Abstract
Whole-cell patch clamp recordings of miniature inhibitory postsynaptic currents (mIPSCs) were obtained in identified abducens motoneurons (aMns) from young rats (P5-P13). Three types of mIPSC were distinguished according to their kinetics and their sensitivity to receptor antagonists: faster decaying events mediated by glycine receptors (glyRs), slower decaying events mediated by GABAA receptors (GABAARs), and mIPSCs displaying two components corresponding to GABA and glycine co-release. Dual component events accounted for ≈30 % of mIPSCs, independently of the rat's age and were also identified during evoked transmitter release. In contrast, the kinetics of glyR- and GABAAR-mediated mIPSCs became faster during development. Monosynaptic inhibitory postsynaptic potentials (IPSPs) were able to fully inhibit motoneuron discharge elicited by current pulses. When the GABAAR-mediated component or the glyR-mediated component of the IPSP was blocked, the inhibition of motoneuron firing was reduced. The 20-80 % rise time and duration of GABAAR-mediated IPSPs were significantly longer than those mediated by glyRs. The time window of inhibition for each component was determined using single postsynaptic action potentials elicited with various delays from the onset of the IPSP. GlyR-mediated IPSPs induced fast transient inhibition whereas GABAAR-mediated IPSPs induced slow sustained suppression of firing. Using a modelling approach, we found that the two components summated non-linearly. We conclude that in developing aMns, co-release of GABA and glycine determines the strength and timing of inhibition through non-linear interactions between the two components, thus optimizing inhibition of motoneuron function.
GABA and glycine are co-accumulated in synaptic vesicles by a common vesicular transporter (Burger et al. 1991; Gasnier, 2000). This transporter is expressed in neurons from various brain areas including brainstem motoneurons that control extra-ocular muscles (Chaudhry et al. 1998). Co-release of fast neurotransmitters has been demonstrated in spinal and hypoglossal motoneurons for GABA and glycine (Jonas et al. 1998; O'Brien & Berger, 1999), and for ATP and GABA (Jo & Schlichter, 1999). However, the functional consequences of neurotransmitter co-release have not been yet considered. In contrast to the possible antagonist action of ATP/GABA co-release on neuronal excitability (Salter & De Koninck, 1999), GABA and glycine are both known to be inhibitory neurotransmitters. Each neurotransmitter activates a different family of ionotropic receptors that are permeable to chloride ions (Bormann et al. 1987; Takahashi & Momiyama, 1991). Therefore, it is expected that both neurotransmitters could cooperate in determining inhibitory strength with possible non-linear interactions between the two components. The kinetics of glycine receptor (glyR)-activated currents are faster than those of GABAA receptor (GABAAR)-activated currents (O'Brien & Berger, 1999) suggesting that the glyR-mediated component of the dual synaptic response could ensure an immediate blockade of motoneuron firing whereas the GABA component could allow a prolonged inhibition.
In abducens motoneurons (aMns), synaptic inhibition is most likely mediated by GABA and glycine (Precht et al. 1973; Spencer et al. 1989; Lahjouji et al. 1996). We therefore used an in vitro preparation of brainstem containing the abducens nucleus to investigate the functional role of this co-inhibition. We show that synaptically co-released GABA and glycine both participate in the inhibition of aMn firing. The complementary nature of the dual inhibition was also seen as a function of time since the inhibition of firing by glycinergic IPSPs was faster and shorter than that produced by GABA IPSPs of the same amplitude.
METHODS
Identification of abducens motoneurons
One day prior to the experiment, 5- to 13-day-old Wistar rats were deeply anaesthetized with chloral hydrate (intraperitoneal, 200 mg kg−1). All animal experiments (surgery and dissection) were performed in accordance with institutional guidelines. A solution of carbocyanin at 15 % (fast DiI, Molecular Probes) was injected behind the eye, close to the rectus lateralis muscle. The day after the injection, animals were killed by decapitation. Transverse brainstem slices (300 μm thick) containing the abducens nucleus were prepared with a vibroslicer (Campden or Leica VT-1000-S). The slicing solution was maintained at 4 °C and contained (mm): 280 sucrose, 26 NaHCO3, 10 d-glucose, 1.3 KCl, 1 CaCl2, and 10 MgCl2. The abducens nucleus was localized using fluorescent microscopy at low magnification (× 4 or × 10). aMns were then identified at higher magnification (× 40) by their fluorescent staining, and visualized with an infrared video-microscopy device. All experiments were performed in a temperature-controlled (34 °C) submerged slice chamber (Luigs & Neumann, Ratingen, Germany).
Electrophysiology
Whole-cell patch clamp recordings were obtained from identified aMns with an Axoclamp-2B amplifier in ‘bridge’ mode or single electrode voltage clamp in the continuous mode. Membrane potentials were filtered (3 kHz low-pass). Digitized signals (18 kHz, Instrutech VR-100B) were stored on videotape for later analysis via the Digidata interface (Axon Instruments; sampling rate, 10-12 kHz) with the use of Acquis1 software (Gérard Sadoc, Biologic/Unité de Neurosciences Intégratives et Computationelles (UNIC)-CNRS). The extracellular solution contained (mm): 125 NaCl, 2.5 KCl, 1.1 NaH2PO4, 26 NaHCO3, 4 CaCl2, and 0.5 MgCl2. GABA and glycine were bath-applied and evoked currents were recorded with a patch pipette containing 130 mm Cl−, in the presence of TTX (1 μM) and kynurenate (2 mm). Monosynaptic IPSPs were evoked in the presence of the excitatory amino acid receptor antagonist kynurenate (2 mm) by extracellular stimulation in the ipsilateral vestibular nucleus. mIPSCs were recorded with CsCl-filled electrodes (140 mm) in the presence of kynurenate (2 mm) and tetrodotoxin (1 μM). Flunitrazepam was added to the extracellular solution in order to prolong the decay time of GABAAR-mediated mIPSCs. As shown previously (Mellor & Randall, 1997), no obvious change in the mIPSC frequency was observed in the presence of flunitrazepam. This compound was not added when studying the kinetics of the GABAA and glycine components, and the time course of inhibition. For evoked IPSP recordings, the internal solution filling the patch pipette contained (mm): 20 KCl, 120 potassium gluconate, 10 Hepes, 10 EGTA, 2 MgCl2, 2 Na2ATP (pH 7.4). In some experiments the concentration of KCl was lowered to 2 mm to obtain hyperpolarizing IPSPs (final Cl− concentration: 4 mm). For mIPSC recordings, the internal solution of the patch pipette contained (mm): 140 CsCl, 5 Hepes, 2 MgCl2, and 2 EGTA (pH 7.4). Neurons were voltage clamped at −70 or −80 mV. Series resistance was in the range of 15-35 MΩ and ≈20 % series resistance compensation was routinely used. Biocytin (0.3 %, Sigma) was added to the solution in some experiments to label the neuron. Biocytin was revealed with avidin-biotin complex coupled to HRP. Strychnine hydrochloride, (-)-bicuculline methiodide and flunitrazepam were from Sigma.
Signal analysis
Postsynaptic currents and potentials were analysed using Acquis1 and DAC2 software (Gérard Sadoc UNIC-CNRS). Individual spontaneously occurring mIPSCs were detected off-line with a semi-automatic procedure. Events were detected above a threshold of the current derivative (dIm/dt). This threshold was adjusted to the smallest value that just allowed detection of synaptic currents emerging from the noise recording. The detection threshold was usually close to 5-10 pA and was twice the baseline noise. The detected events were examined individually; any noise that spuriously met the trigger specifications was rejected. Only events that had stable baselines before the rise and after the end of the decay were kept for analysis. Rise times were determined between 20 and 80 % of the peak amplitude of the mIPSCs. The decay of all mIPSCs was automatically fitted with a bi-exponential function: y = y0 + y1e(-x/τ1) + y2e(-x/τ2) (Acquis1/Fit002; Angulo et al. 1999). Detected mIPSCs were first displayed automatically on the screen at a fast time scale. The length of the analysis was defined by two cursors adjusted manually on each mIPSC and was restricted to the falling phase of the mIPSC. The duration of this analysis window was longer than 60 ms (typically ≈150 ms, see Fig. 2C). When the value of the second exponential was found to be equal to that of the first with an error of 1 %, the fit was considered to be mono-exponential (equation: y = y0 + ze(-x/τ1)) with z = y1 + y2. Thus, the decision to fit a mono- or bi-exponential function was objective and the proportion of single (GABAAR- or glyR-mediated) and dual mIPSCs was determined automatically. Dual mIPSCs exhibited two distinct decay time constants (τ1 and τ2), whereas single mIPSCs exhibited only one decay time constant (τ1). The same analysis was used for evoked asynchronous IPSCs in the presence of Sr2+.
Figure 2. Co-release of GABA and glycine.

A, miniature IPSCs were recorded in the presence of TTX, kynurenate, and flunitrazepam to prolong GABAAR-activated currents. Three types of mIPSCs were observed: fast decaying mIPSCs (*) slow decaying mIPSCs (○) and compound mIPSCs (dual component, arrow). B, fitting procedure for mono- and bi-exponential decays of mIPSCs. Upper and middle traces, mono-exponential fits (dashed lines) with corresponding parameters given beneath the traces. The second term of the equation was equal to 0. Lower trace, bi-exponential fit. C, pharmacological characterization. In the presence of bicuculline (10 μM), only fast mIPSCs were observed. In the presence of strychnine (1 μM), only slow mIPSCs were recorded. D, histograms of decay time constants of compound mIPSCs in control conditions (upper) and under selective GABAAR and glyR antagonists (bottom) in a single aMn. The decay time constant of the faster component of compound mIPSCs (τ1, 14 events) corresponds to that of glyR-mediated mIPSCs (47 events) and the decay time constant of the slower component (τ2, 14 events) corresponds to that of pharmacologically isolated GABAAR-mediated mIPSCs (87 events). E, amplitude histogram of glyR- (612 events, 4 cells) and GABAAR-mediated mIPSCs (225 events, 4 cells). F, estimation of the proportion of GABAAR- and glyR-activated mIPSCs in control conditions. Upper graph, distribution of the decay time constants of mIPSCs in the presence of 10 μM bicuculline (464 events, 3 aMns). 92 % of the events had a decay time constant < 15 ms (vertical arrow). Middle histogram, distribution of the decay time constants of mIPSCs in the presence of 1 μM strychnine (120 events, 3 aMns). 92 % of the events had a decay time constant > 15 ms (vertical arrow). Lower histogram, distribution of decay time constants of single exponential mIPSCs in control conditions (324 events, 3 aMns). Events on the right of the vertical arrow (42 %) are considered to be GABAergic mIPSCs, and those on the left glycinergic mIPSCs (58 %).
Model
Simulations were performed on a compartmental model of an aMn using the Crona software (Kopysova et al. 1996). The aim of the study was focused on the functional consequences of the co-release of GABA and glycine recorded at the soma of aMns. The cell body of aMns receives input from multiple terminals containing GABA and glycine (Lahjouji et al. 1996) and is enriched in postsynaptic GABAA and glycine receptors (Russier et al. 2001). The modelled structure was therefore a simplified version of a canonical aMn based on the morphology of aMns recorded at P6-P8. The length and width of the cell bodies were measured on infrared video-micrographs (mean length: 22.4 ± 1.1 μm; mean width: 15.5 ± 1.4 μm; n = 7 cells). The modelled neuron included the cell body but was devoid of primary dendritic shafts. The cell body was presented as a cylinder with a length of 22.4 μm and a diameter of 15.5 μm. Input resistances and membrane time constants of aMns were measured in a homogeneous population of neurons (P6-P8, mean 7.5) with hyperpolarizing pulses of current. The mean values of input resistance and membrane time constant (Rin = 251 ± 55 MΩ and τm = 23.4 ± 3.8 ms, n = 9 neurons) were used to adjust the parameters of the model. The model had an input resistance at the soma of 250 MΩ and a membrane time constant of 23.3 ms. The membrane contained voltage-gated Na+-, K+-, persistent Na+-, T-type Ca2+- and Ca2+-dependent K+ channels as observed experimentally in hypoglossal (Umemiya & Berger, 1994) and aMns.
Maximal densities of Na+-, K+-, persistent Na+-, T-type Ca2+- and Ca2+-dependent K+ channels were set to 110, 55, 0.2, 20 and 20 mS cm−2, respectively. These values were chosen to closely match the electrical behaviour of aMns observed in vivo (Durand, 1993) and in vitro and were based on the estimations described by others (Brodin et al. 1991; Lipowsky et al. 1996; Aradi & Holmes, 1999). Passive membrane resistivity (Rm) was 15 kΩ cm−2 and specific membrane capacitance (Cm) was 2.52 μF cm−2. A mixed inhibitory synaptic input was placed at the soma and included GABAA- and glycine-like conductances. Synaptic activity was modelled by the outward current Isyn(E,t) = Gsyn(t) × (Em - ECl). To match the time courses of glyR- and GABAAR-mediated IPSPs, the time courses of synaptic conductances were described by the following equations: Gsyn(t) = Gmax × (1-e−t/τ1) × e−t/τ2 (Pongracz et al. 1992), with τ1 and τ2 equal to 0.4 and 12 ms for the glycine-activated conductance and 1.2 and 39 ms for the GABAAR-activated conductance, respectively. Gmax describes the number of postsynaptic channels activated by each type of transmitter and was set to give a maximal value of Gsyn = 5 nS.
RESULTS
Postsynaptic inhibitory receptors
The abducens nucleus (VI) in the brainstem innervates the rectus lateralis muscle. aMns from young rats (P5-P13, mean P7-P8) were identified in the slice after retrograde labelling (Fig. 1A). All labelled neurons exhibited normal electrical activity such as spike discharge and postsynaptic activity. At this stage of development (P7), aMns exhibited a complex dendritic arbor (Fig. 1B).
Figure 1. Identification of aMns.
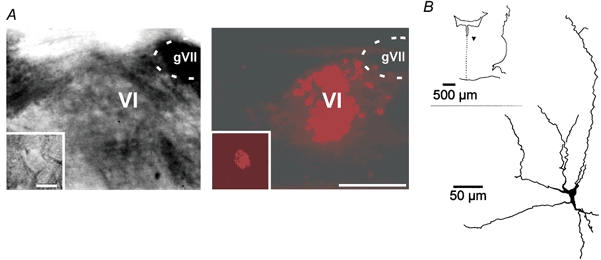
A, infrared video-microscopic (left) and fluorescent (right) pictures of retrogradely labelled aMns. Insets, higher magnification. Calibration bars apply to both panels: 100 μm (main panels) and 25 μm (insets). B, P7 aMn labelled with biocytin (inset, position of the cell (▾) in the brainstem slice).
The response of postsynaptic receptors to inhibitory neurotransmitters was first determined by bath-applying GABA and glycine. The GABAAR antagonist, bicuculline (10-20 μM) blocked postsynaptic currents evoked by 100-400 μM GABA (12 ± 1 % of control amplitude, n = 4 cells) but not those evoked by the same concentration of glycine (87 ± 13 %, n = 3 cells). The glyR antagonist strychnine (1 μM) blocked postsynaptic currents evoked by 100-400 μM glycine (0 %, n = 4 cells) but not those induced by the same concentration of GABA (83 ± 25 %, n = 3 cells). We next examined whether functional GABAB receptors (GABABRs) were present postsynaptically. Neither detectable postsynaptic currents nor conductance changes were evoked by bath application of 30-60 μM of the GABABR agonist, baclofen (n = 5 cells, not shown), indicating that aMns express postsynaptic GABAARs and glyR but lack postsynaptic GABABRs.
GABA and glycine co-release
We first addressed the question of whether both transmitters were co-localized in single vesicles by studying the spontaneous vesicular release of inhibitory transmitter. Miniature inhibitory postsynaptic currents (mIPSCs) were recorded in P5-P13 aMns (n = 11 cells). Flunitrazepam (1 μM) was added to the solution to differentiate between GABAA and glycine components according to their kinetics. Three types of mIPSCs were observed: fast decaying, slow decaying, and mixed events with both fast and slow decay time constants (Fig. 2A). The decay of each mIPSC was fitted off-line (Fig. 2B). The low frequency of mIPSCs (2.0 ± 0.3 Hz, n = 11 cells) rules out the possibility that dual mIPSCs could have arisen from stochastic superimposition of slow and fast decaying events. In the presence of 10 μM bicuculline (Fig. 2C), all events exhibited a fast decay time constant, similar to that of the first component recorded in the same neuron in control conditions (Fig. 2D, τ of GlyR-mediated mIPSCs vs. τ1, Mann-Whitney, P > 0.1). Similar findings were observed in three other aMns. In the presence of 1 μM strychnine (Fig. 2C), all events exhibited a slow decay time constant comparable to that of the second component recorded in the same neuron in control conditions (Fig. 2D, τ of GABAAR-mediated mIPSCs vs. τ2, Mann-Whitney test P > 0.1). Similar findings were observed in three other aMns. These data demonstrate that dual mIPSCs result from the co-release of GABA and glycine from single vesicles.
Amplitudes of GABAAR-mediated mIPSCs were found to be smaller than those of glyR-mediated mIPSCs (15 ± 5 pA, n = 4 and 42 ± 4 pA, n = 4 cells, respectively; Fig. 2E). The mean coefficients of variation were, however, comparable for glyR- and GABAAR-mediated mIPSCs (respectively, 0.57 ± 0.09 and 0.47 ± 0.01, n = 4 cells).
The proportion of pure glycinergic and pure GABAergic events was estimated in three aMns in which the mean decay time constant for each component was homogeneous. In the presence of bicuculline 92 % of glycinergic events had a decay time constant faster than 15 ms (upper histogram in Fig. 2F). In the presence of strychnine, 92 % of the GABAergic events had a decay time constant slower than 15 ms (see middle histogram in Fig. 2F). In control conditions, the total number of events was 426 with 24 % (102/426) of dual mIPSCs. We estimated that single exponential mIPSCs with a decay < 15 ms were glycinergic events and corresponded to 58 % (189/324) of the distribution (see lower histogram in Fig. 2F), i.e. 44 % of the total population of mIPSCs (189/426). Similarly, we estimated that mono-exponential mIPSCs with a decay > 15 ms were GABAergic events and represented 42 % (135/324) of the distribution (see lower histogram in Fig. 2F), i.e. 32 % of the total population of mIPSCs (135/426).
In control conditions, the proportion of mIPSCs exhibiting two components varied between 11 % and 50 % (mean of 30 ± 3 %, n = 11 cells) but was found to be independent of the age (linear regression, y = 0.25x + 27.6; r2 = 0.002; Fig. 3A). The decay time constant of mIPSCs became faster during development (Fig. 3B), as previously reported for other neurons (Takahashi, 1992; Krupp et al. 1994; Singer et al. 1998; Smith et al. 2000). This developmental acceleration was virtually identical to that observed for pharmacologically isolated mIPSCs in the same aMns (see Fig. 3B).
Figure 3. Development of mIPSCs in aMns.

A, percentage of dual component mIPSCs at different postnatal ages. Linear regression: y = 0.25x + 27.6 (r2 = 0.002). B, decay time constants of dual component mIPSCs decreased with age (y = 17e−0.0783x, r2 = 0.18 for τ1 (○) and y = 146e−0.1062x, r2 = 0.59 for τ2 (•)). The decay time constants of glyR- and GABAAR-mediated mIPSCs are superimposed on each distribution (respectively, ▵ and ▴) C, 20-80 % rise time of pharmacologically isolated glyR- and GABAAR-mediated mIPSCs decreased with age (y = 10.3e−0.197x, r2 = 0.79 for glyR-mediated mIPSCs (○) and y = 10.7e−0.1396x, r2 = 0.32 for GABAAR-mediated mIPSCS (•)).
The 20-80 % rise times of GABAAR-mediated mIPSCs were slower than those of glyR-mediated IPSCs (see Fig. 3C). Similarly to the decay time constant, the 20-80 % rise time of both GABAAR- and glyR-mediated mIPSCs became faster during development (P6-8 vs. P11-13: Mann-Whitney, P < 0.001, Fig. 3C), as previously reported for other neurons (see Brickley et al. 1996; Singer et al. 1998; Legendre, 1999; Ali et al. 2000). No correlation was found between the amplitudes and the 20-80 % rise times of mIPSCs (r2 = 0.02 and 0.01 for GABAAR- and glyR-mediated mIPSCs, respectively), indicating a rather limited space clamp error (Ulrich & Lúscher, 1993).
We conclude that in P5-P13 aMns GABA and glycine are co-released from one-third of spontaneously released inhibitory presynaptic vesicles. The kinetics of both GABAAR- and glyR-mediated mIPSCs become faster during development.
Vestibulo-ocular inhibitory pathway involves co-release of GABA and glycine
Dual mIPSCs were observed in all recorded neurons but their involvement in evoked synaptic transmission was not demonstrated. Pharmacologically isolated monosynaptic IPSCs were evoked by extracellular stimulation in the ipsilateral vestibular nucleus. To address the issue of evoked co-release of GABA and glycine from vestibular afferent fibres, de-synchronization of presynaptic release was induced by substituting extracellular calcium with strontium (n = 5 at P8, Fig. 4). The peak IPSC amplitude decreased to 38 ± 6 % of the control in the presence of strontium (4 mm) and the number of asynchronous mIPSCs exhibiting mono- or bi-exponential decay observed within the first 500 ms after the stimulation increased by a factor of two (213 ± 39 %, n = 5, Fig. 4). The parallel decrease of synchronous release and increase of delayed release indicates that asynchronous activity was evoked by the afferent stimulation (Xu-Friedman & Regher, 2000). Among all asynchronous synaptic currents, 23 ± 3 % (n = 5 cells) exhibited two decay time constants. Dual asynchronous mIPSCs showed an amplitude distribution that was virtually identical to that of dual mIPSCs recorded in the presence of TTX (Mann-Whitney test, P > 0.1), indicating that they correspond to the fusion of single evoked vesicles containing both neurotransmitters. The decay time constants of dual asynchronous mIPSCs were virtually identical to those of spontaneous GABAAR- and glyR- mIPSCs at the same age (see Fig. 3B). We conclude that stimulation of vestibular inhibitory fibres evokes co-release of GABA and glycine from ≈25 % of synaptic vesicles in inhibitory terminals.
Figure 4. Evidence for co-release of GABA and glycine from the stimulated pathway.
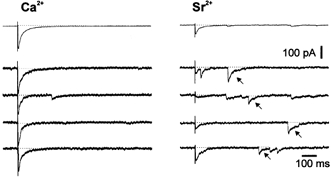
In the presence of external calcium (Ca2+) and flunitrazepam, a 200 pA IPSC exhibiting fast and slow decaying components was evoked by vestibular stimulation (holding potential −80 mV). When Ca2+ was replaced by strontium (Sr2+, 4 mm), the synchronous IPSC amplitude decreased (38 ± 6 %, n = 5) and asynchronous mIPSCs were observed. Among the asynchronous mIPSCs, dual component events were observed (arrows).
Efficacy of GABA and glycine inhibition
What is the functional role of mixed inhibition? We first examined whether the inhibition of motoneuron firing produced by the synergistic action of synaptic co-release of GABA and glycine was more efficient than that produced by the GABAergic or glycinergic system alone. P6-P8 aMns were recorded with a pipette solution containing a low concentration of chloride (4 mm). In these conditions, the reversal potential (E) of evoked IPSPs was −67 ± 6 mV (n = 4). Although the recording conditions might have modified ECl, this reversal potential is only slightly more depolarized than that obtained in P10-P18 neurons recorded with gramicidin (ECl = −73 mV, Singer et al. 1998). Indeed, in brainstem neurons (Singer et al. 1998; see also discussion in Ritter & Zhang, 2001), the developmental shift of ECl towards hyperpolarizing potentials occurs before P9-P10. In any case, ECl was more hyperpolarized than the voltage threshold for action potential (-38 ± 3 mV, n = 7 cells) and slightly more hyperpolarized than the resting membrane potential of these neurons (-59 ± 5 mV).
Motoneuron discharge was fully inhibited by a barrage of IPSPs (100 % inhibition, n = 6 neurons, Fig. 5A and B, upper traces). When the glyR-mediated component of the IPSP was blocked by strychnine, the inhibition of firing was only slightly reduced (74 ± 10 %, n = 3 cells; Fig. 5A and C). In contrast, the inhibition of motoneuron firing dropped to 34 ± 8 % (n = 4 cells) when bicuculline (10 μM) was applied (Figs 5B and D). In the presence of both antagonists, firing was not inhibited (n = 5 cells) and the intrinsic excitability was even slightly increased, as a result of the blockade of tonic inhibition (Brickley et al. 1996). This tonic inhibition would lead to an overestimation of the inhibition mediated by each component and thus its effect was subtracted in each condition. The evoked inhibition was determined in each case (in the presence of strychnine, bicuculline or both) by calculating the percentage of spikes occurring during the time window with and without the synaptic stimulation. These data demonstrate that GABAAR- and glyR-mediated components both determine inhibitory strength. Moreover, GABAAR-mediated inhibition was found to be more powerful than glyR-mediated inhibition (Mann-Whitney test, P < 0.05).
Figure 5. Efficacy of dual inhibition.
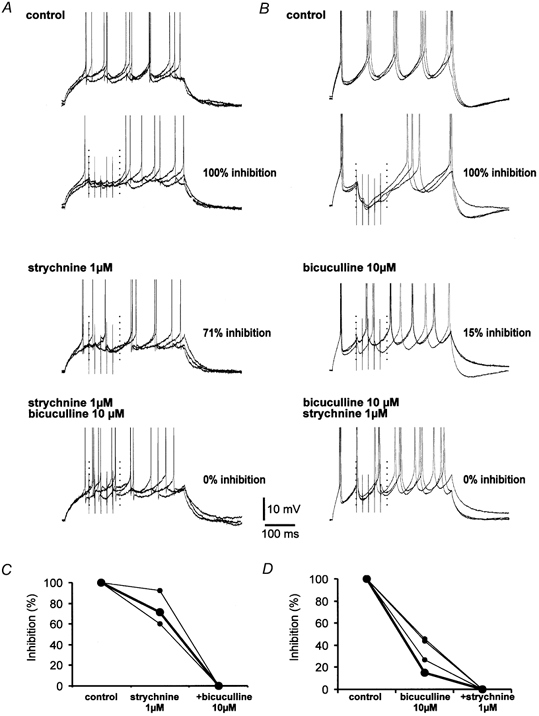
A, pharmacological characterization of the inhibitory power of the IPSP. Motoneuron firing was induced by a depolarizing pulse (≈100 pA, 350-500 ms) at a frequency of 0.3 Hz. For clarity, the APs are truncated. Each second depolarization, the vestibular pathway was activated (five times at 20-50 Hz) in order to test functional inhibition of motoneuron firing. This experimental protocol allowed us to define a control in each experimental condition and to subtract the effect of tonic inhibition due to the blockade of spontaneous and miniature IPSPs. In control conditions, each depolarization evoked five spikes in the motoneuron. The neuron was active during the whole period of depolarization. When the synaptic input was activated, firing was completely abolished (100 % inhibition) in the corresponding stimulation time window (dotted vertical lines, time window ended 30 ms after the last stimulus). All measurements were performed on 40-50 trials. In the presence of strychnine (1 μM), firing in the time window was inhibited to 71 % by GABAAR-mediated IPSPs. When bicuculline (10 μM) was added, the stimulation of the vestibular nucleus did not produce any decrease in the firing activity (0 % inhibition). In some traces, the same depolarizing current even induced six action potentials. B, GlyR-mediated IPSP produced only a weak functional inhibition of aMn firing. In control conditions, the firing of the neuron was fully abolished by activation of the mixed IPSP (100 % inhibition in the defined time window). In the presence of bicuculline (10 μM), firing was inhibited to 15 %. When strychnine was added, the discharge was not inhibited. C, summary of the three experiments in A. D, summary of the four experiments in B. Note that the strychnine-resistant inhibition was much larger than the bicuculline-resistant inhibtion. Large symbols in C and D correspond to the examples illustrated in A and B.
Kinetics of functional inhibition
In addition to the synergy in determining inhibitory strength, the GABAAR- and glyR- mediated components also exhibited temporal complementarity, as a result of their different time courses. Monosynaptic IPSPs were evoked by electrical stimulation in the ipsilateral medial vestibular nucleus. In all recorded motoneurons, we identified a bicuculline-sensitive component and a strychnine-sensitive component, which respectively correspond to GABAAR- and glyR-mediated components (Fig. 6A). The bicuculline-resistant component of the IPSP represented 36 ± 10 % (n = 3 cells) of the control amplitude whereas the strychnine-resistant component accounted for 55 ± 6 % (n = 3 cells), indicating that GABAergic transmission predominates in the vestibulo-ocular inhibitory pathway. The 20-80 % rise time and duration of GABAAR-mediated IPSPs were found to be significantly longer than those mediated by glyRs (20-80 % rise time: 6.2 ± 0.3 ms for GABAAR-mediated IPSPs and 4.1 ± 0.4 ms for glyR-mediated IPSPs, n = 6 cells, duration (onset to end): 240 ± 29 ms for GABAAR-mediated IPSPs and 146 ± 4 ms for glyR-mediated IPSPs, n = 6) (Fig. 3A). We conclude that GABAARs and glyRs mediate IPSPs with slow and fast kinetics, respectively.
Figure 6. Time course of GABAAR- and glyR-mediated inhibition.
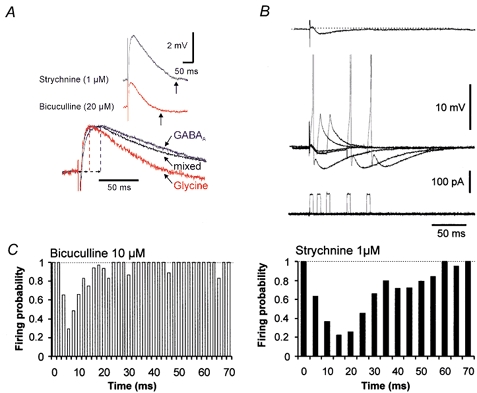
A, kinetics of evoked GABAAR- and glyR-mediated IPSPs recorded with a pipette containing 22 mm Cl−. Vertical arrows indicate the end of the IPSP. Lower traces, scaled glyR-mediated, mixed, and GABAAR-mediated IPSPs. B, protocol for assay of the time window of inhibition by pharmacologically isolated IPSPs recorded with a pipette containing 4 mm Cl−. Single APs were elicited by brief (5 ms) current injections delivered 5-10 times with variable delays after synaptic stimulation (steps of 2 and 5 ms for glyR- and GABAAR-mediated IPSPs, respectively). Typically, the time course of the glycinergic IPSP was determined first since strychnine displayed poor wash out. The recovery of the GABAergic IPSP was usually obtained within 5-10 min of the wash out of bicuculline. The stability of recording was monitored by eliciting, alternately, single APs without synaptic stimulation (not shown). AP generation was inhibited when the depolarizing current pulse was delivered at the peak of the IPSP. C, left, time course of the inhibitory power of glyR-mediated IPSPs (n = 3). The peak of inhibition (firing probability of 0.29 ± 0.18) was obtained at 6 ms. Right, time course of the inhibitory power of GABAAR-mediated IPSPs (n = 3). The peak of inhibition (firing probability of 0.22 ± 0.11) was obtained at 15 ms.
The temporal windows of inhibition induced by evoked GABAAR- and glyR-mediated IPSPs were determined. Hyperpolarizing IPSPs were recorded with a pipette containing 4 mm Cl−. The 20-80 % rise time of the GABAAR-mediated component of the IPSP was still slower than that of the glyR-mediated component (respectively 4.7 ± 0.6 ms and 1.5 ± 0.08 ms, n = 3 cells). The stimulus intensity was adjusted to obtain pharmacologically isolated IPSPs of ≈1 mV at the peak (Fig. 6B). These small IPSPs were, however, sufficient to block the generation of single action potentials (APs) elicited by brief depolarizing current pulses (5 ms, ≈100 pA; Figs 6B and C; n = 3), in ≈75 % of the trials. APs were evoked with various delays from the onset of field stimulation in order to assay the total duration of the evoked IPSP. The peak of inhibition was 6 ms for glyR-mediated IPSPs and 15-20 ms for GABAAR-mediated IPSPs. These values are very close to those reported for strychnine-sensitive and strychnine-insensitive components of the recurrent inhibition in α-motoneurons (Cullheim & Kellerth, 1981). The time window for inhibition was found to be limited to ≈20 ms for glyR-mediated IPSPs but extended up to 60 ms for GABAAR-mediated IPSPs (Fig. 6C). We conclude that co-release of GABA and glycine allows inhibition of motoneuron firing with complementary time courses: sustained inhibition by GABA and fast and transient inhibition by glycine.
Non-linear interactions between GABAAR- and glyR-mediated IPSCs
In our experiments the contribution of the GABAAR-mediated component in evoked IPSPs was found to vary between 40 and 85 %. Using a modelling approach, we examined how the kinetics of the resulting compound IPSP changed as a result of interactions between GABAAR- and glyR-activated currents. As a first approximation, we considered two extreme cases in which the peak conductances of the GABAergic components were 40 and 85 %. The IPSP was found to be fast if the glyR-activated conductance accounted for 60 % of the synaptic response and slow if the GABAAR-activated conductance predominated (85 %, Fig. 7A; total peak conductance of 5 nS). Although the IPSP amplitude remained constant, important modifications in its shape were observed. The half-width of the resulting IPSP was calculated for variable fractional contributions of the GABAAR postsynaptic conductance (Fig. 7B). It was found that the sensitivity of the IPSP half-width to the relative ratio of GABAAR/glyR conductance depended on the value of the postsynaptic conductance. For a large postsynaptic conductance (10 nS), IPSP half-width varied between 24 and 64 ms, for a fractional contribution of GABAAR conductance varying from 0 to 100 %. For a weak postsynaptic conductance (2.5 nS), the IPSP half-width was less affected by the relative composition of the current and varied between 20.5 and 46 ms (Fig. 7B).
Figure 7. Simulation of the non-linear interactions between GABAAR- and glyR-activated currents.
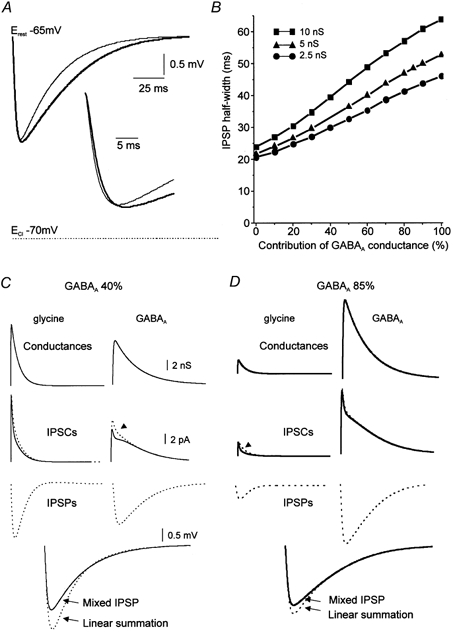
The resting membrane potential is −65 mV and the reversal potential for Cl− is −70 mV. A, time courses of mixed IPSPs depend on the proportion of each component. Thin trace, IPSP with 40 % of GABAAR-activated conductance. Thick trace, IPSP with 85 % GABAAR-activated conductance (maximum of the postsynaptic compound conductance is 5 nS). B, half-width of compound IPSPs as a function of the fractional contribution of GABAAR for three maximal postsynaptic conductances. Modifications of IPSP shape were more limited for small postsynaptic conductances than for large postsynaptic conductances. C and D, comparison of compound IPSPs (continuous lines) and linearly summated IPSPs (dashed lines) for 40 % (C) and 85 % (D) GABAAR-activated conductances (first line). In each case, the compound IPSC can be resolved into a glyR-activated (continuous line, left column) and a GABAAR-activated component (continuous line, right column). The superimposed dashed traces represent the individual IPSCs produced by the same postsynaptic conductance (same number of channels) without the contribution of the complementary currents. In the mixed IPSC, the GABAAR-activated IPSC is reduced by the strong glyR-activated current whereas the glyR-activated current exhibits only a small reduction (arrowhead). Synaptic potentials calculated from these individual IPSCs are shown as dashed traces. Their numerical summation (linear summation) is superimposed on the IPSP resulting from the mixed IPSCs (mixed IPSP). Note that the difference between the continuous and dashed IPSPs is larger for the IPSPs to which GABA and glycine contribute almost equally (C) than for an almost pure GABAAR-mediated IPSP (D).
The observed differences not only reflect the kinetics of the two underlying synaptic currents, but also point to complex interactions between these currents. We compared the glyR- and GABAAR-activated responses in the case of an independent activation and during co-activation of both channels (Figs 7C and D). As in Fig. 7A, the total peak conductance was 5 nS. Pure GABAAR- and pure glyR-activated conductances were set in two different configurations (40 and 60 % in Fig. 7C, and 85 and 15 % in Fig. 7D). Postsynaptic currents and potentials were calculated in two ways. First, they were calculated independently for each component (dashed IPSCs and dashed IPSPs in Figs 7C and D). In a second step, IPSCs and IPSPs were calculated during co-activation of both types of receptor. Compound IPSCs were resolved into glyR- and GABAAR-activated components (continuous IPSCs in Figs 7C and D). For IPSPs with a small GABAAR-mediated component (40 %), the hyperpolarization induced by the glyR-mediated component prevented the full development of the GABAAR-mediated IPSC (arrowhead in Fig. 7C). Although weaker, a reciprocal effect on the glycine current was also seen. In the absence of this mutual inhibition, the IPSCs produced by the same postsynaptic conductances were found to be larger (dashed current traces in Fig. 7C). The linear summation of the GABAAR- and glyR-mediated IPSPs corresponding to these individual IPSCs was found to be significantly larger than the compound IPSP (Fig. 7C). These results reveal the non-linear interactions in GABA-glycine IPSPs.
For IPSPs expressing a large GABAAR-mediated component (85 %), the glyR-activated current was reduced in its late phase; the GABAAR-activated current was also reduced to a lesser extent (see superimposed IPSPs in Fig. 7D). Compared to the previous case, the non-linearity was found to be smaller, indicating that it depends on the fractional contribution of each component. We conclude that variations in the relative proportion of the two inhibitory systems significantly influence the shape of the resulting IPSP, in a non-linear manner.
Role of ECl and membrane time constant on simulated GABAAR- and glyR-mediated IPSPs
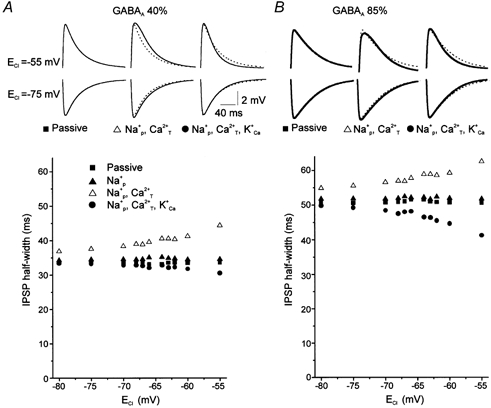
During postnatal development, the reversal potential for chloride shifts from depolarization to hyperpolarization in brainstem motoneurons (Singer et al. 1998). We therefore examined the incidence of a shift of ECl on the kinetics of GABA-glycine IPSP. In all cases, the IPSC kinetics and the morphology of the neuron were kept constant. In a passive model, variation of ECl from −55 mV to −80 mV did not substantially affect the IPSP half-width except for values of ECl in the vicinity of the resting potential (▪ in Figs 8A and B). In the presence of the persistent sodium and the T-type calcium currents, the IPSP half-width however increased for depolarizing ECl (▵ in Figs 8A and B). Addition of K+Ca current in the model shortened the IPSP half-width (• in Figs 8A and B). We conclude that with constant IPSC kinetics, the developmental shift of ECl has important influence on the shape of the resulting IPSP through the activation of intrinsic conductances.
Figure 8.
Role of the reversal potential on the IPSP half-width
A and B IPSP half-width as a function of E Cl in passive and active membranes for two fractional contributions of GABAAR-activated currents (A,40 %;B,85 %). The active membrane contains voltage gated Na+ and K+ conductances (see Methods)in each case but variable expression of sub-threshold conductances (persistent Na+ (Na+p) T-type Ca2+ (Ca2+T) and K+Ca conductances). Four types of modelled membrane have been considered (▪,passive membrane; ▴,active membrane with Na+P; ▵,active membrane with Na+P and Ca2+T; O,active membrane with all conductances (Na+P Ca2+T K+Ca)). Inset,IPSP time courses for depolarizing and hyperpolarizing ECl (ECl =−55 mV and −75 mV,respectively)in different configurations. With the passive model, the IPSP half-width of depolarizing IPSPs was identical to that of hyperpolarizing IPSPs. Addition of Na+P and Ca2+T conductances increased the IPSP half-width for depolarized E Cl (dotted lines represent the passive IPSPs). Addition of K+Ca conductance decreased IPSP half-width.
During development, the membrane time constant (τ) of brainstem motoneurons becomes faster in parallel with a decrease in the input resistance (Rin) (Singer et al. 1998; Cameron et al. 2000). We therefore examined how these two parameters could determine the shape of the GABAAR- and glyR-mediated IPSPs. In particular, we examined whether slow τm could reduce differences in the kinetics of GABAAR- and glyR-mediated IPSPs. The amplitude and kinetics of the postsynaptic conductance (2.5 nS, Fig. 9A) were maintained constant but three values of τm and Rin corresponding to the normal development of brainstem motoneurons (Singer et al. 1998) were considered (τm = 40 ms and Rin = 500 MΩ; τm = 23 ms and Rin = 250 MΩ; τm = 11 ms and Rin = 78 MΩ) (see Fig. 9B). The results show that whatever the values of τm and Rin, the kinetics of GABAAR- and glyR-mediated IPSPs were clearly distinguishable (Fig. 9), thus suggesting that the temporal complementarity of GABAAR- and glyR-mediated IPSPs can be seen at all postnatal stages.
Figure 9. Role of Rin and τm in shaping simulated GABAAR-and glyR-like IPSPs.
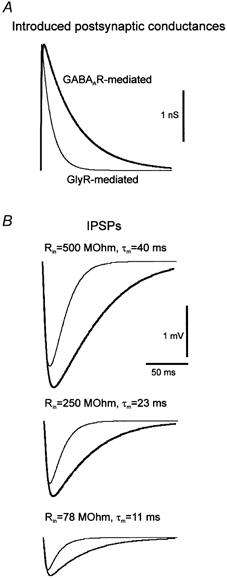
A, time course of the postsynaptic GABAAR-(thick trace)and glyR-like (thin trace)conductances. B,IPSPs for three sets of values of τm and Rin (from the top to the bottom, τm =40 ms and Rin =500 MΩ; τm =23 ms and Rin =250 MΩ; τm =11 ms and Rin =78MΩ). Note that in all cases kinetics are different for GABAAR-(thick trace)and glyR-like (thin trace)IPSPs.
DISCUSSION
Co-release of GABA and glycine
We have shown that GABA and glycine are co-released from one-third of inhibitory presynaptic vesicles and that both neurotransmitters are involved in the functional inhibition of P5-P13 aMns. The proportion of dual release was found to be largely independent of the age of the rats as observed during the early development of spinal neurons (Gao et al. 2001). The remaining inhibition was mediated by vesicular release of GABA and glycine alone. The proportion of pure GABAergic and pure glycinergic mIPSCs has been evaluated and is close to one-third. This estimation must be qualified in two ways. First, the exclusion of unstable data favours fast glycinergic events because traces are more likely to be stable for short periods of time than for long periods. Second, glycinergic events are on average larger than GABAergic events and detection of small GABAergic events may have been underestimated. This estimation is, however consistent with the proportion of terminals that are immunoreactive for GABA and for glycine at the ultra-structural level (Lahjouji et al. 1996). The proportion of co-release found here is also consistent with a previous immunocytochemical study showing that 30 % of the inhibitory presynaptic terminals to adult rat aMns contained GABA and glycine (Lahjouji et al. 1996). One attractive possibility is that only co-release of GABA and glycine occurs specifically at these terminals, although this remains to be demonstrated. In addition, we showed that GABA and glycine co-release was involved in an identified inhibitory pathway originating from the medial vestibular nucleus. These findings are consistent with previous immunocytochemical studies showing that in adult cats GABA and glycine co-localize in some medial vestibular neurons (Walberg et al. 1990).
Predominance of GABAergic over glycinergic transmission
In contrast to lamina I spinal neurons and other brainstem neurons (Jonas et al. 1998; Chery & De Koninck, 1999; Smith et al. 2000; Gao et al. 2001; Keller et al. 2001), inhibition mediated by GABAARs predominates in developing aMns. This dominance was seen with exogenously applied transmitter, in evoked synaptic responses and in the functional inhibition. A significant contribution of GABAARs to the synaptic response can be observed in auditory brainstem neurons and in spinal neurons but the GABAAR-mediated component declines during the first post-natal days (Kotak et al. 1998; Smith et al. 2000; Gao et al. 2001; Keller et al. 2001). For instance, inhibition is entirely mediated by glyRs in inferior olive neurons after P6 (Smith et al. 2000). In other cell types such as neurons from the lamina III of the spinal cord, the situation is rather different: GABAergic transmission persists even at the adult stage (Chéry & De Koninck, 1999). Thus, the developmental shift from GABAergic to glycinergic transmission observed in some neuronal types (Kotak et al. 1998; Smith et al. 2000; Gao et al. 2001; Keller et al. 2001) could non-uniformly affect the different nuclei in the spinal cord and brainstem. Alternatively, the substantial amount of GABAergic transmission remaining at the adult stage in some nuclei could result from initially elevated levels of GABAergic transmission in certain neurons despite a uniform developmental shift towards glycinergic inhibition.
Although such a developmental shift cannot be excluded, GABAergic transmission is clearly involved in inhibition of adult aMns. First, GABAergic transmission is functional in the vestibulo-ocular pathway in vivo (Precht et al. 1973). Second, GABA is present in ≈60 % of inhibitory presynaptic terminals impinging onto adult rat aMns (Lahjouji et al. 1996). Finally, immunostaining of the α1 subunit of the GABAA receptor is co-localized with that of the α1 subunit of the glycine receptor in adult aMns (Russier et al. 2001). Taken together these data strongly suggest that the co-inhibition and co-release we have reported in immature and juvenile aMns (up to P13) may persist in adult aMns. Further work will be required to directly address this issue after overcoming the technical difficulty of visualizing and recording from aMns in rats over 14 days old.
In contrast to exogenously applied transmitter and evoked IPSPs, GABAAR-mediated mIPSCs had smaller amplitudes than glyR-mediated mIPSCs. A similar difference in mIPSCs amplitude has been reported in hypoglossal motoneurons (Donato & Nistri, 2000). The unitary conductance of the GABAAR-channel is on average half that of the glyR-channel (Bormann et al. 1987; Takahashi & Momiyama, 1991) whereas the maximum channel-receptor open probability is rather similar [Po,max = 0.7 with 1 mm glycine (Singer & Berger, 1999) and 0.6 with 3 mm GABA (Perrais & Ropert, 1999)]. Although synaptic receptors might not be fully saturated by the neurotransmitters (Perrais & Ropert, 1999; Suwa et al. 2001), these results support the hypothesis that synaptic and/or extrasynaptic GABAARs are more numerous than glyRs in aMns. Alternatively, extrasynaptically located GABAergic channels could have a higher open probability than synaptic GABAAR channels. Extrasynaptic or perisynaptic GABAAR could be activated by GABA spillover, as suggested previously in the spinal cord (Chéry & De Koninck, 1999; Keller et al. 2001). Thus, the possibility cannot be excluded that the temporal complementarity of GABA-glycine co-inhibition could also result from GABA spillover out of the synaptic cleft. In addition, the discrepancy between the amplitude of the current and the potential may also arise from the filtering of fast glycinergic currents by the postsynaptic membrane. The simulations illustrated in Fig. 9 indeed show that at equal peak conductance, a glycinergic-like conductance produces a smaller IPSP than a slower GABAA-like conductance does.
Functional complementarity of GABA and glycine co-inhibition
Although release from separate vesicles participates in co-inhibition, co-release of GABA and glycine from single presynaptic vesicles allows at least two levels of functional complementarity.
First, the strength of firing inhibition was increased when both inhibitory systems were active. GABA and glycine IPSCs do not, however, summate linearly. This non-linear summation was evidenced by comparing the simulated glyR- and GABAAR-activated responses in the case of an independent activation and during co-activation of both channels. Our model predicts that the linear summation of the GABAAR and glyR-mediated IPSPs corresponding to individual IPSCs will be significantly larger than the compound IPSP. This sub-linear summation will in part be observed because of the higher conductance (shunting effect) in the case of compound IPSC/Ps, but also because both channels are mainly permeable to a single ion (Cl−) with a reversal potential close to the membrane potential. Indeed, the hyperpolarization of the postsynaptic membrane due to the glyR-mediated component reduces the driving force for chloride and thus prevents the full development of the GABAAR-activated response. The model shows that these non-linear interactions profoundly shape the resulting IPSP, especially when the IPSP involves an almost equal contribution of GABAAR- and glyR-mediated components.
Second, we provide evidence that the two types of inhibition are kinetically complementary. As previously reported for mIPSCs (Jonas et al. 1998; Oapos; Brien & Berger, 1999) and minimally evoked IPSCs (Donato & Nistri, 2000), we found that glycinergic IPSC/Ps have faster rise times and decay times than GABAergic IPSC/Ps. Thus, the glycinergic component allows a millisecond-scale inhibition whereas the GABAergic component is involved in sustained inhibition. The kinetics of both GABAAR- and glyR-mediated IPSCs, however, become faster during development as reported previously in rodent motoneurons (Krupp et al. 1994; Singer et al. 1998; Smith et al. 2000). In addition, our modelling study shows that the developmental shift in ECl from depolarized (-55 mV) to hyperpolarized potentials (-80 mV) could also participate in the shaping of synaptic potentials through the activation of intrinsic conductances. It is important to note that this process would only slightly counteract the developmental acceleration of both IPSC kinetics and membrane time constants (Singer et al. 1998).
During development, the membrane time constant of brainstem motoneurons becomes faster in parallel with a decrease in the input resistance (Singer et al. 1998; Cameron et al. 2000). The reduction of the membrane resistance results from an increase in membrane surface area combined with the expression of ionic channels and/or synaptic inputs (review in Cameron & Núñez-Abades, 2000). We simulated the results of normal development by reducing both the input resistance and the membrane time constant in our model. In the different states, significant differences in the rise time and decay time were seen between simulated GABAAR- and glyR-like IPSPs. The IPSP amplitude produced by a fast glycinergic conductance was found to be smaller than that produced by a slow GABAergic conductance as a result of the filtering properties of the membrane (see Johnston & Miao-Sin Wu, 1995). One may note that the kinetics of the postsynaptic conductance and the size of the motoneuron (here the cell body) remained unchanged. Similar differences between the time course of simulated IPSPs have been reported with the injection of IPSC-like current in brainstem and adult motoneurons in vitro (Poliakov et al. 1997; Singer et al. 1998). Thus, taken together, these data confirm that the temporal complementarity of GABAAR- and glyR-mediated inhibition will still be observed independently of the age of development.
The deactivation phase of both GABAAR- (Mellor & Randall, 1998) and glyR-mediated IPSCs (Legendre, 1999) is voltage-dependent but its contribution to shaping the IPSP is expected to be negligible. Indeed, between the resting membrane potential (≈-70 mV) and the spike threshold (-38 mV), the maximal variation of the decay is less than 5 % for GABAAR-mediated IPSCs (Mellor & Randall, 1998) and less than 15 % for glyR-mediated IPSCs (Δτfast ≈ 0.5-1 ms, Legendre 1999). Temporally synergistic inhibition involving GABAARs and GABABRs has been reported in other brain areas such as the cortex or the hippocampus, but the synergistic function of GABA and glycine in motoneurons is unique in the nervous system. In aMns, the cooperative action of the two systems operates at the single vesicle level and does not require the exclusive activation of extrasynaptic receptors by spillover, as demonstrated for GABABRs at hippocampal inhibitory synapses (Scanziani, 2000).
Compared to independent release of two neurotransmitters by different vesicles, co-release of two neurotransmitters confers several advantages. Vesicular release is a stochastic process (Fatt & Katz, 1952) and co-release of two neurotransmitters would ensure reliability of complementary functions that could not be achieved with the same temporal accuracy by independent release from different vesicles. In addition, the location of the postsynaptic inhibition mediated by the co-release of two neurotransmitters is focal and could favour inhibition at a strategic point such as the initial segment of the motoneuron (Lahjouji et al. 1997).
Acknowledgments
We thank Dr T. Bal, Dr H. Bras, Dr L. Cathala, G. Daoudal, Dr A. Destexhe, Dr J. Durand, Professor M. Scanziani, V. Sourdet, Dr S. Tyc-Dumont and Dr P. P. Vidal for helpful discussions and G. Sadoc for help in mIPSC analysis. We thank Dr Y. Frégnac, Professor B. H. Gähwiler, Dr U. Gerber and Dr M. Seagar for helpful comments on the manuscript and L. Fronzaroli for excellent technical assistance. This work was supported by CNRS, the Fondation pour la Recherche Médicale (doctoral fellowship to M.R. and grant to D.D.), the Ministère de la Recherche et de la Technologie (A.C.I. grant to D.D.) and INSERM (‘Avenir’ grant to D.D.).
REFERENCES
- Ali DW, Drapeau P, Legendre P. Development of spontaneous glycinergic currents in the Mauthner neuron of the zebrafish embryo. Journal of Neurophysiology. 2000;84:1726–1736. doi: 10.1152/jn.2000.84.4.1726. [DOI] [PubMed] [Google Scholar]
- Angulo MC, Staiger JF, Rossier J, Audinat E. Developmental changes increase the range of integrative capabilities of an identified excitatory neocortical connection. Journal of Neuroscience. 1999;19:1566–1576. doi: 10.1523/JNEUROSCI.19-05-01566.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aradi I, Holmes WR. Role of multiple calcium and calcium-dependent conductances in regulation of hippocampal dentate granule cell excitability. Journal of Computational Neuroscience. 1999;6:215–235. doi: 10.1023/a:1008801821784. [DOI] [PubMed] [Google Scholar]
- Bormann J, Hamill OP, Sakmann B. Mechanism of anion permeation through channels gated by glycine and gamma-aminobutyric acid in mouse cultured spinal neurones. Journal of Physiology. 1987;385:243–286. doi: 10.1113/jphysiol.1987.sp016493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brickley SG, Cull-Candy SG, Farrant M. Development of a tonic form of synaptic inhibition in rat cerebellar granule cells resulting from persistent activation of GABAA receptors. Journal of Physiology. 1996;497:753–759. doi: 10.1113/jphysiol.1996.sp021806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brickley SG, Cull-Candy SG, Farrant M. Single-channel properties of synaptic and extrasynaptic GABAA receptors suggest differential targeting of receptor subtypes. Journal of Neuroscience. 1999;19:2960–2973. doi: 10.1523/JNEUROSCI.19-08-02960.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brodin L, Traven HG, Lansner A, Wallen P, Ekeberg O, Grillner S. Computer simulations of N-methyl-D-aspartate receptor-induced membrane properties in a neuron model. Journal of Neuroscience. 1991;66:473–484. doi: 10.1152/jn.1991.66.2.473. [DOI] [PubMed] [Google Scholar]
- Burger PM, Hell J, Mehl E, Krasel C, Lottspeich F, Jahn R. GABA and glycine in synaptic vesicles: storage and transport characteristics. Neuron. 1991;7:287–293. doi: 10.1016/0896-6273(91)90267-4. [DOI] [PubMed] [Google Scholar]
- Cameron WE, Núñez-Abades PA. Physiological changes accompanying anatomical remodelling of mammalian motoneurons during postnatal development. Brain Research Bulletin. 2000;53:523–527. doi: 10.1016/s0361-9230(00)00385-3. [DOI] [PubMed] [Google Scholar]
- Cameron WE, Núñez-Abades PA, Kerman IA, Hodgson TM. Role of potassium conductances in determining input resistance of developing brainstem motoneurons. Journal of Neurophysiology. 2000;84:2330–2339. doi: 10.1152/jn.2000.84.5.2330. [DOI] [PubMed] [Google Scholar]
- Chaudhry FA, Reimer RJ, Bellocchio EE, Danbolt NC, Osen KK, Edwards RH, Storm-Mathisen J. The vesicular GABA transporter, VGAT, localizes to synaptic vesicles in sets of glycinergic as well as GABAergic neurons. Journal of Neuroscience. 1998;18:9733–9750. doi: 10.1523/JNEUROSCI.18-23-09733.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chéry N, De Koninck Y. Junctional versus extrajunctional glycine and GABA(A) receptor- mediated IPSCs in identified lamina I neurons of the adult rat spinal cord. Journal of Neuroscience. 1999;19:7342–7355. doi: 10.1523/JNEUROSCI.19-17-07342.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cullheim S, Kellerth J-O. Two kinds of recurrent inhibition of cat spinal α-motoneurones as differentiated pharmacologically. Journal of Physiology. 1981;312:209–224. doi: 10.1113/jphysiol.1981.sp013624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donato R, Nistri A. Relative contribution by GABA or glycine to Cl−-mediated synaptic transmission on rat hypoglossal motoneurons in vitro. Journal of Neurophysiology. 2000;84:2715–2724. doi: 10.1152/jn.2000.84.6.2715. [DOI] [PubMed] [Google Scholar]
- Durand J. Synaptic excitation triggers oscillations during NMDA receptor activation in rat abducens motoneurons. European Journal of Neuroscience. 1993;5:1389–1397. doi: 10.1111/j.1460-9568.1993.tb00925.x. [DOI] [PubMed] [Google Scholar]
- Fatt P, Katz B. Spontaneous subthreshold activity at motor nerve endings. Journal of Physiology. 1952;117:109–128. [PMC free article] [PubMed] [Google Scholar]
- Gao BX, Stricker C, Ziskind-Conhaim L. Transition from GABAergic to glycinergic synaptic transmission in newly formed spinal networks. Journal of Neurophysiology. 2001;86:492–502. doi: 10.1152/jn.2001.86.1.492. [DOI] [PubMed] [Google Scholar]
- Gasnier B. The loading of neurotransmitters into synaptic vesicles. Biochimie. 2000;82:327–337. doi: 10.1016/s0300-9084(00)00221-2. [DOI] [PubMed] [Google Scholar]
- Jo YH, Schlichter R. Synaptic corelease of ATP and GABA in cultured spinal neurons. Nature Neuroscience. 1999;2:241–245. doi: 10.1038/6344. [DOI] [PubMed] [Google Scholar]
- Johnston D, Miao-Sin Wu S. Foundations of Cellular Neurophysiology. Cambridge, MA, USA: MIT Press; 1995. [Google Scholar]
- Jonas P, Bischofberger J, Sandkuhler J. Corelease of two fast neurotransmitters at a central synapse. Science. 1998;281:419–424. doi: 10.1126/science.281.5375.419. [DOI] [PubMed] [Google Scholar]
- Keller AF, Coul JAM, Chéry N, Poisbeau P, De Koninck Y. Region specific developmental specialization of GABA-glycine cosynapses in laminas I-II of the rat spinal dorsal horn. Journal of Neuroscience. 2001;21:7871–7880. doi: 10.1523/JNEUROSCI.21-20-07871.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopysova IL, Korogod SM, Durand J, Tyc-Dumont S. Local mechanisms of phase-dependent postsynaptic modifications of NMDA-induced oscillations in the abducens motoneurons: a simulation study. Journal of Neurophysiology. 1996;76:1015–1024. doi: 10.1152/jn.1996.76.2.1015. [DOI] [PubMed] [Google Scholar]
- Kotak V, Korada S, Schwartz IR, Sanes DH. A developmental shift from GABAergic to glycinergic transmission in the central auditory system. Journal of Neuroscience. 1998;18:4646–4655. doi: 10.1523/JNEUROSCI.18-12-04646.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krupp J, Larmet Y, Feltz P. Postnatal change of glycinergic IPSC decay in sympathetic preganglionic neurons. NeuroReport. 1994;5:2437–2440. doi: 10.1097/00001756-199412000-00008. [DOI] [PubMed] [Google Scholar]
- Lahjouji F, Barbe A, Chazal G, Bras H. Evidence for colocalization of GABA and glycine in afferents to retrogradely labelled rat abducens motoneurones. Neuroscience Letters. 1996;206:161–164. doi: 10.1016/s0304-3940(96)12465-4. [DOI] [PubMed] [Google Scholar]
- Lahjouji F, Bras H, Barbe A, Chmykhova N, Chazal G. Electron microscopic serial analysis of GABA presynaptic terminals on the axon hillock and initial segment of labeled abducens motoneurons in the rat. Neuroscience Research. 1997;27:143–153. doi: 10.1016/s0168-0102(96)01142-x. [DOI] [PubMed] [Google Scholar]
- Legendre P. Voltage dependence of the glycine receptor-channel kinetics in the zebrafish hindbrain. Journal of Neurophysiology. 1999;82:2120–2129. doi: 10.1152/jn.1999.82.5.2120. [DOI] [PubMed] [Google Scholar]
- Lipowsky R, Gillsessen T, Alzheimer C. Dendritic Na+ channels amplify EPSPs in hippocampal CA1 pyramidal cells. Journal of Neurophysiology. 1996;76:2181–2191. doi: 10.1152/jn.1996.76.4.2181. [DOI] [PubMed] [Google Scholar]
- Mellor JR, Randall AD. Frequency-dependent actions of benzodiazepines on GABAA receptors in cultured murine cerebellar granule cells. Journal of Physiology. 1997;503:353–369. doi: 10.1111/j.1469-7793.1997.353bh.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellor JR, Randall AD. Voltage-dependent deactivation and desensitisation of GABA response in cultured murine cerebellar granule cells. Journal of Physiology. 1998;506:377–390. doi: 10.1111/j.1469-7793.1998.377bw.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Brien JA, Berger AJ. Cotransmission of GABA and glycine to brain stem motoneurons. Journal of Neurophysiology. 1999;82:1638–1641. doi: 10.1152/jn.1999.82.3.1638. [DOI] [PubMed] [Google Scholar]
- Perrais D, Ropert N. Effect of zolpidem on miniature IPSCs and occupancy of postsynaptic GABAA receptors in central synapses. Journal of Neuroscience. 1999;19:578–588. doi: 10.1523/JNEUROSCI.19-02-00578.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poliakov AV, Powers RK, Binder MD. Functional identification of the input-output transforms of motoneurones in the rat and cat. Journal of Physiology. 1997;504:401–424. doi: 10.1111/j.1469-7793.1997.401be.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pongracz F, Poolos NP, Kocsis JD, Shepherd GM. A model of NMDA receptor-mediated activity in dendrites of hippocampal CA1 pyramidal neurons. Journal of Neurophysiology. 1992;68:2248–2259. doi: 10.1152/jn.1992.68.6.2248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Precht W, Baker R, Okada Y. Evidence for GABA as the synaptic transmitter of the inhibitory vestibulo-ocular pathway. Experimental Brain Research. 1973;18:415–428. doi: 10.1007/BF00239109. [DOI] [PubMed] [Google Scholar]
- Ritter B, Zhang W. Early postnatal maturation of GABAA-mediated inhibition in the brainstem respiratory rhythm-generating network of the mouse. European Journal of Neuroscience. 2001;12:2975–2984. doi: 10.1046/j.1460-9568.2000.00152.x. [DOI] [PubMed] [Google Scholar]
- Russier M, Barbe A, Bras H. Colocalisation des récepteurs GABAA et de la glycine sur la membrane postsynaptique des motoneurones abducens de rat: une étude en microscopie confocale. Société des Neurosciences. 2001;5:242. [Google Scholar]
- Salter MW, De Koninck Y. An ambiguous fast synapse: a new twist in the tale of two transmitters. Nature Neuroscience. 1999;2:199–200. doi: 10.1038/6296. [DOI] [PubMed] [Google Scholar]
- Scanziani M. GABA spillover activates postsynaptic GABAB receptors to control rhythmic hippocampal activity. Neuron. 2000;25:673–681. doi: 10.1016/s0896-6273(00)81069-7. [DOI] [PubMed] [Google Scholar]
- Singer JH, Berger AJ. Contribution of single-channel properties to the time course and amplitude variance of quantal glycine currents recorded in rat motoneurons. Journal of Neurophysiology. 1999;81:1608–1616. doi: 10.1152/jn.1999.81.4.1608. [DOI] [PubMed] [Google Scholar]
- Singer JH, Talley EM, Bayliss DA, Berger AJ. Development of glycinergic synaptic transmission to rat brain stem motoneurons. Journal of Neurophysiology. 1998;80:2608–2620. doi: 10.1152/jn.1998.80.5.2608. [DOI] [PubMed] [Google Scholar]
- Smith AJ, Owens S, Forsythe ID. Characterisation of inhibitory and excitatory postsynaptic currents of the rat medial superior olive. Journal of Physiology. 2000;529:681–698. doi: 10.1111/j.1469-7793.2000.00681.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer RF, Wenthold RJ, Baker R. Evidence for glycine as an inhibitory neurotransmitter of vestibular. reticular, and prepositus hypoglossi neurons that project to the cat abducens nucleus. Journal of Neuroscience. 1989;9:2718–2736. doi: 10.1523/JNEUROSCI.09-08-02718.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suwa H, Saint-Amant L, Triller A, Drapeau P, Legendre P. High-affinity zinc potentiation of inhibitory postsynaptic glycinergic currents in the zebrafish hindbrain. Journal of Neurophysiology. 2001;85:912–925. doi: 10.1152/jn.2001.85.2.912. [DOI] [PubMed] [Google Scholar]
- Takahashi T. The minimal inhibitory synaptic currents evoked in neonatal rat motoneurones. Journal of Physiology. 1992;450:593–611. doi: 10.1113/jphysiol.1992.sp019145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi T, Momiyama A. Single-channel currents underlying glycinergic inhibitory postsynaptic responses in spinal neurons. Neuron. 1991;7:965–969. doi: 10.1016/0896-6273(91)90341-v. [DOI] [PubMed] [Google Scholar]
- Ulrich D, Lúscher HR. Miniature excitatory synaptic currents corrected for dendritic cable properties reveal quantal size and variance. Journal of Neurophysiology. 1993;69:1769–1773. doi: 10.1152/jn.1993.69.5.1769. [DOI] [PubMed] [Google Scholar]
- Umemiya M, Berger AJ. Properties and function of low- and high-voltage-activated Ca2+ channels in hypoglossal motoneurons. Journal of Neuroscience. 1994;14:5652–5660. doi: 10.1523/JNEUROSCI.14-09-05652.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walberg F, Ottersen OP, Rinvik E. GABA, glycine, aspartate, glutamate, and taurine in the vestibular nuclei: an immunocytochemical investigation in the cat. Experimental Brain research. 1990;79:547–563. doi: 10.1007/BF00229324. [DOI] [PubMed] [Google Scholar]
- Xu-Friedman MA, Regehr WG. Probing fundamental aspects of synaptic transmission with strontium. Journal of Neuroscience. 2000;20:4414–4422. doi: 10.1523/JNEUROSCI.20-12-04414.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]


