Abstract
The noradrenergic neurons of the locus coeruleus (LC) play an important role in modulating arousal and selective attention. A similar function has been attributed to the hypocretin neurons of the hypothalamus which maintain a strong synaptic projection to the LC. As the LC can be difficult to detect in the embryonic and neonatal mouse brain, we used a new transgenic mouse with strong GFP expression in the LC under the regulation of a mouse prion promoter. GFP colocalized with immunoreactive tyrosine hydroxylase in sections and dispersed cultures of the LC, allowing visualization and whole cell or single-unit recording from the LC in early stages of cellular development. GFP expression in the LC had no apparent effect on cellular physiology, including resting membrane potential, input resistance, spike threshold, depolarization-induced spike frequency increase, current-voltage relations, or hypocretin responses. In slices of the mature mouse and rat LC, hypocretin-1 and −2 increased spike frequency, with hypocretin-1 being an order of magnitude more potent. In the postnatal day (P) 0-2 developing mouse slice during a developmental period when spikes could be elicited in some cells, other developing LC neurons showed rhythmic, subthreshold oscillations (≈1 Hz) in membrane potential (2.9-7.4 mV amplitude); others were arrhythmic. Hypocretin-1 depolarized the membrane potential, resulting in the appearance of spikes in developing LC cells that showed no spikes under control conditions. In the presence of TTX and glutamate receptor antagonists, hypocretin-1-mediated inward currents were blocked by substitution of choline-Cl for NaCl, suggesting an excitatory mechanism based on an inward cation current. Hypocretin-1 initiated strong regular membrane voltage oscillations in arrhythmic immature neurons. Hypocretin increased the temporal synchrony of action potentials studied with dual-cell recording in P1-P5 mouse LC slices, consistent with the view that synchrony of LC output, associated with improved cognitive performance, may be increased by hypocretin. Together these data suggest that the hypothalamus, via hypocretin projections, may therefore be in a position to enhance arousal and modulate plasticity in higher brain centres through the developing LC.
The locus coeruleus (LC) provides the noradrenergic innervation to many regions of the brain and is the sole source of noradrenergic fibres to cortical regions including the cerebral and cerebellar cortex and hippocampus (Moore & Bloom, 1979; Levitt & Moore, 1979). Functionally the LC plays an important role in directed attention (Usher et al. 1999) and arousal/sleep modulation (Aston-Jones & Bloom, 1981; Bourgin et al. 2000). The cellular activity of LC neurons is dominated by an intrinsic pacemaker, in part based on an inward non-specific cation current mostly due to Na+, and a strong afterhyperpolarization following spike generation. The pacemaker may be critical for LC functional output and can be modulated by neuromodulators, second messenger systems and drugs of abuse (Nestler et al. 1999). The frequency of the pacemaker increases with LC maturation (Williams & Marshall, 1987).
Through its noradrenergic innervation, the LC plays an important role in targeting attention and modulating levels of awareness. This is accomplished by changes in the firing patterns of LC neurons; higher levels of cellular synchronization correlate with cognitive performance (Usher et al. 1999). Part of the LC cellular synchrony may be due to electrotonic junctions between LC neurons or their dendrites, particularly common during development (Christie et al. 1989; Ishimatsu & Williams, 1996).
Developmentally, LC has been postulated to play a strong role in the modulation of activity of many brain circuits. In rats the LC system is the first monoamine system to mature, and develops very early, with catecholamine-synthesizing enzymes detectable between embryonic day (E) 10 and 13 (Lauder & Bloom, 1974; Specht et al. 1981). Noradrenergic fibres are found in the cortex by E16 and LC neurons can be antidromically activated by E18 (Sakaguchi & Nakamura, 1987). Although LC axons in the mature cortex comprise only about 1 % of the terminals, in the developing brain LC fibres have been suggested to account for more that half of the synapses in some cortical regions, a very substantial level of innervation (Coyle & Molliver, 1977). The initial LC innervation of target areas often precedes many important developmental events, including neuronal migration, final mitosis, synaptogenesis and axonal pathfinding, and is therefore in a position to modulate these events (Lauder & Bloom, 1974; Nakamura & Sakaguchi, 1990). LC noradrenergic fibres have been reported to modulate synapse formation in the developing visual cortex (Blue & Parnavelas, 1982) and to modulate dendritic growth and extension and spine formation in several cortical regions (Maeda et al. 1974; Felten et al. 1982). Functionally, synchronized output of the LC may be important developmentally in the regulation of arousal states.
Hypocretin-1 and −2 (also called orexin A and B) are synthesized exclusively within the CNS in the lateral hypothalamus/perifornical region (de Lecea et al. 1998; Sakurai et al. 1998). Loss of hypocretin appears to be the primary cause of narcolepsy, a disease characterized by excessive day time sleepiness and slow arousal, unusual REM sleep patterns, cataplexy and hypnogogic hallucinations (Nishino et al. 1999; Peyron et al. 2000; Thannickal et al. 2000). Hypocretin fibres innervate a large number of neuronal loci in the brain and spinal cord (Peyron et al. 1998; van den Pol, 1999) and show a particularly strong innervation of the LC (Horvath et al. 1999), consistent with reports of retrograde transport of tracers from the LC to the lateral hypothalamus (Luppi et al. 1995). Hypocretin-1 (Hagan et al. 1999) or hypocretin-2 (Horvath et al. 1999) increases adult LC spike frequency, probably via activation of the hypocretin receptor-1 (orexin-1 receptor) (Trivedi et al. 1998) and mediated by activation of a Gq protein (Sakurai et al. 1998). The present paper examines the actions of hypocretin in the developing LC. A new transgenic mouse that expresses GFP in the embryonic and neonatal LC is described and used to facilitate detection of the LC in the developing brain.
METHODS
Transgenic mice that express GFP in locus coeruleus
We used a mouse prion promoter to drive a cDNA construct of an enhanced, red-shifted, mammalian codon-corrected green fluorescent protein (GFP). The plasmid pEGFP-N2 (BD Biosciences Clontech, Palo Alto, CA, USA) was digested with restriction enzymes Xho1 and Stu1 to isolate a 1.962 kb GFP fragment containing multiple cloning sequences with the Xho1 end at the N-terminal, followed by GFP coding sequences, SV40 poly(A) sequences and the SV40 origin of replication. The mouse prion promoter MoPrp.Xho vector of about 16.3 kb, designed to accept inserts with Xho1 or Sal1 generated ends, was a generous gift of Dr D. Borchelt (Johns Hopkins University) and is described elsewhere (Borchelt et al. 1996). It was digested with Xho1 and gel purified. The Xho1 and Stu1 digested 1.96 kb GFP fragment was gel purified twice to minimize any potential plasmid sequence contamination and first ligated at the Xho1 site of the MoPrp. fragment, phenol:chloroform extracted, alcohol precipitated and then 3′-end filling up was done by Klenow polymerase to make blunt the other Xho1 end of the MoPrp. fragment. This linear construct was then blunt end ligated with the Stu1 end of GFP and Xho1 filled up end of the MoPrp. fragment to circularise the plasmid for bacterial transformation. The Prp.-GFP plasmid construct was introduced by electroporation into competent ElectroMAX DH10B cells (Invitrogen Corp., CA, USA). The clones containing Prp.-GFP sequences in proper orientation were identified by colony-lift hybridization using both GFP and Prp. DNA fragments as probes and restriction enzyme digestion of the constructed plasmid. The Prp.-GFP constructed plasmid was first linearized by digesting the DNA with the restriction enzyme EcoR V, which cuts once in the vector plasmid sequence, and then tested for GFP gene expression on rat primary neuronal cultures transfected by electroporation. To test whether the construct was viable, we transfected primary neuronal cultures by electroporation. Two days after electroporation, we found a small number of neurons or glia (< 0.1 %) that showed GFP expression. A positive control plasmid with a IE1 CMV promoter in place of the prion promoter was also used and transfected a much greater percentage (20 %) of cells.
All animal procedures were conducted in accordance with protocols approved by the Yale University Animal Care and Use Committee. Ova were harvested from pregnant mice anaesthetized with i.p. injections of 100 mg kg−1 ketamine and 10 mg kg−1 xylazine and subsequently killed with carbon dioxide. Ova were injected with a linearized and highly purified Prp-GFP sequence. Eggs were reimplanted into pseudopregnant mice, anaesthetised as described above. Tail biopsies, taken from the caudal fourth of the tail before P4 (see van den Pol & Ghosh, 1998), were used to detect mice expressing the transgene. Positive mice were then bred and brains of progeny were examined after fixation and sectioning to detect GFP-expressing neurons. Tests were also used in which brains of embryonic transgenic mice, taken from pregnant mice anaesthetised as above, and subsequently killed with carbon dioxide, were cultured on glass coverslips to determine if the transgene was expressed in developing cultured cells (van den Pol & Ghosh, 1998). A number of transgenic strains were generated. Of eight strains showing positive protein expression, GFP was found in the LC of two. In the present paper, we focus on one strain (Prp 57) with strong GFP expression in the LC.
Immunocytochemistry
After i.p. Nembutal (100 mg kg−1) administration, transgenic mice were perfused with saline followed by 4 % paraformaldehyde. Thick sections (10-50 μm) of the LC area were cut on a vibratome or freezing microtome. To verify the catecholamine content of GFP-expressing neurons, a rabbit anti-tyrosine hydroxylase antibody was used (1:2000) and this was localized with goat anti-rabbit IgG conjugated to Texas Red. The specificity of the tyrosine hydroxylase antiserum has been described in detail elsewhere (van den Pol et al. 1984). Cultures were fixed in 4 % paraformaldehyde and immunostained in parallel with the histological sections.
Extracellular recording from brain slices
Brain slices were prepared as described previously (Pineda et al. 1996). Briefly, male albino rats (130-180 g, Harlan) or transgenic GFP-expressing mice (18-25 g) were anaesthetized with chloral hydrate (400 mg kg−1, i.p.). Following decapitation, the brains were removed rapidly and trimmed in ice-cold (≈4 °C) artificial cerebrospinal fluid (ACSF) in which sucrose (252 mm) was substituted for NaCl (sucrose-ACSF). A block of tissue containing the LC was dissected and coronal slices (600 μm in rat, 400 μm in mouse) were cut in sucrose-ACSF with an oscillating-blade tissue slicer (DSK Microslicer, Ted Pella. Inc., Redding, CA, USA). A slice containing the LC was positioned on the stage of a fluid-gas interface chamber with the cerebral aqueduct facing an elevated fluid inlet. Fluid was wicked directly from the elevated inlet onto the slice (Burlhis & Aghajanian, 1987). The standard ACSF (pH≈7.35), equilibrated with 95 % O2-5 % CO2, contained (mm): NaCl, 128; KCl, 3; CaCl2, 2; MgSO4, 2; NaHCO3, 24; NaH2PO4, 1.25; and d-glucose, 10. The slices were incubated at 33 ± 0.5 °C and perfused at a rate of ≈1 ml min−1. A 2-3 h recovery period preceded the data collection. Drug solutions, in gassed ACSF, were introduced through a stopcock arrangement to the recording chamber with a latency of ≈20 s. Chemicals were obtained from several different sources: hypocretin-1/orexin A from Phoenix Pharmaceuticals (Belmont, CA, USA), hypocretin-2 from the Stanford University Peptide Facility (Stanford, CA, USA), TTX from Alomone Labs Ltd (Jerusalem, Israel) and met-enkephalin and 6-cyano-7-nitroquinoxaline-2,3-dione (CNQX) from Sigma RBI (St Louis, MO, USA).
Microelectrodes were pulled from filament-containing 1.5 mm glass tubing using a Brown-Flaming pipette puller (Sutter Instrument Co., Novato, CA, USA) and filled with 2 m NaCl. All recordings were made using an Axoclamp 2A amplifier (Axon Instruments, Inc., Union City, CA, USA) in the bridge mode; the output signal was filtered at 10 kHz. Single-unit activity was monitored continuously on an oscilloscope and distinguished with a custom-made window discriminator. The cells selected for study fired spontaneously and the firing could be inhibited by enkephalin (100 μM). The firing rate was continuously recorded on a thermal pen-recorder (Gould TA240, OH, USA).Using pCLAMP V 8.0 (Axon Instruments), the effects of drugs on single-unit activity were sampled by recording 15 s intervals before and during the peak of action of the drugs.
Whole cell recording of GFP-expressing LC cells in culture
Thick (500 μm) slices of LC were cut from E18-19 fetal mice, collected from dams anaesthetised with i.p. injections of 100 mg kg−1 ketamine and 10 mg kg−1 xylazine and subsequently killed with carbon dioxide. The embryonic mice were killed by cooling followed by decapitation. Under an Olympus SZX12 fluorescence microscope, the area of the LC was dissected out, using the GFP fluorescence of the LC as a simple approach to defining the correct cells. Cells were disaggregated by trituration in the presence of trypsin and then plated on poly-l-lysine coated glass coverslips, or directly on the bottom of 35 mm diameter tissue culture dishes. Cultures were maintained in Neurobasal medium from Gibco BRL supplemented with B27. Cells were maintained at 37o C in a Napco incubator in an atmosphere of 5 % CO2 in air.
All culture experiments were performed on neurons after 6-7 days in vitro. The recording chamber was continuously perfused at a rate of 1.5-2 ml min−1 with a bath solution containing (mm): NaCl, 150; KCl, 2.5; CaCl2, 2; MgCl2, 1; Hepes, 10; and glucose, 10; pH adjusted to 7.3 with NaOH. The patch pipette was made of borosilicate glass (World Precision Instruments, FL, USA) using a Narishige puller (PP-83; Narishigi Scientific Instrument Lab., Tokyo, Japan). The tip resistance of recording pipettes was 4-6 MΩ after being filled with pipette solution containing (mm): KCl, 145; MgCl2, 1; Hepes, 10; EGTA, 1.1; Mg-ATP, 2; and Na2-GTP; 0.5, pH adjusted to 7.3 with KOH. The liquid junction potential was between 2 and 4 mV and data were compensated for this here and below in slice recordings. Experiments were performed under current clamp. All data were sampled at 3-10 kHz and filtered at 1 kHz with an Apple Macintosh computer using AxoData 1.2.2 (Axon Instruments).
Whole cell recording in developing LC slices
LC slices (200-300 μm thick) were cut from P0-P2 mice killed by cooling followed by decapitation. The slices were rapidly transferred to a recording chamber perfused at 2 ml min−1 with oxygenated bath solution as described above. Experiments were performed after perfusing LC slices for 30-45 min. The patch pipette was made as described above and had a tip resistance 4-6 MΩ after being filled with pipette solution as described above. Using an Olympus BX51 microscope with fluorescence and infrared differential interference contrast (DIC), the LC area was easily identified and a patch pipette was used for whole cell recording on single identified green LC neurons. All data were sampled at 3-10 kHz and filtered at 1 kHz with an Apple Macintosh computer using AxoData 1.2.2 (Axon Instruments). For experiments examining resting membrane potential, outlier cells with resting membrane potentials more positive than −39 mV were excluded to avoid the possibility that this positive potential may be due to neuronal damage during slice preparation. In the Results, means are reported with s.e.m. unless otherwise noted. Both t tests and analysis of variance were used to compare statistical significance in different experiments.
Dual-cell recording was used to study the synchrony of spikes after hypocretin application. GFP-fluorescent cells in LC slices were detected with a fluorescence and infrared DIC upright Olympus microscope, as described above. Spikes were detected in the first cell with cell attached patch using an EPC9 amplifier (Heka, Lambrecht, Germany) and the second cell was recorded with an extracellular pipette (1-3 MΩ tip resistance) and a DAM50 amplifier (World Precision Instruments). Both signals were simultaneously recorded with Pulse software (Heka).
RESULTS
The first section of the Results describes the expression of GFP in LC neurons in the previously undescribed transgenic mouse used in the present paper. The second section examines the hypothesis that hypocretin enhances the activity and synchronous firing of LC neurons during early stages of development.
GFP expression in mature LC neurons
In live or fixed sections through the hindbrain, the LC was easily detected by its strong expression of GFP fluorescence (Fig. 1A) under regulation by a mouse prion promoter. Cell bodies, dendrites and outgoing axons all showed strong fluorescence. Paraformaldehyde treatment caused a slight decrease in fluorescence, but even after fixation the fluorescence was strong. As a control to verify the cellular identity, sections were immunostained with antisera against tyrosine hydroxylase, an initial enzyme in catecholamine synthesis. In tissue sections, based on examining 100 green LC cells from each of three brains, 98 % of the LC neurons showing GFP expression also showed tyrosine hydroxylase immunoreactivity, as detected with red immunofluorescence. A photomicrograph showing colocalization of GFP and tyrosine hydroxylase is shown in Fig. 1B and C. Successive generations of these transgenic mice (10 generations) consistently showed GFP expression in the LC. Heterozygotes showed GFP expression, suggesting the transgenic gene was dominant, and homozygotes showed a higher level of expression.
Figure 1. GFP expression in the LC.
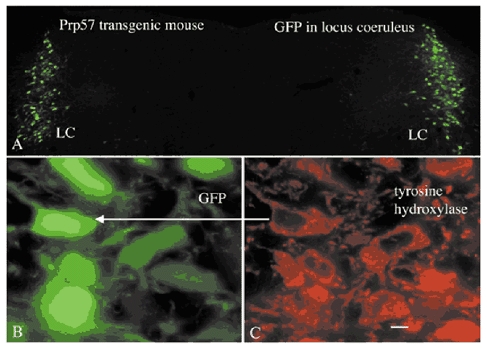
A, in this colour micrograph, GFP expression is seen bilaterally in the LC. Little GFP expression is seen in other cells here. B and C, sections were immunostained with tyrosine hydroxylase (TH) antisera, detected with secondary antiserum conjugated to Texas Red. The green fluorescence in B is due to endogenous expression of GFP and the red (C) shows TH immunoreactivity.
Two-photon microscopy was done using 920 nm laser excitation with a Biorad 2-photon microscope to increase detection of processes in thick sections. LC axons leaving the nucleus showed strong GFP expression, and could be followed a long distance (Fig. 2). In addition, proximal dendrites of LC cells show GFP fluorescence.
Figure 2. Two-photon microscopy was used to examine thick sections of the LC.
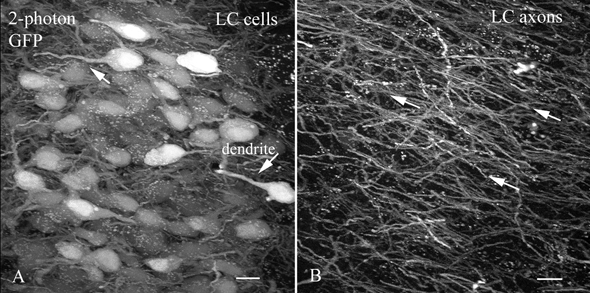
A, GFP expression is seen with 2-photon imaging in the LC. Some of the GFP-filled dendrites are indicated by arrows. There are some small white dots, for instance in the upper right; these are common in older mice, are found in both transgenic and non-transgenic controls, are probably lysosomal, are not related to GFP expression and are not restricted to the LC. Scale bar: 13 μm. B, outside the LC large numbers of LC axons are found coursing in a common direction toward the more rostral brain. Scale bar: 8 μm.
In the general area in which the LC is located, LC neurons stand out selectively due to their GFP fluorescence. GFP expression also occurs in a few other regions of the brain in this transgenic mouse, including some layers of the cerebral cortex, and in some laminae of the spinal cord.
GFP expression appears very early in embryonic LC development
Sections were cut in the LC area from fetal mice. GFP expression was already present by E15 and continued during development (Fig. 3, E 19) through birth and into the adult period. The paired GFP-expressing LC could be located in whole brain with fluorescence microscopy after the cerebellum was removed (Fig. 3B). This strong and selective GFP expression should facilitate detection of the LC for electrophysiological recording in acute in vivo experiments. The oldest mice we examined were ten months old and they had strong GFP fluorescence in the LC. In mature mice, the LC can be identified without the use of GFP fluorescence; in embryonic and young neonatal mice, detection of the noradrenergic cells of the LC was difficult in the absence of the GFP reporter gene.
Figure 3. Embryonic LC shows GFP expression.
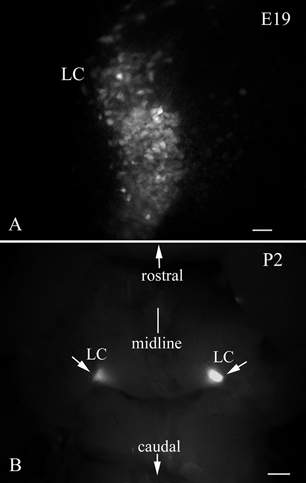
A, GFP expression is found in the LC in embryonic (E19) development. Scale bar: 40 μm. B, after removing the cerebellum from a P2 brain, the bilateral LC can be easily located in this dorsal view of the whole brainstem. Scale bar: 200 μm.
LC cells and processes in culture
The LC was harvested from 8-10 embryonic mice and plated either onto plastic or glass coverslip substrates pretreated with poly-l-lysine. Large GFP expressing neurons were found from 1 to 10 days after plating (Fig. 4) and accounted for about 2-5 % of the cells in culture when large pieces of tissue were used (Fig. 4C); other cells included neurons and glia with no GFP expression. In cultures of smaller pieces containing the LC, the percentage of positive cells was greater. Survival of the GFP-expressing LC cells was enhanced by culturing with other neurons and glia from the same region of the brain. To verify that the GFP-expressing neurons were catecholamine neurons, cultures were immunostained with tyrosine hydroxylase antisera. Large cells showing tyrosine hydroxylase immunostaining (Fig. 4B) also showed GFP fluorescence (Fig. 4A). In positive neurons, long GFP-expressing processes grew out from the cultured perikarya (Fig. 5A). Processes grew out many hundreds of microns (Fig. 5B) and established boutons en passant with unlabelled dendrites of non-LC neurons (Fig. 5C). The fact that the processes of LC cells expressed GFP may prove useful for future studies examining the regulation of axonal growth and pathfinding in these cells and for studying postsynaptic responses to activation of LC neurons.
Figure 4. LC neurons express GFP and tyrosine hydroxylase in culture.
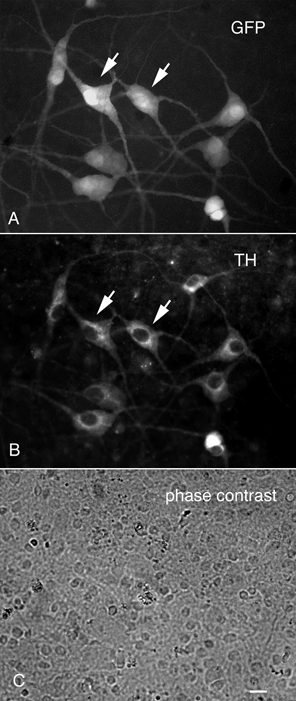
The LC and surrounding brain were dispersed and cultured on glass coverslips. A, LC neurons retained their GFP expression. B, immunostaining for tyrosine hydroxylase (TH) showed that most neurons showing TH immunoreactivity also showed GFP expression. C, a phase contrast micrograph of the area shown in A and B reveals a large percentage of unlabelled cells, many of them glial cells. Scale bar: 5 μm.
Figure 5. GFP in LC neuronal processes.
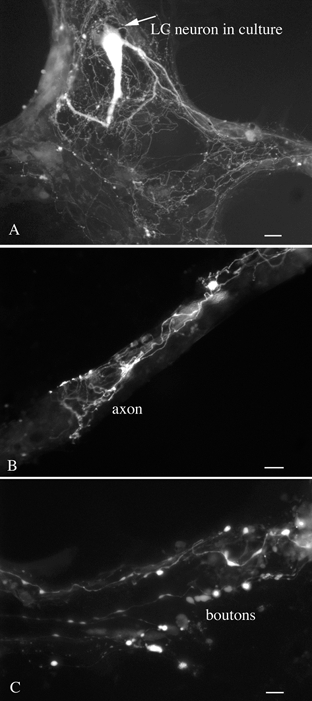
A, a single LC neuron growing on an astrocyte shows GFP fluorescence in its dendritic and axonal processes. Scale bar: 15 μm. B, a higher magnification of an LC axon growing over an unlabelled bundle of neurites. Scale bar: 8 μm. C, Large boutons from an LC neuron are found contacting dendrites of an unlabelled cell. Scale bar: 4 μm.
Membrane properties of GFP expressing LC neurons
To determine whether the GFP expressed by LC neurons in transgenic mice altered the membrane properties of LC cells, we compared the physiological properties of GFP-expressing LC neurons (n = 14) from slices of transgenic mice to non-GFP expressing LC neurons (n = 13) from non-transgenic mice of the same strain at postnatal day 5 ± 2. LC neuron resting membrane potentials (RMP) were −49.2 ± 1.7 mV for GFP expressing slices and −49.8 ± 2.1 mV for non-GFP expressing slices, i.e. not significantly different (P > 0.8). This RMP is slightly more positive than that in reports based on more mature rat LC neurons (53-54 mV; Nieber et al. 1998; Regenold & Illes, 1990), consistent with the view that developing neurons generally show a relatively positive RMP compared with mature neurons (Chen et al. 1996). The input resistances were 219 ± 17 and 236 ± 13 MΩ and spike thresholds were −37.2 ± 1.6 and −38.8 ± 1.9 mV (means ± s.e.m.) for GFP and non-GFP expressing LC neurons, respectively, i.e. neither of these comparisons showed a statistically significant difference. We also compared current-voltage relations in these neurons and found little difference; the mean current-voltage relations for the two groups are shown in Fig. 6C from −20 to +120 pA. Both groups also showed similar increases in spike frequency during depolarization (Fig. 6). In addition, we compared inward currents evoked by hypocretin and found no difference between the two groups, as examined in detail below. We conclude from this set of experiments that the expression of GFP in the LC of the transgenic mice used in the present report does not alter the physiological properties of these neurons in comparison with LC neurons that do not express GFP.
Figure 6. GFP expression does not alter the physiological properties of LC neurons.
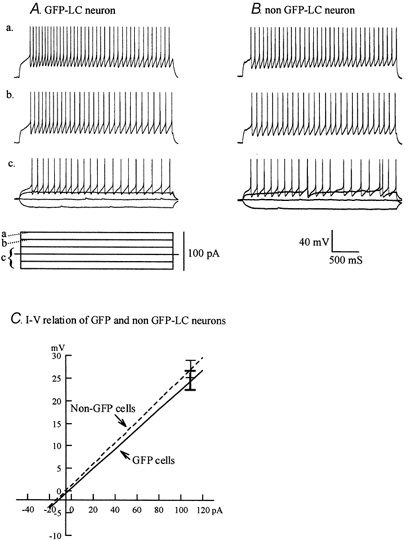
LC cells from transgenic mice that express GFP (A) or from other mice that do not express GFP (B) were compared. A series of current steps (a-c) show that the responses of the LC cells are similar. C, I-V relations from each recorded cell in a group were combined (n = 14 for GFP slices; n = 13 for non-GFP slices). Error bars show overlap in s.e.m. No statistically reliable difference was found between the two groups (P > 0.2).
Dose-response relationship in rat and mouse LC slices
Hypocretin neurons make two neuroactive peptides from preprohypocretin. These are hypocretin-1 (orexin A) and hypocretin-2 (orexin B). The rat LC has been shown to respond to both hypocretin-2 (Horvath et al. 1999; Ivanov & Aston-Jones, 2000) and hypocretin-1 (Hagan et al. 1999). As the primary receptor reported in the LC is the hypocretin receptor-1 (Trivedi et al. 1998) which has been reported to have a greater sensitivity to hypocretin-1 (Sakurai et al. 1998), we examined the firing rate of adult mouse LC cells during stimulation with different concentrations of hypocretin-1 and −2, using single-unit recording in slices. Neurons in the mouse LC, which has not been studied previously in the context of hypocretin responses, showed a substantial increase in spike frequency in response to hypocretin-1 with a mean increase of 234 % for 1 μM hypocretin-1. Spike frequency was increased by the application of hypocretin-1 in a dose-dependent manner. Figure 7A shows typical responses of a single LC neuron to hypocretin-1 and a typical spike is shown in an expanded scale at the bottom of the figure. The dose-response relation is shown in graphic form in Fig. 7B. Based on peptide-induced changes in spike frequency in LC slices, the EC50 dose for hypocretin-1 was 390 nm.
Figure 7. Hypocretin-1 increases firing rate.
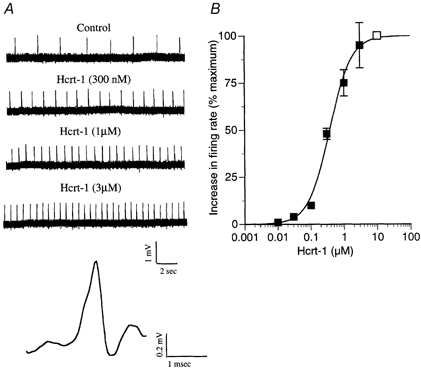
A, the effect of different concentrations of hypocretin-1 (Hcrt-1) on the frequency of action potentials in the mouse LC was tested. A positive correlation was found between concentration and spike frequency. A typical spike is shown at the bottom. B, a dose-response curve is shown. The open square at 10 μM is defined as 100 %; a small increase in spike frequency was found with 30 nm hypocretin-1. The number of neurons recorded for each concentration of the dose-response curve were (starting at lowest concentration on left) n = 4, 4, 5, 15, 15, 3.
In the mouse, hypocretin-1 was considerably more effective than hypocretin-2 (n = 33 neurons); an increase in firing rate was found with 30-100 nm hypocretin-1 but a similar concentration of hypocretin-2 had no detectable effect on firing. However, at higher concentrations hypocretin-2 did increase firing. Met-enkephalin (100 μM) was used as a control. Previous work has shown that LC neurons show a characteristic strong inhibitory response to enkephalin that recovers rapidly upon washout (Williams et al. 1982). As expected, LC neurons were completely inhibited by enkephalin. A dose-response evaluation of the two hypocretin peptides is shown in the bar graph in Fig. 8B. The response to hypocretin-1 showed signs of desensitization even before the peak response was reached, as evidenced by the spontaneous reduction in spike frequency in the constant presence of hypocretin (Fig. 8A). An inherent problem in testing hypocretin dose-response relations in a slice is desensitization to the peptide as the maximal concentration is approached. Thus, the responses found here in LC slices would tend to overestimate the actual concentrations that might generate a specific level of increase in spike frequency.
Figure 8. Mouse LC slices show greater response to hypocretin-1 than to hypocretin-2.
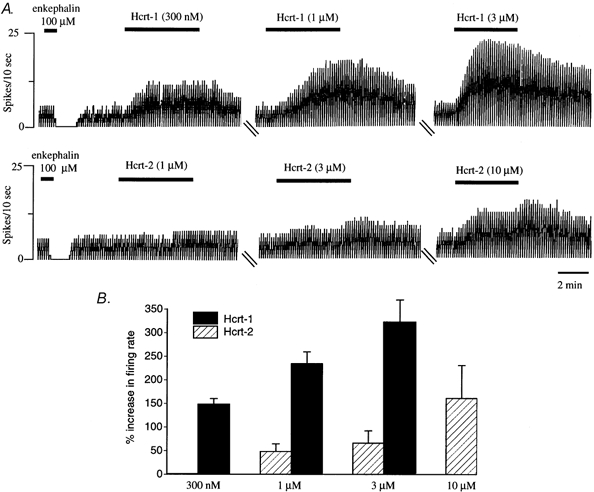
Responses to hypocretin-1 and −2 were tested with single-unit recording. A, neurons showed a complete block of spikes in the presence of enkephalin (100 μM) and then recovered after washout, a feature of LC neurons. Three concentrations of either hypocretin-1 (Hcrt-1) or −2 (Hcrt-2) were tested in the two representative cells shown in this figure. Cells showed an increasingly greater spike frequency as the concentration of the peptides increased. After peptide washout, cells returned toward their baseline spike frequency. At the higher concentrations of peptide, desensitization began soon, potentially obscuring a peak response to the peptide. B, bar graph showing the relative responses to different concentrations of the peptide. No response was found to 300 nm hypocretin-2. Due to the strong desensitization during application of 3 μM hypocretin-1, higher concentrations were not used. n = 15 (300 nm Hcrt-1); n = 12 (300 nm Hcrt-2); n = 15 (1 μM Hcrt-1); n = 12 (1 μM Hcrt-2); n = 3 (3 μM Hcrt-1); n = 5 (3 μM Hcrt-2); n = 3 (10 μM Hcrt-2).
In a further analysis, we compared the response of rat LC slices to mouse LC slices and found them to respond to hypocretin peptides in a qualitatively similar manner. Both rat (n = 18) and mouse (n = 33) showed a similar enhanced sensitivity of LC neurons to hypocretin-1 compared with hypocretin-2. Neurons from both species were excited by 300 nm hypocretin-1, but showed almost no response to the same concentration of hypocretin-2. Both responded to 1 μM hypocretin-2 (Fig. 9).
Figure 9. Comparison of rat and mouse LC.
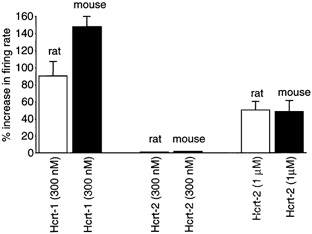
Bar graph showing the relative responses of 51 LC neurons from slices of rat (n = 18 neurons) and mouse (n = 33 neurons), tested in parallel experiments. The responses tend to be similar, with a modest response to hypocretin-2 (Hcrt-2) and a strong response to hypocretin-1 (Hcrt-1).
Hypocretin initiates spikes in non-spiking LC cells during early development
Mouse slices containing the LC were recorded on the day of birth, or within the 2 days after birth, using whole cell current clamp to facilitate study of hypocretin-mediated influences on membrane potential that would not be detectable with the single-unit recording used above. During this developmental period, a number of LC cells showed no spikes whereas other cells did spike. Responses to hypocretin-1 in developing LC slices were excitatory. In some non-spiking neurons (n = 3), hypocretin-1 induced a dramatic membrane depolarization without generating spikes, as shown in Fig. 10A. The depolarization in membrane potential reached its peak immediately after the onset of application of hypocretin-1 and soon desensitized. The depolarized membrane potential returned to baseline after the washout of hypocretin-1. In neurons already showing a low spike baseline, hypocretin-1 increased the frequency of spontaneous spikes without evoking a substantial depolarization (Fig. 10B); the absence of a depolarization in these cells may be due to the strong afterhyperpolarization typical of LC neurons, which would tend to repolarize the membrane potential. In some developing neurons, hypocretin-1 caused a very strong burst of spikes that included a continued depolarization (Fig. 10C). The depolarization induced by hypocretin-1 was from 3.7 to 11.3 mV with a mean of 7.0 ± 1.6 mV (n = 5). The frequency of spontaneous spikes increased by 8-fold, from 0.2 ± 0.1 Hz to 1.6 ± 0.6 Hz, which was significant as suggested by statistical analysis with a t test (P < 0.05, n = 6). Upon washout, neuronal behaviour returned to a less excited level. In eight cells (P1-P5) showing no spikes at resting membrane potential, the membrane potential was depolarized by injection of positive current to 5 to 10 mV positive to rest. As the command depolarization increased all eight cells showed spikes, which then disappeared upon return to resting membrane potential. These data suggest that hypocretin increases spike probability in developing LC neurons by a mechanism that may involve depolarization, as discussed further below.
Figure 10. Hypocretin-1 actions in LC slice at postnatal day 1.
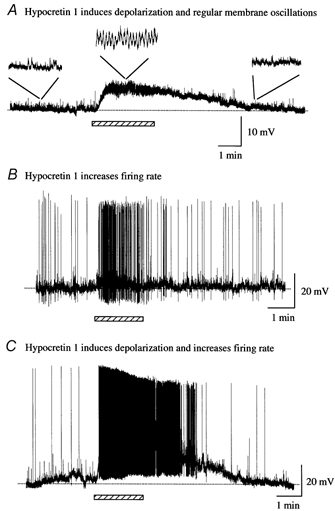
A, hypocretin-1 (1 μM) evoked a strong depolarization of this developing LC cell. In addition, in the presence of hypocretin-1, regular membrane oscillations appeared that were absent in control and washout conditions. B, hypocretin-1 evoked an increase in spike frequency in this developing LC neuron with little membrane depolarization. C, hypocretin-1 depolarized the membrane potential and dramatically increased spike frequency in this developing neuron from an LC slice.
A number of developing LC neurons (n = 4) in slices showed no spikes and no membrane oscillations before hypocretin application. The addition of hypocretin-1 to these cells initiated the appearance of regular oscillations (0.5-1 Hz) in membrane potential of several millivolts (Fig. 10A). Other neurons with no spikes did show baseline cyclical periods of membrane voltage fluctuations with an amplitude, peak to trough, of between 2.9 and 7.4 mV and a mean of 4.5 ± 0.6 mV (n = 5). The frequency of the spontaneous oscillation was between 0.5 and 1.6 Hz with an average of 1.0 ± 0.2 Hz (n = 5). A spontaneous and regular fluctuation in membrane potential has previously been shown to be a consequence of electrotonic coupling with other LC neurons (Christie et al. 1989). Of considerable interest, when hypocretin-1 (1 μM) was applied to these non-spiking cells, an increase in the frequency of these subthreshold membrane oscillations became apparent (Fig. 11). The frequency of oscillations could increase by 300 %, coupled with a depolarization of the membrane potential. Hypocretin-1 (1 μM) could also trigger action potentials out of this spontaneous subthreshold membrane oscillation, as shown in Fig. 11B. After peptide washout, spike frequency returned to baseline levels, and membrane oscillations slowed (Fig. 11C).
Figure 11. Hypocretin-1 initiates spikes and increases frequency of membrane voltage oscillations.
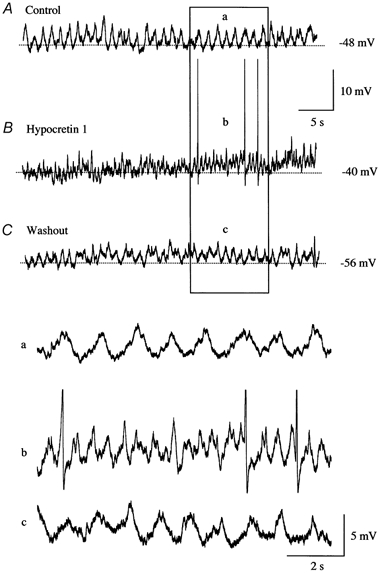
A, this neuron from a mouse LC slice showed no spikes under control conditions, but did show a regular pattern of membrane oscillations. B, when hypocretin-1 was added, the frequency of membrane oscillations increased substantially, and spikes were found. C, after washout of the peptide, spikes were no longer found and the frequency of membrane oscillations returned toward baseline levels. Lower panel, higher resolution traces are shown, corresponding to the boxed area above. Spikes are truncated in b.
The application of hypocretin-2 (1 μM) induced an increase in the frequency of spontaneous spikes in LC slices from P0-P2 mice (Fig. 12). The frequency of spontaneous spikes increased from 3.4 ± 1.0 min−1 to 14.7± 3.1 min−1 during the application of hypocretin-2 in the four neurons tested and returned to 3.0 ± 1.1 min−1 after peptide washout.
Figure 12. Hypocretin-2 increases spike frequency.
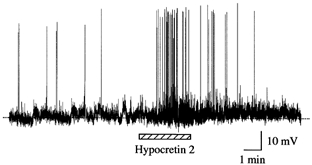
In this postnatal day 1 slice, hypocretin-2 increased the spike frequency and this increase returned to baseline after hypocretin-2 washout.
The increase in excitatory activity induced by hypocretin could result from a decreased K+ current after an action potential (Horvath et al. 1999; Hagan et al. 1999), from an increase in the release of excitatory transmitters (van den Pol et al. 1998) or from a direct depolarization. To study a mechanism that might be involved in the increase in spike frequency, we tested whether membrane depolarization induced by hypocretin-1 was responsible for the increase in spike frequency, using current clamp recording in mouse LC slices. After hypocretin-1 (1 μM) increased spike frequency with a concomitant depolarization, we used a command hyperpolarization in the form of negative current to return the membrane potential back to its pre- hypocretin baseline while in the presence of hypocretin. This eliminated the hypocretin-1 induced spike increase (Fig. 13A) in the seven neurons recorded for this experiment. Prior to hypocretin application, the spontaneous spike frequency, defined as 100 %, was 0.31 ± 0.12 Hz. Hypocretin raised the spike frequency by a factor of 642 ± 135 % greater than baseline; when the cells were brought back to their control level baseline membrane potential by negative current injection, the spike frequency returned to its baseline level of 101 ± 16 %. The hypocretin-mediated increase and the command return to baseline were statistically significant (P < 0.05). Further hyperpolarization to a membrane potential 10 mV negative to the resting membrane potential in the presence of hypocretin (n = 9 neurons) blocked spikes. To further study mechanisms of hypocretin-mediated excitation, we used voltage clamp recording to determine what type of current was evoked by hypocretin (n = 27 neurons). In the presence of TTX (1 μM) to eliminate action potentials and CNQX (10 μM) to block AMPA and kainate-type glutamate responses that otherwise might cause a membrane depolarization, we found that hypocretin-1 (1 μM) evoked a consistent inward (excitatory) current of 28 ± 3 pA (n = 14). For this experiment we compared LC cells from GFP-expressing mice with LC cells from non-GFP expressing mice. We found no differences in the response to hypocretin in the two groups, with a 27 ± 6 pA current in GFP-expressing LC cells (n = 8) and 28 ± 3 pA in non-GFP cells (n = 6), suggesting that GFP expression does not alter the response of LC neurons to this peptide.
Figure 13. Hypocretin activates inward sodium current and depolarization.
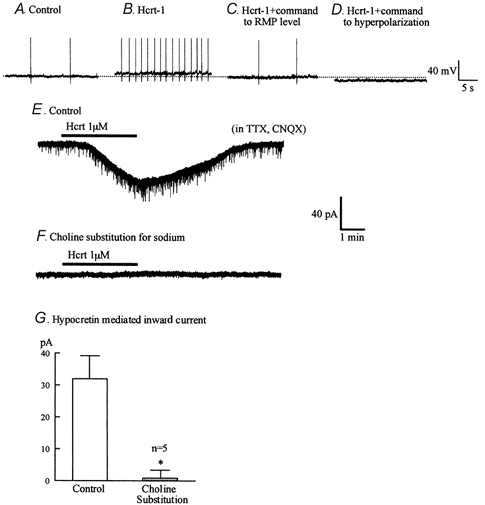
A-D, hypocretin-1 (1 μM) causes a depolarization and dramatic increase in spike frequency. When the membrane potential was returned to its control level (-60 mV) the number of spikes returned to a baseline frequency. When the cell was further hyperpolarized, spikes were stopped. E-F, hypocretin-1 (1 μM) evoked an inward current. When choline was substituted for sodium, hypocretin no longer evoked an inward current in the same cell that had previously responded to hypocretin. Holding potential −60 mV. G, bar graph showing the amplitude of inward current evoked by hypocretin in normal buffer and the same five cells after choline substitution. * Statistically significant difference (P < 0.05).
To test the hypothesis that the inward current was based on sodium, we substituted choline chloride for sodium chloride in the presence of TTX (1 μM) and CNQX (10 μM). Neurons (n = 5) that showed an inward current in response to hypocretin-1 (1 μM) in the presence of sodium (32 ± 7 pA) showed little hypocretin response when the extracellular sodium was replaced with choline (1 ± 2 pA) (Fig. 13D-F). Together these data suggest that hypocretin increases spike frequency at least in part by activating a TTX-insensitive inward sodium based current to depolarize the neuron. A similar inward current activated by substance P has been described previously (Shen & North, 1992).
Hypocretin increases synchronous spikes
To determine if hypocretin might increase the synchrony of spikes recorded simultaneously from different LC neurons, we used dual-cell recording with cell attached recording of one cell and an external glass pipette recording the activity of the second cell in the same LC slice. Electrodes were separated by 200-300 μm and recorded the activity of non-adjacent cells in the LC; GFP fluorescent neurons were detected with a fluorescence-infrared DIC microscope. In 11 of 12 pairs of cells recorded from slices of mice from postnatal day 1 to 5, bath application of hypocretin-2 (5 μM) caused an increase in spike synchrony. An example of one pair of recordings is shown in Fig. 14. To statistically evaluate the change in synchrony, we counted the number of spikes in the second cell that occurred within an interval from the first spike that was < 10 % of the mean interspike interval of the first neuron; if two cells had different initial spike frequencies, the faster one was used as the first cell. From each pair of recorded cells, the number of spikes in the second cell that occurred synchronously with 100 spikes of the first cell was determined. In the control condition prior to hypocretin application, the percentage of synchronized spikes was 15.5 ± 7 %; after hypocretin application, the number of synchronized spikes increased to 257 %, to 39 ± 7 %. This increase in synchronized firing was significant (t test; P < 0.05). The range in increase of synchronized spikes in the 11 pairs showing an increase was from 2- to 8-fold; only one pair with a high level of synchrony in control buffer showed no increase in the presence of the peptide.
Figure 14. Hypocretin increases synchrony of action potentials in developing LC slices.
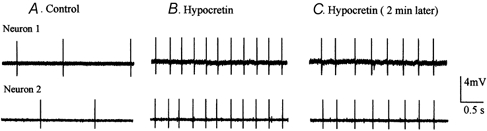
A, during a control period in normal buffer, spikes recorded from two cells in a neonatal mouse LC slice appeared random with respect to each other. B, after hypocretin-2 (5 μM) was added to the bath the action potentials of the two cells showed a substantially higher degree of synchrony. C, 2 min after the trace in B, neuron synchrony remained high. Spikes are truncated.
Tissue culture electrophysiology
Because the LC cells of the transgenic mouse continued to express GFP in culture, we could identify and record from them with whole cell pipettes. In cultured LC neurons (< 7 days in vitro; n = 10) studied with current clamp, spontaneous postsynaptic potentials, but not action potentials, were found. Hypocretin-1 (1 μM) depolarized the membrane potential from −51.9 ± 3.6 mV to −47.2 ± 3.5 mV in four of six tested neurons. The membrane potential returned to −50.6 ± 4.9 mV after the washout of hypocretin-1. Hypocretin-evoked membrane depolarizations could reach the spike threshold, as shown in Fig. 15A. The effect of hypocretin-2 was also tested in cultured LC neurons. Hypocretin-2 (1 μM) caused only a moderate depolarization in membrane potential (Fig.15B). The membrane potential increased from −63.1 ± 2.6 mV to −60.8 ± 1.6 mV during the application of hypocretin-2 and returned to −63.2 ± 1.8 mV after the washout of hypocretin-2 in four tested neurons. These data suggest that LC neurons in culture continue to synthesize GFP and hypocretin receptors.
Figure 15. Hypocretin depolarizes dissociated LC neurons in culture.
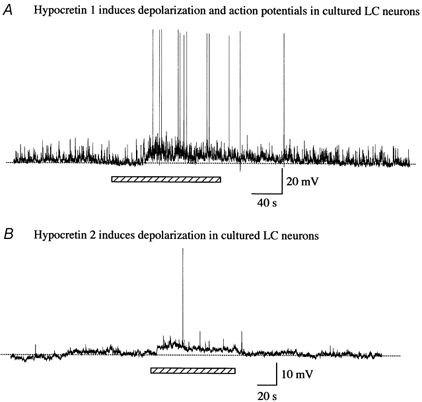
Hypocretin-1, added during the period indicated by the bar, depolarized the membrane potential resulting in the occurrence of action potentials. B, hypocretin-2 also evoked a modest depolarization and slight increase in spike frequency.
DISCUSSION
As indicated in the Introduction, the LC develops early and may play a role in the modulation of development of other neuronal loci that it innervates. From that perspective, it is of interest that the hypocretin system also develops early, that hypocretin receptor expression is found in the embryonic brain and that hypocretin physiological responses are robust on the day of birth in the lateral hypothalamus (van den Pol et al. 2001). In parallel, during embryonic development, long hypocretin immunoreactive fibres are found throughout the CNS.
Hypocretin axons make synaptic contact both with LC perikarya and with long LC dendrites (Horvath et al. 1999). A number of reports have shown that the LC expresses primarily the hypocretin receptor-1 (orexin R-1) (Trivedi et al. 1998; Bourgin et al. 2000). This receptor has been reported to respond primarily to hypocretin-1 (Sakurai et al. 1998). In the present paper we compared rat and mouse LC responses to hypocretin-1 and −2 and found roughly similar response characteristics with dose response evaluations in both species. Hypocretin-1 was substantially more effective, by about an order of magnitude, in generating an increase in spike frequency than hypocretin-2.
LC cells have an intrinsic pacemaker (Andrade & Aghajanian, 1984; Williams et al. 1984) and a regular firing pattern is a signature feature of mature LC cells. Even in the presence of TTX to block voltage-dependent sodium spikes or in the presence of amino acid transmitter receptor antagonists, the pacemaker continues and calcium spikes are generated at regular intervals (Ishimatsu & Williams, 1996). Electrical- and dye-coupling, suggestive of gap junctions, are common in developing LC cells and reduced in frequency with age (Christie et al. 1989; Christie & Jelinek, 1993). In the embryonic LC, cell synchrony can be found (Sakaguchi & Nakamura, 1987). We find during neonatal development that individual cells that normally show no spikes begin to fire in the presence of hypocretin. Furthermore, hypocretin initiates the appearance of regular membrane voltage oscillations in non-oscillating LC cells and substantially increases the synchrony of spike firing tested with dual-cell recording, consistent with the view that the peptide not only increases activity, but also increases the synchrony of output activity. The increase in synchrony may result from excitation mediated by an inward cation current carried primarily by Na+, parallel to an increase in synchrony reported for depolarization induced by glutamate agonists (Ishimatsu & Williams, 1996). These latter results imply that the hypocretin-induced increase in synchrony is secondary to depolarization and increase in spiking activity, rather than to a specific property of hypocretin itself. An increase in synchronized LC output is associated with improved performance on cognitive tasks (Usher et al. 1999). Thus hypocretin would increase the activity of single LC neurons and would also increase the synchronized output during early neonatal development. The early response of the LC to hypocretin suggests that the lateral hypothalamus, acting through hypocretin, could improve task-related cognitive processing, which correlates with synchrony of LC firing, even at a relatively early stage of brain development.
One interesting feature of the developing LC is that it responds to sensory stimuli even during embryonic development (Sakaguchi & Nakamura, 1987). In addition, despite the relative paucity of synaptic input, many cells in the LC respond simultaneously to unimodal sensory input. As it is unlikely that a large percentage of LC neurons would receive specific axonal input from cortical regions during early development, electrotonic coupling may increase the spread of the input within the LC. In that context, during development, hypocretin may act to modulate the general gating of LC neurons responding to sensory input and thereby play a role in early selective attention or arousal. In the context of previous work suggesting that the LC innervation of cortical regions may play a role in synapse formation or the modulation of dendritic growth and spine extension, this hypothetically would give the hypocretin neurons in the lateral hypothalamus (LH) a potential role in modulating development of higher brain regions through an LC intermediate. Hypocretin may provide a mechanism whereby hypothalamic neurons that reduce sleepiness and increase arousal in the mature brain may modulate cortical development through the noradrenergic neurons of the LC.
Interest in hypocretin has been sparked by the finding that it plays a key role in narcolepsy, a disease characterized by unusual REM sleep, increased daytime sleepiness and hypnogogic hallucinations; many narcoleptics also have cataplexy, where a sudden emotion will cause temporary paralysis. Loss of the hypocretin receptor-2 (orexin receptor-2) (Lin et al. 1999) or loss of the peptide (Chemelli et al. 1999) causes narcolepsy in animals. The majority of human narcoleptics have no hypocretin in their CSF (Nishino et al. 2000) and, based on postmortem analysis, have no or only a small number of hypocretin neurons in their brains compared with the brains of non-narcoleptic controls (Peyron et al. 2000; Thannickal et al. 2000). Intraventricular application of hypocretin-1 enhanced arousal (Hagan et al. 1999) and local administration of hypocretin-1 to the LC suppressed REM sleep and increased wakefulness (Bourgin et al. 2000), underlining the potential importance of the hypocretin projection to the LC. The developmentally early response to hypocretin by LC neurons suggests that hypocretin may be in a position to regulate arousal even before normal sleep patterns have matured.
We previously showed that, in the lateral hypothalamic area, hypocretin evoked a strong excitatory response in neonatal rats, increasing spike frequency and synaptic activity. This is consistent with an early high level of expression for mRNA coding for each of the two cloned hypocretin receptors (van den Pol et al. 2001). Hypocretin can enhance activity of developing LC neurons by an increase in TTX-insensitive inward sodium current (as shown here). This current appears similar to the TTX-insensitive non-selective cation current induced in LC neurons by another Gq-coupled protein neuroactive peptide, substance P, a current that was also dependent on extracellular sodium (Shen & North, 1992). As previously reported (Hagan et al. 1999; Horvath et al. 1999), a hypocretin-mediated decrease in K+ currents may also play an excitatory role, probably by increasing the speed of recovery after spiking.
At present, good selective hypocretin receptor antagonists are not available, so it is difficult to examine the physiological or developmental actions of hypocretin released from axon terminals in the LC.
Advantage of GFP expression in LC neurons
The expression of GFP in LC neurons in the transgenic mice described in the present paper has some advantages for several types of experiments. Although in adults the LC can be readily identified without GFP expression, the LC in the embryonic and neonatal mouse is more difficult to find in the absence of the early GFP expression. Most of our recordings were made within a few days of birth, but since GFP expression is found even earlier in the LC, future experiments can address the actions and roles of a number of neuromodulators during early embryonic development. The strong level of GFP expression can also be used to locate the LC in acute in vivo electrophysiological experiments. The LC has become a major model system for the study of cellular mechanisms underlying the action of drugs of abuse, including opiates and anti-depressants (Alreja & Aghajanian, 1993; Nestler et al. 1999), in part based on mechanisms related to long term activation or depression of second messenger molecules such as cAMP. In the past in vitro experiments utilizing extended application of drugs to study cellular mechanisms of dependence, tolerance and habituation with LC neurons were complicated by problems in identifying live LC neurons. Now that LC neurons can be identified in culture by their green fluorescence, novel experiments involving in vitro application of opiates and antidepressants may be explored that otherwise would have not been possible. The use of these mice may also facilitate study of axonal growth and target selection by GFP-expressing LC neurons in vitro or in vivo after transplantation.
Prion promoter
This appears to be the first paper to report the use of the mouse prion promoter with a GFP reporter gene. Transgenic mice could also be generated with a tyrosine hydroxylase or dopamine β-hydroxylase promoter to give selective expression in subsets of catecholamine neurons; however, neurotransmitter-related promoters are sometimes weak, whereas the prion promoter is fairly strong. The strong expression of the reporter in the LC raises the question of whether there is something special about the LC relating to prion disease. First, although some of our other transgenic lines generated with the prion promoter did express in the LC, some did not. Furthermore, as different lines of transgenic mice that we raised showed different patterns of expression, this suggests that the prion promoter, at least as used in the context of the present paper, may be influenced by local gene sequences in the chromosomal region where the transgene was incorporated. The prion promoter has been used in a number of interesting experiments examining the contribution of various agents to neurodegenerative disease in rodent models (e.g. Borchelt et al. 1996; David et al. 1998; Schilling et al. 1999) and expression of those transgenes was found in a number of regions of the brain. It is notable that in the present study transgene expression was found early in development, indicating that the prion promoter can be active in embryonic development. Finally, the prion protein is a common one found throughout the brain; its function in the normal brain is not understood. It appears to play an important role in prion-related neurodegenerative diseases such as kuru, mad cow disease, scrapie and Creutzfeldt-Jakob disease, perhaps related to an infectious ‘scrapie’ form of the prion protein (Prusiner, 1996) which is outside the scope of the present paper.
In summary we show that a new transgenic mouse expresses GFP with a prion promoter in the LC at early stages of development, and that LC neurons continue to express GFP in culture. Using GFP expression as a tool to find the developing LC in the neonatal mouse, we showed a substantial excitatory response to hypocretin, mediated in part by a TTX-insensitive depolarizing cation current, and found that hypocretin also evokes an increase in the synchronized activity of developing LC cells, potentially influencing the LC in its own role in modulating plasticity and function in developing higher brain regions.
Acknowledgments
We thank Y. Yang for excellent technical assistance and Dr D. Borchelt for the prion promoter plasmid. Grant support was provided by NIH NS 10174, NS34887, NS41454 and the National Science Foundation.
REFERENCES
- Alreja M, Aghajanian GK. Opiates suppress a resting sodium-dependent in addition to activating an outward potassium current in locus coeruleus neurons. Journal of Neuroscience. 1993;13:3525–3532. doi: 10.1523/JNEUROSCI.13-08-03525.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrade R, Aghajanian G. Locus coeruleus activity in vitro: intrinsic regulation by a calcium-dependent potassium conductance but not α-2-adrenoceptors. Journal of Neuroscience. 1984;4:161–170. doi: 10.1523/JNEUROSCI.04-01-00161.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aston-Jones G, Bloom FE. Activity o f norepinephrine-containing locus coeruleus neurons in behaving rats anticipates fluctuations in the sleep-waking cycle. Journal of Neuroscience. 1981;1:876–886. doi: 10.1523/JNEUROSCI.01-08-00876.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blue ME, Parnavelas JG. The effect of neonatal 6-hydroxydopamine treatment on synaptogenesis in the visual cortex of the rat. Journal of Comparative Neurology. 1982;205:199–205. doi: 10.1002/cne.902050211. [DOI] [PubMed] [Google Scholar]
- Borchelt DR, David J, Fischer M, Lee MK, Slunt HH, Ratovitsky T, Regard J, Copeland NG, Jenkins NA, Sisodia SS, Price DL. A vector for expressing foreign genes in the brains and hearts of transgenic mice. Genetic Analysis. 1996;13:159–163. doi: 10.1016/s1050-3862(96)00167-2. [DOI] [PubMed] [Google Scholar]
- Bourgin P, Huitron-Resendiz S, Spier AD, Fabre V, Morte B, Criada JR, Sutcliffe JG, Henriksen SJ, De Lecea L. Hypocretin 1 modulates rapid eye movement sleep through activation of locus coeruleus neurons. Journal of Neuroscience. 2000;20:7760–7765. doi: 10.1523/JNEUROSCI.20-20-07760.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burlhis TM, Aghajanian GK. Pacemaker potentials of serotonergic dorsal raphe neurons: contribution of a low-threshold Ca2+ conductance. Synapse. 1987;1:582–588. doi: 10.1002/syn.890010611. [DOI] [PubMed] [Google Scholar]
- Cedarbaum JM, Aghajanian GK. Afferent projections to the rat locus coeruleus as determined by a retrograde tracing technique. Journal of Comparative Neurology. 1978;178:1–16. doi: 10.1002/cne.901780102. [DOI] [PubMed] [Google Scholar]
- Chemelli RM, Willie JT, Sinton CM, Elmquist JK, Scammel T, Lee C, Richardson JA, Williams SC, Xiong Y, Kisanuki Y, Fitch TE, Nakazato M, Hammer RE, Saper CB, Yanagisawa M. Narcolepsy in orexin knockout mice: molecular g enetics of sleep regulation. Cell. 1999;98:437–451. doi: 10.1016/s0092-8674(00)81973-x. [DOI] [PubMed] [Google Scholar]
- Chen G, Trombley P, Van Den Pol AN. Excitatory actions of GABA in developing neurons. Journal of Physiology. 1996;494:451–464. doi: 10.1113/jphysiol.1996.sp021505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christie MJ, Jelinek HF. Dye-coupling among neurons of the rat locus coeruleus during postnatal development. Neuroscience. 1993;56:129–137. doi: 10.1016/0306-4522(93)90568-z. [DOI] [PubMed] [Google Scholar]
- Christie MJ, Williams JT, North RA. Electrical coupling synchronizes subthreshold activity in locus coeruleus neurons in vitro from neonatal rats. Journal of Neuroscience. 1989;9:3584–3589. doi: 10.1523/JNEUROSCI.09-10-03584.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coyle JT, Molliver ME. Major innervation of newborn rat cortex by monoaminergic neurons. Science. 1977;196:444–447. doi: 10.1126/science.850788. [DOI] [PubMed] [Google Scholar]
- Davis JA, Naruse S, Chen H, Eckman C, Younkin S, Price DL, Borchelt DR, Sisodia SS, Wong PC. An Alzheimer's disease-linked PS1 variant rescues the developmental abnormalities of PS1-deficient embryos. Neuron. 1998;20:603–609. doi: 10.1016/s0896-6273(00)80998-8. [DOI] [PubMed] [Google Scholar]
- De Lecea L, Kilduff TS, Peyron C, Gao XB, Foye PE, Danielson PE, Fukuhara C, Battenberg ELF, Gautvik VT, Bartlettii FS, Frankel WN, van den Pol AN, Bloom FE, Gautvik KM, Sutcliffe JG. The hypocretins: two hypothalamic peptides with neuroexcitatory activity. Proceedings of the National Academy of Sciences of the USA. 1998;95:322–327. doi: 10.1073/pnas.95.1.322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felten DL, Hakan H, Jonsson G. Evidence for a neurotrophic role of noradrenaline neurons in the postnatal development of the rat cerebral cortex. Journal of Neurocytology. 1982;11:119–135. doi: 10.1007/BF01258008. [DOI] [PubMed] [Google Scholar]
- Grudt TJ, van den Pol AN, Perl ER. Hypocretin-2 (orexin-B) modulation of superficial dorsal horn activity in rat. Journal of Physiology. 2002;538:517–526. doi: 10.1113/jphysiol.2001.013120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagan JJ, Leslie RA, Patel S, Evans ML, Wattam TA, Holmes S, Benham CD, Taylor SG, Routledge C, Hemmati P, Munton RP, Ashmeade TE, Shah AS, Hatcher JP, Hatcher PD, Jones DN, Smith MI, Piper DC, Hunter AJ, Porter A, Upton N. Orexin A activates locus coeruleus cell firing and increases arousal in the rat. Proceedings of the National Academy of Sciences of the USA. 1999;96:10911–10916. doi: 10.1073/pnas.96.19.10911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horvath TL, Peyron C, Diano S, Ivanov A, Aston-jones G, Kilduff TS, van den Pol AN. Hypocretin (orexin). activation and synaptic innervation of the locus coeruleus noradrenergic system. Journal of Comparative Neurology. 1999;415:145–159. [PubMed] [Google Scholar]
- Ishimatsu M, Williams JT. Synchronous activity in locus coeruleus results from dendritic interactions in the pericoerulear regions. Journal of Ne uroscience. 1996;16:5196–5204. doi: 10.1523/JNEUROSCI.16-16-05196.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivanov A, Aston-jones G. Hypocretin/orexin depolarizes and decreases potassium conductance in locus coeruleus neurons. Neuroreport. 2000;11:1755–1758. doi: 10.1097/00001756-200006050-00031. [DOI] [PubMed] [Google Scholar]
- Jolas T, Aghajanian GK. Neurotensin excitation of serotone rgic neurons in the dorsal raphe nucleus of the rat in vitro. European Journal of Neuroscience. 1996;8:153–161. doi: 10.1111/j.1460-9568.1996.tb01176.x. [DOI] [PubMed] [Google Scholar]
- Lauder JM, Bloom FE. Ontogeny of monoamine neurons in the locus coeruleus, raphe nuclei and substantia nigra of the rat. I. Cell diff erentiation. Journal of Comparative Neurology. 1974;155:469–482. doi: 10.1002/cne.901550407. [DOI] [PubMed] [Google Scholar]
- Levitt P, Moore RY. Development of the noradrenergic innervation of neocortex. Brain Research. 1979;162:243–259. doi: 10.1016/0006-8993(79)90287-7. [DOI] [PubMed] [Google Scholar]
- Lin L, Faraco J, Li H, Kadotani R, Rogers W, Lin X, Qui X, Dejong PJ, Nishino S, Mignot E. The sleep disorder canine narcolepsy is caused by a mutation in the hypocretin (orexin) receptor 2 gene. Cell. 1999;98:365–376. doi: 10.1016/s0092-8674(00)81965-0. [DOI] [PubMed] [Google Scholar]
- Luppi PH, Aston-Jones G, Akaoka H, Chouvet G, Jouvet M. Afferent projections to the rat locus coeruleus demonstrated by retrograde and anterograde tracing with cholera-toxin B subunit and Phaseolus vulgaris leucoagglutinin. Neuroscience. 1995;65:119–160. doi: 10.1016/0306-4522(94)00481-j. [DOI] [PubMed] [Google Scholar]
- Maeda T, Tohyama M, Shimizu N. Modification of postnatal development of neocortex i n rat brain with experimental depreivation of locus coeruleus. Brain Research. 1974;70:515–520. doi: 10.1016/0006-8993(74)90261-3. [DOI] [PubMed] [Google Scholar]
- Masuko S, Nakajima Y, Nakajima S, Yamaguchi K. Noradrenergic neurons from the locus coeruleus in dissociated cell culture: cultures methods, morphology, and electrophysiology. Journal of Neuroscience. 1986;6:3229–3241. doi: 10.1523/JNEUROSCI.06-11-03229.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore RY, Bloom FE. Central catecholamine neuron systems: Anatomy and physiology of the norepinephrine and epinephrine systems. Annual Review of Neur oscience. 1979;2:113–168. doi: 10.1146/annurev.ne.02.030179.000553. [DOI] [PubMed] [Google Scholar]
- Nakamura S, Sakaguchi T. Development and plasticity of the locus coeruleus: a review of recent physiological and pharmacological experimentation. Progress in Neurobiology. 1990;34:505–526. doi: 10.1016/0301-0082(90)90018-c. [DOI] [PubMed] [Google Scholar]
- Nestler EJ, Alreja M, Aghajanian GK. Molecular control of locus coeruleus neurotransmission. Biological Psychiatry. 1999;46:1131–1139. doi: 10.1016/s0006-3223(99)00158-4. [DOI] [PubMed] [Google Scholar]
- Nieber K, Poelchen W, Sieler D, Illes P. Inhibition by ethanol of excitory amin acid receptors in rat locus coeruleus neurons in vitro. Naunyn-Schmiedeberg's Archives of Pharmacology. 1998;357:299–308. doi: 10.1007/pl00005171. [DOI] [PubMed] [Google Scholar]
- Nishino S, Ripley B, Overeem S, Lammers GJ, Mignot E. Hypocretin (orexin) deficiency in human narcolepsy. Lancet. 2000;355:39–40. doi: 10.1016/S0140-6736(99)05582-8. [DOI] [PubMed] [Google Scholar]
- Peyron C, Faraco J, Rogers W, Ripley B, Overeem S, Charnay Y, Nevsimalova S, Aldrich M, Reynolds D, Albin R, Li R, Hungs M, Pedrazzoli M, Padigaru M, Kucherlapati M, Fan J, Maki R, Lammers GJ, Bouras C, Kucherlapati R, Nishino S, Mignot E. A mutation in a case of earl y onset narcolepsy and a generalized absence of hypocretin peptides in human narcoleptic brains. Nature Medicine. 2000;6:991–997. doi: 10.1038/79690. [DOI] [PubMed] [Google Scholar]
- Peyron C, Tighe DK, van den Pol AN, de Lecea L, Heller HC, Sutcliffe JG, Kilduff TS. Neurons containing hypocretin (orexin) project to multiple neuronal systems. Journal of Neuroscience. 1998;1:9996–10008. doi: 10.1523/JNEUROSCI.18-23-09996.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pineda J, Kogan JH, Aghajanian GK. Nitric oxide and carbon monoxide activate locus coeruleus neurons through a cG MP-dependent protein kinase: involvement of a nonselective cationic channel. Journal of Neuroscience. 1996;16:1389–1399. doi: 10.1523/JNEUROSCI.16-04-01389.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prusiner SB. Prions. In: Fields BN, Knipe DM, Howley PM, editors. Fields Virology. Philadelphia: Lippincott-Raven Publishers; 1996. pp. 2901–2950. [Google Scholar]
- Regenold JT, Illes P. Inhibitory adenosine A1-receptors on rat locus coeruleus neurones. An intracellular electophysiological study. Naunyn-Schmiedeberg's Archives of Pharmacology. 1990;341:225–231. doi: 10.1007/BF00169735. [DOI] [PubMed] [Google Scholar]
- Sakaguchi T, Nakamura S. Some in vivo electrophysiological properties of locus coeruleus neurones in fetal rats. Experimental Brain Ressearch. 1987;68:122–130. doi: 10.1007/BF00255239. [DOI] [PubMed] [Google Scholar]
- Sakurai T, Amemiya A, Mishii I, Matsuzaki RM, Chemelli H, Tanaka SC, Williams JA, Richardson GP, Kozlowski S, Wilson JRS, Arch RE, Buckingham AC, Haynes SA, Carr RS, Annan McNulty DE, Liu WS, Terrett JA, Elshourbagy NA, Bergsma DJ, Yanagisawa M. Orexins and orexin receptors: a family of hypothalamic neuropeptides and G protein-coupled receptors that regulate feeding behavior. Cell. 1998;92:573–585. doi: 10.1016/s0092-8674(00)80949-6. [DOI] [PubMed] [Google Scholar]
- Schilling G, Becher MW, Sharp AH, Jinnah HA, Duan K, Kotzuk JA, Slunt HH, Ratovitski T, Cooper JK, Jenkins NA, Copeland NG, Price DL, Ross CA, Borchelt DR. Intranuclear inclusions and neuritic aggregates in transgenic mice expressing a mutant N-terminal fragment of huntingtin. Human Molecular Genetics. 1999;8:397–407. doi: 10.1093/hmg/8.3.397. [DOI] [PubMed] [Google Scholar]
- Shen KZ, North RA. Substance P opens cation channels and closes potassium channels in rat locus coeruleus neurons. Neuroscience. 1992;50:345–353. doi: 10.1016/0306-4522(92)90428-5. [DOI] [PubMed] [Google Scholar]
- Specht LA, Pickel VM, Joh TH, Reis DJ. Light-microscopic immunocytochemical localization of tyrosine hydroxylas e in prenatal rat brain. I. Early ontogeny. Journal of Comparative Neurology. 1981;199:233–253. doi: 10.1002/cne.901990207. [DOI] [PubMed] [Google Scholar]
- Thannickal TC, Moore RY, Nienhuis R, Ramanathan L, Gulyani S, Aldrich M, Cornford M, Siegel JM. Reduced number of hypocretin neurons in human narcolepsy. Neuron. 2000;27:469–474. doi: 10.1016/s0896-6273(00)00058-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trivedi P, Yu H, MacNeil DJ, van der Ploeg LH, Guan XM. Distribution of orexin receptor mRNA in the rat brain. FEBS Letters. 1998;438:71–75. doi: 10.1016/s0014-5793(98)01266-6. [DOI] [PubMed] [Google Scholar]
- Usher M, Cohen JD, Servan-Schreiber D, Rajkowski J, Aston-Jones G. The role of the locus coeruleus in the regulation of cognitive performance. Science. 1999;283:549–554. doi: 10.1126/science.283.5401.549. [DOI] [PubMed] [Google Scholar]
- van den Pol AN. Hypothalamic hypocretin (orexin): robust innervation of the spinal cord. Journal of Neuroscience. 1999;19:3171–3182. doi: 10.1523/JNEUROSCI.19-08-03171.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Pol AN. Narcolepsy, a neurodegenerative disease of the hypocretin system? Neuron. 2000;27:415–418. doi: 10.1016/s0896-6273(00)00050-7. [DOI] [PubMed] [Google Scholar]
- van den Pol AN, Gao XB, Obrietan K, Kilduff T, Belousov A. Pre- and postsynaptic actions and modulation of neuroendocrine neurons by a new hypothalamic peptide, hypocretin/orexin. Journal of Neuroscience. 1998;18:7962–7971. doi: 10.1523/JNEUROSCI.18-19-07962.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Pol AN, Ghosh PK. Selective neuronal expression of green fluorescent protein with cytomegalovirus promoter reveals entire neuronal arbor in transgenic mice. Journal of Neuroscience. 1998;18:10640–10651. doi: 10.1523/JNEUROSCI.18-24-10640.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Pol AN, Herbst RS, Powell JF. Tyrosine hydroxylase-immunoreactive neurons of the hypothalamus: a light and electron microscopic study. Neuroscience. 1984;13:1117–1156. doi: 10.1016/0306-4522(84)90292-6. [DOI] [PubMed] [Google Scholar]
- van den Pol AN, Patrylo PR, Ghosh PK, Gao XB. Lateral hypothalamus: early developmental response to hypocretin (orexin) Journal of Comparative Neurology. 2001;433:349–363. doi: 10.1002/cne.1144. [DOI] [PubMed] [Google Scholar]
- Williams JT, Egan TM, North RA. Enkephalin opens potassium channels on mammalian central neurones. Nature. 1982;299:74–77. doi: 10.1038/299074a0. [DOI] [PubMed] [Google Scholar]
- Williams JT, Marshal KC. Membrane properties and adrenergic responses in locus coeruleus neurons of young rats. Journal of Neuroscience. 1987;7:3687–3694. doi: 10.1523/JNEUROSCI.07-11-03687.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams JT, North RA, Shefner A, Nishi S, Egan TM. Membrane properties of rat locus coeruleus neurones. Neuroscience. 1984;13:137–156. doi: 10.1016/0306-4522(84)90265-3. [DOI] [PubMed] [Google Scholar]


