Abstract
We have investigated the gating properties of the inward rectifier chloride channel (Clir) from mouse parotid acinar cells by external protons () using the whole-cell patch-clamp technique. Increasing the pHo from 7.4 to 8.0 decreased the magnitude of Clir current by shifting the open probability to more negative membrane potentials with little modification of the activation kinetics. The action of elevated pH was independent of the conformational state of the channel. The effects of low pH on Clir channels were dependent upon the conformational state of the channel. That is, application of pH 5.5 to closed channels essentially prevented channel opening. In contrast, application of pH 5.5 to open channels actually increased the current. These results are consistent with the existence of two independent protonatable sites: (1) a site with a pK near 7.3, the titration of which shifts the voltage dependence of channel gating; and (2) a site with pK = 6.0. External H+ binds to this latter site (with a stoichiometry of two) only when the channels are closed and prevent channel opening. Finally, block of channels by Zn2+ and Cd2+ was inhibited by low pH media. We propose that mouse parotid Clir current has a bimodal dependence on the extracellular proton concentration with maximum activity near pH 6.5: high pH decreases channel current by shifting the open probability to more negative membrane potentials and low pH also decreases the current but through a proton-dependent stabilization of the channel closed state.
Inwardly rectifying chloride channels have been described in several tissues (Chesnoy-Marchais, 1983; Thieman et al. 1992; Chesnoy-Marchais & Fritsch, 1994; Komwatana et al. 1994; Arreola et al. 1996; Carew & Thorn, 1996; Fritsch & Edelman, 1996; Kajita & Brown, 1997; Clark et al. 1998; Park et al. 1998; Tarran et al. 2000; Fava et al. 2001; Mohammad-Panah et al. 2001). These native channels share many properties with the cloned ClC-2 channel, including a requirement for strong hyperpolarization to open. ClC-2 cloned from heart and brain (Thieman et al. 1992) is a widely expressed channel that induces the appearance of inwardly rectifying Cl− currents when expressed in Xenopus oocytes. ClC-2 is expressed in many tissues where ClC-2-like currents are present, although the function of this protein in these organs is unclear. The inwardly rectifying Cl− current in Sertoli and Leydig cells requires the ubiquitously expressed Clcn2 gene (Bösl et al. 2001). Moreover, targeted disruption of Clcn2 produces severe postnatal degeneration of photoreceptor cells in the retina and of primary spermatocytes and spermatogonia in the testis, suggesting that ClC-2 plays a critical role in regulating the ionic environment of these cells (Bösl et al. 2001). Using a gene ablation approach, we have recently shown that the inwardly rectifying Cl− current in mouse parotid acinar cells also requires expression of the Clcn2 gene (K. Nehrke, J. Arreola, H.-V. Nguyen, J. Pilato, L. Richardson, G. Okunade, G. Shull, R. Baggs & J. E. Melvin, unpublished observations). Heterologously expressed ClC-2 channels (Jordt & Jentsch, 1997) and native inwardly rectifying (Kajita & Brown, 1997; Ferroni et al. 2000; Tarran et al. 2000) currents in some cells are sensitive to changes in pH. Since cells in the parotid gland are exposed to considerable changes in the pH environment when stimulated to secrete (Melvin et al. 1988; Okada et al. 1991), the H+ sensitivity of this channel may play an important physiological role.
Although no direct evidence exists, several studies suggest that ClC-type Cl− channels undergo conformational changes during gating. For example, following the binding of Cl− ions to the closed state of ClC-0, a rearrangement to a Cl−-bound closed state was proposed to explain the voltage dependence of opening (Chen & Miller, 1996). Also, it was suggested that an increase in affinity of the H+ binding site upon channel opening leads to further activation of ClC-0 by H+ (Hanke & Miller, 1983). The temperature dependence of ClC-0 and ClC-1 also suggests conformational changes of the protein structure during closed to open transitions (Pusch et al. 1997; Bennetts et al. 2001). Finally, using the projection structure of a ClC-type Cl− channel from E. coli, it was reasoned that transmembrane segment 4 lies near the pore entrance in the closed state and possibly undergoes a conformational change during gating to form part of the pore (Mindell et al. 2001).
We have investigated the regulation of the inwardly rectifying Cl− current associated with the Clcn2 gene in mouse parotid acinar cells. We found that pHo had a bimodal effect on this current. Both low and high pHo inhibited channel current, whilst near-neutral pHo facilitated opening. The current inhibition by pHo 8.0 was independent of the conformational state of the channel. Conversely, the inhibition observed at pHo 5.5 occurred only when the channel was in the closed state. These data are consistent with the presence of two independent H+ binding sites, one of which is not available when the channel is in the open state. Channel block by the divalent cations Cd2+ and Zn2+ was H+ dependent, but the data do not allow a determination of whether this effect involves one of the two H+ binding sites that regulate channel gating, or if inhibition is associated with a third protonatable site present on these channels.
METHODS
Single cell dissociation
The single acinar cells were dissociated from mouse parotid glands following a protocol approved by the University of Rochester on Animal Resources, as previously described (Arreola et al. 1996). Briefly, glands were dissected from exsanguinated mice (BlackSwiss × 129/SvJ hybrid mice) after CO2 anaesthesia. Glands were minced in Ca2+-free minimum essential medium (MEM) (Gibco BRL, Gaithersburg, MD, USA) supplemented with 1 % bovine serum albumin (BSA) (Fraction V, Sigma Chemical Co., St Louis, MO, USA). The tissue was treated for 20 min (37 °C) with a 0.02 % trypsin solution (MEM-Ca2+ free + 1 mm EDTA (ethylenediaminetetraacetic acid) + 2 mm glutamine + 1 % BSA). Digestion was stopped with 2 mg ml−1 of soybean trypsin inhibitor (Sigma) and the tissue further dispersed by two sequential treatments of 60 min each with collagenase (100 units ml−1 of type CLSPA, Worthington Biochemical Corp., Freehold, NJ, USA) in MEM-Ca2+ free + 2 mm glutamine + 1 % BSA. The dispersed cells were centrifuged and washed with basal medium Eagle (BME) (Gibco)/BSA-free. The final pellet was resuspended in BME/BSA-free + 2 mm glutamine, and cells were plated onto poly-l-lysine-coated glass coverslips for electrophysiological recordings.
Electrophysiological recordings
Chloride currents were recorded at room temperature (20-22 °C) using the conventional whole-cell patch-clamp configuration (Hamill et al. 1981) and an Axopatch 200B amplifier (Axon Instruments). Patch pipettes were pulled to have a resistance between 2 and 4 MΩ when filled with the standard pipette (internal) solution containing (mm): 140 TEA-Cl, 10 or 20 EGTA and 20 Hepes; pH 7.2. Cells were bathed in the standard external solution containing (mm): 140 NMDG-Cl, 0.5 CaCl2, 50 d-mannitol and 10 Hepes; pH 7.4 (or 8). Other pHo values were adjusted by using 20 mm CAPS (pH 10), AMPSO (pH 9), MOPS (pH 6.75), or MES (pH 6.5, 6.0 and 5.5). The internal and external solutions were designed to have nearly 0 free [Ca2+] and to be slightly hypertonic, respectively, to avoid the activation of Ca2+-dependent and volume-sensitive chloride channels present in mouse parotid acinar cells (J. Arreola, T. Begenisich, K. Nehrke, H.-V. Nguyen, K. Park, L. Richardson, B. Yang, F. Lamb, B. C. Shutte & J. E. Melvin, unpublished observations; Iwatsuki et al. 1985). To assay the effects of [Cl−]o on reversal potentials, glutamate was used for equimolar substitution of Cl−. Chloride channels were activated by delivering square pulses from a holding potential of 0 mV to membrane potentials from −120 to +60 mV in 10 or 20 mV steps. Instantaneous current-voltage relationships were constructed from data collected from tail currents recorded between +60 and −100 mV after opening the channels by a 6 s prepulse potential to −100 mV. Currents were filtered at 1 kHz using an 8 db/decade low-pass Bessel filter and sampled using pCLAMP software (Axon Instruments).
Inward rectifier chloride currents were nearly absent after achieving the whole-cell configuration; instead, they appeared with time and reached a maximum within 15-20 min. This phenomenon has been previously observed in T84 cells and astrocytes and it appears to be related to the presence of intracellular ATP (Fritsch & Edelman, 1996; Fava et al. 2001). However, there is no definitive explanation for this effect and we did not investigate it further. All the data presented in this paper were sampled in the absence of intracellular ATP and after more than 15 min of cell dialysis.
To determine whether the reduction in Clir current induced by changes in [H+]o was state dependent (closed vs. open), dual pulses were given to −100 mV separated by a 5 s depolarization to +60 mV. The first hyperpolarization to −100 mV lasted 50 s and was used to examine whether open channels were sensitive to changes in [H+]o. The second 4 s long hyperpolarization was used to assay the fraction of channels inhibited after being in the closed state for 5 s at pHo of 8.0, 7.4 or 5.5. Means ± s.e.m. of current values without leak correction are given.
Analysis
The dependence of reversal potential shifts (ΔEr) with the external chloride concentration ([Cl−]o) was fitted with the Nernst equation:
| (1) |
where Er([Cl-]o) and Er (141) are the reversal potentials experimentally determined in the presence of X [Cl−]o and 141 mm chloride, respectively, and R, T, z and F have their usual thermodynamic meanings. Whole-cell Cl− conductance (gCl) was calculated as:
| (2) |
where ICl is the whole-cell current, and Vm is the membrane voltage. Whole-cell conductance-voltage relationships were fitted to a Boltzmann distribution function:
| (3) |
where V0.5 is the membrane voltage necessary to obtain 50 % of the maximal conductance (gmax), and parameter s reflects the voltage sensitivity of the conductance. The extrapolated gmax was then used to normalize the whole-cell conductance at each potential to pool normalized conductance from different cells. Time constants of chloride channel activation were estimated by fitting entire raw traces with a double exponential function according to the Levenberg-Marquardt method.
RESULTS
Inward rectifier chloride channels (Clir) in mouse parotid acinar cells
The native inward rectifier chloride channels (Clir) have not been previously described in mouse parotid acinar cells. Therefore, initial studies characterized the inward rectifier chloride currents in this cell type by analysing voltage sensitivity and kinetics. Figure 1A displays inwardly rectifying, whole-cell chloride currents recorded from a representative acinar cell. Upon hyperpolarization, there was an immediate increase in current, which was followed by a further, slow activation with no significant inactivation by the end of the test pulse (up to 50 s, e.g. Fig. 5B). The current jump observed at negative voltages probably represents a small fraction of open channels. No current was detected at positive voltages up to +60 mV with symmetrical [Cl−] solutions, demonstrating that under our experimental conditions both Ca2+-dependent and volume-sensitive outward-rectifying chloride channels present in this cell type were insignificant, and the majority of the current was due to the activation of Clir.
Figure 1. Inward rectifier chloride currents.
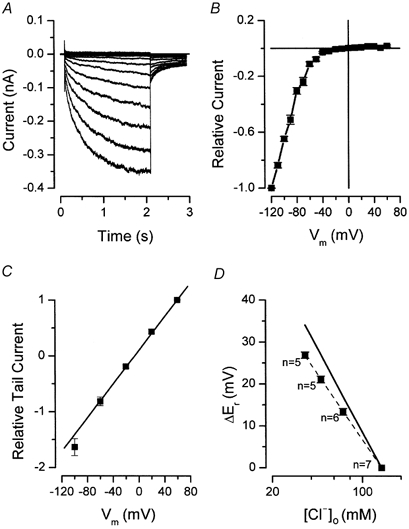
A, whole-cell chloride currents recorded from +60 to −120 mV in 10 mV increments. A 5 s interval was allowed between sweeps. B, normalized current-voltage relationship. Current recorded at −120 mV was used to normalize each curve and then the normalized curves were averaged (n = 12). C, instantaneous current-voltage relationships determined from cells bathed in solutions with pH of 7.4 (n = 6). A prepulse to −100 mV was used to open the channels. Tail currents were generated by repolarizing the membrane to the indicated voltages and its magnitudes were normalized to that obtained at +60 mV. The continuous line is a linear regression. D, reversal potential shifts obtained from tail current experiments are plotted as a function of [Cl−]o. The filled squares are experimental data and the continuous line reflects expected values for a chloride-selective channel. Experimental data were fitted by the Nernst equation with a slope of −45 mV.
Figure 5. State-dependent inhibition of Clir by pHo 5.5.
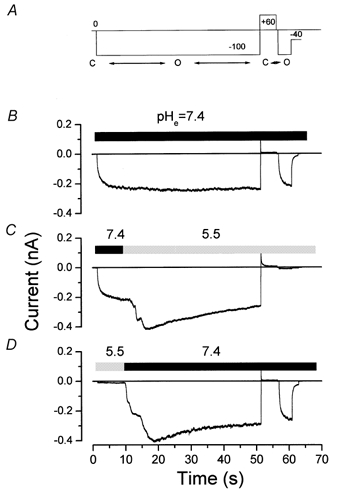
A, pulse protocol used to assess the effects of external H+ on open channels (first 50 s hyperpolarization) and after closing (second 4 s hyperpolarization). Two consecutive hyperpolarizations to −100 mV separated by a depolarization to +60 mV were used as stimuli. The first and longest hyperpolarization allowed the exchange of the bath solution while the channel was open. The second and shortest hyperpolarization evaluated the fraction of channels inhibited after a 5 s pulse to +60 mV that closes the channels. Closed to open confirmation transitions are indicated below the pulse protocol. Bath volume was about 0.2 ml, flow rate was about 4 ml−1, resulting in a typical time for solution exchange of 10-15 s. B, C and D depict traces obtained from the same cell. B shows a control record obtained at pH 7.4 with the protocol shown in A. C shows the effects of changing the bath pH from 7.4 to 5.5. D shows the reversibility of the inhibition by changing bath pH from 5.5 to 7.4.
The strong rectification displayed by Clir is clearly seen in the current-voltage (I-V) relationship shown in Fig. 1B. I-V curves were normalized to the current recorded at −120 mV. The average current at −120 mV was −338 ± 74 pA, whereas the current at +40 mV was +3 ± 0.5 pA (n = 12).
The open channel current-voltage relationship was approximately linear. Tail currents were sampled immediately after a 3 s hyperpolarizing pulse to −100 mV. Instantaneous current-voltage relationships were constructed by plotting the initial magnitude of the tail currents against the repolarizing voltage and were approximately linear (Fig. 1C).
We measured the current reversal potential in solutions in which Cl− was replaced by the ‘impermeant’ anion glutamate. The shifts of the reversal potentials induced by the respective changes in extracellular chloride concentrations are plotted in Fig. 1D. The continuous line is the predicted slope for a chloride-selective channel according to the Nernst equation (eqn (1)), while the squares represent the experimental determinations. Reversal potential shifts exhibited a slope of −45 mV/decade of [Cl−] (dashed line), close to the predicted slope (-58 mV/decade), and consistent with a channel that is predominantly permeable to Cl− ions.
The voltage dependence of channel activation was estimated by calculating the normalized whole-cell conductance as described in Methods (eqn (2)). Figure 2A shows the resulting macroscopic normalized whole-cell conductance, an index of the open probability, as a function of the membrane potential. Clir started to open around −20 to −30 mV, and reached half-maximal activation at −85 mV with a slope factor of 18 mV as deduced from the Boltzmann fit (eqn (3); continuous line). The results of previous studies showed that the kinetics of Clir activation by hyperpolarization have two time constants (Ferroni et al. 1997; Cid et al. 2000). In agreement, a single exponential function did not adequately fit our data. At least two exponential components were needed (data not shown). The resulting values of the fast (τ1, ▪) and slow (τ2, ▴) time constants are plotted in Fig. 2B. The fast and slow time constants were around 100-200 and 1000 ms, respectively, at the most negative voltages. The relative contributions of the fast and slow components to the total currents at −100 mV were 0.34 ± 0.03 and 0.66 ± 0.03 (n = 8), respectively. A modest voltage sensitivity was observed at less negative potentials.
Figure 2. Macroscopic open probability and kinetics of Clir.
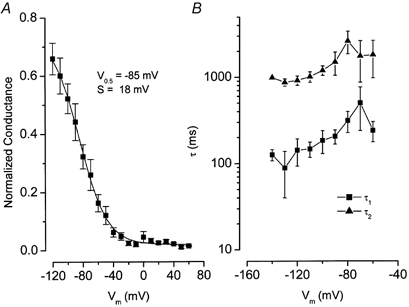
A, the open probability as a function of Vm was estimated by calculating the macroscopic conductance using eqn (2) and normalized to the estimated gmax (see Methods). V0.5 and s parameters were determined by fitting the average normalized conductance curve with the Boltzmann function (eqn (3); n = 12). B, kinetics of Clir. Traces at each membrane potential were fitted with a two-exponential function to estimate both fast (▪, n = 9) and slow (▴, n = 9) time constants.
Effects of elevated external pH on Clir
As described in the Introduction, cloned ClC-2 and some native inwardly rectifying Cl− channels are modulated by external pH. We found that the inwardly rectifying currents in mouse parotid acinar cells were reduced by elevations in external pH with little or no change in gating kinetics. Figure 3A shows whole-cell chloride currents recorded from a cell exposed to a bath solution of pH 7.4 (left-hand traces) and then superfused with a pH 8.0 solution (right-hand traces). The instantaneous current-voltage relationship (not shown) remained linear as for pH 7.4 (Fig. 1C). The reduction in current at pH 8.0 appeared to be a result of a shift of the conductance-voltage relationship along the voltage axis as illustrated in Fig. 3B. Figure 3B shows that increasing the pHo to 8 produced a negative shift of the channel activation of approximately 28 mV, with no apparent change in the voltage sensitivity.
Figure 3. Shift in Clir activation induced by changes in pHo.
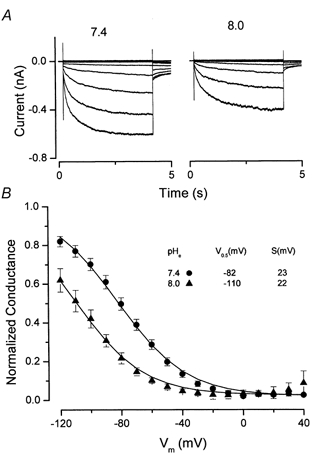
A, cells were bathed in solutions with the pH adjusted to the indicated values. Control currents (pHo = 7.4) are shown in the left-hand panel and test currents are shown in the right-hand panel (pHo = 8.0). Membrane potential was changed from −120 to +40 mV in 20 mV steps. B, voltage dependency of channel activation at pH 7.4 (•, n = 5) and 8.0 (▴, n = 5). The analysis of these curves obtained from paired experiments was as described in Fig. 2B. The resulting average curves were fitted with the Boltzmann function to obtain the parameters V0.5 and s displayed in the inset.
Effects of reduced external pH on Clir
In contrast to the activation shift of high pH solutions, the actions of low pH on the inwardly rectifying Cl− channels were rather more complex. As illustrated in Fig. 4A, modest reduction in pH, e.g. to 6.5, increased current with little kinetic change; pH 6.0 solutions significantly slowed channel gating with little change in magnitude, and very little current was seen in pH 5.5 solutions. We measured the two time constants for channel activation (e.g. Fig. 2B) and found no significant change in solutions of pH between 6.5 and 8.0. In contrast, decreasing the pH to 6.0 slowed fast and slow time constants from 0.21 ± 0.01 to 2.48 ± 1.07 s (n = 6) and from 1.49 ± 0.15 to 7.74 s (n = 8), respectively. At low pHo the kinetics became so slow that even with pulses of 25 s it was not always possible to measure the current amplitude in steady state.
Figure 4. Bimodal regulation of Clir by pHo at −100 mV.
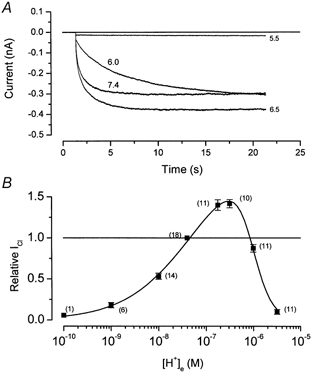
A, recordings obtained from the same cell. External solutions of indicated pH were continuously perfused throughout the experiment. B, normalized current versus[H+]o relationship. Data like those shown in A obtained at −100 mV were normalized to the absolute current value (measured at the end of the pulse) obtained at pH 7.4. The values plotted at pH 6.0 and 5.5 were obtained using 20 or 50 s pulses. Number of observations are indicated in parenthesis. Fitting of data with eqn (4) is shown as continuous line. Parameters obtained from the fit are given in the text.
We measured the amplitude of the current at the end of a pulse to −100 mV in solutions of varying pH. Figure 4B demonstrates that the current amplitude (normalized to the value at pH 7.4) had a bimodal dependence on the external proton concentration with maximum activity near pH 6.5. Reductions in the current amplitude by low pH solutions were much more sensitive to proton concentrations than were changes by high pH solutions.
Actions of pH on Clir: a simple model
In order to begin a quantitative analysis of these data, we consider a simple, generic model in which the channel contains two independent H+ binding sites (S1 and S2):
and
where n1 and n2 represent, in these simple schemes, the number of H+ that interact with the two sites, and k1, k-1, k2 and k-2 are the protonation rate constants. We associate the actions of high pH with deprotonation of site S1 and the actions of low pH with protonation of S2. Thus the bimodal relationship of channel current with pH would be consistent with protonation of site S1 and no protonation of S2. Therefore, the current at any pH would be proportional to:
| (4) |
where [H] is the external proton concentration and K1 (k-1/k1) and K2 (k-2/k2) are the dissociation constants of protonation.
The continuous line in Fig. 4B is the best fit of eqn (4) to the data with values of n1 and n2 of 0.61 and 2.2, respectively, K1 = 5.4 × 10−8m (pK1 = 7.3) and K2 = 9.6 × 10−7m (pK2 = 6.0). The simplest interpretation of this analysis is that channels are ‘activated’ by the binding of a single H+ to a site with an apparent pK of 7.3 and ‘inhibited’ by the binding of 2 H+ to a second site with an apparent pK of 6.0. The requirement for 2 H+ binding to the second site accounts for the increased steepness of the pH sensitivity at low pH values.
State-dependent effects of external pH
We investigated a possible state-dependent action of external pH by applying solutions of different pH at voltages at which the channels were either mostly closed or mostly open. We used a double pulse protocol shown in Fig. 5A. The initial −100 mV pulse lasted 50 s, sufficient time to examine the effects of raising or lowering the external pH on channel activity in the open conformation state. We included a second hyperpolarizing pulse preceded by a 5 s depolarization to +60 mV to evaluate the inhibition of the channel after spending 5 s in the closed state. Figure 5B shows a control trace where the extracellular pH was held constant at 7.4; the channels remained open during the entire 50 s hyperpolarizing pulse to −100 mV, closed when depolarized to +60 mV, and then re-opened normally during the second test pulse to −100 mV. In the following sweep (Fig. 5C), approximately 10 s after the channels were in the open state at −100 mV (the final current is independent of the previous channel state), the pHo was changed from 7.4 to 5.5. Surprisingly, the channel current amplitude was not decreased by this low pH (see pH 5.5 trace in Fig. 4 for comparison). In fact, we consistently observed a relatively fast increase in the current upon decreasing the bath pH, followed by a much slower reduction. After closing the channels in the pH 5.5 solution at +60 mV, reapplication of the −100 mV pulse did not result in the reactivation of current, as if low pH ‘locked’ the channels in the closed conformation. Current recorded during the second hyperpolarization pulse was reduced by 85.6 ± 3.3 % (n = 6) compared with the current during the initial hyperpolarization at pH 7.4. Current inhibition at pHo 5.5 was readily reversed by returning the pHo to 7.4 (Fig. 5D).
We used the same protocol illustrated in Fig. 5A to test for a possible state dependence of the actions of elevated pH on Clir channels. Applying pH 8.0 at −100 mV (when the channels are open) produced the expected reduction in current magnitude (Fig. 6B; 47.6 ± 3.2 %, n = 3). In the continued presence of pH 8.0, reapplication of the −100 mV activating pulse after closing the channels at +60 mV reactivated the current to the same level. The reduction of current at pH 8.0 reversed after returning the cell to pH 7.4 (Fig. 6C).
Figure 6. State-independent inhibition by pHo 8.0 at −100 mV.
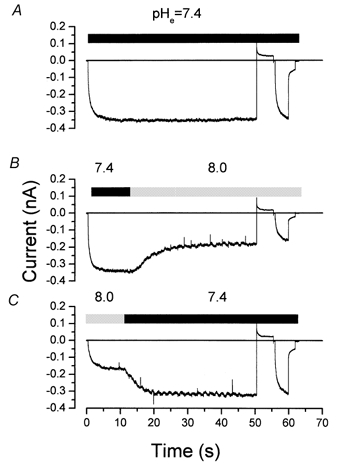
The protocol was as described in Fig. 5A. A, B and C show raw traces obtained from the same cell. A, control trace obtained from cell bathed in a solution with pH 7.4. B, current recorded while changing the bath solution pH from 7.4 to 8.0. C, current recorded while changing the bath solution pH from 8.0 to 7.4.
Thus it appears that the strong inhibitory effects of low pH were state dependent and occurred only if the channels were simultaneously closed and exposed to the low pH solution. In contrast, high pH solutions decreased channel current independently of the conformational state.
pH-dependent actions of divalent cations
Divalent cations block heterologouosly expressed ClC-1 in a pH-dependent manner (Rychokov et al. 1997; Kürz et al. 1999). We tested for this effect on mouse Clir channels. The application of Zn2+ (Fig. 7A) and Cd2+ (Fig. 7B) reversibly inhibited the inward chloride currents from mouse parotid acinar cells at pH 7.4. At −100 mV, 72.1 ± 3.5 and 87.4 ± 1.9 % of the control current was inhibited by 50 μM Zn2+ (n = 5) and 500 μM Cd2+ (n = 4), respectively, comparable to results previously documented in other preparations (Chesnoy-Marchais & Fritsch, 1994; Fritsch & Edelman, 1996; Ferroni et al. 1997; Clark et al. 1998).
Figure 7. Low pHo interferes with Clir inhibition by Zn2+ and Cd2+.
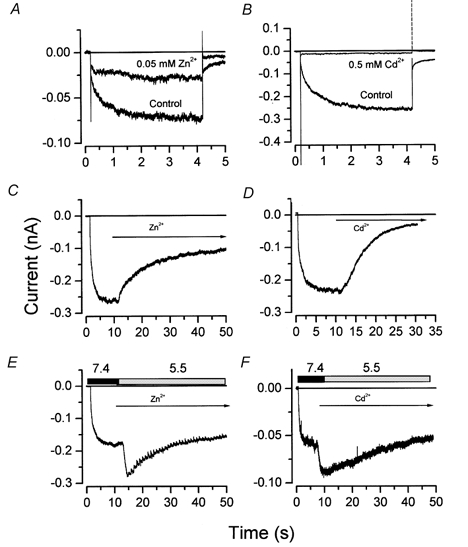
A and B, inhibition of Clir current by 0.05 mm Zn2+ and by 0.5 mm Cd2+, respectively, at −100 mV. Divalent cations were applied in a solution of pH 7.4 while the cell was held at 0 mV. C and D, inhibition of Clir current by 0.5 mm Zn2+ (n = 5) or 0.5 mm Cd2+ (n = 3), respectively, at pH 7.4 and −100 mV. E and F, lack of inhibition by 0.5 mm Zn2+ (n = 5) or 0.5 mm Cd2+ (n = 3), respectively, at pH 5.5 and −100 mV. The bath solution pH in A, B, C and D was 7.4 throughout the experiments. Rectangles in E and F indicate the exchange of the standard pH 7.4 bath solution containing no blocker with a pH 5.5 bath solution containing 0.5 mm of Zn2+ or Cd2+. The arrows in C, D, E and F indicate when the blockers were present.
Taking advantage of our finding that pHo 5.5 does not inhibit the current through open channels, we next evaluated inhibition by Zn2+ and Cd2+ at pH 7.4 and 5.5. We used long hyperpolarizing pulses and exposed the cells to Zn2+ or Cd2+after the channels reached the open state. Figure 7C and D shows whole-cell current traces recorded from two cells bathed in a solution of pH 7.4, and exposed to 500 μM Zn2+ and 500 μM Cd2+, respectively. A relatively rapid inhibition of current (comparable to that in Fig. 7A and B; 61 ± 4.8 % with Zn2+ and 84 ± 1.8 % with Cd2+) was observed after applying the divalent cations. Figure 7E and F shows that these same concentrations of divalent cations had no significant effect on the current when applied in a pH 5.5 solution. That is, the simultaneous application of low pH and divalent cations affected the current no differently than application of only low pH (see Fig. 5C).
DISCUSSION
Targeted disruption of the Clcn2 gene has recently verified that the inwardly rectifying Cl− current in mouse parotid acinar cells is associated with ClC-2 expression (K. Nehrke, J. Arreola, H.-V. Nguyen, J. Pilato, L. Richardson, G. Okunade, G. Shull, R. Baggs & J. E. Melvin, unpublished observations). Thus the hyperpolarization-activated Cl− current in this cell type is almost certainly mediated by the ClC-2 Cl− channel protein, providing an opportunity to characterize this channel in its native environment. Both native and cloned inwardly rectifying chloride channels are extremely sensitive to [H+]o (Jordt & Jentsch, 1997; Kajita & Brown, 1997; Ferroni et al. 2000; Tarran et al. 2000). We found that mouse parotid Clir channels were inhibited by both high and low extracellular pH solutions, demonstrating a novel bimodal mechanism of regulation by external pH. The simplest interpretation of our data suggests the existence of two separate mechanisms for regulating the pH dependency of ClC-2 channel gating. In contrast, Kajita & Brown (1997) found that the pH dependence of ClC-2-like currents from rat choroid plexus cells was monotonic, with inhibition of activity observed at low extracellular pH. They suggested that a single proton acting at an extracellular site blocked the rat choroid plexus channel. The reason for this difference is not clear, but may be due to different gene products, as suggested by the relatively rapid activation kinetics and cAMP dependence of the chloride channels in the choroid plexus.
The inhibition of current magnitude by high pHo can be explained by a shift in the voltage dependency of channel gating. This effect is consistent with changes in membrane surface potential resulting from protonation of a site with pK near 7.3. This type of mechanism has been proposed for the cloned rat ClC-2 channel (Jordt & Jentsch, 1997) and the ClC-2-like currents in cultured rat cortical astrocytes (Ferroni et al. 2000). Alternatively, OH− could conceivably block the channels by tight binding, as has been suggested for Ca2+-dependent Cl− channels (Qu & Hartzell, 2000). A voltage-dependent k-1/k1 ratio in scheme 1 resulting from either mechanism will be sufficient to explain the negative shift in channel activation.
The actions of low pH on the mouse Clir channels were more complex than a shift of channel activation. We found that a pH level of 6.5 increased current amplitude (compared with pH 7.4) and levels below 6.5 decreased the current and substantially slowed channel kinetics. The inhibition of current by low pH required that the channels be in a closed conformation. A detailed mechanistic analysis of these effects is beyond the scope of this investigation. However, we suggest that two protons may bind to a site with a pK near 6.0 and stabilize a closed conformation of the channel. That is, when the channel is closed and the H+ binding site is protonated, hyperpolarization may be unable to move a mobile part of the channel because of the additional positive charge, thus resulting in immobilization of the gating machinery. This mechanism is consistent with the slowing produced by low pH solutions as well as the steep dependence of current magnitude on pH.
We found that divalent cations Zn2+ and Cd2+ decreased Clir channel currents when they are applied at pH 7.4, consistent with previous observations (Chesnoy-Marchais & Fritsch, 1994; Fritsch & Edelman, 1996; Ferroni et al. 1997; Clark et al. 1998). Moreover, low pHo drastically reduced the ability of these divalent cations to inhibit Clir channel currents. This is quite similar to the phenomenon observed for the ClC-1 chloride channel, where important cysteine residues have been identified (Kürz et al. 1999), suggesting that a comparable mechanism may be responsible for this behaviour in ClC-2.
In summary, the inward rectifier ClC-2 Cl− channels in mouse parotid acinar cells were regulated by [H+]o in a bimodal fashion. This regulation may have a physiological consequence. Salivary gland acinar cells are exposed to dramatic changes in their pH environment in response to secretion-inducing agonists (Melvin et al. 1988; Okada et al. 1991). When acinar cells are stimulated, bicarbonate and H+ efflux occurs across the apical and basolateral membranes, respectively (Melvin et al. 1988). If ClC-2 is located in the basolateral membrane of this cell, then low pHo in the range needed for this form of inhibition might occur when Na+-H+ exchange is upregulated during stimulated secretion (Melvin et al. 1988; Evans et al. 1999). Na+-H+ exchanger activity is enhanced several fold by muscarinic stimulation. The extent of the resulting extracellular acidification is unknown, but is probably quite large near the plasma membrane. The secretion of bicarbonate across the apical membrane of acinar cells is likely to produce an alkaline pH near the external apical surface, and if the ClC-2 channel is targeted to this membrane, the resulting alkaline pH would produce negative feedback on channel gating.
Acknowledgments
We thank Dr Patricia Perez-Cornejo for critical reading of this manuscript and Jodi Pilato for excellent technical assistance. This work was supported in part by NIH grants DE09692 and DE13539 (J. E. M.).
REFERENCES
- Arreola J, Park K, Melvin JE, Begenisich T. Three distinct chloride channels control anion movements in rat parotid acinar cells. Journal of Physiology. 1996;490:351–362. doi: 10.1113/jphysiol.1996.sp021149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennets B, Roberts ML, Bretag AH, Rychkov GY. Temperature dependence of human muscle ClC-1 chloride channel. Journal of Physiology. 2001;535:83–93. doi: 10.1111/j.1469-7793.2001.t01-1-00083.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bösl MR, Stein V, Hubner C, Zdebik AA, Jordt SE, Mukhopadhyay AK, Davidoff MS, Holstein AF, Jentsch TJ. Male germ cells and photoreceptors, both dependent on close cell-cell interactions, degenerate upon ClC-2 Cl− channel disruption. EMBO Journal. 2001;20:1289–1299. doi: 10.1093/emboj/20.6.1289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carew MA, Thorn P. Identification of ClC-2-like chloride currents in pig pancreatic acinar cells. Pflügers Archiv. 1996;433:84–90. doi: 10.1007/s004240050252. [DOI] [PubMed] [Google Scholar]
- Chen T-Y, Miller C. Non-equilibrium gating and voltage dependence of the ClC-0 Cl− channel. Journal of General Physiology. 1996;108:237–250. doi: 10.1085/jgp.108.4.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chesnoy-Marchais D. Characterization of a chloride conductance activated by hyperpolarization in Aplysia neurones. Journal of Physiology. 1983;342:277–308. doi: 10.1113/jphysiol.1983.sp014851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chesnoy-Marchais D, Fritsch J. Activation by hyperpolarization and atypical osmosensitivity of a Cl− current in rat osteoblastic cells. Journal of Membrane Biology. 1994;140:173–188. doi: 10.1007/BF00233706. [DOI] [PubMed] [Google Scholar]
- Cid LP, Niemeyer MI, Ramirez A, Sepulveda FV. Splice variants of a ClC-2 chloride channel with differing functional characteristics. American Journal of Physiology. 2000;279:C1198–1210. doi: 10.1152/ajpcell.2000.279.4.C1198. [DOI] [PubMed] [Google Scholar]
- Clark S, Jordt SE, Jentsch TJ, Mathie A. Characterization of the hyperpolarization-activated chloride current in dissociated rat sympathetic neurons. Journal of Physiology. 1998;506:665–678. doi: 10.1111/j.1469-7793.1998.665bv.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans RL, Bell SM, Schultheis PJ, Shull GE, Melvin JE. Targeted disruption of the Nhe1 gene prevents muscarinic-induced upregulation of Na+/H+ exchange in mouse parotid acinar cells. Journal of Biological Chemistry. 1999;274:29025–29030. doi: 10.1074/jbc.274.41.29025. [DOI] [PubMed] [Google Scholar]
- Fava M, Ferroni S, Nobile M. Osmosensitivity of an inwardly rectifying chloride current revealed by whole-cell and perforated-patch recordings in cultured rat cortical astrocytes. FEBS Letters. 2001;492:78–83. doi: 10.1016/s0014-5793(01)02221-9. [DOI] [PubMed] [Google Scholar]
- Ferroni S, Marchini C, Nobile M, Rapisarda C. Characterization of an inwardly rectifying chloride conductance expressed by cultured rat cortical astrocytes. Glia. 1997;21:217–227. doi: 10.1002/(sici)1098-1136(199710)21:2<217::aid-glia5>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- Ferroni S, Nobile M, Caprini M, Rapisarda C. pH modulation of an inward rectifier chloride current in cultured rat cortical astrocytes. Neuroscience. 2000;100:431–438. doi: 10.1016/s0306-4522(00)00272-4. [DOI] [PubMed] [Google Scholar]
- Fritsch J, Edelman A. Modulation of the hyperpolarization-activated Cl− current in human intestinal T84 epithelial cells by phosphorylation. Journal of Physiology. 1996;490:115–128. doi: 10.1113/jphysiol.1996.sp021130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamill OP, Marty A, Neher E, Sakman B, Sigworth F. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflügers Archiv. 1981;391:85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Hanke W, Miller C. Single chloride channels from Torpedo electroplax. Activation by protons. Journal of General Physiology. 1983;82:25–45. doi: 10.1085/jgp.82.1.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwatsuki N, Maruyama Y, Matsumoto O, Nishiyama A. Activation of Ca2+-dependent Cl− and K+ conductances in rat and mouse parotid acinar cells. Japanese Journal of Physiology. 1985;35:933–944. doi: 10.2170/jjphysiol.35.933. [DOI] [PubMed] [Google Scholar]
- Jordt SE, Jentsch TJ. Molecular dissection of gating in the ClC-2 chloride channel. EMBO Journal. 1997;16:1582–1592. doi: 10.1093/emboj/16.7.1582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kajita H, Brown PD. Inhibition of the inward-rectifying Cl− channel in rat choroids plexus by a decrease in extracellular pH. Journal of Physiology. 1997;498:703–707. doi: 10.1113/jphysiol.1997.sp021894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komwatana P, Dinudom A, Young JA, Cook DI. Characterization of the Cl− conductance in the granular duct cells of mouse mandibular glands. Pflügers Archiv. 1994;428:641–647. doi: 10.1007/BF00374588. [DOI] [PubMed] [Google Scholar]
- Kürz LL, Klink H, Jakob I, Kuchenbecker M, Benz S, Lehmann-Horn F, Rudel R. Identification of three cysteines as targets for the Zn2+ blockade of the human skeletal muscle chloride channel. Journal of Biological Chemistry. 1999;274:11687–11692. doi: 10.1074/jbc.274.17.11687. [DOI] [PubMed] [Google Scholar]
- Melvin JE, Moran A, Turner RJ. The role of HCO3− and Na+/H+ exchange in the response of rat parotid acinar cells to muscarinic stimulation. Journal of Biological Chemistry. 1988;263:19564–19569. [PubMed] [Google Scholar]
- Mindell JA, Maduke M, Miller C, Grigorieff N. Projection structure of a ClC-type chloride channel at 6. 5 Å resolution. Nature. 2001;409:219–224. doi: 10.1038/35051631. [DOI] [PubMed] [Google Scholar]
- Mohammad-Panah R, Gyomorey K, Rommens J, Choudhury M, Li C, Wang Y, Bear CE. ClC-2 contributes to native chloride secretion by a human intestinal cell line, Caco-2. Journal of Biological Chemistry. 2001;276:8306–8313. doi: 10.1074/jbc.M006764200. [DOI] [PubMed] [Google Scholar]
- Okada M, Saito Y, Sawada E, Nishiyama A. Microfluorimetric imaging study of the mechanism of activation of the Na+/H+ antiport by muscarinic agonist in rat mandibular acinar cells. Pflügers Archiv. 1991;419:338–348. doi: 10.1007/BF00371116. [DOI] [PubMed] [Google Scholar]
- Park K, Arreola J, Begenisich T, Melvin JE. Functional properties of a voltage-activated chloride channel expressed in a mammalian cell line. Journal of Membrane Biology. 1998;163:87–95. doi: 10.1007/s002329900373. [DOI] [PubMed] [Google Scholar]
- Pusch M, Ludewig U, Jentsch TJ. Temperature dependence of fast and slow gating relaxations of ClC-0 chloride channels. Journal of General Physiology. 1997;109:105–116. doi: 10.1085/jgp.109.1.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qu Z, Hartzell HC. Anion permeation in Ca2+-activated Cl− channels. Journal of General Physiology. 2000;116:825–844. doi: 10.1085/jgp.116.6.825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rychkov GY, Astill D, St J, Bennetts B, Hughes BP, Bretag AH, Roberts ML. pH-dependent interactions of Cd2+ and a carboxylate blocker with the rat ClC-1 chloride channel and its R304E mutant in the Sf-9 insect cell line. Journal of Physiology. 1997;501:335–362. doi: 10.1111/j.1469-7793.1997.355bn.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarran R, Argent BE, Gray MA. Regulation of a hyperpolarization-activated chloride current in murine respiratory ciliated cells. Journal of Physiology. 2000;524:353–364. doi: 10.1111/j.1469-7793.2000.00353.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thieman A, Grunder S, Pusch M, Jentsch TJ. A chloride channel widely expressed in epithelial and non-epithelial cells. Nature. 1992;356:57–60. doi: 10.1038/356057a0. [DOI] [PubMed] [Google Scholar]


