is a general phenomenon, it might be expected that changes in transmission will occur during the conditioning of forelimb circuits.
In the present study, a forelimb flexor reflex classical conditioning paradigm was therefore used in awake cats to investigate: (1) whether facilitations as well as reductions occur in climbing fibre pathway transmission from forelimb afferents during motor learning, (2) whether the time course of the gating is consistent with the signalling of unpredictable sensory events, (3) whether any systematic differences exist between paravermal cortical zones in their patterns of gating during conditioning and (4) whether the modulation is related to flexor muscle activity during the conditioned movement.
METHODS
Animals and implants
Experiments were performed on four purpose-bred adult male cats (4.5-5 kg) in accordance with local animal welfare guidelines and the Animal Scientific Procedures Act (1986). Following behavioural training (see below), implantations were carried out during an aseptic operation under Propofol anaesthesia (0.05 ml min−1i.v.; Schering-Plough, Welwyn Garden City, UK) following premedication with medetomidine hydrochloride (Domitor, 150 μg kg−1s.c.; SmithKline Beecham, Surrey, UK). A surgical level of anaesthesia was maintained throughout the operation, as characterised by general muscle atonia except in the respiratory muscles, strongly or completely depressed withdrawal reflexes and slow, regular breathing. A single dose of atropine (0.5 ml s.c.; Animalcare, Dunnington, UK) was given in order to prevent excessive secretion in the respiratory passages, and an antibiotic (Amfipen LA, Intervet, UK) was administered pre- and post-operatively (three spaced doses of 0.2 mg kg−1). Throughout the operation, the temperature of the animal was kept within physiological limits.
In the left forelimb, two pairs of bipolar cuff electrodes were implanted around the left superficial radial (SR) nerve, for stimulation and nerve volley recording, respectively, and bipolar leads were implanted into the flexor muscle cleidobrachialis (ClB) for monitoring conditioned and reflex responses. The principal function of this muscle is protraction of the shoulder and flexion of the elbow (see for example, Drew, 1993), and previous work in cats has shown that ClB displays a clear burst of EMG activity that coincides with a conditioned forelimb withdrawal response similar to that studied in the present experiments (cf. Kolb et al. 1997). ClB was therefore considered to be a reliable marker of the forelimb-conditioned movement under investigation (see Results). It was also judged reasonable to assume that ClB was efficiently activated by the cutaneous stimulus used, because low-intensity electrical stimulation of the SR nerve results in a short-latency cutaneo-muscular reflex in ClB that is particularly large and synchronous, implying that there is a close functional link between afferent input from the SR nerve and muscle output in ClB (see Fig. 1, see also Drew & Rossignol, 1987). Additional leads were implanted into the left biceps (another elbow flexor) and the right ClB, although the EMG signals from these muscles were not studied as extensively as those from the left (ipsilateral) ClB. Further details of limb implants are as described previously (Apps et al. 1990).
Figure 1. Conditioning of forelimb EMG responses in the awake cat.
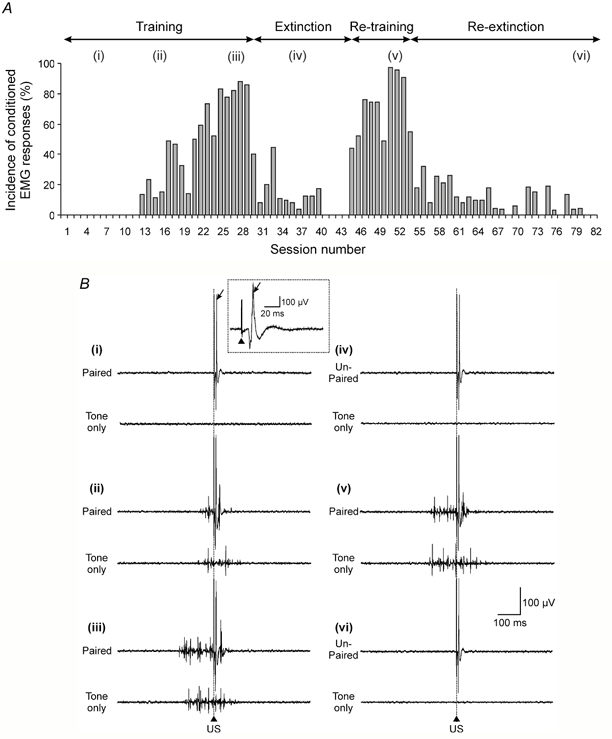
A, bar chart plotting the incidence of conditioned forelimb EMG responses in the ipsilateral cleidobrachialis muscle (ClB) for each recording session (animal CE2) during an initial period of conditioned stimulus (CS)-unconditioned stimulus (US) paired trials (training; i.e. trials when the US occurs at CS offset), followed by a period of CS-US unpaired trials (extinction, i.e. trials when the US occurs at a variable time 5-25 s after CS offset), and during a second period of re-training and re-extinction (based on 45 paired or unpaired trials per session during training and extinction, respectively). B, example EMG traces from ClB for sessions corresponding to those indicated by lower-case roman numerals in A. The upper and lower traces in each pair display the EMG activity in an example CS-US (paired or unpaired) trial and an example CS (tone only) trial, respectively. The EMG traces were obtained (i) during the early stages of the first training period (the arrow indicates the unconditioned response, and in the inset the same response on an expanded time base, while the arrowhead indicates the paired pulse stimulus artefact), (ii) when conditioned responses were beginning to occur, (iii) when conditioned responses were fully acquired, (iv) after a period of extinction, (v) when conditioned responses were re-acquired and (vi) after a second period of extinction. The vertical dotted line and arrowhead indicate the time of offset of the 500 ms CS in paired and tone only trials and time of onset of the US in paired and unpaired trials. See Methods for further details.
A small craniotomy was made to expose the forelimb-receiving area of the cerebellar anterior lobe paravermis on the left side (lobule V), and an array of approximately 20 fine, flexible, platinum-iridium microwires (California Fine Wire, CA, USA), Teflon-insulated except at their tips and 35 μm in total diameter were inserted to a depth of 1-2 mm into the superficial layers of the folia. The point of insertion of each wire was indicated on a scale drawing. The cerebellar surface was then covered with Spongostan (Johnson & Johnson, Ascot, UK) and the wires sealed in position by closing the skull defect with dental acrylic cement (for further details see Apps et al. 1995). The region of the paravermal cortex selected for study was chosen because it is likely to have a major influence on the elbow flexor muscles used to monitor the conditioned withdrawal response. This is because (1) numerous studies have implicated the paravermal cortex of lobule V as being critically involved in the control of forelimb flexion movements (see Armstrong, 1988 for further details and references), (2) the connections of this region of cerebellar cortex with the rubrospinal tract, via nucleus interpositus, means that it is likely to exert a substantial influence on flexor motoneurones of the forelimb (see Cheney et al. 1991 for further details and references, see also Rho et al. 1999 and Horn et al. 2002 as examples of more recent studies that also implicate the rubrospinal tract in the control of forelimb extensors), (3) microstimulation in nucleus interpositus preferentially activates elbow flexors (e.g. Ekerot et al. 1995) and (4) electrical stimulation of the same part of the paravermal C3 zone that was implanted in the present experiments with microwires has been shown to evoke EMG responses in biceps (Hesslow, 1994).
As a precautionary measure, post-operative analgesia was maintained for 24 h with carprofen (Zenecarp, 0.08 ml kg−1i.v.; C-Vet, Leyland, UK). No complications occurred post-operatively and the animals showed no signs of discomfort at any stage of the experiment. Upon termination of the experiment (usually about 6 weeks after the initial operation) the animals were killed with an overdose of barbiturate anaesthetic and perfused transcardially with 4 % paraformaldehyde. The cerebellum was removed and a series of 100 μm sagittal sections prepared for histological examination to verify the folial location of the microwires in the paravermal region of lobule Va-c.
Training procedure
The delay classical conditioning paradigm that was used is similar to that described by Voneida et al. (1990). In brief, cats were trained non-aversively prior to the operation to lie quietly in a box for approximately 30 min with their forelimbs outstretched. Subsequently, during all recording sessions a loud speaker directly above the animal's head (approximately 30 cm above ear level) produced a continuous white noise (70 dB). The same loud speaker produced the conditioned stimulus (CS, a 1 kHz tone, duration 500 ms, 80 dB at ear level) at an inter-trial interval of 30 ± 5 s. The unconditioned stimulus (US) was presented at CS offset and consisted of one or two brief (0.1 ms, 1 kHz) square-wave electrical pulses to the left SR nerve at an intensity of three times the threshold (3T) of the most excitable fibres in the nerve. This intensity of nerve stimulation was considered adequate to obtain classical conditioning because (1), it invariably generated a robust reflex twitch in the stimulated limb, involving a transient elbow flexion and retraction of the forearm, (2) the EMG reflex response in the ipsilateral ClB (and biceps) was typically about five times larger than the peak-to-peak amplitude of the EMG spikes in the conditioned response (see for example Fig. 1B) and (3) in one pilot experiment nerve stimulation at a lower intensity (2T) was used, but this failed to generate any clear conditioned EMG responses.
Each training session comprised a total of 45 CS-US (paired) acquisition trials with presentation of the CS alone every 10th trial, followed by a block of 10 US only (control) trials. At the end of each session, the animal was returned to its pen (shared with other purpose-bred cats), where food was freely available. A training run involved three, evenly spaced training sessions every day until three consecutive sessions displayed conditioned EMG responses in 80 % or more of paired and CS alone trials. No difficulty was found in identifying conditioned responses because baseline EMG activity was usually small (see for example Fig. 1B). In a selection of recording sessions, the EMG data were analysed for number of spikes and integral size (mV ms, see below), but this analysis produced similar results to visual inspection of the EMG trace. The latter method was therefore judged sufficient to identify reliably the occurrence of conditioned responses. In paired trials these were defined as EMG activity occurring within a time period of 300 ms prior to the offset of the CS and lasting up until CS offset. In CS only trials, EMG activity occurring any time within a 300 ms time window before and 100 ms after CS offset was classified as a conditioned response.
Prior to classification of every trial, an initial time window of 500 ms before onset of the CS was also inspected. If the background level of EMG activity in this time window was greater than normal, or intermittent EMG bursts were observed (generated presumably when the animal made small voluntary adjustments to the position of the stimulated limb), then that particular trial was excluded. Typically, this was necessary in one or two trials per session. After each training run all animals received a series of extinction sessions (each session comprising 45 unpaired CS-US trials, followed by 10 US only trials), until none of the unpaired trials elicited a conditioned response in three consecutive sessions. Thereafter, one animal received a second period of acquisition training and another received a second and third period of training.
Data handling
In all animals the afferent volley in the left SR nerve, the extracellular climbing fibre field potentials evoked from one to four different cerebellar cortical recording sites and the EMG activity in the ClB and biceps muscles of the left forelimb were monitored simultaneously throughout all recording sessions. In two animals the ClB in the right forelimb was also monitored. In accordance with many previous studies (e.g. Ekerot & Larson, 1979; Trott & Apps, 1991), individual microwire recording sites were identified prior to (and in most cases after) each training run as located within the C1 or C3 zones of the forelimb-receiving area of the anterior lobe cerebellar cortex by (1) the presence of relatively short-latency climbing fibre field potentials (onset 10.2-13.4 ms) to ipsilateral SR stimulation only and (2) by their location in the medial and lateral parts, respectively, of the paravermal cortex. Recording sites within the C2 zone were identified by (1) the presence of longer-latency climbing fibre field potentials to both ipsilateral (onset 16.0-18.3 ms) and contralateral SR stimulation and (2) by their location in the middle part of the paravermis, usually flanked medially and laterally by sites yielding C1 and C3 zone fields, respectively.
Climbing fibre field potentials were readily distinguishable from responses related to mossy fibre input by a number of criteria. Firstly, their onset latency was always greater than 10 ms and therefore later than any known direct spinal pathway that terminates as mossy fibres (cf. Ekerot & Larson, 1973, 1979). Secondly, they exhibited a highly characteristic waveform, including in some cases one or two secondary peaks on the falling phase at approximately 2 ms intervals, and their duration (ca 5 ms), was always shorter than responses attributable to activity in longer-latency mossy fibre pathways (cf. Kennedy et al. 1966; Morissette & Bower, 1996). Thirdly, they fluctuated in amplitude from trial to trial in response to a constant stimulus. By contrast, responses attributable to mossy fibre input are usually rather more stable in size, especially at the stimulus intensity of 3T used in the present experiments. Finally, the characteristic pattern of response of climbing fibre fields to a ‘paired-pulse’ test helped to distinguish them further from responses evoked by activity in short-latency mossy fibre paths. When two supramaximal stimuli are delivered at inter-stimulus intervals ranging from between 10 and 100 ms, the second climbing fibre field exhibits a prolonged depression, while responses attributable to activation by short-latency mossy fibre paths remain unaffected (Eccles et al. 1966; Armstrong & Harvey, 1968).
Filter settings were 30 Hz-2.5 kHz for recording of the cerebellar fields and the EMG, and 300 Hz-10 kHz for the nerve volley. The signals were digitised on-line by customised software running in a Cambridge Electronic Design (CED) 1401Plus computer interface unit. The sampling rates were 10 kHz for the fields and EMG, and 20 kHz for the nerve volley. The size of individual climbing fibre field potentials was measured by integration (mV ms) of the initial fast component of the climbing fibre field, and by measuring the amplitude of each field (mV, peak-to-peak). These two measures showed a good correspondence (on average r = 0.71, n = 16 comparisons from 4 recording sites, P ≤ 0.001 in each comparison). The results are therefore confined to consideration of changes in field area because this is likely to be a more reliable measure of changes in the overall numbers of Purkinje cells local to the recording tip activated by their climbing fibre input. The amplitude of individual nerve compound action potentials was measured peak-to-peak (for further details see Apps et al. 1990, 1997).
Data analysis
Data from a selection of sites were analysed in detail on a trial-by-trial basis (i.e. all field potentials and the respective nerve volley amplitudes in every session of a training run were measured individually; see for example the data shown in Fig. 6B, C). In the remaining cases, the first three and last three sessions in every run were analysed by the same method, but the complete sequence of changes in mean area of field over the course of each training run was assessed by using a combination of the detailed ‘trial-by-trial’ and a (much less time consuming) ‘computed waveform’ method of analysis. The latter involved measuring the area of a single computer-derived response average waveform for a given training session and therefore lacked information on variance. There was, however, a very close correspondence between the results obtained by the two methods, and given that the standard deviations for the means calculated by the trial-by-trial method were usually rather similar between sessions (see Fig. 6 for example), a combination of the two approaches was used to plot the full series of data points in some training runs (e.g. Fig. 2B).
Figure 6. Climbing fibre field facilitations during training.
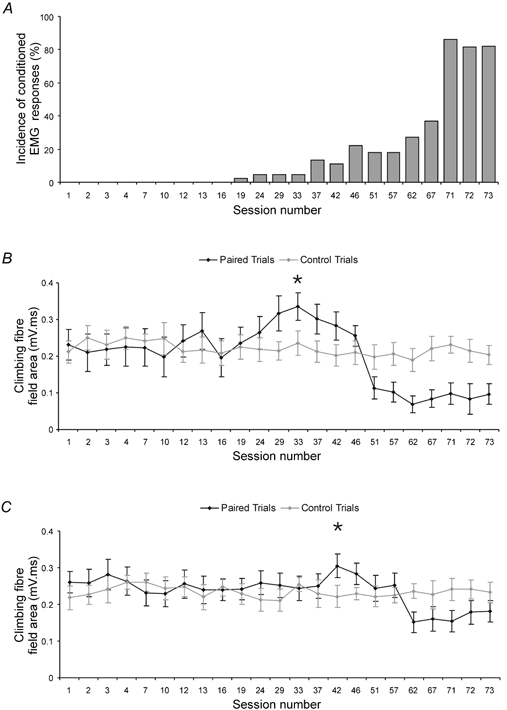
A, acquisition curve (animal CE4) showing the incidence of conditioned EMG responses for each recording session during a training run. B, data from an example recording site (in the C1 zone), plotting for each recording session depicted in A, the mean area (± s.d.) of the climbing fibre field in US only (control) trials (grey line and circles, 10 trials per session) and CS-US (paired) trials (black line and circles, 45 trials per session). C, same as B but for a different C1 zone site recorded simultaneously with the site shown in B. In B and C, an asterisk indicates the session number when fields in paired trials were largest (multiple regression, P ≤ 0.01 in both cases).
Figure 2. Changes in climbing fibre field size during conditioning.
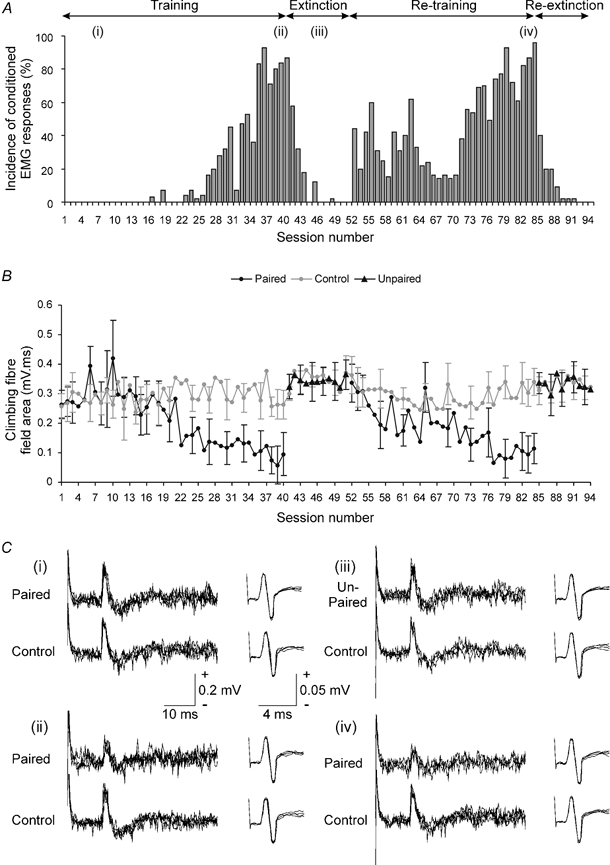
A, bar chart showing the incidence of conditioned EMG responses for each recording session during an initial period of training (animal CE3), followed by a period of extinction and during a period of re-training and re-extinction (based on 45 paired or unpaired trials per session during training and extinction, respectively). Lower-case roman numerals indicate session numbers in which example cerebellar and nerve responses were selected for illustration in C. B, graph plotting the mean area of climbing fibre field potential (± s.d.) evoked at an example cerebellar cortical C3 zone site during the recording sessions shown in A. Mean area of the field evoked in control trials (grey line and circles), paired trials (black line and circles) and unpaired trials (black triangles) is shown (10, 45 and 45 trials per session, respectively). For further details see Methods. C, each set of responses show example C3 zone evoked climbing fibre field potentials (left) and corresponding nerve volley recordings (right; four sweeps superimposed in all traces, US delivered at the start of each trace). The upper and lower records of each set show respectively responses in CS-US (paired or unpaired) and US only (control) trials, obtained (i) early on during training, (ii) after training (iii) during extinction and (iv) after re-training.
In addition, because in previous studies it has been found that some of the variation in climbing fibre field size can be explained by changes in the amplitude of the afferent nerve volley during active movements of the forelimb (Apps et al. 1997), it was considered important to take any trial-by-trial fluctuations in nerve volley size into account within the statistical analysis. A multiple regression model was therefore used as follows: Y = βo + β1X1 + β2X2 + e, where Y is the mean size of the climbing fibre field, βn are regression coefficients, X1 is the mean size of the nerve volley, X2 is the training session number and e is an error term (cf. Apps et al. 1997; Apps & Lee, 1999).
Partial correlation analysis was also employed to assess whether there was any relationship between climbing fibre field size and EMG activity in individual trials (taking any variation in nerve volley amplitude into account), based on a method described previously (Hesslow & Ivarsson, 1996). In the present study, for each session selected for analysis (see Results), the EMG activity prior to US onset in paired trials was divided into a series of 5 ms time bins, and the rectified EMG response size (mV ms) in the time windows 10-20 ms, 10-30 ms, 10-50 ms, 30-40 ms and 40-50 ms prior to US onset were each compared with the size (mV ms) of the corresponding evoked climbing fibre field potential (e.g. Fig. 5).
Figure 5. Variations in climbing fibre field size relative to conditioned EMG.
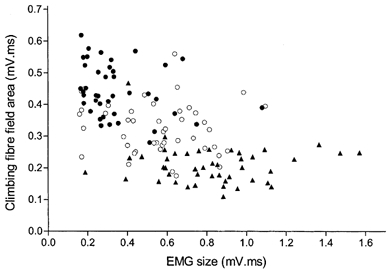
Data from a typical recording site (in the C3 zone) showing the relationship between individual climbing fibre field sizes and ClB EMG amplitudes in the time period 10-20 ms before the US in CS-US paired trials. Data merged from three recording sessions, one obtained early on during training (•), one about mid way through training (○) and one at the end of training (▴). For individual sessions, r = −0.257, −0.126 and −0.063, respectively. Overall, r = −0.561. For further details see text.
RESULTS
EMG responses during conditioning
In each animal (n = 4) the unconditioned EMG response in the ipsilateral elbow flexor muscle ClB was comparable in waveform and onset latency to reflex responses evoked in the same muscle by similar electrical stimuli used in previous experiments (see inset in Fig. 1Bi and compare with Drew & Rossignol, 1987, their Fig. 11B). Likewise, during training, the EMG activity elicited in the ipsilateral ClB by an auditory tone conditioned stimulus (CS) was characteristic of classical associative conditioning (Fig. 1). In addition, insofar as it was possible to gauge from close visual inspection of the animal, whenever there was a conditioned response in the ipsilateral ClB there was also a visible movement of the ipsilateral limb, and when the conditioned withdrawal response was well established, the movement was restricted to that limb. In all sessions in which the contralateral ClB was monitored there was never any EMG activity resembling a conditioned response. Conversely, in trials when there was no conditioned response in the ipsilateral ClB, no movement was observed. Thus, and in agreement with previous studies (Kolb et al. 1997), activity in the ipsilateral ClB was judged to be a reliable indicator of the occurrence of a conditioned movement.
Conditioned responses were at least 1.5 times the size of background EMG and were identified by their timing and duration, occurring within the 300 ms time period before the onset of the US in CS-US (paired) trials and persisting until US delivery in paired trials or occurring around the time when the US would be ‘predicted’ in CS (tone) only trials. In the early stages of an initial training run, such responses were absent (Fig. 1Bi) but started to appear after about 550 paired trials (Fig. 1Bii). They usually displayed a progressive increase in EMG spike frequency after repeated pairings (Fig. 1Biii) and subsequently were always extinguished by repeated presentations of unpaired trials (Fig. 1Biv). In addition, they consistently displayed a rapid re-acquisition when a second training run was performed (Fig. 1Bv) and were always re-extinguished during a second period of unpaired trials (Fig. 1Bvi).
Control experiments
To control for sensitisation (Gormezano, 1966) and pseudoconditioning (Razran, 1971), two of the animals received unpaired presentations of the CS and US prior to their first training run. In both cases, no EMG activity resembling conditioned responses was elicited. To test for discrimination (Pavlov, 1927), the same two animals also received a second conditioned stimulus (CS(B), 3 kHz, 500 ms, 80 dB at ear level), 5-25 s after each paired trial. In one animal, conditioned responses were first elicited in session 16 for the CS and session 17 for the CS(B) tone. By session 38 the responses elicited by the CS had reached asymptotic levels (i.e. they were present in >80 % of tone only trials), while the CS(B) tone-evoked conditioned responses were present in only 9 % of trials. Similarly, in the second animal conditioned responses were first elicited in session 45 for the CS and session 44 for the CS(B) tone. By session 70 the responses elicited by the CS had reached asymptotic levels, while the CS(B) tone evoked conditioned responses in fewer than 6 % of trials. Thus, the progressive increase in the incidence and nature of the flexor EMG responses elicited during training, their reversible elimination during extinction and the failure to produce similar responses during control experiments leaves little doubt that genuine, associatively conditioned EMG responses were obtained in the present study.
Changes in mean size of evoked climbing fibre field potentials
A total of 22 paravermal cerebellar microwire recording sites (ca 20 %) yielded distinguishable climbing fibre field potentials (9 C1, 7 C2 and 6 C3 zone sites). Very similar yields have been obtained using the same method in previous cerebellar studies (see Apps et al. 1995, 1997; Apps & Lee, 1999), presumably because the majority of microwires are not favourably placed relative to the cortical layers. However, the main advantage of the technique is that stable records can be obtained from the same site over many days, and in the present study it was possible to monitor evoked climbing fibre field potentials from all 22 sites during the complete sequence of at least one training run (i.e. over a period of 2 or 3 weeks). For four sites, it was also possible to obtain recordings during a second run.
Figure 2A illustrates example data (obtained from a different animal to that shown in Fig. 1) to show the changes in occurrence of conditioned EMG responses during an initial training run, followed by a period of extinction, and subsequently during a second period of re-training and re-extinction. Figure 2B displays, for the same time period, representative climbing fibre field data (from a C3 zone site) and plots for each recording session the mean area of the field evoked in the paired and corresponding control trials. Inspection of Fig. 2B shows that on average, the climbing fibre fields evoked in the control trials remained similar in size throughout the period of recording. This was supported by statistical analysis, which revealed no significant difference when the mean area of climbing fibre field evoked in controls trials was compared across all sessions of the initial training run (multiple regression analysis, P > 0.05). Note that the peak-to-peak amplitude of the afferent nerve volley is taken into account in this and all subsequent statistical comparisons to control for any possible systematic variations in its size (see Methods for further details). In practice, however, the nerve volley remained remarkably constant in amplitude both within and between recording sessions (multiple regression analysis, P > 0.05 for nerve partial correlation in n = 22 comparisons; see for example Fig. 2C), suggesting that the efficacy of the peripheral afferent input was uniform throughout the experiments.
In marked contrast, the climbing fibre fields evoked during the paired trials showed a progressive reduction in size during the first training run, reaching a minimum in session 39 (Fig. 2B, see also Fig. 2Cii and Fig. 6). However, during a subsequent period of extinction they were similar to their pre-training size (Fig. 2B sessions 41-51, Fig. 2Ciii), but by the end of the second training run they were once again reduced in size, reaching a new minimum in session 77 (Fig. 2B, see also Fig. 2Civ).
Time-series analysis of the data in the first training run in Fig. 2A and B showed that the highest cross correlation (r = −0.74) between mean area of the climbing fibre field and the incidence of conditioned EMG responses in each training session occurred when there was no time lag between the two sets of data, suggesting that the two variables changed in size with a rather similar time course. The two variables appear, therefore, to be inversely related, although it must be emphasised that this does not necessarily imply a causal relationship. This is further emphasised by the fact that the time lag varied somewhat between comparisons. For example, for the data obtained for the same site but for the second training run, the highest cross correlation (r = −0.59) was obtained when there was a time lag of +1, indicating that the best match occurred when the changes (mainly increases) in incidence of conditioned EMG responses in a given training session were compared to the changes (mainly reductions) in mean size of climbing fibre field in the next session. This raises the possibility, but of course does not prove, that climbing fibre field size may be linked in some way to the previous history of conditioned EMG activity (see below).
Additional quantitative analysis of the data shown in Fig. 2 confirmed that the mean area of climbing fibre field evoked in paired trials in the final three sessions of each training run was significantly reduced as compared with the mean area of field evoked in the corresponding control trials (one-way ANOVA, Bonferroni post-test P ≤ 0.001 in both comparisons). By comparison, the mean area of climbing fibre field evoked in paired trials in the first three sessions of each training run was not significantly different from the mean area of field in the corresponding control trials (one-way ANOVA, Bonferroni post-test P > 0.05, in both comparisons). A similar pattern of change in size of climbing fibre fields in paired trials was obtained at the three other sites studied during two successive training runs (with recovery of field size occurring in each case during an intervening period of extinction). In addition, one control animal received unpaired presentations of the CS and US, while a second animal received paired trials over the same time period. The latter displayed a progressive acquisition of conditioned EMG responses over the course of a training run, with a concomitant reduction in mean area of the climbing fibre field in paired trials. By marked contrast, the control animal failed to acquire any EMG responses that resembled conditioned responses, and at three cerebellar recording sites, no significant change in mean area of the climbing fibre field was found. Thus, it seems safe to conclude that the changes in climbing fibre field size observed in paired trials during training were dependent on the animal being conditioned, rather than due to any non-associative learning effects.
In addition, given that no systematic change in size was observed from the first to the last stimulus presentation for any recording site for control responses within a recording session, and for many sites control response size remained stable over the course of an entire training run (see for example Figs 2B, C and 6B, C), it seems reasonable to conclude that for the majority of sites there was unlikely to have been any significant shift over time in microwire recording tip position relative to the cortical layers (cf. Apps et al. 1997). There were, however, a few exceptions in which there was either a progressive (but small) increase or decrease in control response size over the course of a training run (i.e. over the course of many days of recording sessions), presumably because at these sites there was some movement and/or encapsulation of the recording tip as can occur with microwires ‘floating’ in other brain structures (see for example, Palmer, 1978). To take this into account, Fig. 3 shows the results in the final three sessions of each training run expressed in terms of the ratio between the mean area of response in the paired trials and the mean area in the corresponding control trials. For recording sites studied during more than one training run, the data are selected from the first run and the results are presented in a sequence from the least to the most substantial reduction.
Figure 3. Summary of changes in climbing fibre field size after conditioning.
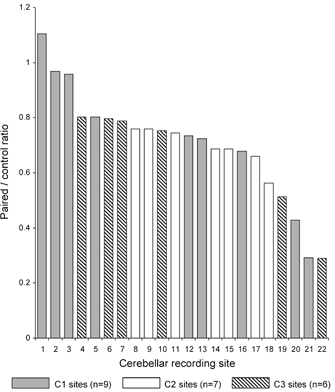
For each of 22 cerebellar paravermal recording sites, bar charts indicate the mean area of the field in CS-US (paired) trials as a ratio of the mean area of the climbing fibre field in the corresponding US only (control) trials. For each site, data are pooled from the last three sessions of a training run (i.e. each ratio is based on 135 paired trials and 30 control trials). Different patterns of shading indicate sites located in the C1 (grey), C2 (white) or C3 (hatched) zones.
Inspection of Fig. 3 shows that by far the most common finding was a reduction in climbing fibre field area in the paired trials after conditioning was well established. Statistical analysis found significant reductions relative to control values at no less than 19 out of 22 (86 %) of recording sites (one-way ANOVA Bonferroni post-test, in each comparison P ≤ 0.05 for field and P > 0.05 for nerve). The degree of this statistically significant reduction did, however, vary considerably between sites, ranging from 80 % to 29 % of the mean area of field evoked in the corresponding control trials (average reduction of 39 %), and at the remaining recording sites (n = 3), no significant difference was found (one-way ANOVA, Bonferroni post-test P > 0.05). By contrast, when the same analysis was confined to the first three sessions of each training run, only 1 out of 22 sites (5 %) displayed a significant reduction, and 3 (14 %) displayed a significant increase, while the large majority (18; 82 %), showed no significant difference between field area in paired and control trials (one-way ANOVA, Bonferroni post-test, P ≤ 0.05, P ≤ 0.05 and P > 0.05, for field data, respectively and P > 0.05 for all nerve data). Thus, in the overwhelming number of available cases, climbing fibre paths arising from the ipsilateral forelimb were found to be open for transmission during the initial stages of conditioning, but were partly closed when conditioning was well established.
For each of the four recording sites monitored over two successive training runs, similar reductions in field area occurred in the final three sessions of the two runs (see for example Fig. 2B), indicating that the changes at a given site were reproducible over time. This raises the possibility that the differences between sites in the extent of change in climbing fibre field size after conditioning may reflect a degree of functional heterogeneity between recording loci within the forelimb-receiving area of the paravermal cortex under study. The observation that all three sites that failed to exhibit a significant reduction in field size after training were located in the C1 zone, while all available C2 and C3 zone sites displayed significant reductions (Fig. 3), provides additional evidence in support of this suggestion.
Furthermore, when a comparison was made between the mean duration of climbing fibre field early on during conditioning and mean duration after conditioning was well established, there was a small but statistically significant reduction. For all 22 sites, mean ± s.d. field duration prior to conditioning was 4.95 ± 1.02 ms and mean ± s.d. duration after conditioning was 4.63 ± 0.99 ms (Student's paired t test, two-tailed P ≤ 0.05). However, on an individual basis, the findings were mixed because 7 sites showed no significant change in response duration, 12 sites showed a small but significant decrease and 3 sites showed a small but significant increase (Student's paired t test, two-tailed P > 0.05, P ≤ 0.05, P ≤ 0.05, respectively). There was no relationship between recording site location and the presence of changes (either increases or decreases) in field duration, nor was there any relationship with the extent to which a particular recording site displayed changes in climbing fibre field size after conditioning. The present findings therefore suggest that changes in synchronicity of climbing fibre input are not likely to play a major role in generating the observed changes (mainly reductions) in field size after conditioning is well established (see Discussion).
Variation in size of individual climbing fibre fields
The tendency for a reduction in field size during the last three sessions of a training run was often evident from the mean values. However, during each recording session there was substantial variability in the size of individual fields evoked by consecutive stimulus presentations (note the size of the s.d. error bars in Fig. 2B). Scatter plots were therefore constructed for a selection of recording sites to assess whether any transient changes in field area occurred within the final session of each run and to assess possible trends in size as a function of stimulus presentation over time. Scatter plots derived from three different recording sites and typical of the data analysed in detail are shown in Fig. 4. This shows that little or no systematic variation in field area occurred within the final training session (linear correlation coefficient r = −0.093, −0.160 and −0.139 for the data shown in Fig. 4A, B and C, respectively; see also Fig. 5). It is noteworthy, however, that while most of the individual fields evoked in paired trials during each session were depressed in size relative to the mean area of field in the control trials (dashed line in Fig. 4A–C), occasionally a field was evoked that was similar in size to control values.
Figure 4. Fluctuations in size of individual climbing fibre fields over time.
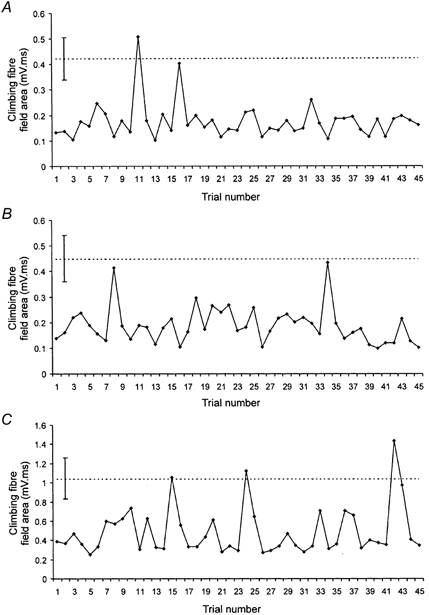
Data obtained in each example from consecutive CS-US (paired) trials during the final recording session of a training run and obtained from three different recording sites. A, data from a C3 zone site (animal CE2). B, data from a C1 zone site (animal CE3). C, data from a different C1 zone site (animal CE4). In each case the dashed horizontal line indicates the mean area of the climbing fibre field in the corresponding control trials (± s.d., 10 trials in each case).
Comparisons between the size of the conditioned EMG responses and the size of evoked climbing fibre fields
Given the evidence for movement-related variations in climbing fibre field size during well rehearsed behaviours such as reaching and walking (see Apps, 1999 for a review) it is possible that the changes in field size observed in the present study were also a movement-related phenomenon. Indeed the results of the cross-correlation comparisons of average field and EMG data between training sessions were compatible with this possibility (as discussed earlier). However, when the analysis was confined to data within individual sessions in which about half the trials contained conditioned responses, it was found that the mean area of climbing fibre field evoked in paired trials in which conditioned EMG responses were present was not significantly different from the mean area of field evoked in trials in the same session in which conditioned responses were absent (Mann-Whitney, P > 0.05 for data from 65 sessions obtained from all sites). This implies that even when the overall trend was for a reasonably good (inverse) relationship between the incidence of conditioned EMG responses and the mean area of climbing fibre field, the changes in climbing fibre field size within individual sessions were not in fact closely related to the presence of the learnt response in the flexor muscle under study (which appeared to be a reliable indicator of the presence or absence of a conditioned movement, as discussed earlier).
To study this issue in more detail, Fig. 5 shows a scatter plot from an example recording site in which data from all paired trials within three different training sessions have been merged to determine whether the size of individual climbing fibre fields is a function of the size of the corresponding conditioned EMG responses (see Methods for further details). The data have been selected from a session early on during training (when conditioned responses were beginning to occur), from a session about midway through training (when about half of paired trials elicited a conditioned response) and from a session near the end of training (when conditioned responses were well established). This is because the relationship between the mean size of the climbing fibre field and the acquisition of conditioned responses sometimes showed three distinct phases: an early phase in which there was little or no change in climbing fibre field size, an intermediate phase in which climbing fibre responses could be facilitated and a late phase in which climbing fibre responses were often depressed (see below and Fig 6).
Overall, and as might be expected from the averaged results (e.g. Fig. 2), there was a weak, but statistically significant negative correlation between the size of individual climbing fibre fields and the size of the conditioned EMG responses (linear regression r2 = 0.315, P ≤ 0.05). Nonlinear regression produced very similar results (the best fit was obtained with a plateau then exponential decay function, R2 = 0.374). By comparison, within individual sessions there was no statistically significant correlation (r2 = 0.066, 0.016 and 0.004; P > 0.05 for each session). For a total of seven different recording sites (four C1, one C2 and two C3 zone sites) the trial-by-trial fluctuations in climbing fibre field size were compared with several different time periods of the EMG (see Methods for details). In all cases there was little or no relationship, with the highest correlation obtained when the comparison was made using the EMG activity in the time window 10-20 ms before the US (for this time window, mean r = −0.155, ranging in individual sessions from r = 0.348 to −0.455; n = 48 comparisons). In other words, at best, only about 21 % of the variance in one parameter could be explained by variation in the other.
Similar negative findings were obtained when conditioned EMG responses in a different flexor muscle (biceps) were used for the analysis (e.g. for the time window 10-20 ms, mean r = −0.157), and also when individual climbing fibre field sizes were compared with the onset latencies of the conditioned response (e.g. r = 0.084 for n = 26 data points obtained from the site illustrated in Fig. 2B). The present findings therefore suggest that there are two processes that change over time: a progressive increase in EMG size (as would be expected as conditioning develops), and a general decline in climbing fibre field size, but that these two processes are not tightly coupled. The latter finding would seem to be at odds with one aspect of the cerebellar classical conditioning hypothesis (Yeo & Hesslow, 1998); namely, that reductions in US-evoked climbing fibre activity during learning are the result of a negative feedback mechanism associated with the conditioned movement (see Discussion).
Periods of climbing fibre field enhancement
Figure 6 shows data from two cerebellar recording sites (obtained from the same animal) in which a transient increase prior to a progressive reduction in the mean area of the climbing fibre field occurred in paired trials during training. In Fig. 6B, C, the largest responses occurred in sessions 33 and 42, respectively (marked with an asterisk), and in both there was a significant difference from the mean area of field evoked in the corresponding control trials (multiple regression, P ≤ 0.05). Overall, periods of field size enhancement occurred during training at a total of 10 out of 22 (45 %) of recording sites (range 121-157 %, mean 130 % of control, multiple regression, P ≤ 0.05). The timing during training when periods of enhancement occurred was variable between sites and no clear relationship was found between the presence of facilitations and cortical location (facilitations occurred at 5/9 C1 zone sites, 2/7 C2 zone sites and 3/6 C3 zone sites). However, facilitations only occurred at sites that subsequently showed reductions in field size.
For all sites at which the climbing fibre fields in one or more sessions were significantly larger than in control trials, the session yielding the largest mean area of climbing fibre field was selected for further analysis. The mean area of field in paired trials in which conditioned EMG responses in ClB were absent was compared to the mean area of field evoked in trials in which they were present, but no significant difference was found in a total of 10 comparisons (Mann-Whitney, P > 0.05 in all cases). Similarly, when a comparison was made between the size of individual climbing fibre fields evoked in paired trials and the corresponding size of the conditioned EMG responses, the correlation was low and in most cases not statistically significant (on average, r = −0.143, range 0.292 to −0.513, n = 7 comparisons from seven recording sites). Thus, with the present experimental arrangements, climbing fibre facilitations, like reductions, would seem not to be closely related to the presence of the conditioned EMG response (and therefore the learnt movement).
DISCUSSION
The present study provides evidence that powerful gating mechanisms operate during classical conditioning of a forelimb flexor reflex in the awake cat. The characteristic timing of the flexor EMG responses both in paired and CS only trials, the absence of such responses after extinction training and the failure to generate similar responses during sensitisation and pseudoconditioning or to a different CS in a discrimination design experiment, leaves little doubt that classical associative conditioning had occurred. The parallel changes in climbing fibre field size (without concomitant changes in the size of the peripheral nerve volley), and the fact that such changes were reversed by extinction training but failed to occur in control experiments, also provides strong evidence that these changes were likely to be dependent upon the occurrence of this conditioning. The present results therefore significantly extend the findings of previous eye-blink conditioning studies (Hesslow & Ivarsson, 1996; Kim et al. 1998) because (1) they demonstrate that central changes in transmission in climbing fibre paths to recording sites within the forelimb-receiving areas of the cerebellar paravermal C1, C2 and C3 zones can occur during a forelimb conditioning paradigm in awake cats, (2) facilitations as well as reductions can occur, (3) such effects would seem not to be closely related to conditioned activity in the flexor muscles under study and (4) a degree of functional heterogeneity exists between sites located in different cortical zones.
Is the gating due to conditioning of climbing fibre pathways by a previous input?
There are a number of inhibitory mechanisms that, at least theoretically, could have played a part in generating the conditioning-related reductions in climbing fibre response size observed in the present experiments (and in previous studies, e.g. Hesslow & Ivarsson, 1996). Collectively, these mechanisms would be activated if the conditioning tone stimulus and/or the EMG response it produced were capable of generating afferent traffic in the 100 ms time period preceding the unconditioned (test) climbing fibre response. It is noteworthy, however, that in the present study there was no significant difference between the size of climbing fibre responses in trials that contained conditioned EMG activity in ClB or biceps, and in trials that did not. This implies that muscle reafference from these flexors was not responsible for generating the observed changes in climbing fibre pathway transmission. In addition, since activity in ClB appeared to be a reliable indicator of the conditioned movement, it seems unlikely that reafference from any other muscles involved in the movement was responsible. Nevertheless, the possibility cannot be entirely excluded that some reafference resulted from activity in other muscles (that was insufficient to produce an observable movement) and this might have played a part in generating the changes in climbing fibre pathway transmission. It is important, therefore, to consider the extent to which climbing fibre pathway inhibitory mechanisms were likely to be in action during the present study.
Of the various mechanisms that have been reported, the best known is the phenomenon in which a conditioning stimulus of sufficient strength to activate a population of olivary cells will leave the same cells unresponsive to a test stimulus during a subsequent 100 ms time window (the paired pulse test, cf. Eccles et al. 1966; Armstrong & Harvey, 1968). If such a process was playing a major role in generating the reductions in transmission observed in the present experiments, then it might be expected that a climbing fibre field would be evident within the 100 ms time period prior to the test climbing fibre response. This is because the conditioned stimulus and/or the conditioned EMG response should have been sufficiently powerful to evoke a climbing fibre field themselves. However, in no case was any evidence for such a response found during conditioning. In addition, given that a robust stimulus intensity of 3T was used for the test stimulus, it seems unlikely that a small, asynchronous climbing fibre response (that might have escaped detection) would have been adequate to generate the substantial depression observed at many of the cerebellar recording sites.
Another mechanism, termed mutual inhibition, occurs when the response of a particular group of olive cells to electrical stimulation of a limb nerve is post-synaptically inhibited following a high-strength (20T) stimulus applied to another nerve that does not itself activate those olive cells (e.g. Andersson, 1984). It seems reasonable to conclude, however, that this type of mechanism would also have had only a negligible effect in the present experiments. This is because the high-strength electrical stimulation required to activate mutual inhibition is likely to produce a much stronger and, most probably, temporally synchronised pattern of discharge in a wider range of low- and high-threshold afferents than would be the case for the conditioning tone stimulus used in the present experiments. In addition, Gellman et al. (1983, see also Oscarsson, 1969) reported that only a very small proportion of cells in the rostral dorsal accessory olive and rostral medial accessory olive (the two olivary regions that supply climbing fibres to the paravermal C zones) have inhibitory responses to tactile or muscle stimulation of the limbs. Thus, inputs from peripheral limb afferents, such as those that may be activated during the conditioned movement studied in the present experiments, are largely ineffective in reducing the spontaneous discharges of olivary cells projecting to the C zones. In addition, given that neither axo-axonal contacts associated with pre-synaptic inhibition, nor inhibitory interneurones have been observed in the course of detailed ultrastructural studies of the olive (see for example de Zeeuw et al. 1998), it seems more likely that any inhibitory mechanism(s) responsible for reducing transmission in climbing fibre pathways targeting these particular cerebellar zones will operate mainly at a pre-olivary level.
In this connection, Lidierth (1991) has obtained evidence that an inhibitory mechanism is indeed present at a pre-olivary relay in the spino-olivocerebellar paths (SOCPs) targeting the paravermal C1 and C3 zones. Low-strength (1.1-1.5T) conditioning electrical stimulation of selected forelimb nerves was found to produce a reduction in climbing fibre responses evoked by subsequent electrical stimulation of a different forelimb nerve. Such a reduction was largely abolished by application of the GABA antagonist bicuculline to the cuneate nucleus, indicating that inhibition at this pre-olivary relay in the relevant SOCPs was contributing to the reduction in climbing fibre pathway transmission. Once again though, it seems unlikely that such a mechanism could have made a significant contribution to the depression of climbing fibre responses reported in the present experiments. Inhibitory effects of the type reported by Lidierth (1991) were found to be largely over-ridden when the intensity of the test stimulus was over 2T, and an intensity of 3T was used in the present study.
In summary, the stimulus protocols used in the present experiments should have minimised any contribution made by the various inhibitory mechanisms outlined to generating the reductions in climbing fibre pathway transmission observed during classical conditioning of a forelimb withdrawal movement. Since the efficacy of the peripheral afferent nerve volley evoked by the US also remained essentially unchanged throughout the course of the experiments, it therefore seems reasonable to conclude that the conditioning-related changes in climbing fibre field size were due mainly to the operation of a central influence on transmission. Nevertheless, to exclude entirely the contribution of climbing fibre pathway inhibitory mechanisms will require further study including, for example, the use of reversible block of excitation- contraction coupling during conditioning to eliminate reafference from muscles such as ClB.
Is the gating related to the conditioned movement?
The underlying neural site(s) and process(es) responsible for the modulation of transmission remain to be determined (for further details and references see Apps, 1999, see also Hansel & Linden, 2000). However, regarding the issue of whether the modulation is a movement-related phenomenon, the present findings suggest that changes in climbing fibre field size are not closely related to the conditioned response in the flexor muscle ClB. Similar negative findings were obtained when a comparison was made between the size of individual fields and EMG activity in a different flexor muscle (biceps), suggesting that the lack of correlation was not confined to the particular muscle under investigation. This finding is perhaps not altogether surprising given that forelimb movements involve a large number of muscles that can be activated in complex ways. However, according to the cerebellar cortical conditioning hypothesis (see Yeo & Hesslow, 1998), a negative correlation might be expected between the size of individual US-evoked climbing fibre fields and the size of the conditioned EMG response preceding the US in paired trials. This is because the excitatory drive for the conditioned motor response is thought to arise from the deep cerebellar nuclei, which also send an inhibitory projection to the inferior olive. When the nuclear cells generate a conditioned response of sufficient size, the nucleo-olivary pathway is thought to inhibit the olive, thereby serving as a negative feedback system to regulate the size of the learnt response. Consistent with this possibility, Hesslow & Ivarsson (1996; see their Fig. 7) showed data from one recording site in the cerebellar cortical C3 zone in which a negative correlation was found between the size of conditioned response EMG activity in the time period 10-20 ms before the US, and the size of US evoked climbing fibre field potentials during eye-blink conditioning in the decerebrate ferret.
The fact that a negative correlation was obtained in eye-blink conditioning but not in the present investigation may be due to a number of factors, including the clear biomechanical and kinematic differences that exist between forelimb and eye-blink movements and the nature and degree to which these effector systems are influenced by the cerebellum during conditioning (for further discussion and references see Bloedel & Bracha, 1995; Kolb et al. 1997). It may also be important that the present study was carried out in intact, alert cats while that of Hesslow & Ivarsson (1996) was performed in a decerebrate ferret preparation. Besides possible differences between species, projections from higher centres are known to have powerful modulatory influences on spinal reflex circuits and in decerebrates, these descending influences are, of course, disrupted. Thus, any links between the size of climbing fibre responses and conditioned muscle activity that may be masked in the awake animal may become unmasked in the decerebrate. It is also possible that descending control of SOCPs targeting the C1 and C3 zones could account for the facilitations in climbing fibre response size observed during conditioning in the present experiments. Step-phase-dependent facilitations have been reported in the same SOCPs during locomotion in intact cats (cf. Apps et al. 1995, see also Apps & Lee, 1999). By contrast, increases in transmission were not found in climbing fibre pathways targeting the C3 zone during eye-blink conditioning in the decerebrate animal (Hesslow & Ivarsson, 1996).
Thus, the discrepancies between the current findings and those reported previously (namely, the presence of facilitations in transmission and the failure to obtain a correlation between the changes in climbing fibre response size and activity in flexor muscles associated with the conditioned movement), could have arisen because there are genuine differences between cerebellar circuits involved in forelimb as compared to eye-blink conditioning. However, before such a conclusion can be fully accepted, a number of alternative possibilities will need to be excluded, including whether a correlation between climbing fibre field size and the conditioned movement might have been revealed if the whole cerebellar cortical region influencing forelimb elbow flexors was sampled.
Possible role of climbing fibres
The findings can be considered in light of the timing hypothesis of olivary function, which is based on the intrinsic electrophysiological properties of olivary neurones, including their ability to fire synchronously (for a review and references see Welsh & Llinas, 1997). Large climbing fibre field potentials imply the synchronous action of many olive cells, while smaller fields could arise because fewer cells discharge and/or there is a lower level of synchrony between them. If changes in synchrony were an important factor in the present experiments, then it might be expected that small field potentials obtained after conditioning would be somewhat longer in duration than larger fields obtained at the same recording site but recorded earlier on during training (because the discharge of individual cells would be less synchronous and thus likely to be more temporally dispersed). However, although the results for different sites were mixed, the average duration of climbing fibre field potential obtained after conditioning was slightly shorter than the duration obtained early on during training, implying that changes in synchrony were not a major factor in generating the observed reductions in field size after conditioning was well established.
Another influential theory of climbing fibre function is the comparator hypothesis, in which climbing fibres are thought to signal ‘unpredicted’ sensory errors to the cerebellar cortex whenever a mismatch occurs between intended and achieved movements (Oscarsson, 1980). The present experiments suggest that more than one process is involved during conditioning of a forelimb reflex to a tone stimulus in awake cats, dividing acquisition into three (not necessarily equal) phases: an initial phase early on during conditioning in which the C zones in lobule V exhibit little or no change in climbing fibre field size; an intermediate phase in which facilitations occur at some loci; and a final phase in which reductions occur at most, but not all loci.
In terms of the comparator hypothesis, the US may be regarded during the initial phase of conditioning as a novel, unpredicted sensory event that is therefore readily transmitted to the cerebellum. During the intermediate phase, the increase in transmission that occurs at some sites may serve to enhance the associative process, while in the final phase, when conditioning is well established, the CS predicts the occurrence of the US and transmission of the US to the cerebellar cortex is gated out, presumably because it has become predictable.
The notion of a comparator is also incorporated into the cerebellar cortical conditioning model that has arisen out of eye-blink experiments, because information concerning the (predicted) CS is thought to be relayed to the cerebellum via the mossy fibres, while information relating to the (unpredicted) US is relayed via the climbing fibres. Convergence of these two inputs is thought to result in the long-term changes in Purkinje cell synaptic efficacy that underlie cerebellar cortical contributions to motor learning (for a review see for example Yeo & Hesslow, 1998). With regard to the present findings, it is important to consider whether mossy fibre signals arising from the auditory CS could interact at paravermal cerebellar recording sites with climbing fibre inputs arising from limb afferents signalling the US. To date, this has not been studied in the awake animal, but long ago Snider & Stowell (1944) charted teleceptive inputs to the cerebellar cortex in anaesthetised cats and in decerebrate animals under light barbiturate anaesthesia. They found that auditory evoked responses were largest in the vermis of lobules VI-VIII, but also that smaller responses could be recorded in the caudal folia of lobule V, including its paravermal regions, where the recording sites in the present study were located. Given that cerebellar auditory responses can be markedly depressed by anaesthesia, even 24 h after a single dose of barbiturate (Wolfe, 1972), it is quite possible that paravermal areas are more responsive to auditory inputs in the unanaesthetised preparation. Consistent with this proposition was the finding in awake monkeys that Purkinje cells in the paravermal region of lobules III-VIII evoked complex spikes in relation to a startle-producing acoustic stimulus (Mortimer, 1973). It may also be relevant to note that Budanur et al. (1999) have found in crus I very powerful simple and complex spike responses to teleceptive (visual) stimulation in the awake cat, despite the fact that Snider & Stowell (1944) mapped only fairly small responses in that part of the cerebellum.
Thus, on the basis of the available evidence, it seems not unreasonable to suggest (in keeping with the cerebellar cortical conditioning hypothesis) that climbing fibre somatosensory inputs could interact with mossy fibre auditory inputs within paravermal lobule V in the awake, behaving animal. One possible interpretation of the present findings would then be that input from the ‘teacher’ is required (even to some extent enhanced) during the early stages of conditioning, but this requirement is reduced as the new association is progressively established, and the excitability of the teaching pathway is then actively lowered by some central regulatory mechanism. Such an arrangement may underlie the finding that Purkinje cells can increase and then subsequently decrease their frequency of discharge of complex spikes over the time course required to learn a new motor task (Gilbert & Thach, 1977). It is noteworthy, however, that in the present experiments the climbing fibre fields evoked at different recording sites were never entirely abolished after conditioning was well established. This partial gating-out may ensure that the associative link between the CS and US is maintained once the new movement has been learnt.
Cerebellar zones and microzones
The results of a wealth of anatomical and physiological studies suggest that an important principle of cerebellar cortical organisation is a division into a series of distinct longitudinally oriented zones (for a review and references see Voogd & Glickstein, 1998). Detailed electrophysiological mapping experiments suggest that individual zones can be further divided into more fundamental units termed microzones (e.g. Ekerot & Larson, 1979, Ekerot et al. 1991). With regard to the C3 zone, approximately 30 microzones relating to the ipsilateral forelimb are thought to exist, and anatomical tract-tracing studies have revealed a related micro-organisation within its olivo-corticonuclear connections (Garwicz et al. 1996; Apps & Garwicz, 2000). By inference, other cerebellar zones are thought to be composed of similar olivo-corticonuclear ‘microcomplexes’, each one serving a distinct function in motor control (cf. Oscarsson, 1979).
In the present investigation, a few sites in the forelimb-receiving area of the paravermis displayed no detectable difference in climbing fibre field size after training, while others varied considerably in the extent of their reduction. This suggests that a degree of functional heterogeneity existed between the different cortical loci, possibly relating to the presence of different forelimb-related microzones. The observation that the three sites exhibiting no change in field size after training were all located in the C1 zone implies that within this zone, one or more microzones exist that were (at least under the current experimental conditions), not subject to the suppression of climbing fibre transmission during conditioning of a forelimb reflex. As to why after conditioning, these particular ‘forelimb’ sites remained open for transmission, while other ‘forelimb’ sites were closed remains an issue for further study.
Acknowledgments
We thank Professor David M. Armstrong for his comments on the manuscript. This work was funded by an MRC Senior Research Fellowship to R.A. S.L. was supported by a Wellcome Trust PhD studentship.
REFERENCES
- Andersson G. Mutual inhibition between olivary cell groups projecting to different cerebellar microzones in the cat. Experimental Brain Research. 1984;54:293–303. doi: 10.1007/BF00236230. [DOI] [PubMed] [Google Scholar]
- Apps R. Movement-related gating of climbing fibre input to cerebellar cortical zones. Progress in Neurobiology. 1999;57:537–562. doi: 10.1016/s0301-0082(98)00068-9. [DOI] [PubMed] [Google Scholar]
- Apps R, Atkins MJ, Garwicz M. Gating of cutaneous input to cerebellar climbing fibres during a reaching task in the cat. Journal of Physiology. 1997;502:203–214. doi: 10.1111/j.1469-7793.1997.203bl.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apps R, Garwicz M. Precise matching of C1-C3 zone olivo-cortical divergence and cortico-nuclear convergence in the paravermal cerebellum. European Journal of Neuroscience. 2000;12:205–214. doi: 10.1046/j.1460-9568.2000.00897.x. [DOI] [PubMed] [Google Scholar]
- Apps R, Hartell NA, Armstrong DM. Step phase-related excitability changes in spino-olivocerebellar paths to the c1 and c3 zones in cat cerebellum. Journal of Physiology. 1995;483:687–702. doi: 10.1113/jphysiol.1995.sp020614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apps R, Lee S. Gating of transmission in climbing fibre paths to cerebellar cortical C1 and C3 zones in the rostral paramedian lobule during locomotion in the cat. Journal of Physiology. 1999;516:875–883. doi: 10.1111/j.1469-7793.1999.0875u.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apps R, Lidierth M, Armstrong DM. Locomotion-related variations in excitability of spino-olivocerebellar paths to cat cerebellar cortical c2 zone. Journal of Physiology. 1990;424:487–512. doi: 10.1113/jphysiol.1990.sp018079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong DM. Review lecture: The supraspinal control of mammalian locomotion. Journal of Physiology. 1988;405:1–37. doi: 10.1113/jphysiol.1988.sp017319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong DM, Harvey RJ. Responses to a spino-olivo-cerebellar pathway in the cat. Journal of Physiology. 1968;194:147–168. doi: 10.1113/jphysiol.1968.sp008399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloedel JR, Bracha V. On the cerebellum, cutaneomuscular reflexes, movement control and the elusive engrams of memory. Behavioural Brain Research. 1995;68:1–44. doi: 10.1016/0166-4328(94)00171-b. [DOI] [PubMed] [Google Scholar]
- Bloedel JR, Bracha V. Current concepts of climbing fiber function. Anatomy Record. 1998;253:118–126. doi: 10.1002/(SICI)1097-0185(199808)253:4<118::AID-AR7>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- Budanur OE, Hollands MA, Marple-horvat DE. Precision signalling of visual events by complex spikes and simple spikes in hemispheral (crus I) Purkinje cells. Society for Neuroscience Abstracts. 1999;25:372. [Google Scholar]
- Chambers WW, Sprague JM. Functional localization in the cerebellum. I. Organisation in longitudinal cortico-nuclear zones and their contribution to the control of posture, both extrapyramidal and pyramidal. Journal of Comparative Neurology. 1955;103:105–129. doi: 10.1002/cne.901030107. [DOI] [PubMed] [Google Scholar]
- Cheney PD, Fetz EE, Mewes K. Neural mechanisms underlying corticospinal and rubrospinal control of limb movements. Progress in Brain Research. 1991;87:213–252. doi: 10.1016/s0079-6123(08)63054-x. [DOI] [PubMed] [Google Scholar]
- De Zeeuw CI, Simpson JI, Hoogenraad CC, Galjart N, Koekkoek SKE, Ruigrok TJH. Microcircuitry and function of the inferior olive. Trends in Neuroscience. 1998;21:391–400. doi: 10.1016/s0166-2236(98)01310-1. [DOI] [PubMed] [Google Scholar]
- Drew T. Motor cortical activity during voluntary gait modifications in the cat. I. Cells related to the forelimbs. Journal of Neurophysiology. 1993;70:179–199. doi: 10.1152/jn.1993.70.1.179. [DOI] [PubMed] [Google Scholar]
- Drew T, Rossignol S. A kinematic and electromyographic study of cutaneous reflexes evoked from the forelimb of unrestrained walking cats. Journal of Neurophysiology. 1987;57:1160–1184. doi: 10.1152/jn.1987.57.4.1160. [DOI] [PubMed] [Google Scholar]
- Eccles JC, Llinas R, Sasaki K, Voorhoeve PE. Interaction experiments on the responses evoked in Purkinje cells by climbing fibres. Journal of Physiology. 1966;182:297–315. doi: 10.1113/jphysiol.1966.sp007825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekerot CF, Garwicz M, Schouenborg J. Topography and nociceptive receptive fields of climbing fibres projecting to the cerebellar anterior lobe in the cat. Journal of Physiology. 1991;441:257–274. doi: 10.1113/jphysiol.1991.sp018750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekerot CF, Jorntell H, Garwicz ML. Functional relation between corticonuclear input and movement evoked on microstimulation in cerebellar nucleus interpositus anterior in the cat. Experimental Brain Research. 1995;106:365–376. doi: 10.1007/BF00231060. [DOI] [PubMed] [Google Scholar]
- Ekerot CF, Larson B. Correlation between sagittal projection zones of climbing and mossy fibre paths in cat cerebellar anterior lobe. Brain Research. 1973;64:446–450. doi: 10.1016/0006-8993(73)90203-5. [DOI] [PubMed] [Google Scholar]
- Ekerot CF, Larson B. The dorsal spino-olivocerebellar system in the cat. I. Functional organization and termination in the anterior lobe. Experimental Brain Research. 1979;36:201–217. doi: 10.1007/BF00238905. [DOI] [PubMed] [Google Scholar]
- Garwicz M, Apps R, Trott JR. Micro-organization of olivocerebellar and corticonuclear connections of the paravermal cerebellum in the cat. European Journal of Neuroscience. 1996;8:2726–2738. doi: 10.1111/j.1460-9568.1996.tb01567.x. [DOI] [PubMed] [Google Scholar]
- Gellman R, Houk JC, Gibson AR. Somatosensory properties of the inferior olive of the cat. Journal of Comparative Neurology. 1983;215:228–243. doi: 10.1002/cne.902150210. [DOI] [PubMed] [Google Scholar]
- Gilbert PFC, Thach WT. Purkinje cell activity during motor learning. Brain Research. 1977;128:309–328. doi: 10.1016/0006-8993(77)90997-0. [DOI] [PubMed] [Google Scholar]
- Gormezano I. Classical conditioning. In: Sidowski JB, editor. Experimental Methods and Instrumentation in Psychology. New York: McGraw-Hill; 1966. pp. 385–420. [Google Scholar]
- Hansel C, Linden DJ. Long-term depression of the cerebellar climbing fiber-Purkinje neuron synapse. Neuron. 2000;26:473–482. doi: 10.1016/s0896-6273(00)81179-4. [DOI] [PubMed] [Google Scholar]
- Hesslow G. Correspondence between climbing fibre input and motor output in eyeblink-related areas in cat cerebellar cortex. Journal of Physiology. 1994;476:229–244. doi: 10.1113/jphysiol.1994.sp020126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hesslow G, Ivarsson M. Inhibition of the inferior olive during conditioned responses in the decerebrate ferret. Experimental Brain Research. 1996;110:36–46. doi: 10.1007/BF00241372. [DOI] [PubMed] [Google Scholar]
- Horn KM, Pong M, Batni SR, Levy SM, Gibson AR. Functional specialization within the cat red nucleus. Journal of Neurophysiology. 2002;87:469–477. doi: 10.1152/jn.00949.2000. [DOI] [PubMed] [Google Scholar]
- Kennedy TT, Grimm RJ, Towe AL. The role of cerebral cortex in evoked somatosensory activity in cat cerebellum. Experimental Neurology. 1966;14:13–32. doi: 10.1016/0014-4886(66)90021-5. [DOI] [PubMed] [Google Scholar]
- Kim JJ, Krupa DJ, Thompson RF. Inhibitory cerebello-olivary projections and blocking effect in classical conditioning. Science. 1998;279:570–573. doi: 10.1126/science.279.5350.570. [DOI] [PubMed] [Google Scholar]
- Kolb FP, Irwin KB, Bloedel JR, Bracha V. Conditioned and unconditioned forelimb reflex systems in the cat: involvement of the intermediate cerebellum. Experimental Brain Research. 1997;114:255–270. doi: 10.1007/pl00005634. [DOI] [PubMed] [Google Scholar]
- Lidierth M. Sensory integration in the spino-olivocerebellar pathways of the anaesthetized cat. Journal of Physiology. 1991;203:1–20. doi: 10.1113/jphysiol.1991.sp018495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormick DA, Steinmetz JE, Thompson RF. Lesions of the inferior olivary complex cause extinction of the classically conditioned eyeblink response. Brain Research. 1985;359:120–130. doi: 10.1016/0006-8993(85)91419-2. [DOI] [PubMed] [Google Scholar]
- Morissette J, Bower JM. Contribution of somatosensory cortex to responses in the rat cerebellar granule cell layer following peripheral tactile stimulation. Experimental Brain Research. 1996;109:240–250. doi: 10.1007/BF00231784. [DOI] [PubMed] [Google Scholar]
- Mortimer JA. Temporal sequence of cerebellar Purkinje and nuclear activity in relation to the acoustic startle response. Brain Research. 1973;50:457–462. doi: 10.1016/0006-8993(73)90751-8. [DOI] [PubMed] [Google Scholar]
- Oscarsson O. Termination and functional organization of the dorsal spino-olivocerebellar path. Journal of Physiology. 1969;200:129–149. doi: 10.1113/jphysiol.1969.sp008685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oscarsson O. Functional units of the cerebellum- sagittal zones and microzones. Trends in Neuroscience. 1979;2:143–145. [Google Scholar]
- Oscarsson O. Functional organization of olivary projection to the cerebellar anterior lobe. In: Courville J, Montigny CD, Lamarre Y, editors. The Inferior Olivary Nucleus: Anatomy and Physiology. New York: Raven; 1980. pp. 279–289. [Google Scholar]
- Palmer C. A microwire technique for recording single neurons in unrestrained animals. Brain Research Bulletin. 1978;3:285–289. doi: 10.1016/0361-9230(78)90129-6. [DOI] [PubMed] [Google Scholar]
- Pavlov IP. Conditioned Reflexes. London: Oxford University Press; 1927. [Google Scholar]
- Razran G. Mind in Evolution: An East-West Synthesis of Learned Behaviour and Cognition. Boston: Houghton Mifflin; 1971. [Google Scholar]
- Rho M-J, Lavoie S, Drew T. Effects of red nucleus microstimulation on the locomotor pattern and timing in the intact cat: a comparison with the motor cortex. Journal of Neurophysiology. 1999;81:2297–2315. doi: 10.1152/jn.1999.81.5.2297. [DOI] [PubMed] [Google Scholar]
- Sears LL, Steinmetz JE. Dorsal accessory inferior olive activity diminishes during acquisition of the rabbit classically conditioned eyelid response. Brain Research. 1991;545:114–122. doi: 10.1016/0006-8993(91)91276-7. [DOI] [PubMed] [Google Scholar]
- Snider RS, Stowell A. Receiving areas of the tactile, auditory and visual systems in the cerebellum. Journal of Neurophysiology. 1944;7:331–358. [Google Scholar]
- Steinmetz JE, Lavond DG, Thompson RF. Classical conditioning in rabbits using pontine nucleus stimulation as a conditioned stimulus and inferior olive stimulation as an unconditioned stimulus. Synapse. 1989;3:225–233. doi: 10.1002/syn.890030308. [DOI] [PubMed] [Google Scholar]
- Thach WT, Goodkin HP, Keating JG. The cerebellum and the adaptive coordination of movement. Annual Review of Neuroscience. 1992;15:403–442. doi: 10.1146/annurev.ne.15.030192.002155. [DOI] [PubMed] [Google Scholar]
- Trott JR, Apps R. Lateral and medial sub-divisions within the olivocerebellar zones of the paravermal cortex in lobule Vb/c of the cat anterior lobe. Experimental Brain Research. 1991;87:126–140. doi: 10.1007/BF00228514. [DOI] [PubMed] [Google Scholar]
- Voogd J, Glickstein M. The anatomy of the cerebellum. Trends in Neuroscience. 1998;21:370–375. doi: 10.1016/s0166-2236(98)01318-6. [DOI] [PubMed] [Google Scholar]
- Voneida TJ, Christie D, Bogdanski R, Chopko B. Changes in instrumentally and classically conditioned limb-flexion responses following inferior olivary lesions and olivocerebellar tractotomy in the cat. Journal of Neuroscience. 1990;10:3583–3593. doi: 10.1523/JNEUROSCI.10-11-03583.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welsh JP, Llinas R. Some organizing principles for the control of movement based on olivocerebellar physiology. Progress in Brain Research. 1997;114:449–461. doi: 10.1016/s0079-6123(08)63380-4. [DOI] [PubMed] [Google Scholar]
- Wolfe JW. Responses of the cerebellar auditory area to pure tone stimuli. Experimental Neurology. 1972;36:295–309. doi: 10.1016/0014-4886(72)90025-8. [DOI] [PubMed] [Google Scholar]
- Yeo CH, Hardiman MJ, Glickstein M. Classical conditioning of the nictitating membrane response of the rabbit. II. Lesions of the cerebellar cortex. Experimental Brain Research. 1985;60:99–113. doi: 10.1007/BF00237023. [DOI] [PubMed] [Google Scholar]
- Yeo CH, Hardiman MJ, Glickstein M. Classical conditioning of the nictitating membrane response of the rabbit. IV. Lesions of the inferior olive. Experimental Brain Research. 1986;63:81–92. doi: 10.1007/BF00235649. [DOI] [PubMed] [Google Scholar]
- Yeo CH, Hesslow G. Cerebellum and conditioned reflexes. Trends in Cognitive Sciences. 1998;2:322–330. doi: 10.1016/s1364-6613(98)01219-4. [DOI] [PubMed] [Google Scholar]


