Abstract
Membrane and discharge properties determine the input-output relationship of neurones and are therefore of paramount importance for the functions of neural circuits. Here, we have tested the hypothesis that neurones in different laminae of the spinal dorsal horn differ in their electrophysiological properties. Whole-cell patch-clamp recordings from dorsal horn neurones in a rat transverse spinal cord slice preparation were used to record active and passive membrane properties. Neurones from superficial dorsal horn laminae had higher membrane resistances and broader action potentials than deep dorsal horn neurones. Action potential thresholds were highest in lamina II neurones, representing low membrane excitability. Five types of firing patterns were identified in response to depolarising current injections. Tonic-firing neurones discharged action potentials at regular intervals throughout the current pulse. Delayed-firing neurones showed a delayed onset of firing in response to current injections that was due to activation of a transient voltage-dependent outward current, presumably an A-current. Another group of neurones fired a short initial burst of action potentials. Single-spiking neurones discharged only one action potential at the onset of a depolarising pulse. Phasic-bursting neurones showed irregular bursts of action potentials. Firing patterns were unequally distributed among laminae. Tonic-firing neurones were numerous in lamina I and deeper laminae but were not found in lamina II. Delayed-firing neurones were encountered in laminae I and II but not in deeper laminae. Most of the neurones showing an initial burst were found in lamina II. These differences in membrane and discharge properties probably contribute to lamina-specific processing of sensory, including nociceptive, information.
Neurones in the central nervous system display a variety of different membrane and discharge properties that modulate the transmission of afferent information to higher-order neurones. In spinal dorsal horn, sensory information from primary afferent Aα/β-, Aδ- and C-fibres is processed by different types of second- and higher-order neurones that relay information to supraspinal structures or to other spinal neurones, e.g. inhibitory interneurones or motoneurones. Based on histological criteria, the rat spinal dorsal horn has been divided into six layers (Molander et al. 1984) similar to the cat spinal dorsal horn (Rexed, 1952). It is now generally accepted that these histological laminae also reflect functional organisation, as termination patterns of Aα/β-, Aδ- and C-fibres, sensory input to dorsal horn neurones and neurotransmitters and their receptors are lamina-specifically distributed (Woolf & Fitzgerald, 1986; Gouarderes et al. 1991; Todd & Spike, 1993; Bohlhalter et al. 1996). However, it is not known if sensory information is encoded in a lamina-specific way by differential membrane and discharge properties. On the other hand, previous studies on membrane properties of spinal dorsal horn neurones mainly used microelectrode recordings that are subject to large current leaks (Yoshimura & Jessell, 1989; Thomson et al. 1989; Lopez-Garcia & King, 1994; Jiang et al. 1995). Tight-seal whole-cell patch-clamp recordings may be a better choice for the study of membrane currents even if cell dialysis and washout of second messengers represent additional problems. Here, we used the whole-cell patch-clamp technique to record membrane and discharge properties from spinal dorsal horn neurones classified according to their location in lamina I, lamina II or deeper laminae. Significant differences in these parameters were found between neurones of different laminae probably contributing to the lamina-specific processing of sensory information in dorsal horn. Voltage-clamp recordings allowed us to characterise some of the membrane currents underlying the different firing patterns. Part of these results has been published in the form of an abstract (Ruscheweyh & Sandkühler, 2001).
METHODS
Preparation of spinal cord slices
Spinal cord slices were obtained from young (18- to 28-day-old) Sprague-Dawley rats. Under deep ether anaesthesia, the spinal cord was exposed by laminectomy and the lumbosacral segments with attached dorsal roots were excised. The rat was then killed by an overdose of ether. Transverse, 500 μm thick spinal cord slices with a long (8-12 mm) dorsal root were cut on a microslicer (DTK-1000, Dosaka EM, Kyoto, Japan). Slices were incubated in a solution that consisted of (mm): NaCl 95, KCl 1.8, KH2PO4 1.2, CaCl2 0.5, MgSO4 7, NaHCO3 26, glucose 15, sucrose 50 and was oxygenated with 95 % O2-5 % CO2; pH 7.4, measured osmolarity 310-320 mosmol l−1. A single slice was then transferred to the recording chamber where it was superfused with oxygenated recording solution at 3 ml min−1. The recording solution was identical to the incubation solution except for (mm): NaCl 127, CaCl2 2.4, MgSO4 1.3 and sucrose 0. Experiments were conducted at room temperature (20-24 °C). All procedures used conformed with guidelines from Regierungspräsidium Karlsruhe.
Recording and stimulation techniques
Standard whole-cell patch-clamp recording techniques were used. Patch pipettes were made from borosilicate glass (Hilgenberg, Malsfeld, Germany) on a horizontal micropipette puller (P-87, Sutter Instruments, Novato, CA, USA). When filled with intracellular solution consisting of (mm): potassium gluconate 120, KCl 20, MgCl2 2, Na2ATP 2, Na-GTP 0.5, Hepes 20, EGTA 0.5, pH 7.28 with KOH, measured osmolarity 300 mosmol l−1 and inserted into the recording solution they had tip resistances of 2-6 MΩ. Biocytin (5 mg ml−1) was added to the intracellular solution to allow subsequent staining of the recorded cells.
Neurones were visualised with Dodt-infrared optics using a × 40, 0.80 NA water-immersion objective on an Olympus BX50WI upright microscope (Olympus, Japan) equipped with a video camera system (PCO, Kehlheim, Germany). Neurones in lamina I, lamina II or deeper dorsal horn laminae were selected for recording. The electrophysiological properties of the recorded neurones were investigated in current- and voltage-clamp modes using an Axopatch 200B amplifier (Axon Instruments, Union City, CA, USA). Data were low-pass filtered at 5 kHz, amplified five times and sampled at 10 kHz.
The dorsal root was stimulated through a suction electrode with a constant current stimulator (World Precision Instruments, Sarasota, FL, USA).
Experimental protocol
The membrane potential measured immediately after establishing the whole-cell configuration was called the ‘resting membrane potential’, even though the membrane potential of a neurone in a slice preparation superfused by artificial cerebrospinal fluid (ACSF), measured in the whole-cell configuration is not equivalent to the situation in the intact animal. Only neurones that had an apparent resting membrane potential more negative than −50 mV were investigated further. Hyperpolarising (-50 and −25 pA) and depolarising (25-350 pA in 25 pA steps) current injections of 1 s duration were applied to determine the firing pattern from resting membrane potential. In some experiments, hyper- or depolarising current prepulses were used to investigate the dependence of the firing pattern on the holding potential. Voltage clamp recordings from different holding potentials were performed to examine the underlying currents. In 73 of the 125 recorded neurones, it was tested if electrical stimulation of the dorsal root evoked excitatory postsynaptic currents (EPSCs) in the recorded cell. Conduction velocities of the responsible afferent fibres were calculated from the dorsal root length and the EPSC latency. EPSCs that displayed low stimulation thresholds (0.05-0.3 mA) and short latencies that are consistent with conduction velocities of afferent fibres in the Aδ-fibre range (2-8 m s−!) were classified to be Aδ-fibre-evoked. EPSCs that had high stimulation thresholds (0.8-2 mA) and long latencies that are consistent with conduction velocities in the C-fibre range (< 2 m s−1) were considered to be C-fibre-evoked. Constant latencies and absence of failures during 10 Hz stimulation (for Aδ-fibres) or 1 Hz stimulation (for C-fibres) were used as criteria for monosynaptic transmission. Membrane resistance, membrane time constant, membrane capacitance, series resistance and leak current were measured by means of a depolarising voltage step from −70 mV to −50 mV at the beginning of the experiment and monitored repetitively throughout the experiment. Series resistance was usually about 20-30 MΩ and results were excluded if it rose above 50 MΩ. No correction for liquid junction potential was made.
At the end of the experiment, much care was taken to classify the neurones correctly as lying either in lamina I, lamina II or deeper laminae (laminae III-VI). This was done visually with a microscope under transmitted light where lamina II could be identified as a translucent band across the dorsal horn. All neurones that could not be clearly assigned to one of the categories were discarded from further analysis. Further subdivision of lamina II into the functionally distinct lamina II outer and inner was attempted by including biocytin in the pipette for later histological location of the recorded neurone. Unfortunately, due to technical problems in recovering the small superficial dorsal horn neurones, successful biocytin staining was obtained for only 20 of the 125 recorded neurones, with not enough lamina II neurones to distinguish between lamina II outer and inner. However, for all of these 20 neurones, biocytin staining confirmed the laminar assignment by visual inspection.
Data analysis
The software package pCLAMP 8 was used for data acquisition and subsequent off-line analysis. Action potential width was measured at the base of the first action potential evoked by the increasing current injections applied to determine the firing pattern. Action potential height and amplitude of the afterhyperpolarisation were measured from the same point. Means ± s.e.m. values are given. The non-parametric Wilcoxon rank-sum test was used for statistical comparisons (Sigma Stat, Version 2.0).
Application of drugs
Tetrodotoxin (TTX, 0.5μM, Tocris, Bristol, UK), 4-aminopyridine (4-AP, 0.5-5 mm, Sigma, Deisenhofen, Germany) or tetraethylammonium (TEA, 20 mm, Sigma) were added to the superfusion solution at defined concentrations as indicated. Stock solutions were prepared by dissolving the drugs in acidic buffer (TTX, pH 4.8) or distilled water (4-AP and TEA) and stored in aliquots at −20 °C.
RESULTS
Whole-cell patch-clamp recordings were obtained from 125 dorsal horn neurones. Of these, 70 neurones were located in lamina I, 28 neurones were recorded in lamina II and 27 neurones were located in deeper laminae (laminae III-VI). Electrical stimulation of the dorsal root produced Aδ- and/or C-fibre-evoked EPSCs in 48 (66 %) of the 73 neurones tested for input. Thirty neurones received input from Aδ-fibres that was monosynaptically evoked in 23 % of cases. C-fibre-evoked EPSCs were encountered in 34 neurones and were monosynaptically evoked in 56 %.
Firing patterns of spinal dorsal horn neurones
The population of neurones was divided into five categories according to their firing pattern in response to 1 s depolarizing current injections of different intensities. Thirteen (10 %) of the recorded neurones could not be classified into any of these categories. Forty-two percent of the recorded neurones were tonic-firing neurones, showing regular firing throughout the current pulse, usually with some degree of frequency adaptation (Fig. 1Aa). The number of action potentials evoked increased sublinearly with the injected current (Fig. 4C) until inactivation of fast Na+ channels occurred. Delayed-firing neurones accounted for 19 % of the recorded neurones and were characterised by a slow ramp depolarisation in response to subthreshold depolarising current pulses. With higher current injections, a delay was seen between the onset of the current pulse and the first action potential that was successively shortened at higher current injections (Fig. 1Ba). Repetitive firing was less regular than in tonic-firing neurones and often slightly accelerating during the current pulse. Action potentials were of more variable height than in tonic-firing neurones. Eight percent of the neurones showed an initial burst of action potentials usually not exceeding 300 ms in response to current injections and were silent during the remainder of the current pulse (Fig. 1Ca). Strong frequency adaptation was apparent during the burst. Half of these cells showed a marked short afterhyperpolarisation after the end of the current pulse (Fig. 1Ca). Single-spiking neurones generated only one or two action potentials at the onset of the current pulse regardless of its intensity and accounted for 6 % of the recorded neurones (Fig. 1Da). Fourteen percent of the recorded neurones were phasic-bursting neurones that were characterised by an irregular firing pattern showing bursts of similar intraburst frequency but variable length and variable interburst intervals during current injections (Fig. 1Ea). Sometimes, a purely tonic firing was generated in response to a given current pulse, but bursting behaviour was evoked at other intensities.
Figure 1. Rat spinal dorsal horn neurones displayed five distinct firing patterns.
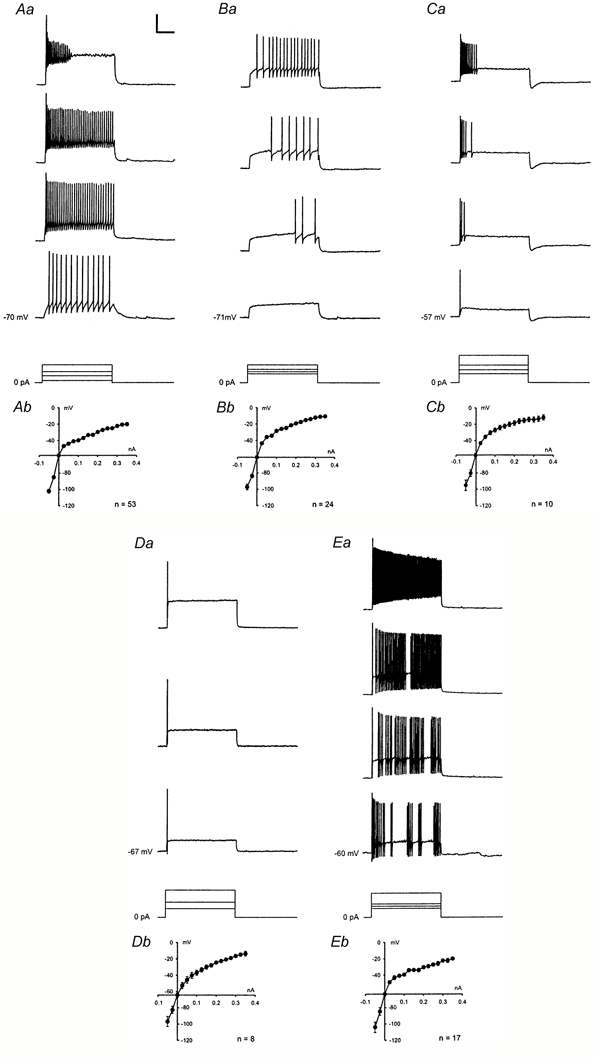
Firing patterns were obtained in response to 1s injections of depolarising current (25-350 pA, 25 pA steps) at resting membrane potential. Selected traces from representative neurones are shown in Aa-Ea. A, tonic-firing neurone. B, delayed-firing neurone. C, neurone showing an initial burst. D, single-spiking neurone. E, phasic-bursting neurone. Bottom traces in A-E, injected currents (superimposed). Calibration: 40 mV, 250 ms, 200 pA. Ab-Eb, averaged current-voltage relations (means ± s.e.m.) from all neurones displaying the respective firing pattern. Current-voltage relations were obtained in current-clamp at the end of 1s hyper- or depolarising pulses. Outward rectification is evident for all types of firing pattern.
Figure 4. Tonic-firing neurones in deeper laminae express higher firing frequencies than tonic-firing neurones in lamina I.
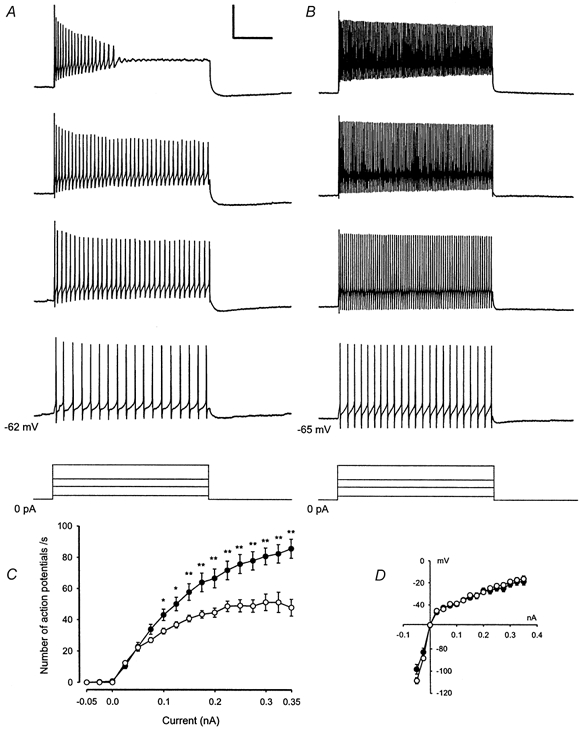
Neurones grouped according to their firing patterns also differed significantly in other membrane properties. These results are summarised in Table 1. For example, tonic-firing neurones showed lower membrane resistances, lower action potential thresholds, narrower and higher action potentials and deeper afterhyperpolarisations following action potentials in comparison to delayed-firing neurones, further confirming that these are two distinct neuronal populations. The shape of the afterhyperpolarisation following action potentials was classified as either monophasic or polyphasic (Fig. 2A and B). While tonic-firing neurones showed predominantly polyphasic afterhyperpolarisations (75 %), monophasic afterhyperpolarisations were typically found in delayed-firing neurones and neurones with an initial burst (83 % and 80 %, respectively). No correlation was found between the different firing patterns and types of afferent fibre input classified as mono- or polysynaptic Aδ- and/or C-fibre-evoked.
Table 1.
Passive and active membrane properties of dorsal horn neurones classified according to their firing patterns
| Tonic-firing (T; n = 53) | Delayed-firing (D; n = 24) | Initial-bursting (IB; n = 10) | Single-spike (S; n = 8) | Phasic-bursting (PB) (PB; n = 17) | |
|---|---|---|---|---|---|
| Resting membrane potential (mV) | −58 ± 1 | −59 ± 1 | −59 ± 3 | −60 ± 3 | −59 ± 2 |
| Membrane resistance (MΩ) | 501 ± 49**D | 644 ± 56**T | 554 ± 105 | 477 ± 87 | 608 ± 116 |
| Membrane capacitance (pF) | 39 ± 3 | 42 ± 7 | 33 ± 5 | 29 ± 3 | 37 ± 5 |
| Membrane time constant (μs) | 705 ± 63 | 915 ± 237 | 581 ± 93 | 608 ± 137 | 756 ± 133 |
| Action potential threshold (mV) | −39 ± 1**D | −31 ± 1**T, **TB, *S | −36 ± 3 | −39 ± 3*D | −38 ± 6**D |
| Action potential width at base (ms) | 2.0 ± 0.1**D | 2.6 ± 0.1**T, **TB, *S | 2.3 ± 0.2 | 2.1 ± 0.2*D | 2.0 ± 0.1**D |
| Action potential height (mV) | 102 ± 3**D, *S | 75 ± 3**T, **TB, **IB | 93 ± 4**D | 84 ± 8*T, *TB | 102 ± 4**D, *S |
| After hyperpolarization depth (mV) | 39 ± 2**D, **IB | 29 ± 2**T | 25 ± 3**T, *TB | 33 ± 4 | 34 ± 3*IB |
Statistical significance is indicated by
(P < 0.05)
(P < 0.01) followed by the abbreviation of the group the comparison was made with. n, number of observations.
Figure 2. Firing patterns of spinal dorsal horn neurones were associated with different shapes of the afterhyperpolarisation following an action potential.
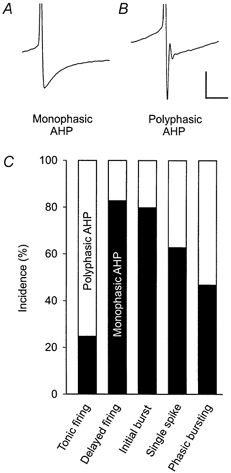
The shape of the afterhyperpolarisation was evaluated at the lowest current injection (25-350 pA, 25 pA steps) that generated action potentials from resting membrane potential. A, example of a monophasic afterhyperpolarisation. B, example of a polyphasic afterhyperpolarisation. Biphasic afterhyperpolarisations were also classified as polyphasic. Calibration for A and B: 20 mV, 20 ms. C, monophasic (black bars) and polyphasic (white bars) afterhyperpolarisations were differentially distributed among firing patterns.
Lamina-specific differences in firing patterns, membrane properties and firing frequencies
Firing patterns were not uniformly distributed among dorsal horn laminae (Fig. 3). Tonic-firing neurones were numerous in lamina I and deeper laminae but were not found in lamina II. Delayed-firing neurones were encountered in laminae I and II but not in deeper laminae. Most of the neurones showing an initial burst were found in lamina II. Moreover, neurones from different laminae also showed significant differences in other membrane properties that are summarised in Table 2. For example, neurones from deeper laminae were characterised by lower membrane resistances, higher membrane capacitances, narrower and higher action potentials and deeper afterhyperpolarisations than neurones from the other laminae. Neurones from lamina II exhibited the highest absolute and relative action potential thresholds; these were on average 26 mV less negative than resting membrane potential, as compared with differences of 21 and 15 mV in neurones of lamina I and deeper laminae, respectively. Neurones from lamina II showed broader action potentials with smaller amplitudes and more shallow afterhyperpolarisations compared with neurones from the other groups.
Figure 3. Lamina-specific distribution of spinal dorsal horn neurone firing patterns.
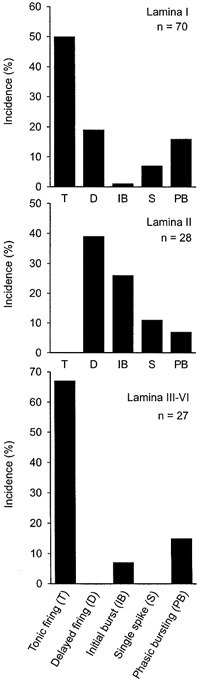
Table 2.
Passive and active membrane properties of dorsal horn neurones from different laminae
| Lamina I (LI; n = 70) | Lamina II (LII; n = 28) | Deeper laminae (DL; n = 27) | |
|---|---|---|---|
| Resting membrane potential (mV) | −58 ± 1 | −60 ± 2*DL | −56 ± 1*LII |
| Membrane resistance (MΩ) | 596 ± 54**DL | 612 ± 50**DL | 404 ± 66**LI, **LII |
| Membrane capacitance (pF) | 37 ± 3*DL | 33 ± 2*DL | 44 ± 4*LI, *LII |
| Membrane time constant (ms) | 679 ± 89 | 678 ± 55 | 847 ± 101 |
| Action potential threshold (mV) | −37 ± 2*DL, *LII | −34 ± 2*LI, **DL | −41 ± 1*LI, **LII |
| Action potential width at base (ms) | 2.2 ± 0.1**DL | 2.5 ± 0.1**DL | 1.6 ± 0.1**LI, **LII |
| Action potential height (mV) | 94 ± 3*LII | 83 ± 4*LI, *DL | 97 ± 4*LII |
| Afterhyperpolarization depth (mV) | 33 ± 1**LII, **DL | 28 ± 2**LI, **DL | 42 ± 3**LI, **LII |
Statistical significance is indicated by
(P < 0.05)
(P < 0.01) followed by the abbreviation of the group the comparison was made with. n, number of observations.
Tonic-firing neurones from deeper laminae (n = 18) exhibited significantly higher firing frequencies in response to defined current injections of different intensities than tonic-firing neurones from lamina I (n = 35, Fig. 4). Supposedly, though, the firing frequency depends more on the actual depolarisation reached during the current pulse than on the amount of current injected, so that differences in membrane resistance of lamina I versus deeper laminae neurones could account for the different firing frequencies. However, neurones in deeper laminae had significantly lower membrane resistances than neurones from lamina I (see Table 2) and thus should need higher amounts of current to reach a defined level of depolarisation. When corrected for the difference in membrane resistance, both groups of neurones had virtually identical current-voltage relations (Fig. 4D). Moreover, when the firing frequencies of the two groups were compared at a fixed level of depolarisation of −30 mV, neurones from deeper laminae still had significantly higher firing frequencies (80 ± 6 action potentials s−1) than neurones from lamina I (44 ± 3 action potentials s−1, P < 0.001). In addition, there were no significant differences in resting membrane potential or action potential thresholds between tonic-firing neurones from lamina I and deeper laminae that could account for the different firing frequencies.
Mechanisms underlying the delayed firing pattern
The characteristic delay to the first action potential occurred only if the depolarising current pulse was applied from holding potentials more negative than approximately −50 mV (Fig. 5A), while tonic firing was generated by the same neurone at more positive holding potentials (n = 20, Fig. 5B). The underlying currents were examined in voltage-clamp mode. All neurones with a delayed firing pattern exhibited a voltage-dependent, rapidly-activating and inactivating outward current when depolarising voltage steps were applied from a holding potential of −80 mV (n = 24). This current was similar to the transient voltage-dependent potassium current called A-current that has been identified in many types of neuronal cells (Yarom et al. 1985; Dekin & Getting, 1987; Klee et al. 1995). In contrast, none of the tonic-firing neurones exhibited an A-like current (n = 53) except two neurones in deeper dorsal horn that showed very small A-like currents that did not cause a delay. Voltage-dependent activation and steady-state inactivation of the A-like current were examined in more detail in five neurones (Fig. 6A). Partial steady-state inactivation is present at voltages less negative than −90 mV, explaining the phenomenon that the delay is more pronounced with more negative holding potentials. At voltages above −50 mV, inactivation of the A-like current is complete, consistent with the observation that the firing pattern of delayed neurones switches to tonic at these holding potentials (Fig. 5B). The A-like current recovered from inactivation with a monoexponential time course with a mean time constant of 15 ± 2 ms (Fig. 6B, n = 5).
Figure 5. Electrophysiological properties of delayed-firing neurones.
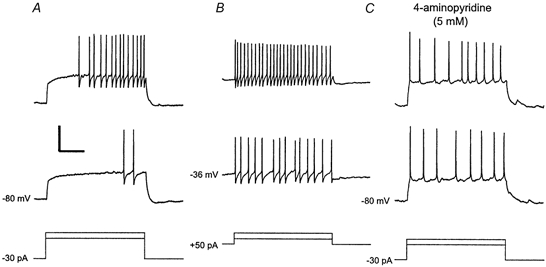
Several second-long prepulses of hyper- or depolarising currents were applied to the neurone to study firing patterns from different holding potentials. A, the neurone was held at a potential of −80 mV by injecting −30 pA. Depolarising current steps from this hyperpolarised holding potential generated the characteristic delay to the first spike. B, the neurone was held at a potential of −36 mV by injecting +50 pA. At this depolarised holding potential, depolarising current steps evoked a tonic firing pattern. Note that the total amount of current injected into the neurone during the steps was the same in A and B. C, during bath application of 4-aminopyridine at a high concentration (5 mm), a tonic firing pattern was also generated from a hyperpolarised holding potential. Bottom traces, injected currents (superimposed). Calibration: 40 mV, 250 ms, 130 pA.
Figure 6. Properties of the A-like current recorded in delayed-firing neurones.
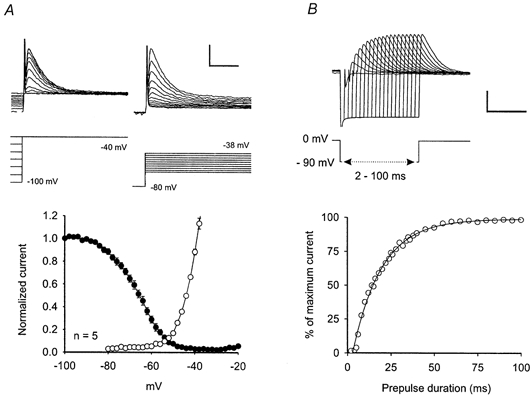
A, activation (open circles) and steady-state inactivation (filled circles) of the A-like current are voltage-dependent. The amplitude of the A-like current was measured by subtracting the steady-state current at the end of a 200 ms voltage pulse from the maximal current evoked by the voltage step. Currents were normalised to the value obtained by a voltage step from −100 mV to −40 mV. Top traces (left), steady-state inactivation was assessed by depolarising voltage steps to - 40 mV applied from different holding potentials. Lower trace, voltage step protocol. Top traces (right), voltage-dependent activation was evaluated by applying defined depolarising voltage steps from a holding potential of −80 mV. Lower trace, voltage step protocol. Calibration: 200 pA, 40 ms, 25 mV. B, recovery from steady-state inactivation of the A-like current followed a monoexponential time course. A typical example is shown. Top traces, recovery from inactivation was assessed by first inactivating the A-like current completely at 0 mV, then applying a hyperpolarising voltage prepulse to −90 mV of varying duration (2-100 ms) before stepping back to 0 mV. TTX (0.5 μM) was present during the recording to prevent action potential generation. Lower trace, voltage protocol. Calibration: 1 nA, 50 ms, 90 mV.
In five experiments in which 4-aminopyridine (4-AP, 5 mm) was added to the bath solution, the delay disappeared and the firing pattern became tonic even if evoked from hyperpolarised holding potentials (Fig. 5C). Consistently, 4-AP (5 mm) greatly reduced the amplitude of the A-like current (Fig. 7, reduction to 18 ± 7 % of control, n = 9, P < 0.001 for the comparison with amplitudes before 4-AP application), measured at a voltage step from −80 mV to −44 mV that was chosen so that no action potentials were evoked in spite of the lowered action potential threshold under 4-AP. A 10-fold lower concentration of 4-AP (0.5 mm) did not affect the delayed firing pattern or the underlying current (n = 3, not shown).
Figure 7. 4-aminopyridine blocks the A-like current.
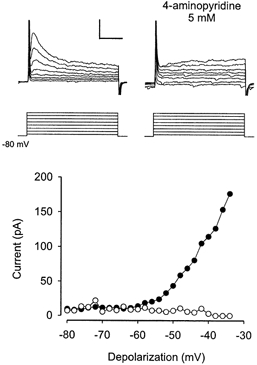
Voltage-dependent activation of the A-like current in one typical experiment before (filled circles) and after (open circles) bath application of 4-aminopyridine (4-AP, 5 mm) is shown. The A-current was evoked by depolarising voltage steps from a holding potential of −80 mV. Top traces (left), original traces before application of 4-AP. Top traces (right), original traces after application of 4-AP. Calibration: 100 pA, 50 ms, 40 mV.
Mechanisms underlying the single-spiking firing pattern
As single-spiking neurones were scarce, detailed examination of this firing pattern could be performed in only two cells. While only single action potentials at the onset of the current pulse could be evoked when injecting current from a hyperpolarised holding potential (Fig. 8A), a short burst of action potentials was elicited when a more depolarised holding potential was used (Fig. 8B). This suggests that, similar to the A-like current for the delayed firing pattern, a transient voltage-dependent outward current may be involved in the generation of the single-spiking firing pattern. Slow activation kinetics of this current would allow for just one or two action potentials before it hyperpolarises the membrane below action potential threshold. At a low concentration, 4-AP (0.5 mm) converted the single-spiking firing pattern to a tonic firing pattern (Fig. 8D). To exclude the possibility that rapid inactivation of fast Na+ channels was responsible for the single-spiking firing pattern, an additional current injection was given during a maintained current pulse that had evoked one action potential at its onset. The additional current was able to generate a burst of action potentials (Fig. 8C), showing that fast Na+ channels were not inactivated.
Figure 8. Electrophysiological properties of single-spiking neurones.
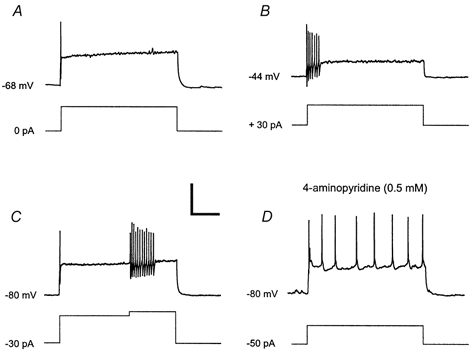
Several second-long prepulses of hyper- or depolarising current were applied to the neurone to study firing patterns from different holding potentials. A, at resting membrane potential, only one spike was generated at the onset of the depolarising current step. B, when the neurone was held at a depolarised potential by constantly injecting positive current, a depolarising current step evoked a burst of spikes. Note that the total amount of current injected during the step was the same in A and B. C, the neurone was held at a hyperpolarised membrane potential by constantly injecting negative current. Additional current injection during an active depolarising current step generated a burst of spikes. D, after application of 4-aminopyridine (0.5 mm), the neurone generated a tonic firing pattern. Bottom traces, current injections. Calibration: 40 mV, 250 ms, 300 pA.
Ionic basis of outward rectification in dorsal horn neurones
Dorsal horn neurones in all laminae showed a marked outward rectification in their steady-state current-voltage relationships irrespective of their firing pattern (Fig. 1A-E, panel b) that was blocked by high concentrations of TEA (20 mm) in all five neurones tested (not shown).
Rebound action potentials in response to release from hyperpolarising current
In six (5 %) of the recorded neurones, release from hyperpolarising current produced a burst of rebound action potentials riding on a depolarising hump. The associated current was a rapidly-activating and inactivating voltage-dependent inward current evident in voltage-clamp recordings (not shown).
DISCUSSION
Characterisation of the membrane and discharge properties of spinal dorsal horn neurones is critical to the understanding of how sensory, including nociceptive information is processed and integrated. In the present study, five types of firing patterns were identified among spinal dorsal horn neurones. Significant differences in active and passive membrane properties were evident between firing patterns. As spinal cord dorsal horn has been shown to be histologically and functionally subdivided into six laminae (Fitzgerald & Woolf, 1980; Molander et al. 1984; Woolf & Fitzgerald, 1986; Todd & Spike, 1993), we classified the neurones according to their location in lamina I, lamina II or deeper laminae. Firing patterns and membrane properties were found to be lamina-specifically distributed among dorsal horn neurones, probably contributing to lamina-specific processing of sensory information. Many of the recorded neurones in laminae I and II received either mono- or polysynaptic input from primary afferent Aδ- and/or C-fibres, suggesting their involvement in processing of nociceptive information.
Electrophysiological properties of dorsal horn neurones
Firing patterns
Five distinct firing patterns were identified in spinal cord dorsal horn neurones, reflecting differences in the input-output functions of the neurones. Tonic-firing neurones are suited to encode both the intensity and the duration of afferent excitation. In contrast, initial-bursting and single-spiking neurones may act as novelty detectors, discharging only at the onset of the afferent excitation. Initial-bursting neurones also encode the strength of the afferent excitation. Delayed-firing neurones have voltage-dependent coding properties and thus are specially suited for the state-dependent integration of information. The overall discharge properties that were found in dorsal horn neurones, with tonic-firing neurones being the most frequently encountered, compare well to the results from other studies on dorsal horn neurones (Thomson et al. 1989; Lopez-Garcia & King, 1994; Hochman et al. 1997; Jo et al. 1998). The phasic-bursting firing pattern has not been described in spinal dorsal horn but a similar firing pattern occurs in cortical neurones (Kawaguchi, 1995; Cauli et al. 1997). As in other studies, rectangular depolarising pulses have been used to evoke and classify firing patterns. Of course, it still has to be investigated how these firing patterns determine the reaction of dorsal horn neurones to natural sensory stimulation in the intact animal. In vivo patch-clamp studies would be the best choice to answer that question. The slice preparation seems much less suited to answer this question since much of the converging afferent input has been cut and natural stimulation is not possible.
Membrane properties
Membrane properties measured in a slice preparation may differ from the in vivo situation because axons and dendrites are severed during the slicing process. However, values for membrane potential, membrane resistance and membrane capacitance were comparable to the results reported from in vivo patch-clamp studies on dorsal horn neurones, suggesting that the transverse spinal cord slice preparation is an appropriate model in which to study membrane properties of dorsal horn neurones (Light & Willcockson, 1999; Furue et al. 1999). At present, no in vivo patch-clamp data are available for action potential properties. Action potential height was in a similar range to that found in other in vitro patch-clamp studies but action potentials were narrower, possibly due to age differences or the use of dissociated neurones in these studies (Huang, 1987; Hochman et al. 1997). Resting membrane potentials and action potential thresholds reported from medullary dorsal horn neurones were about 10 mV more negative than in our population (Li et al. 1999; Li et al. 2000a, b), possibly due to differences in liquid junction potentials. Consistent with our results, tonic-firing neurones have been found to have mostly polyphasic afterhyperpolarisations and narrow action potentials (Lopez-Garcia & King, 1994) while initial bursting neurones show monophasic afterhyperpolarisations (Thomson et al. 1989; Lopez-Garcia & King, 1994).
Delayed-firing neurones
Delayed-firing neurones have been identified previously in spinal cord dorsal horn (Yoshimura & Jessell, 1989; Morisset & Nagy, 1998) but the underlying current has not been completely characterised. In other areas of the CNS, an A-current has been held responsible for the delayed firing pattern (Dekin et al. 1987; Storm, 1988; Fujino et al. 1997; Saito & Isa, 1999). The A-current is a transient voltage-dependent outward potassium current that counteracts fast depolarisations and thus leads to the characteristic delay to the first action potential. The outward current we identified in spinal delayed-firing neurones fulfils the criteria for an A-current including voltage-dependent activation and steady-state inactivation in typical voltage ranges, monoexponential time course of recovery from inactivation with time constants in the range of tens of milliseconds and inhibition by high concentrations of 4-AP (5 mm) (Yoshimura & Jessell, 1989).
Single-spiking neurones
The ionic mechanism underlying the single-spike firing pattern has not been determined conclusively in spinal cord dorsal horn (Jo et al. 1998) or other regions of the CNS (Banks & Smith, 1992; Lopez-Garcia & King, 1994; Zhang & Trussell, 1994; Hochman et al. 1997). We showed that it is not due to inactivation of fast Na+ channels but rather to the expression of a transient voltage-dependent outward current that activates and inactivates more slowly than the previously described A-current and is sensitive to lower concentrations of 4-AP (0.5 mm). These are characteristics of the D-current (Wu & Barish, 1992).
Outward rectification
Different types of steady-state rectification have been reported in spinal dorsal horn neurones (Jiang et al. 1995). In the present study, all neurones showed outward rectification mediated by a TEA (20 mm)-sensitive, voltage-dependent outward current that is usually called delayed rectifier or K-current (Rudy, 1988).
Lamina-specific differences
The histological subdivision of spinal dorsal horn into laminae I-VI (Rexed, 1952; Molander et al. 1984) has been proved to have multiple functional implications. The central endings of primary afferent fibres are lamina-specifically distributed with Aδ-fibres terminating mainly in lamina I and deeper laminae, C-fibres preferring lamina II and Aβ-fibres terminating in deeper laminae (Woolf & Fitzgerald, 1986; Willis & Coggeshall, 1991). Lamina I contains thermoreceptive and nociceptive specific as well as multimodal neurones. Lamina II is divided into an outer part with mainly nociceptive neurones and an inner part with mainly low threshold neurones. Lamina III neurones respond mainly to innocuous mechanical stimuli while deeper dorsal horn neurones are low threshold, nociceptive specific or wide-dynamic range neurones (Willis & Coggeshall, 1991). Lamina I and deeper laminae send projections to the medulla, thalamus, mesencephalon and parabrachial area (Menétrey et al. 1982; Chaouch et al. 1983; Guilbaud et al. 1994) while most of the lamina II neurones are intrinsic spinal cord neurones (Bice & Beal, 1997). Neurotransmitters like GABA and glycine, neuropeptides like substance P and enkephalin and their receptors show lamina-specific distribution (Gouarderes et al. 1991; Todd & Spike, 1993; Littlewood et al. 1995; Bohlhalter et al. 1996). Sensory input has been shown to be modulated in a lamina-specific way by morphine and other neuroactive substances (Kitahata et al. 1971; Kitahata et al. 1973; Fitzgerald & Woolf, 1980). We carefully classified the recorded neurones according to their laminar location as described in Methods and found that firing patterns and membrane properties were unequally distributed among laminae, thus allowing lamina-specific encoding of sensory information. It is tempting to hypothesise that neurones subserving different sensory qualities may exhibit specific firing patterns. Unfortunately, the natural stimulation necessary to answer that question is not possible in our preparation. In the cat, a relationship between morphology in horizontal sections and sensory quality has been found for lamina I neurones (Han et al. 1998) but no such data exist for the rat and distinction of the morphological types known from horizontal or parasagittal sections seems to be difficult in the transverse plane. Dorsal horn projection neurones tend to be larger than intrinsic circuit neurones but again, a reliable distinction based on morphology or size has not been reported for transverse rat spinal cord slices.
Distribution of firing patterns
In lamina I, all five types of firing patterns were present. Medullary dorsal horn lamina I projection neurones exhibited either a tonic or a delayed firing pattern (Li et al. 2000a) as did 69 % of the lamina I neurones in the present study. It is, at present, not known whether the additional firing patterns found in this study are characteristic for local circuit neurones.
In lamina II, we found delayed-firing neurones but no tonic-firing neurones. Tonic-firing neurones have been reported in lamina II of spinal cord and medullary dorsal horn (Yoshimura & Jessell, 1989; Li et al. 1999). However, delayed-firing neurones may be equivocally classified as tonic-firing neurones when the underlying A-current is inactivated at depolarised holding potentials. This may happen when using microelectrode recordings that offer less control over the membrane potential. In addition, lamina II showed initial bursting neurones that may correspond to inhibitory interneurones (Jo et al. 1998), single-spiking and phasic-bursting neurones.
In deeper laminae, we found no delayed-firing neurones, consistent with another study that showed very few delayed-firing neurones (Hochman et al. 1997). In contrast, 40 % delayed-firing neurones were reported in lamina V (Morisset & Nagy, 1998) and some in lamina III of medullary dorsal horn (Li et al. 2000b). The present results suggest that the overall proportion of delayed-firing neurones in deeper dorsal horn laminae is relatively low.
Lamina-specific membrane properties
Lamina-specific membrane properties may have important functional implications. Neurones from superficial laminae had broader action potentials than neurones from deeper laminae, thus allowing a stronger Ca2+ influx through voltage-gated channels during action potentials. The intracellular Ca2+ concentration is known to be crucial to the regulation of many intracellular messenger systems (Berridge, 1998) and likely triggers synaptic long-term plasticity in superficial spinal dorsal horn (Sandkühler, 2000). Enhanced Ca2+ influx during delayed action potential repolarisation may also increase neurotransmitter release and postsynaptic responses (Blumenfeld et al. 1990). The relative action potential threshold was considerably higher in lamina II neurones than in the other groups, representing a lower membrane excitability.
Action potential firing frequencies
The frequency of action potential firing evoked in tonic-firing neurones by a defined current input may determine the intensity coding properties of sensory neurones. Here, deep dorsal horn tonic-firing neurones were found to generate significantly higher firing frequencies in response to a defined depolarisation than lamina I tonic-firing neurones. It has been shown that neither membrane resistance nor rectification are responsible for this difference in firing frequency. Firing frequencies of tonic-firing dorsal horn neurones in previous studies are comparable to the present results (Jiang et al. 1995; Hochman et al. 1997; Jo et al. 1998). One study reported a linear relation between firing frequency and injected current (Jiang et al. 1995), while in the present study, the relation was non-linear for higher currents, possibly due to the marked outward rectification of our neurones.
In conclusion, dorsal horn neurones show a lamina-specific distribution of active and passive membrane properties, discharge patterns and their underlying currents that may contribute to the functional differences among dorsal horn laminae.
Acknowledgments
The authors wish to thank Dr. J. R. Geiger for very helpful hints. This work was supported by grants from the Pain Research Programme of the Medical Faculty of the University of Heidelberg and the Deutsche Forschungsgemeinschaft (grant no. Sa 435/10-2).
REFERENCES
- Banks MI, Smith PH. Intracellular recordings from neurobiotin-labeled cells in brain slices of the rat medial nucleus of the trapezoid body. Journal of Neuroscience. 1992;12:2819–2837. doi: 10.1523/JNEUROSCI.12-07-02819.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge MJ. Neuronal calcium signaling. Neuron. 1998;21:13–26. doi: 10.1016/s0896-6273(00)80510-3. [DOI] [PubMed] [Google Scholar]
- Bice TN, Beal JA. Quantitative and neurogenic analysis of the total population and subpopulations of neurons defined by axon projection in the superficial dorsal horn of the rat lumbar spinal cord. Journal of Comparative Neurology. 1997;388:550–564. doi: 10.1002/(sici)1096-9861(19971201)388:4<550::aid-cne4>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- Blumenfeld H, Spira ME, Kandel ER, Siegelbaum SA. Facilitatory and inhibitory transmitters modulate calcium influx during action potentials in aplysia sensory neurons. Neuron. 1990;5:487–499. doi: 10.1016/0896-6273(90)90088-w. [DOI] [PubMed] [Google Scholar]
- Bohlhalter S, Weinmann O, Mohler H, Fritschy JM. Laminar compartmentalization of GABA A -receptor subtypes in the spinal cord: an immunohistochemical study. Journal of Neuroscience. 1996;16:283–297. doi: 10.1523/JNEUROSCI.16-01-00283.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cauli B, Audinat E, Lambolez B, Angulo MC, Ropert N, Tsuzuki K, Hestrin S, Rossier J. Molecular and physiological diversity of cortical nonpyramidal cells. Journal of Neuroscience. 1997;17:3894–3906. doi: 10.1523/JNEUROSCI.17-10-03894.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaouch A, Menétrey D, Binder D, Besson JM. Neurons at the origin of the medial component of the bulbopontine spinoreticular tract in the rat: an anatomical study using horseradish peroxidase retrograde transport. Journal of Comparative Neurology. 1983;214:309–320. doi: 10.1002/cne.902140308. [DOI] [PubMed] [Google Scholar]
- Dekin MS, Getting PA. In vitro characterization of neurons in the ventral part of the nucleus tractus solitarius. II. Ionic basis for repetitive firing patterns. Journal of Neurophysiology. 1987;58:215–229. doi: 10.1152/jn.1987.58.1.215. [DOI] [PubMed] [Google Scholar]
- Dekin MS, Getting PA, Johnson SM. In vitro characterization of neurons in the ventral part of the nucleus tractus solitarius. I. Identification of neuronal types and repetitive firing properties. Journal of Neurophysiology. 1987;58:195–214. doi: 10.1152/jn.1987.58.1.195. [DOI] [PubMed] [Google Scholar]
- Fitzgerald M, Woolf CJ. The stereospecific effect of naloxone on rat dorsal horn neurones: inhibition in superficial laminae and excitation in deeper laminae. Pain. 1980;9:293–306. doi: 10.1016/0304-3959(80)90044-5. [DOI] [PubMed] [Google Scholar]
- Fujino K, Koyano K, Ohmori H. Lateral and medial olivocochlear neurons have distinct electrophysiological properties in the rat brain slice. Journal of Neurophysiology. 1997;77:2788–2804. doi: 10.1152/jn.1997.77.5.2788. [DOI] [PubMed] [Google Scholar]
- Furue H, Narikawa K, Kumamoto E, Yoshimura M. Responsiveness of rat substantia gelatinosa neurones to mechanical but not thermal stimuli revealed by in vivo patch-clamp recording. Journal of Physiology. 1999;521:529–535. doi: 10.1111/j.1469-7793.1999.00529.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gouarderes C, Beaudet A, Zajac JM, Cros J, Quirion R. High resolution radioautographic localization of 125I FK-33–824-labelled μ-opioid receptors in the spinal cord of normal and deafferented rats. Neuroscience. 1991;43:197–209. doi: 10.1016/0306-4522(91)90427-p. [DOI] [PubMed] [Google Scholar]
- Guilbaud G, Bernard JF, Besson JM. Brain areas involved in nociceptin and pain. In: Wall PD, Melzack R, editors. Textbook of Pain. Edinburgh: Churchill Livingstone; 1994. pp. 113–128. [Google Scholar]
- Han ZS, Zhang ET, Craig AD. Nociceptive and thermoreceptive lamina I neurons are anatomically distinct. Nature Neuroscience. 1998;1:218–225. doi: 10.1038/665. [DOI] [PubMed] [Google Scholar]
- Hochman S, Garraway SM, Pockett S. Membrane properties of deep dorsal horn neurons from neonatal rat spinal cord in vitro. Brain Research. 1997;767:214–219. doi: 10.1016/s0006-8993(97)00578-7. [DOI] [PubMed] [Google Scholar]
- Huang LY. Electrical properties of acutely isolated, identified rat spinal dorsal horn projection neurons. Neuroscience Letters. 1987;82:267–272. doi: 10.1016/0304-3940(87)90267-9. [DOI] [PubMed] [Google Scholar]
- Jiang MC, Cleland CL, Gebhart GF. Intrinsic properties of deep dorsal horn neurons in the L 6-S 1 spinal cord of the intact rat. Journal of Neurophysiology. 1995;74:1819–1827. doi: 10.1152/jn.1995.74.5.1819. [DOI] [PubMed] [Google Scholar]
- Jo YH, Stoeckel ME, Schlichter R. Electrophysiological properties of cultured neonatal rat dorsal horn neurons containing GABA and met-enkephalin-like immunoreactivity. Journal of Neurophysiology. 1998;79:1583–1586. doi: 10.1152/jn.1998.79.3.1583. [DOI] [PubMed] [Google Scholar]
- Kawaguchi Y. Physiological subgroups of nonpyramidal cells with specific morphological characteristics in layer II/III of rat frontal cortex. Journal of Neuroscience. 1995;15:2638–2655. doi: 10.1523/JNEUROSCI.15-04-02638.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitahata LM, Taub A, Kosada Y. Lamina-specific suppression of dorsal horn unit activity by detamine hydrochloride. Anesthesiology. 1973;38:4–11. doi: 10.1097/00000542-197301000-00003. [DOI] [PubMed] [Google Scholar]
- Kitahata LM, Taub A, Sato I. Lamina-specific suppression of dorsal horn unit activity by nitrous oxide and by hyperventilation. Journal of Pharmacology and Experimental Therapeutics. 1971;176:101–108. [PubMed] [Google Scholar]
- Klee R, Ficker E, Heinemann U. Comparison of voltage-dependent potassium currents in rat pyramidal neurons acutely isolated from hippocampal regions CA1 and CA3. Journal of Neurophysiology. 1995;74:1982–1995. doi: 10.1152/jn.1995.74.5.1982. [DOI] [PubMed] [Google Scholar]
- Li YQ, Li H, Kaneko T, Mizuno N. Substantia gelatinosa neurons in the medullary dorsal horn: an intracellular labeling study in the rat. Journal of Comparative Neurology. 1999;411:399–412. [PubMed] [Google Scholar]
- Li YQ, Li H, Yang K, Kaneko T, Mizuno N. Morphologic features and electrical membrane properties of projection neurons in the marginal layer of the medullary dorsal horn of the rat. Journal of Comparative Neurology. 2000a;424:24–36. doi: 10.1002/1096-9861(20000814)424:1<24::aid-cne2>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- Li YQ, Li H, Yang K, Wang ZM, Kaneko T, Mizuno N. Intracellular labeling study of neurons in the superficial part of the magnocellular layer of the medullary dorsal horn of the rat. Journal of Comparative Neurology. 2000b;428:641–655. doi: 10.1002/1096-9861(20001225)428:4<641::aid-cne5>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- Light AR, Willcockson HH. Spinal laminae I-II neurons in rat recorded in vivo in whole cell, tight seal configuration: properties and opioid responses. Journal of Neurophysiology. 1999;82:3316–3326. doi: 10.1152/jn.1999.82.6.3316. [DOI] [PubMed] [Google Scholar]
- Littlewood NK, Todd AJ, Spike RC, Watt C, Shehab SAS. The types of neuron in spinal dorsal horn which possess neurokinin-1 receptors. Neuroscience. 1995;66:597–608. doi: 10.1016/0306-4522(95)00039-l. [DOI] [PubMed] [Google Scholar]
- Lopez-Garcia JA, King AE. Membrane properties of physiologically classified rat dorsal horn neurons in vitro: correlation with cutaneous sensory afferent input. European Journal of Neuroscience. 1994;6:998–1007. doi: 10.1111/j.1460-9568.1994.tb00594.x. [DOI] [PubMed] [Google Scholar]
- Menétrey D, Chaouch A, Binder D, Besson JM. The origin of the spinomesencephalic tract in the rat: an anatomical study using the retrograde transport of horseradish peroxidase. Journal of Comparative Neurology. 1982;206:193–207. doi: 10.1002/cne.902060208. [DOI] [PubMed] [Google Scholar]
- Molander C, Xu Q, Grant G. The cytoarchitectonic organization of the spinal cord in the rat. I. The lower thoracic and lumbosacral cord. Journal of Comparative Neurology. 1984;230:133–141. doi: 10.1002/cne.902300112. [DOI] [PubMed] [Google Scholar]
- Morisset V, Nagy F. Nociceptive integration in the rat spinal cord: role of non-linear membrane properties of deep dorsal horn neurons. European Journal of Neuroscience. 1998;10:3642–3652. doi: 10.1046/j.1460-9568.1998.00370.x. [DOI] [PubMed] [Google Scholar]
- Rexed B. The cytoarchitectonic organization of the spinal cord in the cat. Journal of Comparative Neurology. 1952;96:415–495. doi: 10.1002/cne.900960303. [DOI] [PubMed] [Google Scholar]
- Rudy B. Diversity and ubiquity of K+-channels. Neuroscience. 1988;25:729–749. doi: 10.1016/0306-4522(88)90033-4. [DOI] [PubMed] [Google Scholar]
- Ruscheweyh R, Sandkühler J. Lamina-specific coding properties of rat spinal cord dorsal horn neurones in vitro. Pflügers Archiv. 2001;441:R 158. [Google Scholar]
- Saito Y, Isa T. Electrophysiological and morphological properties of neurons in the rat superior colliculus. I. Neurons in the intermediate layer. Journal of Neurophysiology. 1999;82:754–767. doi: 10.1152/jn.1999.82.2.754. [DOI] [PubMed] [Google Scholar]
- Sandkühler J. Learning and memory in pain pathways. Pain. 2000;88:113–118. doi: 10.1016/S0304-3959(00)00424-3. [DOI] [PubMed] [Google Scholar]
- Storm JF. Temporal integration by a slowly inactivating K+ current in hippocampal neurons. Nature. 1988;336:379–381. doi: 10.1038/336379a0. [DOI] [PubMed] [Google Scholar]
- Thomson AM, West DC, Headley PM. Membrane characteristics and synaptic responsiveness of superficial dorsal horn neurons in a slice presparation of adult rat spinal cord. European Journal of Neuroscience. 1989;1:479–488. doi: 10.1111/j.1460-9568.1989.tb00354.x. [DOI] [PubMed] [Google Scholar]
- Todd AJ, Spike RC. The localization of classical transmitters and neuropeptides within neurons in laminae I-III of the mammalian spinal dorsal horn. Progress in Neurobiology. 1993;41:609–645. doi: 10.1016/0301-0082(93)90045-t. [DOI] [PubMed] [Google Scholar]
- Willis WD, Coggeshall RE. Sensory Mechanisms of the Spinal Cord. New York: Plenum Press; 1991. [Google Scholar]
- Woolf CJ, Fitzgerald M. Somatotopic organization of cutaneous afferent terminals and dorsal horn neuronal receptive fields in the superficial and deep laminae of the rat lumbar spinal cord. Journal of Comparative Neurology. 1986;251:517–531. doi: 10.1002/cne.902510407. [DOI] [PubMed] [Google Scholar]
- Wu RL, Barish ME. Two pharmacologically and kinetically distinct transient potassium currents in cultured embryonic mouse hippocampal neurons. Journal of Neuroscience. 1992;12:2235–2246. doi: 10.1523/JNEUROSCI.12-06-02235.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yarom Y, Sugimori M, Llinas R. Ionic currents and firing patterns of mammalian vagal motoneurons in vitro. Neuroscience. 1985;16:719–737. doi: 10.1016/0306-4522(85)90090-9. [DOI] [PubMed] [Google Scholar]
- Yoshimura M, Jessell TM. Membrane properties of rat substantia gelatinosa neurons in vitro. Journal of Neurophysiology. 1989;62:109–118. doi: 10.1152/jn.1989.62.1.109. [DOI] [PubMed] [Google Scholar]
- Zhang S, Trussell LO. A characterization of excitatory postsynaptic potentials in the avian nucleus magnocellularis. Journal of Neurophysiology. 1994;72:705–718. doi: 10.1152/jn.1994.72.2.705. [DOI] [PubMed] [Google Scholar]


