Abstract
Sympathetic vasoconstriction of muscle vascular beds is important in the regulation of systemic blood pressure. However, vasoconstriction during exercise can also compromise blood flow support of muscle metabolism. This study tested the hypothesis that local factors in exercising muscle blunt vessel responsiveness to sympathetic vasoconstriction. We performed selective infusions of three doses of tyramine into the brachial artery (n = 8) to evoke endogenous release of noradrenaline (norepinephrine) at rest and during moderate and heavy rhythmic handgrip exercise. In separate experiments, tyramine was administered during two doses of adenosine infusion (n = 7) and two doses of sodium nitroprusside (SNP) infusion (n = 8). Vasoconstrictor effectiveness across conditions was assessed as the percentage reduction in forearm vascular conductance (FVC), calculated from invasive blood pressure and non-invasive Doppler ultrasound blood flow measurements at the brachial artery. Tyramine evoked a similar dose-dependent vasoconstriction at rest in all three groups, with the highest dose resulting in a 42-46 % reduction in FVC. This vasoconstriction was blunted with increasing exercise intensity (e.g. tyramine high dose percentage reduction in FVC; rest −43.4 ± 3.7 %, moderate exercise −27.5 ± 2.3 %, heavy exercise −16.7 ± 3.6 %; P < 0.05). In contrast, tyramine infusion resulted in a greater percentage reduction in FVC during both doses of adenosine vs. rest (P < 0.05). Finally, percentage change in FVC was greater during low dose SNP infusion vs. rest (P < 0.05), but not different from rest at the high dose of SNP infusion (P = 0.507). A blunted percentage reduction in FVC during endogenous noradrenaline release in exercise but not vasodilator infusion indicates that sympathetic vasoconstriction is blunted in exercising muscle. This blunting appears to be exercise intensity-dependent.
In exercising muscle, vascular conductance is influenced by the need for increased perfusion to support elevated muscle metabolism. However, when the mass of active muscle is high, systemic blood pressure regulation may require sympathetic restraint of muscle blood flow (Rowell, 1997). Therefore, elucidating the nature of the interaction(s) between local vasodilator influences and sympathetic neural vasoconstrictor influences in muscle is fundamental for understanding vascular control and blood pressure regulation during exercise. In this context, debate continues as to whether sympathetic vasoconstrictor influences on blood vessels in exercising muscle are blunted by local ‘metabolic’ factors.
In the early 1960s Remensnyder et al. (1962) coined the term ‘functional sympatholysis’ to describe the relative insensitivity of the exercising muscle vascular bed to sympathetic activation. Their conclusions have come under repeated criticism, as it has been suggested that interpretation of their data as indicative of functional sympatholysis is based on a ‘mathematical artifact’ when using resistance to compare low (rest) and high (exercise) flow conditions (Rowell, 1993; Rowell, 1997). However, pressure-flow curves in Fig. 7 from their study clearly demonstrate that during carotid sinus hypotension-induced elevations in sympathetic nervous activity, resting limb blood flow was decreased in the face of elevated systemic blood pressure, while exercising muscle blood flow increased proportionally with systemic blood pressure. Since then it has been demonstrated in vitro and in situ that numerous factors associated with muscle contraction, including adenosine and nitric oxide, either impair noradrenaline release (pre-synaptic inhibition) or α-receptor responsiveness (post-synaptic inhibition) (Verhaeghe & Shepherd, 1976; Verhaeghe et al. 1977; Shepherd & Vanhoutte, 1981; Eboute et al. 1987; Lautt et al. 1988; Macedo & Lautt, 1994; Thomas et al. 1997; Buckwalter et al. 1998, 2001; Thomas & Victor, 1998).
Investigations into the phenomenon of functional sympatholysis in conscious exercising animals or in humans have relied primarily on arterial infusion of α-receptor agonists (Buckwalter et al. 1998) or on manoeuvres such as lower body negative pressure (Strandell & Shepherd, 1967; Hansen et al. 1996) or ischaemic exercise (Kagaya et al. 1994; Tschakovsky & Hughson, 1999), which result in systemic activation of the sympathetic nervous system. There are potential limitations to these experimental approaches. With drug infusions luminal and abluminal receptors that are distant from the vascular neuromuscular junction can be stimulated. Additionally, whole body sympathoexcitatory manoeuvres can alter systemic blood pressure and are unable to create an isolated, local endogenous sympathetic activation in vivo.
Therefore, in an attempt to overcome these limitations we elicited endogenous release of noradrenaline in the forearm via selective brachial artery infusions of tyramine at rest, during moderate and heavy rhythmic forearm exercise, and during infusion of putative sympatholytic agents adenosine and nitric oxide (NO) via the NO donor sodium nitroprusside (SNP). Our results support the hypothesis that sympathetic vasoconstriction is blunted in exercising human forearm muscle. This effect depends on the interaction between exercise intensity and the level of sympathetic activation, and nitric oxide may contribute to it.
METHODS
General methods
Subjects
Twenty-three healthy, normotensive, non-smoking subjects (21 men, two women) between the ages of 23 and 40 years participated in the study. The study was approved by the Institutional Review Board at the Mayo Clinic, was performed according to the Declaration of Helsinki and each subject gave written informed consent.
Subject monitoring
Subjects assumed a supine position with the left arm extended laterally at heart level and a 5 cm, 20 gauge brachial artery catheter was placed in the arm at the elbow using sterile techniques after local anaesthesia with 1-2 ml of 1 % lidocaine (lignocaine). A three-port connector was placed in series with a catheter-transducer system so that tyramine alone, adenosine alone, SNP alone, or a combination of adenosine + tyramine or SNP + tyramine could be infused and arterial pressure measured simultaneously (Dietz et al. 1994). A five-lead electrocardiogram was used to monitor heart rate (HR).
Forearm blood flow
Brachial artery blood velocity was measured with a 4 MHz pulsed Doppler probe (Model 500V, Multigon Industries, Mt Vernon, NY, USA) securely fixed to the skin over the brachial artery half way up the upper arm. With this placement and arm position, probe insonation angle relative to the skin is 60 deg and the brachial artery is approximately parallel to the skin surface. A linear 6.0 MHz echo Doppler ultrasound probe (HDI 5000, ATL Ultrasound, Bothell, WA, USA) was sited over the brachial artery immediately proximal to the pulsed Doppler probe and a holder secured to the skin so that the probe could rapidly be re-sited at intervals during the experiment to obtain brachial artery diameter measurements. Forearm blood flow (FBF) was then derived as the product of brachial artery mean blood velocity (MBV) and arterial cross-sectional area. With exercise, a wrist cuff to exclude hand blood flow could not be used due to subject discomfort, so for these subjects there was no wrist cuff at rest or during exercise. Subjects in the vasodilator infusion studies had a wrist cuff inflated to suprasystolic pressure (250 mmHg) at rest and during dilator infusion to occlude arterial blood flow to the hand.
Forearm exercise
Forearm exercise consisted of rhythmic, dynamic handgrip exercise, achieved by lifting and lowering a load suspended over a pulley at a contraction-relaxation duty cycle of 1 s-2 s in time with a signal light. The exercise was dynamic, with ∼0.5 s taken to raise the weight and ∼0.5 s taken to lower the weight. The load used was 6.4 kg for ‘moderate’ and 12.1 kg for ‘heavy’ exercise and was the same for all subjects. Pilot work indicated that the 6.4 kg workload, ∼10-15 % maximal voluntary contraction (MVC), was easily sustainable for at least 30 min in male subjects. The 12.1 kg workload (∼20-25 % MVC) was the maximal workload achievable that did not result in more than minor increases in arterial pressure or fatigue over a 7 min exercise period. This latter characteristic limited the intensity of the heavy work rate that we could examine, but permitted us to study steady-state vascular responses in the exercising forearm across multiple trials. The average increase in blood flow from rest was ∼6-fold for moderate exercise and ∼10-fold for heavy exercise.
Vasodilator infusion
Doses of vasodilators were adjusted for forearm volume. Two doses of adenosine (Low, 6.25 μg (dl forearm volume)−1 min−1; High, 12.5 μg (dl forearm volume)−1 min−1 and two doses of sodium nitroprusside (SNP) (Low, 0.5 μg (dl forearm volume)−1 min−1; High 2.0 μg (dl forearm volume)−1 min−1 were administered separately via the brachial artery catheter. Adenosine doses were chosen based on the dose-responses reported by Radegran & Calbet (2001) in the human leg which resulted in five- and 10-fold increases in flow. SNP doses were chosen to achieve similar increases in flow based on past experience in our laboratory. Actual steady-state flow increases achieved by these doses varied considerably across subjects. Vasodilators were infused for a period of 7 min following a 1 min resting baseline. A rest period of 10-15 min was allowed between infusions for washout of the dilators and a return of baseline forearm blood flow.
Endogenous forearm noradrenaline release
Endogenous noradrenaline release was evoked via infusions of tyramine into the brachial artery. Tyramine causes noradrenaline ‘leakage’ from neuronal vesicles, and consequent diffusion of noradrenaline out of the nerve terminal. Additionally it has no intrinsic vasoconstricting properties (Brandao et al. 1978). To elicit various levels of endogenous noradrenaline release at rest, tyramine was infused for 3 min at three different doses based on forearm volume (2, 4 and 8 μg (dl forearm volume)−1 min−1 for the subjects participating in the exercise protocol, with the order counterbalanced across subjects. For subsequent studies in subjects with vasodilator infusion only the 2 and 8 μg (dl forearm volume)−1 min−1 doses were used. Pilot work indicated these doses provided an appropriate range of vasoconstriction at rest. With exercise and vasodilator infusion, FBF increased above resting levels. The infusion rate of tyramine was increased in proportion to the increase in FBF in order to ensure that the effective arterial concentration of tyramine was the same across experimental conditions within subjects (Buckwalter et al. 1998). Briefly, 1 min of baseline measurements were made before each exercise trial. The subjects then began forearm exercise or received vasodilator infusion and steady-state FBF was reached within 3 min. The appropriate dose of tyramine was infused from 4-7 min of exercise based on the steady-state FBF measured from 3-4 min. This means that at rest and during both levels of exercise each subject received a low, medium and high dose of tyramine and during vasodilator infusion they received a low and a high dose of tyramine. These descriptive terms are used throughout the remainder of the paper.
Specific experimental protocols
We investigated the effect of endogenous noradrenaline release in rest vs. exercise (n = 8), adenosine infusion (n = 7), and SNP infusion (n = 8). Figure 1 illustrates the protocols. Room temperature was held constant at ∼20 °C, and a blanket was used to cover the subject. The exposure of the arm to the cool room temperature served to minimize hand and skin blood flow contribution to the resting and exercising forearm response (Tschakovsky & Hughson, 1999), since we were interested specifically in the muscle response. A constant arterial infusion of saline at 0.5 ml min−1 was maintained throughout the experiments with exercise to maintain catheter patency. Infusion rates for all vasodilator doses was ∼3.5 ml min−1. Infusion syringe concentrations of tyramine for each of the doses were prepared to account for the average increase in FBF in the exercise and vasodilator conditions. Due to the between-subject variation in the vasodilator-induced increase in flow from rest, infusion rates for tyramine ranged from 1-3 ml min−1 across subjects. However, the tyramine infusion rate was virtually identical across trials within each subject.
Figure 1. Experimental protocol.
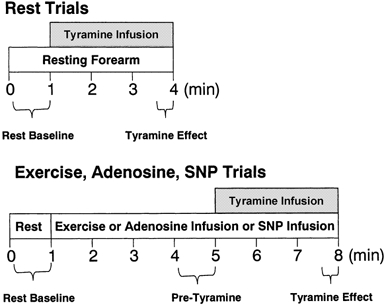
Tyramine dose order was counterbalanced across subjects. Resting trials were separated by 5 min of recovery. Moderate exercise and vasodilator infusion trials were separated by 10-15 min of recovery and heavy exercise trials were separated by 20 min of recovery. Reported values for haemodynamic responses were the mean over the time periods indicated.
Resting trials were always performed first, since calculation for appropriate rates of tyramine infusion during vasodilator infusion and exercise required knowledge of the effective arterial concentration of tyramine at rest. At least 5 min separated each 3 min infusion trial at rest, which was ample time to allow baseline FBF to return to normal (Tables 1, 2 and 3). For vasodilator infusion trials, the order of high and low vasodilator dose was counterbalanced across subjects. For exercise, resting trials were always followed by moderate exercise trials, with heavy exercise trials performed last. This was done because there was the possibility of developing fatigue over the course of the accumulated heavy exercise trials which might then have affected the response to moderate exercise if performed after heavy exercise. Moderate exercise trials were separated by at least 10 min of rest, while heavy exercise trials were separated by 20 min of rest. The order of low and high vasodilator doses were counterbalanced across subjects.
Table 1.
Forearm haemodynamic responses to infusion of tyramine: rest vs. forearm exercise
| Exercise condition | |||||
|---|---|---|---|---|---|
| Variable | Tyramine dose | Time | Rest | Moderate | Heavy |
| FBF (ml min−1) | Low | Rest baseline | 44.6 ± 5.8 | 51.3 ± 4.3 | 106.2 ± 25.5*† |
| Ex. pre-tyr | — | 251.5 ± 17.3 | 434.7 ± 20.2* | ||
| Tyramine | 36.8 ± 5.8‡ | 204.6 ± 19.2‡ | 415.6 ± 23.1*‡ | ||
| Medium | Rest baseline | 42.1 ± 5.7 | 48.4 ± 7.1 | 77.1 ± 10.8*† | |
| Ex. pre-tyr. | — | 251.6 ± 16.5 | 430.3 ± 26.5* | ||
| Tyramine | 29.3 ± 4.3‡ | 189.7 ± 15.7‡ | 389.2 ± 25.8*‡ | ||
| High | Rest baseline | 40.6 ± 5.1 | 49.5 ± 4.4 | 84.6 ± 11.9*† | |
| Ex. pre-tyr. | — | 246.1 ± 14.6 | 444.5 ± 18.5 | ||
| Tyramine | 22.6 ± 2.5‡ | 180.4 ± 12.0‡§ | 384.7 ± 24.2*‡§ | ||
| MAP (mmHg) | Low | Rest baseline | 107.1 ± 4.2 | 114.1 ± 4.3 | 118.5 ± 5.3*† |
| Ex. pre-tyr. | — | 114.5 ± 4.0 | 121.2 ± 4.9* | ||
| Tyramine | 107.9 ± 4.8 | 118.9 ± 4.5 | 124.4 ± 4.7*‡ | ||
| Medium | Rest baseline | 106.4 ± 4.0 | 113.4 ± 4.1 | 116.0 ± 4.5*† | |
| Ex. pre-tyr. | — | 114.5 ± 4.0 | 118.1 ± 4.4* | ||
| Tyramine | 106.3 ± 3.8 | 115.9 ± 3.9 | 122.8 ± 4.8*‡ | ||
| High | Baseline | 104.0 ± 4.5 | 114.0 ± 4.0 | 116.0 ± 4.9*† | |
| Ex. pre-tyr. | — | 115.4 ± 4.4 | 120.1 ± 5.4* | ||
| Tyramine | 104.5 ± 4.8 | 116.7 ± 4.7 | 124.5 ± 5.7*‡ | ||
| FVC (ml min−1 100 mmHg−1) | Low | Rest baseline | 42.4 ± 6.2 | 45.3 ± 4.2 | 91.4 ± 23.4*† |
| Ex. pre-tyr. | — | 223.8 ± 21.2 | 365.7 ± 28.9* | ||
| Tyramine | 35.1 ± 6.0‡ | 176.3 ± 21.1‡ | 341.1 ± 29.7*‡ | ||
| Medium | Rest baseline | 39.5 ± 4.9 | 43.2 ± 6.6 | 68.2 ± 11.2*† | |
| Ex. pre-tyr. | — | 224.5 ± 19.9 | 370.3 ± 31.1* | ||
| Tyramine | 27.8 ± 4.1‡ | 167.0 ± 17.3‡ | 324.8 ± 32.9*‡ | ||
| High | Rest baseline | 39.4 ± 4.9 | 43.6 ± 4.0 | 75.2 ± 12.0*† | |
| Ex. pre-tyr. | — | 217.6 ± 18.5 | 377.2 ± 28.4* | ||
| Tyramine | 22.1 ± 2.8‡ | 157.9 ± 14.1‡§ | 317.2 ± 26.9*‡§ | ||
Values are means ±s.e.m.; n = 8 subjects. Rest baseline, prior to the onset of exercise; Ex. pre-tyr., steady state responses in exercise during 1 min immediately preceding the start of tyramine infusion; Tyramine, response average over the last 30 s of the 3 min tyramine infusion; FBF, forearm blood flow; MAP, mean arterial pressure; FVC, forearm vascular conductance = (FBF/MAP) × 100 mmHg. See text for further details.
Significantly different from moderate exercise condition, P < 0.05.
Significantly different from rest condition, P < 0.05.
Significantly different from pre-tyr. for moderate and heavy exercise conditions, and from rest baseline for resting condition P < 0.05.
Significantly different from tyramine low dose within exercise condition, P < 0.05.
Table 2.
Forearm haemodynamic responses to infusion of tyramine: rest vs. adenosine infusion
| Adenosine infusion condition | |||||
|---|---|---|---|---|---|
| Variable | Tyramine dose | Time | Rest | Low dose | High dose |
| FBF (ml min−1) | Low | Rest baseline | 32.3 ± 3.7 | 33.8 ± 5.7 | 46.8 ± 7.5*† |
| Ado pre-tyr. | — | 165.8 ± 41.2 | 265.5 ± 45.5* | ||
| Tyramine | 23.9 ± 2.4‡ | 52.9 ± 8.8‡ | 98.2 ± 23.9*‡ | ||
| High | Rest baseline | 33.9 ± 3.1 | 32.3 ± 4.6 | 37.6 ± 5.4 | |
| Ado pre-tyr. | — | 148.4 ± 29.1 | 215.0 ± 46.2* | ||
| Tyramine | 19.4 ± 2.0‡ | 32.6 ± 5.8‡§ | 53.0 ± 7.8‡§ | ||
| MAP (mmHg) | Low | Rest baseline | 90.8 ± 3.3 | 92.9 ± 3.7 | 93.4 ± 1.6† |
| Ado pre-tyr | — | 95.4 ± 3.8 | 95.7 ± 1.4 | ||
| Tyramine | 93.5 ± 3.3 | 98.5 ± 4.5‡ | 98.9 ± 2.0‡ | ||
| High | Baseline | 90.6 ± 3.7 | 92.5 ± 4.1 | 95.3 ± 3.8† | |
| Ado pre-tyr. | — | 94.7 ± 4.2 | 97.8 ± 4.0 | ||
| Tyramine | 92.7 ± 3.7 | 98.9 ± 5.2‡ | 100.6 ± 4.4‡ | ||
| FVC (ml min−1 100 mmHg−1) | Low | Rest baseline | 35.9 ± 4.3 | 36.8 ± 6.3 | 49.6 ± 7.0*† |
| Ado pre-tyr. | — | 176.7 ± 43.7 | 276.0 ± 44.2* | ||
| Tyramine | 25.7 ± 2.7‡ | 54.3 ± 9.2‡ | 97.4 ± 22.1*‡ | ||
| High | Rest baseline | 37.9 ± 3.8 | 35.4 ± 5.2 | 39.9 ± 5.7 | |
| Ado pre-tyr. | — | 158.3 ± 31.1 | 223.4 ± 45.7* | ||
| Tyramine | 21.1 ± 2.3‡ | 33.2 ± 6.0‡§ | 53.1 ± 7.5‡§ | ||
Values are means ±s. e. m.; n = 7 subjects. Rest baseline, prior to the onset of adenosine infusion; ado pre-tyr., steady state responses in adenosine infusion during 1 min immediately preceding the start of tyramine infusion; tyramine, response average over the last 30 s of the 3 min tyramine infusion; FBF, forearm blood flow; MAP, mean arterial pressure; FVC, forearm vascular conductance = (FBF/MAP) × 100 mmHg. See text for further details.
Significantly different from adenosine low dose condition, P < 0.05.
Significantly different from rest condition, P < 0.05.
Significantly different from pre-tyr. for adenosine low and high dose conditions, and from rest baseline for resting condition P < 0.05.
Significantly different from tyramine low dose within adenosine dose condition, P < 0.05.
Table 3.
Forearm haemodynamic responses to infusion of tyramine: rest vs. SNP infusion
| SNP infusion condition | |||||
|---|---|---|---|---|---|
| Variable | Tyramine dose | Time | Rest | Low dose | High dose |
| FBF (ml min−1) | Low | Rest baseline | 30.2 ± 2.9 | 25.1 ± 3.6 | 29.1 ± 4.2 |
| SNP pre-tyr. | — | 191.0 ± 38.5 | 284.0 ± 51.7* | ||
| Tyramine | 21.8 ± 1.9‡ | 76.8 ± 12.6‡ | 174.3 ± 25.7*‡ | ||
| High | Rest baseline | 29.5 ± 2.9 | 30.9 ± 6.1 | 49.9 ± 7.0*† | |
| SNP pre-tyr. | — | 190.0 ± 39.8 | 304.5 ± 67.1* | ||
| Tyramine | 16.0 ± 1.8‡ | 52.8 ± 10.7‡§ | 127.9 ± 27.8*‡§ | ||
| MAP (mmHg) | Low | Rest baseline | 87.0 ± 3.1 | 89.1 ± 3.5 | 95.4 ± 2.6*† |
| SNP pre-tyr. | — | 87.9 ± 3.3 | 89.2 ± 2.5¶ | ||
| Tyramine | 89.5 ± 3.4¶ | 92.1 ± 3.3‡¶ | 93.9 ± 2.6 | ||
| High | Baseline | 88.5 ± 3.5 | 93.8 ± 3.2 | 96.9 ± 3.2*† | |
| SNP pre-tyr. | — | 92.5 ± 3.1 | 89.2 ± 3.4¶ | ||
| Tyramine | 90.1 ± 3.6 | 96.6 ± 3.1‡ | 96.3 ± 3.6‡ | ||
| FVC (ml min−1 100 mmHg−1) | Low | Rest baseline | 34.7 ± 2.8 | 27.9 ± 3.6 | 30.5 ± 4.0 |
| SNP pre-tyr. | — | 216.9 ± 42.1 | 320.1 ± 56.4* | ||
| Tyramine | 24.4 ± 1.8‡ | 82.1 ± 11.6‡ | 186.7 ± 26.2*‡ | ||
| High | Rest baseline | 33.2 ± 3.0 | 32.7 ± 6.1 | 50.9 ± 6.5*† | |
| SNP pre-tyr. | — | 210.2 ± 45.3 | 345.0 ± 72.0* | ||
| Tyramine | 17.8 ± 1.9‡ | 54.3 ± 10.1‡§ | 130.8 ± 24.6*‡§ | ||
Values are means ± s.e.m.; n = 8 subjects. Rest baseline, prior to the onset of SNP infusion; SNP pre-tyr., steady state responses in sodium nitroprusside infusion during 1 min immediately preceding the start of tyramine infusion; tyramine, response average over the last 30 s of the 3 min tyramine infusion; FBF, forearm blood flow; MAP, mean arterial pressure; FVC, forearm vascular conductance = (FBF/MAP) × 100 mmHg. See text for further details. Comparisons within FBF, MAP and FVC:
significantly different from SNP low dose condition, P < 0.05
significantly different from rest condition, P < 0.05
significantly different from pre-tyr. for SNP low and high dose conditions, and from rest baseline for resting condition, P < 0.05
significantly different from tyramine low dose within SNP dose condition, P < 0.05. Comparisons within MAP only:
significantly different from rest baseline within SNP dose, P < 0.05.
Data acquisition and analysis
Data were digitised at 200 Hz and stored on computer. The data were analysed off-line with signal processing software (Windaq: Dataq Instruments, Akron, OH, USA). HR was monitored via electrocardiogram. Mean arterial pressure (MAP) was determined electronically from the arterial pressure waveform.
FBF was calculated as:
where the FBF is in ml min−1, the MBV is in cm s−1 and the brachial artery diameter is in cm.
Forearm vascular conductance (FVC) was calculated as:
where FVC is in ml min−1 100 mmHg−1. Flow per 100 mmHg was used so that FVC was quantitatively similar to the standard units for forearm blood flow.
To compare the vasoconstrictor effect of tyramine at rest. vs. exercise or vasodilator infusion we calculated the percentage reduction in FVC (Fig. 3) as:
where FVCpre tyramine was determined from the FBF and MAP averaged over the 30 s immediately preceding the start of tyramine infusion and FVCpost tyramine determined from the FBF and MAP during the last 30 s of tyramine infusion. The onset of tyramine-induced vasoconstriction varied across subjects and was slower at rest than with exercise, but displayed a consistent plateau, i.e. it reached a certain level and then stayed there for the duration of the tyramine infusion.
Figure 3.
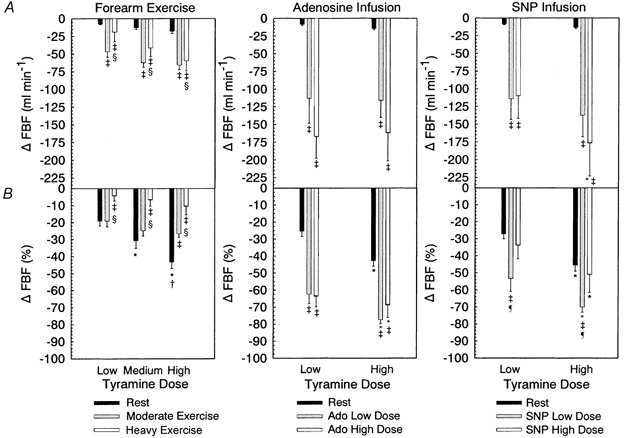
A, change in forearm blood flow (FBF) from baseline for rest, exercise, adenosine infusion and SNP infusion. B, percentage change in FBF, comparison as for A. * Significantly different from low tyramine dose within exercise or vasodilator condition. † Significantly different from medium tyramine dose within exercise condition. ‡ Significantly different from rest within tyramine dose. § Significantly different from moderate exercise within tyramine dose (all P < 0.05). ¶ Significantly different from high SNP dose, P < 0.05.
Statistics
All values are reported as means ± s.e.m. Specific hypothesis testing within each of exercise, adenosine infusion and SNP infusion was performed using two-way repeated measures ANOVA. Level of significance for ANOVA was set at P < 0.05, with significant interaction effects further analysed by Tukey's post hoc test.
RESULTS
Absolute haemodynamic responses: exercise
Table 1 summarises the absolute forearm blood flow (FBF), mean arterial pressure (MAP) and forearm vascular conductance (FVC) during resting, moderate and heavy exercise trials. The resting baseline FBF and FVC were elevated in heavy exercise trials compared to rest and moderate exercise trials (P < 0.001, main effect). Moderate exercise resulted in FBF that was ∼6-fold greater than that during the resting condition, while heavy exercise increased FBF by ∼10-fold, while the increase in FVC from rest was ∼5-fold in moderate and ∼9-fold in heavy exercise. (P < 0.001 for both FBF and FVC, main effect of exercise intensity).
Baseline pre-exercise MAP was elevated vs. resting trials in both moderate and heavy exercise (P < 0.05, main effect). Neither moderate nor heavy exercise increased blood pressure from baseline levels by 4 min of exercise. However, with a further 3 min of exercise combined with tyramine infusion, MAP rose in heavy exercise during all tyramine infusion doses (P < 0.05). It is not clear whether this was due to a systemic effect of tyramine, a progressive effect of heavy exercise, or both. On average, MAP increased by 6-8 mmHg from rest baseline to the end of heavy exercise, but returned to baseline levels between trials.
Absolute haemodynamic responses: adenosine infusion
Table 2 summarizes the absolute FBF, MAP and FVC during resting, ‘low’ and ‘high’ dose adenosine infusion trials. The resting baseline FBF and FVC were elevated for trials in which the subjects were to receive a low tyramine dose during high adenosine dose infusion (P < 0.05), while all other trials had similar baseline FBF and FVC. Steady-state FBF and FVC responses to adenosine infusion were dose dependent (FBF, P = 0.017; FVC P = 0.013, main effects of adenosine dose). While all subjects demonstrated a substantial initial increase in FBF and FVC with adenosine infusion, FBF and FVC did not remain at peak levels and there was considerable variability across subjects in the steady-state FBF and FVC responses reached by 3 min of adenosine infusion (e.g. range of FBF for adenosine high dose: 47-467 ml min−1). However on average, steady state FBF was ∼4-5-fold higher during the low dose adenosine infusion vs. rest and ∼60 % of that observed with moderate exercise. High dose adenosine infusion resulted in an ∼7-9-fold increase in steady-state FBF vs. rest and reached an average flow which was similar to moderate exercise and about 50-60 % of that observed during heavy intensity exercise. Responses for FVC were similar.
Baseline MAP was elevated in the adenosine ‘high’ dose trials compared to rest (P < 0.05, main effect). Adenosine infusion did not alter MAP. Infusion of tyramine at rest and during adenosine infusion did result in an elevation in MAP of ∼2-3 mmHg (P < 0.05, main effect) indicating that there may have been a minor systemic tyramine effect.
Absolute haemodynamic responses: SNP infusion
Table 3 summarises the absolute FBF, MAP and FVC during resting, ‘low’ and ‘high’ dose SNP infusion trials. The resting baseline FBF and FVC were elevated for trials in which the subjects were to receive a high tyramine dose during high SNP dose infusion (P < 0.05), while all other trials had similar baseline FBF and FVC. Steady-state FBF and FVC responses to SNP infusion were dose dependent (FBF, P = 0.002; FVC P = 0.001, main effects of SNP dose). While all subjects demonstrated a substantial initial increase in FBF and FVC with SNP infusion, FBF and FVC did not remain at peak levels and there was considerable variability across subjects in the steady-state FBF and FVC responses reached by 3 min of SNP infusion (e.g. range of FBF for SNP high dose: 120-732 ml min−1). However, on average, steady-state FBF was ∼6-fold higher during the low dose SNP infusion vs. rest and ∼75 % of that observed with moderate exercise. High dose SNP infusion resulted in an ∼10-fold increase in steady-state FBF vs. rest and reached an average flow which was slightly higher than that reached during moderate exercise and ∼70 % of that observed during heavy intensity exercise. Responses for FVC were similar.
Baseline MAP was elevated in the SNP ‘high’ dose trials compared to rest (P < 0.05, main effect). No change in MAP occurred during low dose SNP infusion, while high dose SNP infusion resulted in a drop in blood pressure of ∼6-7 mmHg, suggesting a systemic effect. MAP increased with infusion of low dose tyramine during rest, low and high dose SNP infusion (P < 0.05). MAP increased with high dose tyramine infusion only in the low and high dose SNP infusion trials (P < 0.05). This indicates some degree of systemic effect of tyramine infusion during infusion of SNP.
Vasoconstrictor effect of tyramine infusion: exercise
Figure 2 provides examples of the raw, beat-by-beat brachial artery mean blood velocity tracings prior to and during high dose tyramine infusion. The striking difference in tyramine vasoconstriction between exercise-elevated flow conditions vs. vasodilator infusion-elevated flow conditions is evident.
Figure 2.
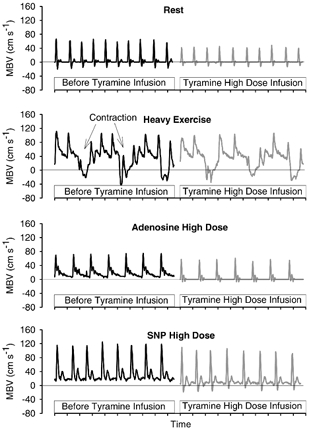
Example of the beat-by-beat arterial inflow blood velocity waveforms in response to high dose of tyramine infusion during rest, heavy exercise, high dose adenosine infusion, and high dose SNP infusion. For each condition, the response is from a subject whose percentage change in FVC was close to the average for that condition.
Quantitative evaluation of the vasoconstrictor effect of tyramine infusion across experimental conditions was assessed by percentage change in FBF and FVC. For percentage change in FBF (Fig. 3) there was an interaction between exercise intensity and tyramine dose (P = 0.031). Post hoc comparisons revealed a clear dose response to tyramine at rest but no significant effect in either moderate or heavy exercise. The percentage change in FBF during moderate exercise was not different from rest at the low (P = 0.998) or the medium (P = 0.202) tyramine dose, but was significantly reduced at the high dose (-26.7 ± 2.1 vs. −43.3 ± 3.7). During heavy exercise the percentage change in FBF across all tyramine doses was blunted compared to rest and moderate exercise (low dose, −4.3 ± 3.0 vs. −19.2 ± 3.3 vs. −19.2 ± 3.3; medium dose, −6.7 ± 3.7 vs. −30.7 ± 4.5 vs. −24.9 ±3.0; high dose, −10.6 ± 4.9 vs. −43.3 ± 3.7 vs. −26.7 ± 2.1, P< 0.05).
There was a main effect of exercise (P < 0.001) on absolute reductions in blood flow with tyramine infusion. Post hoc analysis indicated that the absolute reductions in FBF (ml min−1 ± s.e.m.) at rest were less than those seen with moderate and heavy exercise at all tyramine doses (P < 0.001: low dose, −7.7 ± 1.0 vs. −46.8 ± 7.5 vs. −19.1 ± 13.5; medium dose, −12.8 ± 2.2 vs. −61.9 ± 6.9 vs. −41.3 ± 12.0; high dose, −18.0 ± 3.2 vs. −65.7 ± 6.5 vs. 59.7 ± 14.5, P < 0.05), as would be expected given the much lower flow at rest compared to exercise. However, of particular interest is the observation that the absolute reduction in blood flow with tyramine infusion was blunted in heavy vs. moderate exercise (P = 0.036, main effect of exercise intensity).
For percentage change in FVC there was an interaction between exercise intensity and tyramine dose (P = 0.024; Fig. 4). Post hoc comparisons revealed a clear dose response of percentage change in FVC to tyramine at rest, but no detectable dose response in moderate exercise. In heavy exercise, the percentage change in FVC in response to the high tyramine dose was significantly greater than the response to the low tyramine dose (P = 0.035). Furthermore, heavy exercise substantially blunted the percentage change in FVC across all tyramine doses compared to moderate exercise and rest (low dose, −6.8 ± 3.2 vs. −22.0 ± 3.6 vs. −19.6 ± 3.1; medium dose, −12.8 ± 2.7 vs. −26.1 ± 3.0 vs. −30.7 ± 4.5; high dose, −16.7 ± 3.6 vs. −27.5 ± 2.3 vs. −43.4 ± 3.7; P < 0.05), while the percentage reduction in FVC during moderate exercise was lower than that observed during rest at the high tyramine dose (-27.5 ± 2.3 vs. −43.7 ± 3.7; P = 0.001).
Figure 4.
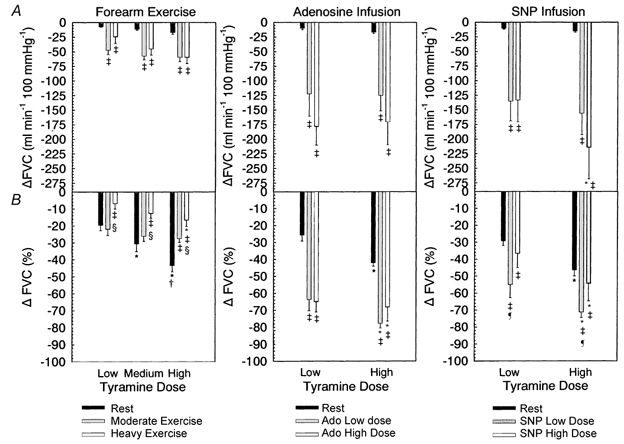
A, change in forearm vascular conductance (FVC) from baseline for rest, exercise, adenosine infusion and SNP infusion. B, percentage change in FVC, comparison as for A. *Significantly different from low tyramine dose within exercise or vasodilator condition. † Significantly different from medium tyramine dose within exercise condition. ‡ Significantly different from rest within tyramine dose. § Significantly different from moderate exercise within tyramine dose (all P < 0.05). ¶ Significantly different from high SNP dose, P < 0.05.
For absolute changes in FVC, there was a main effect of exercise intensity (P < 0.001) but no interaction with tyramine dose (P = 0.389). This means that the absolute reductions in FVC with tyramine infusion at rest were less than those observed during moderate and heavy exercise (low dose, −7.3 ± 0.7 vs. −47.6 ± 7.4 vs. −24.5 ± 11.3; medium dose, −11.6 ± 1.6 vs. −57.5 ± 6.4 vs. −45.5 ± 9.8; high dose, −17.2 ± 2.8 vs. −59.6 ± 7.3 vs. −60.0 ± 10.4; P < 0.05), as would be expected given the much lower flow at rest compared to exercise. The absolute change in FVC in heavy vs. moderate exercise approached statistical significance (P = 0.09). Given the variability of the response, sample size calculations for a statistical power of 0.8 indicated that an n of 15 subjects was required for statistical significance.
Vasoconstrictor effect of tyramine infusion: adenosine and SNP infusion
There was a marked reduction in FBF (Fig. 3) and FVC (Fig. 4) with tyramine infusion during both adenosine and SNP infusion compared to rest (P < 0.001, main effect), but there was no difference in the magnitude of this effect between adenosine doses (FBF, P = 0.417; FVC, P = 0.434). In addition, there was no dose effect of tyramine during adenosine infusion (FBF, P = 0.287; FVC, P = 0.332). In contrast, for both FBF and FVC reduction with tyramine infusion there was an interaction effect between SNP dose and tyramine dose (P = 0.019) such that the reduction in FBF and FVC during SNP high dose infusion was greater with high tyramine dose vs. low tyramine dose (FBF: −176.6 ± 46.5 vs. −109.7 ± 32.0 ml min−1, P < 0.001; FVC: −214.2 ± 54.0 vs. −133.4 ± 37.4 ml min−1 100 mmHg−1).
There was a main effect of tyramine dose (P < 0.001) on percentage reduction in FBF and FVC in the adenosine infusion experiments (Fig. 3 and Fig. 4). The percentage reduction in FBF and FVC with tyramine infusion was significantly greater during low and high dose adenosine infusion compared to rest (P < 0.001 for both, main effect of adenosine infusion) but not different between adenosine doses (FBF, P = 0.335; FVC, P = 0.326). This is in contrast to exercise, where the percentage reduction in FVC was blunted in heavy exercise compared to moderate exercise and rest, and where it was the same or blunted in moderate exercise vs. rest depending on tyramine dose.
There was a main effect of tyramine dose (P < 0.001) on percentage reduction in FBF and FVC in the SNP infusion experiments (Fig. 3 and Fig. 4). However, in contrast to adenosine infusion where there was no difference in the tyramine-induced percentage reduction in FBF and FVC between rest and high dose adenosine infusion (FBF, P = 0.645; FVC, P = 0.507), the percentage reduction in FBF and FVC with tyramine infusion was significantly greater during low dose SNP infusion compared to rest (P = 0.005 for FBF and FVC) and high dose SNP infusion (FBF P = 0.029; FVC, P = 0.05). Therefore, while tyramine-induced vasoconstriction was not affected by adenosine dose, it appeared to be sensitive to the dose of the nitric oxide donor SNP.
Percentage change in FVC: related to baseline forearm vascular conductance?
Finally, we took advantage of the variation in FVC between subjects induced by vasodilator infusion and moderate and heavy exercise to assess whether baseline FVC levels influenced the percentage reduction in FVC with tyramine infusion. Figure 5 and Figure 6 illustrate this relationship. It is clear from these figures that a similar range of percentage reduction in FVC occurred over a wide range of baseline blood flows, indicating that baseline blood flow does not affect the percentage reduction in FVC in response to tyramine infusion.
Figure 5.
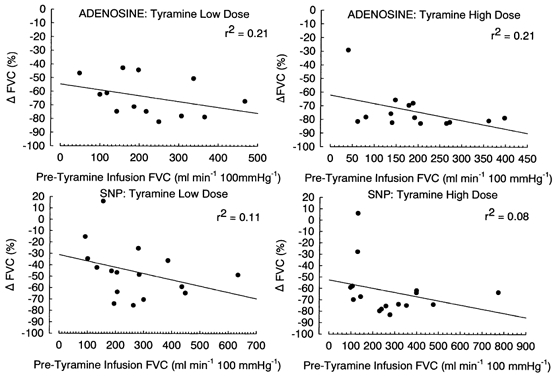
Regression plots of the relationship between baseline forearm vascular conductance (FVC) immediately prior to infusion of tyramine vs. the percentage change in FVC observed with tyramine infusion; r2 values are as indicated. Each data point is the response of an individual subject. Data are pooled across vasodilator dose for each tyramine dose.
Figure 6.
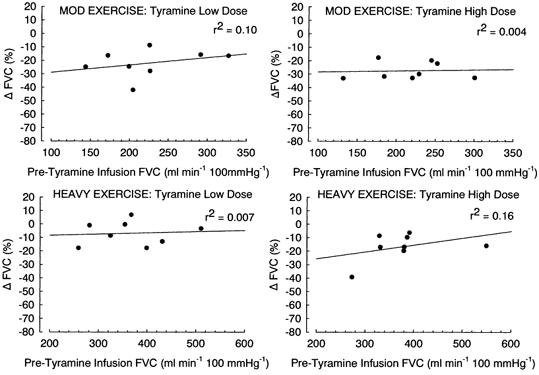
Regression plots of the relationship between baseline forearm vascular conductance (FVC) during moderate and heavy exercise immediately prior to infusion of tyramine vs. the percentage change in FVC observed with tyramine infusion; r2 values are as indicated. Each data point is the response of an individual subject.
DISCUSSION
This study tested the hypothesis that sympathetic vasoconstriction is blunted in exercising vs. resting human muscle in an intensity-dependent manner. The use of selective infusions of tyramine into the brachial artery to evoke endogenous noradrenaline release in the forearm during rest, exercise and vasodilator infusion constitutes a novel approach to this issue. The major new findings of this study are as follows. First, brachial artery infusion of tyramine evoked vasoconstriction during rest, forearm exercise, and selective infusions of the vasodilators adenosine and SNP. Second, the magnitude of vasoconstriction was dependent on exercise intensity, such that vasoconstriction was substantially blunted during heavy forearm exercise. Similar results were obtained when the same experimental approach was used in exercising dogs in a parallel study (Ruble et al. 2002). Third, substantially greater vasoconstriction occurred during adenosine infusion vs. exercise, and this vasoconstriction was not sensitive to adenosine dose. Finally, tyramine infusion during SNP administration also resulted in substantial vasoconstriction compared to exercise, but this effect was blunted with increased SNP dose. These observations support the concept of exercise intensity-dependent functional sympatholysis, and suggest that under some circumstances nitric oxide can blunt vasoconstriction in human forearm muscle.
Assessment of sympathetic vasoconstrictor effectiveness
Controversy exists over which index of vasomotor tone is appropriate for comparison of vasoconstrictor responsiveness between low and high blood flow conditions. We used percentage reduction in FVC as an index of vasoconstrictor effectiveness in this study for the following reasons. Both O'Leary (1991) and Lautt (1989) have pointed out the linear relationship between changes in conductance and blood flow when perfusion pressure remains relatively constant, and Lautt (1989) has suggested that the numerator for calculating vasomotor tone should consist of the variable that is changing more (in the case of our experiments this was blood flow). Using this approach, Thomas et al. (1997) have demonstrated that expressing changes in vasomotor tone as a percentage reduction in vascular conductance from baseline can discriminate blunted vs. preserved vasoconstriction during exercise or drug infusion-induced high flow conditions compared to resting low flow conditions. That is, they observed a significant blunting of the percentage reduction in vascular conductance during exercise but not during isoproterenol-induced vasodilatation.
We observed that the percentage reduction in FVC was reduced in heavy exercise vs. both rest and moderate exercise (Fig. 4), while responses in moderate exercise vs. rest were similar for the low and medium dose of tyramine, but reduced for the high tyramine dose. It may be argued that a smaller percentage reduction in FVC in exercise is simply due to a higher baseline blood flow. However, two key observations support the interpretation that this represents blunted vasoconstriction. First, tyramine infusion during adenosine administration resulted in a much greater percentage change in FVC compared to rest (Fig. 4). Second, there was no systematic difference in the percentage change in FVC with tyramine infusion across a wide range of adenosine- and SNP-induced levels of FVC (Fig. 5) or exercise-induced levels of FVC (Fig. 6).
Exercise intensity dependence of functional sympatholysis
Observations in the literature using a variety of experimental models range from preservation to complete abolishment of sympathetic vasoconstriction during exercise (Strandell & Shepherd, 1967; Thomas et al. 1994; Hansen et al. 1996, 2000; Tschakovsky & Hughson, 1999). The findings of this study and a parallel study in dogs (Ruble et al. 2002) support the general idea that this may be due to an exercise intensity dependence of functional sympatholysis.
Human forearm muscle is mixed, composed of both slow twitch (type I) and fast twitch (type II) fibres (Sadamoto et al. 1992). Type II fibres exhibit greater metabolic sensitivity to reductions in blood flow, and fatigue much more rapidly (Walker et al. 1982; Howlett & Hogan, 2000). Thus, from a teleological standpoint, type II fibres require more protection from sympathetically mediated reductions in muscle blood flow. This idea is consistent with the observation in rats that functional sympatholysis is far more robust in fast twitch muscle (Thomas et al. 1994).
Additionally, in animal models the resistance vessels in fast twitch muscles contain a predominance of post-synaptic vasoconstricting α2-receptors that are especially sensitive to acidosis and hypoxia (McGillivray-Anderson & Faber, 1991; Thomas et al. 1994). Furthermore, data from rat muscle indicate that neuronal NO synthase (nNOS) is preferentially localized in type II fibres (Kobzik et al. 1994). Thus, NO from nNOS could provide a means by which both inadequate blood flow and/or recruitment of type II fibres would evoke functional sympatholysis to protect against sympathetically-mediated restraint of blood flow.
The use of two exercise intensities and the application of three tyramine doses allowed us to assess functional sympatholysis across a range of exercise intensities and sympathetic activation. Our observations of progressively greater blunting of sympathetic vasoconstriction with exercise intensity are consistent with this general scheme.
Sites and mechanisms of functional sympatholysis
We examined a possible contribution of suggested sympatholytic agents adenosine (Lautt et al. 1988; Smits et al. 1991) and nitric oxide (Thomas & Victor, 1998) by selective brachial artery infusion of tyramine during infusion of two doses of adenosine and SNP. In contrast to exercise, tyramine resulted in a greater percentage reduction in FVC during adenosine treatment vs. rest. While this may indicate that adenosine is not responsible for the functional sympatholysis observed in exercise, an alternative explanation is that exogenous delivery did not result in adenosine reaching the site of action at the effective concentrations that would occur with the exercise intensities used in this study.
Similar to adenosine, tyramine evoked a greater percentage reduction in FVC during low dose infusion of SNP vs. rest. However in contrast to adenosine, when a higher dose of SNP was used the percentage reduction in FVC was less than with the low dose. Furthermore, it was not different from rest, which was similar to moderate exercise (Fig. 4). This sensitivity of tyramine-induced vasoconstriction to the dose of SNP suggests that NO might blunt sympathetic vasoconstriction in human muscle under certain conditions.
Our results contrast with those of Smits et al. (1991) who performed similar experiments in humans. These investigators (Smits et al. 1991) demonstrated that the percentage increase in forearm vascular resistance with noradrenaline or tyramine was greater during SNP infusion than adenosine infusion. It is not readily apparent why our results differ. However, the doses of both adenosine and SNP administration used in the study by Smits et al. (1991) were considerably lower than those of the current study. Furthermore, it has been demonstrated by others that blockade of adenosine does not affect the blunting of sympathetic vasoconstriction during exercise while nitric oxide blockade does (Thomas & Victor, 1998).
Implications for whole body exercise
It has been suggested that blunted vasoconstrictor control in exercising muscle would severely impair blood pressure regulation, and humans with defective autonomic nervous systems demonstrate frank hypotension during even mild exercise (Rowell, 1997). Alternatively, blunted but not abolished vasoconstrictor control may in fact permit adequate blood pressure control while minimizing the potentially deleterious effects of the sympathetic nerves restraining blood flow to active muscle (Remensnyder et al. 1962).
How can this occur? The effect of sympathetic vasoconstriction in a given vascular bed on systemic blood pressure is a function of the fraction of the cardiac output directed to, and total flow of, the vascular bed in question (O'Leary, 1991). Such an analysis reveals that the more flow directed to an exercising muscle vascular bed, the smaller the percentage change in vascular conductance required to result in the same change in blood pressure (O'Leary, 1991). This means that since skeletal muscle receives both greater total flow and a higher fraction of the cardiac output during heavy as compared to moderate exercise or rest, its contribution to systemic blood pressure regulation can remain high even when the relative effects of sympathetic vasoconstriction are blunted.
At the same time that blood pressure regulation is being preserved, functional sympatholysis could also protect exercising muscle from sympathetically mediated reductions in blood flow (Thomas et al. 1994). Observations that sympathetic outflow to resting and exercising muscle is the same (Hansen et al. 1994) suggest that functional sympatholysis could potentially optimize the distribution of blood flow between resting and exercising muscle. This same general mechanism would also tend to preserve vasoconstriction in moderately active muscles and favour distribution of blood flow to more metabolically stressed muscles.
Assumptions and limitations of experimental approach
One potential limitation in this study was the inability to confirm the effect of tyramine doses on noradrenaline release both within and across exercise conditions. Simple spillover measurements are confounded by local changes in blood flow, therefore accurate measurement of noradrenaline kinetics requires the use of radiolabelled isotope techniques (Chang et al. 1991) which was not technically feasible in this study. However, a clear dose response of noradrenaline release has been demonstrated over a wide range of tyramine doses (Brandao et al. 1980) and sustained tyramine-induced vasoconstriction has been reported in the human forearm across resting (Frewin & Whelan, 1968) and vasodilator-evoked elevations in flow (Smits et al. 1991). We did observe a clear vasoconstrictor dose response at rest, as has been observed in other studies of the human forearm (Frewin & Whelan, 1968; Jie et al. 1985). Based on the evidence, we believe that increasing doses of tyramine in this study caused proportional, sustained increases in noradrenaline release both at rest and during exercise.
With regard to noradrenaline release across conditions, we adjusted tyramine infusion during exercise and vasodilator-induced elevations in flow to maintain the same arterial tyramine concentration as at rest (Buckwalter et al. 1998) so that the gradient for diffusion of tyramine during exercise was at least that in resting low flow conditions. This approach resulted in a greater percentage reduction in FVC when flow was elevated by vasodilator infusion vs. rest (Fig. 4) and suggests that, in our model, the expected vasoconstrictor response during high flow conditions is manifest as a greater percentage reduction in FVC. It cannot be determined whether this is because the same noradrenaline release induces a greater effect when the starting vessel diameter is larger, or whether our approach resulted in more tyramine accessing nerve terminals during high inflow conditions (net delivery to limb = concentration × blood flow) and initiating a greater noradrenaline release. Regardless, these data reinforce the interpretation that the blunted percentage reduction in FVC during exercise was evidence of functional sympatholysis, and not due to a reduced tyramine-induced noradrenaline release in high flow conditions.
Finally, since tyramine evokes noradrenaline release differently from an action potential (Brandao et al. 1978), we are not able to address the potential for pre-synaptic mechanisms contributing to functional sympatholysis in humans. Thus, it is possible that functional sympatholysis is underestimated in our model.
Conclusions
In summary, we have provided strong evidence that sympathetic vasoconstriction in humans is blunted in exercising muscle and that this blunting is exercise intensity-dependent. This is consistent with functional sympatholysis acting to protect blood flow to metabolically stressed muscle without compromising blood pressure control. Furthermore, we have demonstrated a sensitivity of sympathetic vasoconstriction to infusion dose of the NO donor SNP, indicating that NO may blunt sympathetic vasoconstriction in humans under certain conditions.
Acknowledgments
We would like to thank the subjects involved in this study for their time and effort. We would also like to thank Karen Krucker for excellent technical assistance. This study was supported by NIH Grants HL43695, NS32352 and RR00585. M.T. was supported by a Natural Sciences and Engineering Research Council of Canada Post-Doctoral Fellowship Award.
REFERENCES
- Brandao F, Monteiro JG, Osswald W. Differences in the metabolic fate of noradrenaline released by electrical stimulation or by tyramine. Naunyn-Schmiedeberg's Archives of Pharmacology. 1978;305:37–40. doi: 10.1007/BF00497004. [DOI] [PubMed] [Google Scholar]
- Brandao F, Rodrigues-Pereira E, Guilherme MJ, Osswald W. Characteristics of tyramine induced release of noradrenaline: mode of action of tyramine and metabolic fate of the transmitter. Naunyn-Schmiedeberg's Archives of Pharmacology. 1980;311:9–15. doi: 10.1007/BF00500297. [DOI] [PubMed] [Google Scholar]
- Buckwalter JB, Mueller PJ, Clifford PS. α1-Adrenergic-receptor responsiveness in skeletal muscle during dynamic exercise. Journal of Applied Physiology. 1998;85:2277–2283. doi: 10.1152/jappl.1998.85.6.2277. [DOI] [PubMed] [Google Scholar]
- Buckwalter JB, Naik JS, Valic Z, Clifford PS. Exercise attenuates α-adrenergic-receptor responsiveness in skeletal muscle vasculature. Journal of Applied Physiology. 2001;90:172–178. doi: 10.1152/jappl.2001.90.1.172. [DOI] [PubMed] [Google Scholar]
- Chang PC, Krie E, van der Krogt JA, van Brummelen P. Does regional norepinephrine spillover represent local sympathetic activity? Hypertension. 1991;18:56–66. doi: 10.1161/01.hyp.18.1.56. [DOI] [PubMed] [Google Scholar]
- Eboute Y, Vanhoutte PM, Shepherd JT. Inorganic phosphate inhibits sympathetic neurotransmission in canine saphenous veins. American Journal of Physiology. 1987;252:H131–134. doi: 10.1152/ajpheart.1987.252.1.H131. [DOI] [PubMed] [Google Scholar]
- Frewin DB, Whelan RF. The mechanism of action of tyramine on the blood vessels of the forearm in man. British Journal of Pharmacology. 1968;33:105–116. doi: 10.1111/j.1476-5381.1968.tb00478.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen J, Sander M, Thomas GD. Metabolic modulation of sympathetic vasoconstriction in exercising skeletal muscle. Acta Physiologica Scandinavica. 2000;168:489–503. doi: 10.1046/j.1365-201x.2000.00701.x. [DOI] [PubMed] [Google Scholar]
- Hansen J, Thomas GD, Harris SA, Parsons WJ, Victor RG. Differential sympathetic neural control of oxygenation in resting and exercising human skeletal muscle. Journal of Clinical Investigation. 1996;98:584–596. doi: 10.1172/JCI118826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen J, Thomas GD, Jacobsen TN, Victor RG. Muscle metaboreflex triggers parallel sympathetic activation in exercising and resting human skeletal muscle. American Journal of Physiology. 1994;266:H2508–2514. doi: 10.1152/ajpheart.1994.266.6.H2508. [DOI] [PubMed] [Google Scholar]
- Howlett R, Hogan MC. Differential fatigue rates of muscle fiber types in response to hypoxia. The Physiologist. 2000;43:358. abstract. [Google Scholar]
- Jie K, van Brummelen P, Vermey P, Timmermans PB, van Zwieten PA. Differences between exogenous and endogenous noradrenaline in the effects on vascular post-synaptic alpha 1- and alpha 2-adrenoceptors in man. Journal of Hypertension. 1985;3(suppl. 3):S145–S147. [PubMed] [Google Scholar]
- Kagaya A, Saito M, Ogita F, Shinohara M. Exhausting handgrip exercise reduces the blood flow in the active calf muscle exercising at low intensity. European Journal of Applied Physiology. 1994;68:252–257. doi: 10.1007/BF00376774. [DOI] [PubMed] [Google Scholar]
- Kobzik L, Reid MB, Bredt DS, Stamler JS. Nitric oxide in skeletal muscle. Nature. 1994;372:546–548. doi: 10.1038/372546a0. [DOI] [PubMed] [Google Scholar]
- Lautt WW. Resistance or conductance for expression of arterial vascular tone. Microvascular Research. 1989;37:230–236. doi: 10.1016/0026-2862(89)90040-x. [DOI] [PubMed] [Google Scholar]
- Lautt WW, Lockhart LK, Legare DJ. Adenosine modulation of vasoconstrictor responses to stimulation of sympathetic nerves and norepinephrine infusion in the superior mesenteric artery of the cat. Canadian Journal of Physiology and Pharmacology. 1988;66:937–941. doi: 10.1139/y88-152. [DOI] [PubMed] [Google Scholar]
- Macedo MP, Lautt WW. Nitric oxide suppression of norepinephrine release from nerves in the superior mesenteric artery. Proceedings of the Western Pharmacology Society. 1994;37:103–104. [PubMed] [Google Scholar]
- McGillivray-Anderson KM, Faber JE. Effect of reduced blood flow on alpha 1- and alpha 2-adrenoceptor constriction of rat skeletal muscle microvessels. Circulation Research. 1991;69:165–173. doi: 10.1161/01.res.69.1.165. [DOI] [PubMed] [Google Scholar]
- O'Leary DS. Regional vascular resistance vs. conductance: which index for baroreflex responses? American Journal of Physiology. 1991;260:H632–637. doi: 10.1152/ajpheart.1991.260.2.H632. [DOI] [PubMed] [Google Scholar]
- Radegran G, Calbet JA. Role of adenosine in exercise-induced human skeletal muscle vasodilatation. Acta Physiologica Scandinavica. 2001;171:177–185. doi: 10.1046/j.1365-201x.2001.00796.x. [DOI] [PubMed] [Google Scholar]
- Remensnyder JP, Mitchell JH, Sarnoff SJ. Functional sympatholysis during muscular activity. Circulation Research. 1962;11:370–380. doi: 10.1161/01.res.11.3.370. [DOI] [PubMed] [Google Scholar]
- Rowell LB. Human Cardiovascular Control. New York: Oxford University Press; 1993. [Google Scholar]
- Rowell LB. Neural control of muscle blood flow: importance during dynamic exercise. Clinical and Experimental Pharmacology and Physiology. 1997;24:117–125. doi: 10.1111/j.1440-1681.1997.tb01793.x. [DOI] [PubMed] [Google Scholar]
- Ruble SB, Valic Z, Buckwalter JB, Tschakovsky ME, Clifford PS. Attenuated vascular responsiveness to noradrenaline release during dynamic exercise in dogs. Journal of Physiology. 2002;541:637–644. doi: 10.1113/jphysiol.2001.014738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadamoto T, Mutoh Y, Miyashita M. Cardiovascular reflexes during sustained handgrip exercise: role of muscle fibre composition, potassium and lactate. European Journal of Applied Physiology and Occupational Physiology. 1992;65:324–330. doi: 10.1007/BF00868135. [DOI] [PubMed] [Google Scholar]
- Shepherd JT, Vanhoutte PM. Local modulation of adrenergic neurotransmission. Circulation. 1981;64:655–666. doi: 10.1161/01.cir.64.4.655. [DOI] [PubMed] [Google Scholar]
- Smits P, Lenders JW, Willemsen JJ, Thien T. Adenosine attenuates the response to sympathetic stimuli in humans. Hypertension. 1991;18:216–223. doi: 10.1161/01.hyp.18.2.216. [DOI] [PubMed] [Google Scholar]
- Strandell T, Shepherd JT. The effect in humans of increased sympathetic activity on the blood flow to active muscles. Acta Medica Scandinavia. 1967;472:146–167. doi: 10.1111/j.0954-6820.1967.tb12622.x. [DOI] [PubMed] [Google Scholar]
- Thomas GD, Hansen J, Victor RG. Inhibition of alpha2-adrenergic vasoconstriction during contraction of glycolytic, not oxidative, rat hindlimb muscle. American Journal of Physiology. 1994;266:H920–929. doi: 10.1152/ajpheart.1994.266.3.H920. [DOI] [PubMed] [Google Scholar]
- Thomas GD, Hansen J, Victor RG. ATP-sensitive potassium channels mediate contraction-induced attenuation of sympathetic vasoconstriction in rat skeletal muscle. Journal of Clinical Investigation. 1997;99:2602–2609. doi: 10.1172/JCI119448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas GD, Victor RG. Nitric oxide mediates contraction-induced attenuation of sympathetic vasoconstriction in rat skeletal muscle. Journal of Physiology. 1998;506:817–826. doi: 10.1111/j.1469-7793.1998.817bv.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tschakovsky ME, Hughson RL. Ischemic calf exercise elevates blood flow in non-ischemic exercising human forearm muscles. American Journal of Physiology. 1999;277:H635–642. doi: 10.1152/ajpheart.1999.277.2.H635. [DOI] [PubMed] [Google Scholar]
- Verhaeghe RH, Shepherd JT. Effect of nitroprusside on smooth muscle and adrenergic nerve terminals in isolated blood vessels. Journal of Pharmacology and Experimental Therapeutics. 1976;199:269–277. [PubMed] [Google Scholar]
- Verhaeghe RH, Vanhoutte PM, Shepherd JT. Inhibition of sympathetic neurotransmission in canine blood vessels by adenosine and adenine nucleotides. Circulation Research. 1977;40:208–215. doi: 10.1161/01.res.40.2.208. [DOI] [PubMed] [Google Scholar]
- Walker PM, Idstrom JP, Schersten T, Bylund-Fellenius AC. Metabolic response in different muscle types to reduced blood flow during exercise in perfused rat hindlimb. Clinical Science. 1982;63:293–299. doi: 10.1042/cs0630293. [DOI] [PubMed] [Google Scholar]


