Abstract
The effects of cyclic nucleotide monophosphate (cNMP) in the ciliary cytoplasm of the olfactory receptor cell were examined by using photolysis of caged cNMP loaded from the whole-cell patch clamp pipette. Illumination of the cilia induced an inward current at −50 mV. The current amplitude was voltage dependent and the polarity was reversed at +10 mV. The amplitude of the light-induced current was dependent on both light intensity and duration. The intensity-response relation was fitted well by the Hill equation with a coefficient (nH) of 4.99 ± 2.66 (mean ± s.d., n = 19) and the duration-response relation with a coefficient of 4.03 ± 1.43 (n = 17). The activation time course of adenylyl cyclase was estimated by comparing the light-induced response with the odorant-induced response. Adenylyl cyclase was activated approximately 260 ms later from the onset of the odorant-stimulation. The light-induced current developed very sharply. This could be explained by the sequential openings of cAMP-gated and Ca2+-activated Cl− channels. At +100 mV, where Ca2+ influx is expected to be very small, the current rising phase became less steep. When the cells were stimulated by long steps of either odour or light, the odorant-induced current showed stronger decay than the light-induced response. This observation suggests that the molecular system regulating desensitization is situated upstream of cAMP production.
Olfaction begins at the cilia of the receptor cell in the olfactory epithelium. Odorant binding to receptor proteins on the ciliary surface activates an enzymatic cascade involving cAMP (for review, see Bakalyar & Reed, 1991; Breer & Boekhoff, 1992; Ronnett & Snyder, 1992; Restrepo et al. 1996; Restrepo & Schild, 1998; Firestein, 2001). This enzymatic cascade leads to the opening of two types of ionic channels: cyclic nucleotide-gated (CNG) cationic channels and Ca2+-activated Cl− channels (for review, see Gold & Nakamura, 1987; Firestein, 1992; Reed, 1992; Kleene, 1993; Kurahashi & Yau, 1993; Restrepo et al. 1996). The resulting receptor potential is slow and graded with odorant concentration (Trotier & MacLeod, 1983; Kurahashi, 1989; Firestein et al. 1993), then encoded into spike trains that transmit olfactory information to the brain (see e.g. Imanaka & Takeuchi, 2001).
The transduction system is also modulated by Ca2+ influx through the CNG channel, leading to olfactory adaptation (Kurahashi & Shibuya, 1990; Kurahashi & Menini, 1997; Reisert & Matthews, 1999). The most likely target of cytoplasmic Ca2+ is the CNG channel (Chen & Yau 1994; Kurahashi & Menini, 1997). Other Ca2+-mediated mechanisms of olfactory adaptation include a feedback to the receptor protein (Breer & Boekhoff, 1992), a facilitation of phosphodiesterase (PDE; Borisy et al. 1992) and a feedback to adenylyl cyclase (Wei et al. 1998; Zufall et al. 1999). The recovery from adaptation (reduction of cytoplasmic Ca2+) seems to depend on a Na+-dependent extrusion system (Reisert & Matthews, 1998).
Although the mechanism of olfactory transduction is now qualitatively well understood, quantitative information about the system is still limited. A technical limitation for the study of olfactory transduction is that the transduction takes place at the fine cilia, which makes experimental manipulations extremely difficult. To overcome this difficulty, we employed, in the present work, caged cAMP to replace the action of adenylyl cyclase, without activating odorant-induced enzymatic cascades. The effects of intra-ciliary cyclic nucleotide monophosphates (cNMPs) were examined in cells that retained their intrinsic molecular cascades. This work will present a number of properties of adenylyl cyclase and cytoplasmic cAMP in the olfactory cilia. Furthermore, we found that the response waveforms to a long step of photolysis and odorant stimulation, respectively, were remarkably different; the odorant-induced current showed a more rapid decay than the light-induced response. This observation indicates that the molecular system regulating desensitization during long odorant stimulation exists upstream of the cAMP production site in addition to the feedback to CNG channels.
METHODS
Preparation
Olfactory receptor cells were dissociated enzymatically from the olfactory epithelium of the newt, Cynops pyrrhogasster. Dissociation protocols have been described elsewhere (Kurahashi, 1989). The experiments were performed under the latest ethical guidelines for animal experimentation at Osaka University, based on international experimental animal regulations. The animal was chilled on ice and double pithed. After decapitation, olfactory epithelia were removed and then incubated for 5 min at 37 °C in a solution which contained 0.1 % collagenase (Sigma Chemical Co., St Louis, MO, USA) with no added Ca2+ and Mg2+; the composition was as follows (mm): 110 NaCl, 3.7 KCl, 10 Hepes, 15 glucose, 1 pyruvate. All solutions were adjusted to pH 7.4 with NaOH. The tissue was rinsed three times with a normal Ringer solution (mm): 110 NaCl, 3.7 KCl, 3 CaCl2, 1 MgCl2, 10 Hepes, 15 glucose, 1 pyruvate, and triturated. Isolated cells were plated on the concanavalin A-coated glass coverslip. Cells were maintained at 4 °C until use. In the present study, we selected olfactory receptor cells having more than five cilia.
Recording procedures
Membrane currents were recorded with the whole-cell recording configuration (Hamill et al. 1981). Patch pipettes were made of borosilicate tubing with filament (outer diameter, 1.5 mm; World Precision Instruments, Germany) by using a two-stage vertical patch electrode puller (PP-830, Narishige Scientific Instruments, Tokyo, Japan). The recording pipette was filled with a solution containing 119 mm CsCl (pH was adjusted to 7.4 with Hepes buffer) to suppress K+ channels which cause large current fluctuations. Normal Ringer solution (for composition, see above) was used as the external solution for all recordings. The pipette resistance was 10-15 MΩ. The recording pipette was connected to a patch clamp amplifier (Axopatch 1D, Axon Instruments, Foster City, CA, USA). The signal was low-pass filtered at 0.5 kHz, digitized by an A/D converter (sampling frequency, 1 kHz) connected to a computer (PC9821, NEC, Japan). Simultaneously, signals were monitored on an oscilloscope and recorded on a chart recorder. Light and odour stimuli and the data acquisition were regulated by the same computer using an original program. The results were analysed by an offline computer and plotted by using Microcal Origin 6.0 software (OriginLab Corporation, MA, USA). For curve drawings, data sampled at 1/16 kHz were used. Experiments were performed at room temperature (23-25 °C).
Photolysis of caged compounds
Caged cAMP and caged cGMP (Dojin, Japan) were used in the present study. As described before (Kurahashi & Menini, 1997), we saw no great difference between cAMP and cGMP responses. The only difference was that a lower intensity of light was sufficient to evoke responses by caged cGMP than to evoke responses of similar amplitude by caged cAMP, presumably due to a higher sensitivity of CNG channels to cGMP than to cAMP. Caged cNMP was dissolved in dimethylsulfoxide (DMSO) and stored at −20 °C under complete darkness. Under this condition caged cNMP can be stored up to 180 days without degradation. These stocks were diluted by Cs+-containing pipette solution prior to each experiment, and the solution was filled into the recording pipette. After the establishment of the whole-cell recording configuration, caged compounds were loaded to the cell interior by free diffusion. Since the diffusion coefficient of cAMP within cilia is 2.7 × 10−6 cm2 s−1 (Chen et al. 1999), several tens of seconds were needed for full and uniform distribution of the caged substances within the cell. For one dimensional diffusion, for instance, the average time for cAMP molecules to cover the full length of cilia (20 μm) is 0.56 s derived from the equation x = 2 (Dt/π)1/2, where x is the distance, D is diffusion coefficient and t is the time. During experiments, cell dialysis was confirmed by a Cs+-induced suppression of K+ currents. The ultraviolet (UV) component from a xenon lamp (100 W, XPS-100, Nikon, Japan) was used for photolysis. The timing and duration of light illumination were controlled by a computer-regulated magnetic shutter (Copal, Japan). The intensity of the light was controlled by a neutral-density wedge filter having approximately a two log-unit range. The wedge filter was connected to a pulse motor (UPK569H-NAC, 5 phases, Oriental Motor, Japan). With this system, the angle of the filter can be specified by the number of TTL pulses applied to the motor (1 step = 0.36 deg, range: 0-746 steps). The light intensity was monitored by a photodiode (maximum absorption at 400 nm. G5842, Hamamatsu Photonics, Japan) and was correlated to the angle of the wedge filter. The relation between the step (x) of the pulse motor and light intensity (S) was fitted by a single exponential function: S = kexp(0.0053x), where k is the constant. In the present work, the maximum light intensity (at 746 step position) was referred as 1, resulting in the value of k being 0.019. To avoid vibration from movement of the shutter or the wedge filter, the light source, shutter and wedge filter were mechanically isolated from the microscope, and the light stimulus was guided by a quartz fibre connected to the entrance of the epi-fluorecence system, focused on the cell under recording (objective, 60 ×). By adjusting the diaphragm, the illuminated area was set at 40 μm in diameter, covering only the ciliary region. Light stimuli were applied with > 20 s intervals in order to avoid adaptation of the system and depletion of the caged compound.
Odorant stimulation
Citralva (donated by Takasago Co., Japan) was dissolved in bath solution at 1 mm concentration and applied to the cilia from a puffer pipette having a tip diameter of 1 μm. The tip of the puffer pipette was situated 20 μm from the tip of the dendrite. The pressure (maximum 150 kPa) was controlled by a computer-regulated pressure ejection system developed in our laboratory (Ito et al. 1995). There was a time lag between the actual chemical stimulation of the cell and the application of TTL pulse to the magnetic valve. This time lag, being 60 ms on average, was estimated from the liquid junction current caused by the same application system.
RESULTS
The response induced by the photolysis of caged cyclic nucleotides
Illumination of cilia induced an inward current from all tested cells loaded with 1 mm caged cAMP or cGMP, at −50 mV. With strong illumination, the response had a latency of approximately 100 ms (100.1 ± 28.7 ms, mean ± s.d., n = 31, range 60-156 ms), probably reflecting the time needed for the cytoplasmic concentration of cAMP ([cAMP]i) to become high enough to drive the transduction channels with high cooperativity (see later, also see Lowe & Gold, 1993a). The response latency to odorant stimulation is much longer (200-300 ms, Firestein & Werblin, 1987; Kurahashi, 1989). Thus, it appears that the odorant-activated enzymatic activity takes several hundred milliseconds to accumulate cAMP to the effective concentration (see also later).
The amplitude and polarity of the light-induced current were dependent on the holding voltage (Vh) (Fig. 1). At negative holding voltages, the current polarity was inward. Current reversal occurred at approximately +10 mV. The current-voltage (I-V) relation showed a slight outward rectification (Fig. 1B). The current range, the reversal potential and the shape of the I-V relation were all very similar to those reported for the odorant-activated current measured in the same species of animal (Kurahashi, 1989), and also to the caged cAMP responses observed in the tiger salamander (Lowe & Gold, 1993a).
Figure 1. Voltage dependence of the current response induced by flash photolysis of 1 mm caged cGMP.
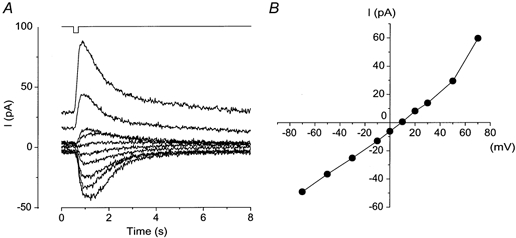
A, whole-cell current recorded from the olfactory receptor cell. Holding voltage (Vh) was changed from −70 to +70 mV. Downward deflection of the upper trace indicates the timing and duration of the light stimulation (intensity, 0.45; duration, 200 ms). B, current-voltage (I-V) relation of the light-induced response. Data from A.
The response amplitude was dependent on both the intensity and duration of light illumination (Fig. 2). With a fixed duration of 200 ms, an increase in the light intensity increased the current amplitude monotonically. One noticeable feature was that the slope of rising phase became steeper as the light intensity was increased (Fig. 2A, inset). The relation between response amplitude and intensity could be fitted by the Hill equation with high cooperativity (Hill coefficient, nH = 4.99 ± 2.66, n = 19), as has been reported in the olfactory receptor cell of the rat (Lowe & Gold, 1993b).
Figure 2. Intensity and duration dependence of the light-induced response.
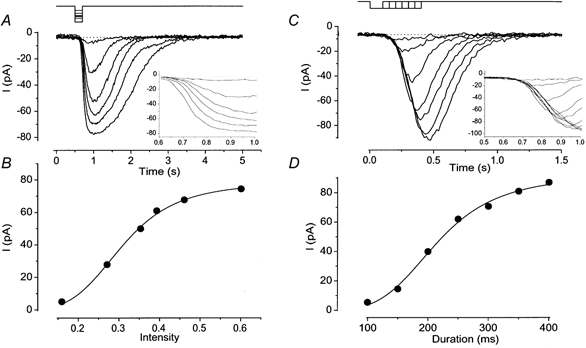
A, membrane currents were recorded from a cell loaded with 1 mm caged cGMP. Light intensity was varied, while the duration was kept constant (200 ms). Downward deflections of the upper trace indicate the timing and duration of the light stimulation. Vh =−50 mV. Inset, expanded time course for the current rising phase. B, intensity-response relation of the light-induced current. Peak amplitudes of responses obtained in A were plotted against the intensity of light. The smooth line was drawn by a least square fitting of the data points by the Hill equation, I =Imax×SnH/(SnH+ K1/2nH), where I is the current, S is the intensity of light, K1/2 is the half-maximum intensity and nH is the Hill coefficient. Imax = 78.2 pA, K1/2 = 0.31 and nH = 4.58. C, light duration was changed, while the intensity was kept constant (0.27). Different cell from A. Vh =−50 mV. Inset, expanded time course for the current rising phase. D, duration-response relation of the light-induced current. Peak amplitudes of the responses obtained in C were plotted against the duration of light stimuli. The smooth line was drawn by a least square fitting of the data points by the Hill equation with Imax = 89.9 pA, K1/2 = 212.3 ms and nH = 4.34.
The amplitude of current responses was also dependent on the duration of the light stimuli at fixed intensity (Fig. 2C). The relation between current amplitude and light duration was also fitted by the Hill equation (Fig. 2D), with a large Hill coefficient (nH = 4.03 ± 1.43, n = 17). In contrast to the experiment in Fig. 2A, however, the slope of the rising phase was constant (Fig. 2C, inset).
Equivalent contributions of intensity and duration on the response amplitude
The experiment in Fig. 2 showed that the intensity and duration dependences expressed similar cooperativities. In the experiment in Fig. 3, we examined the light-induced currents recorded under conditions in which the light intensity and duration were changed but with the time integral of light exposure kept constant. As expected, the amplitude of the light-induced current was exactly identical. The slope of the response rising phase was steeper when the light intensity became stronger. This observation indicates that the cNMP produced by the photolysis varies linearly with the total amount of light absorption, within the time scale examined here.
Figure 3. Responses induced by the same amount of light stimuli.
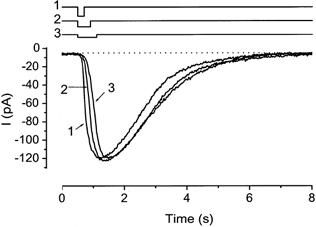
Duration, 200, 400 and 600 ms. Intensity, 0.46, 0.23 and 0.15. Downward deflections of upper traces indicate the timing and duration of the light stimulation. Numbers indicate the sequences of the experiments. The cell was loaded with 1 mm caged cGMP. Vh =−50 mV. Note that the peak values are the same in the various conditions.
Estimation for the time course of odorant-activated adenylyl cyclase
By comparing the odorant-induced and the light-evoked responses, we attempted to estimate the kinetics of the cytoplasmic cAMP production due to the odorant-activated adenylyl cyclase. After recording an odorant-induced response, we adjusted the light intensity and duration in order to produce a response that matched the odorant-induced response in waveform (Fig. 4). One would have thought that there were many combinations of the duration and intensity that could all reproduce the odorant-induced current, because these parameters are interchangeable (Fig. 2). In practice, however, the condition of light stimulation was specified exactly as follows. First, the intensity was set so as to follow the rising phase of the odorant-induced response. As demonstrated above, the rising phase is determined solely by the intensity, but not by the duration (compare Fig. 2A and C). Then the amplitude was adjusted by regulating the duration. The currents induced by both odorant and light stimulation showed a good agreement (Fig. 4), except that the falling phase of the light-induced current was a little faster (n = 4).
Figure 4. Determination of light condition causing a response identical to odorant response.
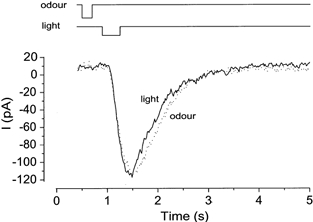
First, odorant stimulation (1 mm citralva) was applied to induce approximately 90 % saturating response. Then, the intensity, timing and duration of light stimulation were adjusted to induce the same waveform of the response. The cell was loaded with 1 mm caged cGMP. Downward deflections of the upper trace indicate the timing and the duration of the light and odour stimuli. Light intensity, 0.08. Vh =−50 mV.
The onset of odour stimulation was faster than the opening of the light shutter by 322 ± 73 ms (n = 4). By subtracting 60 ms needed for the odorant to move from the pipette to the cell, the real time lag was estimated as 260 ms. This time lag was considered to be the time period used for protein reactions or interactions situated upstream to adenylyl cyclase.
To get a good match between the light-evoked and odorant-induced responses, the duration of light stimulation had always to be set longer than that for odorant stimulation. This finding indicates that adenylyl cyclase activity lasts longer than the period that the olfactory receptor cell is exposed to odour. There are three proteins that may be responsible for this prolonged activation: the receptor, G-protein and adenylyl cyclase. However, our experimental protocol did not allow us to identify which of these was most responsible for time amplification.
Rising phase of the cNMP-induced response when stimulated by long steps
So far, the responses were investigated by using brief pulses. With long steps, however, the response behaviour was remarkably changed, presumably due to the delayed expression of feedback system.
In the experiment in Fig. 5, a light step was used to stimulate the cell to induce a current. By stimulation, an inward current developed monotonically to a peak within a few seconds and showed little desensitization thereafter. An aforementioned experiment (Fig. 2C and D) led us to examine whether the rising phase of the light-induced current was fitted by the Hill equation using a similar high nH value, because termination of illumination performed at the experiment in Fig. 2C should be equivalent to the time interruption for the current developing phase. As illustrated in Fig. 5, the rising phase could be fitted by the Hill equation and the averaged nH obtained from the same kind of experiments was 4.22 ± 1.11 (n = 7). In two out of seven preparations, however, the initial part of the current responses showed a remarkable deviation from the Hill fitting when we used a large nH value; this part was fitted instead with a smaller nH value (see e.g. Fig. 8B, arrow). This could be explained if one expects that, at a small current, the Ca2+-activated Cl− channel does not contribute to the net current because of the limited rate of Ca2+ entry; the CNG channel alone expresses a Hill coefficient of approximately two (Kurahashi & Kaneko, 1991).
Figure 5. Rising phase of the current induced by the photolysis.
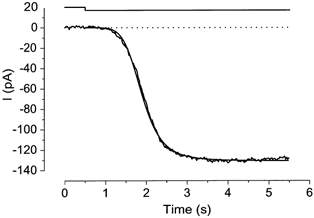
Fitting of the rising phase by the Hill equation. Downward deflection of the upper trace indicates the light step. The cell was loaded with 1 mm caged cAMP. Light intensity, 0.12. Least square fitting was performed on current after the onset of light stimulation. The best fitting was obtained with Imax = 130.6 pA, K1/2 = 1.39 s and nH = 5.67. Vh =−50 mV.
Figure 8. Current reduction from the theoretical curves during a prolonged stimulation.
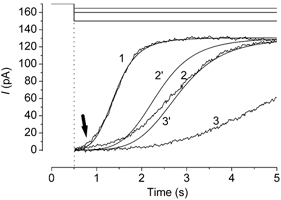
Responses were induced by three different intensities of light steps. The upward deflections of current traces indicate the inward current. Numbers indicate the sequence of the experiments. The cell was loaded with 1 mm caged cAMP. Light intensity, 0.12, 0.07 and 0.06. Vh =−50 mV. Current reduction was observed during long light steps. The current response (1) was fitted by the Hill equation (nH = 5.73, K1/2 = 1.41 s). Two other lines were drawn according to an assumption that the production rate for cAMP increases in proportion to the light intensity (therefore, K1/2 = 2.35 s and 2.82 s. Time zero corresponds to the onset of illumination. An arrow indicates a deviation from the Hill fitting with high nH.
In the experiment of Fig. 2A, the intensity-response relation expressed a large nH value and the high cooperativity is known to be achieved by the additive effect of Cl− and cNMP-induced currents (Lowe & Gold, 1993b). It is therefore likely that the high cooperativity observed with the Hill fitting for the current rising phase is also responsible for the sequential openings of CNG channels and Cl− channels. To confirm this, we examined the Hill fitting for the developing phase at +100 mV where Ca2+ influx is expected to be much slower than that of −50 mV. At +100 mV, the outward current developed more slowly and the time course was fitted using a smaller cooperativity (nH = 2.55 ± 0.10, n = 3; Fig. 6). This result also indicates the possibility that the rising phase of the current response reflects the time course of [cAMP]i increase. In the present study, however, no systematic study was made on this subject.
Figure 6. Voltage dependence of the rising phase.
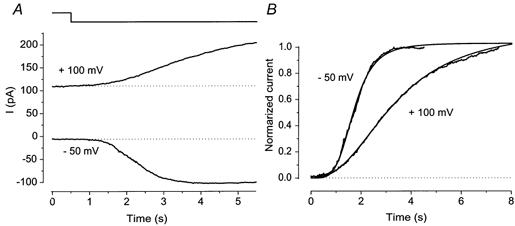
A, light-induced currents obtained at −50 mV and +100 mV. Downward deflection of the upper trace indicates light steps. Note that the rising phase at +100 mV is remarkably slower than that obtained at −50 mV. B, voltage dependence of cooperativity. Current records shown in A were fitted by the Hill equation with nH = 4.52 (-50 mV) and nH = 2.49 (+100 mV). Time zero corresponds to the onset of illumination. The rising phase illustrated in Fig. 1 seems to be constant throughout the varied holding voltages. Note, however, that the experiment in Fig. 1 was carried out using a very bright flash which causes a rapid increase of response, and it was done only up to +50 mV.
Desensitization of responses induced by light and odorant steps
It has been widely accepted that odorant-induced responses show a remarkable reduction in amplitude during prolonged odorant application (Kurahashi & Shibuya, 1990; Zufall et al. 1991). When the cell was stimulated by a prolonged light stimulation, however, the maximum current stayed at a steady level, showing little desensitization (Fig. 5). The experiment of Fig. 7 was carried out to compare the time courses of the odorant-induced and light-induced currents to long duration stimuli in the same cell. As illustrated, the response decay was more dramatic when induced by the odorant. This observation indicates that the desensitization system exists upstream to adenylyl cyclase. This will be described further in the Discussion.
Figure 7. Differences between odour and light responses to prolonged stimuli.
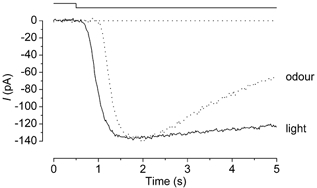
Continuous and dashed lines represent responses induced by light and odorant (1 mm citralva), respectively. Downward deflection of the upper trace indicates both the light and odour stimuli. Light intensity was 0.27. The cell was loaded with 1 mm caged cGMP.
Since the waveform of light-induced current showed little desensitization, one might think that the response even lacked adaptation (for definition, see Discussion), which is expressed by a Ca2+-mediated feedback to CNG channels at negative potential. However, the experiment in Fig. 8 suggests that the response induced by prolonged light stimulation does receive feedback regulation. The cell was stimulated by a light step of different intensities. The response developed within a few seconds would not drive a feedback system, because adaptation observed in the odorant response starts after such time period (see e.g. Kurahashi & Shibuya, 1990). To support this idea, the current developing phase was actually fitted to the Hill equation with a high cooperativity. Using the same parameter of nH, response time courses induced by dimmer light stimuli were predicted based on an assumption that the cAMP production rate was linearly accelerated in proportion to the light intensity. In other words, in this assumption, K1/2 of the Hill fitting would have been increased depending on the reduction of the light intensity. Actually, the current responses induced by dimmer lights developed more slowly than those predicted by this assumption. It is highly likely that the current reduction is, at least in part, due to the negative feedback to the CNG channel (Kleene, 1993; Chen & Yau, 1994; Kurahashi & Menini, 1997). On receiving the feedback, more CNG channels are closed than expected. Therefore, reduced Ca2+ influx will further facilitate the reduction of Cl− current.
DISCUSSION
In the present study we investigated the effect of cNMP on the cytoplasm of olfactory cilia using the photolysis of caged compounds. By comparing the responses evoked by light with odorant responses, cytoplasmic behaviour of cAMP and adenylyl cyclase activities during the odorant perception were estimated. Furthermore, with long steps of stimulation, it was found that the odorant-induced current showed faster decay than the light-induced response. It seems likely that the mechanism causing desensitization during the prolonged stimulation is diverse.
Molecular site expressing the non-linear amplification
It has been widely accepted that the olfactory transduction system shows a high cooperativity establishing signal amplification (Firestein et al. 1993; Lowe & Gold, 1993b; Kurahashi & Menini, 1997). Since in the present study cAMP responses were shown to express a similarly high cooperativity to that of the odorant response, the non-linear boosting is likely to be achieved solely at the channel level (by the combination of both CNG channels and Ca2+-activated Cl− channels; see also Lowe & Gold, 1993b), not by the enzymatic cascade locating upstream to adenylyl cyclase. The signalling process between the receptor and adenylyl cyclase would be almost linear. Recently, Imanaka & Takeuchi (2001) have shown that the spike initiation site also expresses a linear function. Throughout the signal transduction process in the olfactory receptor cell, only the channel level may be responsible for the establishment of non-linear amplification.
Kurahashi & Menini (1997) have reported that Ca2+ feedback expressing adaptation is mainly targeting the CNG channel. Therefore, it might be reasonable to design a feedback system attacking the part underlying a strong amplification.
Olfactory adaptation and desensitization
It was found that the response decay during the prolonged stimulation differed between odorant-induced and light-induced responses; the odorant responses decayed more rapidly than the light-induced response. These differences indicate that desensitization of odorant response occurs upstream to adenylyl cyclase. Seemingly, this result conflicts with the idea suggested by Kurahashi & Menini (1997) who showed that olfactory adaptation occurs at the level of the CNG channel. However, there are a number of points to be considered in further detail as follows.
First, the adaptation and desensitization observed here might not be identical. The sensory adaptation is defined as a shift of the dose-response curve (change in K1/2), which was designed to establish two competitive functions: signal amplification and wide dynamic range. In contrast, desensitization during a long step includes all decaying phenomena. We cannot specify if the response decay is due to the change in K1/2 or Imax on the basis of the experiment using a long stimulation step. It will be necessary to examine whether or not the response decay due to the negative feedback carried out locally upstream to adenylyl cyclase accompanies the change in K1/2. At this point, however, this experiment would be extremely difficult because the decay during the odorant step would have already included a negative feedback to the CNG channel expressing adaptation (see e.g. Fig. 8).
Second, the trigger of feedback systems may be dependent on the stimulus duration. The experiments by Kurahashi & Menini (1997) were performed only with brief pulses. There is a possibility that the feedback system targeting upstream points from adenylyl cyclase works only when the cell was stimulated by a long stimulation step. In fact, Breer & Boekhoff (1992) have shown that the receptor desensitizes during the prolonged stimulation. In addition, it was reported that long odorant pulses cause negative feedback to adenylyl cyclase, but brief pulses do not (Zufall et al. 1999). It will be worthwhile to examine desensitization with various durations of stimuli.
Introduction of cAMP from a whole-cell pipette has been performed previously (Kurahashi, 1990). The waveform of the cAMP-induced current actually expressed a remarkable decay during the dialysis, while photolysis responses presented here did not show significant decay. At this point, we do not have a good explanation for this difference. However, there are three main advantages of using caged compound rather than using cAMP introduction from the whole-cell patch pipette. (1) In photolysis experiments, we are able to stimulate cells repeatedly. (2) We could directly compare the responses induced by odorants and cAMP in the same cell. (3) We could actually adjust the rising phase of responses in both conditions, which allowed us to examine the response kinetics under similar cAMP production rates and time courses in both odorant and photolysis experiments. It is therefore very likely that the decaying time courses observed in the photolysis experiments are physiologically more reliable than those obtained with cAMP introduction from the whole-cell pipette.
Initiation and termination of adenylyl cyclase activities facilitated by the odorant pulse
The rising phase of the light-induced current could be adjusted to the same waveform as that of the odorant-induced current by using the step change of light stimulation. Because the cAMP production rate facilitated by the photolysis is identical to the activity of adenylyl cyclase, this result may indicate that adenylyl cyclase activity increases to a peak value suddenly, and not by a gradual increase. The falling phase of the light-induced current was more rapid than that of the odorant-induced current when the light was terminated suddenly. This observation indicates that the termination of adenylyl cyclase activity is gradual.
In the present study, we investigated several features of adenylyl cyclase and cytoplasmic cAMP change in the olfactory receptor cell during their natural responses. In the photoreceptor cell, enzymatic reactions during the light responses are well investigated. A model proposed by Lamb & Pugh (1992; see also Pugh & Lamb, 1993) has reported that PDE (actually comparable to adenylyl cyclase in the olfactory receptor cell as an effector enzyme) shows a very short (10-20 ms) delay from the activation of receptor protein (Pugh & Lamb, 1993), and that the activity increases linearly with time. The present study does not exclude the possibility that the olfactory system shows the same enzymatic reaction as the photoreceptor cell. It would be worthwhile to investigate how the waveforms of photolysis-induced currents in olfactory cells are changed when we use ramp stimuli.
Furthermore, the rising phase of the olfactory response consists of two kinds of ionic components. One is cationic which flows through the CNG channel, another is anionic passing through the Ca2+-dependent Cl− channel. Combinations of the transduction current make a very rapid rising phase. Therefore, even a small change in [cAMP]i would give rise to a big change in the absolute current. Because of this, experiments like that shown in Fig. 4 may still have room for further investigation. The present study showed that this rapid activation is due to the additive effect of the Cl− current onto the cation current. Blocking the Cl− component may be a useful tool to investigate the transduction machinery more precisely.
Caged compounds in the olfactory cilia
In the present study, we applied caged cNMP to the olfactory receptor cell. It has been reported that the dynamic range of cNMP concentration driving CNG channels in the olfactory receptor cell is from approximately a few micromolar to 100 μM (Kurahashi & Kaneko, 1991). Since we used 1 mm caged compounds in the pipette, the concentration range for the photolysis was less than 10 %. In the present experiment, in addition, uncaging was performed only in the cilia. Caged compounds are continuously supplied from the bulk (the dendrite and the cell body) with a diffusion coefficient of 2.7 × 10−6 cm2 s−1 (Chen et al. 1999). One-dimensional diffusion of cAMP therefore covers the total length of cilia within 0.56 s on average. Furthermore, caged compounds were supplied from the pipette into the cell interior from the whole-cell patch pipette. Thus, depletion of caged compounds during the experiments can be neglected. Actually, double light pulses (a few seconds interval) applied at +100 mV where no Ca2+ feedback is expected caused responses with similar waveforms (H. Takeuchi & T. Kurahashi, unpublished observations).
The present experiments show that it is possible to use caged substances for the quantitative manipulation of cytoplasmic substances. The olfactory cilia have a very fine structure (0.2 μm in diameter), but caged compounds were effective even for such fine processes. The experimental protocols employed here could be a useful tool for investigating the signal transduction system in a wide variety of cells.
Acknowledgments
We thank Drs Akimichi Kaneko, Graeme Lowe and King-Wai Yau for their careful reading of the manuscript. This work was supported by grants from HFSPO, MESSC and JST (to T.K.).
REFERENCES
- Bakalyar HA, Reed RR. The second messenger cascade in olfactory receptor neurons. Current Opinion in Neurobiology. 1991;1:204–208. doi: 10.1016/0959-4388(91)90079-m. [DOI] [PubMed] [Google Scholar]
- Borisy FF, Ronette GV, Cunningham AM, Juilfs D, Beavo J, Snyder SH. Calcium/calmodulin-activated phosphodiesterase expressed in olfactory receptor neurons. Journal of Neuroscience. 1992;3:915–923. doi: 10.1523/JNEUROSCI.12-03-00915.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breer H, Boekhoff I. Second messenger signaling in olfaction. Current Opinion in Neurobiology. 1992;2:439–443. doi: 10.1016/0959-4388(92)90177-m. [DOI] [PubMed] [Google Scholar]
- Chen C, Nakamura T, Koutalos Y. Cyclic AMP diffusion coefficient in frog olfactory cilia. Biophysical Journal. 1999;76:2861–2867. doi: 10.1016/S0006-3495(99)77440-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen T-Y, Yau K-W. Direct modulation by Ca2+-calmodulin of cyclic nucleotide-activated channel of rat olfactory receptor neurons. Nature. 1994;368:545–548. doi: 10.1038/368545a0. [DOI] [PubMed] [Google Scholar]
- Firestein S. Electrical signals in olfactory transduction. Current Opinion in Neurobiology. 1992;4:444–448. doi: 10.1016/0959-4388(92)90178-n. [DOI] [PubMed] [Google Scholar]
- Firestein S. How the olfactory system makes senses of scents. Nature. 2001;413:211–218. doi: 10.1038/35093026. [DOI] [PubMed] [Google Scholar]
- Firestein S, Picco C, Menini A. The relation between stimulus and response in olfactory receptor cells of the tiger salamander. Journal of Physiology. 1993;468:1–10. doi: 10.1113/jphysiol.1993.sp019756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Firestein S, Werblin FS. Gated currents in isolated olfactory receptor neurons of the larval tiger salamander. Proceedings of the National Academy of Sciences of the USA. 1987;84:6292–6296. doi: 10.1073/pnas.84.17.6292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold GH, Nakamura T. Cyclic nucleotide-gated conductances: a new class of ion channels mediates visual and olfactory transduction. Trends in Pharmacological Sciences. 1987;8:312–316. [Google Scholar]
- Hamill OP, Marty A, Neher E, Sakmann B, Sigworth FJ. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflügers Archiv. 1981;391:85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Imanaka Y, Takeuchi H. Spiking properties of olfactory receptor cells in the slice preparation. Chemical Senses. 2001;8:1023–1027. doi: 10.1093/chemse/26.8.1023. [DOI] [PubMed] [Google Scholar]
- Ito Y, Kurahashi T, Kaneko A. Pressure control instrumentation for drug stimulation. Nippon Seirigaku Zasshi. 1995;57:127–133. [PubMed] [Google Scholar]
- Kleene SJ. Origin of the chloride current in olfactory transduction. Neuron. 1993;11:123–132. doi: 10.1016/0896-6273(93)90276-w. [DOI] [PubMed] [Google Scholar]
- Kurahashi T. Activation by odorants of cation-selective conductance in the olfactory receptor cell isolated from the newt. Journal of Physiology. 1989;419:177–192. doi: 10.1113/jphysiol.1989.sp017868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurahashi T. The response induced by intracellular cyclic AMP in isolated olfactory receptor cells of the newt. Journal of Physiology. 1990;430:355–371. doi: 10.1113/jphysiol.1990.sp018295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurahashi T, Kaneko A. A cyclic nucleotide-gated conductance in olfactory receptor cilia. NeuroReport. 1991;2:5–8. [Google Scholar]
- Kurahashi T, Menini A. Mechanism of odorant adaptation in the olfactory receptor cell. Nature. 1997;385:725–729. doi: 10.1038/385725a0. [DOI] [PubMed] [Google Scholar]
- Kurahashi T, Shibuya T. Ca2+-dependent adaptive properties in the solitary olfactory receptor cell of the newt. Brain Research. 1990;515:261–268. doi: 10.1016/0006-8993(90)90605-b. [DOI] [PubMed] [Google Scholar]
- Kurahashi T, Yau K-W. Co-existence of cationic and chloride components in odorant-induced current of vertebrate olfactory receptor cells. Nature. 1993;363:71–74. doi: 10.1038/363071a0. [DOI] [PubMed] [Google Scholar]
- Lamb TD, Pugh EN., Jr A quantitative account of the activation steps involved in phototransduction in amphibian photoreceptors. Journal of Physiology. 1992;449:719–758. doi: 10.1113/jphysiol.1992.sp019111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowe G, Gold GH. Contribution of the ciliary cyclic nucleotide-gated conductance to olfactory transduction in the salamander. Journal of Physiology. 1993a;462:175–196. doi: 10.1113/jphysiol.1993.sp019550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowe G, Gold GH. Nonlinear amplification by calcium-dependent chloride channels in olfactory receptor cells. Nature. 1993b;366:283–286. doi: 10.1038/366283a0. [DOI] [PubMed] [Google Scholar]
- Pugh EN, Jr, Lamb TD. Amplification and kinetics of the activation steps in phototransduction. Biochemica et Biophysica Acta. 1993;1141:111–149. doi: 10.1016/0005-2728(93)90038-h. [DOI] [PubMed] [Google Scholar]
- Reed RR. Signaling pathways in odorant detection. Neuron. 1992;8:205–209. doi: 10.1016/0896-6273(92)90287-n. [DOI] [PubMed] [Google Scholar]
- Reisert J, Matthews HR. Na+-dependent Ca2+ extrusion governs response recovery in frog olfactory receptor cells. Journal of General Physiology. 1998;112:529–535. doi: 10.1085/jgp.112.5.529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reisert J, Matthews HR. Adaptation of the odour-induced response in frog olfactory receptor cells. Journal of Physiology. 1999;519:801–813. doi: 10.1111/j.1469-7793.1999.0801n.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Restrepo D, Schild D. Transduction mechanism in vertebrate olfactory receptor cells. Physiological Reviews. 1998;78:429–466. doi: 10.1152/physrev.1998.78.2.429. [DOI] [PubMed] [Google Scholar]
- Restrepo D, Teeter JH, Schild D. Second messenger signaling in olfactory transduction. Journal of Neurobiology. 1996;30:37–48. doi: 10.1002/(SICI)1097-4695(199605)30:1<37::AID-NEU4>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- Ronnett GV, Snyder SH. Molecular messengers of olfaction. Trends in Neurosciences. 1992;15:508–513. doi: 10.1016/0166-2236(92)90104-g. [DOI] [PubMed] [Google Scholar]
- Trotier D, MacLeod P. Intracellular recordings from salamander olfactory receptor cells. Brain Research. 1983;268:225–237. doi: 10.1016/0006-8993(83)90488-2. [DOI] [PubMed] [Google Scholar]
- Wei J, Zhao AZ, Chan GC, Baker LP, Impey S, Beavo JA, Storm DR. Phosphorylation and inhibition of olfactory adenylyl cyclase by CaM kinase II in neurons: a mechanism for attenuation of olfactory signals. Neuron. 1998;21:495–504. doi: 10.1016/s0896-6273(00)80561-9. [DOI] [PubMed] [Google Scholar]
- Zufall F, Shepherd GM, Firestein S. Inhibition of the olfactory cyclic nucleotide gated ion channel by intracellular calcium. Proceedings of the Royal Society. 1991;B 246:225–230. doi: 10.1098/rspb.1991.0148. [DOI] [PubMed] [Google Scholar]
- Zufall LT, Ma M, Zufall F. Impaired odor adaptation in olfactory receptor neurons after inhibition of Ca2+/calmodulin kinase II. Journal of Neuroscience. 1999;19:1–6. doi: 10.1523/JNEUROSCI.19-14-j0005.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]


