Abstract
Nitric oxide synthase is expressed in the sino-atrial node and animal data suggests a direct role for nitric oxide on pacemaker activity. Study of this mechanism in intact humans is complicated by both reflex and direct effects of nitric oxide on cardiac autonomic control. Thus, we have studied the direct effects of nitric oxide on heart rate in human cardiac transplant recipients who possess a denervated donor heart. In nine patients, the chronotropic effects of systemic injection of the nitric oxide synthase inhibitor NG-monomethyl-l-arginine (l-NMMA) (3 mg kg−1) or increasing bolus doses of the nitric oxide donor, sodium nitroprusside (SNP), were studied. Injection of l-NMMA increased mean arterial pressure by 17 ± 2 mmHg (mean ± s.e.m.; P < 0.001) and also had a significant negative chronotropic effect, lengthening the R-R interval by 54 ± 8 ms (P < 0.001). This bradycardia was not reflex in origin since injection of the non-NO-dependent vasoconstrictor, phenylephrine (100 μg) achieved a similar rise in mean arterial pressure (18 ± 3 mmHg; P < 0.001) but failed to change R-R interval duration (ΔR-R = −3 ± 4 ms). Furthermore, no change in levels of circulating adrenaline was observed with l-NMMA. Conversely, injection of sodium nitroprusside resulted in a positive chronotropic effect with a dose-dependent shortening of R-R interval duration, peak ΔR-R = −25 ± 8 ms with 130 μg (P < 0.01). These findings indicate that nitric oxide exerts a tonic, direct, positive chronotropic influence on the denervated human heart. This is consistent with the results of animal experiments showing that nitric oxide exerts a facilitatory influence on pacemaking currents in the sino-atrial node.
There is increasing evidence that endogenous nitric oxide (NO) modulates many aspects of cardiac physiology, including the regulation of heart rate. It is now clear that the chronotropic effects of NO donors are not just a baroreflex-mediated response to vasodilatation. Direct effects of NO on the autonomic nervous control of heart rate have been shown in animals (for review see Chowdhary & Townend, 1999) and in humans (Chowdhary et al. 2000; Chowdhary et al. 2002). Nitric oxide may also exert a direct influence upon the sino-atrial node (SAN) itself. This is suggested by the presence of nitric oxide synthase (NOS) in SAN cells, their adjacent capillaries and in the innervating neural processes (Klimaschewski et al. 1992; Han et al. 1998). Furthermore, in isolated guinea-pig atria, exogenous NO donors have been shown to produce a significant, positive chronotropic response mediated by activation of the hyperpolarisation-activated inward (If) pacemaker current (Musialek et al. 1997). Results consistent with a similar direct chronotropic effect of NO donors in intact human subjects were reported by Hogan et al. (1999b) but neurally mediated effects could not be excluded with certainty. We have studied cardiac transplant recipients in order to determine, for the first time, in the denervated human heart, the in vivo chronotropic effects of exogenous NO donation and inhibition of endogenous NO production.
METHODS
Subjects
Cardiac transplant recipients who had no evidence of active rejection on cardiac biopsy within 1 month and who maintained sinus rhythm with a low rate (< 2 %) of ectopic beats were recruited. In all patients persistent SAN denervation was confirmed by tilt testing, spectral R-R interval analysis and chronotropic response to phenylephrine bolus injection. Inclusion demanded (i) that heart rate increased by less than 2 beats min−1 during the first 60 s of 70 deg upright tilt; (ii) absence of a low-frequency R-R spectral peak that increased on upright tilt; and (iii) a baroreflex sensitivity calculated by the phenylephrine bolus method of < 0.1 ms mmHg−1. All usual immunosuppressive and antihypertensive treatment was maintained. Patients taking nitrates were excluded. Normal renal and hepatic function was biochemically confirmed and all patients had a blood pressure of < 160/95 mmHg (on treatment with anti-hypertensive drugs). Subjects were asked to abstain from food and drink for at least 2 h prior to study, and from caffeine and alcohol for 24 h. Experimental protocols conformed to the Declaration of Helsinki and were approved by the South Birmingham Research Ethics Committee, with individual written consent obtained.
Screening for chronotropic re-innervation
All subjects attended for an initial acclimatisation and screening visit during which the presence of functional reflex chronotropic responses was rigorously excluded. Supine ECG data was recorded during controlled respiration (at a frequency individually determined for comfort, range = 0.18-0.26 Hz) at rest and during a 10 min period of head up tilt at 70 deg. A time series of R-R interval durations was then subjected to autoregressive spectral analysis in order to determine the presence and magnitude of a low frequency (LF) heart rate variability peak according to previously described methods (Chowdhary et al. 2000). In transplant patients this spectral component is a marker of sympathetic chronotropic re-innervation (Bernardi et al. 1995) validated against improvement of cardiac noradrenaline spillover (Kaye et al. 1993) and neuronal catecholamine uptake and storage measured by positron emission tomography (Ziegler et al. 1996). In some patients this LF peak becomes apparent only during baroreceptor unloading (Bernardi et al. 1995). Our criteria for re-innervation were therefore defined as a LF peak exceeding 5 % normalised power (percentage power > 0.04 Hz) in either the supine or upright tilt (baroreflex unloaded) positions. Additionally, the time course of the heart rate response to upright tilt was also included. A late rise in heart rate was expected due to the intact baroreflex-adrenal axis and the slow release of circulating catecholamines. However, a rise in heart rate that failed to exceed 2 beats min−1 by the end of the first minute of tilt was taken as evidence of an absent rapid neural heart rate response. If both these tests suggested chronotropic denervation the subject attended a first study visit during which the systolic blood pressure/R-R interval duration regression slope was calculated following intravenous bolus injection of phenylephrine, with the study continuing only if the slope (phenylephrine BRS) was < 0.1 ms mmHg−1.
General protocol
Protocols were of a single blind, random order, cross-over design. Each patient was randomly assigned to receive either (i) intravenous NG-monomethyl-l-arginine (l-NMMA; Clinalfa AG; 3 mg kg−1 in 0.9 % saline over 2 min, max 250 mg), or (ii) graded doses of sodium nitroprusside (SNP; Faulding) during the first of two study visits, the remaining agent being given at a subsequent visit 7-14 days later.
Each study was commenced at 2 pm with an ambient temperature maintained at 24 ± 1 °C. Subjects rested semi-supine and signals from a three-lead ECG, a Portapres (TNO Biomedical Instrumentation, Amsterdam, Netherlands) recording continuous arterial pressure and a strain gauge attached around the subjects’ chest quantifying respiratory excursion were displayed on a personal computer (G3 Macintosh). Selected periods from all three signals were digitised at 500 Hz and recorded to disk for off-line analysis. A venous cannula was inserted into an antecubital vein on each arm for drug administration, after which subjects rested for 45 min prior to commencement of the study protocol. Changes in arterial pressure documented by Portapres were confirmed at the end of each recording period by automated arm cuff sphygmomanometry (Omron 711, Omron Healthcare, Hamburg, Germany).
Stored data was analysed off-line by an observer blinded to the agent and the patient under study. ECG data were reviewed and manually edited to remove ectopics or artefact according to previously published techniques (Chowdhary et al. 2000). A consecutive R-R interval tachogram was plotted and the maximal deviation from baseline (an average of a 30 s pre-injection interval) for a running four sample mean was calculated by an automated procedure for each recording. The mean arterial pressure response to each bolus injection was calculated in a similar manner.
Effects of exogenous NO
Once a stable resting haemodynamic state had been achieved, graded boluses of SNP were administered in 5 % dextrose in incremental doses of 50, 70, 100 and 130 μg 10 min apart, provided that the fall in mean arterial pressure with the previous injection had not exceeded 40 mmHg or produced pre-syncopal symptoms (nausea, sweating or dizziness). An equivalent volume of vehicle (5 % dextrose) was injected as a placebo bolus at a randomly determined point in the protocol. To minimise reductions in preload, and the possible electrophysiological consequences of decreased stretch in the region of the SAN, subjects were placed supine with their legs elevated at 20 deg for the duration of the protocol. With each bolus, recordings of the ECG and arterial pressure traces were saved to disk during the 30 s immediately before and 90 s immediately after the injection. The transient hypotension produced by SNP is too short for significant activation of the baroreflex-adrenal axis; however, as an additional safeguard, changes in R-R interval duration were assessed only over the first 60 s after injection.
Effects of endogenous NO
At a separate visit the ECG and arterial pressure waveform were recorded to disk for 30 s before and 10 min after an injection of l-NMMA (3 mg kg−1). The prolonged pressor effect of NOS inhibition could allow responses mediated by the baroreceptor-adrenal axis and changes in circulating catecholamine levels. We did not employ β-adrenoreceptor blockade due to the problem of exaggerated and prolonged haemodynamic responses to this class of drugs in transplant patients. We therefore employed injections of a control vasoconstrictor, the α1-adrenoreceptor agonist phenylephrine (Knoll), to control for any effects of increased arterial pressure. Graded boluses of phenylephrine (80-200 μg in 0.9 % saline) were injected, at intervals of no less than 10 min, at the start of the study to elicit the expected peak change in blood pressure seen with l-NMMA. The time profile of the pressor response to l NMMA was also reproduced in three subjects using a titrated phenylephrine infusion. Additionally, we quantified changes in circulating catecholamine levels. Venous blood was collected into chilled lithium heparin tubes immediately before injection of l-NMMA and again upon stabilisation of any subsequent chronotropic response. Blood was immediately centrifuged at 0 °C and the plasma stored at −70 °C for later analysis. Catecholamines were extracting from plasma onto alumina at pH 8 and then (after several washes with deionised water) eluted with 0.1 m HCL. Catecholamine concentrations within the elutate were then studied by standard HPLC (Jasco PU1580 HPLC pump-Gilson 231 autosampler; coefficient of variation is 5 % for noradrenaline and 10 % for adrenaline).
Statistical methods
Data are represented as means ± s.e.m. Multiple comparisons were made using a repeated measures one-way ANOVA with a Bonferroni's post hoc test. In the case of paired catecholamine data a paired Student's t test was used. Statistical significance was taken as P < 0.05.
RESULTS
Thirteen transplant patients attended for a screening visit. Of these three were excluded as probable cases of cardiac re-innervation, as judged by a rapid heart rate response to upright tilt and by spectral R-R interval analysis in two patients and by a phenylephrine BRS of > 0.1 ms mmHg−1 in the other. A further patient did not proceed to study due to prolonged intercurrent illness. Of the remaining nine patients, the mean age was 52 years (range 38-63) and seven were male. The mean time from transplantation was 2.4 years (range 3 months-6 years). Seven patients had a history of a successfully treated transplant rejection episode an average of 1.4 years previously (mean = 2 rejection episodes per patient) and only one of these was severe. No patient had evidence of transplant vasculopathy as an indication of chronic rejection.
Effects of exogenous NO
Baseline mean arterial pressure was 105 ± 4 mmHg and R-R interval was 690 ± 46 ms. A typical haemodynamic response to injection of SNP is displayed for an individual denervated subject in Fig. 1. The R-R interval is observed to shorten despite the transient nature of the hypotensive response (Fig. 1). Grouped data reveals the dose-dependent nature of this positive chronotropic effect (Fig. 2).
Figure 1. Original recording of R-R interval duration and continuous arterial pressure for a 60-year-old denervated female transplant recipient during administration of SNP.
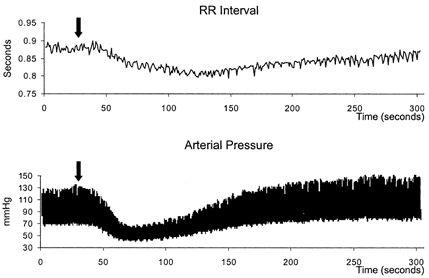
A 100 μg bolus injection of SNP was completed at the point marked by the arrow.
Figure 2. Change in R-R interval duration with increasing doses of SNP bolus.
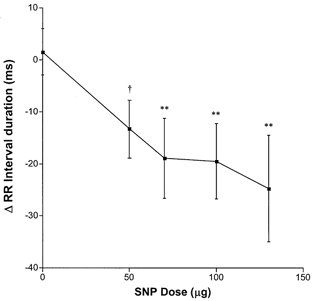
Vehicle (5 % dextrose) injection (n = 9) is shown as 0 μg SNP. SNP injection was tolerated at the 50 μg dose for n = 9, at 70 μg n = 8, at 100 μg n = 8 and at 130 μg n = 6. †P = 0.07, **P < 0.01 vs. vehicle.
Effects of endogenous NO
Mean arterial pressure was 105 ± 6 mmHg and R-R interval was 691 ± 53 ms at baseline. l-NMMA caused a significant rise in mean arterial pressure of 17 ± 2 mmHg that remained elevated for the entire recording period. This rise in mean arterial pressure induced by l-NMMA was most closely reproduced by a 100 μg dose of phenylephrine (Fig. 3).
Figure 3. Change from baseline in mean arterial pressure with bolus injection of l-NMMA, phenylephrine (Phe) or saline.
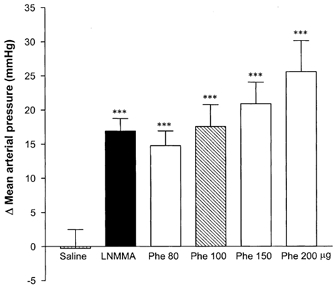
n = 9 for all doses. *** P < 0.001vs. change with saline.
l-NMMA significantly increased R-R interval (negative chronotropic response), whereas no change was seen with either saline or the equipressor 100 μg phenylephrine bolus injection (Fig. 4). Maintaining the pressor effect of phenylephrine over a period equal to that of l-NMMA by use of a continuous infusion also failed to reproduce the lengthening of the R-R interval seen with the NOS inhibitor in any of the three patients studied. A typical response for an individual patient is shown in Fig. 5. Plasma concentrations of adrenaline did not change significantly with l-NMMA (pre bolus = 0.28 ± 0.02 nmol l−1, post bolus = 0.37 ± 0.10 nmol l−1; P = 0.42), while noradrenaline levels tended to fall (pre = 1.62 ± 0.19, post = 1.24 ± 0.25; P = 0.08). Continuous ECG monitoring did not reveal the occurrence of ischaemic changes (alterations in ST level, T wave inversion or increasing ventricular ectopic frequency) with either vasoconstrictor agent.
Figure 4. Change from baseline in R-R interval duration with bolus injection of l-NMMA, phenylephrine 100 μg (Phe) or saline.
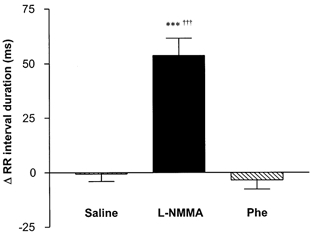
n = 9 for all drugs. *** P < 0.001vs. saline, †††P < 0.001 vs. phenylephrine.
Figure 5. Representative haemodynamic response for an individual patient (50-year-old male, 6 months from transplant) to a bolus of l-NMMA (black line) given at time zero, in comparison to a continuous infusion of phenylephrine (grey line).
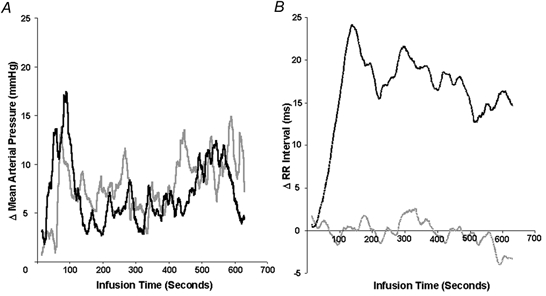
Continuous data are presented as a 30 beat moving average. A, the infusion rate of phenylephrine was titrated to match, over a period of 10 min, the rise from baseline in continuously recorded mean arterial pressure following l-NMMA. B, the resulting rise in R-R interval duration with l-NMMA was not reproduced with the control vasoconstrictor, phenylephrine.
DISCUSSION
In order to determine the functional effects of NO on intrinsic human pacemaker activity we have examined the influence of both endogenous and exogenous NO on heart rate in a cohort of cardiac transplant recipients with objective evidence of cardiac denervation. Our studies have demonstrated that inhibiting endogenous NO generation with l-NMMA results in a significant bradycardia. Conversely, exogenous NO donation with SNP produces a dose-dependent tachycardia. These data show that NO does exert a tonic effect on human heart rate in vivo causing an increase in SAN discharge rate. In addition, the SAN also responds to excess exogenous NO by further increasing pacing rate.
Our interpretation of these results relies upon the assumption that the cardiac transplant recipients in this study had no functional innervation of their SAN. It is recognised, however, that a subset of patients may show incomplete re-growth of sympathetic nerves to the ventricular myocardium from approximately 3 years post transplant (Halpert et al. 1996; Ziegler et al. 1996; Bengel et al. 2001). Reports also exist of a return of functional sympathetic neural chronotropic control (Bernardi et al. 1995; Fagard et al. 1995; Frey et al. 1996; Ziegler et al. 1996), though there is little if any evidence of parasympathetic re-innervation of the SAN (Bernardi et al. 1995; Halpert et al. 1996; Arrowood et al. 1997). We therefore employed multiple tests of neural heart rate control to show that the patients in our study had no functional innervation of their donor SAN.
The vascular effects of systemic NO pathway manipulation may have influenced even the denervated SAN, either by changes in atrial wall tension (and hence stretch on the SAN) due to altered loading conditions or via the baroreceptor-adrenal axis and alteration in circulating catecholamine concentrations. The bradycardic influence of l-NMMA in this study appeared to be independent of its pressor effect, since neither reproduction of the maximum rise in mean arterial pressure with bolus dose phenylephrine nor the entire time-pressure profile with a titrated phenylephrine infusion produced any chronotropic response. The choice of phenylephrine as a control vasoconstrictor is complicated by evidence of a direct positive chronotropic effect in rabbits and dogs (Posner et al. 1984; Talajic et al. 1990). We did not observe a tachycardic response at the dose needed to match the pressor effects of l-NMMA in our patients. Nevertheless, we cannot entirely exclude the possibility that a bradycardic response to phenylephrine may have been masked by a concealed, positive chronotropic action of this agent. Further exclusion of a baroreflex-adrenal axis mediated effect of l-NMMA is provided by the absence of any fall in plasma adrenaline levels. The observed fall in plasma noradrenaline levels reflects a baroreflex-mediated reduction in whole body sympathetic nerve spillover but is unlikely to have led to a chronotropic response as these levels are approximately eight times lower than that required for a hormonal action on the heart (Silverberg et al. 1978).
In considering the effects of exogenous NO on intrinsic heart rate, we administered rapid intravenous bolus doses of SNP and observed chronotropic effects occurring within 60 s. In this manner we avoided sustained baroreflex unloading and quantified changes evoked prior to any possible adrenal medullary activation, thus alleviating the need to use a control vasodilator. Since SNP is a venous as well as an arterial vasodilator, subjects were studied supine with their legs elevated in order to minimise reductions in preload and thus atrial wall tension in the region of the SAN. Reducing traction on the SAN, however, would be expected to reduce firing rate rather than result in the dose-dependent tachycardia that was observed with SNP (Cooper et al. 2000).
Our finding of a tonically active, positive chronotropic influence in humans of endogenous NO, with a further chronotropic influence of exogenous NO, is in agreement with the majority of previous animal data. In isolated rat hearts, low concentrations of SNP produced an increase in spontaneous beating rate whilst a decrease was seen with a NOS inhibitor (Pabla & Curtis, 1995). No significant effect of a NOS inhibitor on heart rate was seen in cats with complete pharmacological autonomic blockade (Yabe et al. 1998); explanations may include species difference or the in vivo nature of the experiment. Other investigators have shown a biphasic, dose-dependent effect of NO in isolated guinea-pig atria which appeared to be due to modulation of the hyperpolarisation-activated inward (If) pacemaking current (Musialek et al. 1997). It was shown that administration of low- and high-dose NO donor resulted in respective increases and decreases in heart rate and also in If recorded from SAN cells. This same group later demonstrated a positive chronotropic effect of NO donors, that was attenuated by a selective If current blocker, in anaesthetised rabbits with cardiac autonomic denervation (Hogan et al. 1999a). Whilst we confirmed a positive chronotropic effect with SNP, we were unable to demonstrate any opposing effect of higher doses as further increments were prohibited by symptomatic hypotension.
Only one previous study has attempted to examine the direct action of NO on heart rate in humans (Hogan et al. 1999b). In a group of healthy, young male athletes the effect of SNP on heart rate was determined using a concomitant infusion of phenylephrine to prevent hypotension and baroreflex unloading. Under these conditions, SNP caused a modest rise in heart rate that was postulated to be intrinsic in nature. As the authors of this paper admit, however, it was not possible to ‘categorically rule out that subtle changes in autonomic activity might have contributed’ to the tachycardia. This acknowledges the fact that study of normally innervated hearts cannot exclude effects mediated by NO on the central and efferent limbs of the cardiac baroreflex (Chowdhary & Townend, 1999; Chowdhary et al. 2000; Chowdhary et al. 2002). Furthermore, despite the normalisation of indirectly measured aortic pressure, there were significant increases in cardiac output and stroke volume and decreases in peripheral resistance, suggesting that reflex neural responses may have been present. The study described here, therefore, is the first assessment of the direct effect of NO on the human SAN with all neural influences excluded. The only previous observation of the effects of SNP in heart transplant patients employed a prolonged (>15 min) infusion, resulting in a notable reflex increase in plasma catecholamine levels that may have driven the observed tachycardic response (Levine et al. 1986).
It should be recognised that effects observed in transplanted hearts may not be representative of normal myocardial physiology. In the normal heart NO generation in the environment of the SAN is entirely from the constitutive NOS isoforms: ‘endothelial’ NOS (eNOS) within the SAN myocytes and the surrounding capillaries and ‘neuronal’ NOS (nNOS) in the innervating neural fibres (Klimaschewski et al. 1992; Han et al. 1998). The high-output isoform, inducible NOS (iNOS), is not basally expressed (Haywood et al. 1996). In the cytokine-enriched environment of the acutely and chronically rejecting cardiac transplant, whilst constitutive NOS activity may be reduced, elevated amounts of NO are produced by iNOS expressed by recipient-derived immune cells infiltrating the myocardium as well as donor-derived vascular smooth muscle cells (Russell et al. 1995; Mugge et al. 1996; Lafond-Walker et al. 1997; Worrall et al. 1999). Expression may also persist in the apparently quiescent donor heart for the first year post transplant (Wildhirt et al. 2001). Whether this would also apply to our older transplants, distant from acute rejection and free of coronary vasculopathy (an indicator of chronic rejection) is unknown. If this were the case then the degree of tonic chronotropic influence of NO (i.e. the ∼6 beats min−1 reduction with l-NMMA) may have been over estimated here due to supra-normal basal NO production. The responses to NO donation may, if anything, be decreased by higher levels of endogenous NO production as the system might potentially be closer to saturation.
In summary, we have shown that endogenous NO exerts a tonic, positive chronotropic effect in the denervated (transplanted) human heart. This mechanism is not basally saturated as a tachycardic effect was also observed with exogenous NO. Nitric oxide can therefore modulate heart rate by a direct action on the SAN, in addition to reflex responses to vasodilatation and neuromodulator influences on cardiac autonomic control (Chowdhary et al. 2000; Chowdhary et al. 2002). The significance of this direct effect to normal physiological control is unclear. Only a modest tonic influence is suggested by this study, but this may be greater in states of increased NO production such as sepsis and inflammatory disease.
Acknowledgments
This work was funded by a grant from the British Heart Foundation. S.C. is a BHF junior research fellow and J.N.T. is a BHF senior lecturer. We are grateful to Professor J. M. Marshall for her helpful comments on this manuscript.
REFERENCES
- Arrowood JA, Minisi AJ, Goudreau E, Davis AB, King AL. Absence of parasympathetic control of heart rate after human orthotopic cardiac transplantation. Circulation. 1997;96:3492–3498. doi: 10.1161/01.cir.96.10.3492. [DOI] [PubMed] [Google Scholar]
- Bengel FM, Ueberfuhr P, Schiepel N, Nekolla SG, Reichart B, Schwaiger M. Effect of sympathetic reinnervation on cardiac performance after heart transplantation. New England Journal of Medicine. 2001;345:731–738. doi: 10.1056/NEJMoa010519. [DOI] [PubMed] [Google Scholar]
- Bernardi L, Bianchini B, Spadacini G, Leuzzi S, Valle F, Marchesi E, Passino C, Calciati A, Vigano M, Rinaldi M, Martinelli L, Finardi G, Sleight P. Demonstrable cardiac reinnervation after human heart transplantation by carotid baroreflex modulation of RR interval. Circulation. 1995;92:2895–2903. doi: 10.1161/01.cir.92.10.2895. [DOI] [PubMed] [Google Scholar]
- Chowdhary S, Nuttall SL, Coote JH, Townend JN. l-arginine augments cardiac vagal control in healthy human subjects. Hypertension. 2002;39:51–56. doi: 10.1161/hy0102.098308. [DOI] [PubMed] [Google Scholar]
- Chowdhary S, Townend JN. Role of nitric oxide in the regulation of cardiovascular autonomic control. Clinical Science. 1999;97:5–17. [PubMed] [Google Scholar]
- Chowdhary S, Vaile JC, Fletcher J, Ross HF, Coote JH, Townend JN. Nitric oxide and cardiac autonomic control in humans. Hypertension. 2000;36:264–269. doi: 10.1161/01.hyp.36.2.264. [DOI] [PubMed] [Google Scholar]
- Cooper PJ, Lei M, Cheng LX, Kohl P. Selected contribution: axial stretch increases spontaneous pacemaker activity in rabbit isolated sinoatrial node cells. Journal of Applied Physiology. 2000;89:2099–2104. doi: 10.1152/jappl.2000.89.5.2099. [DOI] [PubMed] [Google Scholar]
- Fagard R, Macor F, Vanhaecke J. Signs of functional efferent reinnervation of the heart in patients after cardiac transplantation. Acta Cardiologica. 1995;50:369–380. [PubMed] [Google Scholar]
- Frey AW, Dambacher M, Uberfuhr P, Reichart B, Ziegler S, Roskamm H, Schwaiger M. Clinical relevance of heart rate variability changes after heart transplantation. Clinical Science. 1996;91:146–150. doi: 10.1042/cs0910146supp. [DOI] [PubMed] [Google Scholar]
- Halpert I, Goldberg AD, Levine AB, Levine TB, Kornberg R, Kelly C, Lesch M. Reinnervation of the transplanted human heart as evidenced from heart rate variability studies. American Journal of Cardiology. 1996;77:180–183. doi: 10.1016/s0002-9149(96)90592-5. [DOI] [PubMed] [Google Scholar]
- Han X, Kobzik L, Severson D, Shimoni Y. Characteristics of nitric oxide-mediated cholinergic modulation of calcium current in rabbit sino-atrial node. Journal of Physiology. 1998;509:741–754. doi: 10.1111/j.1469-7793.1998.741bm.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haywood GA, Tsao PS, Von DLHE, Mann MJ, Keeling PJ, Trindade PT, Lewis NP, Byrne CD, Rickenbacher PR, Bishopric NH, Cooke JP, McKenna WJ, Fowler MB. Expression of inducible nitric oxide synthase in human heart failure. Circulation. 1996;93:1087–1094. doi: 10.1161/01.cir.93.6.1087. [DOI] [PubMed] [Google Scholar]
- Hogan N, Casadei B, Paterson DJ. Nitric oxide donors can increase heart rate independent of autonomic activation. Journal of Applied Physiology. 1999a;87:97–103. doi: 10.1152/jappl.1999.87.1.97. [DOI] [PubMed] [Google Scholar]
- Hogan N, Kardos A, Paterson DJ, Casadei B. Effect of exogenous nitric oxide on baroreflex function in humans. American Journal of Physiology. 1999b;277:H221–227. doi: 10.1152/ajpheart.1999.277.1.H221. [DOI] [PubMed] [Google Scholar]
- Kaye DM, Esler M, Kingwell B, McPherson G, Esmore D, Jennings G. Functional and neurochemical evidence for partial cardiac sympathetic reinnervation after cardiac transplantation in humans. Circulation. 1993;88:1110–1118. doi: 10.1161/01.cir.88.3.1110. [DOI] [PubMed] [Google Scholar]
- Klimaschewski L, Kummer W, Mayer B, Couraud JY, Preissler U, Philippin B, Heym C. Nitric oxide synthase in cardiac nerve fibers and neurons of rat and guinea pig heart. Circulation Research. 1992;71:1533–1537. doi: 10.1161/01.res.71.6.1533. [DOI] [PubMed] [Google Scholar]
- Lafond-Walker A, Chen CL, Augustine S, Wu TC, Hruban RH, Lowenstein CJ. Inducible nitric oxide synthase expression in coronary arteries of transplanted human hearts with accelerated graft arteriosclerosis. American Journal of Pathology. 1997;151:919–925. [PMC free article] [PubMed] [Google Scholar]
- Levine TB, Olivari MT, Cohn JN. Effects of orthotopic heart transplantation on sympathetic control mechanisms in congestive heart failure. American Journal of Physiology. 1986;58:1035–1040. doi: 10.1016/s0002-9149(86)80034-0. [DOI] [PubMed] [Google Scholar]
- Mugge A, Kurucay S, Boger RH, Bode-Boger SM, Schafers HJ, Wahlers T, Frolich JC, Lichtlen PR. Urinary nitrate excretion is increased in cardiac transplanted patients with acute graft rejection. Clinical Transplantation. 1996;10:298–305. [PubMed] [Google Scholar]
- Musialek P, Lei M, Brown HF, Paterson DJ, Casadei B. Nitric oxide can increase heart rate by stimulating the hyperpolarization-activated inward current, I(f) Circulation Research. 1997;81:60–68. doi: 10.1161/01.res.81.1.60. [DOI] [PubMed] [Google Scholar]
- Pabla R, Curtis MJ. Effects of NO modulation on cardiac arrhythmias in the rat isolated heart. Circulation Research. 1995;77:984–992. doi: 10.1161/01.res.77.5.984. [DOI] [PubMed] [Google Scholar]
- Posner P, Baney R, Prestwich K. The electrophysiological actions of phenylephrine on the rabbit S-A node. Research Communications in Chemical Pathology and Pharmacology. 1984;44:315–318. [PubMed] [Google Scholar]
- Russell ME, Wallace AF, Wyner LR, Newell JB, Karnovsky MJ. Upregulation and modulation of inducible nitric oxide synthase in rat cardiac allografts with chronic rejection and transplant arteriosclerosis. Circulation. 1995;92:457–464. doi: 10.1161/01.cir.92.3.457. [DOI] [PubMed] [Google Scholar]
- Silverberg AB, Shah SD, Haymond MW, Cryer PE. Norepinephrine: hormone and neurotransmitter in man. American Journal of Physiology. 1978;234:E252–256. doi: 10.1152/ajpendo.1978.234.3.E252. [DOI] [PubMed] [Google Scholar]
- Talajic M, Villemaire C, Nattel S. Electrophysiological effects of alpha-adrenergic stimulation. Pacing and Clinical Electrophysiology. 1990;13:578–582. doi: 10.1111/j.1540-8159.1990.tb02071.x. [DOI] [PubMed] [Google Scholar]
- Wildhirt SM, Weis M, Schulze C, Conrad N, Pehlivanli S, Rieder G, Enders G, Von Scheidt W, Reichart B. Coronary flow reserve and nitric oxide synthases after cardiac transplantation in humans. European Journal of Cardiothoracic Surgery. 2001;19:840–847. doi: 10.1016/s1010-7940(01)00681-9. [DOI] [PubMed] [Google Scholar]
- Worrall NK, Misko TP, Botney MD, Sullivan PM, Hui JJ, Suau GM, Manning PT, Ferguson TB., Jr Time course and cellular localization of inducible nitric oxide synthases expression during cardiac allograft rejection. Annals of Thoracic Surgery. 1999;67:716–722. doi: 10.1016/s0003-4975(98)01346-0. [DOI] [PubMed] [Google Scholar]
- Yabe M, Nishikawa K, Terai T, Yukioka H, Fujimori M. The effects of intrinsic nitric oxide on cardiac neural regulation in cats. Anesthesia and Analgesia. 1998;86:1194–1200. doi: 10.1097/00000539-199806000-00010. [DOI] [PubMed] [Google Scholar]
- Ziegler SI, Frey AW, Uberfuhr P, Dambacher M, Watzlowik P, Nekolla S, Wieland DM, Reichart B, Schwaiger M. Assessment of myocardial reinnervation in cardiac transplants by positron emission tomography: functional significance tested by heart rate variability. Clinical Science. 1996;91:126–128. doi: 10.1042/cs0910126supp. [DOI] [PubMed] [Google Scholar]


