Abstract
Neurotransmitters and volatile anaesthetics have opposing effects on motoneuronal excitability which appear to reflect contrasting modulation of two types of subthreshold currents. Neurotransmitters increase motoneuronal excitability by inhibiting TWIK-related acid-sensitive K+ channels (TASK) and shifting activation of a hyperpolarization-activated cationic current (Ih) to more depolarized potentials; on the other hand, anaesthetics decrease excitability by activating a TASK-like current and inducing a hyperpolarizing shift in Ih activation. Here, we used whole-cell recording from motoneurones in brainstem slices to test if neurotransmitters (serotonin (5-HT) and noradrenaline (NA)) and an anaesthetic (halothane) indeed compete for modulation of the same ion channels - and we determined which prevails. When applied together under current clamp conditions, 5-HT reversed anaesthetic-induced membrane hyperpolarization and increased motoneuronal excitability. Under voltage clamp conditions, 5-HT and NA overcame most, but not all, of the halothane-induced current. When Ih was blocked with ZD 7288, the neurotransmitters completely inhibited the K+ current activated by halothane; the halothane-sensitive neurotransmitter current reversed at the equilibrium potential for potassium (EK) and displayed properties expected of acid-sensitive, open-rectifier TASK channels. To characterize modulation of Ih in relative isolation, effects of 5-HT and halothane were examined in acidified bath solutions that blocked TASK channels. Under these conditions, 5-HT and halothane each caused their characteristic shift in voltage-dependent gating of Ih. When tested concurrently, however, halothane decreased the neurotransmitter-induced depolarizing shift in Ih activation. Thus, halothane and neurotransmitters converge on TASK and Ih channels with opposite effects; transmitter action prevailed over anaesthetic effects on TASK channels, but not over effects on Ih. These data suggest that anaesthetic actions resulting from effects on either TASK or hyperpolarization-activated cyclic nucleotide-gated (HCN) channels in motoneurones, and perhaps at other CNS sites, can be modulated by prevailing neurotransmitter tone.
Mechanisms underlying the reversible unconsciousness and immobilization produced by general anaesthetics have yet to be adequately defined, despite widespread use of these agents in surgical procedures for over a century. Although anaesthetics were initially believed to disrupt protein function non-specifically through perturbation of membrane lipid partitioning (Meyer, 1899; Janoff et al. 1981), current theories suggest that direct interactions between anaesthetics and specific membrane protein substrates underlie neuronal effects of anaesthetics. A number of membrane ion channels, which are critical determinants of neuronal excitability, figure prominently in these new theories since they are targeted by general anaesthetics in a clinically appropriate concentration range. These include not only ligand-gated ion channels that mediate fast synaptic transmission (Franks & Lieb, 1994; Mihic et al. 1997), but also other types of voltage-dependent and subthreshold ion channels that regulate neuronal excitability (Nicoll & Madison, 1982; Takenoshita & Steinbach, 1991; Pancrazio et al. 1993; Sirois et al. 1998). A major effort remains to attribute specific anaesthetic actions to these different ion channel targets.
Immobilization, or the absence of movement in response to a painful stimulus, is pre-eminent as an anaesthetic end point (as encompassed in the concept of MAC, the minimum alveolar concentration of anaesthetic at which 50 % of patients are immobilized). In patients, immobilizing anaesthetic effects are associated with decreased motoneuronal excitability (Kawaguchi et al. 1996; Zhou et al. 1997, 1998). In this respect, we found that inhalation anaesthetics act directly on rat brainstem motoneurones at clinically relevant concentrations to cause membrane hyperpolarization and decreased excitability via effects on at least two classes of subthreshold ion channels: anaesthetics enhance a pH-sensitive, TASK-like leak K+ current and inhibit a hyperpolarization-activated cationic current (Ih) (Sirois et al. 1998, 2000). Notably, a number of neurotransmitters that are commonly associated with behavioural arousal and increased motor output (e.g. serotonin (5-HT) and noradrenaline (NA)) target channels with similar properties in motoneurones, but with effects opposite to those of anaesthetics (Larkman & Kelly, 1992; Talley et al. 2000; Rekling et al. 2000). This suggests the possibility that anaesthetics and neurotransmitters converge on the same leak K+ and Ih channels with opposite actions.
Here, we tested this possibility directly by simultaneous co-application of neurotransmitter and anaesthetic - and we determined which effect of these modulators prevails when both are present. We show that neurotransmitters and halothane indeed target the same TASK-like leak K+ channels, and that inhibition by transmitter overcomes channel activation by anaesthetic. Likewise, transmitters and halothane converge on Ih, but in this case the effect of the anaesthetic predominates. Inasmuch as these channels are distributed throughout the CNS, the effects of anaesthetics that we describe - and the modulation of those effects by neurotransmitters - could have implications for anaesthetic action that extend beyond the control of motoneuronal excitability and anaesthetic immobilization.
METHODS
General preparation
Whole-cell recordings were obtained in vitro from brainstem slices using procedures similar to those described previously (Sirois et al. 1998; Talley et al. 2000). All procedures were performed in accordance with National Institutes of Health and University of Virginia Animal Care and Use Guidelines. Briefly, rats (Sprague-Dawley, postnatal day 7-18) were anaesthetized with ketamine/xylazine (200 and 14 mg kg−1, i.m., respectively) and the brainstem removed following decapitation. Transverse slices (200 μm) were cut with a microslicer (DSK-1000, Dosaka, Kyoto, Japan) in an ice-cold solution containing (mm): 260 sucrose, 3 KCl, 5 MgCl2, 1 CaCl2, 1.25 NaH2PO4, 26 NaHCO3, 10 glucose and 1 kynurenic acid. Slices were incubated for ≈1 h at 37 °C in a solution consisting of (mm): 130 NaCl, 3 KCl, 2 MgCl2, 2 CaCl2, 1.25 NaH2PO4, 26 NaHCO3 and 10 glucose. Slices were maintained in this incubation solution at room temperature (23-25 °C) for periods of up to 6 h. Cutting and incubation solutions were bubbled continuously with 95 % O2 and 5 % CO2.
Recording
Patch electrodes with a DC resistance of ≈3-5 MΩ were pulled from borosilicate glass capillaries (Warner Instruments, Hamden, CT, USA) on a two-stage puller (Sutter Instruments, Novato, CA, USA) and coated with Sylgard 184 (Dow Corning, Midland, MI, USA). Slices were submerged in a chamber mounted on a fixed-stage microscope (Zeiss Axioskop FS) and visualized using differential interference (DIC) optics. Hypoglossal motoneurones were identified visually by their location (lateral and ventrolateral to the central canal) and by their characteristic size and shape (Viana et al. 1990). Electrical recordings were performed at room temperature in a bath solution composed of (mm): 130 NaCl, 3 KCl, 2 MgCl2, 2 CaCl2, 1.25 NaH2PO4, 10 Hepes and 10 glucose. The pH of bath solutions was adjusted using either NaOH or HCl. All bath solutions contained tetrodotoxin (0.75 μM) and were perfused continuously over the slice (≈2 ml min−1). Internal solution contained (mm): 120 KCH3SO3, 4 NaCl2, 1 MgCl2, 0.5 CaCl2, 10 Hepes, 10 EGTA, 3 Mg-ATP, 0.3 GTP-Tris, with pH buffered to 7.2 using KOH. In a subset of experiments examining effects of halothane and neurotransmitter on Ih during long term exposure to acidified bath solutions, the internal Hepes concentration was increased to 25 mm in order to minimize changes in intracellular pH that might directly affect Ih (Munsch & Pape, 1999; Zong et al. 2001). Osmolarity was maintained in these experiments by reducing the concentration of KCH3SO3 accordingly. ZD 7288 (40 μM, Tocris Cookson, Ballwin, MO, USA) was added to the internal solution to block Ih in some experiments.
All solutions were bubbled with a room air gas mixture (21 %O2, balance N2). Halothane was added to the perfusate by directing the gas mixture through calibrated vaporizers (Ohmeda, Austell, GA, USA) and equilibrating the bath solution with the halothane-containing gas mixture. Halothane solutions were covered tightly with Parafilm to prevent loss of anaesthetic to the atmosphere. Aqueous concentrations of halothane were measured by gas chromatography from samples collected at the point of solution entry into the slice chamber (Sirois et al. 1998).
Data acquisition and analysis
Voltage commands were applied via an Axopatch 200B patch-clamp amplifier and membrane currents were digitized using a Digidata 1200 analog-to-digital converter (Axon Instruments, Union City, CA, USA). Currents were filtered at 2 kHz with a four-pole, low-pass Bessel filter. Series resistance was typically < 20 MΩ and was compensated by 65-70 %. All data were corrected for a liquid junction potential of 10 mV.
Cells were held at −60 mV and membrane currents in response to a hyperpolarizing pulse (-40 to −50 mV) were recorded at a constant interval (0.1 Hz). Current-voltage (I-V) relationships were determined for various treatment protocols from ‘instantaneous’ currents (i.e. immediately following the capacitive transient, before activation of the time-dependent Ih current) obtained by hyperpolarizing the cell in 10 mV steps (to −130 mV). The maximal amplitude of Ih was quantified as the size of the time-dependent current during a step to −130 mV. The voltage dependence of Ih activation was obtained from tail currents measured during a step to −80 mV that followed the series of hyperpolarizing steps; those data were normalized and fitted with a Boltzmann function (Bayliss et al. 1994). Data analysis was performed using the pCLAMP suite of programs (Axon Instruments) and statistical analysis of data was performed in Microsoft Excel, using Student's paired t test, with significance accepted if P < 0.05.
RESULTS
We examined effects of simultaneous exposure to an inhalational anaesthetic (halothane) and a neurotransmitter (5-HT) on rat brainstem hypoglossal motoneurones under current clamp conditions. As shown in Fig. 1, and consistent with our previous studies (Sirois et al. 1998, 2000), we found that clinical concentrations of halothane (0.35 mm) caused membrane hyperpolarization and decreased motoneuronal excitability. Upon application of 5-HT (2 μM) in the continued presence of halothane, the motoneurone showed a brisk depolarization that evoked repetitive spike discharge, and which dissipated after wash of halothane and 5-HT. In three initially non-spiking cells, halothane induced a 10.7 ± 3.2 mV hyperpolarization from −48.7 ± 1.1 mV; the effect of halothane was completely overcome by 5-HT, which in all cases caused a depolarization well beyond the threshold for repetitive firing. These results illustrate effects on motoneurones that are typical of those described previously for halothane and neurotransmitters (Sirois et al. 1998, 2000; Rekling et al. 2000), and furthermore show that anaesthetic effects on motoneuronal excitability can be reversed by co-application of 5-HT.
Figure 1. Serotonin reverses halothane-induced membrane hyperpolarization in hypoglossal motoneurones.
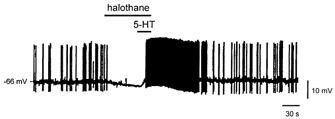
Effect of halothane (0.35 mm) and 5-HT (2 μM) on a hypoglossal motoneurone recorded under current clamp conditions. Halothane caused a membrane hyperpolarization that was reversed by 5-HT, even in the continued presence of halothane. The motoneurone was induced to fire by depolarizing DC injection (150 pA); action potentials were truncated by the chart recorder.
In order to determine if transmitter-induced reversal of anaesthetic effects, such as those just described, result from a convergence of neurotransmitters and anaesthetics on the same ion channels, we studied membrane currents induced by neurotransmitters (5-HT and noradrenaline (NA)) and halothane under whole-cell voltage clamp conditions. First, we characterized effects of neurotransmitters and halothane, either alone or when applied together. Then, we examined interactions between transmitters and anaesthetic on two prominent ion channel targets, a TASK-like, pH-sensitive K+ channel and a hyperpolarization-activated cationic (Ih) channel, each in relative isolation from the other.
Serotonin and halothane elicit opposite effects on a resting K+ current and Ih in motoneurones
At least two ionic conductances contributed to effects of 5-HT on hypoglossal motoneurones, as illustrated in data from the representative cell of Fig. 2. Under voltage clamp, 5-HT (5 μM, applied via the perfusate) produced a robust and reversible inward shift in holding current at the −60 mV holding potential (Fig. 2A), that averaged −250.0 ± 70.0 pA (n = 7). Instantaneous current-voltage (I-V) relationships were obtained under control conditions (Fig. 2A, a) and at the peak of the 5-HT response (Fig. 2A, b) by hyperpolarizing the cell in 10 mV increments from the holding potential (Fig. 2B). The I-V relationship of the current induced by 5-HT was derived by subtracting control currents from those obtained in the presence of 5-HT (Fig 2C, b - a). The 5-HT-sensitive current was weakly rectifying, and associated with a net reduction in membrane conductance that is evident in this cell as a negative slope of the I-V curve. Note, however, the lack of a clear reversal in the I-V relationship, which suggested contributions from currents other than those carried by K+.
Figure 2. Serotonin and halothane elicit opposing actions on two distinct conductances in hypoglossal motoneurones.
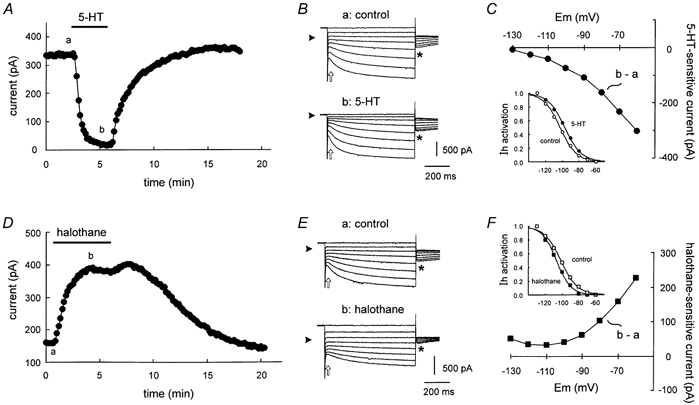
A, representative time series depicting the change in holding current (at −60 mV) induced by serotonin (5-HT, 5 μM); 5-HT caused an inward shift in holding current. B, sample current traces obtained at the times indicated in A in response to hyperpolarizing voltage steps from −60 mV under control conditions (a) and in the presence of 5-HT (b). Arrowheads indicate zero current level and open arrows indicate points used to determine instantaneous I-V relationships. A fixed step to −80 mV followed each test pulse (asterisks) in order to generate the tail currents that were used to derive the voltage dependence of Ih activation. C, the 5-HT-sensitive current was obtained by subtracting control currents (a) from those obtained in the presence of 5-HT (b). This current was associated with a decreased conductance but showed no clear reversal, suggesting modulation of multiple components. Inset, tail currents from control and 5-HT conditions were normalized, plotted with respect to the potential during the preceding step and fitted with a Boltzmann function to obtain the voltage dependence of Ih activation. 5-HT caused a depolarizing shift in Ih activation. D, time series illustrating the outward shift of holding current (at −60 mV) induced by halothane (0.9 mm) in a representative motoneurone. E, current traces obtained at the times indicated in D in response to hyperpolarizing voltage steps from −60 mV under control conditions (a) and in the presence of halothane (b). Note that tail currents are diminished in halothane (asterisks), reflecting decreased Ih amplitude. F, the halothane-sensitive current was obtained by subtracting control currents (a) from those obtained in the presence of halothane (b). This current was associated with an increased conductance, but with no clear reversal. Inset, normalized tail currents from control and halothane were fitted with a Boltzmann function; halothane caused a hyperpolarizing shift in the voltage dependence of Ih activation.
An additional current that could contribute to effects of 5-HT in motoneurones is the hyperpolarization-activated current, Ih. Indeed, previous work suggests that 5-HT enhances Ih, primarily by promoting a depolarizing shift in its voltage dependence of activation (for example see Takahashi & Berger, 1990; Larkman & Kelly, 1992; Hsiao et al. 1997; reviewed in Rekling et al. 2000). In order to test effects of 5-HT on the voltage dependence of Ih activation, tail currents were generated by stepping the membrane potential to −80 mV after activating Ih with voltage steps to increasingly hyperpolarized potentials (Fig. 2B, see asterisks). These Ih tail currents were normalized, plotted as a function of the membrane potential during the initial hyperpolarizing steps, and fitted with a Boltzmann function (Bayliss et al. 1994). As illustrated for this representative cell (Fig. 2C, inset), the Ih activation curve was shifted to more depolarized potentials in the presence of 5-HT (filled circles), compared with the control period before 5-HT application (open circles). These results were borne out in grouped data, where the V1/2 for Ih activation averaged −100.6 ± 2.1 mV in control conditions and −94.4 ± 1.7 mV in 5-HT, a depolarizing shift of ≈6 mV (P < 0.001; n = 7). We found no effect of 5-HT on maximal Ih current amplitude, defined as the difference between steady-state and instantaneous currents at −130 mV test potential (-403 ± 70 pA in control vs. 352 ± 81 pA in 5-HT; P > 0.2, n = 7).
By comparison with effects of 5-HT, the current modulation by halothane in hypoglossal motoneurones was striking inasmuch as it appeared to be reciprocal to that of the neurotransmitter. Thus, halothane (0.9 mm, bath applied) evoked a prominent outward shift in holding current at −60 mV (Fig. 2D) that averaged 225.1 ± 25.5 pA (n = 25). It is important to note that we have previously shown effects of halothane on motoneurones within a clinically relevant concentration range (Sirois et al. 1998, 2000). In most experiments described herein, we chose to use this higher concentration of halothane in order to demonstrate that the overriding effect of neurotransmitters, described in detail below, can occur even in the presence of halothane at supramaximal concentrations.
As described above for 5-HT, the I-V relationship of the halothane-sensitive current component was derived by subtraction of control currents (Fig. 2E, a) from those obtained in the presence of halothane (Fig. 2E, b). This current was outwardly rectifying and associated with a net increase in conductance (Fig. 2F). As with the 5-HT current, however, the lack of a clear reversal in the I-V relationship suggested that additional conductances contribute to the halothane-sensitive current. Accordingly, and opposite to effects of 5-HT, we found that halothane caused a hyperpolarizing shift in voltage dependence of Ih activation (Fig. 2F, inset). On average, the hyperpolarizing shift in Ih activation produced by halothane (ΔV1/2, approximately −4 mV; control: −97.4 ± 1.1 mV; halothane: −101.7 ± 1.2 mV; n = 24, P < 0.001) was of similar magnitude to that reported previously (Sirois et al. 1998). In addition, and in contrast to 5-HT, which did not affect the magnitude of Ih, we found that halothane caused a decrease in Ih amplitude. This is clearly evident in sample records from the representative cell as a decrease in size of Ih tail currents (see asterisks in Fig. 2E); for quantification, maximal Ih amplitude was taken as the time-dependent current at −130 mV, which decreased from −455.7 ± 40.1 pA in control to −326.8 ± 15.2 pA (28 % decrease in current amplitude; n = 24, P < 0.001).
These results indicate, as expected from previous work on these and other neurons (Takahashi & Berger, 1990; Berg-Johnsen & Langmoen, 1990; Larkman & Kelly, 1992; Hsiao et al. 1997; Sirois et al. 1998; reviewed in Pape, 1996; Rekling et al. 2000), that halothane and 5-HT each modulate two distinct conductances in hypoglossal motoneurones - a weakly rectifying K+ current and Ih - but in reciprocal fashion. Thus, 5-HT inhibits a K+ conductance and produces a depolarizing shift in Ih activation, whereas halothane activates a K+ conductance and shifts Ih activation to more hyperpolarized potentials. Effects of halothane and 5-HT were not completely reciprocal because, of the two, only halothane affected maximal Ih current amplitude. Subsequent experiments were designed to examine concurrent regulation of these separate conductances during simultaneous exposure to halothane and neurotransmitters.
Halothane modulates multiple components of neurotransmitter-sensitive current
To test potential interactions in effects of 5-HT and halothane on motoneurones, we employed the experimental protocol depicted for a representative cell in Fig. 3A. An initial application of 5-HT under control conditions (i.e. no halothane) produced an inward current and reduction in conductance, as described above. Following washout of 5-HT, cells were exposed to 0.9 mm halothane, which produced an outward current and increased membrane conductance. At the peak of the halothane response, 5-HT was re-applied in the continued presence of halothane. The resulting 5-HT-induced inward current in the presence of halothane was enhanced compared with that observed under control conditions. On average, as shown in Fig. 3B, the 5-HT current measured in halothane was significantly increased over that in the same cells tested under control conditions (-373.9 ± 102.4 pA in halothane vs. −250.0 ± 69.8 pA; P < 0.05; n = 7). Note, however, that the reduction in membrane current produced by 5-HT in the presence of halothane did not match the initial low level produced by neurotransmitter application under control conditions (Fig. 3A, dashed line). This suggests either that a portion of the halothane-enhanced current is insensitive to 5-HT or, alternatively, that halothane inhibits a separate component of 5-HT current (see below).
Figure 3. Halothane enhances net neurotransmitter-sensitive current.
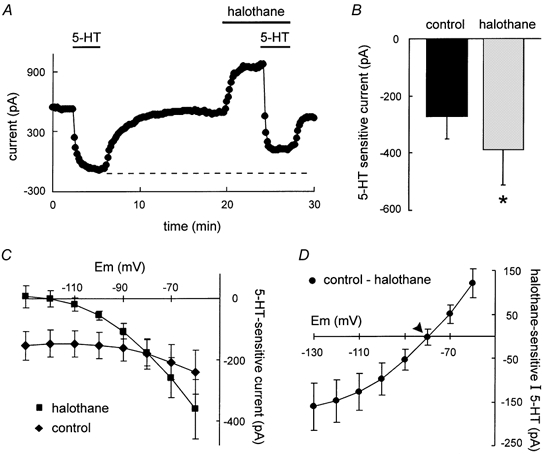
A, time series depicting the effect of halothane on 5-HT-sensitive current. Initially, 5-HT produced an inward shift in holding current (at −60 mV) that was reversible upon wash. Subsequently, halothane induced an outward current and in the continued presence of halothane, the 5-HT-induced inward current shift was enhanced. Note, however, that the absolute current level obtained with both 5-HT and halothane did not reach that observed with 5-HT alone (dashed line). B, averaged data depicting the magnitude of the 5-HT current. As compared with control, the 5-HT current was significantly enhanced in the presence of halothane. C, averaged I-V relationships of 5-HT-sensitive current obtained under control conditions (♦) and in the presence of halothane (▪). In the presence of halothane, the 5-HT current was associated with a greater reduction in conductance. D, mean data depicting the halothane-sensitive component of the 5-HT current. I-V data were derived by subtracting the 5-HT current in the presence of halothane from the control 5-HT current. Note that this current reverses ≈10 mV depolarized to EK (arrowhead).
In averaged data, the I-V relationship of control 5-HT current (Fig. 3C, diamonds) was associated with a small reduction in conductance and did not reverse within the range tested. In the presence of halothane (Fig. 2C, squares), the conductance decrease associated with 5-HT application was enhanced, as expected if 5-HT inhibited the halothane-activated K+ conductance. The halothane-sensitive component of 5-HT current was obtained by subtracting I-V data in halothane from that in control. As shown in Fig. 2D, this component of current was weakly rectifying but with a reversal potential ≈10 mV depolarized to EK (depicted by arrow), which again suggested contributions from additional conductances. The 5-HT-induced depolarizing shift in Ih activation was reduced in the presence of halothane (≈3 vs. ≈6 mV), consistent with the possibility that halothane interferes with 5-HT modulation of Ih. This is explored in greater detail below, under conditions when effects on TASK currents were eliminated.
Halothane also modulated currents evoked by other neurotransmitters, specifically noradrenaline (NA, 5 μM) and thyrotropin-releasing hormone (TRH, 0.1 μM). As with 5-HT, currents elicited by both NA and TRH were enhanced significantly (P < 0.05) in the presence of halothane (INA, −252.2 ± 40.2 pA in control vs. −382.4 ± 71.4 pA in halothane; ITRH, −134.7 ± 22.1 pA in control vs. −235.4 ± 45.8 pA in halothane; n = 5 for both NA and TRH). Like that of 5-HT, the current evoked by NA was characterized by inhibition of a resting K+ conductance coupled with a depolarizing shift in Ih activation (data not shown). Results with TRH were somewhat surprising inasmuch as we did not observe an enhancement of TRH-sensitive current by halothane in an earlier study (Sirois et al. 1998). In that previous work, however, we used a higher concentration of TRH (1 μM), and it is possible that a more pronounced receptor desensitization evoked by those higher TRH concentrations masked any enhancement in TRH current by halothane (Sirois et al. 1998).
A TASK-like component of halothane-sensitive current, revealed in relative isolation after blocking Ih, is inhibited by 5-HT
The joint neurotransmitter- and halothane-sensitive current had properties consistent with inhibition of a K+ conductance, although the current did not reverse at EK. We hypothesized that concomitant effects on Ih might account for this deviation from results expected for modulation of a pure K+ current and tested this possibility by examining effects of 5-HT and halothane after blocking Ih pharmacologically.
Intracellular application of the Ih blocker ZD 7288 (40 μM) produced essentially a complete block of the hyperpolarization-activated, time-dependent current within ≈5-10 min after breaking into the cell (data not shown). Under these conditions, 5-HT again produced an inward current at - 60 mV that was associated with a decrease in conductance (Fig. 4C, diamonds). Similar to experiments described above when Ih was not blocked, the 5-HT-induced inward shift in current in the presence of halothane was enhanced relative to that in control conditions (Fig. 4A and B; −304.4 ± 33.4 pA in halothane vs. −185.6 ± 22.1 pA; n = 9, P < 0.001). Interestingly, however, in these experiments in which Ih was blocked by ZD 7288, the absolute holding current attained in the presence of both 5-HT and halothane was the same as that obtained with 5-HT alone (see dashed line in Fig. 4A). This indicates that essentially all of the halothane current was blocked by 5-HT under these conditions. As shown in Fig. 4C, I-V relationships for 5-HT currents in control conditions (diamonds) and in the presence of halothane (squares) were again consistent with inhibition of a conductance that could be activated by halothane. Subtraction of the 5-HT current in halothane from that in control yielded the halothane-sensitive component of 5-HT current (Fig. 4D), which was well fitted with the Goldman-Hodgkin-Katz (GHK) equation (Hille, 1992), indicating that after blocking Ih with ZD 7288, essentially all of the halothane-sensitive 5-HT current was mediated by inhibition of an open rectifier K+ conductance. These results are consistent with prior data indicating that both neurotransmitters and volatile anaesthetics modulate a pH-sensitive open rectifier K+ current with the properties of TASK-like channels (Sirois et al. 2000; Talley et al. 2000). Moreover, they indicate that inhibitory actions of the neurotransmitter prevail over activating effects of halothane on motoneuronal TASK currents.
Figure 4. Pharmacological block of Ih revealed a TASK-like component of halothane- and neurotransmitter-sensitive current.
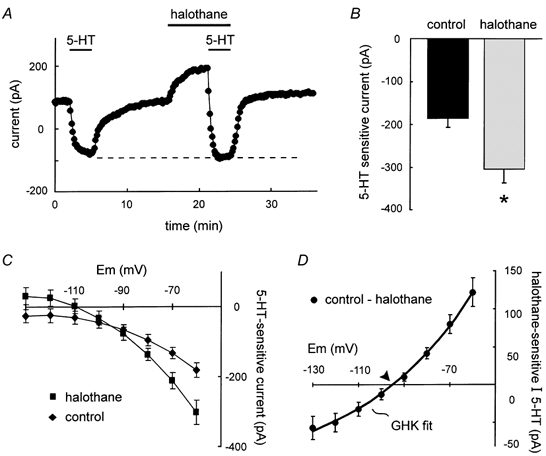
A, when Ih was blocked by ZD 7288 (40 μM, pipette), the 5-HT-induced inward shift in current was enhanced by halothane; 5-HT inhibited all of the halothane-sensitive current (dashed line). B, averaged data depicting the enhanced 5-HT current amplitude in the presence of halothane. C, averaged I-V relationships of 5-HT-sensitive current obtained under control conditions (♦) and in the presence of halothane (▪). D, the halothane-sensitive component of 5-HT current, derived by subtraction of currents induced by 5-HT in the presence of halothane from those under control conditions, was well-fitted using the GHK constant field equation and reversed near EK (arrowhead).
The interaction of anaesthetic and neurotransmitter currents was examined in an additional set of experiments with noradrenaline, again after blocking Ih with ZD 7288 (data not shown). Consistent with results obtained with 5-HT, the average control NA current (-210.6 ± 57.4) was enhanced in the presence of halothane (-349.8 ± 94.6 pA; n = 4, P < 0.05). The I-V profiles of NA-sensitive current in the presence and absence of halothane were similar to those observed with 5-HT, in that the conductance decrease associated with NA application was modest under control conditions and enhanced in the presence of halothane. Furthermore, the halothane-induced component of NA-sensitive current, obtained by subtraction of these two curves, yielded I-V data that were well fitted by the GHK equation. Thus, like 5-HT, NA inhibited a TASK-like, open rectifier K+ conductance activated by halothane.
Halothane modulation of Ih is retained in acidified bath solutions that block the anaesthetic-sensitive leak K+ current
In order to reveal convergent effects of anaesthetics and neurotransmitters on Ih in relative isolation, we performed experiments after blocking the anaesthetic- and transmitter-sensitive TASK currents by using an acidified bath solution (Sirois et al. 2000; Talley et al. 2000). Because decreases in intracellular pH shift the voltage dependence of Ih activation to more hyperpolarized potentials (Munsch & Pape, 1999; Zong et al. 2001), the concentration of Hepes in the internal solution was increased to 25 mm in order to minimize effects of bath acidification on intracellular pH.
Changing bath pH from 7.3 to 6.5 produced an inward shift in holding current at −60 mV (Fig. 5A) and decreased motoneuronal input conductance, as expected from inhibition of pH-sensitive TASK currents (Sirois et al. 2000; Talley et al. 2000). This change in extracellular pH did not appreciably affect maximal Ih amplitude (< 5 % reduction at −130 mV), but we did note a hyperpolarizing shift in Ih activation (V1/2 shifted from −98.7 ± 0.8 mV in pH 7.3 to −101.8 ± 0.8 mV in pH 6.5; n = 20, P < 0.001) perhaps indicative of a slight decrease in intracellular pH (Munsch & Pape, 1999; Zong et al. 2001).
Figure 5. Effects of halothane on Ih are retained in acidified bath conditions that block motoneuronal anaesthetic- and neurotransmitter-sensitive K+ currents.
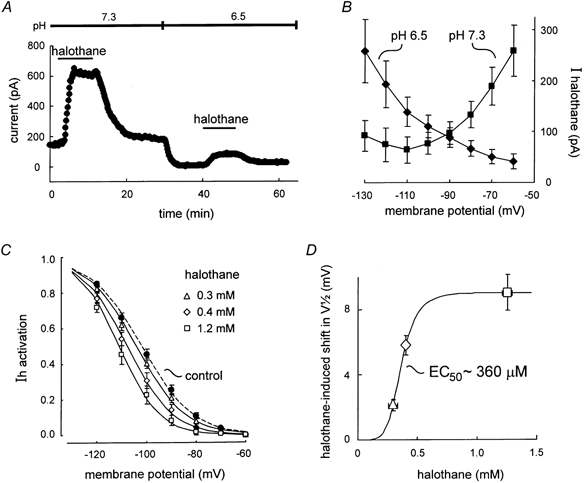
A, the outward shift in holding current induced by halothane at pH 7.3 was reduced following bath acidification to block pH-sensitive K+ currents. B, averaged I-V data depict halothane-sensitive currents obtained at pH 7.3 (▪) and at pH 6.5 (♦); the anaesthetic-sensitive current under acidified conditions increased with hyperpolarization and was associated with a decreased conductance, consistent with a decrease in Ih. C, halothane produced a concentration-dependent hyperpolarizing shift in the voltage dependence of Ih activation. Data were obtained as described in Fig. 2. V1/2 values (mV) were: control (pH 6.5), −101.9 ± 3.2; halothane (0.3 mm), −104. ± 3.4; halothane (0.4 mm), −107.6 ± 3.3; halothane (1.2 mm), −110.8 ± 3.6 (n = 8 for pH 6.5 and 0.3 mm halothane; n = 5 for 0.4 and 1.2 mm halothane). D, concentration-response curve for the halothane-induced shift in voltage dependence of Ih activation. The size of the halothane-induced shift in V1/2 in cells exposed to multiple concentrations of halothane was plotted as a function of measured aqueous halothane concentrations. A least-squares fit of the data with a logistic equation predicted an EC50 of 360 μM.
Blocking the pH-sensitive, TASK-like K+ current reduced the halothane current (Sirois et al. 2000). The averaged I-V relationship of halothane current at pH 7.3 was associated with a net increase in conductance, with currents largest at potentials depolarized to EK (Fig. 5B, squares). By contrast, following bath acidification to pH 6.5, the halothane current was associated with decreased conductance; currents were smallest near rest potentials and enhanced at hyperpolarized potentials (Fig. 5B, diamonds). Thus, the properties of this residual halothane-sensitive current are consistent with inhibition of Ih by the anaesthetic, and are essentially identical to those we reported previously after blocking the halothane-sensitive K+ current with 2 mm Ba2+ (Sirois et al. 1998). Moreover, although additional conductances may contribute slightly to the halothane current measured at the holding potential in acidified bath solutions (e.g. K+ currents not blocked completely at pH 6.5), the residual halothane current is mostly due to Ih inasmuch as it was largely eliminated by ZD 7288 (data not shown; see also Fig. 4A of Sirois et al. 2000).
As in control solutions at pH 7.3, the effect of halothane on Ih in acidified bath solutions included a decrease in maximal current amplitude (data not shown) and a hyperpolarizing shift in the voltage dependence of Ih activation (Fig. 5C). The anaesthetic-induced shift in Ih activation was steeply dependent on the halothane concentration over a range of 0.3-1.2 mm, with an estimated EC50 ≈360 μM (Fig. 5D). Thus, in an acidified bath solution, halothane retained the ability to shift the voltage dependence of Ih activation within a concentration range that is clinically relevant for its anaesthetic effects.
The 5-HT-induced shift in Ih activation, studied in relative isolation after blocking pH-sensitive K+ currents, is diminished by halothane
We characterized combined effects of 5-HT and halothane on Ih in relative isolation by using acidified bath solutions that block anaesthetic- and neurotransmitter-sensitive K+ currents. As expected, 5-HT induced an inward shift in holding current under these conditions (Fig. 6A) that was smaller than that observed at pH 7.3 (see also Talley et al. 2000). The 5-HT current that remained in the acidified bath, however, was associated with a depolarizing shift in Ih activation not unlike that produced by 5-HT at pH 7.3 (≈4 mV; Fig. 6B, from filled diamonds to filled squares). After washout of the neurotransmitter, halothane produced a small outward current (Fig. 6A) that was associated with a hyperpolarizing shift in the V1/2 of Ih activation (Fig. 6B, open circles). In the continued presence of halothane, 5-HT again caused an inward current, but this current was smaller than that observed before halothane (Fig. 6A); in the acidified bath, the averaged 5-HT current was −83.5 ± 17.7 pA before halothane and only −40.1 ± 25.8 pA in the presence of halothane, an ≈50 % reduction (P < 0.05, n = 12). Likewise, the 5-HT-induced depolarizing shift in Ih activation was also reduced ≈50 % by halothane (to ≈2 mV; Fig. 6B, inset). Note also that the V1/2 remained at a more hyperpolarized potential in the presence of halothane and 5-HT (open squares), compared with either the control (filled diamonds) or 5-HT conditions (filled squares). Thus, halothane interferes with modulation by 5-HT of the voltage dependence of Ih activation.
Figure 6. Halothane inhibits effects of 5-HT on Ih activation under acidified bath conditions.
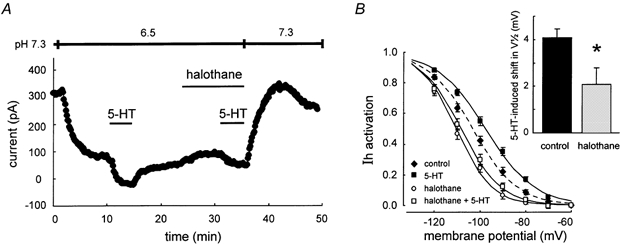
A, representative experiment in an acidified bath solution, when pH-sensitive K+ currents are blocked; under these conditions, halothane diminished the 5-HT-induced current. B, effect of 5-HT on the voltage dependence of Ih activation under control conditions and in the presence of halothane. Compared with control (♦), the V1/2 of Ih activation was shifted to depolarized potentials by 5-HT (▪), but was shifted in the hyperpolarized direction by halothane (○), even when tested in the combined presence of 5-HT (□). Inset, the depolarizing shift in Ih activation produced by 5-HT was significantly reduced by halothane.
DISCUSSION
We tested independent and convergent effects of neurotransmitters (5-HT and NA) and a volatile anaesthetic (halothane) on rat brainstem motoneurones. Independently, the neurotransmitters and halothane each targeted two different types of ion channel - leak K+ channels and Ih channels - and modulated them in opposite directions: 5-HT and NA inhibited a leak K+ current and caused a depolarizing shift in the voltage dependence of Ih activation whereas halothane activated a leak K+ current and induced a hyperpolarizing shift in Ih activation. Co-application revealed that the neurotransmitters and halothane targeted the same leak K+ and Ih channels. Thus, we found a joint halothane- and neurotransmitter-sensitive K+ component of current with properties of TASK currents previously described in motoneurones (Sirois et al. 2000; Talley et al. 2000); this current was activated by halothane, and the halothane-activated K+ current was completely blocked by 5-HT and NA. A second component of current jointly sensitive to halothane and neurotransmitters presented properties that identified it as Ih (i.e. it was a ZD 7288-sensitive, hyperpolarization-activated inward current); halothane caused a concentration-dependent hyperpolarizing shift in Ih activation and diminished effects of 5-HT on Ih gating. These data indicate, therefore, that transmitter action can overcome anaesthetic effects on TASK channels whereas anaesthetic effects dominate those of neurotransmitters on Ih, and further suggest that anaesthetic actions mediated by these channels can be subject to modulation by prevailing neurotransmitter tone.
Joint modulation of a TASK-like resting K+ current by halothane and neurotransmitters
Hypoglossal motoneurones express a prominent leak K+ current that is sensitive to changes in extracellular pH and which has kinetic and voltage-dependent properties expected of the pH-sensitive TASK channels (Sirois et al. 2000; Talley et al. 2000). Previous studies in these motoneurones demonstrated a neurotransmitter-inhibited (Talley et al. 2000) and an anaesthetic-activated K+ current (Sirois et al. 2000), both of which were sensitive to pH and had TASK-like properties, suggesting that neurotransmitters and anaesthetics targeted the same channels. Here, we tested that possibility directly. We showed that leak K+ currents activated by anaesthetic were inhibited by neurotransmitters and that the neurotransmitter- and halothane-sensitive K+ current displayed instantaneous open-rectifier properties characteristic of the cloned TASK-1 (KCNK3) and TASK-3 (KCNK9) channels. It is noteworthy that the pH sensitivity of motoneuronal currents matches precisely that of TASK-1 (Talley et al. 2000), although it is now clear that TASK-3 transcripts are also present in rat motoneurones (Karschin et al. 2001; Talley et al. 2001; Vega-Saenz et al. 2001). Moreover, TASK-3 has similar kinetic and voltage-dependent properties and appears subject to the same type of modulation as TASK-1 (Kim et al. 2000; Meadows & Randall, 2001; E. M. Talley & D. A. Bayliss, unpublished observations). So, although the motoneuronal current appears most like TASK-1 in its pH sensitivity (Talley et al. 2000), a contribution from TASK-3 also seems likely, perhaps together with TASK-1 in a heterodimeric arrangement (Czirjak & Enyedi, 2002).
The mechanisms by which neurotransmitters inhibit TASK channels remain poorly understood. Typically, activation of Gαq-coupled receptors is involved but relevant downstream mediators have not been clearly identified (Duprat et al. 1997; Leonoudakis et al. 1998; Talley et al. 2000; Millar et al. 2000; Lopes et al. 2000; Czirjak et al. 2000, 2001). On the other hand, anaesthetic effects on TASK channels appear to be direct, since halothane can activate TASK-1 in excised membrane patches (Patel et al. 1999). Our results indicate that receptor-activated processes, by whatever mechanism, can completely inhibit the actions of halothane on motoneuronal TASK-like K+ currents. Likewise, in HEK 293 cells we found that activation of TRH-R1 receptors inhibited anaesthetic-activated TASK-1 and TASK-3 channels, in either homomeric or linked heterodimeric configurations (Talley & Bayliss, 2002). Thus, data from both native neuronal and heterologous expression systems suggest that alterations in transmitter tone can affect anaesthetic actions mediated by TASK channels. It is therefore interesting in this respect that treatments which lower noradrenaline and 5-HT levels in the CNS (e.g. reserpine, α-methyldopa and aminergic neurotoxins) increase the potency of halothane, whereas drugs that increase central monoamines (e.g. iproniazid) decrease halothane potency (Miller et al. 1968; Mueller et al. 1975).
Convergent modulation of Ih by halothane and neurotransmitters
Hypoglossal motoneurones express a prominent Ih (Bayliss et al. 1994), a subthreshold hyperpolarization-activated cationic current that can affect neuronal integrative properties in a number of ways (reviewed in Pape, 1996). In motoneurones, 5-HT causes a depolarizing shift in Ih activation via changes in cAMP (Larkman & Kelly, 1997), an effect opposite to the hyperpolarizing shift in Ih gating we observed previously with anaesthetics (Sirois et al. 1998). In the present study, we verified both these findings. Moreover, our data indicate that halothane and neurotransmitters converge on the same Ih channels since ZD 7288 blocked a component of neurotransmitter- and halothane-sensitive current (compare Fig. 3 and Fig. 4) and, more directly, because halothane diminished the neurotransmitter-induced depolarizing shift in Ih activation. Since the ZD 7288-sensitive effects were observed even at a holding potential of −60 mV, it appears that Ih contributes to a persistent current at that potential which is sensitive to both halothane and neurotransmitter (Kjaerulff & Kiehn, 2001).
The cellular/molecular mechanisms responsible for interactive effects of neurotransmitters and anaesthetics on Ih gating have yet to be determined. The HCN gene subfamily of ion channels was revealed recently as the molecular substrate for Ih (Biel et al. 1999; Santoro & Tibbs, 1999; Kaupp & Seifert, 2001), and the presence of a cyclic nucleotide binding domain in the primary structure of HCN channels provides a structural basis for the depolarizing shift in Ih gating by cAMP and by neurotransmitters that activate adenylyl cyclase (Pape, 1996). In motoneurones, HCN1 and HCN2 are the predominant Ih channel subunits expressed (Monteggia et al. 2000; Santoro et al. 2000), perhaps contributing to motoneuronal Ih either as homomeric or heteromeric channels (Chen et al. 2001; Ulens & Tytgat, 2001). Transmitter (and/or cAMP) effects on gating are likely to involve HCN2 subunits, in either a homomeric or heteromeric configuration, since HCN2 is much more sensitive to cAMP than HCN1 in homomeric channels (Biel et al. 1999; Wainger et al. 2001) and it confers cAMP sensitivity on HCN1/HCN2 heteromeric channels (Chen et al. 2001; Ulens & Tytgat, 2001). It is possible that halothane acts to shift Ih gating directly or by inhibiting adenylyl cyclase activity, thereby reducing cAMP levels. Although direct effects of anaesthetics on HCN subunits have not been described, the alternative seems unlikely since clinically relevant concentrations of halothane do not alter basal or NA-stimulated cAMP accumulation in cortical slices (Bazil & Minneman, 1989) and, in general, no consistent effects of general anaesthetics on cAMP or cGMP have been reported (Kress, 1995). Regardless of the mechanisms involved, our results suggest that halothane, and presumably other inhalation anaesthetics that modulate Ih (e.g. isoflurane, sevoflurane; Sirois et al. 2000), will shift the voltage range of Ih activation to hyperpolarized potentials and decrease the ability of transmitters to shift Ih gating into a more depolarized range.
Convergent modulation of a TASK-like current and Ih: implications for general anaesthesia
We have suggested that direct effects of anaesthetics on TASK and Ih channels in brainstem motoneurones provide a hyperpolarizing outward current that can account, at least in part, for the decreased motoneuronal excitability that accompanies anaesthetic-induced immobilization (Sirois et al. 1998, 2000). The present data, however, indicate that increased levels of aminergic (and/or peptidergic) neurotransmitters that are commonly associated with behavioural arousal and increased motor activity (e.g. 5-HT, NA and TRH) can overwhelm the anaesthetic effect. As noted above, such an interaction could contribute to differences in anaesthetic potency associated with pharmacological manipulations of neurotransmitter concentrations in the CNS (Miller et al. 1968; Mueller et al. 1975). In addition, this suggests that anaesthetic potency could also vary with the physiological adjustments in levels of these transmitters that accompany different arousal states. In this respect, however, it is important to point out that anaesthetics also activate TASK-like currents in brainstem aminergic neurons of locus coeruleus and raphe nuclei (Sirois et al. 2000; Washburn et al. 2001), and this could contribute to decreased neuronal activity and neurotransmitter release observed during anaesthesia (e.g. as described for LC neurons and NA release: Saunier et al. 1993; Ohkawa et al. 1995; Anzawa et al. 2001). Thus, although the current data predict that neurotransmitters released from state-dependent aminergic neurons will diminish TASK channel-related anaesthetic actions in motoneurones, a concomitant anaesthetic-induced decrease in activity mediated by TASK channels in those same aminergic neurons would decrease transmitter release - directly disfacilitating motoneurones while also mitigating interactive effects of the transmitters on anaesthetic-activated TASK channels.
Obviously, general anaesthesia involves more than motoneuronal depression and immobilization and the question therefore arises as to whether anaesthetic effects on similar ionic conductances elsewhere in the CNS might contribute to other properties associated with the anaesthetic state (e.g. decreased sensation, loss of consciousness). This seems likely, especially given the widespread distribution of TASK and HCN channels in the brain (Monteggia et al. 2000; Santoro et al. 2000; Talley et al. 2001). One CNS site where joint modulation by neurotransmitters and anaesthetics of TASK and HCN channels may be of particular interest is the thalamus, which regulates information flow to the cortex and participates in generation of thalamocortical oscillations associated with different states of consciousness (for review see McCormick, 1992). As in motoneurones, thalamocortical relay neurons are depolarized by neurotransmitters such as 5-HT and NA via inhibition of a leak K+ current and a depolarizing shift in Ih activation; this depolarization is responsible for the transition from tonic to bursting activity patterns in these cells, which are closely associated with wake and sleep states, respectively (McCormick, 1992). General anaesthetics, on the other hand, suppress thalamic activity in human volunteers (Alkire et al. 2000) and in vitro, thalamocortical relay cells are hyperpolarized by anaesthetics (el-Beheiry & Puil, 1989; Sugiyama et al. 1992). The hyperpolarization involves, at least in part, activation of a leak K+ current (Ries & Puil, 1999) although effects on Ih in thalamocortical neurons have not been reported. Thus, it is possible that the joint modulation by anaesthetics and neurotransmitters of leak K+ and Ih channels that we have shown here for motoneurones could be present also in thalamocortical neurons, and therein contribute to the altered consciousness and sensation that are further characteristics of the anaesthetic state.
Acknowledgments
The authors gratefully acknowledge Ms Jacqueline M. Washington for help with gas chromatography and Dr E. M. Talley for comments and discussion. This work was supported by F33 HL10271 (J.E.S.) and NS33583 (D.A.B.) from the National Institutes of Health.
REFERENCES
- Alkire MT, Haier RJ, Fallon JH. Toward a unified theory of narcosis: brain imaging evidence for a thalamocortical switch as the neurophysiologic basis of anesthetic-induced unconsciousness. Consciousness and Cognition. 2000;9:370–386. doi: 10.1006/ccog.1999.0423. [DOI] [PubMed] [Google Scholar]
- Anzawa N, Kushikata T, Ohkawa H, Yoshida H, Kubota T, Matsuki A. Increased noradrenaline release from rat preoptic area during and after sevoflurane and isoflurane anesthesia. Canadian Journal of Anesthesia. 2001;48:462–465. doi: 10.1007/BF03028309. [DOI] [PubMed] [Google Scholar]
- Bayliss DA, Viana F, Bellingham MC, Berger AJ. Characteristics and postnatal development of a hyperpolarization-activated inward current in rat hypoglossal motoneurons in vitro. Journal of Neurophysiology. 1994;71:119–128. doi: 10.1152/jn.1994.71.1.119. [DOI] [PubMed] [Google Scholar]
- Bazil CW, Minneman KP. Effects of clinically effective concentrations of halothane on adrenergic and cholinergic synapses in rat brain in vitro. Journal of Pharmacology and Experimental Therapeutics. 1989;248:143–148. [PubMed] [Google Scholar]
- Berg-Johnsen J, Langmoen IA. Mechanisms concerned in the direct effect of isofluorane on rat hippocampal and human neocortical neurons. Brain Research. 1990;507:28–34. doi: 10.1016/0006-8993(90)90517-f. [DOI] [PubMed] [Google Scholar]
- Biel M, Ludwig A, Zong X, Hofmann F. Hyperpolarization-activated cation channels: a multi-gene family. Reviews in Physiology, Biochemistry and Pharmacology. 1999;136:165–181. doi: 10.1007/BFb0032324. [DOI] [PubMed] [Google Scholar]
- Chen S, Wang J, Siegelbaum SA. Properties of hyperpolarization-activated pacemaker current defined by coassembly of HCN1 and HCN2 subunits and basal modulation by cyclic nucleotide. Journal of General Physiology. 2001;117:491–504. doi: 10.1085/jgp.117.5.491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czirjak G, Enyedi P. Formation of functional heterodimers between the TASK-1 and TASK-3 two pore domain potassium channel subunits. Journal of Biological Chemistry. 2002;277:5426–5432. doi: 10.1074/jbc.M107138200. [DOI] [PubMed] [Google Scholar]
- Czirjak G, Fischer T, Spat A, Lesage F, Enyedi P. TASK (TWIK-related acid-sensitive K+ channel) is expressed in glomerulosa cells of rat adrenal cortex and inhibited by angiotensin II. Molecular Endocrinology. 2000;14:863–874. doi: 10.1210/mend.14.6.0466. [DOI] [PubMed] [Google Scholar]
- Czirjak G, Petheo GL, Spat A, Enyedi P. Inhibition of TASK-1 potassium channel by phospholipase C. American Journal of Physiology - Cell Physiology. 2001;281:C700–708. doi: 10.1152/ajpcell.2001.281.2.C700. [DOI] [PubMed] [Google Scholar]
- Duprat F, Lesage F, Fink M, Reyes R, Heurteaux C, Lazdunski M. TASK, a human background K+ channel to sense external pH variations near physiological pH. EMBO Journal. 1997;16:5464–5471. doi: 10.1093/emboj/16.17.5464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- el-Beheiry H, Puil E. Postsynaptic depression induced by isoflurane and althesin in neocortical neurons. Experimental Brain Research. 1989;75:361–368. doi: 10.1007/BF00247942. [DOI] [PubMed] [Google Scholar]
- Franks NP, Lieb WR. Molecular and cellular mechanisms of general anaesthesia. Nature. 1994;367:607–614. doi: 10.1038/367607a0. [DOI] [PubMed] [Google Scholar]
- Hille B. Ionic Channels of Excitable Membranes. Sunderland, MA, USA: Sinauer Associates Inc.; 1992. [Google Scholar]
- Hsiao CF, Trueblood PR, Levine MS, Chandler SH. Multiple effects of serotonin on membrane properties of trigeminal motoneurons in vitro. Journal of Neurophysiology. 1997;77:2910–2924. doi: 10.1152/jn.1997.77.6.2910. [DOI] [PubMed] [Google Scholar]
- Janoff AS, Pringle MJ, Miller KW. Correlation of general anaesthetic potency with solubility in membranes. Biochimica et Biophysica Acta. 1981;649:125–128. doi: 10.1016/0005-2736(81)90017-1. [DOI] [PubMed] [Google Scholar]
- Karschin C, Wischmeyer E, Preisig-Muller R, Rajan S, Derst C, Grzeschik KH, Daut J, Karschin A. Expression pattern in brain of TASK-1, TASK-3, and a tandem pore domain K+ channel subunit, TASK-5, associated with the central auditory nervous system. Molecular and Cellular Neuroscience. 2001;18:632–648. doi: 10.1006/mcne.2001.1045. [DOI] [PubMed] [Google Scholar]
- Kaupp UB, Seifert R. Molecular diversity of pacemaker ion channels. Annual Review of Physiology. 2001;63:235–257. doi: 10.1146/annurev.physiol.63.1.235. [DOI] [PubMed] [Google Scholar]
- Kawaguchi M, Sakamoto T, Ohnishi H, Shimizu K, Karasawa J, Furuya H. Intraoperative myogenic motor evoked potentials induced by direct electrical stimulation of the exposed motor cortex under isoflurane and sevoflurane. Anesthesia and Analgesia. 1996;82:593–599. doi: 10.1097/00000539-199603000-00029. [DOI] [PubMed] [Google Scholar]
- Kim Y, Bang H, Kim D. TASK-3, a new member of the tandem pore K+ channel family. Journal of Biological Chemistry. 2000;275:9340–9347. doi: 10.1074/jbc.275.13.9340. [DOI] [PubMed] [Google Scholar]
- Kjaerulff O, Kiehn O. 5-HT modulation of multiple inward rectifiers in motoneurons in intact preparations of the neonatal rat spinal cord. Journal of Neurophysiology. 2001;85:580–593. doi: 10.1152/jn.2001.85.2.580. [DOI] [PubMed] [Google Scholar]
- Kress HG. Effects of general anaesthetics on second messenger systems. European Journal of Anaesthesiology. 1995;12:83–97. [PubMed] [Google Scholar]
- Larkman PM, Kelly JS. Ionic mechanisms mediating 5-hydroxytryptamine- and noradrenaline-evoked depolarization of adult rat facial motoneurones. Journal of Physiology. 1992;456:473–490. doi: 10.1113/jphysiol.1992.sp019347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larkman PM, Kelly JS. Modulation of IH by 5-HT in neonatal rat motoneurones in vitro: Mediation through a phosphorylation independent action of cAMP. Neuropharmacology. 1997;36:721–733. doi: 10.1016/s0028-3908(97)00021-x. [DOI] [PubMed] [Google Scholar]
- Leonoudakis D, Gray AT, Winegar BD, Kindler CH, Harada M, Taylor DM, Chavez RA, Forsayeth JR, Yost CS. An open rectifier potassium channel with two pore domains in tandem cloned from rat cerebellum. Journal of Neuroscience. 1998;18:868–877. doi: 10.1523/JNEUROSCI.18-03-00868.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopes CMB, Gallagher PG, Buck ME, Butler MH, Goldstein SA. Proton block and voltage gating are potassium-dependent in the cardiac leak channel Kcnk3. Journal of Biological Chemistry. 2000;275:16969–16978. doi: 10.1074/jbc.M001948200. [DOI] [PubMed] [Google Scholar]
- McCormick DA. Neurotransmitter actions in the thalamus and cerebral cortex and their role in neuromodulation of thalamocortical activity. Progress in Neurobiology. 1992;39:337–388. doi: 10.1016/0301-0082(92)90012-4. [DOI] [PubMed] [Google Scholar]
- Meadows HJ, Randall AD. Functional characterisation of human TASK-3, an acid-sensitive two-pore domain potassium channel. Neuropharmacology. 2001;40:551–559. doi: 10.1016/s0028-3908(00)00189-1. [DOI] [PubMed] [Google Scholar]
- Meyer H. Welche eigenschaft der anasthetica bedingt ihre narkotische wirkung? Archiv für experimentelle Pathologie und Pharmakologie. 1899;42:108–109. [Google Scholar]
- Millar JA, Barratt L, Southan AP, Page KM, Fyffe REW, Robertson B, Mathie A. A functional role for the two-pore domain potassium channel TASK-1 in cerebellar granule neurons. Proceedings of the National Academy of Sciences of the USA. 2000;97:3614–3618. doi: 10.1073/pnas.050012597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller RD, Way WL, Eger EI. The effects of alpha-methyldopa, reserpine, guanethidine, and iproniazid on minimum alveolar anaesthetic requirement (MAC) Anesthesiology. 1968;29:1153–1158. doi: 10.1097/00000542-196811000-00012. [DOI] [PubMed] [Google Scholar]
- Monteggia LM, Eisch AJ, Tang MD, Kaczmarek LK, Nestler EJ. Cloning and localization of the hyperpolarization-activated cyclic nucleotide-gated channel family in rat brain. Brain Research. Molecular Brain Research. 2000;81:129–139. doi: 10.1016/s0169-328x(00)00155-8. [DOI] [PubMed] [Google Scholar]
- Mueller RA, Smith RD, Spruill WA, Breese GR. Central monaminergic neuronal effects on minimum alveolar concentrations (MAC) of halothane and cyclopropane in rats. Anesthesiology. 1975;42:143–152. doi: 10.1097/00000542-197502000-00006. [DOI] [PubMed] [Google Scholar]
- Munsch T, Pape HC. Modulation of the hyperpolarization-activated cation current of rat thalamic relay neurones by intracellular pH. Journal of Physiology. 1999;519:493–504. doi: 10.1111/j.1469-7793.1999.0493m.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicoll RA, Madison DV. General anesthetics hyperpolarize neurons in the vertebrate central nervous system. Science. 1982;217:1055–1057. doi: 10.1126/science.7112112. [DOI] [PubMed] [Google Scholar]
- Ohkawa H, Kushikata T, Satoh T, Hirota K, Ishihara H, Matsuki A. Posterior hypothalamic noradrenaline release during emergence from sevoflurane anesthesia in rats. Anesthesia and Analgesia. 1995;81:1289–1291. doi: 10.1097/00000539-199512000-00029. [DOI] [PubMed] [Google Scholar]
- Pancrazio JJ, Park WK, Lynch C., III Inhalational anesthetic actions on voltage-gated ion currents of bovine adrenal chromaffin cells. Molecular Pharmacology. 1993;43:783–794. [PubMed] [Google Scholar]
- Pape HC. Queer current and pacemaker: the hyperpolarization-activated cation current in neurons. Annual Review of Physiology. 1996;58:299–327. doi: 10.1146/annurev.ph.58.030196.001503. [DOI] [PubMed] [Google Scholar]
- Patel AJ, Honore E, Lesage F, Fink M, Romey G, Lazdunski M. Inhalational anesthetics activate two-pore-domain background K+ channels. Nature Neuroscience. 1999;2:422–426. doi: 10.1038/8084. [DOI] [PubMed] [Google Scholar]
- Rekling JC, Funk GD, Bayliss DA, Dong X-W, Feldman JL. Synaptic control of motoneuronal excitability. Physiological Reviews. 2000;80:767–852. doi: 10.1152/physrev.2000.80.2.767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ries CR, Puil E. Ionic mechanism of isoflurane's actions on thalamocortical neurons. Journal of Neurophysiology. 1999;81:1802–1809. doi: 10.1152/jn.1999.81.4.1802. [DOI] [PubMed] [Google Scholar]
- Santoro B, Chen S, Luthi A, Pavlidid P, Shumyatsky GP, Tibbs GR, Siegelbaum SA. Molecular and functional heterogeneity of hyperpolarization-activated pacemaker channels in the mouse CNS. Journal of Neuroscience. 2000;20:5264–5275. doi: 10.1523/JNEUROSCI.20-14-05264.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santoro B, Tibbs GR. The HCN gene family: Molecular basis of the hyperpolarization-activated pacemaker channels. Annals of the New York Academy of Sciences. 1999;868:741–764. doi: 10.1111/j.1749-6632.1999.tb11353.x. [DOI] [PubMed] [Google Scholar]
- Saunier CF, Akaoka H, De La Chapelle B, Charlety PJ, Chergui K, Chouvet G, Buda M, Quintin L. Activation of brain noradrenergic neurons during recovery from halothane anesthesia. Persistence of phasic activation after clonidine. Anesthesiology. 1993;79:1072–1082. doi: 10.1097/00000542-199311000-00026. [DOI] [PubMed] [Google Scholar]
- Sirois JE, Lei Q, Talley EM, Lynch C, III, Bayliss DA. The TASK-1 two-pore domain K+ channel is a molecular substrate for neuronal effects of inhalation anesthetics. Journal of Neuroscience. 2000;20:6347–6354. doi: 10.1523/JNEUROSCI.20-17-06347.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sirois JE, Pancrazio JJ, Lynch C, III, Bayliss DA. Multiple ionic mechanisms mediate inhibition of rat motoneurones by inhalation anaesthetics. Journal of Physiology. 1998;512:851–862. doi: 10.1111/j.1469-7793.1998.851bd.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugiyama K, Muteki T, Shimoji K. Halothane-induced hyperpolarization and depression of postsynaptic potentials of guinea pig thalamic neurons in vitro. Brain Research. 1992;576:97–103. doi: 10.1016/0006-8993(92)90613-e. [DOI] [PubMed] [Google Scholar]
- Takahashi T, Berger AJ. Direct excitation of rat spinal motoneurones by serotonin. Journal of Physiology. 1990;423:63–76. doi: 10.1113/jphysiol.1990.sp018011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takenoshita M, Steinbach JH. Halothane blocks low-voltage-activated calcium currents in rat sensory neurons. Journal of Neuroscience. 1991;11:1404–1412. doi: 10.1523/JNEUROSCI.11-05-01404.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talley EM, Bayliss DA. Modulation of TASK-1 (Kcnk3) and TASK-3 (Kcnk9) potassium channels: volatile anesthetics and neurotransmitters share a molecular site of action. Journal of Biological Chemistry. 2002 doi: 10.1074/jbc.M200502200. in the Press. [DOI] [PubMed] [Google Scholar]
- Talley EM, Lei Q, Sirois JE, Bayliss DA. TASK-1,a two-pore domain K+ channel, is modulated by multiple neurotransmitters in motoneurons. Neuron. 2000;25:399–410. doi: 10.1016/s0896-6273(00)80903-4. [DOI] [PubMed] [Google Scholar]
- Talley EM, Solórzano G, Lei Q, Kim D, Bayliss DA. CNS distribution of members of the two-pore-domain (KCNK) potassium channel family. Journal of Neuroscience. 2001;21:7491–7505. doi: 10.1523/JNEUROSCI.21-19-07491.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulens C, Tytgat J. Functional heteromerization of HCN1 and HCN2 pacemaker channels. Journal of Biological Chemistry. 2001;276:6069–6072. doi: 10.1074/jbc.C000738200. [DOI] [PubMed] [Google Scholar]
- Vega-Saenz DM, Lau DH, Zhadina M, Pountney D, Coetzee WA, Rudy B. Kt3. 2 and Kt3.3, two novel human two-pore K+ channels closely related to TASK-1. Journal of Neurophysiology. 2001;86:130–142. doi: 10.1152/jn.2001.86.1.130. [DOI] [PubMed] [Google Scholar]
- Viana F, Gibbs L, Berger AJ. Double- and triple-labeling of functionally characterized central neurons projecting to peripheral targets studied in vitro. Neuroscience. 1990;38:829–841. doi: 10.1016/0306-4522(90)90075-f. [DOI] [PubMed] [Google Scholar]
- Wainger BJ, Degennaro M, Santoro B, Siegelbaum SA, Tibbs GR. Molecular mechanism of cAMP modulation of HCN pacemaker channels. Nature. 2001;411:805–810. doi: 10.1038/35081088. [DOI] [PubMed] [Google Scholar]
- Washburn CE, Sirois JE, Talley EM, Guyenet PG, Bayliss DA. Serotonergic raphe neurons express TASK channel transcripts and a TASK-like pH- and halothane-sensitive K+ conductance. Journal of Neuroscience. 2002;22:1256–1265. doi: 10.1523/JNEUROSCI.22-04-01256.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou HH, Jin T-T, Qin B, Turndorf H. Suppression of spinal cord motoneuron excitability correlates with surgical immobility during isofluorane anesthesia. Anesthesiology. 1998;88:955–961. doi: 10.1097/00000542-199804000-00015. [DOI] [PubMed] [Google Scholar]
- Zhou HH, Mehta M, Leis AA. Spinal cord motoneuron excitability during isofluorane and nitrous oxide anesthesia. Anesthesiology. 1997;86:302–307. doi: 10.1097/00000542-199702000-00005. [DOI] [PubMed] [Google Scholar]
- Zong X, Stieber J, Ludwig A, Hofmann F, Biel M. A single histidine residue determines the pH sensitivity of the pacemaker channel HCN2. Journal of Biological Chemistry. 2001;276:6313–6319. doi: 10.1074/jbc.M010326200. [DOI] [PubMed] [Google Scholar]


