Abstract
It has been proposed that extracellular ATP may be involved in visceral mechanosensory transduction by activating ligand-gated ion channels (P2X receptors). In this study, we have investigated the effects of the P2X3 agonist α,β-methylene ATP (α,β-meATP) and antagonist 2′,3′-O-trinitrophenyl-ATP (TNP-ATP) on pelvic afferents innervating the urinary bladder using an in vitro mouse bladder-pelvic nerve preparation. Intravesical application of α,β-meATP (0.03-1 mm) increased multifibre discharges in a concentration-dependent manner. The agonist potentiated, whereas TNP-ATP (0.03 mm) attenuated, the multifibre responses to bladder distensions. Single-unit analysis revealed that both high threshold (HT) fibres (> 15 mmHg; known to be associated with nociception) and low threshold (LT) fibres (< 15 mmHg; probably associated with non-nociceptive events) could be induced to discharge by intravesical α,β-meATP (1 mm, 0.1 ml). The response of the vast majority (21/22, 95.5 %) of HT fibres to bladder distensions was enhanced with a significantly reduced threshold and an increased peak response after exposure to the agonist. On the other hand, 59.7 % (46/77) of LT fibres showed a greater peak and a slightly reduced threshold for response to bladder distension in the presence of α,β-meATP. An additional 11 ‘silent’ fibres became mechanosensitive after exposure to α,β-meATP. TNP-ATP (0.03 mm) did not affect the threshold of LT fibres, but it reduced the peak response of some (22/51, 43.1 %) LT fibres. Conversely, the antagonist resulted in a markedly elevated threshold and reduced peak activity in the majority (13/16, 81.3 %) of HT fibres. The results support the view that P2X3 receptor-mediated mechanisms contribute to both nociceptive and non-nociceptive (physiological) mechanosensory transduction in the urinary bladder.
There is compelling evidence that extracellular ATP plays a role in sensory transduction via P2X receptors, a family of ligand-gated ion channels (Burnstock, 2000). Seven P2X subtypes (P2X1-7) have been cloned to date (North & Surprenant, 2000) and the mRNA for six of them (P2X1-6) was found in sensory neurons. Among them, P2X2 and P2X3 subtypes are believed to be involved in peripheral sensory transduction, P2X3 subunits being expressed almost exclusively in small diameter nociceptive neurons (Chen et al. 1995) and P2X2 receptors being pH-sensitive (King et al. 1996). Furthermore, P2X agonists have been found to activate sensory neurons in culture (e.g. dorsal root ganglion, nodose ganglion and trigeminal ganglion) (Dunn et al. 2001) and afferent nerve fibres in situ (e.g. cardiopulmonary, mesenteric, trigeminal) (Bland-Ward & Humphrey, 1997; Dowd et al. 1998; Kirkup et al. 1999; Rong et al. 2000; Hamilton et al. 2001).
Cockayne et al. (2000) and Souslava et al. (2000) described the development of P2X3 knockout mice. These mice showed reduced pain-related behaviour induced by injections of formalin although responses to other noxious thermal and mechanical stimuli were unchanged. Furthermore, Cockayne et al. demonstrated that the knockout mice had an increased bladder capacity with reduced voiding frequency and increased voiding volume. Immunohistochemical studies showed that the mouse bladder is heavily innervated by P2X3-immunoreactive sensory fibres terminating in the subepithelial layer of the bladder wall (Cockayne et al. 2000). It has been proposed that ATP released by the cells that line the walls of the bladder, in response to stretch, may activate sensory afferents via an interaction with P2X3 receptors and subsequently initiate the micturition reflex and painful sensation (Burnstock 1999, 2001). This concept is supported by the demonstration that changes in hydrostatic pressure of the bladder released ATP (Ferguson et al. 1997). In a recent study using an in vitro mouse bladder-pelvic nerve model, we further demonstrated the release of ATP induced by distension of the mouse bladder (Vlaskovska et al. 2001). By recording the electrical activity from bundles of pelvic fibres, we found that the P2X agonists (ATP and α,β-meATP) applied intravesically could rapidly activate pelvic afferents and potentiate their response to bladder distension in P2X3 wild-type but not in P2X3 knockout mice. The P2X antagonist pyridoxal phosphate 6-azophenyl-2′,4′-disulfonic acid (PPADS), on the other hand, markedly reduced the mechanosensitive responses in the pelvic afferents of P2X3 wild-type mice.
The urinary bladder receives a dual afferent innervation that travels via the hypogastric and the lumbar splanchnic nerves and also via the pelvic nerves (Cervero, 1994). Investigations in humans indicated that all sensations from the bladder including pain and innocuous filling are signalled mainly by the pelvic afferents (Kuru, 1965). Neurophysiological studies have shown that the bladder afferents are small myelinated Aδ and unmyelinated C fibres and fall into three categories depending on their stimulus-response characteristics. The first group represent the largest population of afferents and respond to bladder distension with a low threshold (LT fibres). The second group respond to distension of the bladder with a high threshold (HT fibres). The third group are normally insensitive to bladder distension but can be sensitised during inflammation to become mechanosensitive (‘silent’ fibres). It has been speculated that the activation of LT fibres triggers micturition whilst the activation of HT and ‘silent’ fibres initiates painful sensation.
In the present report, we attempt to determine which of the above groups of fibres is involved in the purinergic mechanism of sensory transduction. The question is addressed by analysing the effects of the P2X agonist α,β-meATP and the P2X antagonist TNP-ATP on single unit pelvic afferent activity recorded from P2X3 wild-type mice.
METHODS
Animals
Four- to six-month-old P2X3 wild-type mice (6 male, 8 female, 27.6 ± 1.3 g) were used in this study. The breeding of these animals has been described previously (Cockayne et al. 2000). Maintenance and killing of the animals used in this study followed principles of good laboratory animal care and experimentation in compliance with UK national laws and regulations. The mice were killed humanely by exposure to a rising concentration of CO2 and cervical dislocation.
Preparation and nerve recording
The whole urinary tract attached to the surrounding tissues was dissected from the animal and placed in a recording chamber. The tissue was continuously superfused with oxygenated (95 % O2-5 % CO2) Krebs solution (contents, mm: NaCl 120; KCl 5.9; NaH2PO4 1.2; MgSO4 1.2; NaHCO3 15.4; CaCl2 2.5; glucose 11.5). In five experiments using the male mice, a 25G needle was inserted into the bladder and was connected to a three-way Omnifit (Cambridge, UK) to enable infusion/withdrawal of fluid and recording of intravesical pressure. The outlets (the ureters and the urethra) of the bladder were all tied. In the experiments using female mice, a catheter was inserted into the bladder through a small cut on the fundus of the bladder. Another catheter was inserted into the bladder through the urethra. The catheters were secured in place by ligatures with 7-0 sutures. The pelvic nerves were carefully dissected into several fine branches and were recorded using a suction electrode. Electrical activity was picked up by a Neurolog headstage (NL100, Digitimer Ltd, UK), amplified (NL104), filtered (NL125, band pass 300-4000 Hz) and captured by a computer via a power 1401 interface and Spike 2 software (version 3.02, CED, UK).
Experimental protocols
After approximately a 60 min period of stabilisation, the bladder was distended with Krebs solution (0.1 or 0.2 ml min−1) to an intravesical pressure of 40 mmHg or above, followed immediately by evacuation of the fluid. This was repeated several times at intervals of 10-15 min to assess the viability of the preparation and the reproducibility of pressure and neuronal responses to distension. To test the effect of α,β-meATP on the bladder afferents, 0.1 ml test solution (0-1 mm α,β-meATP, lithium salt, Sigma, USA) was injected as a bolus into the bladder. The effects of α,β-meATP on the mechanosensitive property of the bladder afferents were studied by comparing the afferent responses to bladder distensions following pre-exposure to vehicle (0.1 ml) or α,β-meATP (1 mm, 0.1 ml) or by comparing the afferent responses to bladder distensions with vehicle or 0.1 mm α,β-meATP. To determine whether endogenous ATP contributes to mechano-sensory transduction in bladder afferents, the bladder was distended with vehicle or 0.03 mm TNP-ATP.
Data analysis
Multifibre afferent nerve activity was quantified by spike counting using a spike processor (Digitimer D130). Some of the multifibre recordings contained only a few units with sufficiently different spikes to allow off-line single unit discrimination using the Spike 2 software. Baseline afferent activity was obtained by averaging the discharge rate in the 1 min period prior to agonist treatment or bladder distension. The response to a treatment was quantified by averaging the firing rate in every consecutive 10 s from the onset of that treatment. Single units (or nerve branches) were considered as responding to a challenge if the change in discharge rate was greater than 20 %. ‘Silent’ units were considered to respond to a challenge even if only one spike was discharged following that treatment. Where appropriate, data are expressed as means ± s.e.m. Responses were compared by Student's paired t test. Statistical significance was assured at P < 0.05.
RESULTS
Multifibre bladder afferent activity
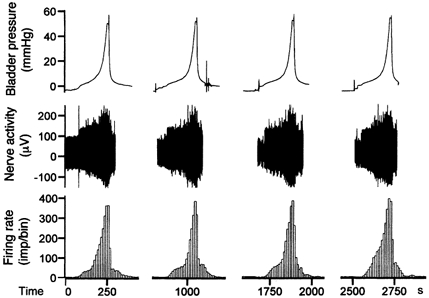
A typical multifibre recording taken from the pelvic nerve demonstrates the activity of mouse bladder afferents (Fig. 1). At rest, with the bladder empty, there was little background activity (< 10 imp s−1). However, as the bladder pressure increased to a threshold level (8.9 ± 1.1 mmHg, range 0.5-11.5 mmHg, n =10), the afferents started to discharge and the firing rate increased progressively as the pressure rose. The nerve responses to bladder distension were fairly consistent for repeated trials (Fig. 1).
Figure 1. Multifibre pelvic afferent activity induced by repeated bladder distensions with Krebs solution (0.2 ml min−1).
Intravesical administration of α,β-meATP (1 mm, 0.1 ml) reliably evoked a marked elevation in multifibre afferent discharge and a modest increase in bladder pressure in all cases (n =10, Fig. 2A). However, the magnitude of the afferent response showed great variability. This may be because of the variable number of active fibres in each multifibre recording or a variable percentage of fibres that express P2X receptors. Consequently, to plot the concentration-response bar graph (Fig. 2B), the afferent responses to different concentrations of the agonist were expressed as a percentage of the response to the highest concentration used (1 mm, 0.1 ml) in each experiment. The plot shows that the afferent responses to α,β-meATP were concentration dependent.
Figure 2. Multifibre afferent activity induced by intravesical application of α,β-meATP.
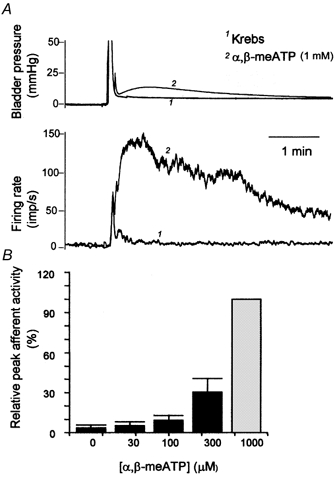
A, superimposed traces of the bladder pressure and rate histogram of a multifibre recording following intravesical injection of vehicle and 1 mm α,β-meATP (0.1 ml). B, relative afferent activity following intravesical application of α,β-meATP (0-1000 μM, 0.1 ml) in five multifibre preparations.
Figure 3 demonstrates the effects of the P2X agonist α,β-meATP and the antagonist TNP-ATP on the multifibre afferent response to bladder distensions. In six multifibre preparations tested, α,β-meATP greatly increased the magnitude of the afferent response to bladder distension in all cases without apparent effect on the threshold of the response. In control distensions with vehicle, the peak firing rate averaged 83.7 ± 17.4 imp s−1, and afferent discharge reached a peak rate of 134.3 ± 26.6 imp s−1 (P < 0.01 vs. control) in distensions with α,β-meATP (0.1 mm). In contrast, TNP-ATP significantly reduced the afferent response to bladder distension, again with no apparent effect on the threshold. The firing rate reached a peak of 85.8 ± 10.6 and 63.2 ± 10.3 imp s−1 (P < 0.01) in control and in the presence of TNP-ATP, respectively. It is noteworthy that the afferent discharges that occurred at lower bladder pressure (< 15 mmHg) were not affected by the antagonist, but the discharges at higher bladder pressure (>15 mmHg), were attenuated (see Fig. 3).
Figure 3. The effects of the P2X agonist and antagonist on multifibre afferent response to bladder distension.
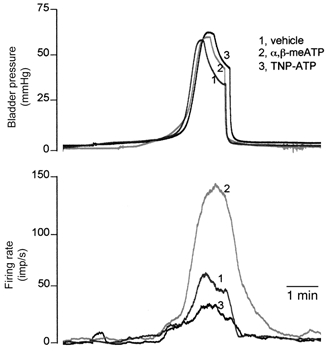
Traces of bladder pressure and rate for multiunit afferent discharge are superimposed for three consecutive bladder distensions with vehicle, 0.1 mm α,β-meATP and 0.03 mm TNP-ATP, respectively. Note the augmented afferent response in the presence of α,β-meATP and the reduced afferent response in the presence of TNP-ATP.
Single fibre afferent activity
The pelvic nerves were dissected further such that each branch only contained a few fibres. In the afferent recording illustrated in Figure 4, six individual units were induced to discharge by bladder distension. These units displayed sufficiently different spike amplitude and shape to allow accurate spike discrimination, as exemplified by the three units illustrated (Fig. 4C). Each had a distinct threshold and markedly different peak firing rate at a given bladder pressure. All three units could be activated by an intravesical application of α,β-meATP (1 mm, 0.1 ml, Fig. 4B). In 19 fibre preparations from 14 mice, a total of 149 individual units were analysed. The majority of these fibres were silent at rest (with the bladder empty) with only 11 (6.5 %) fibres having some ongoing activity (1-5 imp (10 s)−1). Ninety-six units started to discharge at a bladder pressure of < 15 mmHg (mean 7.1 ± 0.4 mmHg) and their activity increased with further increments in bladder pressure (see Fig. 4A, unit 1). These units were designated as low threshold (LT) fibres. Forty-two units were induced to fire at a pressure greater than 15 mmHg (mean 27.3 ± 1.7 mmHg) and were designated as high threshold (HT) fibres (see Fig. 4A, units 2 and 3). Eleven units did not respond to bladder distensions even at pressures of 50 mmHg, but they became sensitive to bladder distensions in the presence of α,β-meATP (e.g. unit 3 in Fig. 5). These units were defined as ‘silent’ units.
Figure 4. Activation of single fibre afferents by bladder distension and α,β-meATP.
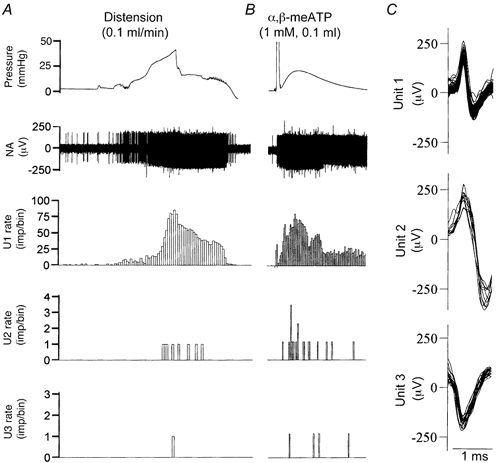
A, afferent discharges evoked by bladder distension. The rates of 3 apparently different units (labelled U1, U2 and U3) are displayed. Note they differ considerably with respect to threshold and discharge rate and can be classified as low threshold (LT, U1) and high threshold (HT, U2 and U3) fibres. B, afferent discharges induced by intravesical application of α,β-meATP (1 mm, 0.1 ml) in the same nerve preparation as in A. Bin = 10 s for A and B. C, superimposed spikes for U1, U2 and U3.
Figure 5. Effects of α,β-meATP on the mechano-sensitive properties of low and high threshold afferent fibres.
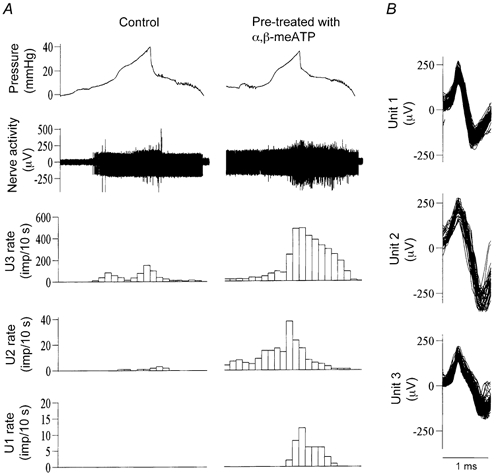
A, left and right columns show afferent activity in response to bladder distension (Krebs solution, 0.1 ml min−1) after pre-exposure to vehicle (0.1 ml) and α,β-meATP (1 mm, 0.1 ml), respectively. Note that a ‘silent’ fibre is sensitised to become mechano-sensitive by the agonist. B, superimposed action potentials for units 1-3. Bin = 10 s for A and B.
Twenty-one of 32 (65.6 %) LT fibres could be induced to discharge by an intravesical injection of α,β-meATP (1 mm, 0.1 ml) after a latency of 1-75 s (see unit 1 Fig. 4B). The maximal response (mean 38.2 ± 11.9 imp (10 s)−1) was observed 30-100 s after application of the agonist. Fourteen of 25 (56 %) HT fibres could also be activated after a latency of 10-175 s and reached peak activity (19.7 ± 4.8 imp (10 s)−1) 50-180 s after application of α,β-meATP (see units 2 and 3 in Fig. 4B).
The effects of α,β-meATP on the mechano-sensory properties of single bladder afferents were studied by comparing their response to bladder distension after pre-exposure to vehicle (Krebs solution) or 1 mm α,β-meATP (0.1 ml). A large proportion (46/77, 59.7 %) of LT fibres showed a greater response to bladder distension in the presence of α,β-meATP (e.g. unit 1 in Fig. 5 and Fig. 8). On average, the peak firing rate of these 46 units during distension was increased from 45.2 ± 11.9 (range 5-210) imp (10 s)−1 in control to 116.4 ± 20.3 (range 13-360) imp (10 s)−1 after exposure to α,β-meATP (P < 0.01). The threshold for the response of LT fibres was decreased only marginally by the agonist (from 8.3 ± 0.5 to 7.1 ± 0.4 mmHg, P < 0.05). On the other hand, the vast majority (21/22, 95.5 %) of HT fibres (threshold range 17-71.8 mmHg) showed a greater response to bladder distension in the presence of α,β-meATP (e.g. unit 2 in Fig. 5 and Fig. 8). The threshold for the response to bladder distension was lowered from an average of 25.6 ± 2.6 to 15.1 ± 1.0 mmHg (P < 0.01), whereas the mean peak firing rate was increased from 57.8 ± 13.9 (range 1-160) imp (10 s)−1 in control to 123.8 ± 29.7 (range 1-284) imp (10 s)−1 (P < 0.01) after the agonist treatment. Furthermore, an additional 11 units, which did not respond to bladder distension in the control situation (‘silent’ fibres), became sensitive to bladder distension after pre-exposure to α,β-meATP (e.g. unit 3 in Fig. 5).
Figure 8. The relationship between bladder pressure and discharge rate for LT and HT fibres.
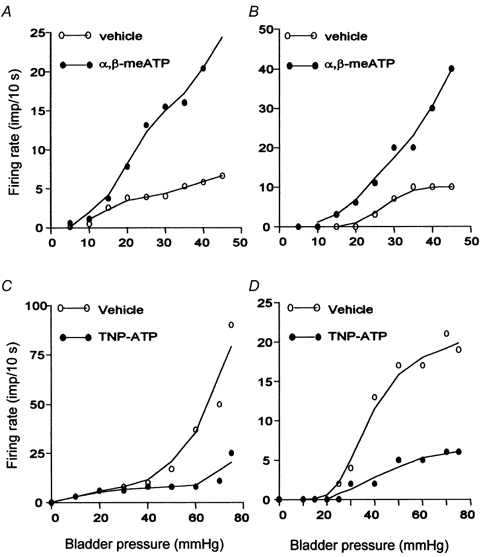
A and B, the rate histogram of a low (A) and a high (B) threshold fibre during bladder distension after pre-exposure to vehicle (0.1 ml) and α,β-meATP (1 mm, 0.1 ml). C and D, the rate histogram of a low (C) and a high (D) threshold fibre during bladder distension with vehicle and 0.03 mm TNP-ATP.
The effects of the P2X antagonist TNP-ATP on the mechano-sensory properties of bladder afferents were analysed in 51 LT and 19 HT fibres, by comparing their response to bladder distensions with vehicle or 0.03 mm TNP-ATP. The antagonist did not affect the threshold for the response of LT fibres to bladder distension (7.05 ± 0.48 vs.7.22 ± 0.55 mmHg, P > 0.05). However, it caused a moderate reduction in the peak response (from 56.3 ± 9.8 to 41.1 ± 9.7 imp (10 s)−1, P < 0.05) of 22 (43.1 %) LT fibres (Fig. 6 and Fig. 8C). The responses of the other 29 (56.9 %) units were unaffected by the antagonist. On the other hand, the antagonist resulted in a markedly elevated threshold (from 25.1 ± 1.9 to 36.4 ± 2.1 mmHg, P < 0.05) and a significantly reduced peak firing rate (from 48.6 ± 13.5 to 19.8 ± 7.2 imp (10 s)−1, P < 0.01) in the majority (13/16, 81.3 %) of HT fibres (Fig. 7, Fig. 8D). The effects of TNP-ATP on the responses of LT and HT fibres were reversible after washout of the antagonist.
Figure 6. The effects of TNP-ATP on the mechano-sensitive properties of a low threshold afferent fibre.
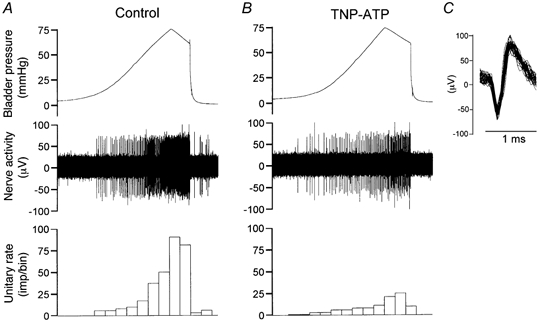
A, single unit activity induced by bladder distension with Krebs solution (0.1 ml min−1). B, single unit activity induced by bladder distension with TNP-ATP (0.03 mm, 0.1 ml min−1). Bin = 10 s for A and B. C, superimposed spikes for the unit.
Figure 7. The effects of TNP-ATP on the mechanosensitive response of a high threshold afferent fibre.
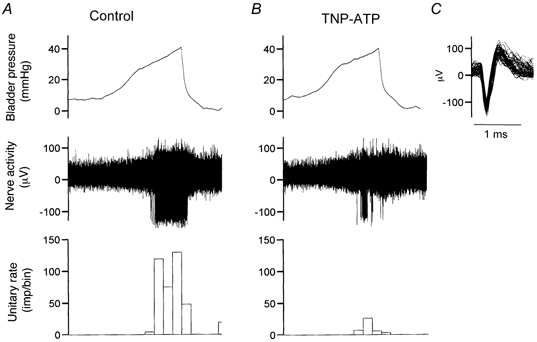
A, afferent discharges induced by bladder distension with Krebs solution (0.1 ml min−1). B, afferent discharges induced by distension with 0.03 mm TNP-ATP (0.1 ml min−1). Bin = 10 s for A and B. C, the superimposed action potential for the unit.
DISCUSSION
The present study has employed an in vitro mouse bladder- pelvic nerve preparation to investigate the effects of the P2X agonist α,β-meATP and the antagonist TNP-ATP on the activity and mechano-sensory properties of bladder afferent fibres. Using this preparation, it was possible to record reproducible afferent responses to bladder distensions over several hours (Fig. 1), thus permitting quantitative analysis of the possible involvement of purinergic mechanisms in sensory transduction in the fibres with afferent endings in the bladder. Our results revealed that intravesically applied α,β-meATP could activate, in a concentration-dependant manner, pelvic afferents and potentiate their response to bladder distension, whereas TNP-ATP reduced responses to bladder distension in most cases (Fig. 2 and Fig. 3).
Types of bladder afferents
Mechano-sensitive afferent fibres innervating the bladder were first described by Evans (1936) in cats and Talaat (1937) in dogs. They recorded the electrical activity of bundles of fibres in hypogastric and pelvic nerves and concluded that they were sensitive to stretch of the bladder wall. In addition, Talaat (1937) also reported that some of the afferent fibres in the hypogastric nerve had high thresholds for response to bladder distension and therefore their function could be related to painful sensations. Iggo (1955) postulated that the pelvic afferent fibres sensitive to bladder distension were tension receptors located in series with the smooth muscle fibres. These afferents have been shown to have a low threshold to passive bladder distension and also to respond to normal bladder contractions associated with voiding (Bahns et al. 1986).
The presence of populations of low (LT) and high threshold (HT) bladder afferents in the pelvic nerve has been well documented in in vivo studies in the cat (Häbler et al. 1990, 1993) and the rat (Sengupta & Gebhart, 1994). In addition, a third population of afferents that were normally insensitive to even noxious bladder distension were identified in inflamed bladder in the cat (Häbler et al. 1990). These were called ‘silent receptors’ or ‘chemosensitive fibres’. As far as we know, the present study is the first attempt to analyse the single unit activity of pelvic afferents in the mouse. In agreement with previous in vivo studies in rats and in cats, bladder afferents were identified in this species with similar stimulus-response characteristics using an in vitro mouse bladder/pelvic nerve preparation (Fig. 4 and Fig. 5). The results demonstrate the existence of LT, HT and ‘silent’ (chemosensitive) bladder afferents in the pelvic nerve in this species.
Activation and sensitisation of bladder afferent by α,β-meATP
Although a considerable amount is known about the stimulus-response properties of the afferents innervating the urinary bladder, very little is known about how mechanical stretch to the bladder wall is actually transduced into a receptor potential in afferent terminals. Studies since the 1980s have demonstrated that many substances, especially irritant chemicals (such as mustard oil and turpentine oil) (Häbler et al. 1988, 1990) and inflammatory mediators (such as bradykinin, serotonin, substance P, histamine, prostaglandin E2 and K+) (Sengupta & Gebhart, 1994) are able to activate these afferents and, more importantly, modulate their mechanosensory properties.
In the present study, we found that a large proportion of LT (21/32, 65.6 %) and HT (14/25, 56 %) fibres could be activated by intravesical application of the selective P2X agonist α,β-meATP (0.1 ml, 1 mm). In addition, 11 ‘silent’ fibres could also be induced to discharge by the agonist. The afferent responses following application of the agonist were obscured by the increase in bladder pressure that resulted from detrusor muscle contraction, possibly due to concomitant activation of P2X1 receptors. However, there was clear evidence for a direct action of the agonist on the afferents, since the increase in pressure was moderate and the afferents discharged more vigorously than could be attributed to the increase in pressure alone (compare afferent activity in Fig. 4A and B).
In addition to activation of the afferents, another important aspect of the effects of intravesically applied α,β-meATP was the potentiation of afferent responses to bladder distensions and the sensitisation of ‘silent’ fibres. In this respect, it is interesting to note that the vast majority (21/22, 99.5 %) of HT fibres exhibited greater sensitivity (lowered threshold and increased peak activity) to bladder distension in the presence of α,β-meATP, whereas a smaller proportion (46/77, 59.7 %) of LT fibres were sensitised by the agonist (increased peak activity) and no change in threshold was observed.
The solution of α,β-meATP applied intravesically contained maximally 3 mm lithium (Li+), but the changes in afferent activity following application of α,β-meATP were unlikely to be mediated by Li+ through epithelial sodium channels. Rather, the changes appeared to be mediated by P2X receptors. We have recently compared multifibre afferent activity following intravesical injection of 0.1 ml vehicle with 3 mm lithium and 0.1 ml solution containing 1 mm α,β-meATP. Whilst α,β-meATP resulted in marked activation of the afferents, there was little change in afferent activity following 3 mm Li+. Furthermore, we have shown in an earlier report that TNP-ATP could effectively antagonise the excitatory action of α,β-meATP on the bladder afferents (Vlaskovska et al. 2001).
P2X receptors have been shown to exist in the urinary bladder (see Burnstock, 2001). P2X1 receptors were found on detrusor muscles (Ferguson, 1999; Lee et al. 2000) whereas P2X3 receptors were localised in the urothelium (Ferguson, 1999). We have previously demonstrated P2X3 immunostaining on afferent fibres (Cockayne et al. 2000; Vlaskovska et al. 2001), whereas Elneil et al. (2001) reported that P2X3 immunostaining in the urothelium (of rat and human bladder) was not associated with CGRP-containing sensory fibres. Thus, the effects of α,β-meATP on the bladder afferents might be mediated by the activation of P2X3 (or P2X2/3) receptors located on afferent terminals. Alternatively, the agonist might activate P2X3 receptors on urothelial cells, which then release substances (including ATP) to stimulate the afferent terminal.
The afferent terminals are located mainly in the subepithelial layer, but some fibres penetrate into the basal layer of the urothelial cells and others terminate in between detrusor muscles. To stimulate the afferents directly, α,β-meATP needs to cross the urothelium and diffuse through to the terminals. We are not sure how readily α,β-meATP can do so. It appears that the agonist can penetrate the urothelium quite fast since the bladder contracted quickly following intravesical administration of the agonist (see Fig. 2), indicating activation of P2X1 receptors on detrusor muscles.
It is not clear whether different receptor mechanisms account for the activation and the sensitisation of bladder afferents. However, the afferent activity induced by intravesical application of α,β-meATP faded quickly despite continued exposure to the agonist (Fig. 2 and Fig. 5), implying an important role of the rapidly desensitising P2X3 subtypes for the activation of the afferents. On the other hand, the agonist continued to sensitise the afferents after cessation of the ongoing activity (e.g. units 1-3 in Fig. 5). This might indicate an involvement of P2X2/3 heteromultimers in the sensitisation of bladder afferents.
Modulation of bladder afferents by endogenous ATP
We applied TNP-ATP to block the action of endogenous ATP on bladder afferents. TNP-ATP has been shown to be a potent and selective antagonist of homomultimeric P2X1, P2X3 and heteromultimeric P2X2/3 receptors with an IC50 of 1-6 nm (Virginio et al. 1998; Burgard et al. 2000). The efficacy of TNP-ATP was reduced in whole tissue experiments (IC50 30 μM, Lewis et al. 1998), possibly due to its breakdown by nucleotidases. We have previously shown that TNP-ATP (30 μM) effectively blocked the excitatory action of α,β-meATP on bladder afferents (Vlaskovska et al. 2001). In the present study, TNP-ATP resulted in a significant reduction in the multifibre afferent discharge at high (>15 mmHg) bladder pressure, indicating an involvement of endogenous ATP in the mechanosensory transduction. Single unit analysis demonstrated that whilst only 43.1 % of LT fibres were affected by TNP-ATP, the majority (81.3 %, 13/16) of HT fibres showed a raised threshold and reduced peak response to bladder distensions. This can probably be explained by the fact that bladder distension induced a pressure-dependent release of ATP (Vlaskovska et al. 2001).
In the human, a sensation of fullness occurs at an intravesical pressure of 5-15 mmHg. This is followed by an urge to void at an intravesical pressure of 20-25 mmHg. The first sign of discomfort occurs when intravesical pressure exceeds 25 mmHg and pain is perceived when micturition is prevented and bladder pressure exceeds 30 mmHg. Our findings with the P2X antagonist suggest that endogenously released ATP may be involved in innocuous as well as nociceptive sensory processing in the bladder.
Perspectives
The involvement of a purinergic mechanism in mechanosensory transduction in the urinary bladder is of considerable interest. Normal bladder function requires co-ordinated contraction and relaxation of detrusor and urethral sphincter muscles and this depends on sensory inputs from the bladder to control centres in the spinal cord, pons and forebrain. There is a high incidence of lower urinary tract symptoms (such as incontinence and pain) that may be associated with afferent nerve activity. Although direct evidence for an involvement of a purinergic sensory mechanism in pathological bladder is still lacking, in theory, increased release of ATP and/or increased expression of P2X3 receptors meant increased afferent activity in LT fibres which may in turn lead to more frequent micturition and increased afferent activity in HT fibres which may cause bladder pain. Indeed, there is now good evidence that P2X3 receptors may be up-regulated in the state of inflammation (Hamilton et al. 1999, 2001; Zhang et al. 2001) and are essential for inflammatory pain (Souslova et al. 2000). There is also evidence that inflamed cells release more ATP (Bodin & Burnstock, 1996). Thus, in our opinion, it is likely that purinergic sensory signalling may be up-regulated and may contribute to the symptoms of bladder inflammation. Agents that reduce ATP release, or block P2X3 (and/or P2X2/3) receptors, may be potentially effective in relieving over-activity and pain of the bladder associated with inflammation.
Acknowledgments
We wish to thank Drs G. Knight and P. Dunn for helpful discussions about this study. The animals used in this study were provided by Drs D. Cockayne and A. P. W. D. Ford (Roche Bioscience, Palo Alto, USA). W.R. is supported by the Wellcome Trust.
REFERENCES
- Bahns E, Ernsberger U, Janig W, Nelke A. Functional characteristics of lumbar visceral afferent fibres from the urinary bladder and the urethra in the cat. Pflügers Archiv. 1986;407:510–518. doi: 10.1007/BF00657509. [DOI] [PubMed] [Google Scholar]
- Bland-Ward PA, Humphrey PP. Acute nociception mediated by hindpaw P2X receptor activation in the rat. British Journal of Pharmacology. 1997;122:365–371. doi: 10.1038/sj.bjp.0701371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodin P, Burnstock G. ATP-stimulated release of ATP by human endothelial cells. Journal of Cardiovascular Pharmacology. 1996;27:872–875. doi: 10.1097/00005344-199606000-00015. [DOI] [PubMed] [Google Scholar]
- Burgard EC, Niforatos W, Biesen TV, Lynch KJ, Kage KL, Touma E, Kowaluk EA, Jarvis MF. Competitive antagonism of recombinant P2X2/3 receptors by 2′,3′-O-(2,4,6-trinitrophenyl) adenosine 5′ -triphosphate (TNP-ATP) Molecular Pharmacology. 2000;58:1502–1510. doi: 10.1124/mol.58.6.1502. [DOI] [PubMed] [Google Scholar]
- Burnstock G. Release of vasoactive substances from endothelial cells by shear stress and purinergic mechanosensory transduction. Journal of Anatomy. 1999;194:335–342. doi: 10.1046/j.1469-7580.1999.19430335.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnstock G. P2X receptors in sensory neurones. British Journal of Anaesthesia. 2000;84:476–488. doi: 10.1093/oxfordjournals.bja.a013473. [DOI] [PubMed] [Google Scholar]
- Burnstock G. Purine-mediated signalling in pain and visceral perception. Trends in Pharmacological Sciences. 2001;22:182–188. doi: 10.1016/s0165-6147(00)01643-6. [DOI] [PubMed] [Google Scholar]
- Cervero F. Sensory innervation of the viscera: peripheral basis of visceral pain. Physiological Reviews. 1994;74:95–138. doi: 10.1152/physrev.1994.74.1.95. [DOI] [PubMed] [Google Scholar]
- Chen CC, Akopian AN, Sivilotti L, Colquhoun D, Burnstock G, Wood JN. A P2X purinoceptor expressed by a subset of sensory neurons. Nature. 1995;377:428–431. doi: 10.1038/377428a0. [DOI] [PubMed] [Google Scholar]
- Cockayne DA, Hamilton SG, Zhu Q, Dunn PM, Zhong Y, Novakovic S, Malmberg AB, Cain G, Berson A, Kassotakis L, Hedley L, Lachnit WG, Burnstock G, McMahon SB, Ford APD. Urinary bladder hyporeflexia and reduced pain-related behaviour in P2X3-deficient mice. Nature. 2000;407:1011–1015. doi: 10.1038/35039519. [DOI] [PubMed] [Google Scholar]
- Dowd E, McQueen DS, Chessell IP, Humphrey PP. P2X receptor-mediated excitation of nociceptive afferents in the normal and arthritic rat knee joint. British Journal of Pharmacology. 1998;125:341–346. doi: 10.1038/sj.bjp.0702080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn PM, Zhong Y, Burnstock G. P2X receptors in peripheral neurons. Progress in Neurobiology. 2001;65:107–134. doi: 10.1016/s0301-0082(01)00005-3. [DOI] [PubMed] [Google Scholar]
- Elneil S, Skepper JN, Kidd EJ, Williamson JG, Ferguson DR. Distribution of P2X1 and P2X3 receptors in the rat and human urinary bladder. Pharmacology. 2001;63:120–128. doi: 10.1159/000056122. [DOI] [PubMed] [Google Scholar]
- Evans JP. Observations on the nerve supply to the bladder and urethra of the cat with a study of their action potentials. Journal of Physiology. 1936;86:396–414. doi: 10.1113/jphysiol.1936.sp003375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson DR, Kennedy I, Burton TJ. ATP is released from rabbit urinary bladder epithelial cells by hydrostatic pressure changes – a possible sensory mechanism? Journal of Physiology. 1997;505:503–511. doi: 10.1111/j.1469-7793.1997.503bb.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson DR. Urothelial function. British Journal of Urology (International) 1999;84:235–242. doi: 10.1046/j.1464-410x.1999.00187.x. [DOI] [PubMed] [Google Scholar]
- Habler HJ, Janig W, Koltzenburg M. A novel type of unmyelinated chemosensitive nociceptor in the acutely inflamed urinary bladder. Agents Actions. 1988;25:219–221. doi: 10.1007/BF01965016. [DOI] [PubMed] [Google Scholar]
- Habler HJ, Janig W, Koltzenburg M. Activation of unmyelinated afferent fibres by mechanical stimuli and inflammation of the urinary bladder in the cat. Journal of Physiology. 1990;425:545–562. doi: 10.1113/jphysiol.1990.sp018117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habler HJ, Janig W, Koltzenburg M. Receptive properties of myelinated primary afferents innervating the inflamed urinary bladder of the cat. Journal of Neurophysiology. 1993;69:395–405. doi: 10.1152/jn.1993.69.2.395. [DOI] [PubMed] [Google Scholar]
- Hamilton SG, McMahon SB, Lewin GR. Selective activation of nociceptors by P2X receptor agonists in normal and inflamed rat skin. Journal of Physiology. 2001;534:437–445. doi: 10.1111/j.1469-7793.2001.00437.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton SG, Wade A, McMahon SB. The effects of inflammation and inflammatory mediators on nociceptive behaviour induced by ATP analogues in the rat. British Journal of Pharmacology. 1999;126:326–332. doi: 10.1038/sj.bjp.0702258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iggo A. Tension receptors in the stomach and urinary bladder. Journal of Physiology. 1955;128:593–607. doi: 10.1113/jphysiol.1955.sp005327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King BF, Ziganshin ALE, Pintor J, Burnstock G. Full sensitivity of P2X2 purinoceptor to ATP revealed by changing extracellular pH. British Journal of Pharmacology. 1996;117:1371–1373. doi: 10.1111/j.1476-5381.1996.tb15293.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkup AJ, Booth CE, Chessell IP, Humphrey PPA, Grundy D. Excitatory effect of P2X receptor activation on mesenteric afferent nerves in the anaesthetised rat. Journal of Physiology. 1999;520:551–563. doi: 10.1111/j.1469-7793.1999.00551.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuru M. Nervous control of micturition. Physiological Reviews. 1965;45:425–494. doi: 10.1152/physrev.1965.45.3.425. [DOI] [PubMed] [Google Scholar]
- Lee HY, Bardini M, Burnstock G. Distribution of P2X receptors in the urinary bladder and the ureter of the rat. Journal of Urology. 2000;163:2002–2007. [PubMed] [Google Scholar]
- Lewis CJ, Surprenant A, Evans RJ. 2′,3′-O-(2,4,6-trinitrophenyl) adenosine 5′-triphosphate (TNP-ATP): a nanomolar affinity antagonist at rat mesenteric artery P2X receptor ion channels. British Journal of Pharmacology. 1998;124:1463–1466. doi: 10.1038/sj.bjp.0702001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- North RA, Surprenant A. Pharmacology of cloned P2X receptors. Annual Review of Pharmacology and Toxicology. 2000;40:563–580. doi: 10.1146/annurev.pharmtox.40.1.563. [DOI] [PubMed] [Google Scholar]
- Rong W, Burnstock G, Spyer KM. P2X purinoceptor-mediated excitation of trigeminal lingual nerve terminals in an in vitro intra-arterially perfused rat tongue preparation. Journal of Physiology. 2000;524:891–902. doi: 10.1111/j.1469-7793.2000.00891.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sengupta JN, Gebhart GF. Mechanosensitive properties of pelvic nerve afferent fibers innervating the urinary bladder of the rat. Journal of Neurophysiology. 1994;72:2420–2430. doi: 10.1152/jn.1994.72.5.2420. [DOI] [PubMed] [Google Scholar]
- Souslova V, Cesare P, Ding Y, Akopian AN, Stanfa L, Suzuki R, Carpenter K, Dickenson A, Boyce S, Hill R, Nebenuis-Oosthuizen D, Smith AJ, Kidd EJ, Wood JN. Warm-coding deficits and aberrant inflammatory pain in mice lacking P2X3 receptors. Nature. 2000;407:1015–1017. doi: 10.1038/35039526. [DOI] [PubMed] [Google Scholar]
- Talaat M. Afferent impulses in the nerves supplying the urinary bladder. Journal of Physiology. 1937;89:1–13. doi: 10.1113/jphysiol.1937.sp003458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Virginio C, Robertson G, Surprenant A, North RA. Trinitrophenyl-substituted nucleotides are potent antagonists selective for P2X1, P2X3, and heteromeric P2X2/3 receptors. Molecular Pharmacology. 1998;53:969–973. [PubMed] [Google Scholar]
- Vlaskovska M, Kasakov L, Rong W, Bodin P, Bardini M, Cockayne DA, Ford AP, Burnstock G. P2X3 knock-out mice reveal a major sensory role for urothelially released ATP. Journal of Neuroscience. 2001;21:5670–5677. doi: 10.1523/JNEUROSCI.21-15-05670.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang YH, Chen Y, Zhao ZQ. Alteration of spontaneous firing rate of primary myelinated afferents by ATP in adjuvant-induced inflamed rats. Brain Research Bulletin. 2001;54:141–144. doi: 10.1016/s0361-9230(00)00422-6. [DOI] [PubMed] [Google Scholar]


