Abstract
This study was designed to test the hypothesis that cardiac receptors tonically inhibit the secretion of renin, arginine vasopressin (AVP) and adrenocorticotropic hormone (ACTH) in late-gestation fetal sheep. Eight chronically catheterised fetal sheep between 122 and 134 days gestation were subjected to injection or infusion of saline or 4 % procaine into the pericardial space. Fetal blood pressure and heart rate were monitored and fetal blood samples were drawn to measure the response to these injections. Injection of procaine into the pericardial space effectively blocked cardiac nerves, as evidenced by a reduction in the variability of fetal heart rate and by the blockade of reflex reductions in fetal heart rate after intravenous injection of phenylephrine (an α-adrenergic agonist which raises blood pressure). Injection of saline had no discernable effects on any of the measured variables. A single injection of procaine, followed by a slow infusion, produced a transient blockade of cardiac nerves. Multiple injections of procaine produced a sustained blockade of cardiac nerves and a sustained rise in fetal plasma renin activity and ACTH. In none of the experiments did procaine significantly alter fetal plasma AVP concentrations. In 11 fetuses between 121 and 134 days gestation, we combined the cardiac nerve blockade with slow haemorrhage to test the cardiac nerves as mediators of the endocrine response to haemorrhage in utero. Cardiac nerve blockade exaggerated the fetal blood gas response to haemorrhage somewhat but did not significantly alter the magnitude of the ACTH, AVP, or plasma renin activity response to haemorrhage. We conclude that cardiac nerves in the late-gestation fetal sheep have minor influences on plasma renin activity and ACTH in normovolaemic fetuses, but that changes in cardiac nerve activity do not mediate the endocrine responsiveness to haemorrhage.
Alterations in blood pressure and volume stimulate reflex homeostatic responses which are appropriate for returning blood pressure and volume to normal levels. In adult animals, neural receptors in the carotid sinus, aortic arch, atria, ventricles, and other receptors in the cardiopulmonary region are important inputs to the central nervous system mediating reflex responses (Gann et al. 1964; Share, 1965, 1967; Brennan et al. 1971; Cryer & Gann, 1974; Thames et al. 1980). Atrial mechanoreceptors with vagal afferent fibres play a well-defined role in the control of arginine vasopressin (AVP), plasma renin activity (PRA), and adrenocorticotropin (ACTH) (Share, 1967; Brennan et al. 1971; Cryer & Gann, 1974). These receptors tonically inhibit the secretion of these three hormonal systems, so that during haemorrhage the reduction in afferent activity allows increases in the rate of secretion. In addition to the atrial receptors, it is likely that other receptors in the cardiopulmonary region also affect the rate of secretion of these hormones (Thames, 1977; Thames et al. 1980).
The fetal sheep differs from the adult both anatomically and functionally. In particular, the fetal blood pressure is regulated at a low level compared to the adult, and there is evidence to suggest that fetal blood pressure is defended mainly by baroreceptors and/or chemoreceptors in the carotid sinus and/or aortic arch (Wood et al. 1989; Chen & Wood, 1992). We have reported that vagal afferent fibres are not involved in the control of hormonal responses to haemorrhage in late-gestation fetal sheep (Wood et al. 1989). We interpreted these results to suggest that the atrial stretch receptors with vagal afferents are not quantitatively important during fetal life. Nevertheless, Jaekle et al. (1995) have reported that constriction of the pulmonary artery proximal to the origin of the ductus arteriosus increased circulating concentrations of vasopressin and renin, in addition to atrial natriuretic peptide. Results of that study suggest a stimulatory effect of cardiac pressure on the secretion of these hormones (since pulmonary artery constriction increases right heart pressures). All of these studies leave unanswered the question of whether there are non-vagal afferent fibres from the heart which affect the secretion of ACTH, AVP and renin during slow haemorrhage. The present study was designed to test this hypothesis.
METHODS
We studied 26 chronically catheterised fetal sheep between 121 and 134 days gestation. The pregnant ewes were of mixed Western and Florida native breeds.
These experiments were approved by the University of Florida Institutional Animal Care and Use Committee. The sheep were anaesthetised for surgery using halothane, delivered at a dose of 0.5-2.0 % in oxygen. At the end of the study, the animals were humanely killed with an overdose of sodium pentobarbital intravenously.
Surgical preparation
The surgeries were performed at least 5 days before the experiments. All fetuses and their mothers were chronically prepared with femoral arterial and venous and amniotic fluid catheters as previously described (Wood, 1989). Each fetus was also subjected to implantation of a catheter in the pericardial space. For placement of the pericardial catheter, the fetus was first positioned by delivery of the fetal head through an incision in the uterus and membranes. The chest and left forelimb were exposed, and the wall of the uterus marsupialised to the fetal skin using Babcock clamps. An incision was made in the third intercostal space, and the fetal heart exposed by retraction of the ribs. A single catheter (i.d. 0.30 inches; o.d. 0.50 inches; polyvinylchloride catheter with a Silastic catheter tip) was inserted into the pericardial space and held in place with a purse-string suture. The vinyl portion of the catheter was secured in place with the sutures used to close the fetal chest. After placement of the catheter, the fetus was returned to the uterus, and the uterus was closed using a double row of sutures.
After closure of the uterus, the vascular catheters were filled with sodium heparin (1000 U ml−1) and plugged. The pericardial catheter and the amniotic fluid catheters were plugged without flushing. All of the catheters were led to the ewe's flank, where they exited through a small incision. The ends of the catheters were held in place in a small disposable pocket sutured to the skin of the ewe. The pocket was protected with expandable bandage stretched around the abdomen of the ewe.
Ampicillin (500 mg) was injected into the amniotic fluid and the same dosage was injected intramuscularly to the mother at the time of surgery as well as twice per day for 5 days after the surgery. The vascular catheters were flushed and re-heparinised at least once every 3 days.
Experimental protocol
In each experiment, fetal arterial and central venous blood pressures, amniotic fluid pressure, and heart rate were monitored continuously.
Study 1. Development of the model
In study 1, we performed experiments which were designed to test the experimental model of cardiac nerve blockade. This study was performed on eight fetal sheep between 122 and 134 days gestation. Blood samples were drawn from one fetal arterial catheter at 30 min intervals for 120 min. The volume of the blood samples was 4 ml; this blood was processed for hormone analysis as described below. At each time point, an additional 1 ml blood sample was drawn anaerobically for measurement of fetal blood gases and haematocrit. In this way, a total of 35 ml of fetal blood was withdrawn in each experiment. Fetuses were subjected to three different experiments. In one experiment (n = 7), 2 ml of 0.9 % physiological saline was injected into the pericardial space at 0 min, then infused at a rate of 0.007 ml min−1 until 120 min. In another experiment (n = 6), 2 ml of 4 % procaine dissolved in 0.9 % physiological saline was injected into the pericardial catheter at 0 min, followed by an infusion of 4 % procaine at a rate of 0.007 ml min−1 for 120 min. In another experiment (n = 5), 2 ml of 4 % procaine was injected into the pericardial catheter at 0 min, followed by injections of 0.5 ml of 4 % procaine into the same catheter at 20 min intervals for 120 min.
Study 2. Application of the model
In study 2, we used the cardiac nerve blockade method to test the role of cardiac nerves in the generation of hormonal responses to haemorrhage. This study was performed on 18 fetal sheep between 121 and 134 days gestation. In this study, we combined intrapericardial injections of procaine or saline (2 ml of 4 % procaine or saline at 0 min, followed by 0.5 ml of 4 % procaine or saline at 20 min intervals) with slow haemorrhage (10 ml of fetal arterial blood at 10 min intervals for 2 h). Each fetus was subjected to two experiments. In the first experiment, the fetus was subjected to pericardial injection of either saline or procaine, and in the second experiment the fetus was subjected to a combination of the same (saline or procaine) injection with slow haemorrhage. We have previously described this haemorrhage protocol, and we have published endocrine responses previously (Wood et al. 1989; Chen & Wood, 1992). This haemorrhage represents a withdrawal of approximately 40 % of the total fetal blood volume (assuming a fetal body weight of approximately 3 kg and a fetal blood volume of approximately 110 ml kg−1), and a withdrawal rate of approximately 0.3 % of total blood volume per minute of haemorrhage (Brace & Cheung, 1986, 1990).
Handling and analysis of blood samples
All blood samples were placed in chilled plastic centrifuge tubes containing 0.05 ml of 0.3 M Na4EDTA (Sigma Chemical Co, St Louis, MO, USA) per ml blood. All tubes were kept on ice until the end of the experiment when they were centrifuged at 3000 g for 20 min in a refrigerated (4 °C) centrifuge. The plasma was stored at −20 °C until hormones were assayed.
Plasma ACTH was measured by radioimmunoassay (RIA) in extracted plasma using anti-ACTH raised in this laboratory and 1-39 hACTH standard purchased from Peninsula Laboratories (Belmont, CA, USA). This antiserum cross-reacts 100 % with (1-24)-ACTH, 11-24 ACTH, and 1-39 ACTH, but does not bind oCRF or methionine enkephalin. The antiserum bound arginine vasopressin 0.5 % relative to 1-39 hACTH. Plasma vasopressin (AVP) concentrations were measured by RIA using anti-vasopressin antiserum produced in this laboratory, [125I]-labelled vasopressin purchased from New England Nuclear (Boston, MA, USA), and synthetic arginine vasopressin from Sigma. Plasma assayed for ACTH was extracted using powdered glass, eluted with HCl and acetone; plasma assayed for AVP was extracted using bentonite, eluted with a 1:1 mixture of 1 m HCl and acetone. Extracts were dried using a Speedvac concentrator (Savant Instruments, Farmingdale, NY, USA). Plasma renin activity (PRA) was measured by radioimmunoassay. For this assay, angiotensin I (ANG I) was generated in buffered (pH 5.7) plasma in vitro for 1 h at 37 °C. At the end of the incubation period the ANG I concentration was measured by RIA. The details of the cortisol, ACTH, vasopressin and plasma renin activity assays have been published elsewhere (Wood et al. 1990, 1993; Bell et al. 1991; Raff et al. 1991).
Fetal blood gases were measured using a Radiometer PHM73 blood gas analyser and BMS3Mk2 blood microsystem (Radiometer, Copenhagen, Denmark). The blood gas analyser was calibrated as recommended by the manufacturer using a Radiometer GMA2 calibrated gas supply.
Measurement of pressures and heart rate
The fetal mean arterial pressure (MAP), central venous pressure (CVP), amniotic pressure and fetal heart rate (HR) were continuously monitored using a Beckman R611 (Fullerton, CA) or Grass Model 7 (Quincy, MA, USA) direct-writing recorder by connecting the fetal catheters to Statham P23Db pressure transducers (Statham Instruments, Oxnard, CA). The variables were sampled and analog-to-digital conversions performed at 2 s intervals using a Keithley 570 analog-to-digital converter and an IBM microcomputer. All fetal intravascular pressures were calculated using amniotic fluid pressure as zero reference.
Statistical analyses
Changes in the values of fetal hormones, haemodynamic variables, mean arterial blood gases and haematocrits (Hct) over time and between groups were assessed using two-way analysis of variance (ANOVA) corrected for repeated measures in one dimension, time (Winer, 1971). A posteriori comparison of individual means was performed using Duncan's multiple range test (Winer, 1971). A significance level of P < 0.05 was used to reject the null hypothesis in all tests.
RESULTS
Study 1. Development of the model: the role of cardiac nerves in normovolaemic fetuses
Procaine injection dramatically reduced the variability in fetal heart rate (Fig. 1). The single injection followed by constant infusion of procaine produced a transient inhibition of the variability in fetal heart rate, probably reflecting a transient blockade of cardiac nerves. The multiple injections of procaine produced a sustained reduction of fetal heart rate variability, most probably reflecting a sustained blockade of the cardiac nerves. Injection of saline or of procaine into the pericardial space did not have any statistically significant effect on the mean levels of mean arterial pressure, heart rate, or central venous pressure (Fig. 2). The effect of pericardial procaine on heart rate variability was most noticeable in the fetuses with distinct heart rate variability (Fig. 1).
Figure 1. Procaine injection into the pericardium reduces the variability in fetal heart rate.
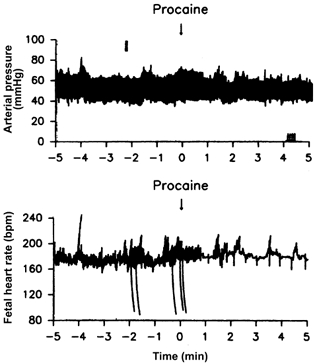
The effect of a single injection of 2 ml of 4 % procaine into the pericardium in a late-gestation fetal sheep.
Figure 2.
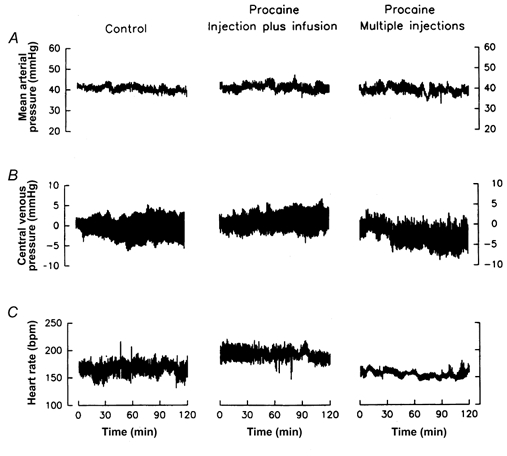
Fetal mean arterial pressure (A), central venous pressure (B), and heart rate (C) before and during administration of saline (left column of panels, n = 7), injection plus infusion of procaine (middle column, n = 6), or multiple injections of procaine (right column, n = 5) into the pericardium.
The efficacy of the blockade of cardiac nerves was tested by injection of phenylephrine intravenously. After injection of saline into the pericardial space, phenylephrine increased fetal blood pressure and stimulated a baroreflex-mediated decrease in fetal heart rate. After injection of procaine, however, the reflex reduction in fetal heart rate after phenylephrine injection was blocked, indicating blockade of the efferent nerves to the heart (Fig. 3). When tested at the end of the experiment, the injection plus infusion of procaine did not produce a sustained blockade, while multiple injections of procaine did.
Figure 3. Procaine injection into the pericardium blocks the reflex heart rate response to injection of phenylephrine.
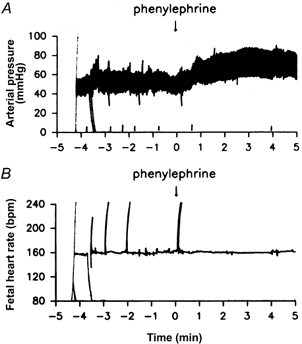
Fetal arterial pressure (A) and heart rate (B) responses to intravenous phenylephrine injection after blockade of cardiac nerves with multiple injections of procaine into the pericardium.
Testing of afferent nerve blockade was attempted in most of the fetal sheep using veratridine. This alkaloid stimulates cardiac C-fibres, and produces a reflex reduction in arterial blood pressure. This test was not feasible, however, in that the reflex response to veratridine is not fully developed in fetuses of this gestational age (Nuwayhid et al. 1975). However, in the fetuses which responded to the veratridine, the injection of procaine blocked the reflex response.
Fetal blood gases were typical of chronically catheterised healthy fetuses (Fig. 4). The injection of saline or of procaine did not significantly alter fetal blood gases, although there tended to be an insignificant decrease in pHa during the infusion of procaine (Fig. 4C, middle panel).
Figure 4.
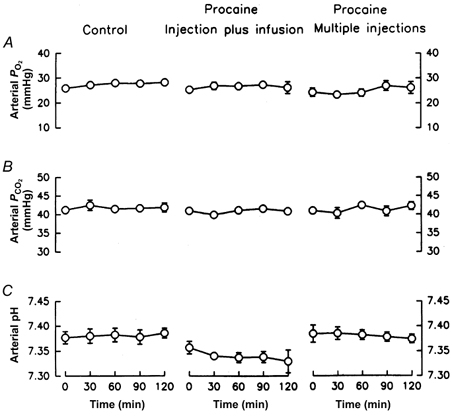
Fetal arterial partial pressure of oxygen (A), arterial partial pressure of carbon dioxide (B), and arterial pH (C) before and during administration of saline (left column of panels, n = 7), injection plus infusion of procaine (middle column, n = 6), or multiple injections of procaine (right column, n = 5) into the pericardium. Data are represented as mean values with vertical bars representing 1 standard error above and below the mean.
Fetal plasma renin activity was not altered by the injection and infusion of saline into the pericardial space (Fig. 5). Injection followed by infusion of procaine did not significantly increase fetal plasma renin activity. There was an apparent (not statistically significant) increase above initial levels (from 2.6 ± 1.2 to 5.0 ± 1.8 ng ml−1 h−1) at 30 min. Multiple injections of procaine produced a sustained statistically significant increase in fetal plasma renin activity from 2.8 ± 1.1 at 0 min to 8.5 ± 2.0 ng ml−1 h−1 at 30 min (Fig. 5). The statistically significant increase in PRA was sustained throughout the 2 h period of study.
Figure 5.
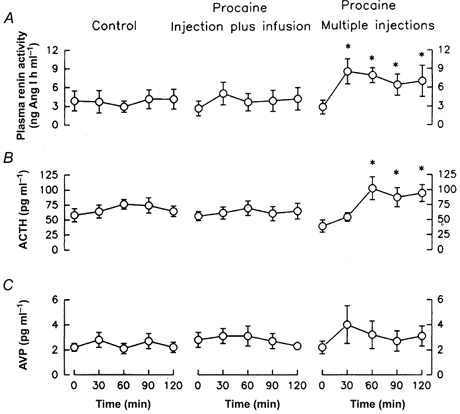
Fetal arterial plasma renin activity (A), and plasma concentrations of ACTH (B), and AVP (C) before and during administration of saline (left, n = 7), injection plus infusion of procaine (middle, n = 6), or multiple injections of procaine (right, n = 5) into the pericardium. Data are represented as mean values with vertical bars representing 1 standard error above and below the mean.
Plasma ACTH concentration was significantly increased from 39 ± 10 to 102 ± 19 pg ml−1 at 60 min in the multiple injection group (Fig. 5). This statistically significant increase was maintained throughout the 2 h of study. Plasma vasopressin concentration was not significantly altered by any of the injections or infusions of saline or procaine (Fig. 5). There was an apparent, but not statistically significant, increase in plasma AVP concentration during multiple injections from 2.2 ± 0.5 to 4.0 ± 1.5 pg ml−1 at 60 min (Fig. 5).
Study 2. Application of the model: the role of cardiac nerves as mediators of the hormonal responses to haemorrhage
Blockade of the cardiac nerves had little effect on the overall blood pressure and heart rate responses to slow haemorrhage (Fig. 6). There was a trend for increased heart rate in the saline-treated haemorrhage fetuses, an apparent increase which was absent in the procaine-treated haemorrhage fetuses. These trends, however, were not statistically significant. Nevertheless, we did measure a statistically significant effect of cardiac nerve blockade on the pHa and Pa,CO2 responses to blood loss. The procaine-treated fetuses had significantly greater increases in Pa,CO2 and decreases in pHa than the saline-treated fetuses (Fig. 7). The Pa,O2 response was similar in the two groups. The initial values of haematocrit were different in the two groups before haemorrhage, but the changes in the two groups were parallel throughout the haemorrhage (Fig. 7).
Figure 6.
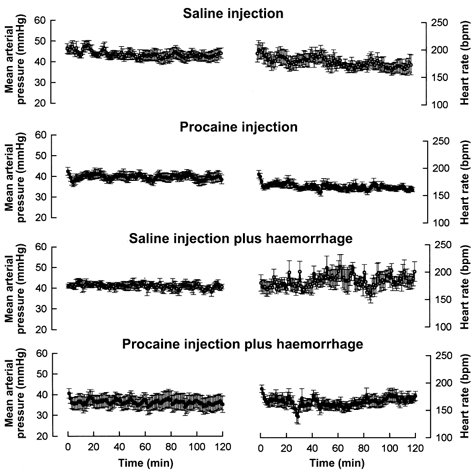
Fetal mean arterial blood pressure (left) and heart rate (right) in response to (from top to bottom) pericardial saline or procaine injection in normovolaemic fetuses and responses to slow haemorrhage in fetuses treated with pericardial saline (n = 7 fetuses were treated with saline, both with and without haemorrhage) or procaine (n = 11 fetuses were treated with procaine, both with and without haemorrhage). Data are represented as mean values with vertical bars representing 1 standard error above and below the mean.
Figure 7.
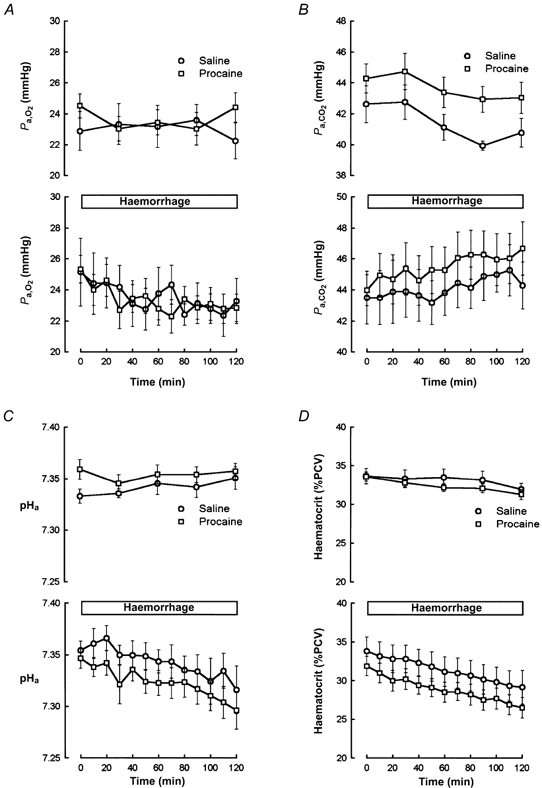
Fetal Pa,O2 (A), Pa,CO2 (B), pHa (C), and haematocrit (D) responses to pericardial saline (n = 7) or procaine (n = 11) injection in normovolaemic fetuses (top graph in each panel) or in fetuses subjected to slow haemorrhage (bottom graph in each panel). In each graph, saline-treated fetuses are represented with open circles and procaine-treated fetuses are represented with open squares.
The endocrine (ACTH, AVP, and renin) responses to the haemorrhage were not significantly affected by the blockade of cardiac nerves (Fig. 8). There was a tendency for the ACTH response to be augmented, and for the AVP response to be augmented in the first half of the haemorrhage, although these differences were not statistically significant.
Figure 8.
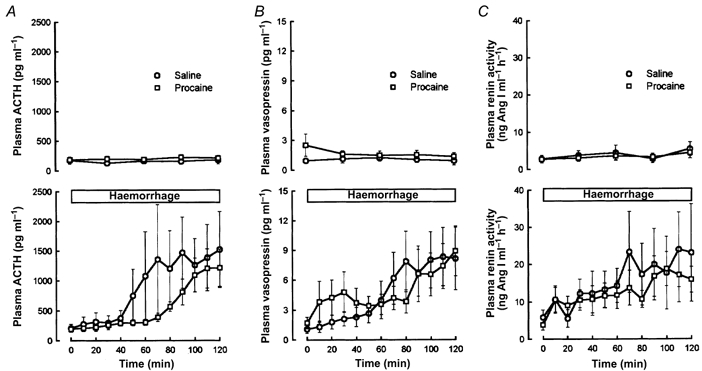
Fetal plasma ACTH (A), vasopressin (B), and plasma renin activity (C) responses to pericardial saline (n = 7) or procaine (n = 11) injection in normovolaemic fetuses (top graph in each panel) or in fetuses subjected to slow haemorrhage (bottom graph in each panel). In each graph, saline-treated fetuses are represented with open circles and procaine-treated fetuses are represented with open squares.
DISCUSSION
The results of these experiments demonstrate that cardiac receptors have relatively minor influence on endocrine (ACTH, AVP, and renin) and cardiovascular (blood pressure, heart rate, and blood gases) function.
Cardiac receptors in adult animals
The results of this study differ somewhat from the results of other studies in adult dogs (O'Donnell et al. 1991). In the adult dog, injection of procaine into the pericardial space produces an increase in arterial blood pressure and no change in the circulating concentrations of renin, AVP, or cortisol. However, when the transient increase in blood pressure was prevented in those experiments with a simultaneous vena caval occlusion, the pericardial procaine increases the rate of secretion of all three hormones (O'Donnell et al. 1992). The results of the present study suggest that cardiac receptors in fetal sheep have little activity with regard to blood pressure control or hormone (ACTH, AVP or renin) secretion. This contrast is also illustrated by the results of other experiments in both adult and fetal animals. In adult dogs vagotomy reduces the magnitude of the AVP and ACTH responses to haemorrhage (Share, 1967; Gann & Cryer, 1973), while in fetal sheep vagotomy does not (Wood et al. 1989). Therefore, it is likely that the atrial receptors become active or otherwise important sometime near the time of or after birth.
Development of the model: efficacy of blockade of cardiac receptors
The method that we used for blockade of cardiac receptors is similar to that used by Korner and coworkers in adult rabbits (Dorward et al. 1983) and by Thrasher and coworkers in adult dogs (O'Donnell et al. 1991, 1992). Procaine blocks both afferent and efferent fibres when injected into the pericardial space (Dorward et al. 1983). In the adult animal, the efficacy of the blockade is tested as the blockade of the reflex heart rate response to intravenous injection of phenylephrine, to test efferent fibres, and as the blockade of the reflex arterial blood pressure response to intravenous injection of veratridine, to test afferent fibres (O'Donnell et al. 1991). We attempted a similar approach in this investigation, and found that procaine effectively blocked the reflex heart rate response to phenylephrine injection (Fig. 3). The procaine also effectively reduced or eliminated the fetal heart rate variability (Fig. 1). Both of these observations demonstrated that efferent fibres to the heart were blocked. However, documenting the assumption that afferent fibres were blocked was more problematic. The Bezold-Jarisch reflex response to veratridine develops in late gestation (Nuwayhid et al. 1975). Therefore, not all of the fetuses tested with veratridine responded with a reflex decrease in arterial blood pressure. We found, however, that in the fetuses which responded to veratridine, the procaine blocked the reflex response. We therefore found no evidence that the procaine was more effective at blocking efferent fibres than afferent fibres. Resultantly we routinely tested blockade by both looking for a reduced degree of variability in fetal heart rate and by testing for blockade of reflex decreases in heart rate after injection of phenylephrine. It is possible, however, that some populations of afferent fibres were not blocked by the procaine. This is, indeed, a possible shortcoming of all blockade or denervation procedures used to investigate reflex control of the cardiovascular system. However, we believe that it is unlikely that the procaine blocked different populations of afferent fibres unevenly.
Modulation of cardiovascular and endocrine responses to haemorrhage
Fetal cardiovascular responses to haemorrhage are now well established. Rapid haemorrhage results in fetal hypotension and bradycardia (Macdonald et al. 1980). The bradycardia associated with acute hypotension in the fetus is the result of peripheral chemoreceptor activity, most likely stimulated by reduced blood flow through the arterial chemoreceptors (Wood, 1989). Slow haemorrhage, as used in this study, produces mild hypotension and only moderate changes in fetal heart rate (Wood et al. 1989). The cardiovascular response to slow haemorrhage differs from that of rapid haemorrhage because of the relatively rapid rate of transcapillary refilling compared to the adult animal, and the time allowed for the process of refilling to occur (Brace & Cheung, 1990). The fetus can tolerate large volumes of blood loss because of the rapid rate of transcapillary refilling (Wood et al. 1989). Rapid haemorrhage does result in the redistribution of fetal combined ventricular output, slowing the flow through the umbilico-placental circulation (Itskovitz et al. 1982). This reduction in umbilico-placental blood flow reduces the rate of gas exchange between maternal and fetal circulations, making the fetus relatively hypercapnic, acidaemic and hypoxic. Changes in umbilico-placental blood flow during slow haemorrhage have not been studied. Although the long time course of slow haemorrhage allows time for transcapillary refilling, and therefore ameliorates the blood loss and reduces the likelihood of reduction of umbilico-placental blood flow, the haemorrhage does produce moderate reduction in arterial blood pressure. The decrease in fetal arterial blood pressure, combined with a relatively weak autoregulation of umbilico-placental blood flow mechanism in the fetal sheep suggests the possibility that one consequence of the haemorrhage is reduced blood flow and gas exchange in this vascular bed. Indeed, Faber et al. have demonstrated that fetal blood volume is a major determinant of umbilico-placental blood flow (Faber et al. 1973). In a previous study from this laboratory, pharmacological support of fetal arterial blood pressure during slow haemorrhage (by infusion of phenylephrine, which constricts the umbilical-plancental vasculature less effectively than elsewhere in the fetus) prevented the hypoxia, hypercapnia, and acidaemia (Chen & Wood, 1992). Exacerbating the hypoxaemia, hypercapnia, and acidaemia which develops during slow fetal haemorrhage is the reduction in fetal haematocrit (a direct consequence of the transcapillary refilling). The relative fetal anaaemia reduces blood oxygen content and tissue oxygen delivery. Anaaemia, by itself, does result in tissue hypoxia (Davis & Hohimer, 1991). Thus, it is possible to view the changes in fetal blood gases and haematocrit as the ultimate consequence of the haemorrhage which reflects both the severity of the haemorrhage and the efficacy of the physiological response. In the present experiments, we found that the blood gas response to the haemorrhage was altered significantly by the blockade of cardiac nerves. Although the effect was small, this result suggests a role for these nerves in the homeostatic response to haemorrhage. It is not likely that this difference is accounted for by blockade of the efferent nerves, since the cardiac nerve blockade did not produce significant differences in blood pressure or heart rate between groups.
Relationship of ACTH and vasopressin
Arginine vasopressin and ACTH responses to cardiovascular stimuli are controlled similarly. Both of these endocrine systems appear to be influenced by carotid sinus baroreceptors as well as by non-baroreceptor-mediated mechanisms (Chen & Wood, 1992; Wood & Tong, 1999). Neither ACTH (Giussani et al. 1994) nor AVP (Raff et al. 1991; Chen & Wood, 1993) is influenced by carotid sinus chemoreceptors. Responses of both hormones to haemorrhage are also qualitatively similar. The responses of both hormones correlate better to changes in fetal arterial blood gases than to changes in either arterial or venous blood pressure, and neither AVP nor ACTH is inhibited by vagotomy (Wood et al. 1989). In a previous study by Jaekle et al. increases in right heart pressure in the fetus increased AVP and renin secretion (ACTH was not measured in that study) (Jaekle et al. 1995). In the present study, procaine injection into the pericardium modestly increased plasma ACTH, and appeared to have a weak stimulatory effect on AVP (although this was not statistically significant). This suggests that at least one population of cardiac receptors tonically exerts a weak inhibition of one (or perhaps both) hormone systems, and that the influence is not as great as in the adult animal. Although there could be some influence of cardiac nerves on both ACTH and AVP in the fetus, the quantitative role of these receptors is not important in generating hormonal responses to hypovolaemia. Both ACTH and AVP responses to slow haemorrhage were not significantly altered by the cardiac nerve blockade.
Renin and its relationship to haemorrhage
The results of the present experiments lead to similar conclusions concerning renin secretion as for ACTH and AVP. We demonstrated a small but measurable effect of the cardiac nerve blockade on plasma renin activity, suggesting some influence of these nerves on the fetal renin-angiotensin system. However, this influence appears to be relatively weak and does not account for the renin response to slow haemorrhage. The mechanism mediating the renin response to slow haemorrhage in the fetus is not clear. The magnitude of the renin response is not altered by sinoaortic denervation (Chen & Wood, 1992) or by bilateral vagotomy (Wood et al. 1989) or (in the present study) by blockade of cardiac nerves. It is possible that the renin response to haemorrhage is dependent upon intra-renal mechanisms, rather than reflex mechanisms.
Conclusions
We conclude that the fetal ACTH, AVP and renin responses to slow haemorrhage are not mediated to any significant extent by cardiac receptors, although the cardiac nerves exert a small influence on plasma renin activity and ACTH in the normovolaemic fetus. This conclusion is consistent with the results of previous experiments in which we demonstrated that vagal afferent fibres do not play a significant role in the mediation of hormonal responses to slow haemorrhage in the fetus.
Acknowledgments
I thank Drs Hong-Gen Chen and Maureen Keller-Wood for their contributions to the design and execution of these experiments. This work was supported by HD20098 from the National Institute of Child Health and Human Development and by an Established Investigator Award from the American Heart Association.
REFERENCES
- Bell ME, Wood CE, Keller-Wood M. Influence of reproductive state on pituitary-adrenal activity in the ewe. Domestic Animal Endocrinology. 1991;8:245–254. doi: 10.1016/0739-7240(91)90060-w. [DOI] [PubMed] [Google Scholar]
- Brace RA, Cheung CY. Fetal cardiovascular and endocrine responses to prolonged fetal hemorrhage. American Journal of Physiology. 1986;251:R417–424. doi: 10.1152/ajpregu.1986.251.2.R417. [DOI] [PubMed] [Google Scholar]
- Brace RA, Cheung CY. Fetal blood volume restoration following rapid fetal hemorrhage. American Journal of Physiology. 1990;259:H567–573. doi: 10.1152/ajpheart.1990.259.2.H567. [DOI] [PubMed] [Google Scholar]
- Brennan LA, Jr, Malvin RL, Jochim KE, Roberts DE. Influence of right and left atrial receptors on plasma concentrations of ADH and renin. American Journal of Physiology. 1971;221:273–278. doi: 10.1152/ajplegacy.1971.221.1.273. [DOI] [PubMed] [Google Scholar]
- Chen HG, Wood CE. Reflex control of fetal arterial pressure and hormonal responses to slow hemorrhage. American Journal of Physiology. 1992;262:H225–233. doi: 10.1152/ajpheart.1992.262.1.H225. [DOI] [PubMed] [Google Scholar]
- Chen H-G, Wood CE. The adrenocorticotropic hormone and arginine vasopressin responses to hypercapnia in fetal and maternal sheep. American Journal of Physiology. 1993;264:R324–330. doi: 10.1152/ajpregu.1993.264.2.R324. [DOI] [PubMed] [Google Scholar]
- Cryer GL, Gann DS. Right atrial receptors mediate the adrenocortical response to small hemorrhage. American Journal of Physiology. 1974;227:325–328. doi: 10.1152/ajplegacy.1974.227.2.325. [DOI] [PubMed] [Google Scholar]
- Davis LE, Hohimer AR. Hemodynamics and organ blood flow in fetal sheep subjected to chronic anemia. American Journal of Physiology. 1991;261:R1542–1548. doi: 10.1152/ajpregu.1991.261.6.R1542. [DOI] [PubMed] [Google Scholar]
- Dorward PK, Flaim M, Ludbrook J. Blockade of cardiac nerves by intrapericardial local anesthetics in the conscious rabbit. Australian Journal of Experimental Biology and Medical Sciences. 1983;61:219–230. doi: 10.1038/icb.1983.20. [DOI] [PubMed] [Google Scholar]
- Faber JJ, Gault CF, Green TJ, Thornburg KL. Fetal blood volume and fetal placental blood flow in lambs. Proceedings of the Society for Experimental Biology and Medicine. 1973;142:340–344. doi: 10.3181/00379727-142-37018. [DOI] [PubMed] [Google Scholar]
- Gann DS, Cryer GL. Feedback control of ACTH secretion by cortisol. In: Brodish A, Redgate ES, editors. Brain-Pituitary-Adrenal Interrelationships. Basel: Karger; 1973. pp. 197–223. [Google Scholar]
- Gann DS, Gould KL, Morley JE, Mumma JV. Effects of vagotomy and of carotid constriction of corticosteroid secretion in the dog. Proceedings of the Society for Experimental Biology and Medicine. 1964;115:944–947. doi: 10.3181/00379727-115-29085. [DOI] [PubMed] [Google Scholar]
- Giussani DA, McGarrigle HH, Moore PJ, Bennet L, Spencer JA, Hanson MA. Carotid sinus nerve section and the increase in plasma cortisol during acute hypoxia in fetal sheep. Journal of Physiology. 1994;477:75–80. doi: 10.1113/jphysiol.1994.sp020172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itskovitz J, Goetzman BW, Rudolph AM. Effects of hemorrhage on umbilical venous return and oxygen delivery in fetal lambs. American Journal of Physiology. 1982;242:H543–548. doi: 10.1152/ajpheart.1982.242.4.H543. [DOI] [PubMed] [Google Scholar]
- Jaekle RK, Sheikh AU, Berry DD, Washburn L, Rose JC. Hemodynamic and hormonal responses to atrial distension in the ovine fetus. American Journal of Obstetrics and Gynecology. 1995;173:694–701. doi: 10.1016/0002-9378(95)90325-9. [DOI] [PubMed] [Google Scholar]
- Macdonald AA, Rose J, Heymann MA, Rudolph AM. Heart rate response of fetal and adult sheep to hemorrhage stress. American Journal of Physiology. 1980;239:H789–793. doi: 10.1152/ajpheart.1980.239.6.H789. [DOI] [PubMed] [Google Scholar]
- Nuwayhid B, Brinkman CR, Su C, Bevan JA, Assali NS. Development of autonomic control of fetal circulation. American Journal of Physiology. 1975;228:337–344. doi: 10.1152/ajplegacy.1975.228.2.337. [DOI] [PubMed] [Google Scholar]
- O'Donnell CP, Keil LC, Thrasher TN. Vasopressin responses to unloading arterial baroreceptors during cardiac nerve blockade in conscious dogs. American Journal of Physiology. 1992;262:R51–60. doi: 10.1152/ajpregu.1992.262.1.R51. [DOI] [PubMed] [Google Scholar]
- O'Donnell CP, Scheuer DA, Keil LC, Thrasher TN. Cardiac nerve blockade by infusion of procaine into the pericardial space of conscious dogs. American Journal of Physiology. 1991;260:R1176–1182. doi: 10.1152/ajpregu.1991.260.6.R1176. [DOI] [PubMed] [Google Scholar]
- Raff H, Kane C, Wood CE. Vasopressin responses to hypoxia and hypercapnia in late-gestation fetal sheep. American Journal of Physiology. 1991;260:R1077–1081. doi: 10.1152/ajpregu.1991.260.6.R1077. [DOI] [PubMed] [Google Scholar]
- Share L. Effects of carotid occlusion and left atrial distention on plasma vasopressin titer. American Journal of Physiology. 1965;208:219–223. doi: 10.1152/ajplegacy.1965.208.2.219. [DOI] [PubMed] [Google Scholar]
- Share L. Role of peripheral receptors in the increased release of vasopressin in response to hemorrhage. Endocrinology. 1967;81:1140–1146. doi: 10.1210/endo-81-5-1140. [DOI] [PubMed] [Google Scholar]
- Thames MD. Reflex suppression of renin release by ventricular receptors with vagal afferents. American Journal of Physiology. 1977;233:H181–184. doi: 10.1152/ajpheart.1977.233.2.H181. [DOI] [PubMed] [Google Scholar]
- Thames MD, Peterson MG, Schmid PG. Stimulation of cardiac receptors with veratrum alkaloids inhibits ADH secretion. American Journal of Physiology. 1980;239:H784–788. doi: 10.1152/ajpheart.1980.239.6.H784. [DOI] [PubMed] [Google Scholar]
- Winer BJ. Statistical Principles in Experimental Design. 2. New York: McGraw-Hill; 1971. pp. 514–603. [Google Scholar]
- Wood CE. Sinoaortic denervation attenuates the reflex responses to hypotension in fetal sheep. American Journal of Physiology. 1989;256:R1103–1110. doi: 10.1152/ajpregu.1989.256.5.R1103. [DOI] [PubMed] [Google Scholar]
- Wood CE, Chen H-G, Bell ME. Role of vagosympathetic fibers in the control of adrenocorticotropic hormone, vasopressin, and renin responses to hemorrhage in fetal sheep. Circulation Research. 1989;64:515–523. doi: 10.1161/01.res.64.3.515. [DOI] [PubMed] [Google Scholar]
- Wood CE, Cudd TA, Kane C, Engelke K. Fetal ACTH and blood pressure responses to thromboxane mimetic U46619. American Journal of Physiology. 1993;265:R858–862. doi: 10.1152/ajpregu.1993.265.4.R858. [DOI] [PubMed] [Google Scholar]
- Wood CE, Kane C, Raff H. Peripheral chemoreceptor control of fetal renin responses to hypoxia and hypercapnia. Circulation Research. 1990;67:722–732. doi: 10.1161/01.res.67.3.722. [DOI] [PubMed] [Google Scholar]
- Wood CE, Tong H. Central nervous system regulation of reflex responses to hypotension during fetal life. American Journal of Physiology. 1999;277:R1541–1552. doi: 10.1152/ajpregu.1999.277.6.R1541. [DOI] [PubMed] [Google Scholar]


