Abstract
Taurine uptake is essential for the maintenance of millimolar intracellular concentrations of taurine, which is released during ischaemia and is thought to be neuroprotective. To determine whether Bergmann glia express functional transporters that can mediate both taurine uptake and efflux, whole-cell patch-clamp recordings were obtained from these cells in rat cerebellar slices. Taurine-induced inward currents can be pharmacologically separated into GABAA receptor and taurine transporter currents. In the presence of GABA receptor blockers, residual taurine currents averaged −28 pA at −70 mV and were strictly inwardly rectifying between −70 and +50 mV. These residual currents were also abolished by external Na+ removal and diminished by reduction of external Cl−, consistent with transport currents. Taurine transport currents were reduced by a taurine transporter inhibitor, guanidinoethyl sulphonate (GES). Other classical inhibitors reduced taurine transport currents with an order of potency (hypotaurine > β-alanine > GES > GABA) similar to that reported for cloned rat taurine transporters. Following intracellular taurine perfusion during the recording, a progressively developing outward current could be observed at −50 mV but not at −70 mV. Intracellular perfusion of taurine also decreased taurine-induced inward currents at both holding potentials. Outward currents induced by intracellular taurine increased in amplitude with depolarization, activated near −50 mV, and were affected by GES. For the first time, these results demonstrate that taurine activates both GABAA receptors and Na+/Cl−-dependent taurine transporters in Bergmann glia in slices. In addition, our data show that taurine transporters can work in reverse and can probably mediate taurine efflux under ischaemic conditions.
In the mammalian brain, taurine (2-aminoethanesulphonic acid) is one of the most abundant free amino acids and is critical for proper brain functioning (Huxtable, 1989, 1992). High levels of taurine, in particular high intracellular levels (about 8 mm; Palkovits et al. 1986), are essential for the functions of taurine. These include osmoregulation (Nagelhus et al. 1993, 1994; Pasantes-Morales et al. 2000) and neuroprotection against glutamate-induced excitotoxicity (El Idrissi & Trenkner, 1999; Saransaari & Oja, 2000a). Although taurine-like immunoreactivity is found in both neurons and astrocytes (Ottersen, 1988; Gragera et al. 1995; Magnusson, 1996), the key enzyme of taurine biosynthesis, cysteinesulphinic acid decarboxylase, is found primarily in glial cells (Almarghini et al. 1991; Tappaz et al. 1994). The very high ratio of intracellular to extracellular taurine concentrations (ratio about 400:1; Jacobson et al. 1985; Lerma et al. 1986) is the result of the functional equilibrium of Na+-dependent taurine uptake, taurine release mechanisms, and a small amount of biosynthesis from cysteine. One mechanism of taurine release is via diffusion through swelling-activated Cl− channels, which is a mechanism by which cells and in particular astrocytes regulate osmolarity (Pasantes-Morales et al. 1990; Nagelhus et al. 1993; Sanchez-Olea et al. 1993; Schousboe & Westergaard, 1995). Another important mechanism of taurine release is via Na+-dependent transport reversal that can occur upon activation of various receptors for neurotransmitters such as glutamate and serotonin in astrocytes (Levi & Gallo, 1995) and following ischaemia and cell damaging conditions in slices (Albrecht et al. 1994; Saransaari & Oja, 1998a, b, 1999). Na+-dependent taurine uptake in astrocytes is important to replenish intracellular taurine levels following taurine efflux, and is thus essential for the maintenance of high intracellular levels of taurine. Pursuant to this idea, taurine uptake has been examined in pure astrocytic cultures (Larsson et al. 1986; Beetsch & Olson, 1993; Petegnief et al. 1995; Tchoumkeu-Nzouessa & Rebel, 1996; Chang et al. 2001). However, in brain slices taurine uptake and release have only been studied in a mixed population of cells (Huxtable, 1989). As a result the properties of taurine transport have not been studied selectively in astrocytes in brain slices. A specific, high-affinity Na+- and Cl−-coupled taurine transporter has been cloned (Liu et al. 1992; Smith et al. 1992; Vinnakota et al. 1997), and the fact that this transporter is electrogenic (Vinnakota et al. 1997) makes it possible to directly record taurine transporter currents in astrocytes in brain slices.
In view of the importance of carrier-mediated taurine transport in astrocytes and its lack of characterization in brain slices thus far, we investigated the properties of in situ taurine transport in Bergmann glia. These cells are the cerebellar radial glia that surround Purkinje cells and share properties with astrocytes such as the expression of glial fibrillary acidic protein, as well as various receptors and transporters for neurotransmitters (Bergles et al. 1997; Clark & Barbour, 1997; Porter & McCarthy, 1997). High levels of taurine and taurine transporters are found in the cerebellum (Palkovits et al. 1986; Ottersen, 1988; Liu et al. 1992; Lake & Orlowski, 1996; Terauchi et al. 1998). In addition, Bergmann glia synthesize taurine (Almarghini et al. 1991) and have the ability to take up taurine released from surrounding neurons (Nagelhus et al. 1993, 1994). However, it remains unknown whether taurine influx in Bergmann glia is mediated by Na+-dependent transporters, and whether these transporters can mediate taurine efflux as well. Thus we ask the following two questions: (1) do Bergmann glia possess functional Na+/Cl−-dependent taurine uptake systems that have the pharmacological properties of the cloned taurine transporter? and (2) can taurine transporters in Bergmann glia work in reverse? Answering the first question will also determine whether taurine activates GABAA receptors present in Bergmann glia (Muller et al. 1994), as has been shown in other cell types (Horikoshi et al. 1988; Kontro & Oja, 1990; Puopolo et al. 1998).
METHODS
Slice preparation
Cerebellar slices were prepared as previously described (Muller et al. 1994; Bordey & Sontheimer, 2000). Briefly, 15- to 30-day-old Sprague-Dawley rats were anaesthetized using pentobarbitone (50 mg kg−1) and decapitated. These procedures were performed in accordance with the Institutional Animal Care and Use Committee, which is guided by USA government Principles for the Utilization and Care of Vertebrate Animals used in Testing, Research and Training. A rapid craniotomy that removed the occipital bone and mastoid processes allowed the cerebellum to be quickly detached, removed and chilled (0-4 °C) in 95 % O2 and 5 % CO2 saturated artificial cerebrospinal fluid (ACSF) containing (mm): NaCl 125, KCl 2.5, CaCl2 2, MgCl2 2, NaHCO3 25 and glucose 10. Next, the cerebellum was glued (cyanoacrylate glue) to the stage of a vibratome and transversal slices (250 μm thick) were cut in cold oxygenated ACSF. After a recovery period of at least an hour in ACSF, slices were placed in a flow-through chamber, held in position by a nylon mesh glued to a U-shaped platinum wire and continuously superfused with oxygenated ACSF at room temperature. The chamber was mounted on the stage of an upright microscope (Olympus BX50) equipped with a ×60 water immersion objective and infrared optics.
Whole-cell recordings and drug application
Whole-cell patch-clamp recordings were obtained as previously described (Bordey & Sontheimer, 2000). Patch pipettes were pulled from thin-walled borosilicate glass (o.d. 1.55 mm; i.d. 1.2 mm; WPI, TW150F-40) on a PP-83 puller (Narishige, Japan). Pipettes had resistances of 6-8 MΩ when filled with the following solutions (mm): KCl 140 or CsCl 140 when noted, CaCl2 1.0, MgCl2 2.0, ethylene glycol-bis (aminoethyl ether)-N,N,N ′,N ′-tetraacetic acid (EGTA) 10 and Hepes 10; pH adjusted to 7.2 with NaOH. The osmolarities of the intracellular and extracellular solutions were 295-300 and 305-310 mosmol l−1, respectively. The osmolarity of all solutions was measured with a vapour pressure osmometer 5500 (Wescor) and was adjusted by addition of water or d-mannitol. In some experiments extracellular Na+ was replaced by an equimolar amount of choline or Li+. For experiments lowering extracellular Cl− concentration, a high molarity agar bridge made out of a bent glass pipette was used. When voltage steps were applied to the recorded cell, 5 mm Cs+ and 40 mm TEA were added to the extracellular solution in exchange for an equimolar amount of Na+ to suppress voltage-activated and time-dependent outward K+ currents present in Bergmann glia (Bordey & Sontheimer, 2000). To permit the reversal of taurine transport, Hepes sodium salt was used instead of Hepes in some glia and 10 mm taurine replaced an equal amount of KCl. Solutions containing picrotoxin were prepared daily. Usually 15 mg of picrotoxin were added to 50 ml of extracellular solution and sonicated for approximately 20 min. For morphological identification, 0.1 % Lucifer Yellow (LY, dilithium salt) was added to the pipette solution. Whole-cell recordings were performed using an Axopatch-200B amplifier (Axon Instruments). Current signals were low-pass filtered at 2-5 kHz and digitized on-line at 5-20 kHz using a Digidata 1320 digitizing board (Axon Instruments) interfaced with an IBM-compatible computer system. Data acquisition, storage and analysis were performed using pCLAMP version 8.0.2 software (Axon Instruments). Settings were determined by compensating the transients of a small (5 mV) 10 ms hyperpolarizing voltage step. The capacitance reading of the amplifier was used as the value for the whole-cell capacitance. Capacitive and leak conductances were not subtracted. Peak currents were determined using Clampfit (Axon Instruments), and statistical values (means ± s.d., with n being the number of cells tested) were evaluated with a statistical graphing and curve-fitting program (Origin, MicroCal). Statistical comparison of means was performed with Student's t test. In the graphs, values are represented as means ± s.e.m.
Both bath and pressure applications were used. Receptor and transporter inhibitors were diluted in ACSF and applied by a rapid bath application system. Receptor and transporter substrate agonists were pressure applied by a computer-controlled pressure ejection system. Inhibitors were diluted in ACSF in which Hepes replaced NaHCO3 and the pH was adjusted to 7.4 by NaOH. When NaCl was replaced by choline or another chemical, similar changes were performed in the pressure pipette solution. The pressure ejection pipettes were standard unpolished patch-electrodes with resistances of 6-8 MΩ for local agonist application and were positioned just above the slice. The applied pressure was 3-6 p.s.i. For the application of two drugs to the same cell, a theta glass with one drug in each compartment was used.
Intracellular perfusion of taurine during the recording
Intracellular perfusion of a taurine transporter blocker was performed as previously reported by others for single or multiple drug applications (Tang et al. 1990). We used a straight pipette holder with a perfusion port (EH-U2, E. W. Wright, CT, USA). Through the perfusion port, a polyethylene tube (i.d. 0.86 mm; o.d. 1.27 mm) was introduced sufficiently far to reach well into the patch pipette solution. A 1 ml syringe containing the LY-filled intracellular solution to be perfused during the recording was connected to the polyethylene tube via an elongated and thinned plastic pipette tip. Before adding the patch pipette, positive pressure was manually applied to fill the tube all the way to the end, remove air bubbles, and visualize efflux of solution. Then, after applying negative pressure to prevent any solution leakage but without adding an air bubble to the end of the tube, the patch pipette was inserted into the holder. To perfuse the LY-filled solution containing taurine, positive pressure was manually applied to add sufficient solution to double the volume in the patch pipette (about 20 μl). The concentrations of taurine and Na+ were doubled to obtain the intended final concentrations in the cell.
Chemicals were purchased from Sigma (St Louis, MO, USA), unless otherwise stated.
RESULTS
Whole-cell recordings were obtained from 98 visually identified Bergmann glia in cerebellar slices from 15- to 30-day-old rats. Bergmann glia were identified by the location of their cell body in the Purkinje cell layer and by the small size of their somata (8-12 μm diameter) in comparison to the large Purkinje cell bodies (≥ 30 μm in diameter). Each recorded cell was filled with Lucifer Yellow (LY) and identified as a Bergmann glial cell by its typical morphology characterized by three or more long, parallel processes extending through the molecular layer toward the pial surface (de Blas, 1984; Reichenbach et al. 1995). Figure 1A shows a representative example of a LY-filled Bergmann glial cell. In addition, cells recorded with a KCl-based intracellular solution had a characteristically low mean input resistance of 38.8 ± 10.5 MΩ (n = 87, mean ± s.d.) and a mean hyperpolarized resting membrane potential (VR) of −77.7 ± 5.8 mV (n = 87). These values were determined in the first 3 min of whole-cell recording. The remaining eleven cells were recorded in CsCl-based intracellular medium. Cells exhibiting a cell capacitance smaller than 30 pF were disregarded. Mean cell capacitance (Cm) was 51.8 ± 12.9 pF (n = 98).
Figure 1. Taurine activates GABAA receptors and transporters in Bergmann glia in situ.
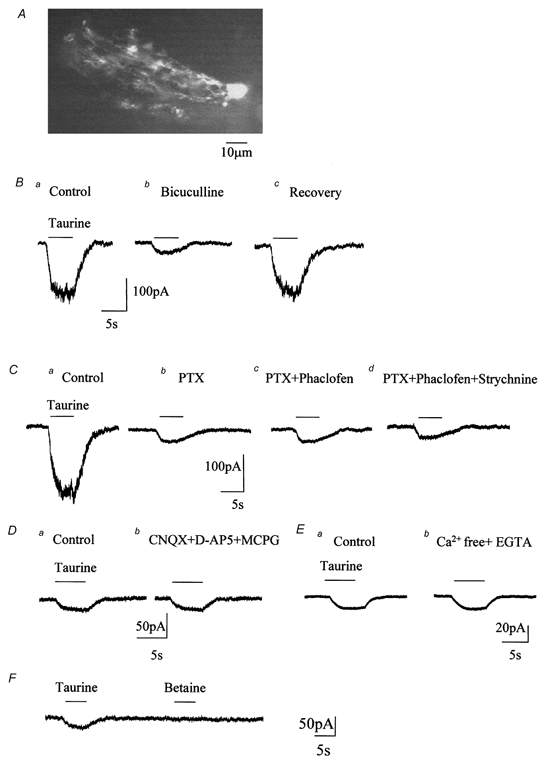
A, photograph of a Lucifer Yellow-filled Bergmann glial cell in a 250 μm slice from a 16-day-old rat. B, a puff of taurine (1 mm) induced an inward current (a) reduced by bath application of 250 μM bicuculline (b), a blocker of GABAA receptors. C, taurine-induced currents were also reduced by picrotoxin (PTX, 500 μM, b), a blocker of GABAA/C receptors. The PTX-insensitive current was not affected by 100 μM phaclofen, a blocker of GABAB receptors (c). The residual current was not affected by 100 μM strychnine, a blocker of glycine receptors (d). D and E, the PTX-, phaclofen- and strychnine-insensitive currents were not affected by bath application of glutamate receptor blockers, including d-AP5 (50 μM), CNQX (20 μM) and (S)-α-methyl-4-carboxyphenylglycine (MCPG, 200 μM) to block AMPA/kainate, NMDA and metabotropic glutamate receptors, respectively (D) nor by a 0 Ca2+/EGTA extracellular solution (E). F, taurine and betaine (1 mm each) were pressure applied onto the same cell using a theta glass pipette. All the recordings were performed from a holding potential of −70 mV with a KCl-based internal solution.
Bergmann glia possess functional Na+-dependent taurine transport systems
For all of the following experiments, Bergmann glia were recorded at a holding potential of −70 mV in the presence of 1 μM TTX in the external solution to prevent action potential-induced neurotransmitter release from surrounding neurons.
Taurine activates both GABAA receptors and Na+-dependent transporters
Pressure applications (puff) of taurine (1 mm, 5 s) induced inward currents in all cells tested (n = 73). Taurine-induced currents could be the result of taurine activation of GABAA/B receptors, glycine (GLY) receptors (Horikoshi et al. 1988; Kontro & Oja, 1990), and/or Na+-dependent taurine transport systems (Liu et al. 1992; Smith et al. 1992; Clark & Amara, 1994; Borden et al. 1995b; Borden, 1996). In 8 of 19 (42 %) Bergmann glia recorded under control conditions (in the absence of receptor blockers), taurine-induced currents were accompanied by an increase in noise. In these eight cells, taurine-induced currents had a mean amplitude of −208.5 ± 45.8 pA (Fig. 1B and C). Because an increase in noise is characteristic of ionotropic receptor currents, and GABAA receptors are known to be expressed in Bergmann glia (Muller et al. 1994), we first tested the effects of GABA receptor antagonists on taurine-induced currents in these nineteen cells. Bath application of bicuculline (250 μM, Fig. 1Bb) or picrotoxin (PTX, 500 μM, Fig. 1Cb), two blockers of GABAA receptors, reversibly reduced taurine-induced currents by 84 ± 4 % (n = 3/8) and 87 ± 4 % (n = 5/11), respectively. No block of taurine-induced currents by bicuculline or PTX could be observed in the remaining 5/8 and 6/11 cells, respectively. Such concentrations of PTX and bicuculline have been used previously to block GABAA receptors and isolate GABA transporter currents (Cammack & Schwartz, 1993; Dong et al. 1994). In the presence of bicuculline or PTX, residual currents were small in amplitude and were not accompanied by an increase in noise, as one would expect for metabotropic receptor or transporter currents. These residual taurine-induced currents were unaffected by both the GABAB receptor antagonist phaclofen (100 μM) (-27.6 ± 2.3 pA in control and −27.4 ± 1.8 pA in phaclofen, n = 7; Fig. 1Cc) and by the glycine receptor antagonist strychnine (100 μM) (-25.6 ± 5.6 pA in control and −25.2 ± 6 pA in strychnine, n = 4; Fig. 1Cd). For the following experiments, phaclofen and strychnine were routinely applied along with PTX in the extracellular solution. PTX was used instead of bicuculline because PTX blocks both GABAA receptors and GABAC receptors (Feigenspan & Bormann, 1994; Bormann & Feigenspan, 1995). Residual taurine-induced currents were unaffected by an extracellular solution containing glutamate receptor blockers, including d-AP5 (50 μM), CNQX (20 μM) and MCPG (200 μM) to block AMPA/ kainate, NMDA and metabotropic glutamate receptors, respectively (Fig. 1D). Taurine-induced currents were −26.2 ± 6.0 pA in the control and −25.7 ± 7.6 pA in the presence of glutamate receptor blockers (n = 4). This result rules out the possibility that taurine-induced glutamate release activates glutamate receptors in the recorded Bergmann glia (Muller et al. 1996; Bergles et al. 1997) and generates the recorded currents. Glutamate could be released by reversed uptake driven by the Na+ accumulation that taurine uptake produces. In addition, residual taurine-induced currents were unaffected by an extracellular solution containing 0 Ca2+/1 mm EGTA to prevent vesicular release of glutamate following depolarization of presynaptic terminals by taurine transporter activation (-28.0 ± 2.6 pA in control and −27.6 ± 2.5 pA in 0 Ca2+/EGTA, n = 3, Fig. 1E). When recorded with a KCl-based intracellular solution (Fig. 1), the mean residual taurine current amplitude averaged −27.8 ± 5.2 pA (n = 62). This mean amplitude was similar to the mean current amplitude of −28 ± 2.1 pA (n = 11) when cells were recorded with a CsCl-based intracellular solution (data not shown). This result was expected because K+ is not known to be required for taurine transport or the transport of other inhibitory amino acids via Na+/Cl−-dependent transporters such as GABA transporters (Borden, 1996). The possibility that the taurine-evoked current is generated by glutamate transporters is ruled out by the fact that this current would be reduced by about 40 % when intracellular K+ is replaced by Cs+ (Barbour et al. 1991) and by the strong dependence of the current on external Cl− which is described below. The mean amplitude of residual taurine currents averaged −29.6 ± 5.3 pA (n = 11) in the presence of a blocker of swelling-sensitive Cl− channels, 5-nitro-2-(3-phenyl-propylamino)-benzoate (NPPB, 100 μM) (Jackson & Strange, 1993; Bres et al. 2000). The taurine current amplitude in the presence of NPPB was not significantly different from that in control conditions. Such a result rules out the possibility that an influx of taurine (zwitterion at physiological pH) and a concurrent efflux of Cl− through chloride channels (Sanchez-Olea et al. 1993) generate the inward currents recorded at −70 mV. Finally, we pressure-applied taurine and betaine (1 mm each) onto the same cell using a theta glass pipette (n = 3) (Fig. 1F). Betaine is a substrate for one of the GABA transporters, BGT-1 (Borden, 1996), and is not supposed to be taken up via taurine transporters. Taurine induced an inward current while betaine failed to induce a current, suggesting that our method of application did not generate an artifactual current. Taken together, these data suggest that taurine activates GABAA receptors and that residual taurine currents in the presence of GABA receptor and GLY receptor blockers may be mediated by taurine transport into Bergmann glia.
We then determined whether the residual taurine-induced currents were voltage dependent and compared their voltage dependence to that of taurine-induced currents recorded in the absence of GABA receptor blockers. When the cell membrane was progressively depolarized from −70 to +50 mV, taurine-induced currents in the absence of GABA receptor blockers decreased progressively in amplitude with depolarization and reversed between 0 and 10 mV (2.9 ± 0.1 mV, n = 3), as is expected for GABAA receptor currents in our recording conditions (Fig. 2A and B). In the presence of GABA receptor and GLY receptor blockers, taurine-induced currents displayed prominent inward rectification and did not reverse in our recording conditions (Fig. 2C and D, n = 8). Because Bergmann glia are coupled by gap junctions (Muller et al. 1996), the resulting voltage non-uniformity could contribute to the presence of inward currents at positive potentials. However, our data show that the reversal potential of GABAA receptor-mediated currents (≈3 mV) is similar to that predicted by the equilibrium potential of Cl− ions (≈2 mV). This result argues against the contribution of voltage non-uniformity to the I-V curve of residual taurine-induced currents. In addition, similar current-voltage (I-V) relationships have been reported for other Na+/Cl−-dependent transporters in similar recording conditions (Cammack & Schwartz, 1993; Biedermann et al. 1994; Dong et al. 1994; Takahashi et al. 1996). Because taurine uptake is dependent on extracellular Na+ and Cl− (Liu et al. 1992; Smith et al. 1992) we removed Na+ and reduced Cl− in the extracellular solution (Borden et al. 1992, 1995a). When choline (Fig. 3A) or Li+ (data not shown) was substituted for external Na+ (n = 8), GABA receptor and GLY receptor blocker-insensitive taurine currents were reversibly and completely blocked, as is expected for taurine-induced transport currents. Lowering extracellular Cl− from 136 to 11 mm (NaCl replaced with sodium gluconate) reduced taurine-induced transporter currents by 67.3 + 3.0 % (n = 4) (Fig. 3B).
Figure 2. Voltage dependence of taurine-induced responses.
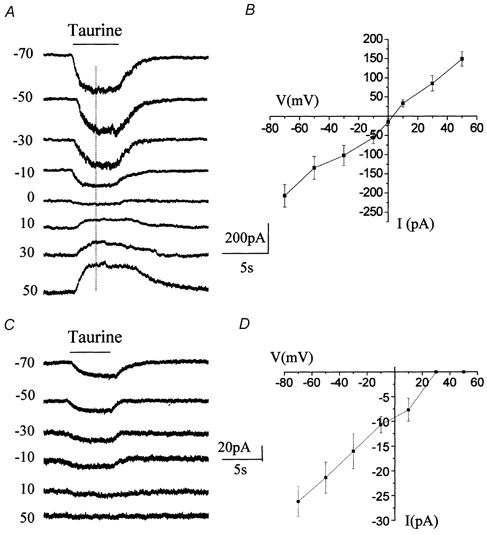
A, taurine-induced inward currents recorded at distinct holding potentials in normal recording conditions (no GABA receptor blockers). The current reversed in polarity close to 0 mV. B, mean current-voltage curve of taurine-induced inward currents (n = 3) recorded without GABA receptor blockers. C, taurine-induced currents recorded at different holding potentials in the presence of GABAA/B/C and glycine receptor blockers in the bath. These currents inwardly rectified and did not reverse for positive membrane potentials. D, mean current-voltage curve of taurine-induced inward currents (n = 8) recorded with GABA and glycine receptor blockers.
Figure 3. Ion dependence of taurine-induced currents in the presence of GABA and glycine receptor blockers.
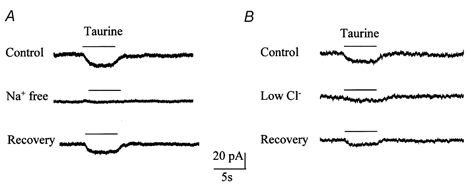
A, taurine-induced current was reversibly abolished by replacing external Na+ by choline. B, taurine-induced current was reversibly diminished by reduction of external Cl− (11 mm instead of 136 mm).
Together, these data suggest that taurine-induced currents in the presence of GABA receptor and GLY receptor blockers are generated by taurine and Na+ being taken up into Bergmann glia.
Pharmacological characterization of taurine transport currents
All of the following experiments were performed in the presence of GABAA/B/C receptor blockers (500 μM PTX and 100 μM phaclofen) and a GLY receptor blocker (100 μM strychnine), except for the experiments where the effect of these blockers was tested on hypotaurine-induced currents. We first tested the effects of guanidinoethyl sulphonate (GES, 100 μM) on taurine transport currents, because GES is a selective competitive antagonist of taurine transporters at the concentration used (Liu et al. 1992; Smith et al. 1992; Borden, 1996). The IC50 of GES has been reported to be 2500 μM to inhibit GABA uptake into synaptosomes (Li & Lombardini, 1990), and 4840, 696, 915 and 4800 μM to inhibit the GABA transporter subtypes GAT-1, GAT-2, GAT-3 and BGT-1 expressed in COS-7 cells (Borden, 1996). These values suggest that 100 μM GES should not significantly affect the currents generated by taurine uptake that can possibly occur via GABA transporters (Borden et al. 1995b; Borden, 1996). GES reversibly reduced taurine-induced transport currents by 72.3 ± 13.2 % (n = 14, Fig. 4A), suggesting that these currents are taurine transporter currents. In 8 out of 19 cells, GES induced inward currents that averaged −6.8 ± 1.8 pA (range 5-9 pA), suggesting that GES is weakly transported by taurine transporters. The commonly tested and most potent transportable inhibitors of taurine uptake are hypotaurine and β-alanine (Liu et al. 1992; Smith et al. 1992). Figure 4 shows that hypotaurine completely and reversibly blocks taurine transport currents (Fig. 4B, n = 4) and β-alanine blocks 84.5 ± 3.8 % (n = 4) of taurine transport currents (Fig. 4C). Consistent with the action of hypotaurine and β-alanine as competitive transportable blockers, bath application of hypotaurine and β-alanine (each at 100 μM) induced inward currents that averaged −19 ± 2.6 pA (n = 4) and −21.2 ± 3.5 pA (n = 4), respectively, as shown by the deviation of the holding current in the presence of hypotaurine or β-alanine from the control current (Fig. 4Bb and Cb, dotted line). Because hypotaurine and β-alanine are also substrates for two subtypes of GABA transporters (rat GAT-2 and GAT-3) (Borden et al. 1992; Liu et al. 1993; Borden et al. 1995b; Borden, 1996), these inward currents could result from both taurine transporter as well as GABA transporter activation. GABA was also tested on taurine transport currents because it is a weak blocker of taurine transporters cloned from rat brain (Smith et al. 1992). GABA (100 μM) reduced taurine transport currents by 70.7 ± 2.1 % (n = 4) (Fig. 4D). Like hypotaurine and β-alanine, GABA induced a mean inward current of −22.7 ± 2.2 pA (n = 4) in the presence of a non-transportable blocker of the GABA transporter subtype GAT-1 (SKF89976-A, 100 μM, Fig. 4D). A GAT-1 blocker was applied because Bergmann glia have been shown to stain positive for GAT-1 (Swan et al. 1994; Morara et al. 1996). In addition, GABA-induced currents recorded in the presence of GABAA receptor blockers are Na+ dependent (data not shown), which strongly suggests that these currents are mediated by GABA uptake into Bergmann glia. In the presence of a GAT-1 blocker, GABA-induced currents are probably due to GABA uptake via the two rat transporter subtypes, GAT-2 and/or GAT-3, and less likely via taurine transporters (Borden et al. 1992; Smith et al. 1992; Borden et al. 1995b; Borden, 1996). Although taurine is not transported by GAT-1 (Borden, 1996), we tested the effect of SKF89976-A on taurine transport currents. The mean amplitude of taurine-induced currents was not affected by bath application of 100 μM SKF89976-A (-28.0 ± 2.2 and −25.2 ± 0.9 pA, before and after SKF89976-A, respectively, n = 4), which confirms that taurine is not transported by GAT-1 transporters. To further characterize taurine transport currents in Bergmann glia, we pressure-applied hypotaurine and studied the GES sensitivity and Na+- and voltage-dependence of the resulting currents. We chose to apply hypotaurine because it is the most potent blocker of taurine-induced transport currents and because it is an endogenous substrate of taurine transport and a precursor of taurine (Oja et al. 1981; Kontro et al. 1984; Huxtable, 1992). A puff of hypotaurine (1 mm) (Smith et al. 1992) induced an inward current of −24.0 ± 4.1 pA (n = 8/8) in the presence of GABA receptor and GLY receptor blockers (Fig. 5). GES (100 μM) reversibly reduced hypotaurine-induced currents by 68.2 ± 5.3 % (n = 5, Fig. 5Ab), which suggests that most of the hypotaurine-induced current is due to uptake into Bergmann glia via taurine transporters. When Na+ was replaced by choline, hypotaurine-induced currents were reversibly and completely blocked (n = 3, Fig. 5Bb). However, hypotaurine-induced currents were unaffected by the GABAA/B/C receptor antagonists PTX and phaclofen (n = 3, Fig. 5Cb) or by the GLY receptor antagonist strychnine (n = 3, Fig. 5Cc). In addition, the voltage dependence of hypotaurine-induced currents was strictly inwardly rectifying up to +50 mV as observed for taurine-induced transport currents (n = 3, Fig. 5D and E, and see Fig. 2C and D). Thus hypotaurine-induced currents are generated by hypotaurine being taken up into Bergmann glia.
Figure 4. Pharmacological profile of taurine transport currents in Bergmann glia.
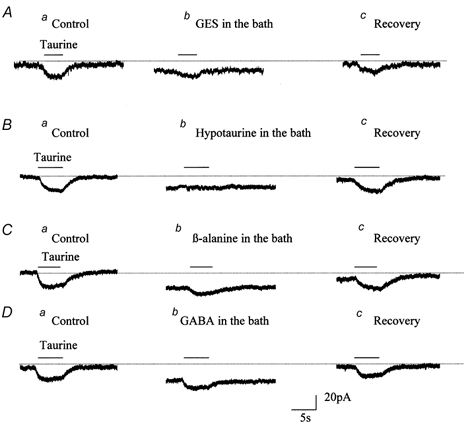
Effect of distinct blockers of taurine transport, GES (A, 100 μM), hypotaurine (B, 100 μM), β-alanine (C, 100 μM), and GABA (D, 100 μM), on taurine-induced currents recorded in the presence of GABAA/B/C receptor blockers (500 μM PTX and 100 μM phaclofen) and a GLY receptor blocker (100 μM strychnine). For each treatment, data show the current induced by a 1 mm puff of taurine before (a), during (b), and after the washout (c) of the drug.
Figure 5. Pharmacology, voltage- and ion-dependence of hypotaurine-induced currents.
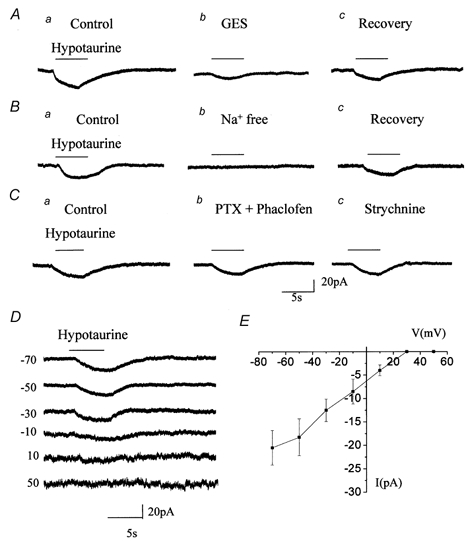
A, B and C, hypotaurine-induced currents (a) were reversibly reduced by 100 μM GES (Ab), were abolished by replacing external Na+ with choline (Bb), but were not affected by bath application of 500 μM PTX with 100 μM phaclofen (Cb) or by 100 μM strychnine in the presence of PTX and phaclofen (Cc). D, records of hypotaurine-induced currents obtained at different holding potentials from −70 to +50 mV. E, mean current-voltage curve of hypotaurine-induced currents (n = 3). For all the recordings, hypotaurine was pressure-applied at 1 mm to cells recorded at −70 mV. Experiments in A, B and D were performed in the presence of GABAA/B/C receptor blockers (500 μM PTX and 100 μM phaclofen) and a GLY receptor blocker (100 μM strychnine).
These data suggest that taurine is taken up into Bergmann glia mostly by classical GES-sensitive taurine transporters that have a high affinity for β-alanine and hypotaurine (Liu et al. 1992; Smith et al. 1992). Because taurine can also be taken up weakly by GAT-2 and GAT-3, and because hypotaurine and β-alanine are high-affinity substrates for GAT-2/3 (Borden, 1996), it is possible that taurine-induced currents in Bergmann glia are also due to uptake via GAT-2 and/or GAT-3.
Carrier-mediated taurine efflux from Bergmann glia
For the following experiments, whole-cell recordings were initially obtained in a normal external solution containing GABAA/B/C receptor and GLY receptor blockers as previously mentioned, and subsequently in an external solution containing 0 Ca2+/1 mm EGTA and 1 μM TTX to block Ca2+- and action potential-dependent taurine efflux, respectively (Andjelkovic et al. 1999). TEA (40 mm) and Cs+ (5 mm) were also included in the external solution to block K+ currents. To study taurine transport reversal, we perfused 20 mm taurine + 10 mm Na+ intracellularly during the recording (10 mm Na+ being already included in the patch pipette). Intracellular taurine and Na+ concentrations were thus about 10 mm each, assuming that taurine and Na+ concentrations were diluted 2-fold upon perfusion in the patch pipette (see Methods). In addition, NPPB was included in the bath solution to prevent a possible taurine and Cl− efflux through swelling-sensitive Cl− channels (Sanchez-Olea et al. 1993) upon intracellular taurine perfusion, which would result in an inward current at −50 or −70 mV. In these recording conditions, cells had a mean input resistance of 71.3 ± 13.2 MΩ (n = 15) and a mean resting membrane potential (VR) of −54.6 ± 5.2 mV (n = 10). To first test for taurine transport reversal, we intracellularly perfused taurine between two successive pressure-applications of taurine. When the cell was held at −70 mV, inward uptake currents induced by pressure-applications of taurine were decreased by 23.4 ± 4.1 % (n = 3, Fig. 6A) after intracellular taurine perfusion. When the cell was held at −50 mV, the inward uptake current was decreased by 57.1 ± 6.2 % (17.6 ± 2.0 pA before and 7.6 ± 1.6 pA after intracellular taurine perfusion, n = 4, Fig. 6B), which was a significantly greater reduction than that obtained for the inward currents recorded at −70 mV. This decrease in taurine uptake current was probably due to an intracellular competitive block of taurine uptake by taurine. In addition, when the cell was held at −50 mV, an outward current of 6.3 ± 1.05 pA (n = 4/4) developed upon intracellular perfusion of taurine (Fig. 6B), while no outward current was observed when the cell was held at −70 mV. Such an outward current was not observed at −50 mV when a similar solution without internal taurine was intracellularly perfused (n = 4, Fig. 6C). Similarly, an outward current was not observed when taurine without Na+ was intracellularly perfused and the cell was recorded with an intracellular solution containing no Na+. The time dependence of the development of the outward current is shown on a plot of the current amplitude at −50 mV as a function of the recording time in Fig. 6D. This outward current induced by intracellular taurine probably represents an outward taurine efflux current due to transport reversal. To further characterize the voltage dependence of intracellular taurine-induced outward currents, 20 mV increment voltage steps of 150 ms were applied from −100 to +60 mV from a holding potential of −70 mV. In response to voltage steps, Bergmann glia generated transient (capacitive) and steady-state currents (Fig. 7A). Intracellular perfusion of 20 mm taurine during the recording (Na+ being already included internally) induced an increase in the steady-state currents without affecting capacitive currents (Fig. 7B, cf. Fig. 7A). Point-by-point subtraction of the currents in the presence and absence of intracellular taurine isolated outward currents that averaged 7.4 ± 0.9 pA at −50 mV (n = 4, Fig. 7E). This mean amplitude is similar to the amplitude of the outward currents obtained upon intracellular perfusion of taurine in the cells held at −50 mV (Fig. 6A). The I-V curves of these currents show that they are voltage dependent, outwardly rectifying, and could be clearly detected at −50 mV (Fig. 7E). Subsequent bath application of GES (100 μM) reversibly affected the steady-state outward currents (n = 4, Fig. 7C and D). Point-by-point subtraction of the traces in the presence and absence of GES illustrates the effect of GES (Fig. 1F). As a control for taurine intracellular perfusion, LY was routinely added in the intracellularly perfused solution but not in the recording solution to confirm that the solution intracellularly perfused during the recording was diffusing into the cell. In all of these experiments, LY diffused into the cell (data not shown).
Figure 6. Intracellular taurine perfusion during the recording to study taurine transport reversal.
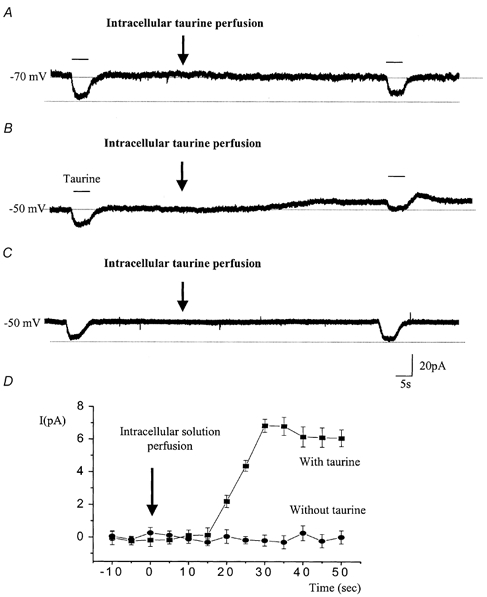
A and B, 20 mm taurine was intracellularly perfused during the recording as indicated by the arrow, and 1 mm taurine was pressure-applied before and after intracellular taurine perfusion. Intracellular taurine perfusion induced an outward current in cells held at −50 mV (B) but not in those held at −70 mV (A). However, in both conditions the inward currents induced by a puff of taurine were diminished by intracellular taurine perfusion. C, a solution containing no taurine was intracellularly perfused during the recording (arrow) at −50 mV. Taurine (1 mm) was pressure-applied before and after intracellular taurine perfusion. D, plot of the mean current amplitude (±s.e.m.) as a function of the recording time for cells that were recorded at −50 mV and intracellularly perfused with either a solution containing taurine (n = 4, ▪) or a solution without taurine (n = 4, •). The baseline currents were considered as a zero current. These experiments were performed in the presence of GABAA/B/C receptor and GLY receptor blockers, as previously mentioned.
Figure 7. Voltage dependence of taurine transport reversal.
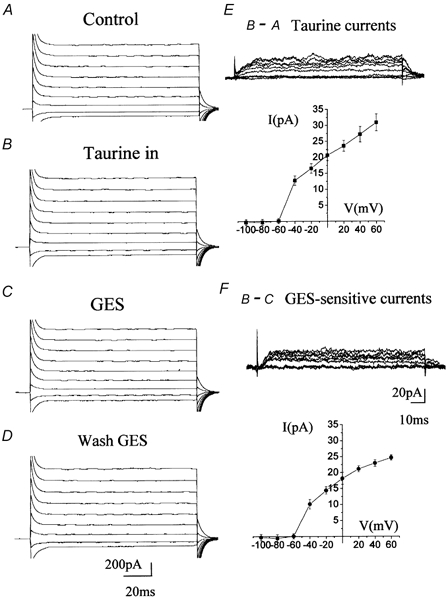
Voltage step records for taurine efflux. A, B, C and D, current traces following 20 mV increment voltage steps of 150 ms applied from −100 to +0 mV from a holding potential of −70 mV under control conditions (A) and after intracellular perfusion of 20 mm taurine (B). Following intracellular taurine perfusion, the cells were recorded in the presence of GES (C) and following washout of GES (D). E, traces in A were subtracted from traces in B to reveal taurine efflux currents (top panel) and their respective I-V curve (lower panel). F, traces in C were subtracted from traces in B to illustrate the effect of GES and its magnitude by showing the respective I-V curve (lower panel). All these experiments were performed in the presence of GABAA/B/C receptor and GLY receptor blockers.
DISCUSSION
For the first time, the present study reports the presence and properties of functional taurine transporters in Bergmann glia recorded in brain slices. More specifically, our data show that: (1) taurine activates taurine transporters in addition to GABAA receptors in Bergmann glia, (2) taurine transport currents display a pharmacological profile similar to that of cloned rat taurine transporters, and (3) taurine transporters can mediate electrogenic taurine efflux from Bergmann glia.
Taurine activates taurine transporters in addition to GABAA receptors in Bergmann glia
Isolation and identification of taurine transport currents
. Our data show that in a subset of Bergmann glia, a large proportion of the amplitudes of taurine-induced currents (about 86 %) are sensitive to PTX and bicuculline and are thus likely to be mediated by GABAA receptor activation. In addition, taurine-induced currents reverse around 3 mV, as one would expect for GABAA/C receptor-mediated currents in our recording conditions (the equilibrium potential of Cl− ions is ≈2 mV). Activation of GABAA receptors by taurine is in agreement with previous studies showing the functional presence of GABAA receptors in Bergmann glia (Muller et al. 1994) and the activation of GABAA receptors by taurine in other cell types (Horikoshi et al. 1988; Kontro & Oja, 1990). Taurine-induced GABAA receptor responses, which were accompanied by an increase in noise, were detected in 42 % of the recorded cells. This result can be explained in two ways: (1) taurine has a very low affinity for GABAA receptors (EC50 in the millimolar range) (Bureau & Olsen, 1991; Dominguez-Perrot et al. 1996) and the presence of functional taurine uptake systems in cerebellar slices would decrease the actual concentration that reaches the cells recorded at ≤ 50 μm deep in the tissue, and (2) GABAA receptor expression progressively decreases during neonatal development in animals older than postnatal day 10 (P10) (Muller et al. 1994). In the presence of GABAA receptor blockers, taurine induced small currents (mean amplitude of 28 pA) in every recorded cell. These currents were not accompanied by an increase in noise and were not affected by blockers of GLY receptors and GABAB receptors, although taurine has been shown to activate these receptors in other cell types (Horikoshi et al. 1988; Kontro & Oja, 1990). Residual taurine-induced currents were voltage dependent and strictly inwardly rectifying, which is a characteristic of transporter currents in our recording conditions (no internal Na+ and taurine) (Cammack & Schwartz, 1993; Mager et al. 1993; Dong et al. 1994; Takahashi et al. 1996). In addition, residual taurine-induced currents were reduced by reduction of external Cl− and were blocked by removal of external Na+. This result identifies taurine-induced currents insensitive to GABA receptor and GLY receptor blockers as transport currents due to Na+/Cl−-dependent taurine uptake into Bergmann glia (Liu et al. 1992; Smith et al. 1992; Borden, 1996).
Pharmacology of taurine transport currents
Taurine can be taken up via high-affinity taurine transporters (Smith et al. 1992) and to a very small extent via two subtypes of GABA transporters (GAT-2 and GAT-3) (Borden et al. 1995b; Borden, 1996). A 70 % block of taurine transport currents by GES, a selective inhibitor of taurine transporters at the concentration used (100 μM) (Liu et al. 1992; Smith et al. 1992; Borden, 1996), indicates that taurine is taken up via taurine transporters. Although taurine transporters share a high amino acid homology (61 %) with GAT-2 and GAT-3, high concentrations of taurine (1.3 and 2.9 mm) are required to inhibit 50 % of GABA uptake via GAT-2 and GAT-3, respectively (Borden, 1996). Because we pressure-applied taurine at 1 mm, we cannot exclude the possibility that a portion of taurine transport currents is due to taurine uptake via GAT-2 and/or GAT-3. The effects of different competitive antagonists that are also substrate agonists for taurine transporters and GAT-2/3 were tested on taurine transport currents. Taurine transport was inhibited by taurine transport inhibitors with the following order of potency: hypotaurine (complete block) > β-alanine > GES > GABA. This order of potency is similar to that reported for taurine transporters cloned from rat brain (Smith et al. 1992). We then studied GES sensitivity, and Na+- and voltage-dependence of hypotaurine-induced currents, because hypotaurine is a better substrate agonist for taurine transporters than for GAT-2 and GAT-3, whereas β-alanine has a higher affinity for GAT-2 and GAT-3 than for taurine transporters (Borden et al. 1992; Liu et al. 1993; Borden et al. 1995b; Borden, 1996). In addition, hypotaurine is an endogenous substrate of taurine transport and a precursor of taurine (Oja et al. 1981; Kontro et al. 1984; Huxtable, 1992). Like taurine, hypotaurine induced small, Na+-dependent currents. In addition, a 68 % block of hypotaurine-induced currents by GES suggests that hypotaurine transport currents are predominantly due to hypotaurine being taken up by taurine transporters. (Borden et al. 1992, 1995a). GABA is a transportable blocker of taurine transporters cloned from rat brain (Smith et al. 1992), though it displays only modest potency at this site (IC50 ≈700 μM for uptake of 50 nm taurine) (Borden, 1996). However, we obtain a 70 % block of taurine transport by 100 μM GABA. It is also likely that GABA would block part of the current mediated by GAT-2 and/or GAT-3 activation. It is possible that GABA induces taurine release from surrounding cells presumably by activating Na+-dependent GABA transporters and inducing taurine transport reversal. Indeed, it has been previously reported that GABA can induce taurine release (Saransaari & Oja, 2000b). An incomplete block of taurine transport into Bergmann glia by 100 μM GABA (a saturating concentration for GAT-2 and GAT-3) strengthens the deduction that taurine is indeed transported by taurine transporters. Together, these data indicate that taurine is being transported into Bergmann glia via classical taurine transporters and possibly via GAT-2 and/or GAT-3.
Taurine transporters can work in reverse
To study taurine transport reversal we recorded cells with a pipette containing internal Na+ at 10 mm and intracellularly perfused taurine (20 mm) + Na+ (10 mm) during the recording to obtain intracellular concentrations of taurine and Na+ of about 10 mm each. We first tested the effects of intracellular perfusion of taurine on the inward current amplitudes induced by pressure applications of taurine and on the baseline current. At both −70 and −50 mV, taurine-induced inward currents were reduced in amplitude after intracellular taurine perfusion. These data suggest that intracellular taurine influences the uptake of extracellular taurine. Such an interaction between intracellular and extracellular transporter substrate has been observed for glutamate and glycine transporters (Takahashi et al. 1996; Roux & Supplisson, 2000). This finding substantiates the conclusion that the Na+-dependent inward currents induced by a puff of taurine are due to the uptake of Na+ and taurine into the cell. No change in the baseline current upon taurine perfusion could be detected at −70 mV. However, at −50 mV an outward current progressively developed upon intracellular taurine perfusion in every cell tested. Such a current was not observed upon intracellular perfusion of a solution without taurine or a solution with taurine but without Na+. These data suggest that taurine transport systems can work in reverse, and transport taurine out upon internal taurine perfusion and glial cell depolarization. These data also suggest that the taurine-induced outward current is not a non-specific leakage current. To obtain the I-V curves of the outward currents we studied the reversal of taurine transport systems by applying voltage steps. The I-V curve of intracellular taurine-induced outward currents showed that these currents were voltage dependent, outwardly rectifying, activated near −60 mV, and were clearly detectable at −50 mV. Consistent with the effect of GES on taurine uptake currents, GES affected intracellular taurine-induced outward currents, which substantiates the presence of taurine transporters. The observation of taurine transporter reversal around −60 mV is in good agreement with previous studies on Na+/Cl−-dependent transporters (Attwell et al. 1993), in particular GABA transporters (Schwartz, 1987). Indeed, in a study on retinal neurons in vitro, Schwartz could detect carrier-mediated GABA efflux currents at −60 mV. It was also previously reported that taurine release via Na+-dependent transporter reversal can occur in brain slices (Saransaari & Oja, 1999, 2000a) and cells in vitro (Levi & Gallo, 1995; Ohkuma et al. 1996). Because astrocytes have been shown to have about 10-16 mm intracellular Na+, a 10 mm concentration of intracellular Na+ is physiological (Rose & Ransom, 1996). The intracellular taurine concentration was chosen to be 10 mm because it was previously reported that brain cells have high intracellular taurine levels (about 8 mm) (Palkovits et al. 1986). In addition, Bergmann glia have the ability to synthesize taurine (Almarghini et al. 1991).
Together, these data indicate that taurine transporters can work in reverse and are likely to release taurine upon glial cell depolarization.
Functional implications of functional taurine transporters in Bergmann glia
Taurine uptake in Bergmann glia is essential for two main reasons: (1) taurine uptake into Bergmann glia is necessary to maintain a very (Bardakdjian et al. 1979) high ratio of intracellular to extracellular taurine concentrations (ratio about 400 : 1; Jacobson et al. 1985; Lerma et al. 1986) that is necessary for glia to respond to hyposmotic shock. For example, volume regulation is impaired in taurine-deficient cultured astrocytes (Moran et al. 1994). (2) Taurine transport is probably involved in the neuronal-glial exchanges of taurine in response to hypo-osmotic stress (Nagelhus et al. 1994). This reversible cellular redistribution of taurine from neurons to glia following an acute hyposmotic stress can protect neurons from swelling-induced damage. Regarding carrier-mediated taurine release, it is unclear whether carrier reversal can occur under physiological conditions. However, our data argue that taurine transporter reversal can occur under hypoxic/ischaemic conditions during which cells are depolarized to more than −50 mV and intracellular Na+ is estimated to rise to 39 mm (Attwell et al. 1993). In these circumstances, taurine transport reversal will allow an increase in extracellular levels of taurine, which is known to have neuroprotective effects against excitotoxicity (Trenkner et al. 1996; El Idrissi & Trenkner, 1999; Saransaari & Oja, 2000a). Overall, our study characterizes taurine transporters in Bergmann glia in situ and is an important step in defining the role of taurine transporters and taurine in a neuroglial network.
Acknowledgments
This work was supported by NIH P01-NS39092-03. We thank Keith Gipson for helpful suggestions.
REFERENCES
- Albrecht J, Bender AS, Norenberg MD. Ammonia stimulates the release of taurine from cultured astrocytes. Brain Research. 1994;660:288–292. doi: 10.1016/0006-8993(94)91301-3. [DOI] [PubMed] [Google Scholar]
- Almarghini K, Remy A, Tappaz M. Immunocyto-chemistry of the taurine biosynthesis enzyme, cysteine sulfinate decarboxylase, in the cerebellum: evidence for a glial localization. Neuroscience. 1991;43:111–119. doi: 10.1016/0306-4522(91)90421-j. [DOI] [PubMed] [Google Scholar]
- Andjelkovic AV, Kerkovich D, Shanley J, Pulliam L, Pachter JS. Expression of binding sites for β chemokines on human astrocytes. Glia. 1999;28:225–235. [PubMed] [Google Scholar]
- Attwell D, Barbour B, Szatkowski M. Non-vesicular release of neurotransmitter. Neuron. 1993;11:401–407. doi: 10.1016/0896-6273(93)90145-h. [DOI] [PubMed] [Google Scholar]
- Barbour B, Brew H, Attwell D. Electrogenic uptake of glutamate and aspartate into glial cells isolated from the salamander (Ambystoma) retina. Journal of Physiology. 1991;436:169–193. doi: 10.1113/jphysiol.1991.sp018545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bardakdjian J, Tardy M, Pimoule C, Gonnard P. GABA metabolism in cultured glial cells. Neurochemical Research. 1979;4:517–527. doi: 10.1007/BF00964645. [DOI] [PubMed] [Google Scholar]
- Beetsch JW, Olson JE. Taurine transport in rat astrocytes adapted to hyperosmotic conditions. Brain Research. 1993;613:10–15. doi: 10.1016/0006-8993(93)90447-u. [DOI] [PubMed] [Google Scholar]
- Bergles DE, Dzubay JA, Jahr CE. Glutamate transporter currents in Bergmann glial cells follow the time course of extrasynaptic glutamate. Proceedings of the National Academy of Sciences of the USA. 1997;94:14821–14825. doi: 10.1073/pnas.94.26.14821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biedermann B, Eberhardt W, Reichelt W. GABA uptake into isolated retinal Muller glial cells of the guinea-pig detected electrophysiologically. NeuroReport. 1994;5:438–440. doi: 10.1097/00001756-199401120-00017. [DOI] [PubMed] [Google Scholar]
- Borden LA. GABA transporter heterogeneity: pharmacology and cellular localization. Neurochemistry International. 1996;29:335–356. doi: 10.1016/0197-0186(95)00158-1. [DOI] [PubMed] [Google Scholar]
- Borden LA, Smith KE, Gustafson EL, Branchek TA, Weinshank RL. Cloning and expression of a betaine/GABA transporter from human brain. Journal of Neurochemistry. 1995a;64:977–984. doi: 10.1046/j.1471-4159.1995.64030977.x. [DOI] [PubMed] [Google Scholar]
- Borden LA, Smith KE, Hartig PR, Branchek TA, Weinshank RL. Molecular heterogeneity of the γ-aminobutyric acid (GABA) transport system. Cloning of two novel high affinity GABA transporters from rat brain. Journal of Biological Chemistry. 1992;267:21098–21104. [PubMed] [Google Scholar]
- Borden LA, Smith KE, Vaysse PJ, Gustafson EL, Weinshank RL, Branchek TA. Re-evaluation of GABA transport in neuronal and glial cell cultures: correlation of pharmacology and mRNA localization. Receptors and Channels. 1995b;3:129–146. [PubMed] [Google Scholar]
- Bordey A, Sontheimer H. Ion channel expression by astrocytes in situ: comparison of different CNS regions. Glia. 2000;30:27–38. doi: 10.1002/(sici)1098-1136(200003)30:1<27::aid-glia4>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- Bormann J, Feigenspan A. GABAC receptors. Trends in Neurosciences. 1995;18:515–519. doi: 10.1016/0166-2236(95)98370-e. [DOI] [PubMed] [Google Scholar]
- Bres V, Hurbin A, Duvoid A, Orcel H, Moos FC, Rabie A, Hussy N. Pharmacological characterization of volume-sensitive, taurine permeable anion channels in rat supraoptic glial cells. British Journal of Pharmacology. 2000;130:1976–1982. doi: 10.1038/sj.bjp.0703492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bureau MH, Olsen RW. Taurine acts on a subclass of GABAA receptors in mammalian brain in vitro. Euorepean Journal of Pharmacology. 1991;207:9–16. doi: 10.1016/s0922-4106(05)80031-8. [DOI] [PubMed] [Google Scholar]
- Cammack JN, Schwartz EA. Ions required for the electrogenic transport of GABA by horizontal cells of the catfish retina. Journal of Physiology. 1993;472:81–102. doi: 10.1113/jphysiol.1993.sp019938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang RC, Stadlin A, Tsang D. Effects of tumor necrosis factor α on taurine uptake in cultured rat astrocytes. Neurochemistry International. 2001;38:249–254. doi: 10.1016/s0197-0186(00)00082-6. [DOI] [PubMed] [Google Scholar]
- Clark BA, Barbour B. Currents evoked in Bergmann glial cells by parallel fibre stimulation in rat cerebellar slices. Journal of Physiology. 1997;502:335–350. doi: 10.1111/j.1469-7793.1997.335bk.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark JA, Amara SG. Stable expression of a neuronal γ-aminobutyric acid transporter, GAT-3, in mammalian cells demonstrates unique pharmacological properties and ion dependence. Molecular Pharmacology. 1994;46:550–557. [PubMed] [Google Scholar]
- De Blas AL. Monoclonal antibodies to specific astroglial and neuronal antigens reveal the cytoarchitecture of the Bergmann glia fibers in the cerebellum. Journal of Neuroscience. 1984;4:265–273. doi: 10.1523/JNEUROSCI.04-01-00265.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dominguez-Perrot C, Feltz P, Poulter MO. Recombinant GABAA receptor desensitization: the role of the γ2 subunit and its physiological significance. Journal of Physiology. 1996;497:145–159. doi: 10.1113/jphysiol.1996.sp021756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong CJ, Picaud SA, Werblin FS. GABA transporters and GABAC-like receptors on catfish cone- but not rod-driven horizontal cells. Journal of Neuroscience. 1994;14:2648–2658. doi: 10.1523/JNEUROSCI.14-05-02648.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El Idrissi A, Trenkner E. Growth factors and taurine protect against excitotoxicity by stabilizing calcium homeostasis and energy metabolism. Journal of Neuroscience. 1999;19:9459–9468. doi: 10.1523/JNEUROSCI.19-21-09459.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feigenspan A, Bormann J. Differential pharmacology of GABAA and GABAC receptors on rat retinal bipolar cells. European Journal of Pharmacology. 1994;288:97–104. doi: 10.1016/0922-4106(94)90014-0. [DOI] [PubMed] [Google Scholar]
- Gragera RR, Muniz E, De Esteban G, Alonso MJ, Martinez-Rodriguez R. Immunohistochemical demonstration of taurine in the rat cerebellar cortex. Evidence for its location within mossy fibers and Golgi axons. Journal für Hirnforschung. 1995;36:269–276. [PubMed] [Google Scholar]
- Horikoshi T, Asanuma A, Yanagisawa K, Anzai K, Goto S. Taurine and β-alanine act on both GABA and glycine receptors in Xenopus oocyte injected with mouse brain messenger RNA. Brain Research. 1988;464:97–105. doi: 10.1016/0169-328x(88)90002-2. [DOI] [PubMed] [Google Scholar]
- Huxtable RJ. Taurine in the central nervous system and the mammalian actions of taurine. Progress in Neurobiology. 1989;32:471–533. doi: 10.1016/0301-0082(89)90019-1. [DOI] [PubMed] [Google Scholar]
- Huxtable RJ. Physiological actions of taurine. Physiological Reviews. 1992;72:101–163. doi: 10.1152/physrev.1992.72.1.101. [DOI] [PubMed] [Google Scholar]
- Jackson PS, Strange K. Volume-sensitive anion channels mediate swelling-activated inositol and taurine efflux. American Journal of Physiology. 1993;265:C1489–1500. doi: 10.1152/ajpcell.1993.265.6.C1489. [DOI] [PubMed] [Google Scholar]
- Jacobson I, Sandberg M, Hamberger A. Mass transfer in brain dialysis devices - a new method for the estimation of extracellular amino acid concentration. Journal of Neuroscience Methods. 1985;15:263–268. doi: 10.1016/0165-0270(85)90107-4. [DOI] [PubMed] [Google Scholar]
- Kontro P, Marnela KM, Oja SS. GABA, taurine and hypotaurine in developing mouse brain. Acta Physiologica Scandinavica. 1984;537(supplement):71–74. [PubMed] [Google Scholar]
- Kontro P, Oja SS. Interactions of taurine with GABAB binding sites in mouse brain. Neuropharmacology. 1990;29:243–247. doi: 10.1016/0028-3908(90)90008-f. [DOI] [PubMed] [Google Scholar]
- Lake N, Orlowski J. Cellular studies of the taurine transporter. Advances in Experimental Medicine and Biology. 1996;403:371–376. doi: 10.1007/978-1-4899-0182-8_39. [DOI] [PubMed] [Google Scholar]
- Larsson OM, Griffiths R, Allen IC, Schousboe A. Mutual inhibition kinetic analysis of γ-aminobutyric acid, taurine, and β-alanine high-affinity transport into neurons and astrocytes: evidence for similarity between the taurine and β-alanine carriers in both cell types. Journal of Neurochemistry. 1986;47:426–432. doi: 10.1111/j.1471-4159.1986.tb04519.x. [DOI] [PubMed] [Google Scholar]
- Lerma J, Herranz AS, Herreras O, Abraira V, Martin DR. In vivo determination of extracellular concentration of amino acids in the rat hippocampus. A method based on brain dialysis and computerized analysis. Brain Research. 1986;384:145–155. doi: 10.1016/0006-8993(86)91230-8. [DOI] [PubMed] [Google Scholar]
- Levi G, Gallo V. Release of neuroactive amino acid from glia. In: Kettenmann H, Ransom BR, editors. Neuroglia. New York: Oxford University Press; 1995. pp. 815–826. [Google Scholar]
- Li YP, Lombardini JB. Guanidinoethanesulfonic acid - inhibitor of GABA uptake in rat cortical synaptosomes. Brain Research. 1990;510:147–149. doi: 10.1016/0006-8993(90)90742-t. [DOI] [PubMed] [Google Scholar]
- Liu QR, Lopez-Corcuera B, Mandiyan S, Nelson H, Nelson N. Molecular characterization of four pharmacologically distinct γ-aminobutyric acid transporters in mouse brain. Journal of Biological Chemistry. 1993;268:2106–2112. [PubMed] [Google Scholar]
- Liu QR, Lopez-Corcuera B, Nelson H, Mandiyan S, Nelson N. Cloning and expression of a cDNA encoding the transporter of taurine and β-alanine in mouse brain. Proceedings of the National Academy of Sciences of the USA. 1992;89:12145–12149. doi: 10.1073/pnas.89.24.12145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mager S, Naeve J, Quick M, Labarca C, Davidson N, Lester HA. Steady states, charge movements, and rates for a cloned GABA transporter expressed in Xenopus oocytes. Neuron. 1993;10:177–188. doi: 10.1016/0896-6273(93)90309-f. [DOI] [PubMed] [Google Scholar]
- Magnusson KR. Distributions of taurine, glutamate, and glutamate receptors during post-natal development and plasticity in the rat brain. Advances in Experimental Medicine and Biology. 1996;403:435–444. doi: 10.1007/978-1-4899-0182-8_47. [DOI] [PubMed] [Google Scholar]
- Moran J, Maar TE, Pasantes-Morales H. Impaired cell volume regulation in taurine deficient cultured astrocytes. Neurochemical Research. 1994;19:415–420. doi: 10.1007/BF00967318. [DOI] [PubMed] [Google Scholar]
- Morara S, Brecha NC, Marcotti W, Provini L, Rosina A. Neuronal and glial localization of the GABA transporter GAT-1 in the cerebellar cortex. NeuroReport. 1996;7:2993–2996. doi: 10.1097/00001756-199611250-00039. [DOI] [PubMed] [Google Scholar]
- Muller T, Fritschy JM, Grosche J, Pratt GD, Mohler H, Kettenmann H. Developmental regulation of voltage-gated K+ channel and GABAA receptor expression in Bergmann glial cells. Journal of Neuroscience. 1994;14:2503–2514. doi: 10.1523/JNEUROSCI.14-05-02503.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller T, Moller T, Neuhaus J, Kettenmann H. Electrical coupling among Bergmann glial cells and its modulation by glutamate receptor activation. Glia. 1996;17:274–284. doi: 10.1002/(SICI)1098-1136(199608)17:4<274::AID-GLIA2>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- Nagelhus EA, Amiry-Moghaddam M, Lehmann A, Ottersen OP. Taurine as an organic osmolyte in the intact brain: immunocytochemical and biochemical studies. Advances in Experimental Medicine and Biology. 1994;359:325–334. doi: 10.1007/978-1-4899-1471-2_33. [DOI] [PubMed] [Google Scholar]
- Nagelhus EA, Lehmann A, Ottersen OP. Neuronal-glial exchange of taurine during hypo-osmotic stress: a combined immunocytochemical and biochemical analysis in rat cerebellar cortex. Neuroscience. 1993;54:615–631. doi: 10.1016/0306-4522(93)90233-6. [DOI] [PubMed] [Google Scholar]
- Ohkuma S, Katsura M, Chen DZ, Kuriyama K. Nitric oxide-evoked [3H]taurine release is mediated by reversal of the Na+-dependent carrier-mediated taurine transport system. Advances in Experimental Medicine and Biology. 1996;403:417–425. doi: 10.1007/978-1-4899-0182-8_45. [DOI] [PubMed] [Google Scholar]
- Oja SS, Korpi ER, Kontro P. Potassium-stimulated release of taurine, hypotaurine, and GABA from brain tissue in vitro. Advances in Biochemical Psychopharmacology. 1981;29:175–181. [PubMed] [Google Scholar]
- Ottersen OP. Quantitative assessment of taurine-like immunoreactivity in different cell types and processes in rat cerebellum: an electronmicroscopic study based on a post-embedding immunogold labelling procedure. Anatomy and Embryology. 1988;178:407–421. doi: 10.1007/BF00306047. [DOI] [PubMed] [Google Scholar]
- Palkovits M, Elekes I, Lang T, Patthy A. Taurine levels in discrete brain nuclei of rats. Journal of Neurochemistry. 1986;47:1333–1335. doi: 10.1111/j.1471-4159.1986.tb00761.x. [DOI] [PubMed] [Google Scholar]
- Pasantes-Morales H, Franco R, Torres-Marquez ME, Hernandez-Fonseca K, Ortega A. Amino acid osmolytes in regulatory volume decrease and isovolumetric regulation in brain cells: contribution and mechanisms. Cellular Physiology and Biochemistry. 2000;10:361–370. doi: 10.1159/000016369. [DOI] [PubMed] [Google Scholar]
- Pasantes-Morales H, Moran J, Schousboe A. Volume-sensitive release of taurine from cultured astrocytes: properties and mechanism. Glia. 1990;3:427–432. doi: 10.1002/glia.440030514. [DOI] [PubMed] [Google Scholar]
- Petegnief V, Lleu PL, Gupta RC, Bourguignon JJ, Rebel G. Taurine analog modulation of taurine uptake by two different mechanisms in cultured glial cells. Biochemical Pharmacology. 1995;49:399–410. doi: 10.1016/0006-2952(94)00390-8. [DOI] [PubMed] [Google Scholar]
- Porter JT, McCarthy KD. Astrocytic neuro-transmitter receptors in situ and in vivo. Progress in Neurobiology. 1997;51:439–455. doi: 10.1016/s0301-0082(96)00068-8. [DOI] [PubMed] [Google Scholar]
- Puopolo M, Kratskin I, Belluzzi O. Direct inhibitory effect of taurine on relay neurones of the rat olfactory bulb in vitro. NeuroReport. 1998;9:2319–2323. doi: 10.1097/00001756-199807130-00031. [DOI] [PubMed] [Google Scholar]
- Reichenbach A, Siegel A, Rickmann M, Wolff JR, Noone D, Robinson SR. Distribution of Bergmann glial somata and processes: implications for function. Journal für Hirnforschung. 1995;36:509–517. [PubMed] [Google Scholar]
- Rose CR, Ransom BR. Intracellular sodium homeostasis in rat hippocampal astrocytes. Journal of Physiology. 1996;491:291–305. doi: 10.1113/jphysiol.1996.sp021216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roux MJ, Supplisson S. Neuronal and glial glycine transporters have different stoichiometries. Neuron. 2000;25:373–383. doi: 10.1016/s0896-6273(00)80901-0. [DOI] [PubMed] [Google Scholar]
- Sanchez-Olea R, Pena C, Moran J, Pasantes-Morales H. Inhibition of volume regulation and efflux of osmoregulatory amino acids by blockers of Cl− transport in cultured astrocytes. Neuroscience Letters. 1993;156:141–144. doi: 10.1016/0304-3940(93)90458-w. [DOI] [PubMed] [Google Scholar]
- Saransaari P, Oja SS. Mechanisms of ischemia-induced taurine release in mouse hippocampal slices. Brain Research. 1998a;807:118–124. doi: 10.1016/s0006-8993(98)00793-8. [DOI] [PubMed] [Google Scholar]
- Saransaari P, Oja SS. Release of endogenous glutamate, aspartate, GABA, and taurine from hippocampal slices from adult and developing mice under cell-damaging conditions. Neurochemical Research. 1998b;23:563–570. doi: 10.1023/a:1022494921018. [DOI] [PubMed] [Google Scholar]
- Saransaari P, Oja SS. Characteristics of ischemia-induced taurine release in the developing mouse hippocampus. Neuroscience. 1999;94:949–954. doi: 10.1016/s0306-4522(99)00384-x. [DOI] [PubMed] [Google Scholar]
- Saransaari P, Oja SS. Taurine and neural cell damage. Amino Acids. 2000a;19:509–526. doi: 10.1007/s007260070003. [DOI] [PubMed] [Google Scholar]
- Saransaari P, Oja SS. Taurine release modified by GABAergic agents in hippocampal slices from adult and developing mice. Amino Acids. 2000b;18:17–30. doi: 10.1007/s007260050002. [DOI] [PubMed] [Google Scholar]
- Schousboe A, Westergaard N. Transport of neuroactive amino acids in astrocytes. In: Kettenmann H, Ransom BR, editors. Neuroglia. New York: Oxford University; 1995. pp. 246–258. [Google Scholar]
- Schwartz EA. Depolarization without calcium can release γ-aminobutyric acid from a retinal neuron. Science. 1987;238:350–355. doi: 10.1126/science.2443977. [DOI] [PubMed] [Google Scholar]
- Smith KE, Borden LA, Wang CH, Hartig PR, Branchek TA, Weinshank RL. Cloning and expression of a high affinity taurine transporter from rat brain. Molecular Pharmacology. 1992;42:563–569. [PubMed] [Google Scholar]
- Swan M, Najlerahim A, Watson RE, Bennett JP. Distribution of mRNA for the GABA transporter GAT-1 in the rat brain: evidence that GABA uptake is not limited to presynaptic neurons. Journal of Anatomy. 1994;185:315–323. [PMC free article] [PubMed] [Google Scholar]
- Takahashi M, Sarantis M, Attwell D. Postsynaptic glutamate uptake in rat cerebellar Purkinje cells. Journal of Physiology. 1996;497:523–530. doi: 10.1113/jphysiol.1996.sp021785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang JM, Wang J, Quandt FN, Eisenberg RS. Perfusing pipettes. Pflügers Archiv. 1990;416:347–350. doi: 10.1007/BF00392072. [DOI] [PubMed] [Google Scholar]
- Tappaz M, Almarghini K, Do K. Cysteine sulfinate decarboxylase in brain: identification, characterization and immunocytochemical location in astrocytes. Advances in Experimental Medicine and Biology. 1994;359:257–268. doi: 10.1007/978-1-4899-1471-2_26. [DOI] [PubMed] [Google Scholar]
- Tchoumkeu-Nzouessa GC, Rebel G. Regulation of taurine transport in rat astrocytes by protein kinase C: role of calcium and calmodulin. American Journal of Physiology. 1996;270:C1022–1028. doi: 10.1152/ajpcell.1996.270.4.C1022. [DOI] [PubMed] [Google Scholar]
- Terauchi A, Nakazaw A, Johkura K, Yan L, Usuda N. Immunohistochemical localization of taurine in various tissues of the mouse. Amino Acids. 1998;15:151–160. doi: 10.1007/BF01345288. [DOI] [PubMed] [Google Scholar]
- Trenkner E, El Idrissi A, Harris C. Balanced interaction of growth factors and taurine regulate energy metabolism, neuronal survival, and function of cultured mouse cerebellar cells under depolarizing conditions. Advances in Experimental Medicine and Biology. 1996;403:507–517. doi: 10.1007/978-1-4899-0182-8_55. [DOI] [PubMed] [Google Scholar]
- Vinnakota S, Qian X, Egal H, Sarthy V, Sarkar HK. Molecular characterization and in situ localization of a mouse retinal taurine transporter. Journal of Neurochemistry. 1997;69:2238–2250. doi: 10.1046/j.1471-4159.1997.69062238.x. [DOI] [PubMed] [Google Scholar]


